Abstract
We report the largest retrospective, phase IV non-interventional, observational study of ofatumumab therapy in heavily pre-treated patients with poor-prognosis chronic lymphocytic leukemia. Total number of patients was 103; median age was 65 years (range 39–85). Median number of prior lines of therapy was 4 (range 1–13), including, in most cases, rituximab-, fludarabine- and alemtuzumab-based regimens; 13 patients had been allografted. Of 113 adverse events, 28 (29%) were considered to be directly related to ofatumumab. Grade 3–4 toxicities included neutropenia (10%), thrombocytopenia (5%), anemia (3%), pneumonia (17%), and fever (3%). Two heavily pre-treated patients developed progressive multifocal leukoencephalopathy. On an intention-to-treat analysis, the overall response rate was 22% (3 complete response, 1 incomplete complete response). Median progression-free and overall survival times were 5 and 11 months, respectively. This study confirms in a daily-life setting the feasibility and acceptable toxicity of ofatumumab treatment in advanced chronic lymphocytic leukemia. The complete response rate, however, was low. Therefore, treatment with ofatumumab should be moved to earlier phases of the disease. Ideally, this should be done in combination with other agents, as recently approved for ofatumumab plus chlorambucil as front-line treatment for patients unfit for fludarabine. This study is registered at clinicaltrials.gov identifier:01453062.
Introduction
Treatment of chronic lymphocytic leukemia (CLL) has dramatically improved. In 2010, the combination of fludarabine, cyclophosphamide and rituximab (FCR), developed by the MD Anderson group,1 was shown in a randomized trial conducted by the German CLL Study Group to result in a higher response rate, a longer progression-free-survival (PFS), and better overall survival (OS) than fludarabine plus cyclophosphamide in untreated, fit patients.2 These results were corroborated by observational studies.3–5 As a result, chemoimmunotherapy (CIT), particularly FCR, is considered the treatment of choice for CLL. However, only 40%–60% of patients obtain complete remission (CR), and most patients eventually relapse. Furthermore, FCR produces considerable hematologic toxicity, particularly among patients over 70 years of age. This has generated an interest in strategies to improve both the efficacy and tolerability of CIT, including the development of new anti-CD20 monoclonal antibodies.
Ofatumumab (HuMax-CD20; Arzerra [GSK/Genmab]) is a fully human IgG1 2nd-generation antiCD20 MAb with a molecular weight of 150 KDa. It was produced by immunizing HCo7 and KM mice with a murine cell line transfected with human heavy and light chain genes. Ofatumumab binds to a different epitope on the CD20 molecule than rituximab, which is located on the smaller extracellular loop of CD20 and then releases itself very slowly from the target (reduced off-rates). As compared to rituximab, ofatumumab in vitro achieves higher surface density, efficiently activates in vitro complement-dependent cytotoxicity (CDC), and shows improved antibody-dependent cell-mediated phagocytosis (ADCC) even when the CD20 expression is low.6–9
The results of a pivotal study10 led to the approval of ofatumumab in October 2009 by the US Food and Drug Administration (FDA) for the treatment of CLL refractory to both fludarabine and alemtuzumab, and likewise, in April 2010, the European Medicines Agency (EMA) issued a conditional marketing authorization for the same indication although this required further clinical data in a daily-life setting.11,12 In this context, the European Research Initiative on CLL (ERIC) conducted a non-interventional, observational phase IV study to determine the safety and efficacy of ofatumumab in patients with pre-treated and poor prognosis CLL in daily practice, outside clinical trials.
Methods
Diagnosis, patients and study design
The diagnosis of CLL was made according to WHO/IWCLL criteria.13,14 Data from patients treated with ofatumumab outside phase II or III ofatumumab-based trials were included in the study. The presence of bulky lymphadenopathy, fludarabine- and alemtuzumab-refractoriness was registered as recorded by participating investigators. Severity of adverse events (AEs) was graded according to the NCI Common Terminology Criteria for Adverse Events (v.3.0). One hundred and three patients from 25 centers in Europe were accrued. The median number of patients treated in each center was 3 (range 1–15). Data collection started on 30 September 2011 and was completed on 24 November 2012. Twenty-seven patients had been previously reported.15 All patients were evaluated on an intention-to-treat basis, independently of the number of cycles of therapy received and whether or not all planned therapy was given. Response to therapy was evaluated according to the IWCLL recommendations,14 changes occurring in clinical stage16 and modifications in individual parameters. Computed tomography (CT) scans were not used to assess disease status.
Study end points
The main study end points were safety and effectiveness. Progression-free-survival (PFS) was defined as the time from ofatumumab initiation to disease progression or death due to any cause. Overall survival (OS) was defined as the time from ofatumumab initiation to death due to any cause or to last date of contact. PFS and OS were analyzed according to the Kaplan-Meier method. Multivariate analyses were performed using Cox proportional hazard method. Correlation with clinical parameters and adverse events were summarized using descriptive statistics. P<0.05 was considered statistically significant.
All parameters were registered by participating investigators in an ad hoc electronic form and centrally reviewed for consistency. The study was approved by the ethical committees of participating institutions and was in agreement with the recommendations of the Declaration of Helsinki.
Results
Demographics, prior therapy, and patients’ characteristics at ofatumumab treatment
Details of patients’ main clinical characteristics at start of ofatumumab treatment are shown in Table 1. Median age was 65 years (range 39–85) and 69% were males. Median time from diagnosis to ofatumumab treatment was 92 months (range 8–297). Most patients presented with advanced, symptomatic disease. The best response rate ever achieved before ofatumumab therapy was CR in 24 (23%) patients, incomplete CR in 4 (4%), and partial response (PR) in 55 (53%).
Table 1.
Demographics, prior treatment, and patients’ characteristics at ofatumumab treatment.

Treatment dose and schedule
In most cases, ofatumumab was given according to the approved prescribing information, i.e. 300 mg ofatumumab for the first infusion and 2000 mg thereafter for 8 consecutive weekly infusions, followed 4–5 weeks later by 4 monthly infusions every four weeks. Ofatumumab pre-medication was given according to procedures of each participating center. Median number of cycles of ofatumumab administered was 9 (range 1–16). Thirteen patients (13%) received other cytotoxic agents (mainly corticosteroids or chlorambucil) along with ofatumumab, 64% received prophylactic antibiotics, 62% antivirals, 17% granulocyte colony-stimulating factor (G-CSF), and 6% erythropoietin.
Toxicity and adverse events
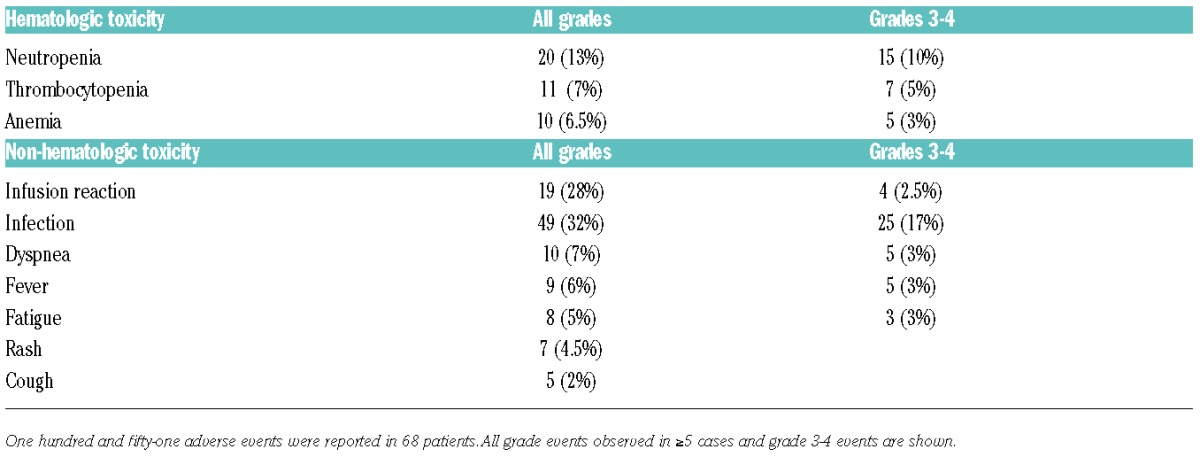
One hundred and fifty-one adverse events were reported in 68 patients, ranging from 1 to 12 events per patient. Infusion-related events occurred in 19 subjects (28%), and were grade 3–4 in 4 patients. No infusion-related deaths or tumor lysis syndrome were reported. Main observed toxicities and adverse events reported at least on five occasions are shown in Table 2. The relationship between ofatumumab and toxicity/adverse events was considered to be: 28 (19%) definitely related, 63 (42%) possibly related, 22 (15%) probably related, 22 (15%) 36 (23%) unlikely, 2 (1%) unknown. Treatment was delayed or modified because of toxicity in 30 (29%) patients, 16 (15.5%) received G-CSF, 7 (6%) erythropoietin, 28 (27%) were admitted to hospital, and 42 (41%) received antibiotics. Moreover, 2 patients had progressive multifocal leukoencephalopathy (PML) diagnosed at six and 11 months after starting therapy, respectively; these 2 patients had received extensive prior therapy including fludarabine, rituximab and alemtuzumab. Ten (10%) patients were reported to develop autoimmune hemolytic anemia, 4 (4%) Richter syndrome, and 3 (3%) a second neoplasia. Before starting ofatumumab, 4 of 65 and 2 of 66 patients investigated were known to be HBV and HCV positive, respectively. No case of viral reactivation upon treatment was reported. Toxicity was registered as the cause for discontinuing therapy in 5 (6%) patients.
Table 2.
Toxicity and adverse events.
Response to treatment
In 41 patients (40%), treatment could be given as initially planned. In 81 cases, treatment was discontinued because of disease progression (36%), toxicity (6%), patient decision (2%), or other reasons (16%).
On an intention-to-treat analysis, the overall response rate (ORR) was 22%. Three patients (3%) achieved CR, one (1%) incomplete CR, and 19 (18%) PR (Tables 3 and 4). CR response rates and Binet clinical stages pre- and post-ofatumumab therapy are shown in Tables 3 and 4. Twenty-seven of 101 evaluable patients (27%) entered CR (n=4) or were down-staged as a result of therapy. No patient with adverse cytogenetic features [del(17p), del(11q)] achieved a durable response. Due to the large proportion of patients having received prior rituximab (81%), it was not feasible to make a meaningful analysis of response depending on this variable.
Table 3.
Response to treatment.

Table 4.
Response to treatment by changes in Binet stage.

Progression-free and overall survival
Median follow-up times from diagnosis and from ofatumumab administration were of 104 and 9.4 months, respectively. At the time of closing the study, 67 patients (65%) had died. Main causes of death were disease progression (61%), infection (28%), CLL unrelated (1%), and unknown (9%). Projected PFS and OS at 12 months were 18% (95%CI: 9–29) and 47% (95%CI: 37–57), respectively. Median PFS and OS were of 5 (95%CI: 3–6) and 11 (95%CI: 9–13) months, respectively. Although the study was not powered to detect differences in patients’ subgroups, in exploratory multivariate analysis including age, sex, clinical stage, response to therapy, number of prior lines of therapy (≥4; ≥3), fludarabine- and alemtuzumab-refractoriness, the only variables associated with a shorter OS were clinical stage (advanced vs. early) (HR: 1.50; P<0.05), no response to therapy (HR: 1.45; P<0.05) and prior refractoriness to fludarabine (HR: 1.73 (P=0.018). Cytogenetics was not investigated because of the large number of missing data. Patients responding to ofatumumab had a longer OS than those who failed to therapy (median 24 vs. 10 months; P=0.007). At last contact, 29 (41%) patients categorized as not refractory to fludarabine nor alemtuzumab were alive as compared to 3 (16%) and 5 (23%) of those refractory to fludarabine or alemtuzumab.
Discussion
The main objectives of this study were to determine feasibility, safety and also effectiveness of ofatumumab in patients with unfavorable CLL treated outside clinical trials. This was a requirement of the EMA in its provisional approval in 2010 of ofatumumab therapy for fludarabine-and alemtuzumab-refractory CLL (EMA/CHMP/195135/2010) based on phase I and II studies. The results presented here need, therefore, to be compared with those obtained in these studies.
In spite of all necessary caveats when comparing different series of patients, the characteristics of patients included in our study were comparable, if not worse, than those in a phase I/II trial reported by Coiffier et al.17 and in the pivotal phase II trial by Wierda et al.10,18 The vast majority of our patients presented with advanced, symptomatic stage, and adverse clinical and biological features. Also, the time between last therapy and ofatumumab administration was very short, indicating progressive disease. As anticipated, patients had been extensively pre-treated, prior therapy mainly consisting of fludarabine and alemtuzumab, and also rituximab-based regimens; moreover, 13 patients had been allografted. Therefore, this series can be considered as representative of patients with very poor prognosis CLL.
Regarding safety, Coiffier et al.17 investigated the toxicity of ofatumumab in 33 patients with relapsed or refractory CLL. In 27 patients, 246 adverse events were registered, of which only 7% were reported as grade 3 or 4. Altogether 61% of adverse events were considered to be related to treatment, the majority of which were registered on the day of infusion. Infections were observed in 51% of the patients. In turn, in the study reported by Wierda et al.10,18 including 206 patients, the most common adverse events were first infusion-related reactions with an incidence of around 40%, decreasing with subsequent infusions; the most common grade 3–4 adverse events were infections (24%), neutropenia (12%), anemia (5%) and thrombocytopenia (4%). Infections were frequent and often severe. In total, 6 of 59 FA-ref and 5 of 79 BF-ref patients died from infection, including one patient with promyelocytic leumekia (PML). As in all retrospective studies, the appraisal of toxicity in our study could have been hampered by under-reporting and differences in clinical interventions and interpretation of events among participating centers. As an example, the assessment of ofatumumab-infusion related events was blurred by the variability in the medications accompanying ofatumumab administration (e.g. corticosteroids, acetaminophen). Nevertheless, some form of infusion reaction was reported in 19 subjects (28%) but this was of grade 3 or 4 in only 4 patients. However, there were no cases of infusion-related deaths or tumor lysis syndrome. Importantly, only 6% of patients had to discontinue treatment because of toxicity directly related to treatment. However, in 30 cases (29%), treatment had to be delayed because of toxicity. From the hematologic standpoint, main toxicities included infusion-related events, neutropenia, thrombocytopenia. Main non-hematologic toxicities were anemia and infection, dyspnea, fever, fatigue, rash, and cough. Also, 2 heavily pre-treated patients developed PML, a complication known to occur in CLL and well recognized in subjects receiving anti-CD20 monoclonal antibodies.19 Recently, concern has been raised about the possibility that ofatumumab could trigger hepatitis B virus (HBV) reactivation. In our series, no case of viral reactivation upon treatment was reported in 4 patients known to be anti-HBs positive.
As far as effectiveness is concerned, Coiffier et al.17 reported an ORR of 44% at 19 weeks after therapy. Among patients previously treated with rituximab (7 of 33), alemtuzumab (6 of 33) and or fludarabine (20 of 33), 7 responded to treatment. Importantly, however, only 9 patients (27%) had sustained responses. Median PFS was 106 days. In turn, Wierda et al. reported a phase II study including 206 patients refractory to both fludarabine and alemtuzumab (FA-ref) or refractory to fludarabine with bulky lymphadenopathy (BF-ref). According to a planned interim analysis including 138 patients, the ORR was 58% and 47% in the FA-ref and BF-ref groups, respectively. Median PFS and OS were six and 14 months in the FA-ref group, and six and 15 months in the BF-ref group, respectively. One complete response (CR) was observed in the BF-group, all other responses being partial.10 In contrast to the Coiffier et al. study, 17 cytogenetic information was available, with del(17p) being the only factor associated with a lower response rate in the BF-ref group (ORR: 14% vs. 55%; P<0.05). The final analysis of 206 patients demonstrated an ORR of 51% for the FA-ref group and 44% for the BF-ref group, independently of prior rituximab administration.10,18 In our series, the large proportion of patients previously treated with rituximab (81%) precluded a meaningful analysis of the response based on this variable. Notably, the ORR was inferior to that previously reported in phase I and phase II trials10,16,18 (22% vs. approx. 50%), most likely due to differences in the time points at which response was assessed, the intention-to-treat analysis, and patients’ characteristics. Importantly, 27 of 101 evaluable patients (27%) entered CR (n=4) or were down-staged as a result of therapy, Of particular interest is that 21 of 64 patients (33%) in Binet stage C before ofatumumab shifted to Binet A or B stage after treatment, mainly due to the recovery of hemoglobin (Hb) levels and platelet counts (data not shown). Although criteria for treatment-refractoriness were not as robust (investigators’ assessment) as in phase I-II trials, patients considered refractory to fludarabine and/or alemtuzumab showed poorer treatment results. Concerning PFS, our results were better than those reported by Coiffier et al.17 (5 months vs. 106 days) and similar to those reported by Wierda et al. (6 months).10 Information on median overall survival was only available in the Wierda et al. study (14 months), this being slightly longer than in our series (11 months).
In conclusion, the present report includes the largest series of patients with poor-prognosis CLL treated with ofatumumab on a routine basis, outside trials. From this study it can be concluded that results regarding feasibility, toxicity, and effectiveness of ofatumumab in daily life are comparable to those obtained in pioneer phase I and phase II trials.10,16,18 Unfortunately, the response rate and outcome in all these studies, including ours, has been poor. This observation has been confirmed in a randomized phase III trial comparing ibrutinib versus ofatumumab (ORR: 4%; median PFS: 8 months in the ofatumumab arm).20 Ofatumumab, therefore, joins other anti-CD20 monoclonal antibodies such as rituximab and obinutuzumab in the armatorium for CLL treatment. Looking forward to the future, several studies have, not surprisingly, shown much better results in untreated patients and when ofatumumab has been given in combination with other agents,21–25 fulfilling the paradigms that combination therapy is better than single agents and that effective treatments should be given before the disease becomes refractory to therapy. It is important, therefore, to extend treatment with ofatumumab, particularly in combination with other agents, to earlier phases of CLL. The recent approval by the FDA and EMA of the combination ofatumumab plus chlorambucil as front-line therapy for patients with CLL unfit for fludarabine25 is an important step in that direction. In line with this, the availability of signal transduction inhibitors, such as the BTK inhibitor ibrutinib and the PI3κδ inhibitor idelasilib, and also BCL2 antagonists (ABT199), will make it possible to study the effectiveness of ofatumumab given not only alongside standard cytotoxic agents but also with new non-cytotoxic compounds.
Acknowledgments
Thanks are due to all participating investigators and institutions, as well as to patients and their relatives. Carmen Torres, Martin Fox, and Dan Wylde provided most valuable secretarial support. Thanks are also due to Bradley Healthcare Associates and to Dimitri Bennett (GSK, USA) and his team for their support in the logistics of the study. European Research Initiative on CLL (ERIC) activities are supported by unrestricted grants from Gilead (Spain), ViviaBiotech (Spain), Celgene (USA), and Pharmacyclics (USA). ERIC is a European Hematology Association (EHA), and European Leukemia Network (ELN) working group. ERIC is the sole responsible for this study, including data collection, analysis, and interpretation.
Footnotes
Funding
This study was funded by Glaxo-Smith-Kline (GSK, USA) to meet an EMA requirement for post-authorization real world data (ClinicalTrials.gov NCT01453062).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. [DOI] [PubMed] [Google Scholar]
- 3.Kristinsson SY, Dickman PW, Wilson WH, Caporaso N, Björkholm M, Landgren O. Improved survival in chronic lymphocytic leukemia in the past decade: a population-based study including 11,179 patients diagnosed between 1973–2003 in Sweden. Haematologica. 2009;94(9):1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danese MD, Griffiths RI, Gleeson M, et al. An observational study of outcomes after initial infused therapy in Medicare patients diagnosed with chronic lymphocytic leukemia. Blood. 2011;117(13):3505–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado J, Ghita G, Bumann T, et al. Rituximab-based chemoimmunotherapy prolongs survival of patients with chronic lymphocytic leukemia independently of the time of administration. Clin Lymphoma Myeloma Leuk. 2014;14(1):73–79. [DOI] [PubMed] [Google Scholar]
- 6.Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104(6):1793–1800. [DOI] [PubMed] [Google Scholar]
- 7.Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177(1):362–371. [DOI] [PubMed] [Google Scholar]
- 8.Beum PV, Lindorfer MA, Beurskens F, et al. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol. 2008;181(1):822–832. [DOI] [PubMed] [Google Scholar]
- 9.Bologna L, Gotti E, Da Roit F, et al. Ofatumumab is more effective than rituximab in B chronic lymphocytic leukemia cells in whole blood and in combination with chemotherapy. J Immunol. 2013;190(1):231–239. [DOI] [PubMed] [Google Scholar]
- 10.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as a single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leucemia. J Clin Oncol. 2010;28(10):1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemery SJ, Zhang J, Rothmann MD, et al. U.S. Food and Drug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab. Clin Cancer Res. 2010;16(17):4331–4338. [DOI] [PubMed] [Google Scholar]
- 12.Gravanis I, Ersboll J, Skovlund E, Abadie E, Marty M, Pignatti F. The European Medicines Agency review of ofatumumab (Arzerra®) for the treatment of chronic lymphocytic leukemia in patients refractory to fludarabine and alemtuzumab: summary of the scientific assessment of the European medicines agency committee for medicinal products for human use. Oncologist. 2010;15(12):1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller-Hermelink HK, Montserrat E, Catovsky D, Campo E, Harris NL, Stein H. Chronic lymphocytic leukaemia/small lymphocytic lymphoma. In “WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues”, IARC, Lyon: 2008, pp. 180–182. [Google Scholar]
- 14.Hallek M, Cheson BD, Catovsky D, et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(9):4446–4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhury O, Varghese A, Pattison J, et al. Ofatumumab in advanced stage chronic lymphocytic leukaemia: results of the UK named patient compassionate use programme. Br J Haematol. 2011;155(4):509–533. [DOI] [PubMed] [Google Scholar]
- 16.International Workshop on Chronic Lymphocytic Leukemia. Chronic lymphocytic leukemia: recommendations for diagnosis, staging, and response criteria. Ann Intern Med. 1989;110(3):236–238. [DOI] [PubMed] [Google Scholar]
- 17.Coiffier B, Lepretre S, Pedersen LM, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 study. Blood. 2008;111(3):1094–1100. [DOI] [PubMed] [Google Scholar]
- 18.Wierda WG, Padmanabhan S, Chan GW, et al. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood. 2011;118(19):5126–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113(20):4834–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrd JC, Brown JR, O’Bien S, et al. Ibrutinib vs. ofatumumab in previously treated chronic lymphocytic leukemia. N Engl J Med. 2014;371(3):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wierda WG, Kipps TJ, Dürig J, et al. Chemoimmunotherapy with ofatumumab, fludarabine, and cyclophosphamide (O-FC) in previously untreated patients with chronic lymphocytic leukemia. Blood. 2011;117(24):6450–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Offner F, Panagiotidis P, Afanasyev B, et al. Ofatumumab and bendamustine combination therapy in patients with untreated and relapsed chronic lymphocytic leukemia: initial results of the phase II study OMB115991. XV iwCLL Abstracts 2013 4.29 [Google Scholar]
- 23.Shanafelt T, Lanasa MC, Call TG, et al. Ofatumumab-based chemoimmunotherapy is effective and well tolerated in patients with previously untreated chronic lymphocytic leukemia. Cancer. 2013;119(21):3788–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ujjani C, Ramzi P, Gehan E, Wang H, Wang Y, Cheson BD. Ofatumumab and bendamustine in previously treated chronic lymphocytic leukemia and small lymphocytic lymphoma. Leuk Lymphoma. 2014;6:1–6 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Hillmen P, Robak T, Janssens A, et al. Ofatumumab plus chlorambucil versus chlorambucil alone in patients with untreated chronic lymphocytic leukemia: results of the phase III study Complement 1 (OMB110911). Blood. 2013;122(21) Abstract 528. [Google Scholar]


