Abstract
The Nordic Lymphoma Group has conducted a phase ll trial in newly diagnosed primary central nervous system lymphoma patients applying an age-adjusted multi-agent immunochemotherapy regimen, which in elderly patients included temozolomide maintenance treatment. Patients aged 18–75 years were eligible. Thirty-nine patients aged 18–65 years and 27 patients aged 66–75 years were enrolled. The median age of the two age groups was 55 and 70 years, respectively. The overall response rate was 73.8% for the entire cohort: 69.9% in the younger and 80.8% in the elderly subgroup. With a median follow up of 22 months, the 2-year overall survival probability was 60.7% in patients aged 65 years or under and 55.6% in patients aged over 65 years (P=0.40). The estimated progression-free survival at two years was 33.1% (95%CI: 19.1%–47.9%) in patients aged under 65 years and 44.4% (95%CI: 25.6%–61.8%) in the elderly subgroup (P=0.74). Median duration of response was ten months in the younger subgroup, and not reached in the elderly patient subgroup (P=0.33). Four patients aged 64–75 years (6%) died from treatment-related complications. Survival in the two age groups was similar despite a de-escalation of induction treatment in patients aged over 65 years. Duration of response in elderly patients receiving maintenance temozolomide was longer than in the younger age subgroup. While toxicity during induction is still of concern, especially in the elderly patients, we conclude from these data that de-escalation of induction therapy in elderly primary central nervous system lymphoma patients followed by maintenance treatment seems to be a promising treatment strategy. (clinicaltrials.gov identifier:01458730)
Introduction
Primary central nervous system lymphoma (PCNSL) is a separate group of non-Hodgkin lymphoma confined to the central nervous system (CNS). Incidence has increased during the last decades, particularly in elderly patients.1,2 Median age at diagnosis has increased from 60.5 to 65 years during the last 20 years.3 In 2002, the Nordic Lymphoma Group published a phase ll trial investigating whether an age-adjusted regimen consisting of chemotherapy alone could induce durable remissions in PCNSL patients.4 The responses achieved were of short duration. Due to profound toxicity, the study was closed after the enrolment of only 30 patients. Although treatment results in PCNSL patients have improved during recent years, the prognosis still remains poor in the majority of patients, especially in the elderly. The first prospective phase ll trial designed with special attention to elderly patients reported a median overall survival (OS) of 14.3 months obtained by a chemotherapy-alone regimen (high-dose methotrexate (HD-MTX), lomustine, procarbazine and dexamethasone).5 HD-MTX-based chemotherapy combined with whole brain radiotherapy produced a median survival time of 50 months in patients aged under 60 years and 21.8 months in patients aged over 60 years.6 Promising results were obtained in younger patients by an HD-MTX and HD-AraC-based multi-agent regimen including intraventricular treatment and omitting radiotherapy (hereafter referred to as the Bonn protocol) yielding an OS at five years of 75% in patients younger than 61 years as compared to only 19% in patients older than 60 years.7
The aim of the present study was to investigate the efficacy and safety of a systemic HD-MTX/HD-AraC-based multi-agent immunochemotherapy regimen with cerebrospinal fluid (CSF) targeted treatment but without radiotherapy in newly diagnosed PCNSL patients. The goal of our study was to find a treatment concept with improved outcome and/or less toxicity than those reported hitherto for this patient group. In the extension of the Bonn study,7 we added the following modifications. 1) Rituximab was added to the induction as the majority of PCNSL are CD20+ diffuse large B-cell lymphomas (DLBCL) and it was previously shown that the addition of rituximab to CHOP chemotherapy has improved the prognosis in DLBCL outside the brain.8 2) The intraventricular MTX/cytarabine treatment of the Bonn protocol requiring an Ommaya reservoir was replaced by intraspinal administration of liposomal cytarabine in view of the expected lower risk of procedure-related infections. 3) The infusion time of MTX was reduced from 24 h to 3 h as a higher drug penetration has been achieved by a shorter infusion time.9 4) Importantly, given the cited literature, the treatment was age-adjusted with patients aged over 65 years having cyclophosphamide of the second and fifth and ifosfamide of the fourth chemotherapy cycle replaced by the less toxic agent temozolomide (TZM). Vincristine was also not part of the study treatment for the elderly patients. 5) To improve disease control in responding patients aged over 65 years in whom induction intensity was reduced, maintenance treatment with TZM was added. This was done because TZM, an oral alkylating agent, can penetrate the intact blood brain barrier,10 and because it has shown activity and a favorable toxicity profile in PCNSL patients.11
Methods
Study design
This was a prospective multicenter phase ll trial.
Eligibility
Eligibility criteria were: immunocompetent patients aged 18–75 years with newly diagnosed histologically-confirmed PCNSL. Patients pre-treated with steroids were eligible. There were no limitations for inclusion with regard to Eastern Co-operative Oncology Group (ECOG) performance score. Exclusion criteria were: lymphoma outside the CNS, HIV positivity, inadequate bone marrow function, liver disease, creatinine clearance less than 60 mL/min, organ transplantation, prior radiotherapy to the brain, previous malignancy unless disease free for at least five years, pregnancy or lactation. The study was approved by the local ethical committees and conducted in agreement with the Declaration of Helsinki and Good Clinical Practice Guidelines. Written informed consent from all patients or guardians was required.
Base-line evaluation
Pre-treatment evaluation, performed according to international guidelines,12 included magnetic resonance imaging (MRI) of the brain performed before and after stereotactic biopsy/operation. Ocular involvement was ruled out by slit lamp examination and systemic involvement by total body computed tomography, bone marrow aspirate and biopsy. Ultrasonic scan of testes was performed in male patients aged over 60 years. Cerebrospinal fluid (CSF) was analyzed for protein, cell count, cytology and immunophenotype.
Study treatment
Patients aged 18–65 years and patients aged 66–75 years received immunochemotherapy as shown in Online Supplementary Tables S1 and S2, respectively. Drug infusion time was 3 h for HD-MTX and HD-AraC. Maintenance treatment with TZM 150 mg/m2 days 1–5 at an interval of 28 days was administered to elderly patients who responded to induction therapy. Maintenance treatment was started one month after completion of induction and continued for one year or until relapse/progression. Additional treatment in case of ocular lymphoma was not part of our protocol.
Response assessment
Response was assessed after cycles 2, 4 and 6 and evaluated according to international response criteria.12 Patients not responding to therapy and patients with later disease progression were taken off study. In case of stable disease (SD) at the first response assessment (after two HD-MTX cycles) the study treatment was continued as planned. Patients not achieving complete response (CR) at completion of planned therapy went off study and were followed for survival only. During follow up, MRI of the brain was performed every three months the first year, twice a year for four years, and once a year thereafter. Toxicity was graded according to Common Toxicity Criteria (CTC, version 2.0). Neurotoxicity was evaluated by means of Mini Mental State Examination (MMSE) and Functional Independent Measure (FIM) at baseline and at each response assessment.
Statistical analysis
All patients enrolled (n=66) were included in the final analyses. The primary end point was OS and the secondary end points were: response rate (RR), progression-free survival (PFS), systemic toxicity, and neurotoxicity. OS was calculated from the date of diagnosis to death or latest follow up. PFS was calculated from the date of diagnosis to the date of progression, death of any cause or latest date of follow up. Duration of response (DOR) was calculated from the date of first documentation of CR to the date of progression, death or latest follow up.
Results
Patients’ characteristics
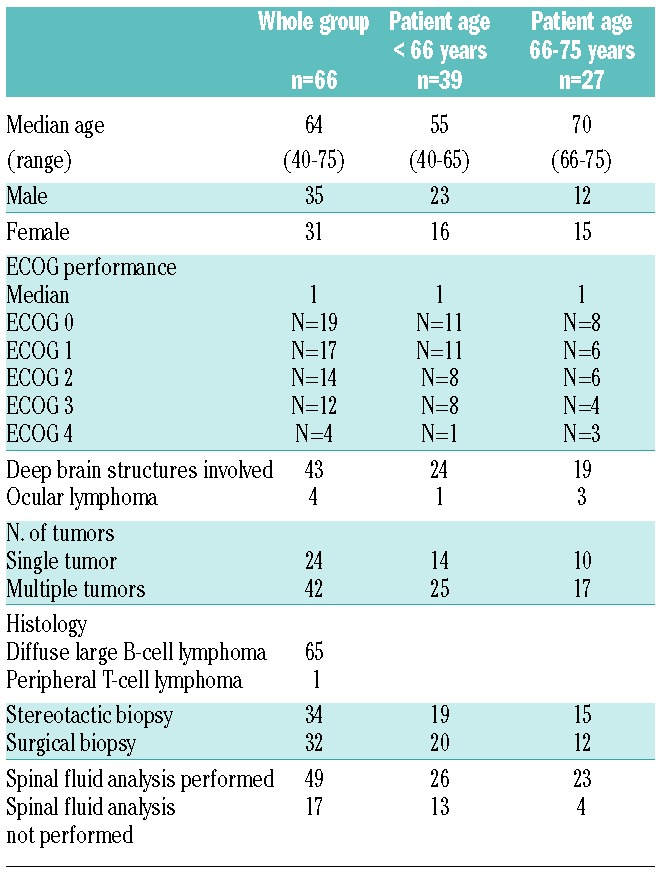
Sixty-six patients (35 male and 31 female patients) from twelve centers in Sweden, Norway, Denmark and Finland were enrolled from May 2007 to October 2010. Patients’ characteristics are summarized in Table 1. Median age was 64 years for the entire patient cohort (range 40–75 years), 55 years in 39 younger patients (range 40–65 years), and 70 years in 27 elderly patients (range 66–75 years). Median ECOG performance status was 1 (range 0–4). Histology was DLBCL in all patients but one who had a peripheral T-cell lymphoma. Ocular lymphoma was found in 4 cases. None of the male patients aged 60 years or older was diagnosed with testicular lymphoma. According to the Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic model,13 9 patients were class 1 (13.6%), 31 patients class 2 (47.0%), and 26 patients class 3 (39.4%).
Table 1.
Patients’ characteristics.
Cerebrospinal fluid findings
Cerebrospinal fluid (CSF) analysis was performed at diagnosis in 49 patients and not performed in 17 patients. The cell number was normal in 22 cases, increased in 18, and not evaluated in 9 cases. Cytology was positive for lymphoma involvement in 8 patients, negative in 36, and not examined in 5 patients. Flow cytometry was positive in only 2 of 30 cases examined. CSF protein was elevated in 24 cases, normal in 15 cases, and not examined in 10 cases.
Treatment
All patients started treatment with a median time of 10 days (0–42 days) from diagnosis to first day of therapy. Forty-two patients (63.6%) received the planned six courses of induction chemotherapy. A reduced number of chemotherapy cycles (n=1–5) were delivered to the remaining 24 patients due to poor performance status in 6, toxicity in 9 and early progression in 8 patients. The reasons for discontinuation of chemotherapy are summarized in the Online Supplementary Table S3. One patient with a partial response (PR) went off study after four chemotherapy cycles due to lack of additional response to the third and fourth cycle. Treatment delay was defined as a chemotherapy delivery more than a week behind schedule. Treatment was delayed for 8–53 days (median 14 days) in 15 of 62 patients. Intraspinal treatment was liposomal cytarabine 50 mg administered by lumbar puncture at day 2 during the four HD-MTX courses. A total of 200 liposomal cytarabine treatments, (median 4, range 1–4) were delivered to 66 patients. Maintenance treatment with temozolomide was started in 15 of 27 elderly patients and not started in 12 patients due to toxicity during the induction therapy in 6, early progression in 3 and poor performance in 3 patients. Median number of TZM treatments delivered was 11 (range 1–12).
Response to induction treatment
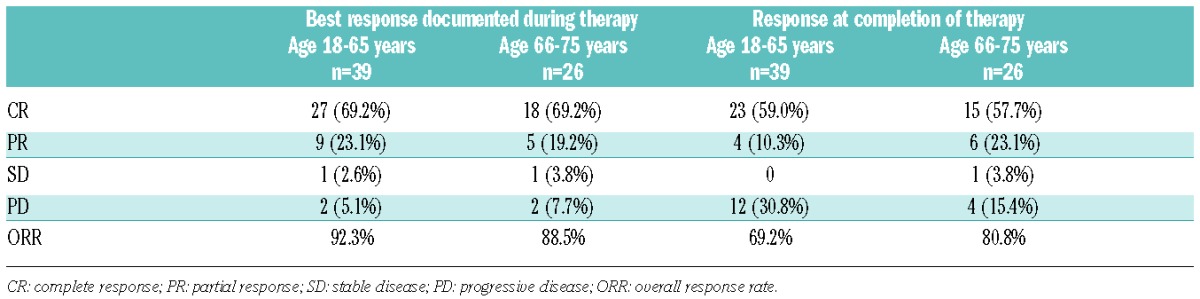
In one of 66 patients, response to induction chemotherapy could not be assessed as the treatment was terminated after the first cycle due to poor clinical condition. In addition, 3 patients completed only one chemotherapy cycle due to progressive disease (PD) after the first cycle. These 3 patients were included in the response analysis. Best response documented during therapy was CR in 42 (64.6%) and PR in 17 patients (26.2%); overall response rate (ORR) 90.8%. Six patients (9.2%) did not respond to induction chemotherapy (SD 2, PD 4). However, in 11 patients who initially responded to therapy (achieved either CR or PR after 2 or 4 chemotherapy courses), the final response assessment at completion of therapy showed PD. When these cases are included in the analysis as PD, the overall response rate for the entire cohort was 73.8%; 69.2% in the younger and 80.8% in the elderly subgroup (Table 2). At a median follow up of 22 months, relapse after achieving CR occurred in 17 of 27 patients (63%) under the age of 66 years and in 5 of 18 elderly patients (28%). All 4 patients with ocular lymphoma went off study after 1–4 cycles of chemotherapy because of progression or poor clinical condition.
Table 2.
Response to maintenance treatment
Disease status at start of maintenance was PR in 3 patients and CR in 12 patients. During maintenance treatment, 4 CR patients progressed, 2 PR patients achieved CR. One PR patient achieved CR at month 9 of follow up.
Survival
Median follow up was 22 months (range 1–57 months). The estimated 2-year OS rate for the entire patient cohort was 58.7% (95%CI: 45.8%–69.5%) (Figure 1A). The estimated 2-year OS in patients under the age of 66 years was 60.7% (95%CI: 43.3%–74.2%) and 55.6% in patients older than 65 years (95%CI: 35.2%–71.8%; P=0.40). The median OS has not yet been reached in either age group (Figure 1B). The estimated PFS at two years was 37.8% (95%CI: 26.3%–49.3%) for the whole group (Figure 1C), 33.1% (95%CI: 19.1%–47.9%) in patients under the age of 66 years and 44.4% (95%CI: 25.6%–61.8%; P=0.74) in the elderly subgroup (Figure 1D). Relapse-free survival after CR at two years was 44.1% (95%CI: 29.3%–57.9%) (Figure 2A) with no significant difference between the two age groups (P=0.33) (Figure 2B). Median DOR for those patients who achieved CR was 15 months: 10 months in the younger patients and not reached in the elderly age group (P=0.33). Outcome in terms of OS was significantly better in patients in MSKCC class 1 or 2 as compared to MSKCC class 3 (data not shown) (P=0.004).
Figure 1.
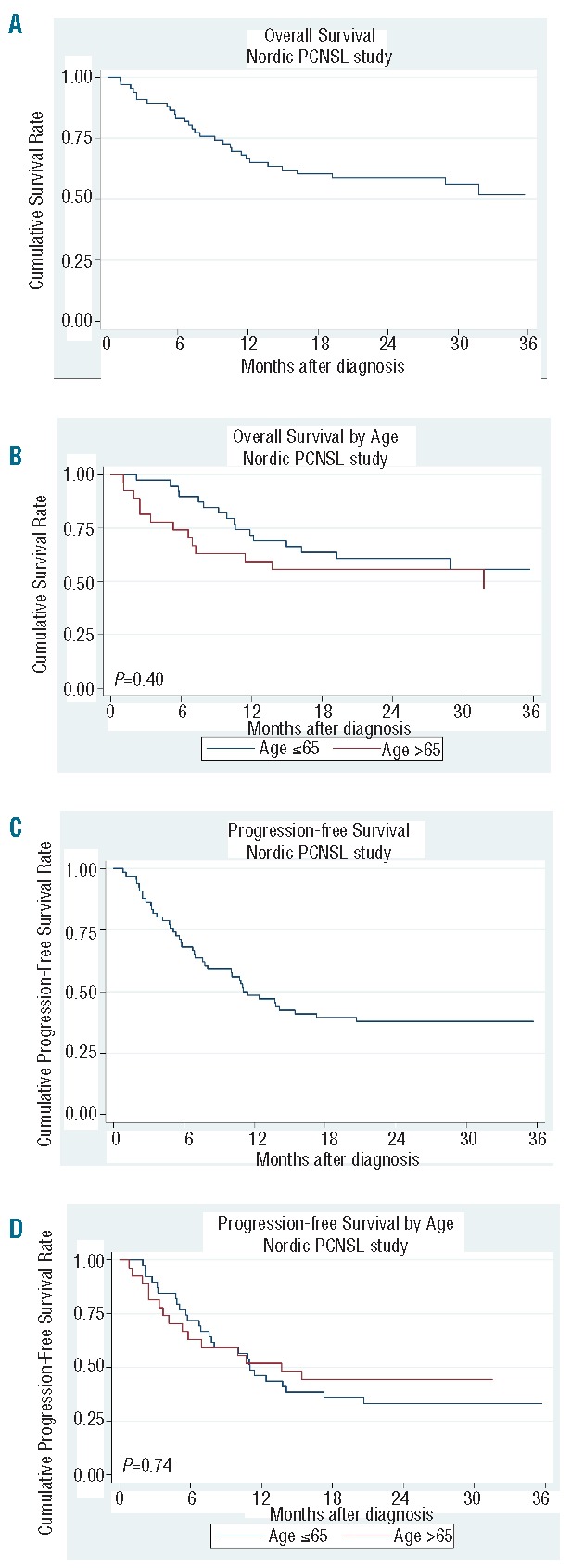
(A) Overall survival for the entire patient cohort. (B) Overall survival according to age. (C) Progression-free survival for the entire patient cohort. (D) Progression-free survival according to age.
Figure 2.
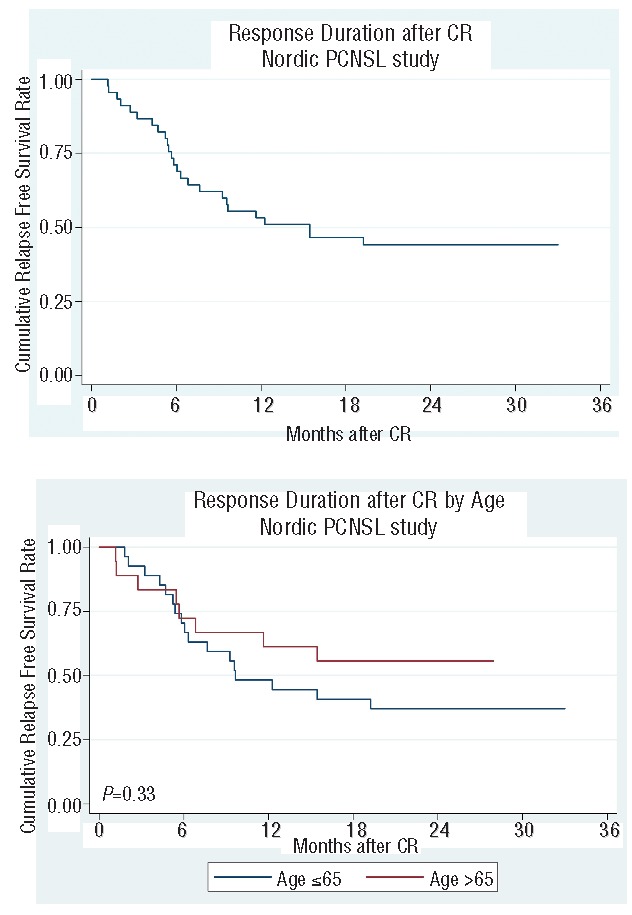
(A) Response duration after complete remission. (B) Response duration after complete remission according to age.
Toxicity
Myelosuppression was the most common side effect and occurred mainly after the HD-AraC cycles. Subsequent to HD-AraC grade 3–4 anemia, neutropenia or thrombocytopenia was reported in 16.1%, 89.3% and 80.9% of the cases, respectively. HD-MTX was relatively well tolerated with anemia, or grade 3–4 neutropenia or thrombocytopenia (Common Toxicity Criteria, v.2.0) reported in 1.5%, 25.8% and 10.6% of the cases, respectively. Impaired kidney function (grade 2–3) occurred in 4 patients (6.1%), and deep venous thrombosis in 7 patients (10.6%; grade 3–4 in 4). Four patients (6%) aged 64, 66, 73 and 74 years died as a result of treatment. All treatment-related deaths occurred after the first HD-AraC course due to neutropenia and sepsis-induced multi-organ failure. With respect to neurotoxicity during treatment, 16 grade 2–4 events were reported in 15 patients during induction therapy (n=13) or maintenance therapy (n=2). The clinical condition, onset and duration of symptoms and medication before the onset of symptoms are summarized in Online Supplementary Table S4. Twelve patients recovered. In 9 patients, the planned treatment was continued, but further liposomal cytarabine was omitted in 5 patients. Three patients did not recover and went off study.
Discussion
In this study, we have shown that the survival in elderly patients was not compromised, and possibly even may have been improved, by de-escalating induction and adding maintenance therapy. The number of elderly patients was too low, however, to allow any definite conclusions to be drawn. We found equally favorable response rates in elderly and younger patients in spite of the fact that patients with poor performance status (ECOG 3–4) were included in our study. It is widely accepted that the regimens in PCNSL should contain HD-MTX as the most important agent of induction. Even though radiotherapy increases the remission rate, several studies have investigated the efficacy of HD-MTX alone or in combination with other drugs in order to avoid radiotherapy due to the risk of long-term neurotoxicity.14 These studies have shown improvement in response rates as compared to HD-MTX alone regimens.6,14–16 The present study provides additional evidence of this benefit.
An important strategy of this study was the stratification of therapy according to age, which has been identified as the most important prognostic risk factor in PCNSL together with performance status.13,17 The main finding of our data, and in contrast to other studies,7,14 is that patients in the elderly age group responded equally well as younger patients in terms of ORR, OS and PFS, indicating benefit of temozolomide. These findings suggest an improvement in the elderly when compared with a report by Pels et al. who reported age-related treatment outcome after chemotherapy only with an estimated OS at five years of 19% in 31 patients older than 60 years in contrast to 75% in 30 younger patients.7
Comparison of survival outcomes between different studies is generally difficult due to different study design, different treatment modalities, and great variability in patients’ characteristics. The entire cohorts treated in the Bonn study7 and in the present study were similar in terms of median age (62 vs. 64 years) and performance status (Karnofsky 70 vs. ECOG 1). Also, the study treatment with regard to younger patients was similar with the exception of the differences in the intrathecal treatment: administration route (intraventricular vs. intraspinal), number of treatments (6 vs. 4), and composition of treatment (MTX, AraC, methylprednisolone vs. liposomal cytarabine). Although the ORR was similar in both studies, and median OS was not reached in either, they provided different results regarding median response duration, which was only ten months in patients aged under 66 years in the present study, and not reached in the Bonn study after a median follow up of 26 months. This difference might be due in part to the different upper age limit of the younger subgroup in these two studies: 65 years in the present study versus 60 years in the Bonn study. Notably, more than one-third of the younger patients in the present study were 61–65 years old and duration of response was especially short in these patients, as 8 of 14 responding patients relapsed early (median 6.3 months). An up-dated analysis of the Bonn study has shown that the median OS was 34 months in the elderly subgroup, but still not reached in younger patients after a follow up of a median 100 months.18 Age was, however, not found to be a risk factor in a recently published Cancer and Leukemia Group B (CALGB) study in which 44 patients with median age of 61 years (12–76 years) were treated with HD-MTX, rituximab and TZM induction followed by etoposide and HD-AraC consolidation without CSF-targeted chemotherapy.19 The median PFS in the CALGB cohort was similar in older and younger patients. Estimated 2-year OS of 70% for the entire CALGB cohort was superior to the results of the present trial in which the estimated 2-year OS survival was 58.7%. However, only patients with ECOG performance of of 2 or less than 2 were included in the CALGB study, which could well account for the superior survival, since we also included patients with an ECOG performance status of 3–4.
The treatment–related mortality (TRM) of our study of 6% is comparable with other studies using HD-MTX and HD-AraC-based chemotherapy. Pels et al.7 reported a TRM of 9% in patients treated with basically the same systemic chemotherapy as that used in our study. Of 41 patients enrolled in a phase II study applying the HD-MTX and HD-AraC combination published by Ferreri et al.,21 4 patients (9.7%) died due to treatment-related complications. In a phase II randomized trial published in 2009,21 40 patients were randomized to HD-MTX and 39 patients to HD-MTX+HD-AraC. Three of 4 treatment-related deaths occurred in the combination arm (7.7%). These three studies7,20,21 did not report TRM according to age. Of note, all 4 treatment-related deaths in our study occurred in patients aged 64 years or older. Thus, the TRM in the elderly age subgroup in our study was 11.1% as compared to 2.6% in the younger patients. Given that HD-AraC-related toxicity was much more pronounced than toxicity related to HD-MTX, and in particular given that all 4 treatment-related deaths in the present study occurred after HD-AraC, it seems reasonable to suggest that this agent should be omitted from the treatment regimen, especially for elderly patients. It is an open question, however, as to whether AraC should be replaced by another agent or not.
Another feature of interest in PNSCL is the need for local treatment of the CSF compartment as a prerequisite for disease control. Here, the Bonn group reported early relapses and a median duration of response of ten months in younger patients (median age 53, range 27–59 years), who did not receive intraventricular treatment, but otherwise the same systemic therapy as in the original Bonn regimen, arguing for the need of CSF-targeted therapy.22 While the collective experience from the two Bonn studies7,22 indicates the need for intraventricular treatment, two retrospective analyses did not find that intrathecal chemotherapy had any impact on survival.23,24 Moreover, Omuro et al.25 reported disappointing results of a retrospective study of 64 patients with a median age of 47 years who had a median PFS of 12 months only, despite the fact that intrathecal treatment consisting of the same agents (MTX, AraC and methylprednisolone) was part of both the induction treatment and a maintenance phase. These observations pinpoint a crucial feature in the PNSCL patients, that a way needs to be found to obtain local tumor control. In our treatment design, we applied intraspinal administration of liposomal cytarabine, which has previously been used in the treatment of leptomeningeal cancer metastasis,26 including lymphomatous meningitis.27 It should be stressed, however, that the efficacy of liposomal cytarabine in PCNSL has still not been determined. Our study was not designed to evaluate a single agent of the regimen and our results do not allow any conclusion to be drawn as to the efficacy of liposomal cytarabine. Given the small number of cases with lymphoma detected in the spinal fluid in the present study, we cannot draw any conclusions as to whether liposomal cytarabine was effective or even necessary to eradicate lymphoma cells from the spinal fluid compartment. We found also a discrepancy between cytological and flow cytometry analysis of the spinal fluid. It is well documented in the literature that flow cytometry is a more sensitive method than cytology,28 but flow cytometry in our study was positive in only 2 cases as compared to 8 cases detected by cytology. The analysis of spinal fluid was not centralized and was, therefore, performed at several different laboratories, which is a drawback of the multicenter design of our study.
Furthermore, concerns have been raised regarding neurotoxicity related to liposomal cytarabine, administered either alone or in combination with systemic chemotherapy.29 In the present study, a total of 200 liposomal cytarabine treatments were delivered to 66 patients, with 15 patients experiencing neurological symptoms grade 2–4, 11 of whom had received liposomal cytarabine within two weeks before onset of symptoms. However, the neurological complications experienced during therapy in the present study cannot be ascribed exclusively to liposomal cytarabine, since other agents, in particular HD-MTX and ifosfamide used concomitantly, can also give rise to drug-related neurotoxicity. Moreover, 4 of the 15 patients mentioned above were not exposed to liposomal cytarabine prior to the development of neurological symptoms.
Ocular lymphoma, reported to occur in 3%–14% of PCNSL patients,25,30 was found in 4 of 66 patients in our study (6%). None of them completed treatment due to poor clinical condition, toxicity or progressive disease. Additional treatment in case of ocular lymphoma besides the study treatment planned for all patients was not part of our protocol. Optimal treatment for intraocular lymphoma remains to be defined.
Both the route and the timing of MTX administration should be considered as factors influencing the treatment results. In most studies, HD-MTX has been administered at intervals no longer than 21 days. The HD-MTX schedule of the present study (Table 1) entailed no MTX delivery through six weeks (i.e. from the second to the third HD-MTX cycle). This was also the case in the Bonn regimen when given without intraventricular therapy.22 In comparison, intraventricular MTX, AraC and methylprednisolone was delivered during all six induction chemotherapy courses in the original Bonn regimen using the same systemic HD-MTX schedule, thus avoiding a 6-week period of no MTX delivery. Theoretically, the inferior results of the Bonn regimen without intraventricular treatment, as well as the results in the present study as compared to the original Bonn regimen, could be due to lymphoma growth or leptomeningeal seeding during the time when the tumor was not exposed to MTX. Consequently, in future studies, attention should be paid to the drug intensity of MTX delivery whatever the route of administration.
In conclusion, the main feature of the present study is its focus on elderly patients, who have so far not received much attention. Our approaches of de-escalating induction and introduction of maintenance therapy have provided results which are among the most favorable ever reported in elderly PCNSL patients.5,30,31,32 Thus, moving the focus to the maintenance phase seems to indicate that temozolomide maintenance has been of benefit regarding sustained remission, since relapse after achieving CR occurred in only 5 of 18 elderly patients (28%) as compared to 17 of 27 patients (63%) in the younger subgroup who did not receive maintenance treatment. On the other hand, the induction regimen applied in the elderly subgroup was still too toxic, given that 3 of 4 treatment-related deaths occurred in this age group. We propose a maintenance treatment in all responding patients not fit for more aggressive therapies in order to improve disease control and further de-escalation of induction in elderly patients to reduce toxicity.
Acknowledgments
The authors would like to thank Professor Peter Hokland for helpful advice and M.Sc. Leif Spange Mortensen for statistical assistance and for commenting on the manuscript.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was funded by grants from Nordic Cancer Union, Norpharma, Roche, Schering-Plough, and Inge og Jørgen Larsens Mindelegat.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Olson JE, Janney CA, Rao RD, et al. The Continuing Increase in the Incidence of Primary Central Nervous System Non-Hodgkin Lymphoma. A Surveillance, Epidemiology, and End Results Analysis. Cancer. 2002;95(7):1504–1510. [DOI] [PubMed] [Google Scholar]
- 2.Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bessell EM, Dickinson P, Dickinson S, Salmon J. Increasing age at diagnosis and worsening renal function in patients with primary central nervous system lymphoma. J Neurooncol. 2011;104(1):191–193. [DOI] [PubMed] [Google Scholar]
- 4.Christina Goldkuhl, Tor Ekman, Tom Wiklund, Ragnar Tellhaug for the Nordic Lymphoma Group. Age-adjusted Chemotherapy for Primary Central-Nervous System Lymphoma. A Pilot Study. Acta Oncol. 2002;41(1):29–36. [DOI] [PubMed] [Google Scholar]
- 5.Hoang-Xuan K, Taillandier L, Chinot O, et al. Chemotherapy Alone as Initial Treatment for Primary CNS Lymphoma in Patients Older Than 60 Years: A Multicenter Phase II Study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol. 2003;21(14):2726–2731. [DOI] [PubMed] [Google Scholar]
- 6.DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ. Combination Chemotherapy and Radiotherapy for Primary Central Nervous System Lymphoma: Radiation Therapy Oncology Group Study 93–10. J Clin Oncol. 2002;20(24):4643–4648. [DOI] [PubMed] [Google Scholar]
- 7.Pels H, Schmidt-Wolf IGH, Glasmacher A, et al. Primary Central Nervous System Lymphoma: Result of a Pilot and Phase ll Study of Systemic and Intraventricular Chemotherapy With Deferred Radiotherapy. J Clin Oncol. 2003;21(24):4489–4495. [DOI] [PubMed] [Google Scholar]
- 8.Coiffier B, Lepage E, Brière J, et al. CHOP Chemotherapy Plus Rituximab Compared With CHOP Alone In Elderly Patients With Diffuse Large B-Cell Lymphoma. N Engl J Med. 2002;346(4):235–242. [DOI] [PubMed] [Google Scholar]
- 9.Hiraga S, Arita N, Ohnishi T, et al. Rapid infusion of high-dose methotrexate resulting in enhanced penetration into cerebrospinal fluid and intensified tumor response in primary central nervous system lymphomas. J Neurosurg. 1999;91(2):221–230. [DOI] [PubMed] [Google Scholar]
- 10.Wedge SR, Newlands ES. 06-Benzylguanine enhances the sensitivity of a glioma xenograft with low 06-alkylgua-nine-DNA alkyltransferase activity to temozolomide and BCNU. Br J Cancer. 1996;73(9):1049–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enting RH, Demopoulos A, DeAngelis LM, Abrey LE. Salvage therapy for primary CNS lymphoma with a combination of Rituximab and temozolomide. Neurology. 2004;63(5):901–903. [DOI] [PubMed] [Google Scholar]
- 12.Abrey LE, Batchelor TT, Ferreri AJM, et al. Report of an International Workshop to Standardize Baseline Evaluation and Response Criteria for Primary CNS Lymphoma. J Clin Oncol. 2005;23:5034–5043. [DOI] [PubMed] [Google Scholar]
- 13.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary Central Nervous System Lymphoma: The Memorial Sloan-Kettering Cancer Center Prognostic Model. J Clin Oncol. 2006;24(36):5711–5715. [DOI] [PubMed] [Google Scholar]
- 14.Abrey LE, Yahalom J, DeAngelis LM. Treatment for Primary CNS Lymphoma: The Next Step. J Clin Oncol. 2000;18(17):3144–3150. [DOI] [PubMed] [Google Scholar]
- 15.Yang SH, Lee KS, Kim IS, et al. Long-term survival in primary CNS lymphoma treated by high-dose methotrexate mono-chemotherapy: role of STAT6 activation as prognostic determinant. J Neurooncol. 2009;92(1):65–71. [DOI] [PubMed] [Google Scholar]
- 16.Batchelor T, Carson K, O’Neill A, et al. Treatment of Primary CNS Lymphoma With Methotrexate and Deferred Radiotherapy: A Report of NABTT 96–07. J Clin Oncol. 2003;21(6):1044–1049. [DOI] [PubMed] [Google Scholar]
- 17.Ferreri AJM, Blay JY, Reni M, et al. Prognostic Scoring System for Primary CNS Lymphomas: The International Extranodal Lymphoma Study Group Experience. J Clin Oncol. 2003;21(2):266–272. [DOI] [PubMed] [Google Scholar]
- 18.Juergens A, Pels H, Rogowski S, et al. Long-Term Survival with Favorable Cognitive Outcome after Chemotherapy in Primary Central Nervous System Lymphoma: Ann Neurol. 2010;67(2):182–189. [DOI] [PubMed] [Google Scholar]
- 19.Rubenstein JL, His ED, Johnson JL, et al. Intensive Chemotherapy and Immunotherapy in Patients With Newly Diagnosed Primary CNS Lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31(25):3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreri AMJ, Dell’Oro S, Foppoli M, et al. MATILDE regimen followed by radiotherapy is an active strategy against primary CNS lymphomas. Neurology. 2006;66(9):1435–1438. [DOI] [PubMed] [Google Scholar]
- 21.Ferreri AJM, Reni M, Foppoli M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374(9700):1512–1520. [DOI] [PubMed] [Google Scholar]
- 22.Pels H, Juergens A, Glasmacher A, et al. Early relapses in primary CNS lymphoma after response to polychemotharapy without intraventricular treatment. J Neurooncol. 2009;91(3):299–305. [DOI] [PubMed] [Google Scholar]
- 23.Khan RB, Shi W, Thaler HT, DeAngelis LM, Abrey L. Is intrathecal methrotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol. 2002;58(2):175–178. [DOI] [PubMed] [Google Scholar]
- 24.Ferreri AJM, Reni M, Pasini F, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58(10):1513–1520. [DOI] [PubMed] [Google Scholar]
- 25.Omuro A, Taillandier L, Chinot O, et al. On behalf of the ANOCEF Group (French Neuro-Oncology Association). Primary CNS lymphoma in patients younger than 60: can whole-brain radiotherapy be deferred? J Neurooncol. 2011;104(1):323–330. [DOI] [PubMed] [Google Scholar]
- 26.Glantz MJ, Jaeckle KA, Chamberlain MC, et al. A Randomized Conrolled Trial Comparing Intrathecal Sustained- release Cytarabine (DepoCyt) to Intrathecal Methotexate in Patients with Neoplastic Meningitis from SolidTumors. Clin Cancer Res. 1999;5(11):3394–3402. [PubMed] [Google Scholar]
- 27.Glantz MJ, LaFollette S, Jaeckle KA, et al. Randomized Trial of a Slow-release Versus a Standard Formulation of Cytarabine for the Intrathecal Treatment of Lymphomatous Meningitis. J Clin Oncol. 1999;17(10):3110–3116. [DOI] [PubMed] [Google Scholar]
- 28.Hegde U, Filie A, Little RF, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood. 2005;105(2):496–502. [DOI] [PubMed] [Google Scholar]
- 29.Ostermann K, Pels H, Kowoll A, Kuhnhenn J, Schlegel U. Neurologic complications after intrathecal liposomal cytarabine in combination with systemic polychemotherapy in primary CNS lymphoma. J Neurooncol. 2011;103(3):635–640. [DOI] [PubMed] [Google Scholar]
- 30.Illerhaus G, Marks R, Muller F, et al. High-dose methotrexate combined with procarbazine and CCNU for primary CNS lymphoma in the elderly: results of a prospective pilot and phase II study. Ann Oncol. 2009;20(2):319–325. [DOI] [PubMed] [Google Scholar]
- 31.Omuro AMP, Taillandier L, Chinot O, Carnin C, Barrie M, Hoang-Xuan K. Temozolomide and methotrexate for primary central nervous system lymphoma in the elderly. J Neurooncol. 2007;85(2):207–211. [DOI] [PubMed] [Google Scholar]
- 32.Fritsch K, Kasenda B, Hader C, et al. Immunochemotherapy with rituximab, methotrexate, procarbazine, and lomustine for primary CNS lymphoma (PCNSL) in the elderly. Ann Oncol. 2011;22(9):2080–2085. [DOI] [PubMed] [Google Scholar]


