Abstract
The mammalian target of rapamycin plays an important role in multiple myeloma. The allosteric mammalian target of rapamycin inhibitor everolimus has long been approved for immunosuppression and has shown activity in certain cancers. This investigator-initiated phase I trial explored the use of everolimus in relapsed and/or refractory multiple myeloma patients who had received two or more lines of prior treatment. Following a dose-escalation design, it called for a fixed dose of oral everolimus. Blood drug levels were monitored and the biological activity of everolimus was evaluated in bone marrow. Seventeen patients were enrolled (age range, 52 to 76 years). All had been previously treated with stem cell transplantation and proteasome inhibitors and almost all with immunomodulatory drugs. No dose-limiting toxicity was observed and the intended final daily dose of 10 mg was reached. Only one severe adverse event was assessed as possibly related to the study drug, namely atypical pneumonia. Remarkably few infections were observed. Although the trial was mainly designed to evaluate feasibility, anti-myeloma activity, defined as clinical benefit, was documented in ten of 15 evaluable patients at every dose level including eight patients with stable disease, one patient with minor remission and one with partial remission. However, the median time to progression was 90 days (range, 13 to 278 days). The biomarker study documented on-target activity of everolimus in malignant plasma cells as well as the microenvironment. The observed responses are promising and allow further studies to be considered, including those testing combination strategies addressing escape pathways. This trial is registered with EudraCT number 2006-002675-41.
Introduction
The mammalian target of rapamycin (mToR), a serine (Ser)/threonine (Thr) kinase, plays an important role in multiple myeloma (MM) since it is involved in the phosphoinositol-3 kinase (PI3K)-AKT pathway activated by essential growth and survival factors such as interleukin-6 (IL-6) as well as the insulin-like growth factor-1, by mutated oncogenes such as Ras, by vascular endothelial growth factor or by loss of the phosphatase and tensin homolog (PTEN).1–6 mToR is part of the mToR1 complex (mToRC1), which is controlled by prolinerich AKT substrate of 40 kDa (PRAS40) and tuberous sclerosis (TSC)2, both inhibited by activated AKT (when phosphorylated on Thr308 and Ser473). The mToRC1 acts as an ATP and amino acid sensor to balance nutrient availability and cell growth by regulating metabolism, translation and autophagy.7–12 Rapamycin, a bacterial product from Streptomyces hygroscopicus with antifungal, immunosuppressive and antitumor activity, is first in a class of allosteric mToR inhibitors (rapalogues).13,14 Preclinical data showed the anti-myeloma activity of these drugs alone or in combination with dexamethasone.15–18 Thus, it was tempting to test the anti-myeloma activity of mToR inhibitors in MM patients who had failed to benefit from the available treatment options extended in the last decade by the introduction of proteasome inhibitors such as bortezomib and immunomodulating drugs.19–22
The rapalogue everolimus (RAD001) is approved for immunosuppression and for the treatment of several malignancies such as metastatic renal cell cancer, gastroenteropancreatic neuroendocrine tumor and subependymal giant cell astrocytoma.23–30 In this investigator-initiated phase I study the safety and activity of everolimus in relapsed or refractory MM were evaluated.
Methods
Study design
The trial CRAD001C2455 was designed as an open-label, multicenter phase I trial of continuous, escalating doses of everolimus once daily in patients aged ≥18 years who had relapsed or refractory MM after two prior treatment lines. The primary objective was to determine the maximum tolerated dose of everolimus and dose-limiting toxicities. Secondary objectives included an assessment of the tolerability and clinical activity of everolimus. At least three patients were to be included at each dose level until the maximum tolerated dose was reached. The treatment was planned for six 28 day cycles if no progressive disease or dose-limiting toxicity was observed. However, an additional treatment with study drug was allowed for patients achieving clinical benefit after six cycles.
Patients
Adult patients with relapsed or refractory MM of Salmon and Durie stage ≥ II after failure of at least two prior treatment regimens were enrolled. The study was designed in accordance with the International Conference on Harmonisation (ICH) Harmonised Tripartite Guidelines for Good Clinical Practice, with applicable local regulations and the ethical principles of the Declaration of Helsinki. The protocol was approved by the Institutional Review Board/Independent Ethics Committee/Research Ethics Board at each study site, and informed consent was obtained from all patients (EudraCT number: 2006-002675-41).
Key inclusion criteria were WHO performance status ≤ 2, measurable disease markers, adequate bone marrow function, and adequate liver function.
Pharmacokinetic analyses and drug adjustment
The everolimus concentrations in whole blood were determined by a validated liquid chromatography method (Central Laboratory of the University Hospital of Göttingen, Germany). In cohort 1 of the dose escalation phase, the starting dose was 5 mg everolimus, which was followed by cohorts given doses of 7.5 mg and 10 mg. If a patient showed a significant deviation from the intended blood drug level (defined as ± 50%), the dose had to be adjusted without, however, exceeding the dose of 10 mg/day. To investigate the correlation of the median achieved drug level and the M protein response, the Pearson product-moment correlation coefficient was calculated (2-tailed significance, SPSS 13).
Bone marrow assessment
Bone marrow aspirates and biopsies were performed during screening, 28 days into treatment, at the end of study and when clinically indicated. Immunohistochemical staining for mToR, for the downstream targets 4E-binding protein 1 and phosphorylated S6 ribosomal protein (S6) were performed using antibodies from Cell Signaling (Danvers, USA), as described previously.31
Effect on the immune system
To elucidate the effect of everolimus on the immune system of MM patients the levels of the not involved immunoglobulin classes were determined by turbidimetric measurement (Cobas c system, Roche Diagnostics, Mannheim, Germany) and lymphocyte subsets were assessed by FACS staining as described previously (antibodies provided by Beckman Coulter, Krefeld, Germany).32 The values determined at baseline were compared to the last values achieved during treatment and tested for significant changes using the paired two-tailed t-test (SPSS 13). In two patients under treatment, cytotoxic activities of natural killer (NK) cells were determined (compared to those of a healthy volunteer) in a standard 51Cr release assay performed as described previously.33
Results
Patients
Seventeen patients (13 males, 4 females) aged from 52 to 73 years with relapsed or refractory MM were enrolled in this interventional clinical trial (for details see Table 1). They had received a median of three (range, 2 to 9) prior lines of therapy and all had undergone one or more autologous stem cell transplant; one patient had also undergone allogeneic stem cell transplantation. Sixteen of 17 patients had received prior treatment with both bortezomib and immunomodulatory drugs. In addition, most patients received some form of radiation therapy during the course of their disease.
Table 1.
Patients’ characteristics.
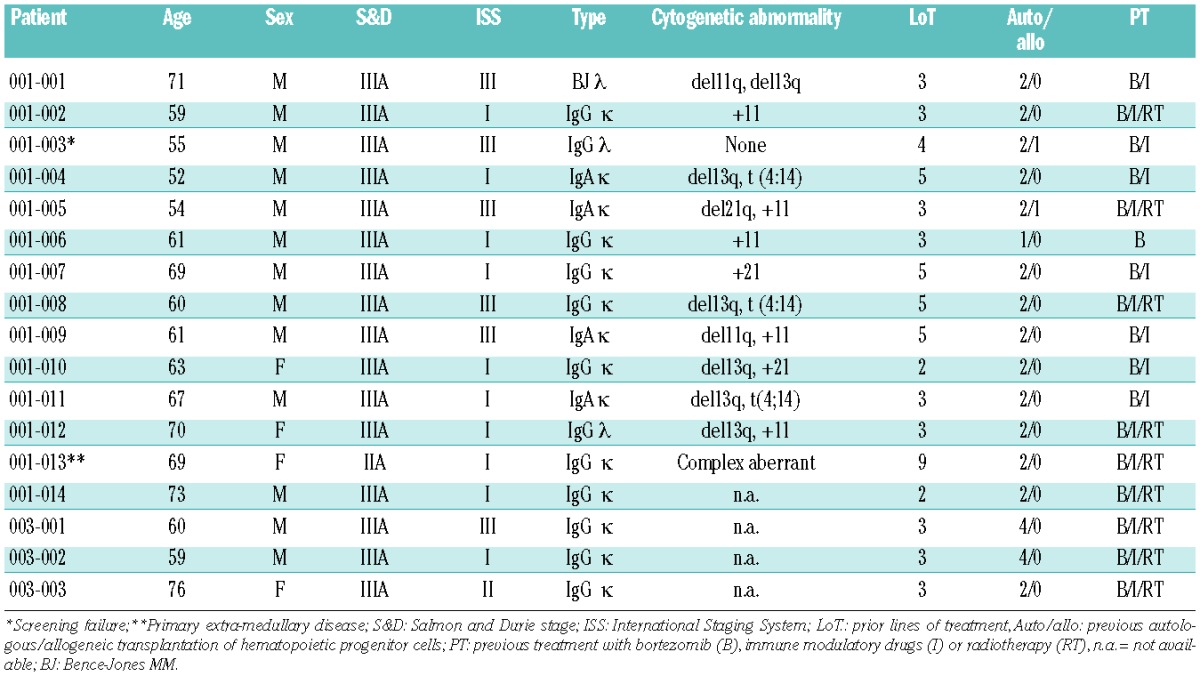
The initial cohort treated with 5 mg everolimus had to be expanded with two additional patients due to one screening failure and one patient in whom the everolimus dose had to be increased to 10 mg daily according to protocol to achieve the intended blood level. While the patients in cohort 2 were given 7.5 mg everolimus as planned, three patients in the third cohort of 10 mg were replaced because of early termination of the trial due to progressive disease or withdrawal of consent in one case. In total three patients, one at the dose level of 7.5 mg and two patients receiving 10 mg completed the planned 6 months of study treatment.
Pharmacokinetic analysis
The median blood level achieved in the first two cohorts was in the intended range, whereas six of nine of the third cohort did not achieve the intended level (Online Supplementary Data). However, at this highest planned dose level no dose changes were performed. Notably, the median blood level of all three cohorts was comparable at day 28 and no correlation between dose and blood levels was shown.
Safety
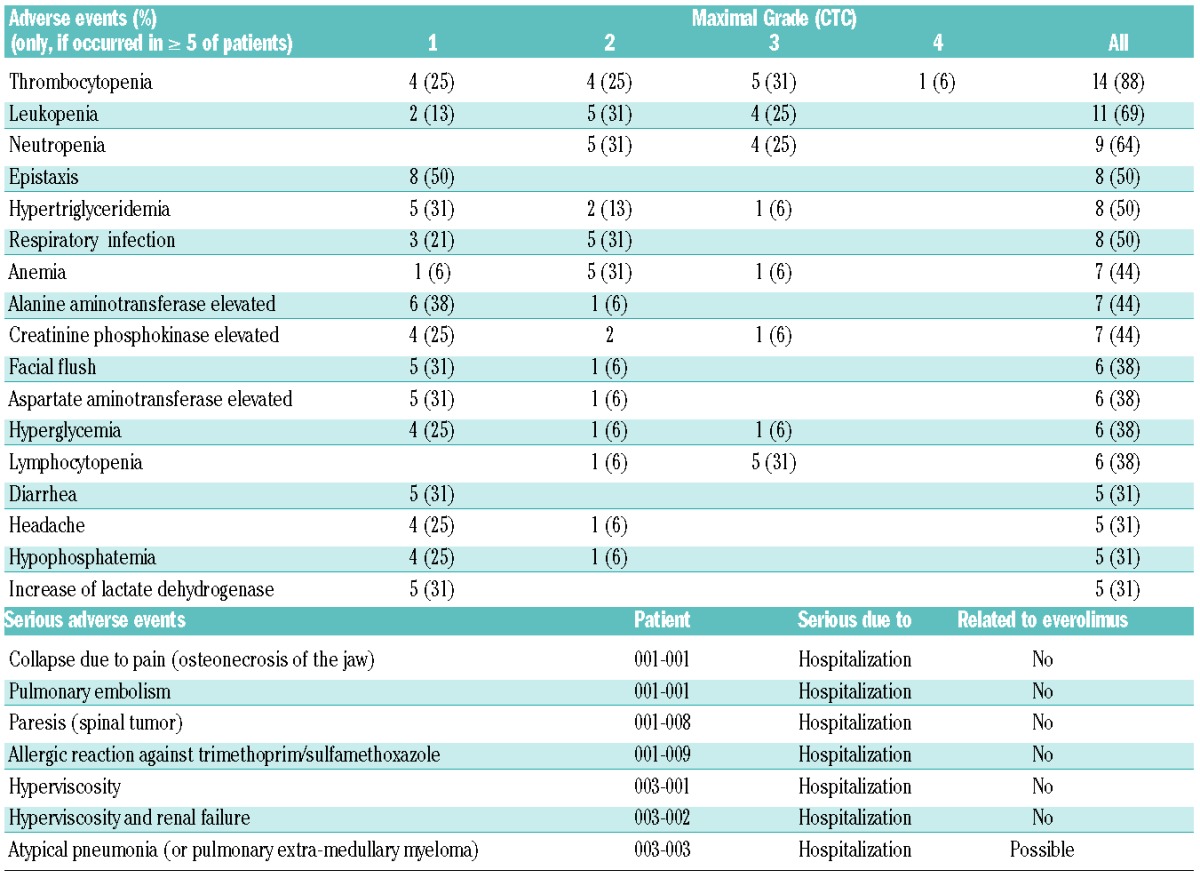
Adverse events were mostly mild to moderate (Table 2). Thrombocytopenia was the most frequent hematologic adverse event, followed by leukopenia and anemia. Metabolic changes as well as elevated muscle and liver enzymes were the most frequent non-hematologic events followed by several mostly mild gastrointestinal events. Interestingly, infectious complications and bleeding were rare events and when they did occur, they were mild. According to protocol, if relevant toxicity was observed, the everolimus dose had to be reduced or the drug withheld for up to 7 days at the investigator’s discretion. However, none of the adverse events led to dose reduction or permanent withholding of the study drug. During the trial eight serious adverse events were reported, all being serious because hospitalization was required. However, only one serious adverse event, namely atypical pneumonia (extra-medullary MM in the lung was also discussed), was considered by the investigators to be possibly related to the study drug, although it did not resemble a drug-induced pneumonitis. Most of the other serious adverse events were caused by progressive disease or were clearly related to other causes such as an allergic reaction to trimethoprim/sulfamethoxazole or pain due to osteonecrosis of the jaw. Pulmonary embolism occurred in one patient without a previous history of thromboembolic events.
Table 2.
Tolerability of everolimus.
Determination of maximum tolerated dose
By protocol dose-limiting toxicity was defined as follows: any febrile neutropenia, any grade ≥3 neutropenia or grade 4 thrombocytopenia lasting more than 7 days, any grade ≥3 pneumonitis, any grade 4 non-hematologic events, and any two or more events requiring a reduction in the dose of everolimus or any adverse event resulting in more than a 4-week interruption or delay of study treatment. Since none of these was observed, the highest tested dose of 10 mg daily (or above) was considered to be the maximum tolerated dose.
Immunomodulation
Despite everolimus acting as an immunosuppressive, the rate of infectious complications was comparable to that reported with other treatment regimens used in patients with advanced MM. No significant alteration of lymphocyte subsets was noted during treatment (Online Supplementary Data). Functional tests of NK cells did not reveal differences in comparison to controls (Figure 1A). To evaluate immunosuppressive effects of everolimus on normal immunoglobulin levels, non-involved immunoglobulin classes were monitored. While most patients had secondary antibody deficiency at baseline, slight declines of IgM and IgG, but not of IgA, were noted (Figure 1B).
Figure 1.
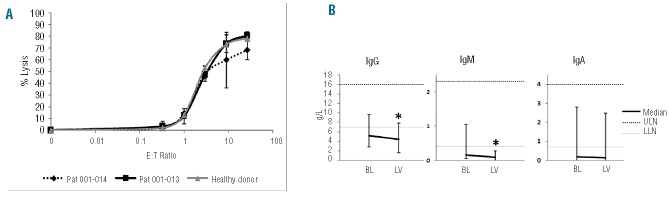
The NK cells of two MM patients treated at the highest dose level (everolimus 10 mg daily) were compared to NK cells of a healthy donor regarding their ability to lyse K562 cells (A, values represent the mean of triplicate experiments). No alterations in NK cell function were observed (E:T = effector to target cell ratio). The immunoglobulin levels not involved in myeloma disease were monitored to measure the impact of everolimus on normal B and plasma cells (B). The medians of immunoglobulin levels of informative patients at baseline (BL) and at the end of the treatment (last available value, LV) are shown. Bars indicate minimal and maximal values, dotted lines the range of normal values (ULN= upper limit of normal; LLN= lower limit of normal). Most patients had secondary antibody deficiency. The level of IgM declined significantly over the treatment period (paired two-tailed t-test: P=0.029; * indicates statistical significance), while the decline of IgG was of borderline significance (P=0.048) and IgA was not changed significantly (P=0.057).
Efficacy and pharmacokinetics
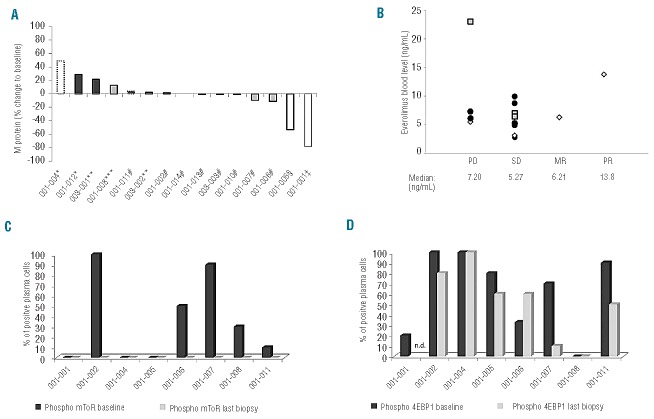
Fifteen patients in the dose escalation phase were available for the planned efficacy assessment every 4 weeks or showed early progressive disease within the first cycle (two cases). The median time to progression was 90 days (range, 13 to 278 days). The clinical response of all patients is shown in Figure 2A. The majority of patients (10 out of 15; 67%) achieved a clinical benefit during the trial. One patient (7 %) had a partial remission after four cycles of treatment. Bone marrow biopsy revealed that reduction of M-protein levels in the serum correlated with a decrease in malignant plasma cells. Another patient (7%) in the first cohort diagnosed with Bence-Jones MM qualified for a minor response with a greater than 50% reduction of urinary M protein. Eight patients (80%) achieved stable disease (for details see also Online Supplementary Data). Three of 15 patients reached the endpoint of the study at 6 months with stable disease. One of these remained under treatment for 278 days until progression occurred. Therefore, based on the pre-defined efficacy criteria, everolimus was found to be active with good tolerability. The drug levels achieved in the blood showed considerable intra-individual variability, leading to comparable blood levels in all cohorts. Although the patient who achieved a partial response showed a rather high blood level, no correlation between blood level and clinical efficacy was established (Figure 2B). In addition, no significant association was found between the median everolimus blood level and the achieved M-protein response (Pearson r = −0.07, P=0.81).
Figure 2.
The waterfall plot shows the best response at time of assessment of the 12 available patients (A). The shading of the bars indicates the different drug level cohorts: white is cohort 1 (5 mg), light gray cohort 2 (7.5 mg) and dark gray cohort 3 (10 mg). Black frames represent serum M protein, black spotted frames total IgA (in cases without measurable serum M protein) and a gray frame represents urinary M protein as a disease marker. Seven patients had clinical benefit, achieving stable disease (#), minor response (ǂ) or partial response (§). Five patients showed progressive disease as defined by IMWG criteria (*), early withdrawal due to hyperviscosity (**) or growing spinal tumor (***). The everolimus blood levels of patients (average of all measurements in a particular patient) grouped according to the best clinical status achieved (B). Although the patient with a partial response had the second highest blood level, the data show no obvious correlation between drug level and efficacy as indicated by comparable median blood levels for all groups [white diamond: dose level 1 (5 mg daily), gray quadrangle: dose level 2 (7.5 mg daily), dark gray circle: dose level 3 (10 mg daily)]; PD=progressive disease, SD=stable disease, MR= minor response, PR= partial response). Bone marrow was stained for the phosphorylated forms of mToR (C) and 4EBP1 (D) demonstrating a wide variety in activation of the pathway in plasma cells at baseline (dark gray bars). However, under treatment phosphorylation of mToR was abolished in all investigated samples (last biopsy: light gray bars) whereas activation of the downstream target 4EBP1 was only slightly decreased (n.d.= not done).
Biomarker analysis
In order to access biomarkers for everolimus activity, the phosphorylation of key proteins in the mToR pathway, particularly mToR, 4EBP1 and S6 protein, was investigated. The biopsies at baseline showed a wide variety of activation of the pathway in the tumor cells (Figure 2C). Irrespectively of the dose level and the response to treatment, mToR phosphorylation was abolished in all biopsies obtained under treatment. However, the downstream targets 4EBP1 (Figure 2D) and S6 showed residual phosphorylation in plasma cells as well as in bystander cells (see also Online Supplementary Data for immunohistology).
Discussion
Since the mToR pathway plays a crucial role in MM pathophysiology the purpose of this trial was to evaluate tolerability as well as the clinical benefit of the oral rapalogue everolimus in relapsed and/or refractory MM. The trial showed that the drug had an acceptable safety profile in this heavily pretreated group of patients. The rate of infections was within the expected range. Pulmonary embolism was observed in a single case. Since thromboembolism due to other reasons is not infrequent in MM, a relationship with the study drug could not be firmly established.
The most frequent toxicities were thrombocytopenia and leukopenia, gastrointestinal events and metabolic changes. All of those were manageable, without any treatment changes due to side effects. In a recent trial in patients with renal cancer a proportional absorption of everolimus was found.34 In our study, no clear correlation was observed between everolimus dose and serum drug levels achieved, perhaps because of the higher rate of anti-infectious prophylaxis used in MM patients interacting with drug metabolism or the limited patient number.
Since the main objective of the trial was to assess the tolerability of everolimus, the efficacy data should be viewed with caution. Despite these limitations the anti-myeloma activity of everolimus was considerable: one patient achieved a partial remission, one had a minor response, and eight patients were clinically stable for up to 9 months. However, the median time of 90 days until progression indicates that monotherapy may be insufficient for long-term control of advanced disease. The rapalogue temsirolimus administered intravenously had previously also shown some activity in patients with advanced MM with a trend for better outcome in patients achieving higher drug levels.35 In our trial such a correlation could not be established, although the patient with the best response did have a rather high level of everolimus in the blood. The weak dose-efficacy relationship of rapalogues in MM might be explained by our observation that mToR activation in plasma cells was blocked even at the lowest dose level.
Inhibition of mToRC1 induces negative feedback loops, such as inhibition of insulin receptor substrate by p70/S6 kinase.36,37 The evaluation of bone marrow during everolimus treatment revealed a reduction but not abrogation of the mToR downstream target 4EBP1 and S6 protein. This was irrespective of the dose level and despite abolished mToR phosphorylation. In vitro experiments showed that rapalogues failed to block cell growth completely even at high doses.17,38 However, the observed activity of everolimus shows that mToRC1 is an essential target in myeloma patients. Notably, activation of the ToRC2 pathway by overexpression of DEP domain containing mTOR interacting protein (DEPTOR)39 or activation of the ERK pathway may limit the dependency of PI3K and mToRC1.40 The observed down-regulation of the mToR pathway in bystander cells may have contributed to the clinical activity of everolimus, since the PI3K/AKT/mToR pathway plays a critical role in osteoclasts, possibly supporting the malignant plasma cells.41 The anti-angiogenic activity of rapalogues, via inhibition of vascular endothelial growth factor, also has to be taken into account.42
The rate of adverse events was low in this highly pretreated group of patients suggesting that everolimus could potentially be combined with other drugs. As an alternative, catalytic mToR kinase inhibitors block the signaling of both mToR complexes also affecting the mToRC2- dependent AKT activation. Notably, this class of drugs showed greater anti-myeloma activity in vitro when compared to rapalogues.38,43 However, in contrast to rapalogues these compounds activate the ERK pathway as a feedback loop that may limit activity.44 However, current data suggest that the combination of PI3 kinase inhibitors added to rapalogues could be useful given that the feedback loop of S6 protein to PI3K will drive tumor cells in dependency on this pathway.45 Another rationale is to explore synergistic activity in vitro, similar to that of the combination of rapalogues and histone deacetylase inhibitors such as panobinostat.46 However, in a phase I trial combining everolimus with panobinostat in lymphoma patients, severe thrombocytopenia occurred.47 Recently, based on in vitro synergism,48 the combination of everolimus and lenalidomide was explored in a phase I trial and was found to have promising activity and acceptable tolerability in patients with relapsed MM.49 Interestingly, weekly bortezomib combined with temsirolimus showed promising activity in relapsed MM, with responses in patients who had been refractory to prior bortezomib-containing regimens.50
Since everolimus as a single agent showed promising activity, with a limited rate of side effects, in relapsed and/or refractory MM patients, efforts should be made to evaluate optimal combination partners to enhance the anti-myeloma activity and to target the PI3K-AKT-mToR signaling network at multiple sites.
Acknowledgments
The excellent assistance of the study nurses Mrs. Angelika Ziegler, Anja Modlich and Tina Heinig is acknowledged. In addition, we thank all patients and their families.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Pene F, Claessens YE, Muller O, et al. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene. 2002;21(43):6587–6597. [DOI] [PubMed] [Google Scholar]
- 2.Chang H, Qi XY, Claudio J, Zhuang L, Patterson B, Stewart AK. Analysis of PTEN deletions and mutations in multiple myeloma. Leuk Res. 2006;30(3):262–265. [DOI] [PubMed] [Google Scholar]
- 3.Brown EJ, Albers MW, Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756–758. [DOI] [PubMed] [Google Scholar]
- 4.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20(42):5991–6000. [DOI] [PubMed] [Google Scholar]
- 5.Hu L, Shi Y, Hsu JH, Gera J, Van Ness B, Lichtenstein A. Downstream effectors of oncogenic ras in multiple myeloma cells. Blood. 2003;101(8):3126–35. [DOI] [PubMed] [Google Scholar]
- 6.Attar-Schneider O, Drucker L, Zismanov V, Tartakover-Matalon S, Rashid G, Lishner M. Bevacizumab attenuates major signaling cascades and eIF4E translation initiation factor in multiple myeloma cells. Lab Invest. 2012;92(2):178–190. [DOI] [PubMed] [Google Scholar]
- 7.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40(2):310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem. 2005;280(25):23433–23436. [DOI] [PubMed] [Google Scholar]
- 9.Sancak Y, Thoreen CC, Peterson TR, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25(6):903–915. [DOI] [PubMed] [Google Scholar]
- 10.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15(7):807–826. [DOI] [PubMed] [Google Scholar]
- 11.Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294(5544):1102–1105. [DOI] [PubMed] [Google Scholar]
- 12.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294(5548):1942–1945. [DOI] [PubMed] [Google Scholar]
- 13.Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo). 1975;28(10):727–732. [DOI] [PubMed] [Google Scholar]
- 14.Sehgal SN. Rapamune (Sirolimus, rapamycin): an overview and mechanism of action. Ther Drug Monit. 1995;17(6):660–665. [DOI] [PubMed] [Google Scholar]
- 15.Stromberg T, Dimberg A, Hammarberg A, et al. Rapamycin sensitizes multiple myeloma cells to apoptosis induced by dexamethasone. Blood. 2004;103(8):3138–3147. [DOI] [PubMed] [Google Scholar]
- 16.Guenther A, Burger R, Klapper W, et al. mToR and PI3K inhibitors block myeloma cell growth in a synergistic manner. ASH Annual Meeting Abstracts. 2009;114(22):1843. [Google Scholar]
- 17.Frost P, Moatamed F, Hoang B, et al. In vivo antitumor effects of the mTOR inhibitor CCI-779 against human multiple myeloma cells in a xenograft model. Blood. 2004;104(13):4181–4187. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, Gera J, Hu L, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62(17):5027–5034. [PubMed] [Google Scholar]
- 19.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. [DOI] [PubMed] [Google Scholar]
- 20.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. [DOI] [PubMed] [Google Scholar]
- 21.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357(21):2133–2142. [DOI] [PubMed] [Google Scholar]
- 22.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. [DOI] [PubMed] [Google Scholar]
- 23.Sedrani R, Cottens S, Kallen J, Schuler W. Chemical modification of rapamycin: the discovery of SDZ RAD. Transplant Proc. 1998;30(5):2192–2194. [DOI] [PubMed] [Google Scholar]
- 24.Vitko S, Tedesco H, Eris J, et al. Everolimus with optimized cyclosporine dosing in renal transplant recipients: 6-month safety and efficacy results of two randomized studies. Am J Transplant. 2004;4(4):626–635. [DOI] [PubMed] [Google Scholar]
- 25.Vitko S, Margreiter R, Weimar W, et al. Everolimus (Certican) 12-month safety and efficacy versus mycophenolate mofetil in de novo renal transplant recipients. Transplantation. 2004;78(10):1532–1540. [DOI] [PubMed] [Google Scholar]
- 26.Kovarik JM, Kaplan B, Tedesco Silva H, et al. Exposure-response relationships for everolimus in de novo kidney transplantation: defining a therapeutic range. Transplantation. 2002;73(6):920–925. [DOI] [PubMed] [Google Scholar]
- 27.Kovarik JM, Eisen H, Dorent R, et al. Everolimus in de novo cardiac transplantation: pharmacokinetics, therapeutic range, and influence on cyclosporine exposure. J Heart Lung Transplant. 2003;22(10):1117–1125. [DOI] [PubMed] [Google Scholar]
- 28.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–4265. [DOI] [PubMed] [Google Scholar]
- 29.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381(9861):125–132. [DOI] [PubMed] [Google Scholar]
- 31.Schrader C, Janssen D, Meusers P, et al. Repp86: a new prognostic marker in mantle cell lymphoma. Eur J Haematol. 2005;75(6):498–504. [DOI] [PubMed] [Google Scholar]
- 32.Repp R, Schaekel U, Helm G, et al. Immunophenotyping is an independent factor for risk stratification in AML. Cytometry B Clin Cytom. 2003;53(1):11–19. [DOI] [PubMed] [Google Scholar]
- 33.Kellner C, Maurer T, Hallack D, et al. Mimicking an induced self phenotype by coating lymphomas with the NKp30 ligand B7-H6 promotes NK cell cytotoxicity. J Immunol. 2012;189(10):5037–5046. [DOI] [PubMed] [Google Scholar]
- 34.Xu B, Wu Y, Shen L, et al. Two-dose-level confirmatory study of the pharmacokinetics and tolerability of everolimus in Chinese patients with advanced solid tumors. J Hematol Oncol. 2011;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farag SS, Zhang S, Jansak BS, et al. Phase II trial of temsirolimus in patients with relapsed or refractory multiple myeloma. Leuk Res. 2009;33(11):1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrington LS, Findlay GM, Gray A, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166(2):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14(18):1650–1656. [DOI] [PubMed] [Google Scholar]
- 38.Hoang B, Frost P, Shi Y, et al. Targeting TORC2 in multiple myeloma with a new mTOR kinase inhibitor. Blood. 2010;116(22):4560–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson TR, Laplante M, Thoreen CC, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137(5):873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramakrishnan V, Kimlinger T, Haug J, et al. Anti-myeloma activity of Akt inhibition is linked to the activation status of PI3K/Akt and MEK/ERK pathway. PLoS One. 2012;7(11):e50005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugatani T, Hruska KA. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J Biol Chem. 2005;280(5):3583–3589. [DOI] [PubMed] [Google Scholar]
- 42.Frost P, Shi Y, Hoang B, Lichtenstein A. AKT activity regulates the ability of mTOR inhibitors to prevent angiogenesis and VEGF expression in multiple myeloma cells. Oncogene. 2007;26(16):2255–2262. [DOI] [PubMed] [Google Scholar]
- 43.Maiso P, Liu Y, Morgan B, et al. Defining the role of TORC1/2 in multiple myeloma. Blood. 2011;118(26):6860–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoang B, Benavides A, Shi Y, et al. The PP242 mammalian target of rapamycin (mTOR) inhibitor activates extracellular signal-regulated kinase (ERK) in multiple myeloma cells via a target of rapamycin complex 1 (TORC1)/eukaryotic translation initiation factor 4E (eIF-4E)/RAF pathway and activation is a mechanism of resistance. J Biol Chem. 2012;287(26):21796–21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4(10):1533–1540. [DOI] [PubMed] [Google Scholar]
- 46.Simmons JK, Patel J, Michalowski A, et al. TORC1 and class I HDAC inhibitors synergize to suppress mature B cell neoplasms. Mol Oncol. 2014;8(2):261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oki Y, Buglio D, Fanale M, et al. Phase I study of panobinostat plus everolimus in patients with relapsed or refractory lymphoma. Clin Cancer Res. 2013;19(24):6882–6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raje N, Kumar S, Hideshima T, et al. Combination of the mTOR inhibitor rapamycin and CC-5013 has synergistic activity in multiple myeloma. Blood. 2004;104(13):4188–4193. [DOI] [PubMed] [Google Scholar]
- 49.Yee AJ, Hari P, Marcheselli R, et al. Outcomes in patients with relapsed or refractory multiple myeloma in a phase I study of everolimus in combination with lenalidomide. Br J Haematol. 2014;166(3):401–409. [DOI] [PubMed] [Google Scholar]
- 50.Ghobrial IM, Weller E, Vij R, et al. Weekly bortezomib in combination with temsirolimus in relapsed or relapsed and refractory multiple myeloma: a multicentre, phase 1/2, open-label, dose-escalation study. Lancet Oncol. 2011;12(3):263–272. [DOI] [PubMed] [Google Scholar]