Ibrutinib is an oral irreversible inhibitor of the bruton tyrosine kinase (Btk) which has shown promising efficacy and excellent tolerability in patients with chronic lymphocytic leukemia (CLL) and other B-cell malignancies.1 Ibrutinib inhibits BCR signaling, impairs chemokine-mediated adhesion and migration, and induces moderate levels of apoptosis on CLL cells.2,3 It also reduces TCR downstream activation on Th2 cells by targeting another kinase, the interleukin-2–inducible kinase (Itk).4 The combination of ibrutinib with different targeted therapies is now being explored. In a single arm study, the combination of ibrutinib and rituximab in patients with high-risk CLL was generally well-tolerated and resulted in an overall response (OR) rate of 95% and a progression-free survival (PFS) of 78% at 18 months.5 The added effect of rituximab to the ibrutinb therapy is being tested in a randomized trial testing ibrutinb versus ibrutinb and rituximab (clinicaltrials.gov identifier 02007044). Given that macrophages are the most important effector cells in the anti-CD20-therapeutic effect in murine models,6,7 and that they probably play a key role in human anti-CD20 therapy,8,9 we asked whether ibrutinib could interfere with the capacity of human macrophages to mediate phagocytosis of rituximab-coated CLL cells. Our results showed that ibrutinib impairs the phagocytosis of rituximab-opsonized CLL cells by human macrophages.
Macrophages differentiated from healthy peripheral blood monocytes were treated with or without ibrutinib for 30 min and then cultured for 1, 2 or 3 h with CFSE-labeled CLL cells or rituximab-coated CFSE-labeled CLL cells. Cells were then trypsinized and the proportion of macrophages that have taken up CFSE-labeled CLL cells (CFSE+ macrophages) were scored by flow cytometry and verified using confocal microscopy, as previously described.10 As expected, we found that the cultures with rituximab-coated CLL cells showed the highest percentage of CFSE+ macrophages, which increase in a time-dependent manner (Figure 1A, open circles). Ibrutinib was able to reduce these values in all the times evaluated (Figure 1A, solid circles). Low percentages of CFSE+ macrophages were obtained in cultures with uncoated CLL cells, which were not modified by ibrutinib (Figure 1A, open and solid squares). In addition, we found that ibrutinib diminishes the percentage of CFSE+ macrophages in the cultures with rituximab-coated cells in a dose-dependent manner (Figure 1B), which was not associated to a decreased viability of the macrophages (data not shown). Moreover, the inhibitory effect of ibrutinib was not limited to rituximab since comparable results were obtained when campath-coated CFSE-labeled CLL cells were employed (Figure 1C). Similar results were found when macrophages from CLL patients were used: mean±SE of the percentage of CFSE+ macrophages: 26.8±2.1 versus 17.3±27 versus 10.8±0.7 for macrophages cultured with rituximab-coated CFSE-labeled CLL cells alone, with 0.5 μM or 5 μM of ibrutinib (n=6). Representative dot plots are shown in Figure 1D. The results obtained by flow cytometry analysis were validated by confocal microscopy quantifying the number of macrophages that engulfed at least one tumor target cell (Figure 1E). A representative experiment is shown in Figure 1F. In addition, by performing a binding assay at 4°C, we confirmed that ibrutinib did not reduce the binding of rituximab-coated CFSE-labeled CLL cells to macrophages (Figure 1G). It should be mentioned that although ibrutinib 5 μM seems to increase the binding of CLL to macrophages, this difference was not statistically significant.
Figure 1.
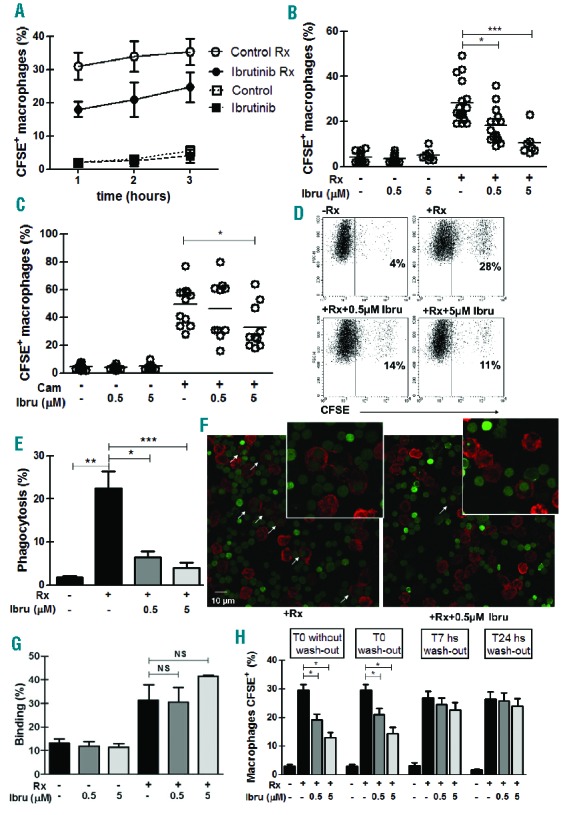
Ibrutinib impairs rituximab (Rx)-coated CLL cells phagocytosis by human macrophages. Human macrophages were obtained by culturing monocytes from healthy donors’ peripheral blood (AC and E–H) or from chronic lymphocytic leukemia patients’ peripheral blood (D) in RPMI 1640 medium supplemented with 10% FCS and with GM-CSF (100 ng/mL) for five days. Purified CLL cells were labeled with 1 μM of CFSE (Invitrogen, Life Technologies, Carlsbad, CA, USA) and then coated or not with 50 μg/mL of rituximab (Roche Diagnostics GmbH, Mannheim, Germany) in RPMI 1640 medium for 30 min at 4°C. (A) For the phagocytosis assay macrophages were cultured for 30 min in 48-well plates in RPMI 1640 medium supplemented with 10% FCS in the presence of 0.5 μM of Ibrutinib (MedKoo Biosciences Inc, Chapel Hill, NC, USA) or the vehicle of the drug, DMSO (Control). Then, uncoated CFSE-labeled CLL cells or rituximab-coated CFSE-labeled CLL (Rx) were added to the culture. The phagocytosis was evaluated after 1, 2, and 3 h of culture when macrophages were trypsinized and analyzed by using a FACScan flow cytometer (BD Immunocytometry Systems, San Jose, CA, USA). The graph shows the percentage of macrophages (determined by morphology in the FSC-H and SSC-H dot plot) that have taken up CLL cells as the percentage of CFSE+ macrophages (n=4). (B and C) The phagocytosis assay was performed in the presence of DMSO (control) or ibrutinib at 0.5 μM and 5 μM, with uncoated CFSE-labeled CLL cells or coated with rituximab (B) (P=0.0001, Kruskal-Wallis test followed by Dunn’s multiple comparison test *P=0.01 to 0.05 and ***P<0.001) or campath (Cam) (C) (P=0.0467, Kruskal-Wallis test followed by Dunn’s Multiple Comparison Test *P<0.05). The percentage of CFSE+ macrophages was analyzed by flow cytometry after 1 h of culture.
(D) The same phagocytosis assay was performed with macrophages derived from CLL patients, dot plots showing FSC-H vs. CFSE of a representative experiment and the percentages of CFSE+ macrophages are shown. (E) The phagocytosis assay was performed as previously described and macrophages were stained with anti-CD14-PE mAb after trypsinization. Then, cells were centrifuged onto cytospin slides and coverslips were mounted on the microscope slide using Fluoromount G. Immunofluorescence images were acquired with a FluoView FV1000 confocal microscope (Olympus, Tokio, Japan) using a Plapon 60 × 1.42 NA oil immersion objective and images were analyzed using the Olympus FV10-ASW software. The phagocytosis was evaluated by a double-blind method, with a separate operator counting at least 200 cells for each experimental condition, and calculating the percentage of macrophages that engulfed at least one tumor target cell with respect to total macrophages. (Kruskal-Wallis test followed by Dunn’s Multiple Comparison Test *P=0.01 to 0.05, **P=0.01 to 0.001 and ***P<0.001). (F) Images from a representative experiment are shown. Macrophages that have phagocyte CFSE+ CLL cells are indicated with white arrows. 3X magnification from the original images are shown in the insert. (G) To analyze if Ibrutinib could modify macrophage-binding of rituximab-coated CLL cells, macrophages were cultured with Ibrutinib for 90 min, then detached with cold PBS-EDTA and incubated with uncoated or rituximab-coated CFSE-labeled CLL cells for 15 min at 4°C, to avoid the internalization of the cells. Afterwards cells were washed with PBS and analyzed by flow cytometry. The graph shows the percentage of macrophages that bind CFSE+ cells (n=5). (H) Macrophages were treated for 2 h with ibrutinib, then some of them were washed out and drug-free medium was added to the culture (wash-out) while others were left with the drug present in the culture and the phagocytosis assay was performed as described before (T0 without wash-out). Washed-out macrophages were then used to perform the phagocytosis assay immediately after ibrutinib was removed (T0 wash out) or after 7 or 24 h of culture in drug-free medium (T7 h or T24 h wash out). *P<0.05, Kruskal-Wallis test followed by the Dunn’s Multiple Comparison Test.
We next asked if macrophages could recover their phagocytic capacity once ibrutinib was removed from the culture. To this aim, macrophages were treated with ibrutinib for 2 h and then either washed- or not washed-out, and immediately used for the phagocytosis assay. In addition, washed-out macrophages were cultured in ibrutinib-free medium for 7, 24 and 48 h before performing the phagocytosis assay. As shown in Figure 1H, the phagocytosis of rituximab-coated CLL cells by macrophages was impaired when ibrutinib was present (T0 without wash-out) or immediately after ibrutinib was removed (T0 wash-out). Interestingly when ibrutinib was washed out, macrophages recovered their phagocytic capacity after 7 h (T 7 h washout), 24 h (T 24 h wash-out) and 48 h of culture (data not shown) in drug-free medium (Figure 1H), showing a reversible effect on macrophages. In contrast, we confirmed a previous report by Honigberg et al.11 showing that ibrutinib was able to irreversibly inhibit BCR-mediated activation of CLL cells (data not shown). Thus, while ibrutinib binds to Btk in an irreversible manner on CLL cells, its inhibitory effect on the phagocytosis of rituximab-coated CLL cells by macrophages was reversible and needed the continuous presence of the drug, suggesting the existence of a reversible target, other than Btk. In line with this, ibrutinib was shown to inhibit other kinases11 including Hck, Fgr, and Lyn, which were described to be involved in Fc-mediated phagocytosis on macrophages.12 So it is possible that ibrutinib impairment of macrophage phagocytosis involves binding to one or more of these kinases in a reversible manner.
Our results are in line with those recently reported by Kohrt et al.13 showing that ibrutinib prevented natural killer cell-mediated cytotoxicity of antibody-coated CLL cells in vitro. They also found that the concurrent treatment with ibrutinib and rituximab or trastuzumab reduces the therapeutic efficacy of both anti-CD20 antibodies in a mouse model, while the sequential treatment with ibrutinib and rituximab restored its anti-lymphoma activity. Moreover, similar results showing ibrutinib inhibition of rituximab-dependent functions on macrophages and neutrophils were published in this Journal while this manuscript was under evaluation.14 Although the combination of rituximab and ibrutinib has been shown to be active and safe in high-risk CLL patients,5 our results and those obtained by Kohrt et al.13 and by Da Roit et al.14 suggest that the sequential administration of ibrutinib followed by rituximab, and not the concurrent treatment with these agents, might enhance their anti-tumor activity in vivo and improve CLL therapy.
Acknowledgments
The authors would like to thank Beatriz Loria, Gabriela Biscochea and María Tejeda for technical assistance. We also thank CLL patients who participated in the study.
Footnotes
Funding: this work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Davids MS, Brown JR. Ibrutinib: a first in class covalent inhibitor of Bruton’s tyrosine kinase. Future Oncol. 2014;10(6):957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590–2594. [DOI] [PubMed] [Google Scholar]
- 3.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15(10):1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchida J, Hamaguchi Y, Oliver JA, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199(12):1659–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taskinen M, Karjalainen-Lindsberg ML, Nyman H, Eerola LM, Leppa S. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin Cancer Res. 2007;13(19):5784–5789. [DOI] [PubMed] [Google Scholar]
- 9.Canioni D, Salles G, Mounier N, et al. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J Clin Oncol. 2008;26(3):440–446. [DOI] [PubMed] [Google Scholar]
- 10.Rafiq S, Butchar JP, Cheney C, et al. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J Immunol. 2013;190(6):2702–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107(29):13075–13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzer-Attas CJ, Lowry M, Crowley MT, et al. Fcgamma receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med. 2000;191(4):669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohrt HE, Sagiv-Barfi I, Rafiq S, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood. 2014;123(12):1957–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Roit F, Engelberts PJ, Taylor RP, et al. Ibrutinib interferes with the cell-mediated anti-tumour activities of therapeutic CD20 antibodies: implications for combination therapy. Haematologica. 2015;100(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


