Waldenström macroglobulinemia (WM) is a rare form of lymphoma characterized by an infiltration of lymphoplasmacytic cells in bone marrow and immunoglobulin M monoclonal gammopathy.1 Despite the introduction of several therapeutic approaches, WM remains incurable.2 Therefore, a better understanding of the mechanisms underlying the immune surveillance around this pathology is still needed to help to develop novel treatment strategies. Natural killer (NK) cells were initially identified through their ability to lyse tumor cells. They express an array of inhibitory, activating, adhesion and cytokine receptors that enable them to kill targets while sparing normal cells.3 NK cells also express the low-affinity Fc receptor (FcγRIIIA/CD16), enabling them to detect antibody-coated target cells and to exert antibody-dependent cell cytotoxicity (ADCC).3 In this study, we focused our attention on NK cells relative to the presence of a circulating B-cell clone in WM patients.
The 47 WM patients in the study were diagnosed at the WM French Reference Center Pitié-Salpêtrière Hospital, Paris, France, according to classical biological criteria.1 This study was approved by the institutional ethic committee at the Pitié-Salpêtrière Hospital (CPPIDF6) and all patients provided informed consent prior to participation.
Characteristics of the WM patients are summarized in Table 1. They were untreated or had received no treatment during the two years prior to the study. Patients were compared to 20 healthy blood donors from the Etablissement Français du Sang. In 26 of 47 patients, circulating B-cell clone was detected with a frequency ranging from 0.7% to 63.0% of lymphocytes, corresponding to 0.1% to 17.0% of leukocytes. NK cells, and B and T lymphocytes from freshly harvested whole blood samples were analyzed on the CD45+ lymphocytic gate after staining with an appropriate antibody cocktail (Online Supplementary Appendix), as previously described.4,5 At least 100,000 leukocytes were analyzed on a FACSCanto II (BD Biosciences) to assess the distribution of NK cells, B and T lymphocytes from WM patients, relative to the presence of circulating B cells (as defined in the Online Supplementary Appendix), and compared with healthy controls. A similar absolute number of whole CD3+ T and CD19+ B cells in healthy controls and WM samples are observed (Figure 1A). The absolute value of B cells correlated significantly with the percentage of a circulating B-cell clone (Figure 1B). There was no difference in the distribution of CD3‒CD56+ NK cells and both CD56dim and CD56bright subsets between the study groups (Figure 1C and D), indicating that WM does not disturb this immune subset, compared with other hematologic diseases.5–8
Table 1.
Main characteristics of WM patients.
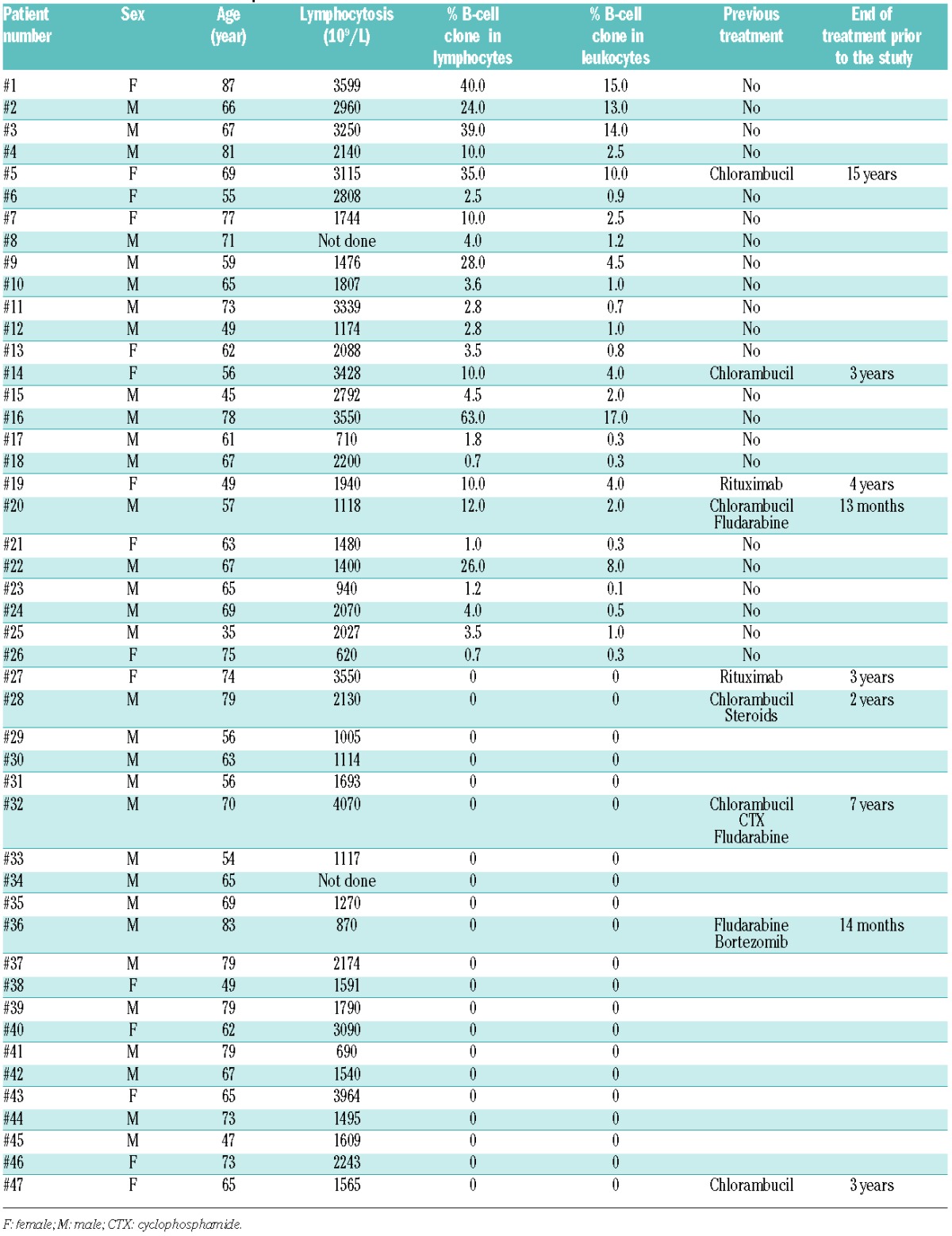
Figure 1.
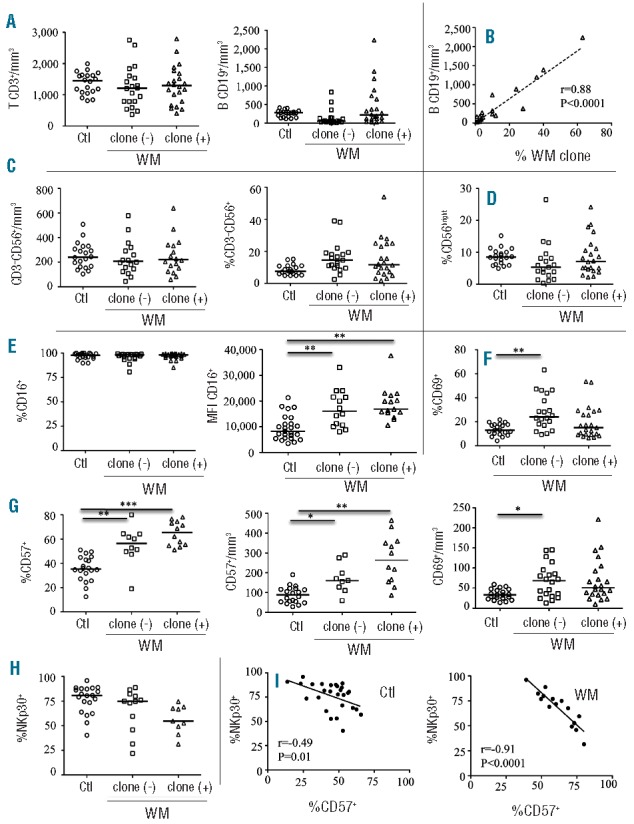
Distribution of lymphocyte subsets and characteristics of natural killer (NK) cells from Waldenström macroglobulinemia (WM) patients with or without circulating B-cell clones. (A) Absolute values of CD3+ T and CD19+ B cells from peripheral blood. (B) Linear regression between absolute value of CD19+ B cells and the percentage of circulating B-cell clones (WM clone). (C) Absolute value and frequency of CD3‒CD56+ NK cells from peripheral blood. (D) Expression of CD56bright gated on CD3‒CD56+ NK cells. (E) Frequency and mean fluorescence intensity (MFI) of CD16 on CD3‒CD56dim NK cells. (F) Frequency and absolute value of CD69 on CD3‒CD56dim NK cells. (G) Frequency and absolute value of CD57 on CD3‒CD56dim NK cells. (H) Frequency of NKp30 on CD3‒CD56dim NK cells. Cells were collected from healthy donors (Ctl; circles) and WM patients without [(clone (−); squares) or with (clone (+); triangles)] circulating B-cell clones (WM clone). Horizontal bars represent median values. Intergroup comparisons were assessed with Kruskal Wallis test and Dunn post test; *P<0.05, **P<0.001, ***P<0.0001. (I) Linear regression between CD57 and NKp30 frequencies on CD3‒CD56dim NK cells in healthy donors (Ctl, left panel) and WM patients (right panel).
The phenotypic frequency of the CD3‒CD56dim cytotoxic NK-cell subset was further investigated on a Gallios (Beckman-Coulter) flow cytometer, while gating out CD3‒CD56bright cell. The frequency of CD56dim NK cells from WM patients was indistinguishable from those of controls in terms of the cell-surface expression of NK receptors (FcγRIIIA/CD16, NKG2A, NKG2D, NKG2C, ILT-2, LAIR1, DNAM-1, NKp80, and 2B4) (Figure 1E and data not shown). However, it is important to note that mean of fluorescence intensity (MFI) values for CD16 are significantly increased in WM patients, regardless of the presence or not of circulating B-cell clone, as compared to controls (Figures 1E). In addition, the early activation marker CD69 is increased in WM both in absolute value and percentage (Figure 1F), whereas it is decreased in chronic lymphocytic leukemia (CLL).5 Notably, the expression of each tested receptor seemed remarkably stable over a 1-year period (Online Supplementary Appendix). More importantly, CD57, a marker associated with terminal NK differentiation,9 was profoundly increased in the WM patients, both in percentage and absolute value, and particularly in those with a circulating B-cell clone (Figure 1G). These data are consistent with those reported by Li et al.10 showing that CD57+ cytotoxic T cells are increased in patients with WM. CD57 was also frequently reported in the association with several cancers, and was strongly linked to a less severe disease and a better outcome, in sharp contrast to what was observed for CD8+ T cells.11 We also observed a weaker expression of the activating NK receptors NKp30 in WM patients compared with controls (Figures 1H). In addition, Figure 1H shows an inverse correlation between CD57 and NKp30 expressions, which is highly more significant in WM patients (Spearman test: r=−0.91, P<0.0001), than in healthy controls (Spearman test: r=−0.49, P=0.01), and that had been previously reported as being related to differentiation of NK cells. Taken together, these data suggest that CD56dim NK cells in WM are more differentiated in patients with a circulating B-cell clone. Similar modulations of the NK-cell repertoire have been described in elderly individuals4 and in patients with acute leukemia,12 but not with CLL.5
We further assessed the overall cytolytic activity of NK cells from WM patients. Direct cytotoxicity was performed against K562 target cells. NK cells were purified from fresh peripheral blood mononuclear cells (PBMCs) at the Flow Cytometry Core CyPS (Pitié-Salpêtrière hospital) with a cell-sorting BD FACSAria II flow cytometer. The purification of CD3‒CD56+ NK cells was more than 98%. Direct cytotoxicity was assessed on purified NK cells before or after IL-2 stimulation (1,000 U/ml, Proleukin, Chiron) for 48 h with a standard51Cr-release assay against the K562 cell line, at a different effector/target (E:T) cell ratio.5,6 A slight but non-significant decrease in the cytotoxicity mediated by non- or IL2-activated NK cells was observed in the group of WM patients with a circulating B-cell clone compared with two other groups (Figure 2A). Of note, granzyme-B and perforin were expressed at equivalent levels in all samples (Online Supplementary Appendix).
Figure 2.
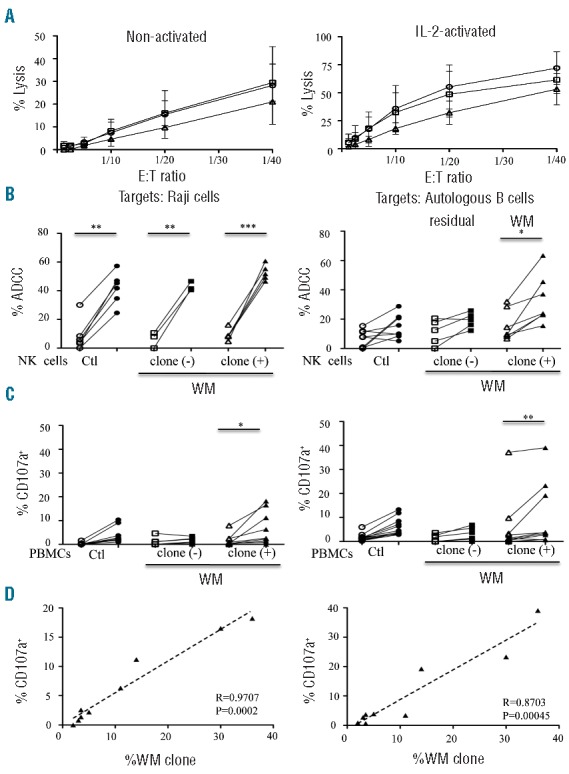
Cytolytic activities of natural killer (NK) cells from Waldenström macroglobulinemia (WM) patients with or without circulating B-cell clones. (A) Direct lysis of non-activated (left panel) and IL2-activated (right panel) NK cells from healthy donors (Ctl; circles; n=6) and WM patients without [clone (-); squares; n=4] or with [clone (+); triangles; n=3] circulating B-cell clones. Cytotoxicity was measured using standard 51Cr release-assays against K562 target cells at different effector:target cell (E:T) ratios. Mean percentage values±s.d. (vertical bars) are shown. (B) Comparison of NK cell ADCC in the presence of rituximab (open symbols) or ublituximab (closed symbols) against Raji cells at 1 ng/mL of anti-CD20 (left panel) or against purified peripheral blood autologous B cells at 100 ng/mL of anti-CD20 (right panel). Assays were performed with purified NK cells from healthy donors (Ctl; circles) and from WM patients without [clone (−); squares] or with [clone (+); triangles] circulating B-cell clones (WM clone) (E:T ratio at 1:10). (C) Degranulation response determined using CD107a expression on CD3‒CD56+ NK cells in the presence of 10 ng/mL (left panel) or 1,000 ng/mL (right panel) of rituximab (open symbols) or ublituximab (closed symbols). Assays were performed on fresh peripheral blood mononuclear cells (PBMC) from healthy donors (Ctl; circles) or WM patients without [clone (−); squares] or with [clone (+); triangles] circulating B-cell clones (WM clone). P values were calculated with the Wilcoxon rank sum test and refer to the comparison between rituximab and ublituximab; *P<0.05, **P<0.001, ***P<0.0001. (D) Linear regression between the percentage of CD107a expression on CD3‒CD56+ NK cells and the percentage of circulating B-cell clones (WM clone) after treatment with 10 ng/mL (left panel) or 1000 ng/mL (right panel) of ublituximab. All data relative to ADCC and CD107a expression are presented after subtraction of the values obtained in the absence of any antibody.
Antibody-dependent cellular cytotoxicity (ADCC) assays were performed with purified non-activated NK cells in the presence of 51Cr-labeled Raji target cells or purified autologous peripheral blood B cells, at an effector ratio of 1:10 in the presence of anti-CD20 rituximab (Roche Ltd.) or ublituximab (LFB Biotech), an optimized anti-CD20 mAb characterized by a low fucose content in its Fc region that improves FcγRIIIA (CD16) binding and consequently increases ADCC activity.13 ADCC against Raji cells was significantly increased in the presence of 1 ng/mL of ublituximab, compared with rituximab, in all groups (Figure 2B, left panel). However, ADCC against autologous B cells was significantly increased in WM patients with a circulating B-cell clone only in the presence of ublituximab, and not of rituximab (Figure 2B, right panel). The comparison of ADCC mediated in the presence of Raji cells and autologous B cells revealed that ADCC efficacy is more dependent on the intrinsic resistance to the tumor target cells than on the NK functionality. Additional support for the findings of the ADCC assays was provided from the degranulation capacities of NK cells, which are highlighted by CD107a cell-surface expression by flow cytometry in the presence of these two anti-CD20 mAbs, as described.6 Indeed, regardless of the concentration, ublituximab triggered better effector functions to clear WM cells in patients with a circulating B-cell clone compared with rituximab (Figure 2C), which is consistent with findings in CLL patients.5,6 Importantly, the percentage of CD107a+ NK cells significantly correlated with the frequency of circulating B-cell clones only in the presence of ublituximab (Figure 2D).
Taken together, these data show that the efficacy of NK cells against B-cell clones is greater with ublituximab than with rituximab, allowing the NK phenotypic features observed in WM to be bypassed.
Finally, our findings provide additional insights into the cellular mechanisms underlying the clinical activity of optimized anti-CD20 mAbs. An ongoing phase I/II study of ublituximab (NCT01647971) to evaluate monotherapy in rituximab-relapse and refractory B-cell lymphoid malignancies, including WM, shows promising early results.14 However, it would be important to perform side-by-side in vivo experiments in human, testing both ublituximab and rituximab efficacy in WM. In parallel, further combination of optimized anti-CD20 mAbs with novel chemo-free drugs that target B-cell receptor signaling such as bruton tyrosine kinase (BTK) inhibitor (ibrutinib) and phosphatidylinositol-3-kinase delta (PI3Kδ) inhibitor (idelalisib) may achieve complete remission in WM patients.15
Acknowledgments
The authors would like to thank the patients for providing the research samples used in this study. We also thank C. Blanc and B. Hoareau from the Flow Cytometry Core CyPS (UPMC, Univ Paris 06, Paris, France) for the cell sorting. J. Decocq, M. Brissard, S. Gueguen and A. Grelier are acknowledged for their technical assistance. This study was supported in part by funds from the Laboratoire Français de Fractionnement et des Biotechnologies (LFB, Les Ulis, France), the association la Ligue Contre le Cancer (RS08/75-4) and INSERM. V. Vieillard is recipient of a Contrat Hospitalier de Recherche Translationnelle (CHRT).
Footnotes
The online version of this article has a Supplementary Appendix.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leblond V, Johnson S, Chevret S, et al. Results of a randomized trial of chlorambucil versus fludarabine for patients with untreated Waldenstrom macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic lymphoma. J Clin Oncol. 2013;31(3):301–317. [DOI] [PubMed] [Google Scholar]
- 3.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12(4):239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Garff-Tavernier M, Beziat V, Decocq J, et al. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9(4):527–535. [DOI] [PubMed] [Google Scholar]
- 5.Le Garff-Tavernier M, Decocq J, de Romeuf C, et al. Analysis of CD16+CD56dim NK cells from CLL patients: evidence supporting a therapeutic strategy with optimized anti-CD20 monoclonal antibodies. Leukemia. 2011;25(1):101–109. [DOI] [PubMed] [Google Scholar]
- 6.Le Garff-Tavernier M, Herbi L, de Romeuf C, et al. Antibody-dependent cellular cytotoxicity of the optimized anti-CD20 monoclonal antibody ublituximab on chronic lymphocytic leukemia cells with the 17p deletion. Leukemia. 2014;28(1):230–233. [DOI] [PubMed] [Google Scholar]
- 7.Farnault L, Sanchez C, Baier C, Le Treut T, Costello RT. Hematological malignancies escape from NK cell innate immune surveillance: mechanisms and therapeutic implications. Clin Dev Immunol. 2012;2012:421702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lion E, Willemen Y, Berneman ZN, Van Tendeloo VF, Smits EL. Natural killer cell immune escape in acute myeloid leukemia. Leukemia. 2012;26(9):2019–2026. [DOI] [PubMed] [Google Scholar]
- 9.Bjorkstrom NK, Riese P, Heuts F, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116(19):3853–3864. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Sze DM, Brown RD, et al. Clonal expansions of cytotoxic T cells exist in the blood of patients with Waldenström macroglobulinemia but exhibit anergic properties and are eliminated by nucleoside analog therapy. Blood. 2010;115(17):3580–3588. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen CM, White MJ, Goodier MR, Riley EM. Functional Significance of CD57 Expression on Human NK Cells and Relevance to Disease. Front Immunol. 2013;4:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fauriat C, Just-Landi S, Mallet F, et al. Deficient expression of NCR in NK cells from acute myeloid leukemia: Evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2007;109(1):323–330. [DOI] [PubMed] [Google Scholar]
- 13.de Romeuf C, Dutertre CA, Le Garff-Tavernier M, et al. Chronic lymphocytic leukaemia cells are efficiently killed by an anti-CD20 monoclonal antibody selected for improved engagement of FcgammaRIIIA/CD16. Br J Haematol. 2008;140(6):635–643. [DOI] [PubMed] [Google Scholar]
- 14.Deng C, Effie Amengual J, Schreeder MT, et al. A phase I dose-escalation trial of ublituximab (TG-1101), a novel anti-CD20 monoclonal antibody (mAb), for rituximab relapsed/refractory B-cell lymphoma patients. J Clin Oncol. 2013;31(15):8575. [Google Scholar]
- 15.Ghobrial IM. Choice of therapy for patients with Waldenstrom macroglobulinemia. J Clin Oncol. 2013;31(3):291–293. [DOI] [PubMed] [Google Scholar]


