Abstract
The prevalence of periodontal disease (POD) among adults aged 30 years and older in the United States is reported to be more than 47%, with higher prevalence seen among patients with diabetes mellitus (DM). POD has been associated with systemic inflammation, a known risk factor for cardiovascular and bone disease, both of which are more common in patients with DM. However, there is mixed evidence that treatment of POD reduces inflammation, improves DM control, and reduces DM complications. Our study objectives are to assess factors associated with POD in patients with DM and determine the impact of POD treatment on inflammation and bone turnover biomarkers associated with complications of DM.
In this pilot study, we will first recruit 200 patients with DM to complete a 48-item investigator-administered questionnaire designed to assess socio-economic status, oral health status, adequacy of oral care, glycemic control and presence of DM complications. Responses will be verified by individual chart review. Then, using a crossover design, a subgroup of 24 subjects with responses suggestive of POD will be assigned to undergo POD treatment for three months followed by three months of routine dental care (Group 1) or be followed for three months during routine dental care then receive POD treatment for three months (Group 2). Outcome measures will be collected before and after POD treatment and include glycemic control and inflammatory and bone turnover biomarkers.
We hypothesize that the prevalence of POD among DM patients will be associated with inadequate glycemic control and greater DM complications.
Keywords: Periodontal disease, glycemic control, inflammation biomarkers, diabetes complications and bone turnover biomarkers
Introduction
The 2012 National Diabetes Statistics Report estimates that 29.1 million people (9.3% of the U.S. population) are living with diabetes mellitus (DM) [1]. With increasing incidence and longer life expectancy, the prevalence of DM will double by the year 2050 [2]. The prevalence of periodontal disease (POD) among adults 30 years or older in the National Health and Nutrition Examination Survey (NHANES) is 47% [3]. A higher prevalence of POD is seen among patients with DM [1, 4-7]. Systemic inflammation is a common finding among patients with DM and POD [7-9]. The explanation for this increase in inflammation relates to the presence of chronic periodontal bacterial infection causing continuous release of inflammatory mediators in the systemic circulation [7-9]. Inflammation has been implicated in the pathogenesis of both cardiovascular disease (CVD) [10-12] and bone disease [13, 14]. There is existing evidence that links POD to adverse CVD outcomes [4, 7-9]. Patients with POD have been reported to have higher levels of bone turnover markers [14]. The reasoning behind this relationship is not well understood but it has been postulated that chronic inflammation from POD causes increased bone loss, which is reflected in the biomarkers [14]. In addition, those patients with DM have an increased risk for fractures [15]. In spite of these findings, evidence supporting improved DM control and reduction in DM complications including osteoporosis after both surgical and non-surgical treatment of POD in patients with DM is mixed [4, 7, 16]. This may be partly responsible for the lack of specific guidelines and weak emphasis on prevention and treatment of POD in the clinical care of patients with DM [17, 18].
Our goals in this pilot study are to determine the factors associated with POD in patients with DM and to determine if treatment of POD in patients with DM will result in changes in glycemic control as well as inflammation and bone turnover markers. Our long-term goal is to begin to establish the necessary evidence base for development of guidelines for the management of POD in patients with DM that will positively impact the morbidity, mortality, and health care costs in this patient population.
Material and Methods
Study design
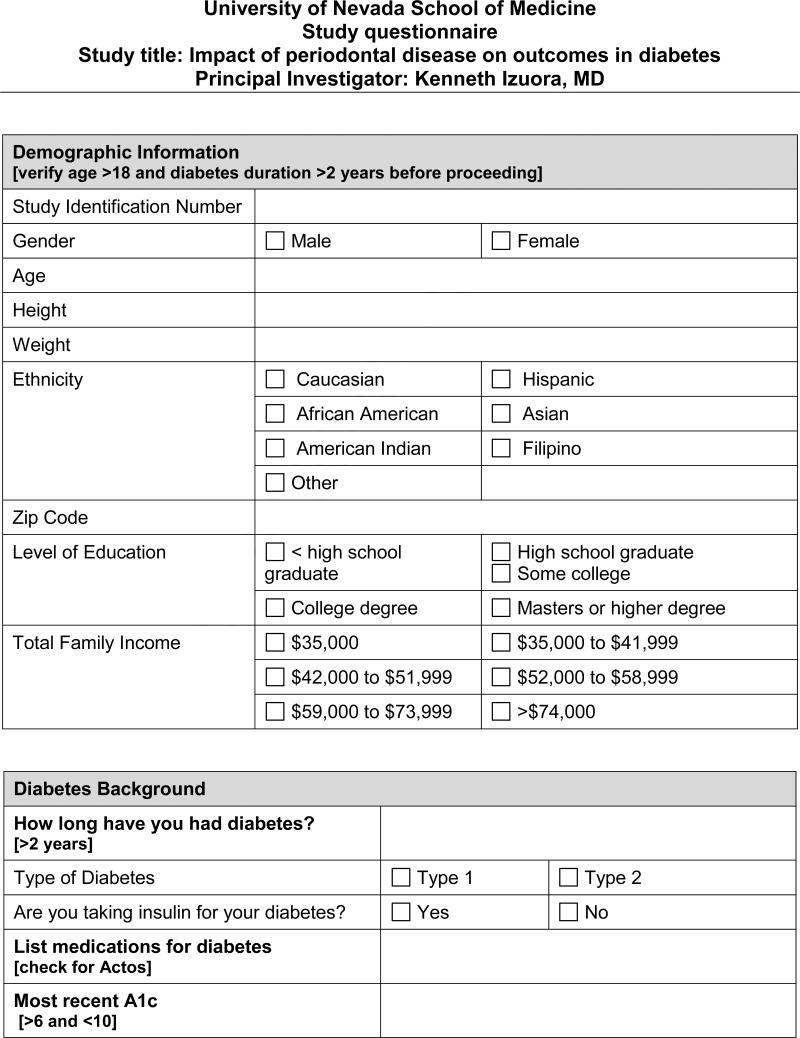
In the cross-sectional part of the study, we will administer a questionnaire (Appendix) to 200 consecutive patients with DM (both type 1 and type 2) attending an urban medical school-affiliated clinic. We will collect data on demographic information, socio-economic status, oral health status, dental care, DM history (duration, control and complications) and bone health.
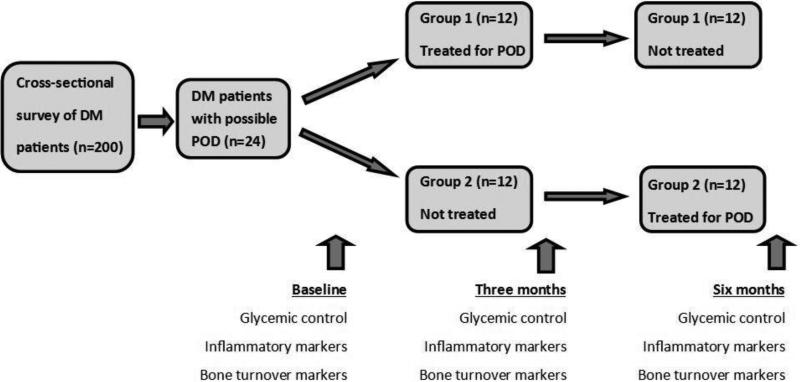
For the pilot intervention part of the study, a subgroup consisting of the first 24 participants with survey responses suggestive of POD [19-21] (i.e. one or more affirmative answers to receipt of deep cleaning, loose teeth, tooth sensitivity, and/or gum bleeding) will be assigned to two groups (group 1 will contain the odd-numbered patients, and group 2 the even-numbered) according to their sequence of enrollment (Figure 1). Based on a simple power calculation, and on systemic inflammation biomarker C-reactive protein (CRP) levels found in Pejcic [22], sample sizes of 11 for each subgroup will enable us to detect an effect size of 1.1 between the mean subgroup CRP levels in our study with sufficient statistical power (80%) at a one-sided significance level of p<0.05. Effect size is computed as the difference in subgroup means versus the pooled standard deviation of the two samples. Similarly, using the baseline diabetic control hemoglobin A1c (A1c) levels specified in Rudolph, et al. [23], the power calculation shows that we will be able to detect an effect size of 1.1 between mean subgroup A1c levels with sufficient statistical power (80%) at a one-sided significance level of p<0.05, using a sample size of 11 for each subgroup. This converts to an 18% decline in mean A1c levels before and after treatment for POD. Group 1 will receive intensive treatment for POD for three months followed by an observation period of three months with routine dental care (twice daily toothbrushing, regular interdental flossing and regular use of mouthwash), while group 2 will begin with routine dental care for three months followed by intensive treatment for POD for three months. We will compare their mean glycemic control, inflammation marker and bone turnover marker levels at baseline, at three months and at six months follow-up for both subgroups.
Figure 1.
Flow diagram of group assignment and collection of outcome measures. DM = diabetes mellitus; POD = periodontal disease.
This study was approved by the Institutional Review Board of our institution, and the study protocol was registered at http://www.clinicaltrials.gov (NCT02289066).
Study population
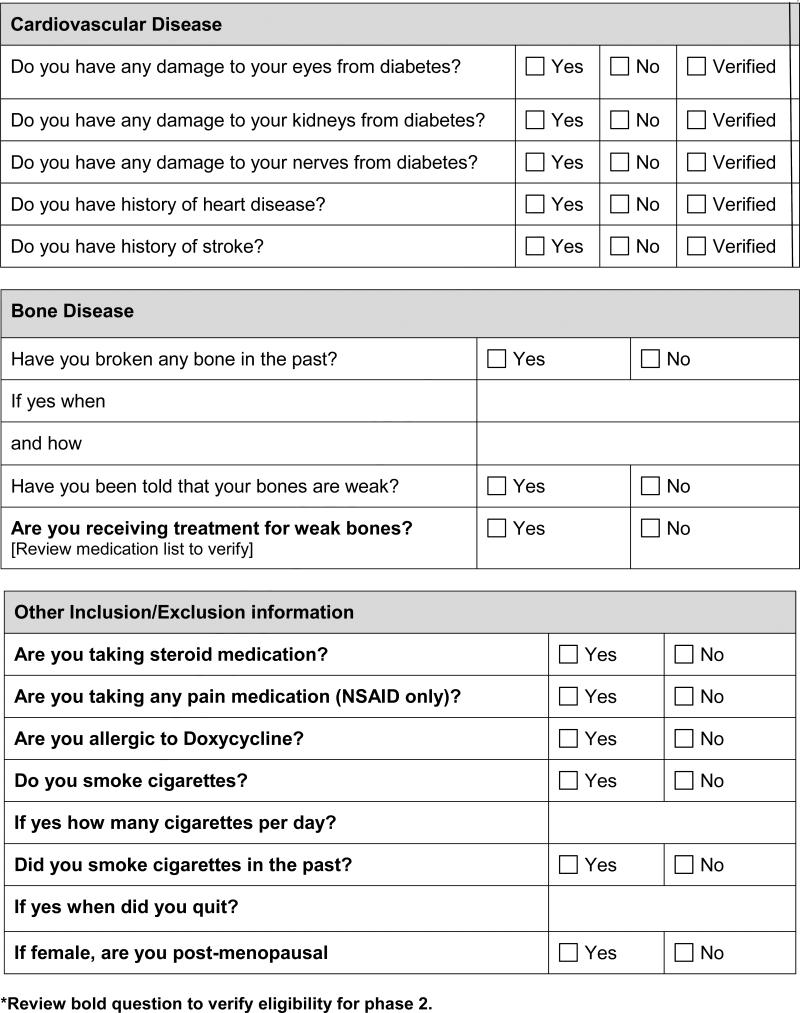
Adult patients (18 years or older) with DM (Type 1 or 2) for at least two years who present for routine DM clinic visit will be approached for enrollment in the study, which will cease when a sample size of 200 enrollees is reached. A subgroup of the first 24 subjects with survey responses suggestive of POD will be selected to undergo intervention. This subgroup will be selected based on the following inclusion and exclusion criteria:
Inclusion criteria
Exclusion criteria
Treatment with anti-inflammatory medications
Cigarette smoking
Treatment with thiazolidinediones
Previous diagnosis of osteoporosis or treatment for osteoporosis with FDA-approved agents.
Current treatment for POD
Those subjects who are not selected for POD treatment will be given the recommendation to continue routine dental care per American Diabetes Association guidelines. There will be no study-related follow-up of subjects not in the POD treatment subgroup of 24, beyond completion of the questionnaire.
Enrollment process
Our clinic has more than 1000 patients being followed for their routine DM care. We will recruit 200 consecutive patients who present for their regular clinic visit. Informed consent and HIPAA authorization will be obtained during the clinic visit. Using the aforementioned questionnaire, we will gather the following information by interviewing the patient directly and through a review of their clinic charts:
Demographic information (age, gender, race/ethnicity)
Socio-economic status (average household income, level of education, zip codes)
DM history (type, duration, treatment)
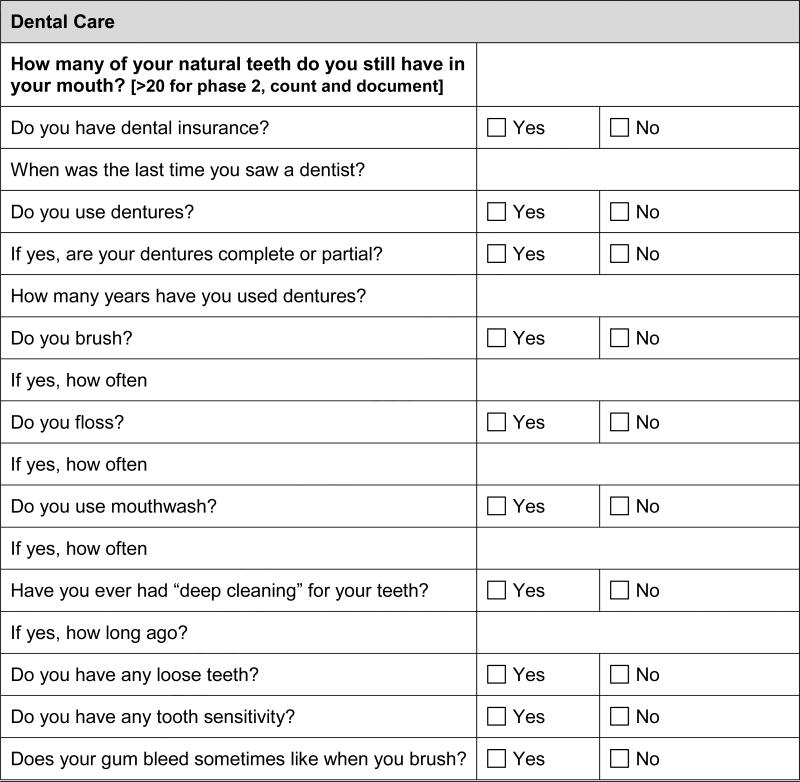
Oral health history (loose teeth, dental loss, gum disease/bleeding, frequency of brushing/flossing/mouthwash use, frequency of dentist visits)
Smoking status and history
Complications of DM (retinopathy, nephropathy, neuropathy, coronary artery disease, stroke, peripheral vascular disease)
Bone health (history of fractures, height loss, spine deformity, known diagnosis of osteoporosis)
Study procedures
After signing consent, subjects (n=200) will complete the questionnaire at the time of their routine DM clinic visit. Subjects with survey responses suggestive of POD (n=24) will be examined by a single study dentist to confirm the presence of POD before receiving treatment. Periodontal examination will include: clinical attachment loss (based on pocket depths and free gingival margin), bleeding upon probing, furcation involvement, tooth mobility, and panoramic oral radiographs. Final periodontal diagnosis will be determined by clinical attachment loss and bleeding upon probing. Treatment for POD will consist of scaling and root planing (subgingival curettage), removal of supragingival calculus and plaque, and oral doxycycline 20mg twice daily for 90 days. Additionally, any dental condition that might be a contributory cause of periodontal inflammation will be addressed per patient acceptance (e.g. extractions or endodontic emergency treatment). Periodontal examination parameters will be measured again at 6 weeks after initiation of treatment as part of the standard of care and at three months after the completion of therapy to determine improvements in periodontal status. Outcomes related to this study (glycemic control, inflammatory and bone turnover markers) will only be measured before POD treatment (baseline) and after the completion of POD treatment (at 3 months and 6 months). Both subgroups will be provided mouthwash, toothbrush, toothpaste and dental floss samples during the study.
The principal investigator or study coordinator will collect fasting blood (venous) and clean-catch urine samples from both subgroups for assessment of biomarkers at baseline, three months and six months of follow up, during their scheduled study visits. Samples for glucose will be collected using Sodium Fluoride tubes, A1c will be collected in EDTA tubes and the samples for the rest of the biomarkers will be collected in heparin tubes. A total of 3 – 5 ml of blood will be collected for each of the tubes. Except for A1c samples, all other samples will be centrifuged with separation of the plasma. Urine samples will be collected in standard sterile specimen cups. All samples will be stored at -70 degrees centigrade until they are assayed. The biomarkers that will be measured will include:
DM control: A1c, blood glucose and insulin levels
Inflammation: Serum high-sensitivity C-reactive protein (CRP) and tumor necrosis factor alpha
Bone turnover: Urinary C-terminal telopeptide and serum bone-specific alkaline phosphatase
All samples will be analyzed at the Clinical and Research Laboratory of the Division of Endocrinology, Diabetes and Metabolism, University of Tennessee, Memphis (http://www.uthsc.edu/endolab/). This lab is familiar to the principal investigator and will perform the assays at a reasonable cost.
During the three months before (group 2) and after (group 1) intensive treatment for POD (i.e., the “not treated” portion of the crossover), the subjects will continue to receive reminders during their routine DM follow up visits to obtain regular dental care according to current ADA standards [17].
Participant Safety
We will explain the rationale for the study and the potential risks and benefits to all patients approached for enrollment. Adequate time will be given to answer all questions and to ensure voluntary participation. Informed consent and HIPAA authorization will be obtained to access the patients’ records for the study. The 24 participants in the subgroup that will be undergoing POD intervention will also be informed of potential additional risks and discomfort associated with blood sample collection and treatment for POD, including bruising or infection from dental cleaning, and adverse effects of doxycycline (photosensitivity, gastointestinal upset, blood disorders and, rarely, hepatotoxicity). There is also the risk of exposure to x-rays during the course of their dental evaluation. These risks are not more than the usual risk involved in treatment for POD outside of the research environment. These risks are considered to be minimal, since we will follow the standard evidence-based approach, as in normal clinical settings.
The risk for the patients in the non-intervention, cross-sectional part of the study is minimal, as their participation is limited to completion of the questionnaire. For the group undergoing intervention, any unanticipated adverse events during the course of their treatment will be evaluated promptly in the appropriate clinical setting and will be reported to the overseeing institutional review board.
We will emphasize that participation will be voluntary, and patients will be assured that they can withdraw from the study at any time without any impact on their regular care with their provider. Protected Health Information (PHI) will be de-identified, and the key will be stored in a password- protected computer. Paper records will be stored in a locked cabinet in the primary investigator's office. Electronic records that need to be deleted will be overwritten and saved as blank files, while paper records will be destroyed using a cross-cut shredder. Only the primary investigator and the study coordinator will have access to PHI.
Statistical analyses
To determine whether the prevalence of POD among patients with DM is statistically significantly higher than in the general population, we will perform a simple hypothesis test of proportions. We would expect approximately 47% of the general population to have POD, based on the NHANES data [3]. Thus, for every 100 patients with DM enrolled, we expect notably more than 47 patients to have POD. A test of proportions will show whether this difference is statistically significant.
To determine whether biomarker levels are statistically different between the two intervention subgroups, standard Student's t-tests will be performed. To measure the impact of POD treatment on glycemic control and inflammatory and bone turnover marker levels, standard Student's t-tests will be performed on pre- and post-treatment biomarker levels. To determine which factors predict the presence of POD in DM patients, a multivariate logistic regression analysis will be performed on the questionnaire data, with the adjustment of the co-variables of age, gender, DM type, BMI, and smoking in the regression model.
Discussion
An estimated 29.1 million (9.3%) Americans were living with DM, with direct costs estimated at 176 billion dollars in 2012 [1]. This is an increase from 25.8 million people in 2010 [6]. With the increasing rate of obesity in the United States, the incidence of DM is also expected to rise and this increase, along with the improved longevity that accompanies better DM care, will result in an estimated doubling in the prevalence of DM by the year 2050 [2]. The burden of disease from the complications of DM is also expected to increase. Several of the complications of DM (micro- and macro-vascular) have been shown to have clear associations with DM control, and evidence-based interventions are available for prevention of these complications. However, there is less evidence of the role of POD and the impact of POD treatment on DM control and on preventing vascular complications and bone disease that are more prevalent in DM patients.
The relative risk for hip fracture in type 1 and type 2 DM patients compared to normal subjects at the same bone density T-score was estimated to be 6.3 and 1.7 respectively [15]. Systemic inflammation is associated with increased risk for cardiovascular disease [10-12] but also associated with increased risk for fractures [13]. POD is associated with dental loss and increased systemic inflammation [7-9]. The prevalence of POD among adults 30 years or older in the NHANES was 47% [3]. The prevalence of POD among patients with DM is higher than in the general population [1, 4-7]. In non-diabetic patients, there is some association between POD and osteoporosis [24]. Hence, the presence of POD, with its associated increase in systemic inflammation, in patients with DM may be one of the contributing factors to the relatively higher risk for fracture found in DM patients as compared to normal patients.
Our study will provide initial evidence to explore the prevalence of POD in our DM patient population and the association between POD, inflammation and bone turnover markers in these patients. By first looking at a cross-section of our DM patient population, we will identify the disease pattern of POD among our patients across different age, socio-economic and ethnic groups. This information will be useful in designing future studies and effective implementation of interventions. Then, results from our pilot study will assist with the exploration of associations between different DM complications and their potential causes, in order to strengthen the evidence for intervention directed at these causes. This may result in reduction of the impact of these complications on patients with DM. With regard to oral health, the American Diabetes Association and the International Diabetes Federation currently lack specific recommendations for more aggressive oral care in patients with DM [17, 18]. The recommended approach for oral care in patients with DM is similar to that for the general population, despite the higher risk and worse outcomes associated with POD in DM patients. This is likely a reflection of the insufficient/mixed evidence showing benefit of treatment.
By demonstrating the potential impact of treatment of POD via changes in biomarkers following treatment, we can lay the foundation for larger randomized trials that have the potential of providing stronger evidence on which to base clearer practice guidelines that will lead to improved outcomes for patients with DM and POD.
Study Limitations
Our proposed study has several limitations. Since it is a single-site study using a sample population from the Las Vegas area, there is the potential that environmental factors peculiar to our locality may contribute to the outcomes of interest. However, because our patient population has similar demographic characteristics and rate of complications compared to national figures, we believe our findings can be generalized.
Another limitation is that changes in bone turnover markers suggest bone loss or bone formation but do not necessarily equate to fracture risk. A bone mineral density (BMD) assessment would be ideal; however, changes in BMD take longer to occur, and we are unlikely to observe those changes within the timeframe of a pilot study. However, we expect that the data from this pilot will be a useful foundation for a larger study that will look at bone mineral density and fractures as outcome measures.
Lastly, our study may not achieve statistical significance with our small sample size if we encounter a high degree of participant attrition over the six month follow-up period. Even though we do not anticipate high participant attrition, we increased our sample size to include a 10% attrition rate. We will also carefully collect participant's contact information during enrollment, so as to minimize attrition. Because the participants will be followed regularly for their DM care, they will receive the follow-up study appointment reminders.
Current Study Status
We have enrolled 202 subjects in the questionnaire arm of the study, and, from this population, we have completed enrollment of the 24 subjects in the subgroup undergoing POD treatment. All of the subjects in this subgroup have completed the study with > 90% follow up rate; however, two subjects’ data will be excluded from analysis, based on discovery that they met exclusion criteria. Blood and urine samples have been collected for all completed study visits and are currently being analyzed.
Acknowledgements
This work is being conducted with support from the National Institutes of Health/National Institute of General Medical Sciences (1U54GM104944-01). The authors also acknowledge the assistance of Gayle Allenback, Clinical/Translational Research Data Analyst in the Office of Medical Research at the University of Nevada School of Medicine – Las Vegas, for her work in the manuscript editing and submission process.
Appendix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention . National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. U.S. Department of Health and Human Services; Atlanta, GA: 2014. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. [Google Scholar]
- 2.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. http://dx.doi.org/10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–20. doi: 10.1177/0022034512457373. http://dx.doi.org/10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 4.Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Periodontol. 2013;84(4 Suppl):S153–69. doi: 10.1902/jop.2013.1340017. http://dx.doi.org/10.1902/jop.2013.1340017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16(1):329–34. http://care.diabetesjournals.org/content/16/1/329. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. [Google Scholar]
- 7.Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. 2008;14(3):191–203. doi: 10.1111/j.1601-0825.2008.01442.x. http://dx.doi.org/10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- 8.D'Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83(2):156–60. doi: 10.1177/154405910408300214. http://dx.doi.org/10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- 9.D'Aiuto F, Ready D, Tonetti MS. Periodontal disease and C-reactive protein-associated cardiovascular risk. J Periodontal Res. 2004;39(4):236–41. doi: 10.1111/j.1600-0765.2004.00731.x. http://dx.doi.org/10.1111/j.1600-0765.2004.00731.x. [DOI] [PubMed] [Google Scholar]
- 10.Jeevanantham V, Singh N, Izuora K, D'Souza JP, Hsi DH. Correlation of high sensitivity C-reactive protein and calcific aortic valve disease. Mayo Clin Proc. 2007;82(2):171–4. doi: 10.4065/82.2.171. http://dx.doi.org/10.4065/82.2.171. [DOI] [PubMed] [Google Scholar]
- 11.Izuora KE, Chase HP, Jackson WE, Coll JR, Osberg IM, Gottlieb PA, et al. Inflammatory markers and diabetic retinopathy in type 1 diabetes. Diabetes Care. 2005;28(3):714–5. doi: 10.2337/diacare.28.3.714. http://dx.doi.org/10.2337/diacare.28.3.714. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–9. doi: 10.1056/NEJM199704033361401. http://dx.doi.org/10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 13.Koh JM, Khang YH, Jung CH, Bae S, Kim DJ, Chung YE, et al. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int. 2005;16(10):1263–71. doi: 10.1007/s00198-005-1840-5. http://dx.doi.org/10.1007/s00198-005-1840-5. [DOI] [PubMed] [Google Scholar]
- 14.Yoshihara A, Deguchi T, Hanada N, Miyazaki H. Relation of bone turnover markers to periodontal disease and jaw bone morphology in elderly Japanese subjects. Oral Dis. 2009;15(2):176–81. doi: 10.1111/j.1601-0825.2008.01511.x. http://dx.doi.org/10.1111/j.1601-0825.2008.01511.x. [DOI] [PubMed] [Google Scholar]
- 15.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495–505. doi: 10.1093/aje/kwm106. http://dx.doi.org/10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 16.Engebretson SP, Hyman LG, Michalowicz BS, Schoenfeld ER, Gelato MC, Hou W, et al. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA. 2013;310(23):2523–32. doi: 10.1001/jama.2013.282431. http://dx.doi.org/10.1001/jama.2013.282431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. http://dx.doi.org/10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Diabetes Federation Clinical Guidelines Task Force Guideline on oral health for people with diabetes. International Diabetes Federation. 2009 http://www.idf.org/guidelines/diabetes-and-oral-health/guideline.
- 19.Dietrich T, Kaiser W, Naumann M, Stosch U, Schwahn C, Biffar R, et al. Validation of a multivariate prediction rule for history of periodontitis in a separate population. J Clin Periodontol. 2009;36(6):493–7. doi: 10.1111/j.1600-051X.2009.01400.x. http://dx.doi.org/10.1111/j.1600-051X.2009.01400.x. [DOI] [PubMed] [Google Scholar]
- 20.Eke PI, Genco RJ. CDC Periodontal Disease Surveillance Project: background, objectives, and progress report. J Periodontol. 2007;78(7 Suppl):1366–71. doi: 10.1902/jop.2007.070134. http://dx.doi.org/10.1902/jop.2007.070134. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Weng H, Lin X. Self-reported questionnaire for surveillance of periodontitis in Chinese patients from a prosthodontic clinic: a validation study. J Clin Periodontol. 2013;40(6):616–23. doi: 10.1111/jcpe.12103. http://dx.doi.org/10.1111/jcpe.12103. [DOI] [PubMed] [Google Scholar]
- 22.Pejcic A, Kesic L, Milasin J. Association between Periodontopathogens and CRP Levels in Patients with Periodontitis in Serbia. J Dent Res Dent Clin Dent Prospects. 2011;5(1):10–6. doi: 10.5681/joddd.2011.003. http://dx.doi.org/10.5681/joddd.2011.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudolph JW, Hirsch IB. Assessment of therapy with continuous subcutaneous insulin infusion in an academic diabetes clinic. Endocrinol Pract. 2002;8(6):401–5. doi: 10.4158/EP.8.6.401. http://dx.doi.org/10.4158/EP.8.6.401. [DOI] [PubMed] [Google Scholar]
- 24.Wactawski-Wende J. Periodontal diseases and osteoporosis: association and mechanisms. Ann Periodontol. 2001;6(1):197–208. doi: 10.1902/annals.2001.6.1.197. http://dx.doi.org/10.1902/annals.2001.6.1.197. [DOI] [PubMed] [Google Scholar]