Abstract
Objective
To evaluate vasopressin vs dopamine as initial therapy in ELBW infants with hypotension during the first 24 hours of life.
Study design
Hypotensive ELBW infants ≤ 30 weeks’ gestation and ≤ 24 hours old randomly received treatment with vasopressin or dopamine in a blinded fashion. Normotensive infants not receiving vasopressor support served as a comparison group.
Results
Twenty hypotensive ELBW infants received vasopressin (n=10) or dopamine (n=10), and 50 were enrolled for comparison. Mean gestational age was 25.6 ± 1.4 weeks and birth weight 705 ± 154 g. Response to vasopressin paralleled that of dopamine in time to adequate mean BP (Kaplan-Meier curve, p=0.986); 90% of infants in each treatment group responded with adequate BP. The vasopressin group received fewer doses of surfactant (p<0.05), had lower PaCO2 values (p<0.05), and were not tachycardic (p<0.001) during vasopressin administration, compared with the dopamine group.
Conclusions
Vasopressin in ELBW infants as the initial agent for early hypotension appeared safe. This pilot study supports a larger randomized controlled trial of vasopressin vs dopamine therapy in ELBW infants with hypotension.
Keywords: neonatal, preterm, extremely low birth weight infant, vasopressors, blood pressure
Hypotension in extremely low birth weight (ELBW, birth weight ≤1000 g) infants is common and close to half of the infants receive vasopressors for its treatment.1, 2 Hypotensive preterm infants have a higher incidence and increased severity of intraventricular hemorrhage, periventricular leukomalacia, retinopathy of prematurity, chronic lung disease, and death compared with normotensive preterm infants.3-7 Additionally, they have poorer neurodevelopmental outcome.2, 8
Dopamine is traditionally used as the initial agent for treatment of neonatal hypotension because of its inotropic and vasoconstrictive actions.9 Additional vasopressors and/or hydrocortisone are often administered to increase blood pressure (BP) to the desired range. Unfortunately, when more than one agent is used, mortality may be higher in hypotensive ELBW infants.10 Dopamine has variable dose-related activation of D1, D2, β1, β2, α1, and α2 receptors,11 but it is unclear whether similar receptor activation occurs in ELBW infants. Moreover, dopamine increases pulmonary arterial pressure12 and inhibits thyrotropin, growth hormone, and gonadotropins.11, 13
A few comparative effectiveness studies between dopamine and other vasopressors as initial treatment of hypotension in preterm infants have been performed.14-18 Epinephrine was as effective as dopamine in increasing BP, however, there were more short-term side effects with epinephrine (ie, tachycardia, increased lactate levels, hyperglycemia).15 Additionally, dopamine was more effective for treating hypotension than dobutamine in five trials.19 Thus, if a medication is to supplant dopamine for initial treatment of hypotension in preterm infants, it must be more effective than dopamine in reaching goal BP, or at least as effective in treating hypotension with fewer adverse effects.
Vasopressin has minimal to no inotropic or chronotropic effects,20 maintains cardiac function,21 may cause pulmonary vasodilation,22-24 and its vasoconstrictive effects are preserved during hypoxia and severe acidosis.25 Thus, vasopressin is a potentially desirable vasopressor to compare with dopamine as initial therapy for the treatment of hypotension in ELBW infants. Vasopressin has mostly been reported to increase BP in hypotension refractory to other vasopressors without significant adverse effects in case reports and retrospective reviews including populations of ELBW infants up to adolescents.20, 26-29 Therefore, we conducted a pilot trial to compare blood pressure responses, safety, and side effect profile of vasopressin vs dopamine. Our hypothesis was that vasopressin was safe and at least as effective in increasing BP as dopamine.
Methods
This prospective randomized, double-blinded study compared clinical responses to continuous infusions of vasopressin vs dopamine for the treatment of early hypotension in ELBW infants with sample size of 10 subjects per treatment group. The study was approved by the Baylor College of Medicine Institutional Review Board, and it was conducted at Texas Children’s Hospital NICU between May 2011 and May 2013. ELBW infants <24 hours of age and gestational age of ≤30 weeks were eligible with informed parental consent. Outborn ELBW infants who met eligibility criteria and did not receive treatment for hypotension before transfer were also eligible. Infants were designated as cases if they developed hypotension defined as mean BP ≤4 mmHg below gestational age (in weeks) or mean BP ≤ gestational age plus evidence of hypoperfusion (i.e., increasing blood lactate, delayed capillary refill) during the first 24 hours of life. We chose to study early hypotension, to avoid hypotension associated with symptomatic patent ductus arteriosus or late-onset sepsis as an etiology. BP was measured invasively with umbilical artery or peripheral arterial catheters in hypotensive infants. Non-invasive BP monitoring was used in non-hypotensive comparison infants when arterial access was not available. Infants with major congenital defects or chromosomal abnormalities, hydrops fetalis, hypovolemia (perinatal history consistent with decreased circulating blood volume plus clinical signs of hypovolemia), or hypotension associated with air leaks, lung overdistention, and metabolic abnormalities were excluded. Comparison infants were consented ELBW infants who did not meet study criteria for hypotension and, therefore, did not receive therapy for hypotension during the first 24 hours of life.
At enrollment, hypotensive subjects randomly (using a computer-generated random number table) received vasopressin or dopamine as initial treatment by continuous infusion through an umbilical venous or peripherally inserted central catheter. Caregivers and researchers were blinded to study drug as the research pharmacy prepared medication syringes with transparent solutions and similar volumes. Each 0.2 ml/kg/hr increase in infusion rate was equal to 0.01 units/kg/hr for vasopressin (maximum dose 0.04 units/kg/hr), and 5 mcg/kg/min for dopamine (maximum dose 20 mcg/kg/min). Study drugs were increased stepwise every 20 minutes until adequate mean BP was attained and maintained for 60 minutes (responders). Adequate mean BP was defined as 2 mmHg above gestational age (in weeks) and/or improvement in signs of hypoperfusion. If adequate mean BP was not reached after the highest study drug dose, one dose of intravenous hydrocortisone (1 mg/kg) was administered.
The primary outcome measures were percentage of infants in each treatment group who achieved adequate mean BP and the time taken to reach adequate mean BP. Mean BP measurements and vital signs were collected at baseline and every 20 minutes after study drug initiation until adequate mean BP was reached with drug titration. After adequate BP was reached, mean BP and vital signs were recorded every 20 minutes for 1 hour of stable BP and then hourly for 96 hours. For infants who reached adequate BP after hydrocortisone, the study continued in a blinded fashion and the study drug was reduced by the clinical care team in full or half increments as necessary. For those who did not respond after the addition of hydrocortisone (non-responders), the study concluded. At the end of the study, the assigned medications were continued open label at the discretion of the attending physician.
Secondary outcome measures examined between the groups were differences in heart rate; systolic, diastolic, and mean BP; arterial oxygen saturation; acid-base status; lactate, glucose, and sodium levels; urine output; presence of necrotizing enterocolitis or spontaneous intestinal perforation; respiratory variables (presence of chronic lung disease, time on ventilator, any respiratory support, oxygen, number of surfactant doses received); presence and treatment for patent ductus arteriosus; cranial ultrasound abnormalities; presence and treatment for retinopathy of prematurity; and mortality. Clinical variables were abstracted from the medical record and recorded prospectively.
For data analysis, the vasopressin group was compared with the dopamine group, and the treatment groups individually were compared with the infants without hypotension. Statistical analysis of the primary outcome variable–percentage of infants in each treatment group who achieved and maintained adequate mean BP was performed using Fisher’s exact test. A Kaplan-Meier survival curve was used to evaluate time to adequate mean BP in the treatment groups. Categorical variables were compared using a Fisher exact test or chi-square test, where appropriate. Continuous variables were analyzed using a t-test or ANOVA when normally distributed and Mann-Whitney U or Kruskal-Wallis test when not.
Results
Seventy subjects were enrolled; 10 in the vasopressin group, 10 in the dopamine group, and 50 in the comparison group. Mean gestational age and birth weight for the entire cohort was 25.6 ± 1.4 weeks and 705 ± 154 g, respectively.
Feasibility
Feasibility outcomes that were evaluated during this pilot study included willingness of parents to allow their infants to be randomized, willingness of clinicians to allow their patients to participate in research, number of eligible subjects and consent rate, withdrawal rate during the study, and pharmacy process for study drug administration. For the project to be feasible, we a priori decided that a 75% success rate for willingness of parents and the treatment team to allow study participation and an 80% consent rate with <20% withdrawal rate would be necessary. We found no barriers to enrollment among parents and clinicians and exceeded the 80% consent rate target. There were no withdrawals and the pharmacy process was timely.
Comparison between the Treatment Groups
Subject characteristics were similar between the treatment groups (Table I). Infants in the vasopressin group had lower mean PaCO2 (p<0.05, using arterial blood gases only during study drug administration) and received fewer doses of surfactant (p<0.05; Table II). Moreover, heart rate did not change (0 ± 10 beats per minute) in the vasopressin group compared with an increase of 31 ± 19 beats per minute for infants receiving dopamine (p<0.001). Fluid intake and urine output were similar between the vasopressin and dopamine groups. There were no differences between the treatment groups in all other morbidities of prematurity and mortality (Table II). Six subjects died prior to hospital discharge: 1 from each treatment group during study drug administration and 4 infants died remote from treatment of hypotension (3 from the vasopressin group and 1 from the dopamine group).
Table 1.
Subject Characteristics
| Characteristic | Vasopressin Group (N=10) | Dopamine Group (N=10) | Comparison Group (N=50) | p-value |
|---|---|---|---|---|
| Birth weight (grams) | 640 ± 109 | 675 ± 148 | 723 ± 161 | .25 |
|
| ||||
| Gestational age (weeks) | 25.7 ± 2 | 25.1 ± 1 | 25.6 ± 1 | .53 |
|
| ||||
| Male sex (%) | 60 | 50 | 44 | .64 |
|
| ||||
| Race (%) | .17 | |||
| White | 50 | 50 | 66 | |
| Black | 40 | 40 | 32 | |
| Other | 10 | 10 | 2 | |
|
| ||||
| Ethnicity | ||||
| Hispanic/Latino (%) | 40 | 30 | 32 | .87 |
|
| ||||
| Multiple gestation (%) | 20 | 20 | 14 | .82 |
|
| ||||
| Vaginal delivery (%) | 20 | 60 | 36 | .17 |
|
| ||||
| Inborn (%) | 100 | 80 | 76 | .22 |
|
| ||||
| Antenatal steroids (%) | 80 | 60 | 86 | .20 |
|
| ||||
| IUGR (%) | 30 | 10 | 20 | .54 |
|
| ||||
| 1 minute APGAR Median (IQR) | 2 (1,2.75) | 2.5 (1,3) | 5 (3,6.75) | <.001*, .01† |
|
| ||||
| 5 minute APGAR Median (IQR) | 5.5 (5,6) | 5.5 (4.25,7) | 7 (6,8) | .002* |
|
| ||||
| Initial mean BP (mmHg) | 21.3 ± 3.1 | 20.5 ± 3.4 | 31.8 ± 8.1 | <.001* <.001† |
|
| ||||
| Initial hematocrit (%) | 40.8 ± 6.8 | 39.4 ± 6.0 | 41.3 ± 6.3 | .680 |
|
| ||||
| Initial creatinine (mg/dL) | .84 ± 0.18 | .85 ± 0.17 | .91 ± 0.24 | .602 |
Significant between Vasopressin and Comparison Group
Significant between Dopamine and Comparison Group
Table 2.
Clinical Variables
| Variable | Vasopressin Group (n=10) | Dopamine Group (n=10) | Comparison Group (n=50) | p- value |
|---|---|---|---|---|
| Blood gases§ | ||||
| pH | 7.20 ± .07 | 7.18 ± .07 | 7.25 ± .09 | .016† |
| PaCO2 (mmHg) | 44.7 ± 6.8 | 53.7 ± 10.4 | 47.1 ± 8.8 | .026‡, .034† |
| PaO2 (mmHg) | 61.1 ± 13.3 | 59.2 ± 9.1 | 60.5 ± 15.4 | .956 |
| Bicarbonate (mEq/L) | 17.5 ± 2.8 | 19.5 ± 1.4 | 20.4 ± 2.8 | .002* |
| Base Deficit (mEq/L) | 10.3 ± 3.3 | 8.9 ± 1.7 | 6.7 ± 3.2 | .001*, .045† |
|
| ||||
| Surfactant doses received | 2.3 ± 1 | 3.1 ± 1 | 1.7 ± 1 | .02‡, .001† |
|
| ||||
| Hyponatremia (%) | 30 | 30 | 22 | .785 |
|
| ||||
| Hyperglycemia (%) | 30 | 20 | 32 | .738 |
|
| ||||
| Urine output (ml/kg/hr) | 3.5 ± 1.4 | 4.4 ± 1.4 | 3.9 ± 1.4 | .384 |
|
| ||||
| Fluid intake (ml/kg/day) | 142 ± 21.3 | 154.4 ± 30.3 | 150.4 ± 28.8 | .600 |
|
| ||||
| Necrotizing enterocolitis n (%) | 0 (0) | 1 (10) | 2 (4) | .801 |
|
| ||||
| Sepsis (%) | .155 | |||
| Early-onset | 0 | 0 | 6 | |
| Late-onset | 50 | 40 | 14 | |
|
| ||||
| Retinopathy of prematurity (%) | ||||
| Stage 3 or higher | 20 | 30 | 4 | .078 |
| Treatment (Avastin or laser) | 20 | 30 | 8 | .118 |
|
| ||||
| Ventilator days in survivors Median (IQR) | 45.5 (23.5,56) | 52 (37,160) | 17.5 (1,38) | .04† |
|
| ||||
| Hospital length of stay in survivors (days) Median (IQR) | 112 (110,139) | 123 (116,225) | 103 (89,117) | .004† |
|
| ||||
| Death or BPD (%) | 80 | 100 | 78 | .261 |
ANOVA with post-hoc pairwise comparison LSD (p<.05)
Significant between Vasopressin and Comparison Groups
Significant between Dopamine and Comparison Groups
Significant between Vasopressin and Dopamine Groups
Ten subjects received a normal saline bolus (10 ml/kg) before study drug initiation (as directed by the attending neonatologist)—seven in the vasopressin vs three in the dopamine group (p =0.179). Study drug was started at a mean age of 6.5 ± 0.2 hours of life. Treatment was successful in 90% of hypotensive subjects (nine subjects in each treatment group were responders). Three responders also received hydrocortisone (two from the vasopressin group and one from dopamine group). The percentage of subjects with adequate mean BP at 24 hours of life was 90% and 80% for the vasopressin and dopamine groups, respectively (p=1.000). There was no difference between the two groups in time taken to reach adequate mean BP (p=0.986; Figure). Responders received vasopressin and dopamine for 2.2 ± 2.1 days and 4.5 ± 4.1 days, respectively (p=0.154).
Figure.
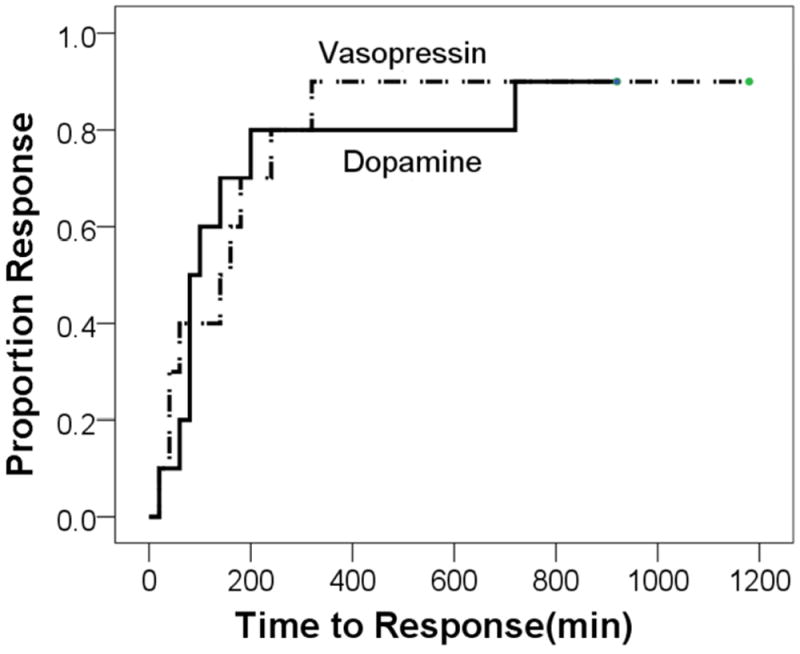
Time to Adequate Mean BP in Treatment Groups
Comparison between the Treatment Groups and infants without hypotension
Apgar scores and initial BP (mean, systolic, and diastolic) were higher in the comparison infants than the study drug infants. Specifically, comparisons had higher mean BP (31.8 ± 8.1 mmHg) upon admission than the vasopressin (21.3 ± 3.1 mmHg, p<0.001) and dopamine groups (20.5 ± 3.4 mmHg, p<0.001; Table I). Incidence of antenatal conditions (receipt of antenatal steroids, chorioamnionitis, preeclampsia, and prolonged (>24 hours) rupture of the membranes) were similar among the three groups. In addition, there were no significant differences between the three groups in neonatal variables (early- and late-onset sepsis, patent ductus arteriosus, necrotizing enterocolitis, intestinal perforations, hyponatremia, or hyperglycemia). There were no differences between the three groups in the presence of severe IVH (grades 3 and 4) on the initial or subsequent cranial ultrasounds (worst grade) during the hospitalization.
The three groups did, however, differ in respiratory status (Table II). The vasopressin group had lower mean bicarbonate and higher mean base deficit, whereas the dopamine group had lower mean pH, higher mean PaCO2, and higher mean base deficit on arterial blood gases compared with the comparison group in the first 96 hours of life. In addition, the dopamine group received more surfactant doses than the comparison group (clinical decision of the attending neonatologist not involved in the study), and there was no difference between the vasopressin and comparison groups.
Characteristics of survivors (to hospital discharge) of the three groups were also examined (Table II). There were no significant differences in days taken to reach full feeds or days spent on nasal continuous positive airway pressure. Survivors in the dopamine group, but not the vasopressin group, had longer hospital length of stay and spent more days on mechanical ventilation than the comparison group survivors.
Discussion
Although a small sample size, this pilot study is a head-to-head, randomized, double-blinded, controlled trial examining the effects of using vasopressin vs dopamine as initial therapy for hypotension in ELBW infants. Although there were no differences between the two treatment groups with regard to the primary efficacy outcomes (ie, percent responders and time to adequate mean BP), the infants who were randomized to vasopressin had lower mean PaCO2 levels, received fewer doses of surfactant, and did not have tachycardia during study drug administration compared with the dopamine infants. The respiratory benefits and absence of tachycardia in infants who received vasopressin may have resulted from the pulmonary vasodilatory effects and lack of cardiac effects of vasopressin. Although we cannot conclude from our small trial that vasopressin is superior to dopamine for the treatment of hypotension in ELBW infants, the results support a larger phase II or III trial.
Dopamine is the most studied medication in neonatology for hypotension which likely contributes to the frequency of its use as the initial agent in neonatal hypotension. Its use in both children and adults, however, has decreased over the years.30-32 Dopamine can increase systemic oxygen consumption33 and it has other cardiovascular, renal, and endocrine effects that may be contraindicated in preterm infants,11 such as tachycardia, hyponatremia, and decreased thyrotropin secretion.13 Vasopressin, on the other hand, has no known inotropic or chronotropic effects, and may improve pulmonary vasoconstriction.22-24, 34 Its use in neonatal hypotension, however, has been reported only in case reports and reviews demonstrating efficacy in refractory cases.20 We recently showed that vasopressin use has increased in NICUs during the last decade.10 From the results of this small pilot trial, it is encouraging that vasopressin was as efficacious as dopamine in treating hypotension in ELBW infants, perhaps may have some pulmonary benefits, and did not cause tachycardia.
Although the main limitation of this pilot study is the small sample size, it is a head-to-head trial of vasopressors that found that vasopressin had equal efficacy to dopamine, with perhaps pulmonary benefits and lack of cardiac adverse effects. Concentrations of vasopressin and dopamine were not measured during study drug administration to determine pharmacokinetics, because the blood volume required for the commercial assays was too large for ELBW infants. We have since developed a micro-volume assay35 that will be used in future studies.
Although the definition of neonatal hypotension, its etiology, and need for treatment remain unclear and are important unresolved issues in neonatology, it was not the purpose of this study to address these issues, but to focus on patients in whom therapy was deemed clinically necessary. And as with many other trials of neonatal hypotension, the definition of hypotension we employed was “clinically-derived” and not evidenced-based. Thus, the primary medical team decided which hypotensive ELBW infants required treatment and when to initiate treatment. There were some variations in the initial mean BP value and clinical symptoms present in the subjects at randomization. However, the study protocol had strict entrance criteria which helped to minimize variations in treatment. Another limitation is that we were unable to directly evaluate the pharmacodynamics of these medications on cardiovascular, renal, or cerebral circulations. We used indirect observations, such as, vital signs, urine output, and oxygen saturations to infer effects on perfusion, but did not directly measure organ perfusion during study drug administration nor did we determine the etiology of hypotension.
Acknowledgments
We thank Pamela Gordon, RNC-NIC, Geneva Shores, RNC-LRN, Cindy Bryant, CCRN-Neo, and Jennifer Lynds, PharmD, E. O’Brian Smith, PhD, for statistical analysis, and the support of the TCH neonatologists, NICU nurses, fellows, residents, and advanced nurse practitioners are gratefully appreciated. D.R. acknowledges mentorship by Lisa Bomgaars, MD, Eric Eichenwald, MD, and Al Gest, MD, for this project.
Funded by Thrasher Research (9162). D.R. was supported by BCM Section of Neonatology and The Bad Pants Open fund, National Institutes of Health (M01-RR00188) to the BCM General Clinical Research Center, Texas Children’s Hospital CRC.
Registered with ClinicalTrials.gov: NCT01318278
Abbreviations
- BP
blood pressure
- ELBW
extremely low birth weight
- NICU
neonatal intensive care unit
- TCH
Texas Children’s Hospital
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Pellicer A, Valverde E, Elorza MD, Madero R, Gayá F, Quero J, et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: A randomized, blinded, clinical trial. Pediatrics. 2005;115:1501–12. doi: 10.1542/peds.2004-1396. [DOI] [PubMed] [Google Scholar]
- 2.Fanaroff JM, Wilson-Costello DE, Newman NS, Montpetite MM, Fanaroff AA. Treated hypotension is associated with neonatal morbidity and hearing loss in extremely low birth weight infants. Pediatrics. 2006;117:1131–5. doi: 10.1542/peds.2005-1230. [DOI] [PubMed] [Google Scholar]
- 3.Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012 Dec;4:e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore GP, Lemyre B, Barrowman N, Daboval T. Neurodevelopmental outcomes at 4 to 8 years of children born at 22 to 25 weeks’ gestational age: A meta-analysis. JAMA Pediatr. 2013;167:967–74. doi: 10.1001/jamapediatrics.2013.2395. [DOI] [PubMed] [Google Scholar]
- 5.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol. 2000;5:89–106. doi: 10.1053/siny.1999.0001. [DOI] [PubMed] [Google Scholar]
- 6.Lemons JA, Bauer CR, Oh W, Korones SB, Papile L-A, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development neonatal research network, January 1995 Through December 1996. Pediatrics. 2001;107:e1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 7.Fanaroff AA, Hack M, Walsh MC. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Semin Perinatol. 2003;27:281–7. doi: 10.1016/s0146-0005(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 8.Fanaroff AA, Fanaroff JM. Short- and long-term consequences of hypotension in ELBW infants. Semin Perinatol. 2006;30:151–5. doi: 10.1053/j.semperi.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Noori S, Seri I. Neonatal blood pressure support: The use of inotropes, lusitropes, and other vasopressor agents. Clin Perinatol. 2012;39:221–38. doi: 10.1016/j.clp.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Rios DR, Moffett BS, Kaiser JR. Trends in pharmacotherapy for neonatal hypotension. J Pediatr. 2014;165:697–701. doi: 10.1016/j.jpeds.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Seri I. Cardiovascular, renal, and endocrine actions of dopamine in neonates and children. J Pediatr. 1995;126:333–44. doi: 10.1016/s0022-3476(95)70445-0. [DOI] [PubMed] [Google Scholar]
- 12.Liet J-M, Boscher C, Gras-Leguen C, Gournay V, Debillon T, Rozé J-C. Dopamine effects on pulmonary artery pressure in hypotensive preterm infants with patent ductus arteriosus. J Pediatr. 2002;140:373–5. doi: 10.1067/mpd.2002.123100. [DOI] [PubMed] [Google Scholar]
- 13.Dong BJ. How medications affect thyroid function. West J Med. 2000;172:102–6. doi: 10.1136/ewjm.172.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osborn D, Evans N, Kluckow M. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J Pediatr. 2002;140:183–91. doi: 10.1067/mpd.2002.120834. [DOI] [PubMed] [Google Scholar]
- 15.Valverde E, Pellicer A, Madero R, Elorza D, Quero J, Cabañas F. Dopamine versus epinephrine for cardiovascular support in low birth weight infants: Analysis of systemic effects and neonatal clinical outcomes. Pediatrics. 2006;117:e1213–e22. doi: 10.1542/peds.2005-2108. [DOI] [PubMed] [Google Scholar]
- 16.Klarr JM, Faix RG, Pryce CJE, Bhatt-Mehta V. Randomized, blind trial of dopamine versus dobutamine for treatment of hypotension in preterm infants with respiratory distress syndrome. J Pediatr. 1994;125:117–22. doi: 10.1016/s0022-3476(94)70137-7. [DOI] [PubMed] [Google Scholar]
- 17.Greenough A, Emery EF. Randomized trial comparing dopamine and dobutamine in preterm infants. Eur J Pediatr. 1993;152:925–7. doi: 10.1007/BF01957532. [DOI] [PubMed] [Google Scholar]
- 18.Roze CJ, Tohier C, Maingueneau C, Lefevre M, Mouzard A. Response to dobutamine and dopamine in the hypotensive very preterm infant. Arch Dis Child. 1993;69:59–63. doi: 10.1136/adc.69.1_spec_no.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subhedar NV, Shaw N. Dopamine versus dobutamine for hypotensive preterm infants. Cochrane Database Syst Rev. 2003;3 doi: 10.1002/14651858.CD001242. [DOI] [PubMed] [Google Scholar]
- 20.Meyer S, Gortner L, McGuire W, Baghai A, Gottschling S. Vasopressin in catecholamine-refractory shock in children. Anaesthesia. 2008;63:228–34. doi: 10.1111/j.1365-2044.2007.05317.x. [DOI] [PubMed] [Google Scholar]
- 21.Cheung D, Gill R, Liu J-Q, Manouchehri N, Sergi C, Bigam D, et al. Vasopressin improves systemic hemodynamics without compromising mesenteric perfusion in the resuscitation of asphyxiated newborn piglets: a dose–response study. Intensive Care Med. 2012;38:491–8. doi: 10.1007/s00134-011-2437-4. [DOI] [PubMed] [Google Scholar]
- 22.Mohamed A, Nasef N, Shah V, McNamara PJ. Vasopressin as a rescue therapy for refractory pulmonary hypertension in neonates: case series. Pediatr Crit Care Med. 2014;15:148–54. doi: 10.1097/PCC.0b013e31829f5fce. [DOI] [PubMed] [Google Scholar]
- 23.Evora PR, Pearson PJ, Schaff HV. Arginine vasopressin induces endothelium-dependent vasodilatation of the pulmonary artery. V1-receptor-mediated production of nitric oxide. Chest. 1993;103:1241–5. doi: 10.1378/chest.103.4.1241. [DOI] [PubMed] [Google Scholar]
- 24.Acker SN, Kinsella JP, Abman SH, Gien J. Vasopressin improves hemodynamic status in infants with congenital diaphragmatic hernia. J Pediatr. 2014;165:53–8.e1. doi: 10.1016/j.jpeds.2014.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunton LL, Lazo JS, Parker KL. Goodman’s & Gilman’s the pharmacological basis of therapeutics. 11. New York: McGraw-Hill; 2006. [Google Scholar]
- 26.Lechner E, Hofer A, Mair R, Moosbauer W, Sames-Dolzer E, Tulzer G. Arginine-vasopressin in neonates with vasodilatory shock after cardiopulmonary bypass. Eur J Pediatr. 2007;166:1221–7. doi: 10.1007/s00431-006-0400-0. [DOI] [PubMed] [Google Scholar]
- 27.Meyer S, Gottschling S, Baghai A, Wurm D, Gortner L. Arginine-vasopressin in catecholamine-refractory septic versus non-septic shock in extremely low birth weight infants with acute renal injury. Crit Care. 2006;10:R71. doi: 10.1186/cc4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikegami H, Funato M, Tamai H, Wada H, Nabetani M, Nishihara M. Low-dose vasopressin infusion therapy for refractory hypotension in ELBW infants. Pediatr Int. 2010;52:368–73. doi: 10.1111/j.1442-200X.2009.02967.x. [DOI] [PubMed] [Google Scholar]
- 29.Bidegain M, Greenberg R, Simmons C, Dang C, Cotten CM, Smith PB. Vasopressin for refractory hypotension in extremely low birth weight infants. J Pediatr. 2010;157:502–4. doi: 10.1016/j.jpeds.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinman ME, Chameides L, Schexnayder SM, Samson RA, Hazinski MF, Atkins DL, et al. Pediatric advanced life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics. 2010;126:e1361–e99. doi: 10.1542/peds.2010-2972D. [DOI] [PubMed] [Google Scholar]
- 31.Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S729–S67. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 32.Dellinger RP, Levy M, Rhodes A, Annane D, Gerlach H, Opal S, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Zhang G, Holtby H, Humpl T, Caldarone CA, Van Arsdell GS, et al. Adverse effects of dopamine on systemic hemodynamic status and oxygen transport in neonates after the norwood procedure. J Am Coll Cardiol. 2006;48:1859–64. doi: 10.1016/j.jacc.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 34.Stathopoulos L, Nicaise C, Michel F, Thomachot L, Merrot T, Lagier P, et al. Terlipressin as rescue therapy for refractory pulmonary hypertension in a neonate with a congenital diaphragmatic hernia. J Pediatr Surg. 2011;46:e19–e21. doi: 10.1016/j.jpedsurg.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Rios DR, Tam V, Chow DS-L. Development and validation of a highly sensitive LC-MS/MS assay for the quantification of arginine vasopressin in human plasma and urine: Application in preterm neonates and child. J Pharm Biomed Anal. 2014;99:67–73. doi: 10.1016/j.jpba.2014.07.001. [DOI] [PubMed] [Google Scholar]


