Abstract
Objective
To determine whether exposure to diabetes in utero affects resting energy expenditure (REE) and fuel oxidation in infants.
Study design
At 35 ± 5 days after birth, body composition and REE were measured in full-term offspring of Native American and Hispanic women with either well-controlled diabetes (13 girls, 11 boys) or normal healthy pregnancies (18 girls, 17 boys).
Results
Control of dysglycemia during gestation in the women with diabetes mellitus met current clinical standards shown by average glycated hemoglobin (5.9 ± 0.2%; 40.6 ± 2.3 mmol/mol). Infant body mass (offspring of women with diabetes: 4.78 ± 0.13, control offspring: 4.56 ± 0.08 kg) and body fatness (offspring of women with diabetes: 25.2 ± 0.6, control offspring: 24.2 ± 0.5 %) did not differ between groups. REE, adjusted for lean body mass, was 14% lower in offspring of women with diabetes (41.7 ± 2.3 kJ/h) than control offspring (48.6 ± 2.0, p=0.025). Fat oxidation was 26% lower in offspring of women with diabetes (0.54 ± 0.05 g/h) than control offspring (0.76 ± 0.04, p < 0.01) but carbohydrate oxidation did not differ. Thus, fat oxidation accounted for a lower fraction of REE in the offspring of women with diabetes (49 ± 4%) than control offspring (60 ± 3%, p = 0.022). Mothers with diabetes were older and had higher pre-pregnancy BMI than control mothers.
Conclusion
Well-controlled maternal diabetes did not significantly affect body mass or composition of offspring at 1-month old. However, infants with diabetic mothers had reduced REE and fat oxidation, which could contribute to adiposity, and future disease risk. Further studies are needed to assess the impact differences in age and higher prepregnancy BMI.
Keywords: fetal origins, obesity, metabolic rate, indirect calorimetry
A mother’s health and her lifestyle during pregnancy, including her nutrition, physical activity, smoking, or the presence of metabolic disease, can have both immediate and long- lasting effects on offspring (1). Diabetes during pregnancy has a particularly important impact on offspring health as shown by the 10-fold higher rate of childhood obesity (2) and 4-fold higher risk of developing impaired glucose tolerance (pre-diabetes) during adolescence (3). Cardiovascular risk factors are also increased in otherwise healthy children exposed to diabetes in utero compared with unexposed children (4). All of those outcomes appear to be due, at least in part, to in utero exposure to maternal diabetes (5). The risk for offspring to develop obesity and insulin resistance has been reported to increase in association with elevated maternal glucose in some studies (6, 7), suggesting that the maternal diabetes state per se is responsible, possibly through epigenetic programming in utero (8, 9). Although increased surveillance and more aggressive management of dysglycemia in pregnant women has substantially reduced neonatal morbidity, it remains to be shown whether such advances in prenatal care affect subsequent development of obesity, diabetes, and metabolic syndrome in offspring exposed to maternal diabetes (5, 10-12).
Low resting energy expenditure (REE) and rates of fat oxidation are positive predictors for future weight gain in adults (13, 14). Likewise, adults with type 2 diabetes have been shown to oxidize less fat and more carbohydrate while at rest (part of a phenotype called metabolic inflexibility) compared with people without diabetes, and this could contribute to the deposition of excess fat mass (15). Whether these metabolic features are transmissible from mother to baby or are evident early in life is unclear. In the current study we postulated that such metabolic alterations might be implicated in the subsequent development of obesity and/or diabetes, and may serve as markers for risk of future disease early in children born to mothers with diabetes during pregnancy. To test this we measured energy expenditure in one-month old offspring of Hispanic and Native American women, members of racial/ethnic groups at increased risk for diabetes and obesity (2).
METHODS
Self-declared Native American and Hispanic women experiencing a normal, uncomplicated pregnancy (Control group, N = 34) or pregnancy accompanied by diabetes mellitus (gestational or pre-existing type 2 diabetes, diabetes group, N = 27) were recruited before the birth of their child. The current analyses are from a larger ongoing investigation of maternal diabetes on offspring. Native American mothers (N = 25 Controls, N = 17 with diabetes) received the majority of their prenatal care and completed delivery at the Chickasaw Nation Medical Center (Ada, OK), the Choctaw Nation Healthcare Center (Talihina, OK), or the University of Oklahoma Medical Center (Oklahoma City, OK). Hispanic mothers (N = 9 Controls, N = 10 with diabetes) received the majority of their prenatal care and completed delivery at the University of Oklahoma Medical Center (Oklahoma City, OK). All mothers provided informed consent for their child to participate in the study in accordance with the Institutional Review Boards of the Chickasaw Nation, Choctaw Nation of Oklahoma, and the University of Oklahoma Health Sciences Center, respectively, which approved the study. Control offspring and offspring of women with diabetes were excluded from the study if they were born prior to 37 weeks of gestation, or there was evidence of known or presumed congenital birth defects, including major physical malformations, severe persistent nervous system deficit, severe birth asphyxia (defined as 5 minute APGAR score of < 6 or umbilical cord pH < 7), or congenital infection, or metabolic or endocrine disease. Infants born to mothers with pre-eclampsia or other potentially confounding health conditions other than diabetes were also excluded from these analyses. The approaches used for diabetes management were insulin (11 mothers), glyburide (7 mothers), metformin (1 mother), both insulin and metformin (2 mothers), both insulin and glyburide (1 mother), and lifestyle/no medication (5 mothers).
Study personnel at each clinic site used standardized protocols to acquire estimates of gestational age, measurements of maternal health characteristics and the weight and length of the baby at birth. All anthropometric, body composition and metabolic measurements performed on the one-month old infants were conducted during a single visit to the CMRI Pediatric Metabolic Research Center at the University of Oklahoma Health Sciences Center, Oklahoma City, when the infants were between 26-46 days old (mean ± standard deviation = 35 ± 5 days). Previous experience in our center has shown that feeding infants within 30-60 minutes before performing body composition measurements helps keep them relaxed during testing and therefore increases data acquisition. Therefore, we did not restrict food intake of the infants prior to the measurements in this study. Because infants at this age eat frequently throughout the day they are in a near-continuous post-prandial state.
Body length (cm) was measured from crown to heal using a horizontal stadiometer and body mass (kg) was measured using an infant scale. Ponderal index was calculated as: body mass (kg)/height (m3). Body mass was also expressed as a percentile on the age- and sex-specific growth charts available from the United States Centers for Disease Control. Body composition was measured using dual energy X-ray absorptiometry (Lunar iDXA v11-30.062, GE- Healthcare, Fairfield, CT) similar to the approach used by our group for 6-months-old babies (16). Briefly, the infant, wearing only a disposable diaper, was swaddled in a light cotton blanket, the overhead lights were dimmed, and noise was minimized during the ~4-minute-long procedure. The fat and lean mass of the whole body (excluding the head), trunk, and appendicular regions was calculated using the pediatric whole body analysis enCore 2007 software package from the instrument manufacturer. All measurements were performed and analyzed by the same investigator using standardized protocol that included daily calibration of the instrument.
Resting energy expenditure (REE) and the relative portion of fat and carbohydrate oxidation were measured using an indirect calorimetry system with a flow-through canopy placed over the head while the baby was resting supine (TrueOne 2400, ParvoMedics, Sandy, UT). Measurements were performed for approximately 20-25 minutes. Data were summarized each minute with the first 5 minutes excluded from analysis. Short time segments (1-2 minutes) were also excluded from analyses if the baby moved excessively. Tests in which the baby began to cry or engaged in continuous movement were also excluded from analyses. Rates of oxygen consumption and carbon dioxide production were used to calculate REE and fuel oxidation using the Weir equations (17). Volumetric flow rate and gas concentration analyzers were calibrated daily, and ethanol burn tests were used to confirm optimal performance of the instrument.
Statistical analyses
Summary statistics are presented as mean ± SEM. Student’s unpaired t-tests were used to compare results between groups. Strength of association among selected variables was calculated using Pearson’s correlation coefficient. Covariate adjustment of REE and fuel oxidation results for lean body mass was performed as previously described (18). Multivariate regression modeling, using stepwise approaches for variable entry and removal, was used to determine the best set of predictor variables for the REE and fuel oxidation outcomes in the infant. P<0.05 was considered statistically significant for all analyses.
RESULTS
Characteristics of the mothers during pregnancy and the offspring at birth are presented in Table I. The diabetes group was comprised of 9 women who had type 2 diabetes before becoming pregnant and 18 women diagnosed with gestational diabetes mellitus (GDM). Mothers with diabetes were older, had higher parity and pre-pregnancy body mass index (BMI), and had slightly shorter gestation than the control group mothers. Glycated hemoglobin (HbA1c) was 5.9 ± 0.2% (40.6 ± 2.3 mmol/mol) in the mothers with diabetes, demonstrating good glycemic control. Maternal blood pressure and infant birth weight did not differ between Control and diabetes groups, although there was a trend for offspring of women with diabetes to be larger at birth compared with control offspring. Mothers with pre-pregnancy type 2 diabetes (N = 9) had higher (p = 0.032) HbA1c (6.7 ± 0.5%, 49.7 ± 5.3 mmol/mol) than mothers with gestational diabetes (N = 18, 5.4 ± 0.1%, 35.9 ± 1.0 mmol/mol) but there were no other differences between those subgroups in age, pre-pregnancy BMI, parity, blood pressure, or the gestational age or birth weight of their offspring (not shown).
Table 1.
Pregnancy characteristics (mean ± SEM)
| Control | Diabetes | % diff | p-value | |
|---|---|---|---|---|
| Maternal age, y | 24.2 ± 0.7 | 31.9 ± 1.3 | 32 | 0.001 |
| Previous live births, # | 0.8 ± 0.2 | 2.2 ± 0.4 | 116 | 0.029 |
| Pre-pregnancy BMI, kg/m2 | 28.5 ± 1.2 | 33.1 ± 1.1 | 17 | 0.003 |
| Systolic BP, mmHg | 123 ± 2 | 117 ± 3 | −4 | 0.149 |
| Diastolic BP, mmHg | 75 ± 2 | 70 ± 2 | −6 | 0.108 |
| Gestational age, weeks | 39.7 ± 0.2 | 38.6 ± 0.1 | −3 | 0.001 |
| Infant birth weight, kg | 3.48 ± 0.07 | 3.71 ± 0.10 | 7 | 0.069 |
% diff, difference between groups; BMI, body mass index; BP, blood pressure for mother during pregnancy. P-values are for t-test comparison between groups.
The physical and body composition characteristics of the infants at 1-month old are shown in Table II. There were no significant differences between groups for those outcomes, although there was a non-significant trend for higher total and appendicular fat mass in the group of offspring of women with diabetes. There was also no difference in the type of nutrition the infant received during their first month. In the control offspring group 50% were formula-fed, 15% were breast fed, and 30% received both formula and breast milk (data missing for 1 infant). In the group of offspring of women with diabetes, 48% were formula-fed, 19% were breast fed, and 30% received both formula and breast milk (data missing for 1 infant). Although the intent of the study was to test all of the infants at one month after birth, the practical challenges of caring for a newborn, and long travel distances for some participants caused some visits to be delayed. However, there was no difference in infant age at the time of the measurements between the control (35 ± 1 days) and diabetes (35 ± 1 days) offspring groups. In the Control group 71% of the infants were tested between 28-37 days after birth, while in the Diabetes group 74% were tested between 26-36 days. Age was also not a significant predictor variable in regression analyses described below.
Table 2.
Infant characteristics at 1 month old (mean ± SEM)
|
Control
(19F/15M) |
Diabetes
(14F/13M) |
% diff | p-value | |
|---|---|---|---|---|
| Age and body size | ||||
| Age, days since birth | 35 ± 1 | 34 ± 1 | −3 | 0.425 |
| Body mass, kg | 4.55 ± 0.08 | 4.75 ± 0.11 | 5 | 0.146 |
| Body length, cm | 53.9 ± 0.4 | 54.2 ± 0.4 | 0 | 0.658 |
| Ponderal index, kg/m3 | 29.0 ± 0.5 | 29.9 ± 0.7 | 3 | 0.323 |
| Body mass, percentile | 63 ± 4 | 73 ± 5 | 16 | 0.126 |
| Body composition | ||||
| Total lean mass, kg | 3.53 ± 0.06 | 3.62 ± 0.08 | 2 | 0.376 |
| Total fat mass, kg | 1.15 ± 0.03 | 1.26 ± 0.05 | 9 | 0.085 |
| Total fat mass, % | 24.2 ± 0.5 | 25.2 ± 0.6 | 4 | 0.161 |
| Trunk lean mass, kg | 1.81 ± 0.04 | 1.87 ± 0.04 | 3 | 0.271 |
| Trunk fat mass, kg | 0.38 ± 0.02 | 0.42 ± 0.02 | 11 | 0.148 |
| Appendage lean mass, kg | 0.85 ± 0.03 | 0.90 ± 0.06 | 6 | 0.393 |
| Appendage fat mass, kg | 0.54 ± 0.02 | 0.60 ± 0.03 | 11 | 0.118 |
| Indirect calorimetry | ||||
| VO2, ml/min | 40 ± 2 | 35 ± 2 | −12 | 0.070 |
| VCO2, ml/min | 32 ± 1 | 29 ± 2 | −8 | 0.267 |
| RER | 0.81 ± 0.01 | 0.84 ± 0.01 | 4 | 0.023 |
Number of female (F) and male (M) participants is listed under the group heading. % diff, difference between groups; VO2, oxygen consumption rate; VCO2, carbon dioxide elimination rate; RER, respiratory exchange ratio (= VCO2 / VO2); % diff: difference between groups. P-values are for t-test comparison between groups.
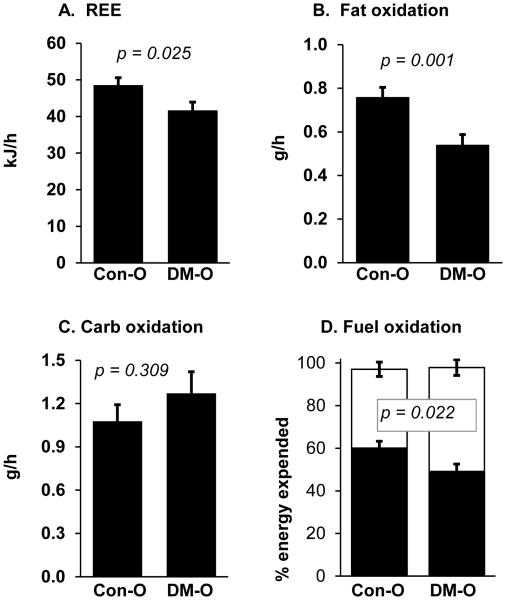
The REE calculated from indirect calorimetry measurements was positively correlated with lean body mass (r = 0.56, p < 0.01). After covariate adjustment for lean body mass, the REE was significantly (14%) lower in the group of offspring of women with diabetes (Figure 1, A). Rates of oxygen consumption (VO2) and carbon dioxide elimination (VCO2) did not differ significantly between groups, although the respiratory exchange ratio (VCO2 / VO2) was 4% higher in the group of offspring of women with diabetes, indicating a shift in fuel metabolism away from fat and toward carbohydrate (Table II). The rate of fat oxidation was 26% lower in the group of offspring of women with diabetes than the control offspring group (Figure, B). Carbohydrate oxidation did not differ between groups (Figure, C). Thus, the proportion of energy expenditure attributable to fat oxidation was significantly lower in the offspring of women with diabetes versus the control infants (Figure, D).
Figure 1.
Resting energy expenditure (REE) and fuel oxidation in 1-month old offspring from mothers who had diabetes mellitus during pregnancy compared with offspring of non-diabetic mothers. A) REE after adjustment for lean body mass; B) Fat oxidation rate; C) Carbohydrate (Carb) oxidation rate; D) Relative oxidation rates of fat (black bars) and carbohydrate (white). P-values are for t-test comparisons between groups.
Because mothers in the diabetic group were older, had higher pre-pregnancy BMI, had more prior pregnancies, we examined the impact of those attributes on the metabolic outcomes in the infants. First, we performed multivariate regression modeling (with both groups combined) to determine the best set of predictors for REE and fat oxidation rate. The list of potential predictor variables included maternal age, race/ethnicity, pre-pregnancy BMI, number of prior births, group designation (Control, diabetes), infant birth weight, feeding mode (breast, formula, combined), and age, anthropometric measurements, and DXA-derived body composition at the 1-month measurement visit. REE (after adjustment for lean body mass) was best predicted by a set of three variables, in order of entry into the stepwise regression: mother’s pre-pregnancy BMI, mother’s age, and infant’s birth weight (R2 = 0.278, p = 0.001). Fat oxidation was best predicted by a set of four variables, in order of entry into the model: group designation, mother’s pre-pregnancy BMI, infant’s birth weight, and infant ponderal index at 1-month (R2 = 0.467, p < 0.001). Because mother’s age and pre-pregnancy BMI differed between the control and diabetes groups and were co-variant with group designation, we examined the correlations between those variables and the infant REE and fat oxidation separately within the diabetes or Control groups. None of those correlations were statistically significant (r < 0.22, p > 0.05).
DISCUSSION
The main new finding is that offspring from diabetic mothers have lower REE and fat oxidation than infants from mothers with a non-diabetic, uncomplicated pregnancy. The lower REE in the group of offspring of women with diabetes is primarily explained by reduced fat oxidation because carbohydrate oxidation did not differ between groups. These metabolic alterations occurred in the face of near optimal control of maternal diabetes as evidenced by glycated hemoglobin that was 5.9% on average in mothers with diabetes and marginal differences in infant birth weights between control and diabetic offspring.
Our findings are in agreement with studies of adults that reported that REE and fat oxidation rate are reduced in men and women with a family history of diabetes (19, 20), and in adult offspring of women who had diabetes during pregnancy (21). Other studies of adults showed that reduced REE and/or reduced rate of fat oxidation are predictors of accelerated future weight gain (13, 14, 22). Those data in adults fit well with findings that offspring of diabetic mothers are prone to develop obesity during childhood (12, 23). The current findings also align with evidence from a mouse model of beta cell dysfunction that causes females to develop diabetes during pregnancy (24). The offspring of those diabetic dams had lower energy expenditure and higher respiratory exchange ratio while young. Furthermore, the altered fuel metabolism preceded the divergence in growth curves and the higher accrual of body fat in the diabetic offspring compared with control animals, suggesting that reduced REE and fat oxidation contribute to future obesity (24).
In the current study the mothers with diabetes were older and had higher BMI on average compared with the control group of women. Applying multivariate regression analyses to the entire group to assess the potential impact of these confounders showed that mother’s pre- pregnancy BMI and age were significantly related to REE. However, because diabetes prevalence increases with age and BMI, these associations could simply reflect the demographics of diabetes, rather than age or adiposity per se. Additionally, the effect of a difference in pre-pregnancy maternal BMI on infant REE in the current study is likely small because the women in the control group were overweight on average, while those with diabetes were obese. Previously, Rising and Lifshitz (25) reported that 4-month old infants from overweight or obese mothers had lower REE than infants from normal weight mothers, but there was no difference in REE between the overweight and obese groups. In support of a predominant effect of diabetes, the associations of age and maternal BMI were not observed when control and diabetes groups were analyzed separately and group designation was an independent influence on fat oxidation rate in the combined analysis.
The underlying mechanisms responsible for the differences in REE and fat oxidation in the current study are not yet clear. Although we ascribe the lower REE and fat oxidation in the group offspring of women with diabetes to the effects of maternal diabetes, the mothers with diabetes had relatively well-controlled glycemia during pregnancy, especially when considered against historical standards, and supported by the non-significant difference in birth weight compared with control offspring. Still, an average HbA1c of 5.9% is above the normal range for non-pregnant women of 4.0 - 5.6%, and even more significant considering that maternal HbA1c values are typically lower during gestation. (26, 27). Furthermore, data from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study of more than 20,000 non-diabetic pregnancies showed that maternal HbA1c > 5.5% doubles the risk for a birth weight > 90th percentile in offspring, implying even minimal hyperglycemia may have an effect (28). Thus, it is possible that mild maternal hyperglycemia in the present study is at least partly responsible for the effects on infant metabolic rate.
There is evidence that improving glycemic control during pregnancy may favorably affect some adverse outcomes that are associated with GDM, but may be insufficient to prevent measures of long-term risk for obesity and diabetes. Gillman et al (29) conducted an intervention with mothers with GDM that reduced the rate of macrosomia at birth compared with standard-of-care, but by age 4-5 years old the rates of overweight and obesity in the offspring were unaffected by the intervention, suggesting that even mild dysglycemia may produce long lasting effects. Alternatively, the differences between groups in the present study may result from underlying genetic variation that is covariant with GDM. It has been shown that the inherent metabolic rate and fuel oxidation have a high degree of heritability because REE rates cluster within families (22, 30, 31) and have been shown to associate with some genetic variants (14). The current study was not designed to explore the genetic versus environmental contributions to REE and fuel oxidation in the infants. A larger, family-based study would be required to distinguish the relative contributions of diabetes and family heritability on infant metabolism. Additionally, diabetes is accompanied by alterations in lipids or other circulating metabolites that were not measured in the current study. Like glycemia, those or other factors may not be fully corrected in the mothers with diabetes and could therefore have effects on the infant metabolic outcomes.
There are few corroborating data on metabolic rate available for young children. Rising and Lifshitz (25) reported that at 4 months of age, infants from overweight or obese mothers had lower REE compared with infants from normal weight mothers, but they did not include women with diabetes in their study. Salbe et al. (32) reported that both resting and total daily energy expenditure was unaltered by maternal diabetes during pregnancy in Pima Indian children who were tested at age 5 years old. The discrepancy between the latter findings and ours may be attributable to the difference in age at which the children studied and/or the very high risk for diabetes and obesity inherent in Pima Indians.
The strengths of this study are application of detailed and standardized measures of body composition and metabolism applied to a population at high risk for subsequent metabolic disease, and the novel finding of reduced fat oxidation in 1-month old infants born to women with typical diabetes and management. Limitations of the study are primarily related to expected difference in anthropometry between control and mothers with diabetesand the relatively small number of participants, which precluded a more detailed analysis of other factors that potentially contribute to the metabolic state. We could not adequately assess the effects of diabetes severity, duration, or therapy as we included patients with either pre-existing or GDM who were treated with different modalities. We did not detect any differences in metabolic rates between those diabetes subgroups, supporting the postulate above that correcting glycemia may not be sufficient to normalize the impact of maternal diabetes on infant metabolism. However a study with larger sample size is required to fully account for the relative impact of these factors. In addition we suspect that maternal obesity plays a role, independent of in utero glycemia, on infant metabolism, but a different study design and a larger number of participants would be needed to formally examine this question.
Acknowledgments
The authors thank Mary Ayn Tullier (Choctaw Nation of Oklahoma), Justin Fowler (Chickasaw Nation), and Lancer Stephens, Jennifer Chadwick, Shelly Hopper, and Olufolake Olufowote (all of University of Oklahoma Health Sciences Center) for their efforts to recruit participants and perform data collection.
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK089034), the National Institute on Minority Health and Health Disparities/National Institutes of Health (P20 MD000528), the American Diabetes Association (1-10-CT-09), and the CMRI Metabolic Research Program, Department of Pediatrics, OUHSC.
Footnotes
The authors declare no conflicts of interest.
Portions of the sutyd were presented as an abstract at the Pediatric Endocrinology annual meeting, Washington, DC, May <>, 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 1983;308:242–5. doi: 10.1056/NEJM198302033080502. [DOI] [PubMed] [Google Scholar]
- [3].Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care. 1995;18:611–7. doi: 10.2337/diacare.18.5.611. [DOI] [PubMed] [Google Scholar]
- [4].West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 2011;54:504–7. doi: 10.1007/s00125-010-2008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes. 2011;60:1849–55. doi: 10.2337/db11-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chandler-Laney PC, Bush NC, Rouse DJ, Mancuso MS, Gower BA. Maternal glucose concentration during pregnancy predicts fat and lean mass of prepubertal offspring. Diabetes Care. 2011;34:741–5. doi: 10.2337/dc10-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Regnault N, Gillman MW, Rifas-Shiman SL, Eggleston E, Oken E. Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care. 2013;36:3045–53. doi: 10.2337/dc13-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seki Y, Williams L, Vuguin PM, Charron MJ. Minireview: Epigenetic programming of diabetes and obesity: Animal models. Endocrinology. 2012;153:1031–8. doi: 10.1210/en.2011-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bouchard L, Thibault S, Guay S-P, Santure M, Monpetit A, St-Pierre J, et al. Leptin gene epigenetic adaptation to impaired glucose metabolism during pregnancy. Diabetes Care. 2010;33:2436–41. doi: 10.2337/dc10-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. 2009;94:2464–70. doi: 10.1210/jc.2009-0305. [DOI] [PubMed] [Google Scholar]
- [11].Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. High prevelance of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes. Diabetes Care. 2008;31:340–6. doi: 10.2337/dc07-1596. [DOI] [PubMed] [Google Scholar]
- [12].Srinivasan SR, Frontini MG, Berenson GS. Longitudinal changes in risk variables of insulin resistance syndrome from childhood to young adulthood in offspring of parents with type 2 diabetes: The Bogalusa Heart Study. Metabolism. 2003;52:443–50. doi: 10.1053/meta.2003.50065. [DOI] [PubMed] [Google Scholar]
- [13].Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62:4043–51. doi: 10.2337/db13-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Astrup A. The relevance of increased fat oxidation for body-weight management: metabolic inflexibility in the predisposition to weight gain. Obes Rev. 2011;12:859–65. doi: 10.1111/j.1467-789X.2011.00894.x. [DOI] [PubMed] [Google Scholar]
- [15].Zurlo F, Lillioja S, Puente AED, Nyomba BL, Raz I, Saad MF, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol Endocrinol Metab. 1990;259:E650–E7. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- [16].Fields DA, Demerath EW, Pietrobelli A, Chandler-Laney PC. Body composition at 6 months of life: comparison of air displacement plethysmography and dual-energy X-ray absorptiometry. Obesity. 2012;20:2302–6. doi: 10.1038/oby.2012.102. [DOI] [PubMed] [Google Scholar]
- [17].Weir JBDV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286:E92–E101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- [19].Carstens MT, Goedecke JH, Dugas L, Evans J, Kroff J, Levitt NS, et al. Fasting substrate oxidation in relation to habitual dietary fat intake and insulin resistance in non-diabetic women: a case for metabolic flexibility. Nutr Metab. 2013;10 doi: 10.1186/1743-7075-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].De Pergola G, Pannacciulli N, Minenna A, Martina RA, Cannito F, Giorgino R. Fuel metabolism in adult individuals with a wide range of body mass index: effect of a family history of type 2 diabetes. Diab Nutr Metab. 2003;16:41–7. [PubMed] [Google Scholar]
- [21].Lattuada G, Costantino F, Caumo A, Scifo P, Ragogna F, De Cobelli F, et al. Reduced whole-body lipid oxidation is associated with insulin resistance, but not with intramyocellular lipid content in offspring of type 2 diabetic patients. Diabetologia. 2005;48:741–7. doi: 10.1007/s00125-005-1686-6. [DOI] [PubMed] [Google Scholar]
- [22].Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WGH, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318:467–72. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- [23].Crume TL, Ogden L, Daniels S, Hamman RF, Norris JM, Dabelea D. The Impact of in utero exposure to diabetes on childhood body mass index growth trajectories: The EPOCH Study. J Pediatr. 2011;158:941–6. doi: 10.1016/j.jpeds.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lau SM, Lin SJ, Stokes RA, Cheng K, Baldock PA, Enriquez RF, et al. Synergistic effects of genetic beta cell dysfunction and maternal glucose intolerance on offspring metabolic phenotype in mice. Diabetologia. 2011;54:910–21. doi: 10.1007/s00125-010-1998-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rising R, Lifshitz F. Lower energy expenditure in infants from obese biological mothers. Nutrition Journal. 2008;7:15. doi: 10.1186/1475-2891-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nielsen LR, Ekbom P, Damm P, Glümer C, Frandsen MM, Jensen DM, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200–1. doi: 10.2337/diacare.27.5.1200. [DOI] [PubMed] [Google Scholar]
- [27].Mosca A, Paleari R, Dalfra MG, Di Cianni G, Cuccuru I, Pellegrini G, et al. Reference intervals for hemoglobin A1c in pregnant women: data from an Italian multicenter study. Clin Chem. 2006;52:1138–43. doi: 10.1373/clinchem.2005.064899. [DOI] [PubMed] [Google Scholar]
- [28].Lowe LP, Metzger BE, Dyer AR, Lowe J, McCance DR, Lappin TRJ, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care. 2012;35:574–80. doi: 10.2337/dc11-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestastional diabetes mellitus on obesity in the next generation. Diabetes Care. 2010;33 doi: 10.2337/dc09-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bogardus C, Lillioja S, Ravussin E, Abbott W, Zawadzki JK, Young A, et al. Familial dependence of the resting metabolic rate. N Engl J Med. 1986;315:96–100. doi: 10.1056/NEJM198607103150205. [DOI] [PubMed] [Google Scholar]
- [31].Bosy-Westphal A, Wolf A, Bührens F, Hitze B, Czech N, Mönig H, et al. Familial influences and obesity-associated metabolic risk factors contribute to the variation in resting energy expenditure: the Kiel Obesity Prevention Study. Am J Clin Nutr. 2008;87:1695–701. doi: 10.1093/ajcn/87.6.1695. [DOI] [PubMed] [Google Scholar]
- [32].Salbe AD, Fontvielle AM, Pettitt DJ, Ravussin E. Maternal diabetes status does not influence energy expenditure or physical activity in 5-year-old Pima Indian children. Diabetologia. 1998;41:1157–62. doi: 10.1007/s001250051045. [DOI] [PubMed] [Google Scholar]