SUMMARY
Proteomic studies have revealed many potential functions of cytoplasmic lipid droplets and recent activity has confirmed that these bona fide organelles are central not only for lipid storage and metabolism, but for development, immunity, and pathogenesis by several microbes. There has been a burst of recent activity on the assembly, maintenance and turnover of lipid droplets that reveal fresh insights. This review summarizes several novel findings in initiation of lipid droplet assembly, protein targeting, droplet fusion, and turnover of droplets through lipophagy.
Cytoplasmic lipid droplets comprise a central hub for metabolism and cellular homeostasis. They are found in most if not all nucleated cells and even in several prokaryotes [1]. Tissues dedicated to their function exist in higher plants and animals, where they provide energy for the organism, and, at least in mammals, regulate appetite and energy metabolism at distant sites through release of adipokines [2]. Lipid droplets go far beyond providing fuel and regulating its use: the neutral lipids within their cores, triacylglycerols and steryl esters plus a variety of other lipids depending on tissue and cell type, are the source of hormones, secondary messengers, and plasmalogens [3,4]. They protect the cell from fatty acid-induced lipotoxicity [5]. The proteins in the surrounding phospholipid monolayer have roles not only in lipid metabolism but also in interorganellar communication [6], development [7], and immunity [8,9]. Lipid droplets are likely linked to ER-mediated protein degradation [10], and they are essential for assembly of viruses and for providing energy for their replication [9,11]. Awakening to the importance of this organelle, the effort to understand their structure, function, birth and death, have become areas of intensive research. There have been several outstanding reviews in the past 2–3 years on droplet cell biology (among them are [12–14]). This mini-review will touch upon a few very recent findings and the controversies they address and raise.
Initiation of droplet assembly
Lipid droplets originate in the endoplasmic reticulum; the terminal enzymes in the synthetic pathways that generate neutral lipids – mainly triacylglycerols (TG) and steryl esters (SE) are localized there [15]. Since these acyltransferases have their active sites facing either the cytosolic or luminal side of the bilayer, neutral lipids can enter the bilayer from either direction [16,17]. Because model phospholipid bilayers can support a few mole percent in neutral lipids without sacrificing stability [18], it seems plausible that the ER membrane has a low level of neutral lipids freely diffusing within its bilayer. As saturation is reached, droplets will form. Recent application of emulsion chemistry to droplet formation is consistent with a spontaneous model for droplet formation, with the surrounding phospholipids (presumably derived from the ER outer leaflet) serving as the emulsifying agent [19]. It is likely, however, that proteins play a role in development of the nascent droplet. For example, by embedding into the cytosolic side of the membrane they can stabilize or enhance the initial convex curvature to ensure that droplets bud from the cytosolic membrane leaflet and not into the ER lumen. Plin3, which binds to nascent droplets on the ER surface, is a good candidate for this function [20]. Proteins containing helical hairpins, such as GPAT4 or DGAT2, which traffic from the ER to droplets [21,22] may also contribute to vectorial budding. The initial generation of the bud may be promoted by FIT2, an ER protein that binds to triacylglycerols, [23]; seipin, mutations in which cause severe lipodystrophy [24], may also be a player. Lipid droplet formation is delayed in the absence of seipin, leading to accumulation of neutral lipids in the ER and blebbing out into inappropriate sites such as the nucleus [25]. Curvature-producing lipids also may contribute to droplet formation. The outer leaflet of the ER membrane must deform generating both a convex surface over the bud and a concave surface at the ER-bud interface. Diacylglycerol (DG), which would support convex curvature, has already been shown to promote droplet budding[20], and yeast lipin, which generates DG from phosphatidic acid (PA), is required to prevent a large accumulation of neutral lipid in the ER, even in the absence of TG synthesis [26]. This finding suggests that DG rather than PA (both of which promote shape change in the same direction) is more important for droplet assembly. In this regard, Fei et al. have observed an increase in PA in the ER in the absence of seipin in yeast [27], and our group has seen PA puncta in these cells (Han and Goodman, unpublished), suggesting that PA accumulation caused by an absence of seipin may have an inhibitory effect on droplet formation. The salutary role of DG in droplet formation, therefore, may involve more than its membrane-curvature properties.
A long-standing question is whether droplet formation occurs at fixed sites in the ER, or whether these sites are random. In mammalian models of adipogenesis, droplets often first develop in the cell periphery, where the ER is rather sparse, and then migrate towards the nucleus [28]. To address whether sites on the ER are marked for droplet formation, Kassan et al. expressed in COS cells a 50-amino acid fusion peptide containing minimal ER and droplet targeting motifs. In starved cells this peptide formed puncta on the ER, even though droplets were not visible by normal staining methods. Upon addition of oleic acid, which rapidly becomes incorporated into neutral lipid, these puncta, termed pre-LDs, marked the sites of the first wave of droplet formation [29]. Ultrastructural studies suggested the pre-LDs were tiny droplets of ~250 nm in these starved cells which were apparently stable over time. Interestingly, the pre-LDs contain the acyl-CoA synthase ASCL3, but not perilipins 2 or 3, nor DGAT2 [29]. Thus, a limited population of droplet precursors on the ER may always present to promote rapid lipid storage.
Another unresolved question is whether droplets, as they mature, separate from the ER. Early electron micrographic studies revealed the close association of the ER with droplets [30]. In yeast our group reported that 94% of droplets could not be resolved from the ER by fluorescence microscopy [31], as if they were attached. There is beauty in a permanent link between droplets and the ER, since bridges between the two organelles, which recently have been visualized [21], could serve as a large buffer to changes in phospholipid mass on droplets during lipolysis or growth of droplets. Furthermore, protein trafficking between the ER and droplets would be facilitated by such bridges. However, an alternative mechanism involving COPI for removal of phospholipids from shrinking droplets has been proposed (see below), obviating the requirement of the ER as a sink. Moreover, GPAT4 only targets from the ER to a subset of droplets, suggesting a subpopulation disconnected from the ER [21]. Droplets also can move rapidly on microtubules in animals or on actin-based cables in yeast [32,33], suggesting their independence from other organelles. However, the ER could accompany lipid droplets during their movement on cytoskeletal elements, as it does with mitochondria [34]. Another argument for independent lipid droplets is a difference in phospholipid composition on droplets compared to ER-derived microsomes [35,36]. However, the composition of such microsomes may be an average from various ER subdomains, or there may be remodeling of droplet or ER phospholipids without free diffusion at the interorganellar junction. While closely associated ER is generally characteristic of lipid droplets, whether all droplets are attached to the ER, and whether this attachment always involves continuity of the outer ER leaflet with the lipid droplet monolayer remain open questions.
Lipid droplet maintenance
Protein trafficking to lipid droplets
While progress has been made regarding the trafficking of proteins to and from lipid droplets, several gaps in our understanding remain. Several small sequences have been identified that are necessary and sufficient for targeting of proteins to droplets [21,37–39]. Typically they consist of a hydrophobic stretch (often a hairpin) and a positive or amphipathic helix. Targeting of GPAT4, which is transported from the ER, was recently examined in detail [21]. The hairpin in GPAT4 was found to be sufficient for targeting to the ER (in the absence of oleate) and to droplets (in the presence of oleate). Substitution of the hairpin with an irrelevant hydrophobic sequence resulted in loss of targeting to the ER but not droplets. Several aspects of the ER-droplet pathway still remain obscure: Do proteins enter the ER through the classical SRP-Sec61 pathway or is there a novel route? Is trafficking from ER to droplets, which can occur over several minutes [22], spontaneous or protein-catalyzed? What is the mechanism of droplet retention of these proteins?
Other proteins destined for droplets originate in the nucleus (CTP:phosphocholine cytodylyltransferase [40]) or the cytosol (the “exchangeable” PLIN proteins [41]). For proteins originating outside the ER, trafficking is likely to involve a combination of affinities to droplet lipids and proteins.
Role of COPI
Many proteomics studies since 2004 have shown the presence of enzymes involved in both synthesis and metabolism of core neutral lipid in lipid droplets [42]. Besides these enzymes, components of COPI were found in early genetic screens for factors involved in droplet morphology. COPI, which coats vesicles that traverse the Golgi and returns cargo to the ER, could be important in indirect ways for droplet maintenance, for example, in retaining important factors in the ER compartment. However, evidence is building that its function is more specific. It was found that COPI is important for transporting ATGL to droplets [43] and that this may be mediated through Arf1 and its exchange factor GBF1 [44]. More recently, COPI was implicated in removing phospholipid from the droplet during periods of lipolysis. In an inverted oil-aqueous system in which water drops exist in a bulk oil solvent with a phospholipid leaflet separating the two phases, the addition of COPI and Arf1 (loaded with GTPγS) into the aqueous phase result in release of oil nanodrops, lowering the interfacial concentration of phospholipids [45]. Such a release could also result in trafficking of proteins from the droplet to other organelles. More recently, the generation of nanodrops from isolated lipid droplets upon supplementation with COPI and Arf1 was demonstrated. Interestingly, the trafficking of GPAT4 from ER to droplets was inhibited by COPI knockdown. This was interpreted as a requirement of COPI for the formation or maintenance of ER/LD bridges [46], although more evidence is needed to demonstrate a direct link between COPI and ER-LD bridge formation.
Droplets and ERAD
An area of controversy has been whether droplets play a role in ER-activated protein degradation (ERAD). This idea was stimulated by the presence of several ERAD proteins in the droplet proteome. Indeed, a small fraction of HMG CoA reductase during its degradation resided in the lipid droplet fraction [47]. However, the presence of TG and SE, and therefore visible droplets, was found not to be necessary for ERAD [48]. This finding, which involved knocking out the acyltransferases required for TG and SE synthesis, cannot rule out that other droplet components that exist on the ER in the absence of droplets may still promote ERAD. Alternatively, ERAD may occur in an ER subcomponent adjacent to, but independent of, droplets. In fact, ubiquitination of HMG CoA reductase was recently found to occur on droplet-associated ER membranes [10]. Whether this indicates a novel ER compartment for ERAD, whether droplet components are important for mammalian but not yeast ERAD, and whether ERAD is an important mechanism for turning over lipid droplet proteins, are presently unanswered questions.
Function of Fsp27
Fat-specific protein of 27 kDa (also known as CIDEC) is a lipid droplet component, induced during adipogenesis, that has been established to negatively regulate lipolysis [49]. Two mechanisms for regulating lipolysis have recently been elucidated. First, Fsp27 binds directly to ATGL and inhibits lipolysis, both basal and hormone-stimulated, in an ATGL-dependent manner [50]. Second, Fsp27, a fraction of which resides in the nucleus, directly potentiates the negative regulation of Egr1 at the ATGL promoter [51]. ChIP analysis reveals both proteins bound to this promoter. Besides regulating ATGL through these two modalities, the protein also stimulates droplet fusion. Fusion is energetically favorable in the absence of any protein factors if the number of surrounding phospholipids became diminished. Fsp27, however, appears to work by an independent mechanism. This protein concentrates at lipid droplet contact sites, where it promotes emptying of the contents of small droplets into larger ones, promoting fusion [52]. Recent evidence has uncovered two modes of Fsp27 regulation of droplet fusion. First is the activation of Fsp27 by perilipin 1 through direct binding to the N-terminal domain (CIDE-N) [53]; this mechanism, which stabilizes the Fsp27 active dimer, is responsible for the large unilocular droplets seen in adipose cells. The second mechanism is through Rab8a. Interestingly, the active form is the GDP-bound state, which is promoted by the GTPase-activing-protein AS160 [54]. Virtually the same region of Fsp27 controls lipolysis and droplet fusion. It is not yet clear how these two processes mechanistically relate to each other (for example, Rab8a may be important for ATGL inhibition), how the Fsp27-AS160-Rab8a complex causes bilayer fusion, and whether Plin1 and Rab8a function together at the same contact site.
Lipophagy
The combustion of lipid stores for energy classically begins with the action of lipases on the lipid droplet monolayer. Recently however, autophagy has also been implicated in neutral lipid utilization from droplets under starvation conditions, a process called lipophagy [55]. Similar to autophagy of other cellular components, lipophagy may involve the formation of a double membrane around the lipid droplet to form an autophagosome that precedes fusion with the lysosome (macroautophagy), or the direct engulfment of lipid droplets by lysosomal membranes (microautophagy). Both types involve subsets of core autophagic proteins (Atgs).
The engulfment of lipid droplets by double-membraned vesicles (macrolipophagy) was first observed in electron micrographs by Singh et al. in mouse hepatocytes [56]. Consistent with this, the group also reported that deletion of the core autophagic protein Atg7 resulted in a blockage of lipophagy and the accumulation of lipid droplets. Other studies have since detected the involvement of lipophagy in consumption of lipid droplets, upon starvation [57,58]. A control point was revealed when exposure of animal cells to fat resulted in an inhibition of lipophagy at the autophagosome-lysosome fusion step, perhaps by a change in lysosome membrane composition [59]. Regeneration of the lysosome following macrolipophagy was found to depend on the GTPase dynamin-2; its inhibition by both genetic and pharmacological means lead to accumulation of lipid droplets within autolysosomes as observed in electron micrographs [60]. Whether dynamin-2 is equally involved in resolution of autolysosomes upon autophagy of other types of organelles is unknown but seems likely.
In contrast to mammalian cells, microlipophagy has been documented in Saccharomyces cerevisiae, in which the lipid droplet is trafficked to the vacuole by microtubules and not by Atg proteins [57]. However, the latter are still needed for catalyzing interactions between the lipid droplet and the vacuole, as is seen by the formation of aggregates of larger droplets, in their absence. Microlipophagy utilizes the special adaptor proteins Vac8 and Trs85 in a mechanism selective for lipid droplets. Atg11, a component of other specialized autophagic machinery (pexophagy and cvt pathway) also increases the efficiency of microlipophagy. Moreover, in the stationary phase, the vacuole was shown to uptake lipid droplets through its specific sterol enriched sites, implicating a feed-forward loop, whereby lipophagy, by providing sterols to the vacuole, promoted the phase-separation required for further droplet uptake and digestion [58].
Lipid droplet maintenance and lipophagy seem at opposite ends of lipid metabolism, but both lipid droplets and autophagosomes share Atg2A, a protein essential for autophagy in mammalian cells [61]. Knockdown of Atg2A and its homolog Atg2B not only results in a block in autophagy, but it also causes aggregation of large droplets in the cytoplasm, suggesting a coupling of droplet morphology with lipophagy to regulate lipid metabolism. The mechanism by which lipophagy is regulated and whether there is cross-talk between lipophagy and lipolysis on lipid droplets outside the lysosome, are two questions that remain incompletely understood.
As the momentum of research in droplet biology continues to increase, many long-held secrets of this organelle are coming to light. However, many basic aspects of droplet biogenesis, maintenance, and destruction, let alone the regulation of these processes, remain obscure. But at the rate in which progress is currently occurring, one expects to learn considerably more in the next few years.
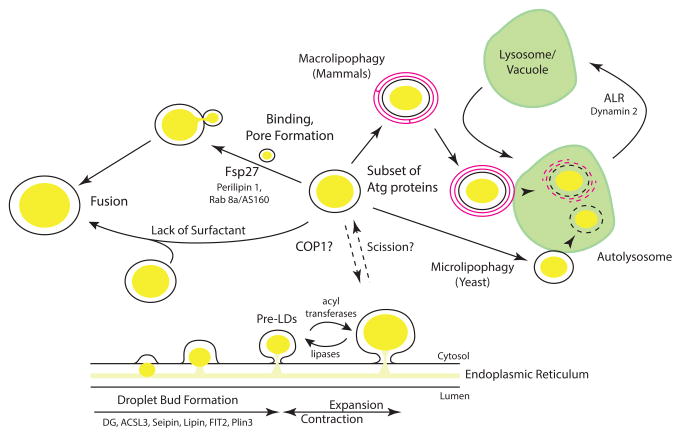
Figure 1. The life cycle of a lipid droplet.
Important events in the lipid droplet life cycle are shown. Recent advances include a pre-droplet organelle and associated ACSL3, a possible cycling of droplets associated and released from the ER mediated by COP1, the dual role of Fsp27 in lipolysis and droplet fusion, and two specific pathways of lipophagy, one common in mammals, the other in yeast. ALR, autophagic lysosome reformation. Although droplets may exist that are disconnected from the ER, the figure is not meant to imply that droplet expansion, fusion, or autophagy is limited to ER-connected or -dissociated droplets.
Acknowledgments
The authors are grateful for funding from NIH GM084210 and ADA 7-13-BS-055 to support our research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Noted* and Outstanding** papers
- 1.Waltermann M, Steinbuchel A. Neutral lipid bodies in prokaryotes: recent insights into structure, formation, and relationship to eukaryotic lipid depots. J Bacteriol. 2005;187:3607–3619. doi: 10.1128/JB.187.11.3607-3619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 3.Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RG, Liu P, Chapman KD. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res. 2007;48:837–847. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Bozza PT, Viola JP. Lipid droplets in inflammation and cancer. Prostaglandins Leukot Essent Fatty Acids. 2010;82:243–250. doi: 10.1016/j.plefa.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Kohlwein SD. Obese and anorexic yeasts: experimental models to understand the metabolic syndrome and lipotoxicity. Biochim Biophys Acta. 2010;1801:222–229. doi: 10.1016/j.bbalip.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 6**.Wang H, Sztalryd C. Oxidative tissue: perilipin 5 links storage with the furnace. Trends Endocrinol Metab. 2011;22:197–203. doi: 10.1016/j.tem.2011.03.008. The paper demonstrated the importance of vacuolar lipid domains in lipophagy and a feed-forward loop for its regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Li Z, Thiel K, Thul PJ, Beller M, Kuhnlein RP, Welte MA. Lipid droplets control the maternal histone supply of Drosophila embryos. Curr Biol. 2012;22:2104–2113. doi: 10.1016/j.cub.2012.09.018. Demonstrates the importance of droplets in buffering a subclass of histones in development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Anand P, Cermelli S, Li Z, Kassan A, Bosch M, Sigua R, Huang L, Ouellette AJ, Pol A, Welte MA, et al. A novel role for lipid droplets in the organismal antibacterial response. Elife. 2012;1:e00003. doi: 10.7554/eLife.00003. Provides evidence that histones stored on lipid droplets protect insect cells from bacterial attack. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saka HA, Valdivia R. Emerging roles for lipid droplets in immunity and host-pathogen interactions. Annu Rev Cell Dev Biol. 2012;28:411–437. doi: 10.1146/annurev-cellbio-092910-153958. [DOI] [PubMed] [Google Scholar]
- 10*.Jo Y, Hartman IZ, DeBose-Boyd RA. Ancient ubiquitous protein-1 mediates sterol-induced ubiquitination of 3-hydroxy-3-methylglutaryl CoA reductase in lipid droplet-associated endoplasmic reticulum membranes. Mol Biol Cell. 2013;24:169–183. doi: 10.1091/mbc.E12-07-0564. While the role of droplets in ERAD is controversial, this work demonstrates that ERAD components are enriched in a droplet fraction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaton NS, Randall G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe. 2010;8:422–432. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pol A, Gross SP, Parton RG. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J Cell Biol. 2014;204:635–646. doi: 10.1083/jcb.201311051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Wilfling F, Haas JT, Walther TC, RV Lipid droplet biogenesis. Curr Opin Cell Biol. 2014;29C:39–45. doi: 10.1016/j.ceb.2014.03.008. Provides evidence for a role of COP1 in phospholipid removal from droplets and a possible connection to ER-LD protein trafficking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimoto T, Parton RG. Not just fat: the structure and function of the lipid droplet. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buhman KK, Chen HC, Farese RV., Jr The enzymes of neutral lipid synthesis. J Biol Chem. 2001;276:40369–40372. doi: 10.1074/jbc.R100050200. [DOI] [PubMed] [Google Scholar]
- 16.Choudhary V, Jacquier N, Schneiter R. The topology of the triacylglycerol synthesizing enzyme Lro1 indicates that neutral lipids can be produced within the luminal compartment of the endoplasmatic reticulum: Implications for the biogenesis of lipid droplets. Commun Integr Biol. 2011;4:781–784. doi: 10.4161/cib.17830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wurie HR, Buckett L, Zammit VA. Evidence that diacylglycerol acyltransferase 1 (DGAT1) has dual membrane topology in the endoplasmic reticulum of HepG2 cells. J Biol Chem. 2011;286:36238–36247. doi: 10.1074/jbc.M111.251900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton JA. Interactions of triglycerides with phospholipids: incorporation into the bilayer structure and formation of emulsions. Biochemistry. 1989;28:2514–2520. doi: 10.1021/bi00432a025. [DOI] [PubMed] [Google Scholar]
- 19*.Thiam AR, Farese RV, Jr, Walther TC. The biophysics and cell biology of lipid droplets. Nat Rev Mol Cell Biol. 2013;14:775–786. doi: 10.1038/nrm3699. Provides evidence for a novel hypothesis of the role of COPI in droplet phospholipid trafficking using a reconstituted system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skinner JR, Shew TM, Schwartz DM, Tzekov A, Lepus CM, Abumrad NA, Wolins NE. Diacylglycerol enrichment of endoplasmic reticulum or lipid droplets recruits perilipin 3/TIP47 during lipid storage and mobilization. J Biol Chem. 2009;284:30941–30948. doi: 10.1074/jbc.M109.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Frohlich F, et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell. 2013;24:384–399. doi: 10.1016/j.devcel.2013.01.013. Documents the trafficking of lipid biosynthetic enzymes from ER to droplets and their role in droplet expansion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Jacquier N, Choudhary V, Mari M, Toulmay A, Reggiori F, Schneiter R. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Sci. 2011;124:2424–2437. doi: 10.1242/jcs.076836. Demonstrates discrete lipid domains on the surface of the yeast vacuole. [DOI] [PubMed] [Google Scholar]
- 23.Gross DA, Zhan C, Silver DL. Direct binding of triglyceride to fat storage-inducing transmembrane proteins 1 and 2 is important for lipid droplet formation. Proc Natl Acad Sci U S A. 2011;108:19581–19586. doi: 10.1073/pnas.1110817108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cartwright BR, Goodman JM. Seipin: from human disease to molecular mechanism. J Lipid Res. 2012;53:1042–1055. doi: 10.1194/jlr.R023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cartwright BR, Binns DD, Hilton CL, Han S, Gao Q, Goodman JM. Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology. Mol Biol Cell. 2014 doi: 10.1091/mbc.E14-08-1303. mbc.E14–08-1303. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adeyo O, Horn PJ, Lee S, Binns DD, Chandrahas A, Chapman KD, Goodman JM. The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J Cell Biol. 2011;192:1043–1055. doi: 10.1083/jcb.201010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fei W, Shui G, Zhang Y, Krahmer N, Ferguson C, Kapterian TS, Lin RC, Dawes IW, Brown AJ, Li P, et al. A role for phosphatidic acid in the formation of “supersized” lipid droplets. PLoS Genet. 2011;7:e1002201. doi: 10.1371/journal.pgen.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolins NE, Skinner JR, Schoenfish MJ, Tzekov A, Bensch KG, Bickel PE. Adipocyte protein S3–12 coats nascent lipid droplets. J Biol Chem. 2003;278:37713–37721. doi: 10.1074/jbc.M304025200. [DOI] [PubMed] [Google Scholar]
- 29**.Kassan A, Herms A, Fernandez-Vidal A, Bosch M, Schieber NL, Reddy BJ, Fajardo A, Gelabert-Baldrich M, Tebar F, Enrich C, et al. Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains. J Cell Biol. 2013;203:985–1001. doi: 10.1083/jcb.201305142. This remarkable work demonstrated the existence of pre-LDs and the importance of ACSL3 in mediating lipogenesis at these sites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, Rondinone CM, Theodorakis JL, Greenberg AS, Londos C. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res. 1995;36:1211–1226. [PubMed] [Google Scholar]
- 31.Szymanski KM, Binns D, Bartz R, Grishin NV, Li WP, Agarwal AK, Garg A, Anderson RG, Goodman JM. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci U S A. 2007;104:20890–20895. doi: 10.1073/pnas.0704154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welte MA, Gross SP, Postner M, Block SM, Wieschaus EF. Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell. 1998;92:547–557. doi: 10.1016/s0092-8674(00)80947-2. [DOI] [PubMed] [Google Scholar]
- 33.Wolinski H, Kolb D, Hermann S, Koning RI, Kohlwein SD. A role for seipin in lipid droplet dynamics and inheritance in yeast. J Cell Sci. 2011;124:3894–3904. doi: 10.1242/jcs.091454. [DOI] [PubMed] [Google Scholar]
- 34.Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J Cell Biol. 2010;190:363–375. doi: 10.1083/jcb.200911024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grillitsch K, Connerth M, Kofeler H, Arrey TN, Rietschel B, Wagner B, Karas M, Daum G. Lipid particles/droplets of the yeast Saccharomyces cerevisiae revisited: lipidome meets proteome. Biochim Biophys Acta. 2011;1811:1165–1176. doi: 10.1016/j.bbalip.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, Daum G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol. 1991;173:2026–2034. doi: 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zehmer JK, Bartz R, Liu P, Anderson RG. Identification of a novel N-terminal hydrophobic sequence that targets proteins to lipid droplets. J Cell Sci. 2008;121:1852–1860. doi: 10.1242/jcs.012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zehmer JK, Bartz R, Bisel B, Liu P, Seemann J, Anderson RG. Targeting sequences of UBXD8 and AAM-B reveal that the ER has a direct role in the emergence and regression of lipid droplets. J Cell Sci. 2009;122:3694–3702. doi: 10.1242/jcs.054700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingelmo-Torres M, Gonzalez-Moreno E, Kassan A, Hanzal-Bayer M, Tebar F, Herms A, Grewal T, Hancock JF, Enrich C, Bosch M, et al. Hydrophobic and basic domains target proteins to lipid droplets. Traffic. 2009;10:1785–1801. doi: 10.1111/j.1600-0854.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Krahmer N, Guo Y, Wilfling F, Hilger M, Lingrell S, Heger K, Newman HW, Schmidt-Supprian M, Vance DE, Mann M, et al. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP: phosphocholine cytidylyltransferase. Cell Metab. 2011;14:504–515. doi: 10.1016/j.cmet.2011.07.013. Demonstrated control of phosphatidylcholine synthesis by translocation of a critical enzyme from nucleus to droplet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 42.Hodges BD, Wu CC. Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J Lipid Res. 2010;51:262–273. doi: 10.1194/jlr.R003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soni KG, Mardones GA, Sougrat R, Smirnova E, Jackson CL, Bonifacino JS. Coatomer-dependent protein delivery to lipid droplets. J Cell Sci. 2009;122:1834–1841. doi: 10.1242/jcs.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellong EN, Soni KG, Bui QT, Sougrat R, Golinelli-Cohen MP, Jackson CL. Interaction between the triglyceride lipase ATGL and the Arf1 activator GBF1. PLoS One. 2011;6:e21889. doi: 10.1371/journal.pone.0021889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thiam AR, Antonny B, Wang J, Delacotte J, Wilfling F, Walther TC, Beck R, Rothman JE, Pincet F. COPI buds 60-nm lipid droplets from reconstituted water-phospholipid-triacylglyceride interfaces, suggesting a tension clamp function. Proc Natl Acad Sci U S A. 2013;110:13244–13249. doi: 10.1073/pnas.1307685110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilfling F, Thiam AR, Olarte MJ, Wang J, Beck R, Gould TJ, Allgeyer ES, Pincet F, Bewersdorf J, Farese RV, Jr, et al. Arf1/COPI machinery acts directly on lipid droplets and enables their connection to the ER for protein targeting. Elife. 2014;3:e01607. doi: 10.7554/eLife.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartman IZ, Liu P, Zehmer JK, Luby-Phelps K, Jo Y, Anderson RG, DeBose-Boyd RA. Sterol-induced dislocation of 3-hydroxy-3-methylglutaryl coenzyme A reductase from endoplasmic reticulum membranes into the cytosol through a subcellular compartment resembling lipid droplets. J Biol Chem. 2010;285:19288–19298. doi: 10.1074/jbc.M110.134213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olzmann JA, Kopito RR. Lipid droplet formation is dispensable for endoplasmic reticulum-associated degradation. J Biol Chem. 2011;286:27872–27874. doi: 10.1074/jbc.C111.266452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu L, Zhou L, Li P. CIDE proteins and lipid metabolism. Arterioscler Thromb Vasc Biol. 2012;32:1094–1098. doi: 10.1161/ATVBAHA.111.241489. [DOI] [PubMed] [Google Scholar]
- 50*.Grahn TH, Kaur R, Yin J, Schweiger M, Sharma VM, Lee MJ, Ido Y, Smas CM, Zechner R, Lass A, et al. Fat-specific protein 27 (FSP27) interacts with adipose triglyceride lipase (ATGL) to regulate lipolysis and insulin sensitivity in human adipocytes. J Biol Chem. 2014;289:12029–12039. doi: 10.1074/jbc.M113.539890. Demonstrates that the effect of Fsp27 on lipolysis is through direct interaction with ATGL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh M, Kaur R, Lee MJ, Pickering RT, Sharma VM, Puri V, Kandror KV. Fat-specific Protein 27 Inhibits Lipolysis by Facilitating the Inhibitory Effect of Transcription Factor Egr1 on Transcription of Adipose Triglyceride Lipase. J Biol Chem. 2014;289:14481–14487. doi: 10.1074/jbc.C114.563080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Gong J, Sun Z, Wu L, Xu W, Schieber N, Xu D, Shui G, Yang H, Parton RG, Li P. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol. 2011;195:953–963. doi: 10.1083/jcb.201104142. The important paper demonstrated a novel function for Fsp27 in allowing neutral lipid flux between droplets and eventual fusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Sun Z, Gong J, Wu H, Xu W, Wu L, Xu D, Gao J, Wu JW, Yang H, Yang M, et al. Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat Commun. 2013;4:1594. doi: 10.1038/ncomms2581. Shows that Fsp27 and perilipin 1 promote the unilocular nature of droplets in adipocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Wu L, Xu D, Zhou L, Xie B, Yu L, Yang H, Huang L, Ye J, Deng H, Yuan YA, et al. Rab8a-AS160-MSS4 Regulatory Circuit Controls Lipid Droplet Fusion and Growth. Dev Cell. 2014;30:378–393. doi: 10.1016/j.devcel.2014.07.005. Shows the regulation of Fsp27 by a specific small GTPase. [DOI] [PubMed] [Google Scholar]
- 55.Singh R, Cuervo AM. Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol. 2012;2012:282041. doi: 10.1155/2012/282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Zutphen T, Todde V, de Boer R, Kreim M, Hofbauer HF, Wolinski H, Veenhuis M, van der Klei IJ, Kohlwein SD. Lipid droplet autophagy in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 2014;25:290–301. doi: 10.1091/mbc.E13-08-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang CW, Miao YH, Chang YS. A sterol-enriched vacuolar microdomain mediates stationary phase lipophagy in budding yeast. J Cell Biol. 2014;206:357–366. doi: 10.1083/jcb.201404115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koga H, Kaushik S, Cuervo AM. Altered lipid content inhibits autophagic vesicular fusion. The FASEB Journal. 2010;24:3052–3065. doi: 10.1096/fj.09-144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Schulze RJ, Weller SG, Schroeder B, Krueger EW, Chi S, Casey CA, McNiven MA. Lipid droplet breakdown requires dynamin 2 for vesiculation of autolysosomal tubules in hepatocytes. J Cell Biol. 2013;203:315–326. doi: 10.1083/jcb.201306140. Shows the importance of dynamin-2 in recycling components of the autolysosome after lipophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Velikkakath AK, Nishimura T, Oita E, Ishihara N, Mizushima N. Mammalian Atg2 proteins are essential for autophagosome formation and important for regulation of size and distribution of lipid droplets. Mol Biol Cell. 2012;23:896–909. doi: 10.1091/mbc.E11-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]