Abstract
Rationale
The mechanism of functional restoration by stem cell therapy remains poorly understood. Novel manganese-enhanced MRI and bioluminescence reporter gene imaging (BLI) were applied to follow myocardial viability and cell engraftment, respectively. Human-placenta-derived amniotic mesenchymal stem cells (AMCs) demonstrate unique immunoregulatory and pre-cardiac properties. In this study, the restorative effects of three AMC-derived sub-populations were examined in a murine myocardial injury model: 1) unselected AMCs (uAMCs), 2) ckit+AMCs (c+AMCs), and 3) AMC-derived iPSCs (MiPSCs).
Objective
Determine the differential restorative effects of the AMC-derived sub-populations in the murine myocardial injury model using multi-modality imaging.
Methods and Results
SCID mice underwent left anterior descending artery ligation and were divided into 4 treatment arms: 1) normal saline control (n=14), 2) uAMCs (n=10), 3) c+AMCs (n=13), and 4) MiPSCs (n=11). Cardiac MRI assessed myocardial viability and left ventricular (LV) function while BLI assessed stem cell engraftment over a four-week period. Immunohistological labeling and RT-PCR of the explanted myocardium were performed. The uAMC and c+AMC treated mice demonstrated transient LV functional improvement. However, the MiPSCs exhibited a significantly greater increase in LV function compared to all the other groups during the entire four-week period. LV functional improvement correlated with increased myocardial viability and sustained stem cell engraftment. The MiPSCs treated animals lacked any evidence of de novo cardiac differentiation.
Conclusion
The functional restoration seen in MiPSCs was characterized by increased myocardial viability and sustained engraftment without de novo cardiac differentiation, indicating salvage of the injured myocardium.
Keywords: Amniotic mesenchymal stem cells, manganese-enhanced MRI, bioluminescence, ischemic heart disease, acute myocardial infarction, stem cell imaging, cardiac magnetic resonance imaging, stem cell
INTRODUCTION
Multiple human stem cell populations have demonstrated the ability to improve left ventricular ejection fraction (LVEF) after myocardial damage. These cells include pluripotent human embryonic stem cells (ESCs), human induced pluripotent stem cells (iPSCs)1, 2, bone marrow stem cells, mesenchymal stem cells (MSCs), and cardiac progenitor cells (CPCs)1, 3–6. Initial studies hypothesized that the improved LVEF was due to the spontaneous cardiac differentiation of the stem cells and regeneration of the injured myocardium (regeneration hypothesis)7. However, increasing evidence has refuted differentiation of the engrafted stem cells8, 9. Instead, careful analysis of the data suggested that the paracrine effects salvaged the injured myocardium without de novo cardiac differentiation or myocardial regeneration (salvage hypothesis)3, 8, 9. In support of the salvage hypothesis, multiple studies have demonstrated similar improvements with conditioned media, secreted cell products, or cell lysis when compared to intact stem cells3, 10–12.
The pluripotency states of the stem cell are theorized to correlate directly with myocardial restoration potential. However, few studies have conducted head-to-head comparisons of the restorative processes of different stem cell populations and assessed their direct effects on the myocardial viability in vivo. In a comparison study of murine ESCs vs. MSCs in post-ischemic injury, the ESCs demonstrated greater functional recovery when compared to MSCs13. The investigation suggested that the greater restorative potential of the ESCs was due to the increased paracrine signaling with enhanced production of VEGF, IL-10 and IGF-1 in the ESC-treated hearts. However, the direct effect of paracrine signals on myocardial viability and the biological role of stem cell engraftment were not evaluated.
We examined the therapeutic effects of three sub-populations of AMCs derived from the human placenta. AMCs are derived from the inner cell mass of the embryo, which differentiate into the epiblast and the hypoblast on days 8–9 of embryologic development. The epiblast gives rise to the extraembryonic mesoderm-like AMCs in the amniotic membrane, which retain pluripotent gene expression14. These stem cells differentiate predominantly along the mesodermal lineage and have propensity for cardiac lineage specification by the expression of the ckit+ cell surface marker associated with CPCs14. In addition, these cells line the amniotic membrane situated at the maternal-fetal interface, conferring the critical immuno-modulatory properties for the fetus14. Three cell sub-populations were generated from this common lineage to directly compare their therapeutic potential: 1) unselected AMCs (uAMCs), 2) ckit+AMCs (c+AMCs), and 3) AMC-derived induced pluripotent stem cells (MiPSCs). This study hypothesized that the MiPSCs would have the greatest cardiac restorative potential due to their pluripotency.
Manganese-enhanced MRI (MEMRI) enables viability-specific evaluation of the myocardium. This novel technique was integrated with delayed-enhanced MRI (DEMRI) to measure the direct therapeutic impact of the stem cells on myocardial viability and to correlate with sensitive in vivo bioluminescence imaging (BLI) of stem cell engraftment15, 16. This integrated in vivo imaging platform allowed real-time evaluation of the direct biological effects of the engraftment of AMC-derivatives on the viable, injured, and non-viable myocardium volume at high temporal and spatial resolution. This study demonstrated that myocardial viability paralleled differential engraftment of each AMC sub-population and correlated with the degree of salvage of the injured myocardium.
METHODS
Detailed methods are provided in the online supplement.
Isolation of AMCs from the human placentas
A placenta from one healthy subject was obtained. uAMCs were enzymatically isolated from the amniotic membrane.
Fluorescent Activated Cell Sorting (FACS)
The uAMCs underwent 2-step FACS with ckit and SSEA-4 antibodies. The sorted cells were labeled c+AMCs.
BLI Reporter Gene (RG) virus generation
A BLI RG plasmid DNA (courtesy of Joseph Wu, Stanford University17) was isolated using the plasmid Maxi-kit (Qiagen Inc., CA, USA). 293FT cells were then transfected. The supernatant was collected and centrifuged to obtain the pellets used for transduction.
RG virus transduction
5×105 AMCs per one-well were plated in 6-well plates one day before transduction. On the day of transduction, the cells were washed once with PBS and then incubated overnight in total volume of 250 mL of OptiMEM (Invitrogen) with BLI RG virus pellets and 10 μg/mL of polybrene (Sigma, MO, USA). BLI signal detected after 3 days assured effective transduction.
Virus production and iPSC (MiPSC) generation
The plasmid of pHAGE2EF1-OKSM (courtesy of G. Mostoslavsky, Boston University) was employed to generate the virus14. 293FT cells were then transfected. The virus was harvested over 3 days and concentrated by centrifugation. AMCs were transduced with the concentrated virus. These cells were then passaged on day 6 and cultured on plates pre-seeded with irradiated mouse fibroblasts. The cells were grown until the formation of spontaneous colonies.
Permanent LAD ligation
Animal care and interventions were done in accordance with the Laboratory Animal Welfare Act and all animals received humane care and treatment in accordance with the “Guide for the Care and Use of Laboratory Animals” (www.nap.edu/catalog/5140.html). Immunotolerant SCID-beige male mice (90–120 days; Charles River Laboratories, Inc, MA, USA) were anesthetized in an isofluorane inhalational chamber and endotracheally intubated with a 20-gauge angiocatheter (Ethicon Endo-Surgery, Inc, OH, USA). Ventilation was maintained with a Harvard rodent ventilator (Harvard Apparatus, Inc, MA, USA). Acute myocardial injury (AMI) was created by ligation of the mid left anterior descending coronary artery (LAD) through a left thoracotomy. The mice were randomly allocated to four groups. The mice received one of the following cell types or normal saline (NS) directly into the peri-infarct region immediately following induction of AMI: 1) NS control (n=9), 2) uAMCs (n=9), 3) c+AMCs (n=8), and 4) MiPSCs (n=9). The cells were injected into the peri-infarct region with a Hamilton syringe containing 250,000 cells suspended in a 20 μL volume of a 1:5 mixture of matrigel (BD Biosciences, CA, USA) and cell dissociation buffer (Sigma) to prevent clumping of cells. Cell dissociation buffer was used to prevent cell clumping. The chest was closed in two layers with 5-0 Vicryl suture (Ethicon).
In vivo magnetic resonance imaging
Mice were serially imaged by cardiac MRI (CMR) at weeks 1, 2 and 4 after LAD ligation and treatment. Mice were anesthetized during the exam and electrocardiographic gating obtained with two subcutaneous precordial leads. LV function was evaluated with fast spoiled gradient echo sequences. MEMRI and DEMRI utilized fast gradient echo inversion recovery sequences. MEMRI was obtained after intraperitoneal injection (IP) of manganese solution. The following day, DEMRI was obtained after IP injection of gadopentetate dimeglumine. Images were analyzed offline. Peri-infarct region was defined as the region of overlap between DEMRI enhancement and MEMRI defect, representing the area of viable myocardium (MEMRI) in the region of non-viable myocardial scar (DEMRI) as shown previously15. Percent MEMRI scar volume = (MEMRI defect volume × 100)/total LV mass volume; % MEMRI viable myocardial volume = (MEMRI enhancement volume × 100)/total LV mass volume; % DEMRI scar volume = (DEMRI scar volume × 100)/total LV mass volume; % DEMRI-MEMRI peri-infarct volume = (DEMRI-MEMRI peri-infarct volume × 100)/total LV mass volume.
In vivo optical Bioluminescence Imaging (BLI)
Optical BLI was performed 15–25 minutes after d-luciferin IP injection (400 mg/kg; Xenogen, MA, USA) with 3–5 minute acquisition scans on a charge-coupled device camera (IVIS 200; Xenogen). Peak signal from a fixed region of interest was evaluated with Living Image 3.2 software (Xenogen).
Immunohistology
Hearts were flushed with normal saline, fixed with paraformaldehyde and embedded in paraffin blocks. 4 μm slices were sectioned and then stained with hematoxylin and eosin.
Unstained paraffin embedded sections were deparaffinized and rehydrated. Sections underwent antigen unmasking, treatment with Triton-X 100 and blocking with BSA and normal goat serum. MiPSCs and c+AMCs of human origin were detected in murine myocardium utilizing primary antibodies specific for human mitochondria. Primary antibodies specific for cardiomyocytes and endothelial cells were also detected using a Goat Anti-Human IgG1 AlexaFluor 488 Ab. A rabbit polyclonal antibody to ckit was used and visualized with Goat Anti-Rabbit IgG AlexaFluor 488. The cellular cytoskeleton was counterstained with AlexaFluor 568 Phalloidin. Finally, luciferin was immunostained by incubating rabbit anti-firefly luciferase and anti-human nuclear antigen and visualized with anti-rabbit AlexaFluor 488 and anti-mouse Cy3 secondary antibodies.
Luminex immunoassay
The human 63-plex magnetic bead kit (eBiosciences/Affymetrix, CA, USA) was used according to the manufacturer’s recommendations with modifications as described below. Briefly, the beads were added to a 96 well plate and washed in a washer solution (BioTek ELx405, VT, USA). Samples were added to the plate containing the mixed antibody-linked beads and incubated at room temperature for 1 hour followed by overnight shaking incubation at 4°C. Cold and room temperature incubation steps were performed on an orbital shaker at 500–600 rpm. Following overnight incubation, the plates were washed and then biotinylated with detection antibody for 75 minutes at room temperature. The plate was washed as described above and streptavidin-PE was added. After incubation for 30 minutes at room temperature, another wash was performed and reading buffer was added to the wells. Fluorescence intensity of each sample was measured in duplicate. Plates were read using a Luminex 200 instrument with a lower bound of 50 beads per sample per cytokine. For quality control, custom assay control beads (Radix Biosolutions, TX, USA) were added to all wells.
Real-time reverse transcription-polymerase chain reaction
Total RNA was extracted from the 3 cell sub-populations (uAMCs, c+AMCs, and MiPSCs) using Trizol reagent (Invitrogen) according to the manufacturer’s recommendation. 2 ug of total RNA was transcribed into cDNA using Superscript first strand synthesis system (Invitrogen). The PCR products were size fractionated by 2% agarose gel electrophoresis (Invitrogen). Genes were amplified using iQ SYBR Green Supermix (Applied Biosystems, MA, USA) and StepOne Plus Real-Time PCR Detection System (Applied Biosystems). All genes were amplified for 40 cycles. Specific gene expression was first normalized to GADPH and then compared to control groups. Relative quantitation for human primers was performed for the following: KLF4, NANOG, EBAF, MYC, SOX2, TDGF1, OCT4, GATA4, ANP, CTNT, CTNI, alpha-MHC, NKX2.5 (Invitrogen). KLF4, NANOG, EBAF, MYC, SOX2, TDGF1, and OCT4 are previously described ESC pluripotency genes17. NKX2.5 and GATA4 are transcription factors associated with heart field specific progenitors and embryonic cardiomyocytes18 while ANP, CTNT, CTNI and alpha-MHC are mature cardiac specific cell marker genes19, 20.
In addition, total mRNA was isolated from the cell-treated and control myocardial tissue at the end of the study. Total mRNA was then reverse-transcribed into cDNA. Real-time quantitative PCR was run on a 96 well real-time PCR thermocycler using Power SYBR Green master mix (Applied Biosystems), according to the manufacturer’s recommendations. Relative quantitation for mouse primers was performed for the following: collagen I, collagen III, connective tissue growth factor (CTGF), TGF, fibronectin, and Akt. The GADPH housekeeping gene was used as reference for the relative quantification of the genes of interest21.
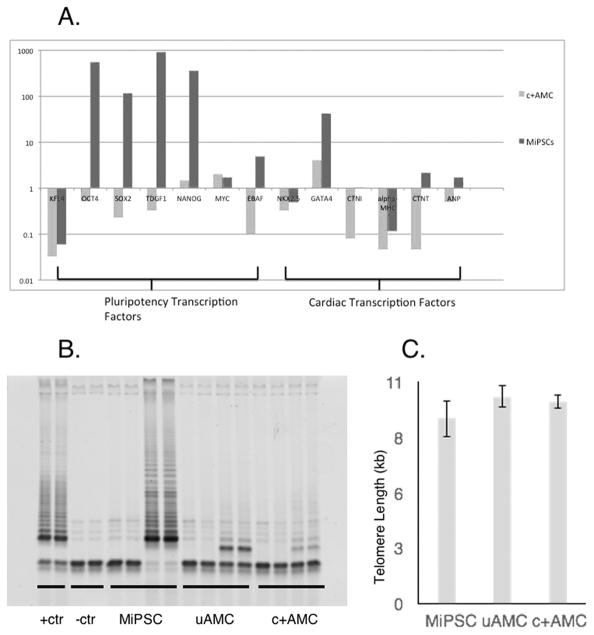
Telomerase activity and telomere length
Telomerase activity was measured using the non-radioisotopic method of the TRAPeze Telomerase Detection kit (S7700; EMD Millipore, MA, USA). The products were stained with SYBR Gold Nucleic Acid Gel Stain (Life Technologies, NY, USA) and run on a 15% polyacrylamide gel in 1X TBE. Telomere length was measured by SpectraCell Laboratories, Inc. (TX, USA). Genomic DNA was extracted from 500,000 cells using phenol chloroform and quantified using the Quant-iT™ PicoGreen® dsDNA Assay Kit (Life Technologies). Telomere length analysis was performed at SpectraCell Laboratories Inc. using a CLIA approved, high throughput qPCR assay22, 23. The assay determines a relative telomere length by measuring the factor by which the sample differs from a reference DNA sample in its ratio of telomere repeat copy number to singe gene (36B4) copy number. All samples were run in quadruplicate with at least one negative control and two positive controls of two different known telomere lengths (high and low). The results were reported as a telomere score equivalent to the average telomere length in kb.
Statistical analysis
Results are mean +/− standard error of mean. Significant differences (p<0.05) were tested using analysis of variance and Bonferroni post-test for more than two groups or time points, and Student’s unpaired t-test for two groups. The Pearson correlation coefficient was assessed between LVEF and viable myocardium by MEMRI, LVEF and myocardial scar by DEMRI and LVEF and stem cell engraftment by BLI.
RESULTS
LV systolic function correlates with stem cell engraftment
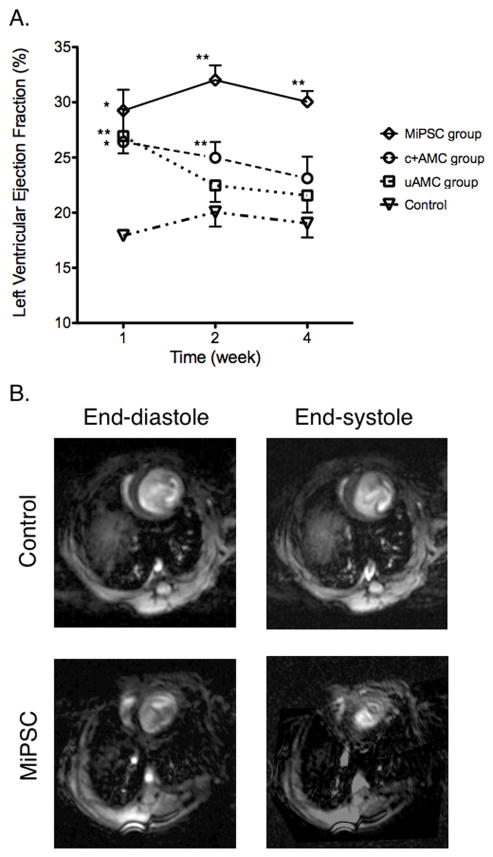
One week after AMI, all treated mice demonstrated improved LVEF compared to control (Figure 1A). However, the initial LVEF increase in the uAMC group (26.9±1.5% vs. 17.9±0.2%, week 1, p<0.01) did not persist and was not significantly different from control (NS) at weeks 2 and 4. The initial functional improvement and subsequent decline paralleled the engraftment pattern of the transplanted uAMCs as measured by BLI (Figure 2A). The c+AMC group exhibited significant improvement at weeks 1 and 2 but did not persist through week 4 compared to the control group (23.1±2.0% vs. 19.0±1.3%, week 4, p=0.1). This intermediate restorative capability also paralleled the engraftment of the c+AMCs (Figure 2B). Conversely, the MiPSC treated mice had sustained improvement in LVEF throughout the 4 weeks compared to control (30.0±0.98% vs. 19.0±1.3%, week 4, p<0.01, Figure 1B). Furthermore, the MiPSC group demonstrated significantly greater improvement of LVEF when compared to the uAMC group. The MiPSC mediated improvement in LVEF also paralleled the engraftment of the transplanted stem cells by BLI (Figure 2C). Finally, there was a non-significant trend towards decreased end-systolic volumes in the c+AMC and significant decrease in the MiPSC group compared to control (not shown). No significant difference in the end-diastolic volumes was seen between the treated and control groups.
Figure 1. Effects of uAMCs, c+AMCs, and MiPSCs on LVEF. Stacks of short axis images acquired by CMR were analyzed offline to determine the LVEF for weeks 1, 2 and 4.
(A) All groups treated with stem cells demonstrated improved LVEF initially compared to control. However, only the MiPSC group demonstrated sustained improvement through week 4. The c+AMC group demonstrated an intermediate restorative effect with significantly improved LVEF compared to control through weeks 1 and 2. The control group showed severely depressed LVEF that was unchanged throughout the study. (B) Short axis acquisitions are shown during end-diastole and end-systole at the mid-LV. The MiPSC treated mouse demonstrated increased contractility compared to control. (*p < 0.05, **p < 0.01 vs. control by unadjusted Student’s t-test.)
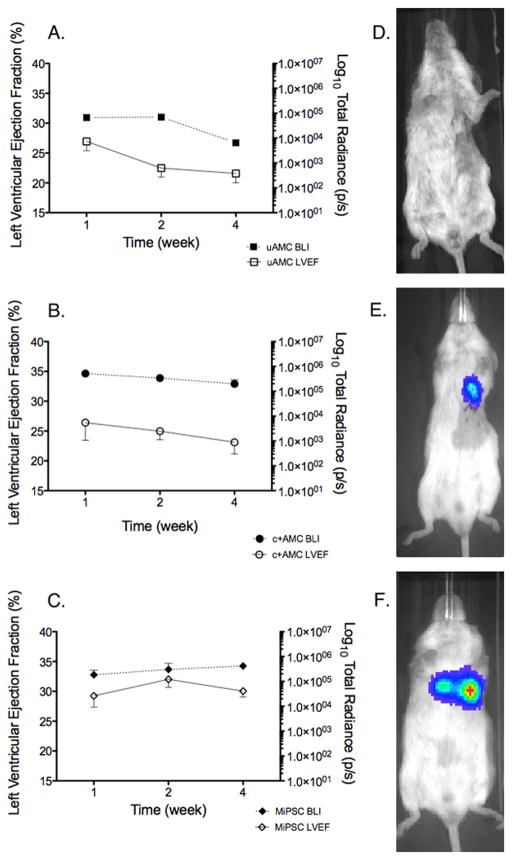
Figure 2. Effects of engraftment of uAMCs, c+AMCs and MiPSCs on LVEF demonstrate correlation between mean engraftment by BLI signal and LVEF by CMR.
(A) Decreased stem cell engraftment and reduced LVEF in the uAMC group. (B) Decreased stem cell engraftment and reduced LVEF in the c+AMC group. (C) In contrast, sustained engraftment and improved LVEF in the MiPSC group throughout the 4 weeks. (D–F) BLI signal observed at week 4 for mice treated with uAMCs, c+AMCs, and MiPSCs respectively.
MEMRI of viable myocardium and DEMRI of infarct scar
To quantify the contribution of the stem cells to the myocardium, myocardial viability was assessed directly by MEMRI and infarct scar by DEMRI. The uAMC treated group had a significant increase in percent viable myocardial volume at week 1 compared to the control arm (85.6±0.6% vs. 75.2±1.0%, p<0.01, Figure 3A). However, this difference did not persist to week 4. Similarly, DEMRI measurement of percent scar volume was decreased significantly compared to control at week 1 (27.6±1.2% vs. 32.6±0.7%, p<0.01, Figure 3C) but not sustained through week 4. Myocardial viability and infarct scar size paralleled the engraftment kinetics of the uAMCs. There was no significant difference between the peri-infarct volumes when comparing the uAMC vs. control groups at week 4.
Figure 3. Effects of cell therapy as determined by MEMRI and DEMRI.
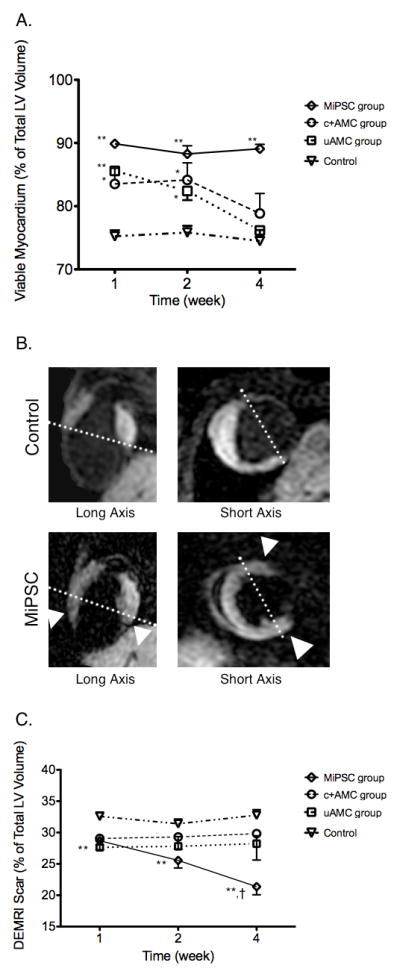
(A) All groups treated with stem cells demonstrated increased viable myocardium by MEMRI, initially, compared to control. At week 4, only the MiPSC group demonstrated sustained increase in myocardial viability compared to control. The c+AMC and uAMC groups had decreasing viability that paralleled their reduced LVEF. (B) Representative MEMRI images from a control mouse and an MiPSC treated mouse are shown in the corresponding long- axis and short- axes. Increased myocardial viability was seen in the anterior and inferior walls of the MiPSC treated mouse (white arrowhead). (C) MiPSC group demonstrated significantly decreased myocardial scar by DEMRI while the uAMC and c+AMC groups demonstrated a trend towards decreased myocardial scar compared to control through the 4 week period. The difference between week 4 compared to week 1 was significant for the MiPSC group (†p = 0.01, *p < 0.05, **p < 0.01 vs. control.)
The c+AMC treated mice initially had increased percent viable myocardial volume up to week 2 as measured by MEMRI (84.2±2.7% vs. 75.8±1.1%, p<0.05, Figure 3A) but this increase did not persist to week 4 (78.9±3.2% vs. 74.5±1.7%, p=0.16). DEMRI measurement of scar volume trended towards decreased scar volume at week 4 (31.0±5.2% vs. 32.8±0.9%, p=0.75, Figure 3C). These findings also paralleled the engraftment kinetics of c+AMCs. No significant difference in the DEMRI-MEMRI peri-infarct volumes was observed between the c+AMC and control groups at week 4 (9.8±3.2 vs. 7.3±1.3, p=0.49).
On the other hand, the MiPSC arm demonstrated a significant and persistent increase in the direct MEMRI measurement of percent viable myocardial volume compared to the control arm throughout the four-week period (89.1±0.7% vs. 74.5±1.7%, week 4, p<0.01, Figure 3A–B). DEMRI scar volume was also significantly decreased compare to the control arm up to week 4 (21.4±1.3% vs. 32.8±0.9%, p<0.01, Figure 3C). These findings paralleled the sustained engraftment of MiPSCs. The peri-infarct volume, however, demonstrated no significant difference when compared to the controls (10.5±2.0% vs. 7.3±1.3%, p=0.21).
Finally, significant correlation was found when analyzing the relationship between the 3 imaging parameters (MEMRI, BLI, and DEMRI) and functional restoration (LVEF) in the 3 treatment groups. First, there was a positive correlation between viable myocardium measured by MEMRI and LVEF with a Pearson correlation coefficient of 0.78 (p<0.01). Second, there was a positive correlation between stem cell engraftment demonstrated by BLI and LVEF with a Pearson correlation coefficient of 0.74 (p<0.01). Third, there was a negative correlation between myocardial scar by DEMRI and LVEF with a Pearson correlation coefficient of −0.66 (p<0.05).
Immunohistological validation of stem cell engraftment signal
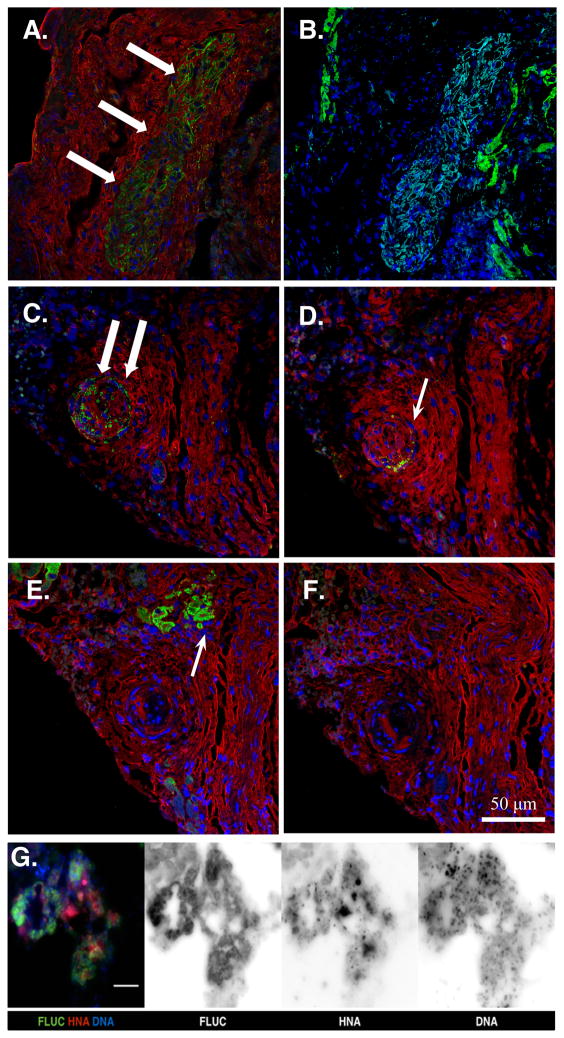
Immunohistology of the MiPSC-treated heart tissue sections at week 4 demonstrated successful engraftment of the MiPSCs with positive staining of human mitochondrial antibody (Figure 4A). Immunohistology using human anti-cardiac troponin T and anti-CD31 (PECAM-1) antibodies did not demonstrate evidence of cardiac or endothelial differentiation, respectively (Figure 4B). Immunohistology of the c+AMC-treated heart tissue sections at week 4 demonstrated successful engraftment of the c+AMCs with positive staining of both human mitochondrial antibody and ckit receptor antibody (Figure 4C–D). However, immunohistology using anti-cardiac troponin T and anti-CD31 antibodies did not demonstrate evidence of cardiac or endothelial differentiation, respectively (Figure 4E–F). The uAMC group did not demonstrate immunohistological evidence of engraftment. In order to confirm the origin of the BLI signal from the human cells, the MiPSCs were co-stained with anti-luciferase antibody and human nuclear antibody (Figure 4G). Robust co-localization of the 2 immunostains confirms the origin of the BLI signal from the transplanted human stem cells.
Figure 4. Immunohistology at the site of cell injection in MiPSC and c+AMC treated mice.
(A) MiPSCs showed successful engraftment by the detection of human mitochondrial antibody (green, white arrows) at the site of cell injection. (B) Immunostaining with cardiac troponin T antibody (green) did not demonstrate any signal within the engrafted MiPSCs (light blue) and labeled only the native murine myocardium. (C) c+AMCs showed successful engraftment by human mitochondrial antibody (green, white arrow), (D) Persistent ckit+ expression was confirmed by ckit+ receptor antibody (green, white arrow). (E–F) However, immunostaining using cardiac troponin T (green, white arrow) and PECAM antibodies did not co-localize with the human mitochondrial or ckit+ stain demonstrating no evidence of cardiac or endothelial differentiation, respectively. Nuclear counterstain (blue) and F-actin (red) antibodies were used to visualize the cellular cytoskeleton. (G) Merged and monochrome images of the MiPSCs co-stained with anti-luciferase antibody (FLUC), human nuclear antibody (HNA), and Hoechst 33342 (DNA). Robust co-localization of the 3 immunostains confirms the origin of the BLI signal from the transplanted human stem cells.
Of note, hematoxylin and eosin stained sections of the myocardium demonstrated teratoma formation in all the mice in the MiPSC group as expected. The teratoma, however, were less invasive by gross histological visualization compared to our prior experience with murine and human ESCs (Figure 5). There was no teratoma formation seen in the other groups.
Figure 5. Bright-field light micrograph of a teratoma at the peri-infarct cell injection site in a MiPSC treated mouse at 4 weeks.
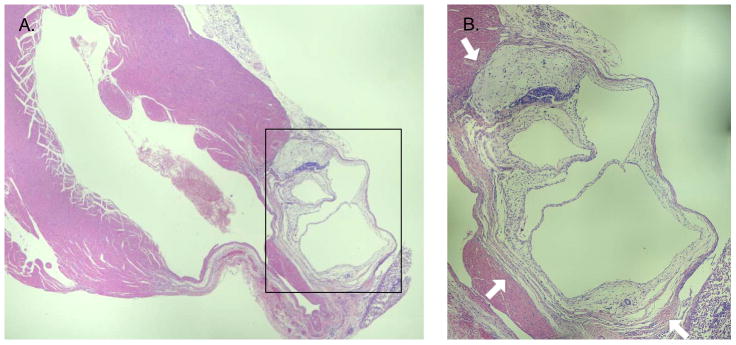
This micrograph was representative of teratomas observed in all explanted hearts of MiPSC treated mice. Three germ layers were identified confirming the presence of a teratoma. The teratoma formed from MiPSCs was loosely packed masses without intra-cavitary invasion typically seen with human ESCs. (A) 5X and (BB) 10X zoom.
Ex-vivo analysis of paracrine factors
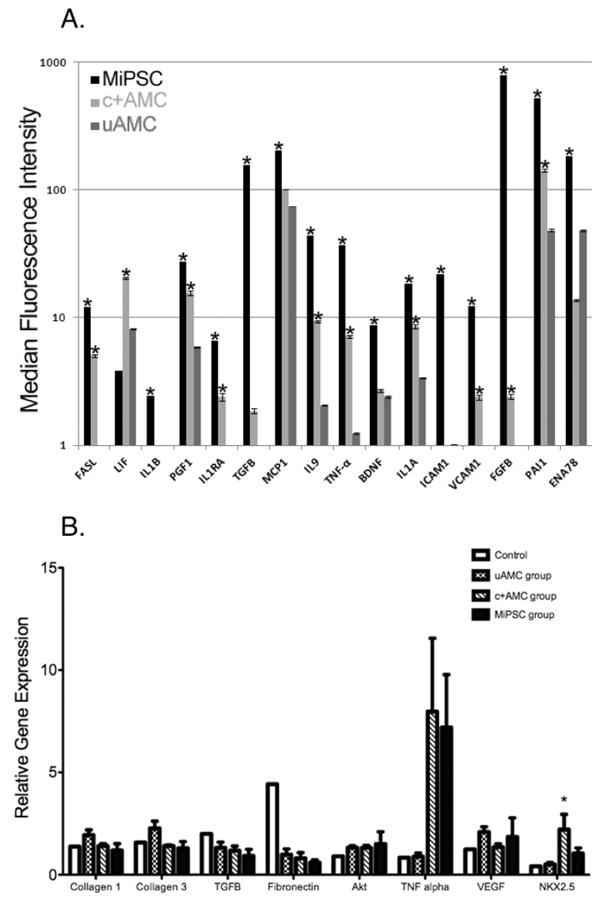
In order to better elucidate the myocardial salvage mechanism, the supernatant of each sub-population underwent 63-plex Luminex Immunoassay of human cytokines. Significant differences in the production of 15 cytokines in the supernatant of MiPSCs and 10 cytokines in c+AMCs were detected when compared to uAMCs (p<0.05, Figure 6A). The significant production of cytokines in the MiPSCs could be classified into the following categories: 1) anti-apoptosis (FASL, IL9, BDNF), 2) anti-fibrosis (PAI1, TGF-B), 3) pro-angiogenesis (VEGF, FGF-B, PGF1), and 4) anti-inflammation (IL1A, IL1B, IL1RA, ICAM1, VCAM1, TNF-a, MCP1, ENA78). Specifically, IL1B, TGF-B, IL9, TNF-a, ICAM1, VCAM1, FGF-B, PAl1, and ENA78 are significantly up-regulated in MiPSCs compared to c+AMCs. These findings underlie the enhanced engraftment of MiPSCs. Similarly, c+AMCs showed increased production of 11 cytokines when compared to uAMCs: FASL, LIF, PGF1, IL1RA, TGF-B, IL9, TNF-a, IL1A, VCAM1, FGF-B, PAI1. These findings may explain their higher engraftment potential when compared to the uAMCs.
Figure 6.
(A) The 63-plex Luminex Immunoassay of human cytokines detected significant increase in the production of 15 cytokines in the supernatant of MiPSCs and of 10 cytokines in c+AMCs when compared to uAMCs (*p<0.05, Figure 6A). The significant production of cytokines in the MiPSCs was involved in anti-apoptosis (FASL, IL9, BDNF), anti-fibrosis (PAI1, TGF-B), pro-angiogenesis (VEGF, FGF-B, PGF1), and anti-inflammation (IL1A, IL1B, IL1RA, ICAM1, VCAM1, TNF-a, MCP1, ENA78). Similarly, c+AMCs showed increased production of 11 cytokines when compared to uAMCs : FASL, LIF, PGF1, IL1RA, TGF-B, IL9, TNF-a, IL1A, VCAM1, FGF-B, PAI1 (*p<0.05). (B) Effects of cell therapy on para-crine factor gene expression in the explanted myocardium as assayed by RT-PCR. Expression of fibrotic (collagen 1, collagen 3, fibronectin, Akt), apoptotic (TNF-alpha), angiogenic (VEGF), inflammatory (TGF-B), and cardiac specific (NKX2.5) genes were evaluated by RT-PCR at 4 weeks. Fibronectin, TNF-alpha, VEGF and NKX2.5 showed a trend towards differential gene expression in cell-based therapy treated groups compared to control. Only NKX2.5 gene expression in the c+AMC group demonstrated a significant increase compared to control (p = 0.04). *p < 0.05 vs. control.
Corresponding in vivo expression of molecular markers of apoptosis (Akt), fibrosis (collagen I, collagen III, CTGF, TGF-B, and fibronectin), angiogenesis (VEGF), inflammation (TNF-a), and early cardiac differentiation (Nkx2.5), were measured at week 4 from the myocardial tissue in the four groups. Consistent with the Luminex assay, RT-PCR demonstrated a trend towards increased expression of TNF-a in both MiPSC and c+AMC groups. In addition, NKX2.5 was significantly increased in the c+AMC group (Figure 6B).
Pluripotency, telomerase activity and telomere length
RT-PCR was performed on MiPSCs, c+AMCs, and uAMCs (Figure 7A). Gene expression values are shown in relative units compared to uAMCs. MiPSCs demonstrated high expression of pluripotency genes (OCT4, SOX2, TDGF1, NANOG, MYC, and EBAF) compared to uAMCs and c+AMCs, confirming their pluripotent status. There was no differential pattern of expression with respect to cardiac specific transcription factors (NKX2.5, CTNI, alpha-MHC, CTNT, and ANP) when comparing the MiPSCs and c+AMCs to uAMCs. Furthermore, the telomerase activity and telomere length were measured for the 3 cell sub-populations. There was a significant increase in the telomerase activity for the MiPSC group, providing an additional parameter to explain for the prolonged engraftment. Telomere lengths were not significantly different for the 3 groups (Figure 7B–C).
Figure 7.
(A) Stem cells demonstrated varying levels of pluripotency and cardiac lineage specification by RT-PCR. The relative gene expressions compared to uAMCs are shown. MiPSCs demonstrated significantly increased expression of early transcription factors compared to uAMCs, including OCT4, SOX2, TDGF1, NANOG, MYC, and EBAF. (B) Detection of telomerase activity in the MiPSCs by TRAP assay while uAMCs and c+AMCs did not exhibit increased telomerase activity (4,000 cells were used in each sample). (C) Mean telomere lengths in MiPSCs, uAMCs, and c+AMCs as measured by qPCR did not demonstrate any significant difference.
DISCUSSION
Cardiomyocyte death or dysfunction after AMI results in pathologic remodeling of the left ventricle and eventual heart failure. Despite reports of restorative potential of cell-based therapies for the injured myocardium, real-time in vivo monitoring of regeneration of de novo myocardium or salvage of the in situ myocardium has not been possible16, 21, 24–26. In this study, we evaluated in vivo myocardial viability and stem cell engraftment directly to track the salvage of the injured myocardium using a novel multi-modality imaging approach. Regeneration requires functional restoration, enhanced myocardial viability, and sustained engraftment with evidence of de novo cardiac differentiation8. Conversely, salvage requires functional restoration, enhanced myocardial viability, and/or engraftment signal without evidence of de novo cardiac differentiation8, 27. This study tested the salvage hypothesis that the enhanced stem cell engraftment will increase myocardial viability and restore the function of the injured myocardium. The three sub-populations derived from a common stem cell lineage were studied: 1) uAMCs, 2) c+AMCs, and 3) MiPSCs. These cells were transduced with luciferase RG, which enabled reliable detection of stem cell engraftment by BLI. MEMRI and DEMRI were used to directly quantify myocardial viability and scar size. MEMRI employs the unique property of manganese (Mn2+), which enters only the metabolically active, viable cells through voltage-gated calcium channel and induces a T1- shortening effect to generate positive MRI contrast. This property complements DEMRI, which relies on non-specific distribution of gadolinium in the extra-cellular space to delineate the scar15.
The three AMC sub-populations demonstrated variable functional improvement of the injured myocardium during the four-week period. In the uAMC-treated group, the initial recovery in function and viability was only seen transiently, which paralleled the non-engraftment or death of the transplanted stem cells. The c+AMC-treated mice demonstrated intermediate improvement in both LVEF and myocardial viability. While these cells exhibited increased up-regulation of NKX2.5, consistent with early cardiac progenitor phenotype28, significant functional improvement, myocardial viability and cellular engraftment did not persist throughout the 4-week duration of the study. Sustained restoration was seen only in the MiPSC group, which exhibited significant increase in the viable myocardium by MEMRI and continued cell engraftment by BLI during all four weeks. This persistent increase in myocardial viability and cell engraftment without any evidence of cardiac differentiation in the MiPSC group is consistent with myocardial salvage as opposed to regeneration as the mechanism underlying sustained improvement in LVEF.
In support of salvage of the myocardium by the stem cells, the human cytokine 63-plex Luminex analysis demonstrated a significant increase in paracrine factors, including 15 anti-inflammatory, anti-apoptotic, anti-fibrotic, and pro-angiogenic factors from the MiPSCs and 10 from the c+AMCs. Specifically, there were over 9 factors (IL1B, TGF-B, IL9, TNF-a, ICAM1, VCAM1, FGF-B, PAl1, and ENA78), which MiPSCs expressed more than 2-fold increase when compared to c+AMCs. These findings were substantiated by the PCR analysis of the explanted myocardium, which exhibited a similar trend towards up-regulation of the related genes. Specifically, TNF-a, a cytokine with multiple effects, which includes the anti-apoptotic effects via activation of NF-kB, had increased signal by both cytokine and gene expression29. The sustained MiPSC engraftment and enhanced myocardial viability remained steady throughout the 4-week period, which most likely led to persistent paracrine effect to improve the LVEF. The findings that uAMCs show minimal while c+AMCs show intermediate evidence of salvage are consistent with our model that the decreased production of paracrine factors is commensurate with their limited engraftment. Finally, increased telomere activity was observed which does not necessarily correlate with telomere length. Telomere length is regulated by factors in addition to telomerase activity30–33, including cis-acting regulators of association of telomerase with telomeres34, mitotic rate, and oxidative stress35. Telomere length is the net outcome of competing telomere shortening and lengthening events over the history of a cell lineage, and thus may not correlate with telomerase activity at one time point. iPSC-derived cells may have variable telomere length but the presence of increased telomerase activity in the MiPSCs provides definitive evidence of reprogramming of the cells30, 31. Based on these data, this study confirms that the MiPSCs enhance stem cell engraftment that leads to increased production of the paracrine factors to salvage the injured myocardium without any histological evidence of cardiac differentiation.
Limitation
The three cell sub-populations, derived from identical cell lineage, provided an optimal model to compare the restorative impact on the myocardium by stem cells with varying pluripotency states. However, the major limitation of this study is the absence of a MiPSC-derived cardiomyocytes. Since this study investigated the effect of stem cell engraftment and myocardial viability on the restorative potential of distinct cell types, the MiPSCs, which represent the highest pluripotency state, were selected to demonstrate myocardial salvage. Studies are underway to purify MiPSC-derived cardiomyocytes to assess the restorative potential of the differentiated cardiac cells when compared to the MiPSCs and their engraftment in an immunocompetent mice model. The teratoma formation by the MiPSCs is a clear contraindication to clinical use. However, additional experiments to understand the paracrine mechanism of the cell products derived from the MiPSCs are planned. The exosomes and their associated miRNAs will be identified to elucidate the underlying biological pathway in the production of the cytokines involved in myocardial salvage. The functional effects of the cytokines, their corresponding recombinant human analogs and siRNAs, and the exosomes will be studied in pre-clinical animal models. If significant restoration is confirmed by these MiPSC derivatives, a potential clinical application of the MiPSCs may be to generate these cell products in vitro.
In summary, the MiPSC treated mice had sustained improvement in myocardial viability and LVEF compared to the uAMCs, c+AMCs and control groups. These findings were coupled with sustained engraftment of MiPSCs, which enhanced the paracrine effects to salvage the injured myocardium. In contrast, the relatively modest improvements of the uAMC and c+AMC groups were attributed to limited engraftment and the subsequent attenuated release of paracrine factors, providing only transient enhancement of myocardial viability.
Supplementary Material
Novelty and Significance.
What Is Known?
The mechanism of functional restoration with stem cell therapy remains poorly understood.
The pluripotency states of the stem cell are thought to directly correlate with myocardial restoration potential after acute myocardial injury.
Novel multi-modality imaging using manganese- and delayed-enhanced MRI (MEMRI and DEMRI) and bioluminescence imaging (BLI) allows for serial in vivo evaluations of myocardial function and viability in relation to stem cell engraftment.
What New Information Does This Article Contribute?
Amniotic stem cell derived iPSCs (MiPSCs) demonstrated significantly greater increase in myocardial function and viability compared with the non-reprogrammed native populations of lesser cell potency.
Improved myocardial function and viability correlated with the degree of stem cell engraftment.
Amniotic stem cell derived iPSCs demonstrated increased production of paracrine factors without evidence for cardiac or endothelial differentiation, consistent with myocardial salvage.
The underlying myocardial biology of functional restoration by stem cell therapy has been described as either regeneration or salvage of the injured myocardium. Using novel multi-modality imaging with MEMRI, DEMRI and BLI, we demonstrate that MiPSC-treated mice have a significant and sustained improvement in myocardial function and viability that are not seen in other amniotic stem cell derived sub-populations of lesser cell potency. This correlated with the degree of stem cell engraftment by BLI. Further analyses demonstrated increased production of paracrine factors without evidence for cardiac or endothelial differentiation, consistent with myocardial salvage.
Acknowledgments
We greatly appreciate the assistance with evaluation of teratomas from Dr. Donna M. Bouley, the preparation of histological sections by Ms. Pauline Chu and the generous use of facilities at the Stanford Center for Innovation in In-Vivo Imaging (SCI3).
SOURCES OF FUNDING
NIH/NHLBI 1R01HL095716-01 (PCY), NIH/NHLBI 5UM1 HL113456-02 (PCY)
Nonstandard Abbreviations and Acronyms
- AMC
amniotic mesenchymal stem cells
- AMI
acute myocardial infarction
- BLI
bioluminescence imaging
- c+AMC
ckit+AMC
- CMR
cardiac MRI
- CPC
cardiac progenitor cell
- DEMRI
delayed-enhanced MRI
- ESC
embryonic stem cell
- FACS
fluorescent activated cell sorting
- fGRE-IR
fast gradient echo inversion recovery
- FOV
field of view
- IP
intraperitoneal
- LAD
left anterior descending artery
- LV
left ventricle
- LVEF
left ventricular ejection fraction
- MEMRI
manganese-enhanced MRI
- MiPSC
AMC-derived iPSC
- MSC
mesenchymal stem cell
- NS
normal saline
- RG
reporter gene
- uAMC
unselected AMC
Footnotes
This manuscript was sent to Mark A. Sussman, Consulting Editor, for review by expert referees, editorial decision, and final disposition.
In January 2015, the average time from submission to first decision for all original research papers submitted to Circulation Research was 14.7 days.
DISCLOSURES
None.
References
- 1.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes S, Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. Journal of molecular and cellular cardiology. 2010;49:941–9. doi: 10.1016/j.yjmcc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, Tendera M, Ratajczak J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26:1166–73. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- 4.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–40. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welt FG, Gallegos R, Connell J, Kajstura J, D’Amario D, Kwong RY, Coelho-Filho O, Shah R, Mitchell R, Leri A, Foley L, Anversa P, Pfeffer MA. Effect of cardiac stem cells on left-ventricular remodeling in a canine model of chronic myocardial infarction. Circ Heart Fail. 2013;6:99–106. doi: 10.1161/CIRCHEARTFAILURE.112.972273. [DOI] [PubMed] [Google Scholar]
- 6.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–16. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 8.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 9.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 10.Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–53. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- 11.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem cell research. 2010;4:214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Merling RK, Sweeney CL, Choi U, De Ravin SS, Myers TG, Otaizo-Carrasquero F, Pan J, Linton G, Chen L, Koontz S, Theobald NL, Malech HL. Transgene-free iPSCs generated from small volume peripheral blood nonmobilized CD34+ cells. Blood. 2013;121:e98–107. doi: 10.1182/blood-2012-03-420273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crisostomo PR, Abarbanell AM, Wang M, Lahm T, Wang Y, Meldrum DR. Embryonic stem cells attenuate myocardial dysfunction and inflammation after surgical global ischemia via paracrine actions. Am J Physiol Heart Circ Physiol. 2008;295:H1726–35. doi: 10.1152/ajpheart.00236.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge X, Wang IN, Toma I, Sebastiano V, Liu J, Butte MJ, Reijo Pera RA, Yang PC. Human amniotic mesenchymal stem cell-derived induced pluripotent stem cells may generate a universal source of cardiac cells. Stem Cells Dev. 2012;21:2798–808. doi: 10.1089/scd.2011.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dash R, Chung J, Ikeno F, Hahn-Windgassen A, Matsuura Y, Bennett MV, Lyons JK, Teramoto T, Robbins RC, McConnell MV, Yeung AC, Brinton TJ, Harnish PP, Yang PC. Dual manganese-enhanced and delayed gadolinium-enhanced MRI detects myocardial border zone injury in a pig ischemia-reperfusion model. Circ Cardiovasc Imaging. 2011;4:574–82. doi: 10.1161/CIRCIMAGING.110.960591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung TC, Suzuki Y, Urashima T, Caffarelli A, Hoyt G, Sheikh AY, Yeung AC, Weissman I, Robbins RC, Bulte JW, Yang PC. Multimodality evaluation of the viability of stem cells delivered into different zones of myocardial infarction. Circ Cardiovasc Imaging. 2008;1:6–13. doi: 10.1161/CIRCIMAGING.108.767343. [DOI] [PubMed] [Google Scholar]
- 17.Sun N, Lee A, Wu JC. Long term non-invasive imaging of embryonic stem cells using reporter genes. Nat Protoc. 2009;4:1192–201. doi: 10.1038/nprot.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamanaka S, Takahashi K. Induction of pluripotent stem cells from mouse fibroblast cultures. Tanpakushitsu Kakusan Koso. 2006;51:2346–51. [PubMed] [Google Scholar]
- 19.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111:344–58. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 21.Wang IN, Wang X, Ge X, Anderson J, Ho M, Ashley E, Liu J, Butte MJ, Yazawa M, Dolmetsch RE, Quertermous T, Yang PC. Apelin enhances directed cardiac differentiation of mouse and human embryonic stem cells. PloS one. 2012;7:e38328. doi: 10.1371/journal.pone.0038328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic acids research. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic acids research. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendry SL, 2nd, van der Bogt KE, Sheikh AY, Arai T, Dylla SJ, Drukker M, McConnell MV, Kutschka I, Hoyt G, Cao F, Weissman IL, Connolly AJ, Pelletier MP, Wu JC, Robbins RC, Yang PC. Multimodal evaluation of in vivo magnetic resonance imaging of myocardial restoration by mouse embryonic stem cells. J Thorac Cardiovasc Surg. 2008;136:1028–1037 e1. doi: 10.1016/j.jtcvs.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 25.Arai T, Kofidis T, Bulte JW, de Bruin J, Venook RD, Berry GJ, McConnell MV, Quertermous T, Robbins RC, Yang PC. Dual in vivo magnetic resonance evaluation of magnetically labeled mouse embryonic stem cells and cardiac function at 1.5 t. Magn Reson Med. 2006;55:203–9. doi: 10.1002/mrm.20702. [DOI] [PubMed] [Google Scholar]
- 26.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. Faseb J. 2007;21:1345–57. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 28.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–31. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Suhr ST, Chang EA, Rodriguez RM, Wang K, Ross PJ, Beyhan Z, Murthy S, Cibelli JB. Telomere dynamics in human cells reprogrammed to pluripotency. PloS one. 2009;4:e8124. doi: 10.1371/journal.pone.0008124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JC, Warner JK, Erdmann N, Lansdorp PM, Harrington L, Dick JE. Dissociation of telomerase activity and telomere length maintenance in primitive human hematopoietic cells. Proc Natl Acad Sci U S A. 2005;102:14398–403. doi: 10.1073/pnas.0504161102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci U S A. 1995;92:4818–22. doi: 10.1073/pnas.92.11.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryan TM, Englezou A, Dunham MA, Reddel RR. Telomere length dynamics in telomerase-positive immortal human cell populations. Experimental cell research. 1998;239:370–8. doi: 10.1006/excr.1997.3907. [DOI] [PubMed] [Google Scholar]
- 34.Teixeira MT, Arneric M, Sperisen P, Lingner J. Telomere length homeostasis is achieved via a switch between telomerase- extendible and -nonextendible states. Cell. 2004;117:323–35. doi: 10.1016/s0092-8674(04)00334-4. [DOI] [PubMed] [Google Scholar]
- 35.von Zglinicki T. Oxidative stress shortens telomeres. Trends in biochemical sciences. 2002;27:339–44. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.