Abstract
When the field of tissue engineering first arose, scaffolds were conceived of as inert 3-dimensional structures whose primary function was to support cellularity and tissue growth. Since then, advances in scaffold and biomaterial design have evolved to not only guide tissue formation, but also to interact dynamically with and manipulate the wound environment. At present, these efforts are being directed towards strategies that directly address limitations in endogenous wound repair, with the goal of reprogramming the local wound environment (and the cells within that locality) from a state that culminates in an inferior tissue repair into a state in which functional regeneration is achieved. This review will address this approach with a focus on recent advances in scaffold design towards the resolution of tears of the knee meniscus as a case example. The inherent limitations to endogenous repair will be discussed, as will specific examples of how biomaterials are being designed to overcome these limitations. Examples will include design of fibrous scaffolds that promote colonization by modulating local ECM density and delivering recruitment factors. Furthermore, we will discuss scaffolds that are themselves modulated by the wound environment to alter porosity and modulate therapeutic release through precise coordination of scaffold degradation. Finally, we will close with emerging concepts in local control of cell mechanics to improve interstitial cell migration and so advance repair. Overall, these examples will illustrate how emergent features within a biomaterial can be tuned to manipulate and harness the local tissue microenvironment in order to promote robust regeneration.
Keywords: Meniscus, Biomaterials, Wound Healing, Regeneration, Scaffold, Extracellular Matrix
Introduction
The soft tissues of the musculoskeletal system (e.g., dense connective tissues such as tendon, ligament, cartilage, and the knee meniscus) are vital for the efficient and pain free execution of the activities of daily living. However, and as a direct consequence of their central role in load bearing, these tissues are commonly injured and present a frustrating scenario in clinical practice. Namely, all of these tissues have a healing response to injury in adults that ranges from ‘poor’ to ‘nonexistent’. For example, torn tendons (which normally transmit forces from muscle to bone) can mount a modest repair response; yet, this process most often culminates in the formation of ‘scar’ tissue that is disordered, has lower mechanical properties than the original tissue, and is prone to re-injury [1]. Other tissues, such as the articular cartilage lining joint surfaces, mount almost no postnatal repair response [2]. Indeed, a single area of cartilage damage not only fails to heal on its own, but can precipitate more widespread degeneration of the entire joint surface.
Poor healing of dense connective tissues in adults arises in part from the challenging mechanical environment in which healing takes place and is exacerbated by the high density and precise ordering of the extracellular matrix (ECM) as well as the relative paucity of cells in these tissues. Quite interestingly, these same tissues can heal via regeneration (i.e. complete restoration of structure and function) during adolescence [2, 3]. In this earlier developmental state, tissue density is much lower and the number of cells is much higher. As will be described below, our studies in the knee meniscus, for example, have shown that tissue mechanics and matrix density increase with aging, while repair capacity decreases [4]. Defining the critical tissue characteristics that separate these non-healing from healing states may direct new therapies and biomaterial-based interventions.
To address deficits in the repair of dense connective tissues in the adult, we and others have developed a number of tissue engineering strategies with the goal of promoting tissue regeneration. In our case, these approaches are structurally motivated, in that they are based on organized nanofibrous scaffolds composed of ultra-fine biodegradable and biologic fibers [5, 6]. These scaffolds can be fabricated in such a way as to recreate the order of typical dense connective tissues [6], and can also serve as a three dimensional micro-pattern that directs cells at the repair site to produce new matrix with order and directionality comparable to the native tissue [7]. We have used these materials to query basic mechanisms of cell response to scaffold architecture, fiber mechanics, and mechanical deformation [8-10] and have begun to translate these findings towards clinical practice by testing in large animal models of tissue repair. Expanding the potential of these materials, we have also devised novel fabrication strategies to generate scaffolds that are both dynamic and multi-functional [11], and whose bulk mechanical properties can be tailored to match that of the native tissues they are to replace [12]. In the context of fibro-cartilaginous tissues (with a focus on the knee meniscus), we will identify the limitations to endogenous repair, as well as illustrate how such scaffold templates can be engineered to overcome these limitations.
Meniscus Structure, Mechanics, Injury, and Repair
In order to appreciate the inherent challenges to meniscus repair, it is essential to define the function, organization, and macromolecular constituents of the tissue, as each of these factors plays a role in healing response and outcomes. The menisci are semilunar, wedge-shaped structures in the knee that are rich in collagens and proteoglycans (PGs) and function to transfer load from the femur to the tibia [13-15]. Collagen content and directionality (where most fibers are circumferentially oriented) is paramount for meniscus function [16]. When compressive loads impinge on the wedge shaped meniscus, the PG-rich inner zone projects this load outward to engage the collagen fibers [17]. These fibers resist extrusion and enforce joint congruency to promote load transmission [18]. Because the menisci are central to knee function, injury is very common. Meniscus tears may arise acutely (coincident with a traumatic event such as ACL rupture) or with degeneration. Small longitudinal ‘bucket handle’ tears can progress to involve a significant portion of the meniscus, and loose fragments can displace and interrupt knee function. Tears in the avascular zone (inner 2/3rd) have a poor long-term prognosis, even with suture repair [19]. As a consequence, removal of the damaged portion (partial meniscectomy) is the most common treatment option for meniscal tears. However, the volume of meniscus removal scales with increasing stress on the underlying cartilage and predisposes the joint to osteoarthritic changes [13, 20]. Despite this, partial meniscectomy is performed >750,000 times each year in the US [19], does not provide definitive improvements relative to untreated lesions [21], and predisposes patients to the development of osteoarthritis (OA) [22]. Clearly, there is a need for new therapies that can improve symptoms while preserving meniscus structure and function, particularly with a focus on promoting endogenous repair.
Impediments to Meniscus Repair
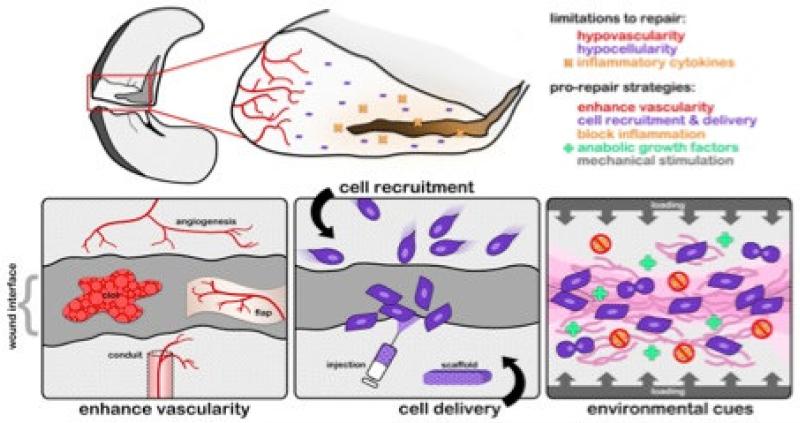
In order to develop regenerative approaches to improve meniscus repair, it is important to consider the natural processes of wound repair and impediments to endogenous healing that are present in adult meniscus tissue (Figure 1). It is generally understood that the overall low cellularity (of both endogenous meniscus cells and meniscus progenitors), the dense extracellular matrix, the poor vascularity, and the inflammatory environment present at the wound site, all contribute to failure of meniscus healing and regeneration [23-25]. Based on this understanding, it may be possible to develop and deliver therapeutics to specifically address these limitations through novel biomaterial-based technologies. Of further note, since many dense fibrous connective tissues (including tendon, ligament, and the intervertebral disc) have a limited healing capacity in adults [4, 26, 27], lessons learned in one tissue (the meniscus) might have applicability across many dense connective tissues. In the following sections we discuss the salient features of the meniscus that change with development that may contribute to a failure of repair in the adult.
Figure 1. Impediments to meniscus repair.
Schematic illustration of a meniscus defect and the underlying impediments to adult meniscus repair, including a lack of vascularity, a loss of cellularity, alterations in ECM density and mechanics, and inflammatory factors in the wound environment. These factors converge to limit endogenous repair and have been incorporated into pro-repair strategies aimed at improving regeneration. Adapted from [96] with permission.
Tissue Aging and Maturation
From a clinical perspective, meniscal tears are rarely seen in children but are common in adults [28, 29]. Moreover, increasing patient age correlates with worse clinical outcomes after meniscal repair, including higher rates of repair failure [30-33]. Importantly, patients >40 years of age with meniscal tears have significantly fewer meniscus cells at the wound interface than younger patients [34]. Meniscal fibrochondrocytes (MFCs) are the native cell type within the meniscus and are responsible for maintaining tissue homeostasis [15, 35]. These same cells respond to injury or altered loading by up-regulating production of matrix proteins and/or enzymes to affect repair [36-39]. However, cellularity decreases progressively with age, reaching very low cell densities in adult tissue [4, 28]. Since healing is characterized by cellular migration to the defect, proliferation, synthesis of new matrix to bridge the wound gap, and eventually tissue remodeling and maturation [40], the lack of reparative cells at the wound interface in adults may constitute a significant impediment to repair.
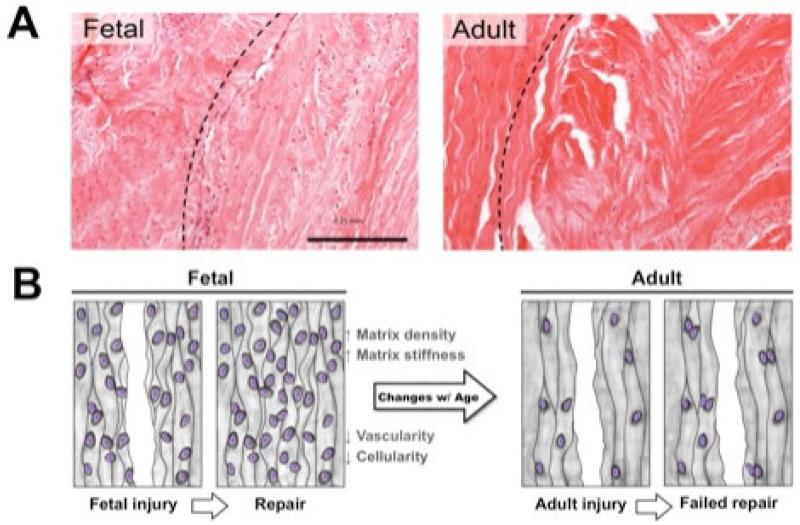
It has also long been thought that deficits in vascularity play a role in the poor healing of the adult meniscus (where nascent vessels serve as a conduit for the delivery of regenerative cells and growth factors to the wound site). Indeed, blood vessels penetrate only the peripheral 1/3rd in the adult [41, 42], while they are present throughout the fetal meniscus. Recent data shows though that fetal meniscal explants cultured in vitro (in the absence of a vascular supply) still exhibit superior integration compared to adult specimens. For example, utilizing an in vitro explant model [4], we showed that both fetal and juvenile meniscus repair constructs formed a robust repair over 8 weeks, while a considerable gap remained in the adult meniscus, Figure 2A. Consistent with these findings, others have shown that when adult and fetal ovine tendons are injured, removed, and implanted into the subcutaneous space of adult mice, healing is superior in fetal tissues, and proceeds in a similar manner as in the fetus [3]. Together, these findings suggest that the paucity of repair is not inherent to the adult environment or solely due to the lack of blood supply per se, but rather suggest that impediments to repair originate within the tissue itself as it matures.
Figure 2. Intrinsic meniscus repair decreases with tissue maturation.
A) Histological sections (H&E staining) showing near complete and seamless repair of a fetal meniscus segment 8 weeks post injury, compared to persistent defects, fissures, and clefts in an adult tissue repair construct cultured similarly. B) Schematic illustration of the dynamic processes of fetal tissue repair and the intrinsic changes to the tissue that limit repair in the adult. Adapted from [4] and [96] with permission.
Meniscus ECM with Tissue Maturation and Aging
What then changes with tissue maturation that might limit endogenous repair? One obvious alteration with tissue maturation is the density and structure of the extracellular matrix (ECM) [28]. Meniscus ECM density increases markedly with maturation and load bearing use, resulting in higher bulk and local mechanical properties in the tissue [4]. Given the already small pore size within the ECM of this dense connective tissue, increasing matrix density may impede cell migration to the wound site, a requirement for tissue repair [43]. Indeed, unlike migration in 2D, where matrix stiffness directly modulates migration speed [44, 45], cells in 3D interpret not just the adhesive and mechanical features of their microenvironment, but also must migrate through the steric hindrances presented by the ECM itself [46-48]. Interstitial cell migration can occur through either cell-mediated degradation of the matrix (to enable tunneling) or direct migration through the small matrix pores [49, 50], or a combination of the two. As the pores within the matrix become progressively smaller, migration rates decline and cells are eventually rendered immobile [49]. The same is true in very stiff and/or non-degradable artificial matrices, where the matrix cannot be effectively remodeled to allow cell passage [47, 51, 52]. Thus, steric impediments may arise naturally as a consequence of the tissue specialization that enables mechanical function, while these changes may reduce endogenous healing potential, Figure 2B.
Based on this understanding of native meniscus structure, function, and healing capacity, our team has developed novel scaffolds to promote regeneration and repair. Clearly, organization is a critical feature of a functional regenerate meniscus tissue, and this must be one of the first and foremost considerations. However, the cellularity of the healing interface is just as critical, if not more for functional resolution of a defect. Without cellular colonization of the implant and/or wound site, then little if any new tissue can be formed. As will be detailed below, these lessons learned from native tissue healing have informed our scaffold designs, while at the same time we have incorporated lessons learned from the scaffold design and colonization to develop novel strategies to improve native tissue healing.
Engineered Scaffolds to Enhance Meniscus Tissue Formation and Integration: Controlling Porosity
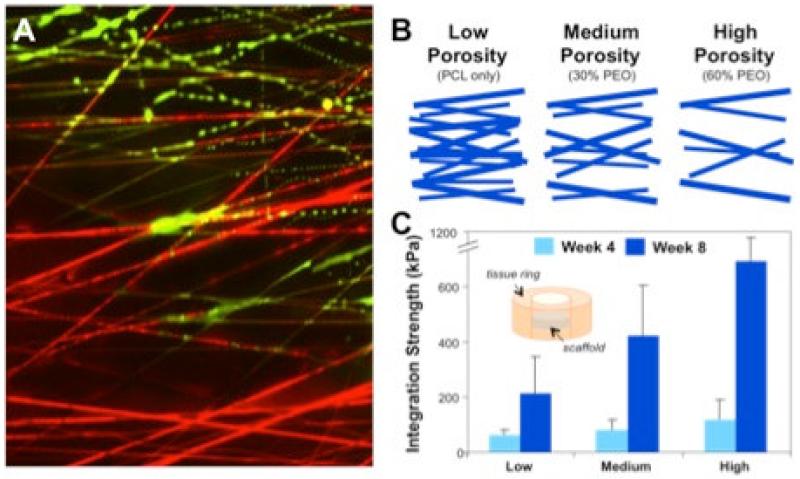
As a starting concept in our scaffold designs, it was clear that the dense and organized nature of the native meniscus was essential for recapitulation, but also that these same network features might pose impediments to repair. This led to the development of a new class of fibrous networks that possessed alignment reminiscent of the native tissue [53-55], along with controllable and emergent porosity. These nanofibrous composites were engineered to include at the time of fabrication ‘sacrificial’ fiber fractions, where the emergence of porosity and change in mechanics was dictated by the solubility and degradation characteristics of fiber populations within the composite structure [12, 56-58], Figure 3. In in vitro studies, wherein cells were seeded atop these structures, cell colonization and matrix deposition in the scaffold could be tuned by altering the amount and type of sacrificial fiber [59]. Likewise, when these scaffolds were apposed to native meniscus tissue, using an annular/ring integration model [60], scaffold porosity modulated the rate and degree to which cells from the native tissue invested the biomaterial network. When these scaffolds with tunable porosity were coupled to native meniscus, histology and mechanical testing revealed that increasing the sacrificial PEO fiber fraction (and thus pore size) increased the strength of the repair ([61], Figure 3). Taken together, these studies showed that an enabling context for 3D interstitial cell migration could be established by tuning the pore size within the fibrous biomaterial framework, and that this feature could be used to improve both engineered meniscus tissue formation and integration with native tissue.
Figure 3. Scaffold porosity modulates cell infiltration and integration.
A) Dynamic composite fibrous scaffold with a stable poly(ε-caprolactone) (PCL) fiber fraction (red) and a water-soluble poly(ethylene oxide) (PEO) fiber fraction (green). Here, the composite is undergoing a transition in porosity as a hydration front advances from the bottom to the top of the image, with the sacrificial PEO fibers being removed. B) Schematic of fiber composites engineered to present differing levels of porosity based on the fraction of sacrificial PEO fibers included in the network at the time of fabrication. C) Integration strength as a function of time and scaffold porosity, where scaffolds with higher porosity integrate with native tissue with higher mechanical strength than those with lower porosity. Adapted form [59] and [61] with permission.
Reprogramming Local Tissue to Enable Colonization of the Wound Margin: Local Delivery of Degradative Enzymes
The above studies focusing on manipulating scaffold porosity to improve cell colonization and integration led naturally to considerations of what else might be done to further enhance repair. As noted above, the density of adult meniscus ECM likely presents physical impediments to 3D cell migration, dampening endogenous repair capacity by limiting the number of cells that can migrate through the native tissue to the wound interface. Since the sacrificial elements of the scaffold were designed to dissolve and/or degrade soon after apposition with native tissue, these fibers also provide a useful vehicle from which to deliver drugs, growth factors, and other biological agents [62]. As such, we next considered whether fibers within these composites could be harnessed to deliver factors to reprogram the local tissue environment to a state that would better support interstitial cell migration. In the cartilage literature, it had been shown that partial digestion of the tissue wound edge, via exposure to solutions containing proteolytic enzymes, enhanced tissue-to-tissue integration, with a greater number of cells migrating through the dense matrix to the wound site [63].
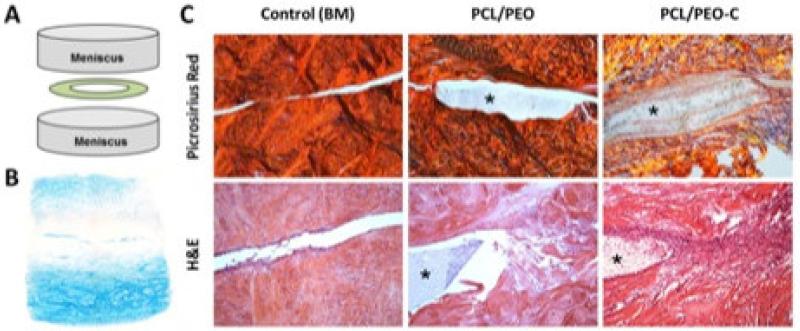
To develop this concept in terms of biomaterial-mediated meniscus repair, we first validated that integration was improved in the adult meniscus when repair segments were exposed to soluble collagenase prior to apposition [64]. In that study, using the annular/ring defect model, in vitro culture showed cells and collagen fibrils closing ~92% of the wound gap in adult meniscus repair constructs that were pre-treated with a high dose of collagenase before assembly and culture for 8 weeks. Repair in these samples reached levels were higher than samples treated with a lower collagenase dose (74% closure) and basal media controls (43% closure). To enable biomaterial mediated and targeted enzyme delivery, PEO nanofibers containing active collagenase (PEO-C) were next fabricated. When these collagenase-releasing PEO-C/PCL composite scaffolds (with two fiber fractions) were placed into a juvenile bovine meniscus defect, loss of PG was apparent at the wound edge within 6 hours, with decreased staining intensity persisting through day 7 (Figure 4). This loss of matrix did not extend to the periphery of treated samples, and was not observed in controls, suggesting a local action of the delivered enzyme. Moreover, when these repair constructs (inclusive of two meniscus segments with an interposed bioactive biomaterial) were implanted subcutaneously in nude rats and evaluated over a 4 week period, composite scaffolds delivering collagenase from the PEO fiber fraction (PCL/PEO-C) showed the greatest amount of ECM deposition both within and at the boundaries of the implanted scaffold [64]. DAPI staining of these same constructs also showed marked increases in cellularity in the tissue surrounding the PEO-C scaffolds. Taken together, these studies demonstrated that the storage and rapid release of collagenase from sacrificial PEO nanofibers, when tuned appropriately, could increase cell migration to and matrix deposition at the wound margin as well as cellularity in the adjacent tissue margins. These studies established that by reprogramming the adult tissue ECM towards a more immature state (via partial digestion) one could overcome some of the inherent limitations of endogenous repair.
Figure 4. Bioactive scaffolds modulate local ECM density to improve repair.
A) Schematic of meniscus repair construct and B) demonstration of matrix degradation (removal of PGs) at the wound interface (Alcian Blue staining, 2X magnification) with local delivery of collagenase from the PEO fiber fraction (PEO-C). C) Improved tissue integration as a result of biomaterial-mediated delivery of collagenase (PEO-C) to the wound interface in a subcutaneous model of meniscus repair. Picrosirius red staining of collagen (viewed under polarized light) and H&E staining of the repair interface at 4 weeks (10× magnification). Asterisk indicates scaffold. Adapted form [64] and [96] with permission.
Recruiting Endogenous Cells to Wound Margin: Homing and Growth Factor Release from Scaffolds
Beyond simply enabling MFC migration through dense ECM via matrix degradation, it may also be essential to provide directional cues, such as chemotactic gradients, to guide endogenous cells to the injury site. Recent studies have shown that simply allowing migration may not in and of itself provide sufficient motivation to drive colonization of the wound site. For example, Greiner et al. showed that a soluble chemical gradient was essential for inducing cell migration through small pores and Mao and colleagues showed in vivo cartilage regeneration only when TGF was released from implanted biomaterials [65, 66]. Others have delivered BMP from alginate hydrogels to increase stem cell recruitment to critically sized bone defects [51]. Interestingly, these studies also highlighted the requirement that the scaffold provide a permissive environment for cell migration along with chemotactic cues to guide cell migration to the wound site.
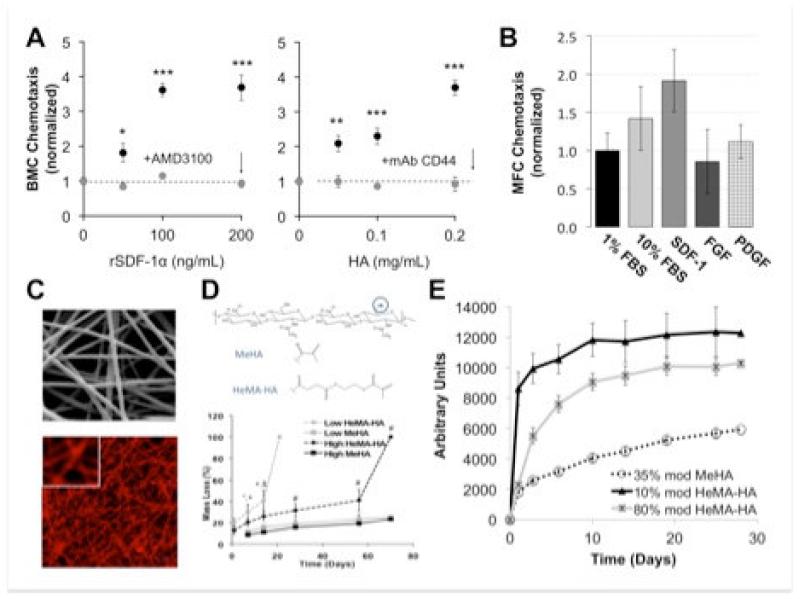
While a number of molecules have been implicated in meniscus cell migration and biosynthetic activity [67], several recent studies have shown a role for stromal-derived factor-1 alpha (SDF). SDF is a critical regulator of bone marrow progenitor cell (BMC) mobilization and local engraftment at injury sites, is regulated through the SDF/CXCR4 axis [68, 69], and may play a role in meniscus repair. Indeed, in a recent rat meniscus injury model, the SDF/CXCR4 axis was implicated in homing of injected progenitor cells to the site of injury [70]. Due to its short half-life however, SDF is often delivered from biomaterials to enable a sustained response. In recent studies, we used a hydrolytically degradable hyaluronic acid (HA) hydrogel to deliver SDF [71]. In these studies, released SDF, as well as released HA itself, stimulated BMC chemotaxis over a period of 7 days (Figure 5A). In follow on in vitro studies, we queried MFC migration in response to several potential factors, and showed that SDF engendered significantly more meniscus cell migration than either fibroblast growth factor (bFGF) or platelet derived growth factor (PDGF) alone, Figure 5B. Building from these observations, we modified our nanofiber fabrication methods to enable electrospinning of HA fibers [11, 72, 73], Figure 5C. Because of the versatile crosslinking chemistry available with HA, these fibers could be tuned to degrade at different times and so controllably release large molecules at pre-determined rates, Figure 5D, 5E. These novel materials and fabrication methods enable the release of active biologic factors from nanofibrous composites in a controlled fashion, providing yet another means by which endogenous meniscus repair may be improved.
Figure 5. Chemotactic cues and novel delivery methods to improve colonization of the wound site.
A) Bone marrow cell (BMC) chemotaxis in response to SDF and HA release from degradable HA hydrogels. B) Meniscus fibrochondrocyte (MFC) chemotaxis in response to serum (FBS), SDF, FGF, and PDGF (normalized to 1% FBS controls). C) SEM and fluorescent images of HA nanofibers. D) Crosslinking chemistries providing stable (MeHA) and hydrolytically degradable (HeMA-HA) HA-based materials (gels and fiber networks). E) FITC-conjugated BSA release from HA-based nanofibers as a function of time and degree of modification and type of crosslink. Adapted from [71] and [11, 77] with permission.
Instruction Upon Arrival at the Wound Margin: Scaffold-Directed Differentiation
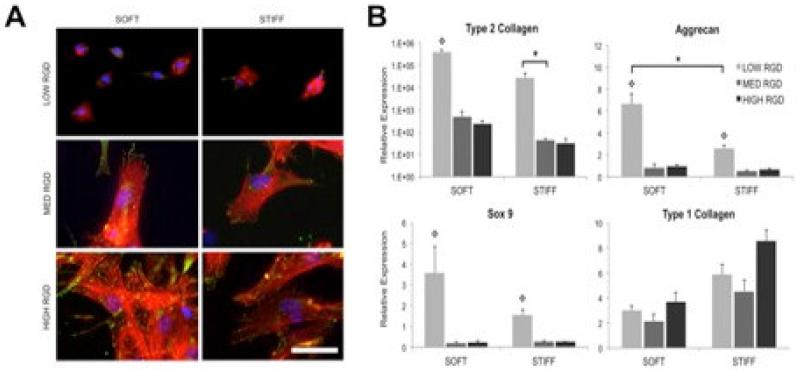
In addition to providing a local environment that is amenable to integration and cell migration, as well as chemotactic signals to recruit cells to the defect site, it may be necessary to provide the appropriate biologic and mechanical context for cell differentiation upon arrival at the wound margin. While we and others have released biologic factors from scaffolds to drive differentiation of cells arriving at the wound interface [62, 74], it is now well understood that cells, and particularly stem cells, are also influenced by the local adhesive and biophysical properties [75]. This includes the mechanical properties of the local environment, as well as the amount and distribution of ligands available for integrin binding [76]. To this end, we recently developed tunable fibrous composites in which the fiber mechanics and level of ligand density (i.e., RGD) could be independently altered to investigate the role that these features play in guiding progenitor cell differentiation (e.g., chondrogenesis of human mesenchymal stem cells) [11, 77]. In these studies, fiber mechanics was altered by changing the crosslinking density within each individual fiber, while adhesive ligand presentation was controlled by defining the degree of ligand incorporation in the fiber backbone. As expected, the level of spreading and focal adhesion formation increased as the RGD density on the fibers increased, which in turn influenced the magnitude of cell engagement and pulling on the fibers (Figure 6A). Generally, this increase in ligand density led to a decrease in the level of chondrogenic gene expression and an increase in fibrous gene expression (Figure 6B). Interestingly, for the levels investigated, the mechanical properties of the fibers themselves did not influence chondrogenesis to any appreciable extent, though stiffer fibers increased expression of the fibrous tissue marker type I collagen. This data demonstrates that, upon arrival, the biophysical properties of the material scaffold, and how cells interact with it, can play a role in the phenotypic decisions made by cells that have migrated to the wound margin. These features might be manipulated to promote fibro-cartilaginous differentiation and matrix production by both endogenous meniscus cells and progenitor cells recruited to the wound site.
Figure 6. Scaffold mediated instruction upon arrival.
A) Vinculin localization (green), actin cytoskeleton (red), and nuclei (blue) staining of human mesenchymal stem cells (MSCs) cultured for 24 hours on fibrous HA scaffolds with varied RGD density and fiber modulus. Scale bar: 50 μm. B) MSC expression of chondrogenic markers after 14 days of culture in chondrogenic medium on fibrous HA scaffolds with varied RGD density and fiber modulus. *denotes significance (p<0.05) between groups, and  denotes significance (p<0.05) compared to other RGD densities within the same fiber stiffness condition. Adapted with permission from [11].
denotes significance (p<0.05) compared to other RGD densities within the same fiber stiffness condition. Adapted with permission from [11].
Looking Forward: Emerging Concepts in Meniscus Repair
In addition the progress described above in addressing some of the inherent limitations to meniscus repair, other key features are present in the wound environment that must be considered. These include the inflammatory state of the wound margin, as well as the physical properties of the cells within the tissue itself. Each of these factors may provide an additional target for modification to improve repair.
Inflammation and Meniscus Repair
While cell migration to the wound site is crucial for new tissue formation, and newly arrived cells must be instructed to adopt the appropriate phenotype, the inflammatory environment also plays a significant role in meniscus repair by regulating new tissue formation and integration of the wound margins. It is well established that joint injury and degeneration are accompanied by increased synovial inflammation, thus increasing the proteolytic enzyme burden in the synovial fluid [78-80], with matrix metalloproteinase-1 (MMP-1) specifically increased with joint injury [81]. Studies have shown that MMP levels in OA joints are a predictor of future joint space narrowing [79], and that synovial inflammation is a predictor of poor knee function post-meniscectomy [82]. Together, these data suggest that the ‘joint as an organ’ [82-85] cannot be ignored in strategies to promote meniscus repair. This is particularly relevant at the local level, where in vitro studies have shown that even picomolar levels of inflammatory cytokines (TNFα and IL-1β) reduce or eliminate integration between apposed meniscus edges [25, 86, 87], while provision of broad spectrum MMP-inhibitors in this inflammatory context can restore integration capacity [88, 89]. Approaches that limit these proteolytic events, especially at the wound interface, may therefore improve meniscus repair.
The field of biomaterials design has matured significantly over the past decade and we are now able to design triggered responsiveness into scaffolds, including response to local proteases, such as MMPs [72, 73]. We have recently used such MMP-degradable materials to control stem cell behavior [90, 91] and to promote repair in a sheep cardiac infarct model [92, 93]. As with meniscus injury, cardiac injury results in a local increase in MMP activity. Delivery of MMP-cleavable hydrogels to infarcted regions had the effect of decreasing local protease activity, likely through competitive inhibition or quenching of local MMPs, thus promoting repair. Furthermore, inclusion of the MMP inhibitor TIMP-3 as a delivered factor within the MMP-sensitive hydrogel resulted in further recovery of cardiac function [94, 95]. Here, release of the TIMP-3 was governed by material degradation, providing ‘on demand’ release profiles that could be tuned by the local inflammatory environment. Studies are now underway to explore this new class of materials and mechanism of action in the context of meniscus repair and regeneration.
Cell Mechanics in Interstitial Cell Migration: Applications to Meniscus Repair
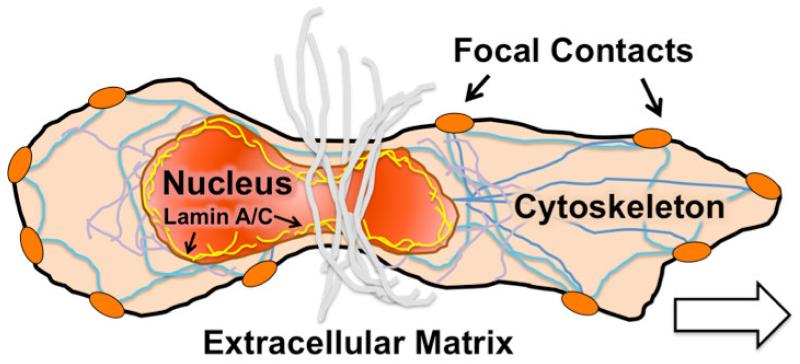
As a final point, our recent studies using partial digestion of the meniscus wound interface to loosen the ECM network have shown definitive improvements in wound edge cellularity in vitro and in vivo [64, 96]. While this finding implicates the dense meniscus ECM as an impediment to repair, an ideal repair strategy would preserve existing ECM (and tissue volume) while promoting integration. Migration in 3D is dependent on the deformability of cells and cell sub-structures [97]. As such, age-related changes in cell mechanics may play a role in meniscus healing. The cell nucleus is considered the rate-limiting organelle in 3D migration, given its large size and stiffness relative to the rest of the cell (2-4 times higher) [98], Figure 7. Nuclear stiffness is determined in part by the amount of heterochromatin (condensed DNA) [99-101], and in part by the filamentous nucleoskeletal network [102]. This filamentous network is comprised of a number of proteins, most prominent of which is the type V intermediate filament protein Lamin A/C, which provides structure and stability to the nuclear envelope [103-110]. Interestingly, while cells with stiff nuclei have limited migratory capacity inside dense collagen matrices [49], cells with compliant nuclei lacking Lamin A/C (such as leukocytes and certain cancer cells) remain highly mobile [49, 97, 111, 112]. Similarly, cells expressing a mutant isoform of Lamin A/C (progerin) that have stiffer nuclei cannot migrate through a constrictive array of micro-posts [113], while reduction of Lamin A/C content enhances migration through small pores [65, 110, 114]. Likewise, modulation of heterochromatin levels influence nuclear deformability [115-117] and cell migration through small pores [118]. Interestingly, both stem cell differentiation and increased tissue micromechanics have been implicated in higher Lamin A/C expression [119], elevated levels of heterochromatin [101, 120-124], and increased nuclear stiffness [110, 119]. Collectively, these data suggest that barriers to interstitial cell migration posed by the dense ECM may be overcome if the endogenous cell nuclei can adopt a ‘softer’ phenotype, deforming more readily through the small pores within the dense ECM. Materials designed to deliver factors to manipulate the nuclear mechanics of these endogenous cells may therefore promote cell migration to the wound margin, while preserving as much of the existing ECM as is possible.
Figure 7. Emerging concepts: manipulating endogenous cell mechanics to improve migration to the wound site.
Schematic illustration of interstitial 3D cell migration in dense fibrous networks. Nuclear mechanics, mediated by the amount and distribution of nucleo-structural filamentous components such as Lamin A/C, mediate the ability of cells to squeeze through small pores in dense connective tissues.
Conclusions
In current clinical practice, endogenous repair of dense connective tissues culminates in a state in which the regenerate tissue is of inferior quality than the native tissue, and is prone to failure. This failure in repair presents difficulties for the effective treatment of the meniscus, as well as other dense connective tissues such as articular cartilage, tendons, ligaments, and the annulus fibrosus of the intervertebral disc. Over the last decade, and guided by a deeper understanding of the limitations in endogenous repair of these tissues, biomaterials have been developed to reprogram this microenvironment from a state of failed repair towards one that enables true regeneration of tissue structure and function. Based on the predicate of providing an organized template for new tissue formation, and with lessons learned from the population and maturation of such structures in a tissue engineering context, new strategies have been developed to use biomaterials to reprogram the wound microenvironment. These include advances in material design that provide dynamic and multifunctional scaffolds that can lessen local ECM density to improve interstitial cell migration, delivery of chemotactic cues to draw cells from the native tissue to the wound site, and materials that influence cell fate decisions upon arrival via their biophysical properties and adhesive ligand presentation. In essence, these materials are designed to enable and direct cell colonization of the wound site and instruct cell behavior upon arrival so as to influence the trajectory of repair. Additionally, new concepts are now being developed to further reprogram the repair environment, using the materials themselves to control the inflammatory context of repair and to deliver agents that are tailored to manipulate the underlying cell mechanics to improve repair outcomes. These advanced materials, through their specific attendance to the inherent limitations of dense connective tissue repair, coupled with rehabilitation regimens that dynamically interact and synergize with the state of the repair itself, may one day turn an intractable clinical situation into one that is readily resolved. Improving dense connective tissue repair in the adult will have a profound impact on musculoskeletal health, and restore function to the millions of patients suffering from connective tissue failure.
Acknowledgements
This work was supported with grants from the National Institutes of Health (R01 EB008722 and AR056624) and the Department of Veterans Affairs (I01 RX000700 and RX000174). The authors gratefully acknowledge Dr. Brendon Baker, Dr. Matt Fisher, Dr. Lara Ionescu, Dr. Iris Kim, Dr. Brendan Purcell, and Ms. Sylvia Qu for their helpful input, graphical design, and in depth discussions on this topic.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to report with respect to the submitted manuscript.
References
- [1].Dourte LM, Kuntz AF, Soslowsky LJ. Twenty-five years of tendon and ligament research. J Orthop Res. 2008;26:1297–305. doi: 10.1002/jor.20646. [DOI] [PubMed] [Google Scholar]
- [2].Namba RS, Meuli M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am. 1998;80:4–10. doi: 10.2106/00004623-199801000-00003. [DOI] [PubMed] [Google Scholar]
- [3].Favata M, Beredjiklian PK, Zgonis MH, Beason DP, Crombleholme TM, Jawad AF, et al. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J Orthop Res. 2006;24:2124–32. doi: 10.1002/jor.20271. [DOI] [PubMed] [Google Scholar]
- [4].Ionescu LC, Lee GC, Garcia GH, Zachry TL, Shah RP, Sennett BJ, et al. Maturation state-dependent alterations in meniscus integration: implications for scaffold design and tissue engineering. Tissue Eng Part A. 2011;17:193–204. doi: 10.1089/ten.tea.2010.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–21. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- [6].Mauck RL, Baker BM, Nerurkar NL, Burdick JA, Li WJ, Tuan RS, et al. Engineering on the straight and narrow: the mechanics of nanofibrous assemblies for fiber-reinforced tissue regeneration. Tissue Eng Part B Rev. 2009;15:171–93. doi: 10.1089/ten.teb.2008.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nerurkar NL, Baker BM, Sen S, Wible EE, Elliott DM, Mauck RL. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater. 2009;8:986–92. doi: 10.1038/nmat2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nathan AS, Baker BM, Nerurkar NL, Mauck RL. Mechano-topographic modulation of stem cell nuclear shape on nanofibrous scaffolds. Acta Biomater. 2010;7:57–66. doi: 10.1016/j.actbio.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heo SJ, Nerurkar NL, Baker BM, Shin JW, Elliott DM, Mauck RL. Fiber stretch and reorientation modulates mesenchymal stem cell morphology and fibrous gene expression on oriented nanofibrous microenvironments. Ann Biomed Eng. 2011;39:2780–90. doi: 10.1007/s10439-011-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Han WM, Heo SJ, Driscoll TP, Smith LJ, Mauck RL, Elliott DM. Macro- to Microscale Strain Transfer in Fibrous Tissues is Heterogeneous and Tissue-Specific. Biophys J. 2013;105:807–17. doi: 10.1016/j.bpj.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim IL, Khetan S, Baker BM, Chen CS, Burdick JA. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials. 2013;34:5571–80. doi: 10.1016/j.biomaterials.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baker BM, Nerurkar NL, Burdick JA, Elliott DM, Mauck RL. Fabrication and modeling of dynamic multipolymer nanofibrous scaffolds. J Biomech Eng. 2009;131:101012. doi: 10.1115/1.3192140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rath E, Richmond JC. The menisci: basic science and advances in treatment. Br J Sports Med. 2000;34:252–7. doi: 10.1136/bjsm.34.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Adams ME, Hukins DWL. The extracellular matrix of the meniscus. In: Mow VC, Arnoczky SP, Jackson DW, editors. Knee meniscus: basic and clinical foundations. Raven Press, Ltd.; New York: 1992. pp. 15–28. [Google Scholar]
- [15].McDevitt CA, Webber RJ. The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop Relat Res. 1990:8–18. [PubMed] [Google Scholar]
- [16].Petersen W, Tillmann B. Collagenous fibril texture of the human knee joint menisci. Anat Embryol (Berl) 1998;197:317–24. doi: 10.1007/s004290050141. [DOI] [PubMed] [Google Scholar]
- [17].Shrive NG, O’Connor JJ, Goodfellow JW. Load-bearing in the knee joint. Clin Orthop. 1978:279–87. [PubMed] [Google Scholar]
- [18].Proctor CS, Schmidt MB, Whipple RR, Kelly MA, Mow VC. Material properties of the normal medial bovine meniscus. J Orthop Res. 1989;7:771–82. doi: 10.1002/jor.1100070602. [DOI] [PubMed] [Google Scholar]
- [19].Greis PE, Holmstrom MC, Bardana DD, Burks RT. Meniscal injury: II. Management. J Am Acad Orthop Surg. 2002;10:177–87. doi: 10.5435/00124635-200205000-00004. [DOI] [PubMed] [Google Scholar]
- [20].Petrosini AV, Sherman OH. A historical perspective on meniscal repair. Clin Sports Med. 1996;15:445–53. [PubMed] [Google Scholar]
- [21].Sihvonen R, Paavola M, Malmivaara A, Itala A, Joukainen A, Nurmi H, et al. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369:2515–24. doi: 10.1056/NEJMoa1305189. [DOI] [PubMed] [Google Scholar]
- [22].Ahmed AM. The load-bearing role of the knee meniscus. In: Mow VC, Arnoczky SP, Jackson DW, editors. Knee meniscus: basic and clinical foundations. Raven Press, Ltd.; New York: 1992. pp. 59–73. [Google Scholar]
- [23].Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32:7411–31. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Qu F, Fisher MB, Mauck RL. The Basic Science of Meniscus Repair: Endogenous Limitations and Emerging Regenerative Strategies. In: Kelly JD, editor. Meniscal Surgery: Management and Techniques. Springer Science+Business Media, LLC; 2014. [Google Scholar]
- [25].McNulty AL, Guilak F. Integrative repair of the meniscus: lessons from in vitro studies. Biorheology. 2008;45:487–500. [PMC free article] [PubMed] [Google Scholar]
- [26].Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowsky LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31:1143–52. doi: 10.1114/1.1616931. [DOI] [PubMed] [Google Scholar]
- [27].Provenzano PP, Hayashi K, Kunz DN, Markel MD, Vanderby R., Jr Healing of subfailure ligament injury: comparison between immature and mature ligaments in a rat model. J Orthop Res. 2002;20:975–83. doi: 10.1016/S0736-0266(02)00036-0. [DOI] [PubMed] [Google Scholar]
- [28].Clark CR, Ogden JA. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J Bone Joint Surg Am. 1983;65:538–47. [PubMed] [Google Scholar]
- [29].Andrish JT. Meniscal Injuries in Children and Adolescents: Diagnosis and Management. J Am Acad Orthop Surg. 1996;4:231–7. doi: 10.5435/00124635-199609000-00001. [DOI] [PubMed] [Google Scholar]
- [30].Barrett GR, Field MH, Treacy SH, Ruff CG. Clinical results of meniscus repair in patients 40 years and older. Arthroscopy. 1998;14:824–9. doi: 10.1016/s0749-8063(98)70018-0. [DOI] [PubMed] [Google Scholar]
- [31].Tenuta JJ, Arciero RA. Arthroscopic evaluation of meniscal repairs. Factors that effect healing. Am J Sports Med. 1994;22:797–802. doi: 10.1177/036354659402200611. [DOI] [PubMed] [Google Scholar]
- [32].Eggli S, Wegmuller H, Kosina J, Huckell C, Jakob RP. Long-term results of arthroscopic meniscal repair. An analysis of isolated tears. Am J Sports Med. 1995;23:715–20. doi: 10.1177/036354659502300614. [DOI] [PubMed] [Google Scholar]
- [33].Vanderhave KL, Moravek JE, Sekiya JK, Wojtys EM. Meniscus tears in the young athlete: results of arthroscopic repair. Journal of pediatric orthopedics. 2011;31:496–500. doi: 10.1097/BPO.0b013e31821ffb8d. [DOI] [PubMed] [Google Scholar]
- [34].Mesiha M, Zurakowski D, Soriano J, Nielson JH, Zarins B, Murray MM. Pathologic characteristics of the torn human meniscus. Am J Sports Med. 2007;35:103–12. doi: 10.1177/0363546506293700. [DOI] [PubMed] [Google Scholar]
- [35].Natsu-Ume T, Majima T, Reno C, Shrive NG, Frank CB, Hart DA. Menisci of the rabbit knee require mechanical loading to maintain homeostasis: cyclic hydrostatic compression in vitro prevents derepression of catabolic genes. J Orthop Sci. 2005;10:396–405. doi: 10.1007/s00776-005-0912-x. [DOI] [PubMed] [Google Scholar]
- [36].Upton ML, Hennerbichler A, Fermor B, Guilak F, Weinberg JB, Setton LA. Biaxial strain effects on cells from the inner and outer regions of the meniscus. Connect Tissue Res. 2006;47:207–14. doi: 10.1080/03008200600846663. [DOI] [PubMed] [Google Scholar]
- [37].Upton ML, Chen J, Guilak F, Setton LA. Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res. 2003;21:963–9. doi: 10.1016/S0736-0266(03)00063-9. [DOI] [PubMed] [Google Scholar]
- [38].Baker BM, Shah RP, Huang AH, Mauck RL. Dynamic tensile loading improves the functional properties of mesenchymal stem cell-laden nanofiber-based fibrocartilage. Tissue Eng Part A. 2011;17:1445–55. doi: 10.1089/ten.tea.2010.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McNulty AL, Estes BT, Wilusz RE, Weinberg JB, Guilak F. Dynamic loading enhances integrative meniscal repair in the presence of interleukin-1. Osteoarthritis Cartilage. doi: 10.1016/j.joca.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kambic HE, Futani H, McDevitt CA. Cell, matrix changes and alpha-smooth muscle actin expression in repair of the canine meniscus. Wound Repair Regen. 2000;8:554–61. doi: 10.1046/j.1524-475x.2000.00554.x. [DOI] [PubMed] [Google Scholar]
- [41].Day B, Mackenzie WG, Shim SS, Leung G. The vascular and nerve supply of the human meniscus. Arthroscopy. 1985;1:58–62. doi: 10.1016/s0749-8063(85)80080-3. [DOI] [PubMed] [Google Scholar]
- [42].Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90–5. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- [43].Friedl P, Sahai E, Weiss S, Yamada KM. New dimensions in cell migration. Nat Rev Mol Cell Biol. 2012;13:743–7. doi: 10.1038/nrm3459. [DOI] [PubMed] [Google Scholar]
- [44].Lange JR, Fabry B. Cell and tissue mechanics in cell migration. Exp Cell Res. 2013;319:2418–23. doi: 10.1016/j.yexcr.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol. 2005;204:198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- [46].Doyle AD, Petrie RJ, Kutys ML, Yamada KM. Dimensions in cell migration. Curr Opin Cell Biol. 2013;25:642–9. doi: 10.1016/j.ceb.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wolf K, Friedl P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 2011;21:736–44. doi: 10.1016/j.tcb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- [48].Schmidt S, Friedl P. Interstitial cell migration: integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res. 2010;339:83–92. doi: 10.1007/s00441-009-0892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wolf K, Te Lindert M, Krause M, Alexander S, Te Riet J, Willis AL, et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol. 2013;201:1069–84. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A. 2006;103:10889–94. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kolambkar YM, Dupont KM, Boerckel JD, Huebsch N, Mooney DJ, Hutmacher DW, et al. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32:65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bott K, Upton Z, Schrobback K, Ehrbar M, Hubbell JA, Lutolf MP, et al. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials. 2010;31:8454–64. doi: 10.1016/j.biomaterials.2010.07.046. [DOI] [PubMed] [Google Scholar]
- [53].Li WJ, Mauck RL, Cooper JA, Yuan X, Tuan RS. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2007;40:1686–93. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28:1967–77. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fisher MB, Henning EA, Soegaard N, Esterhai JL, Mauck RL. Organized nanofibrous scaffolds that mimic the macroscopic and microscopic architecture of the knee meniscus. Acta Biomater. 2013;9:4496–504. doi: 10.1016/j.actbio.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Baker BM, Gee AO, Metter RB, Nathan AS, Marklein RA, Burdick JA, et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29:2348–58. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ifkovits JL, Wu K, Mauck RL, Burdick JA. The influence of fibrous elastomer structure and porosity on matrix organization. PLoS ONE. 5:e15717. doi: 10.1371/journal.pone.0015717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Metter RB, Ifkovits JL, Hou K, Vincent L, Hsu B, Wang L, et al. Biodegradable fibrous scaffolds with diverse properties by electrospinning candidates from a combinatorial macromer library. Acta Biomater. 6:1219–26. doi: 10.1016/j.actbio.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Baker BM, Shah RP, Silverstein AM, Esterhai JL, Burdick JA, Mauck RL. Sacrificial nanofibrous composites provide instruction without impediment and enable functional tissue formation. Proc Natl Acad Sci U S A. 2012;109:14176–81. doi: 10.1073/pnas.1206962109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Moretti M, Wendt D, Schaefer D, Jakob M, Hunziker EB, Heberer M, et al. Structural characterization and reliable biomechanical assessment of integrative cartilage repair. J Biomech. 2005;38:1846–54. doi: 10.1016/j.jbiomech.2004.08.021. [DOI] [PubMed] [Google Scholar]
- [61].Ionescu LC, Mauck RL. Porosity and cell preseeding influence electrospun scaffold maturation and meniscus integration in vitro. Tissue Eng Part A. 2013;19:538–47. doi: 10.1089/ten.tea.2012.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ionescu LC, Lee GC, Sennett BJ, Burdick JA, Mauck RL. An anisotropic nanofiber/microsphere composite with controlled release of biomolecules for fibrous tissue engineering. Biomaterials. 2010;31:4113–20. doi: 10.1016/j.biomaterials.2010.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].van de Breevaart Bravenboer J, In der Maur CD, Bos PK, Feenstra L, Verhaar JA, Weinans H, et al. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res Ther. 2004;6:R469–76. doi: 10.1186/ar1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Qu F, Lin JM, Esterhai JL, Fisher MB, Mauck RL. Biomaterial-mediated delivery of degradative enzymes to improve meniscus integration and repair. Acta Biomater. 2013;9:6393–402. doi: 10.1016/j.actbio.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Greiner AM, Jackel M, Scheiwe AC, Stamow DR, Autenrieth TJ, Lahann J, et al. Multifunctional polymer scaffolds with adjustable pore size and chemoattractant gradients for studying cell matrix invasion. Biomaterials. 2014;35:611–9. doi: 10.1016/j.biomaterials.2013.09.095. [DOI] [PubMed] [Google Scholar]
- [66].Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 376:440–8. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage. 2004;12:736–44. doi: 10.1016/j.joca.2004.05.007. [DOI] [PubMed] [Google Scholar]
- [68].Dai M, Yang Y, Omelchenko I, Nuttall AL, Kachelmeier A, Xiu R, et al. Bone marrow cell recruitment mediated by inducible nitric oxide synthase/stromal cell-derived factor-1alpha signaling repairs the acoustically damaged cochlear blood-labyrinth barrier. Am J Pathol. 2010;177:3089–99. doi: 10.2353/ajpath.2010.100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, et al. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–20. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- [70].Shen W, Chen J, Zhu T, Chen L, Zhang W, Fang Z, et al. Intra-Articular Injection of Human Meniscus Stem/Progenitor Cells Promotes Meniscus Regeneration and Ameliorates Osteoarthritis Through Stromal Cell-Derived Factor-1/CXCR4-Mediated Homing. Stem Cells Transl Med. 2014 doi: 10.5966/sctm.2012-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Purcell BP, Elser JA, Mu A, Margulies KB, Burdick JA. Synergistic effects of SDF-1alpha chemokine and hyaluronic acid release from degradable hydrogels on directing bone marrow derived cell homing to the myocardium. Biomaterials. 2012;33:7849–57. doi: 10.1016/j.biomaterials.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat Commun. 2012;3:1269. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- [73].Wade RJ, Burdick JA. Engineering ECM Signals into Biomaterials. Materials Today. 2012;15:454–9. [Google Scholar]
- [74].Kim IL, Fisher MB, Baker BM, Mauck RL, Burdick JA. Tunable Fibrous Hyaluronic Acid Scaffolds for Cartilage Tissue Engineering; Transactions of the Society for Biomaterials Annual Meeting; 2014. [Google Scholar]
- [75].Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [76].Connelly JT, Garcia AJ, Levenston ME. Interactions between integrin ligand density and cytoskeletal integrity regulate BMSC chondrogenesis. J Cell Physiol. 2008;217:145–54. doi: 10.1002/jcp.21484. [DOI] [PubMed] [Google Scholar]
- [77].Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials. 32:8771–82. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Struglics A, Hansson M, Lohmander LS. Human aggrecanase generated synovial fluid fragment levels are elevated directly after knee injuries due to proteolysis both in the inter globular and chondroitin sulfate domains. Osteoarthritis Cartilage. 2011;19:1047–57. doi: 10.1016/j.joca.2011.05.006. [DOI] [PubMed] [Google Scholar]
- [79].Lohmander LS, Brandt KD, Mazzuca SA, Katz BP, Larsson S, Struglics A, et al. Use of the plasma stromelysin (matrix metalloproteinase 3) concentration to predict joint space narrowing in knee osteoarthritis. Arthritis Rheum. 2005;52:3160–7. doi: 10.1002/art.21345. [DOI] [PubMed] [Google Scholar]
- [80].Struglics A, Larsson S, Pratta MA, Kumar S, Lark MW, Lohmander LS. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthritis Cartilage. 2006;14:101–13. doi: 10.1016/j.joca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- [81].Tchetverikov I, Lohmander LS, Verzijl N, Huizinga TW, TeKoppele JM, Hanemaaijer R, et al. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis. 2005;64:694–8. doi: 10.1136/ard.2004.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Scanzello CR, Albert AS, DiCarlo E, Rajan KB, Kanda V, Asomugha EU, et al. The influence of synovial inflammation and hyperplasia on symptomatic outcomes up to 2 years post-operatively in patients undergoing partial meniscectomy. Osteoarthritis Cartilage. 2013;21:1392–9. doi: 10.1016/j.joca.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–57. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63:391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Interleukin-1 and tumor necrosis factor alpha inhibit repair of the porcine meniscus in vitro. Osteoarthritis Cartilage. 2007;15:1053–60. doi: 10.1016/j.joca.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hennerbichler A, Moutos F, Hennerbichler D, Fermor B, Weinberg JB, Guilak F. Inhibition of integrative repair of the meniscus in vitro by interleukin-1 and tumor necrosis factor alpha. Trans ORS. 2006;31:1038. [Google Scholar]
- [88].McNulty AL, Weinberg JB, Guilak F. Inhibition of Matrix Metalloproteinases Enhances In Vitro Repair of the Meniscus. Clin Orthop Relat Res. 2008 doi: 10.1007/s11999-008-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].McNulty AL, Moutos FT, Weinberg JB, Guilak F. Enhanced integrative repair of the porcine meniscus in vitro by inhibition of interleukin-1 or tumor necrosis factor alpha. Arthritis Rheum. 2007;56:3033–42. doi: 10.1002/art.22839. [DOI] [PubMed] [Google Scholar]
- [90].Khetan S, Burdick JA. Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials. 2010;31:8228–34. doi: 10.1016/j.biomaterials.2010.07.035. [DOI] [PubMed] [Google Scholar]
- [91].Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Natl Mater. 2013;12:458–65. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellars KN, et al. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat Mater. 2014;13:653–61. doi: 10.1038/nmat3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellars KH, et al. Injectable and Bioresponsive Hydrogels for On-Demand Matrix Metalloproteinase Inhibition. Nat Mater. doi: 10.1038/nmat3922. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Eckhouse SR, Purcell BP, McGarvey JR, Lobb D, Logdon CB, Doviak H, et al. Local hydrogel release of recombinant TIMP-3 attenuates adverse left ventricular remodeling after experimental myocardial infarction. Sci Transl Med. 2014;6:223ra21. doi: 10.1126/scitranslmed.3007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Purcell BP, Lobb D, Spinale FG, Burdick JA. On-demand Delivery of TIMP-3 from Injectable and MMP Degradable Hydrogels for Infarct Repair. Trans Soc Biomat. 2014 [Google Scholar]
- [96].Qu F, Pintauro MP, Haughan JE, Henning EA, Esterhai JL, Schaer TP, et al. Repair of dense connective tissues via biomaterial-mediated matrix reprogramming of the wound interface. Biomaterials. 2015;39:85–94. doi: 10.1016/j.biomaterials.2014.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 2011;23:55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Guilak F, Tedrow JR, Burgkart R. Viscoelastic properties of the cell nucleus. Biochem Biophys Res Commun. 2000;269:781–6. doi: 10.1006/bbrc.2000.2360. [DOI] [PubMed] [Google Scholar]
- [99].Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys J. 2005;89:2855–64. doi: 10.1529/biophysj.105.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Krause M, Te Riet J, Wolf K. Probing the compressibility of tumor cell nuclei by combined atomic force-confocal microscopy. Physical biology. 2013;10:065002. doi: 10.1088/1478-3975/10/6/065002. [DOI] [PubMed] [Google Scholar]
- [101].Talwar S, Kumar A, Rao M, Menon GI, Shivashankar GV. Correlated spatio-temporal fluctuations in chromatin compaction states characterize stem cells. Biophys J. 2013;104:553–64. doi: 10.1016/j.bpj.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Martins RP, Finan JD, Guilak F, Lee DA. Mechanical regulation of nuclear structure and function. Annu Rev Biomed Eng. 2012;14:431–55. doi: 10.1146/annurev-bioeng-071910-124638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ho CY, Lammerding J. Lamins at a glance. J Cell Sci. 2012;125:2087–93. doi: 10.1242/jcs.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Lammerding J. Mechanics of the nucleus. Compr Physiol. 2011;1:783–807. doi: 10.1002/cphy.c100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Rowat AC, Lammerding J, Herrmann H, Aebi U. Towards an integrated understanding of the structure and mechanics of the cell nucleus. Bioessays. 2008;30:226–36. doi: 10.1002/bies.20720. [DOI] [PubMed] [Google Scholar]
- [106].Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res. 2008;102:1307–18. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem. 2006;281:25768–80. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- [108].Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–8. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lammerding J, Lee RT. The nuclear membrane and mechanotransduction: impaired nuclear mechanics and mechanotransduction in lamin A/C deficient cells; Novartis Found Symp.; 2005; pp. 264–73. discussion 73-8. [PubMed] [Google Scholar]
- [110].Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci U S A. 2007;104:15619–24. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Rowat AC, Jaalouk DE, Zwerger M, Ung WL, Eydelnant IA, Olins DE, et al. Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions. J Biol Chem. 2013;288:8610–8. doi: 10.1074/jbc.M112.441535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zwerger M, Jaalouk DE, Lombardi ML, Isermann P, Mauermann M, Dialynas G, et al. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum Mol Genet. 2013;22:2335–49. doi: 10.1093/hmg/ddt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Booth-Gauthier EA, Du V, Ghibaudo M, Rape AD, Dahl KN, Ladoux B. Hutchinson-Gilford progeria syndrome alters nuclear shape and reduces cell motility in three dimensional model substrates. Integr Biol (Camb) 2013;5:569–77. doi: 10.1039/c3ib20231c. [DOI] [PubMed] [Google Scholar]
- [114].Harada T, Swift J, Irianto J, Shin JW, Spinler KR, Athirasala A, et al. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J Cell Biol. 2014 doi: 10.1083/jcb.201308029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Heo SJ, Thorpe SD, Driscoll TP, Hashmi SK, Lee DA, Mauck RL. Rapid and Sustained Changes in Nuclear Architecture and Mechanics in Mesenchymal Stem Cells in Response to Dynamic Stretch. Trans Orthop Res Soc. 2014 [Google Scholar]
- [116].Heo SJ, Driscoll TP, Thorpe SD, Han WM, Elliott DM, Lee DA, et al. Rapid Chromatin Condensation Increases Stem Cell Nuclear Mechanics and Mechanosensitivity. Trans Orthop Res Soc. 2014 [Google Scholar]
- [117].Masaeli M, Tse HTK, Gossett DR, Gupta D, Di Carlo D. Multi-Parameter High-Throughput Mechanic Phenotyping. Freiburg, Germany: 2013. pp. 383–5. [Google Scholar]
- [118].Fu Y, Chin LK, Bourouina T, Liu AQ, VanDongen AM. Nuclear deformation during breast cancer cell transmigration. Lab Chip. 2012;12:3774–8. doi: 10.1039/c2lc40477j. [DOI] [PubMed] [Google Scholar]
- [119].Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell. 2006;10:105–16. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Krejci J, Uhlirova R, Galiova G, Kozubek S, Smigova J, Bartova E. Genome-wide reduction in H3K9 acetylation during human embryonic stem cell differentiation. J Cell Physiol. 2009;219:677–87. doi: 10.1002/jcp.21714. [DOI] [PubMed] [Google Scholar]
- [122].Bartova E, Krejci J, Harnicarova A, Kozubek S. Differentiation of human embryonic stem cells induces condensation of chromosome territories and formation of heterochromatin protein 1 foci. Differentiation. 2008;76:24–32. doi: 10.1111/j.1432-0436.2007.00192.x. [DOI] [PubMed] [Google Scholar]
- [123].Spivakov M, Fisher AG. Epigenetic signatures of stem-cell identity. Nat Rev Genet. 2007;8:263–71. doi: 10.1038/nrg2046. [DOI] [PubMed] [Google Scholar]
- [124].Bhattacharya D, Talwar S, Mazumder A, Shivashankar GV. Spatio-temporal plasticity in chromatin organization in mouse cell differentiation and during Drosophila embryogenesis. Biophys J. 2009;96:3832–9. doi: 10.1016/j.bpj.2008.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]