Introduction
Cells within connective tissues reside in a dynamic microenvironment that provides both a three-dimensional scaffold “housing” and a milieu of biophysical signals. This scaffolding, termed the extracellular matrix (ECM), is primarily composed of basement membrane and interstitial connective tissue and actively stimulates resident cells on both the macroscopic (global) and the microscopic (local) level. Within the context of the lung, macroscopic regulatory roles include providing structural support to facilitate gas exchange and prevent airway collapse; developing a hierarchical, branched tissue architecture separating the epithelium, endothelium and interstitium; and also providing a scaffold capable of imparting global mechanical forces onto resident cells (e.g., stretch from breathing)82. On the cellular level, the ECM also provides a “biochemical and mechanical language” that governs fundamental processes such as cell signaling pathways10, cell shape and function2, changes in cytoskeletal organization and differentiation15, formation of focal adhesions and stress fibers14, alterations of proliferation and migration90, stimulation of polarity and gene expression94, induction of metastatic activity58, response to growth factors16, and information regulating appropriate location of specific cell contingencies within the matrix11. Further, cell-matrix interactions play critical roles throughout life: during embryonic development and organogenesis21, 79, angiogenesis23, wound healing74, disease and metastasis62.
The importance of matrix biology in the context of regenerative medicine has resulted in substantial efforts to define the scope of matrix effects on cells, determine the underlying mechanisms of this regulation, and harness this relationship to facilitate the production of tissue engineered organs. One notable advancement has been the development of decellularized scaffolds20. These “repurposed biomaterials” serve as an attractive source of microscopic, tissue-specific matrix constituents and a means to recreate physiological conditions for in vitro or ex vivo studies. Decellularized matrix is currently used in 2D cell culture systems, as a model for organ development or disease, and as a potential platform for the creation of organ replacements.
A brief overview of lung matrix composition
Lung matrix is generally composed of collagen and elastin fibers that are interwoven with glycosaminoglycans (GAGs), fibronectin fibrils, proteoglycans (PGs), and water sequestered by PGs and GAGs. (For a more thorough review of lung ECM composition please refer to Dunsmore et al22.) Other essential ECM constituents include laminin, heparan sulfate, nidogen/entactin, hyaluronate, chondroitin sulfate and matricellular proteins such as thrombospondin, tenascin X, and tenascin-C9, 22. Given that our knowledge of ECM composition in the lung is still evolving, it is highly likely that some variants of these common matrix structural proteins have not yet been characterized.
The major collagen subtypes that populate the lung are types I, III, IV, and V. Of these subtypes, the interstitial collagens (I and III) play the principle load-bearing role in the parenchyma, while type IV is a key basement membrane component, and assists in barrier function22. Elastin, the matrix component that is largely responsible for the intrinsic recoil property of lung tissue, is a highly flexible and crosslinked protein that can withstand up to 200% strain29. Elastin is particularly resilient and has a half-life that approximates the life expectancy of the organism (80 or more years for humans)75. PGs are proteins that are located on the surface of cell membranes, and also within intracellular vesicles, and are incorporated throughout the ECM22. These proteins are composed of glycosaminoglycans (GAGs), a family of highly charged polysaccharides, and a protein core. PGs, with their attached GAGs, sequester water, ions, growth factors, and directly control macromolecular and cellular movement across the basal lamina63. The mechanical nature of the lung, characterized by viscoelastic stress-strain patterns and elastic recoil, enables ventilation and ultimately gas exchange. (For an excellent review of lung matrix mechanics please refer to reviews by Suki and colleagues82, 83, and for a thorough review of vascular composition and mechanics refer to Mecham and colleagues91.) These properties are not simply a reflection of the summation of individual protein contributions, but rather a combination of coupled interactions between matrix components, and regional organization of the tissue. Therefore the depletion or damage of one matrix protein (e.g., elastin depletion as seen in emphysema) has an impact on the function of the neighboring matrix proteins as well29, 95.
Although fibronectin and laminin may not contribute greatly to the mechanical nature of the lung, they are essential proteins for cell adhesion and survival85. Fibronectin is a cell-adhesive glycoprotein that is of particular importance for the adherence of a variety of pulmonary cell types to the extracellular matrix88 and interacts with cells to impact their morphology, motility, and differentiation31, 35, 42. Fibronectin is also important for growth factor storage, a feature of particular importance during states of remodeling74.
1) Tissue elasticity
Over the last several decades it has become increasingly clear that matrix stiffness, a mechanical property inherit to the ECM, has an influential role in numerous cell functions and is as important as chemical composition in regulating cell behaviors such as migration (durotaxis)46, formation of focal adhesions84, cell proliferation4, apoptosis56, growth factor or surfactant production4, and stem cell differentiation25. Culturing type II alveolar cells on stiffer substrates results in enhanced laminin and fibronectin assembly, upregulation of the α3 laminin subunit, and a change in morphology from the rounded to a flattened shape that is more typical of type I cells24. Matrix stiffness can also underpin pathological outcomes; interstitial cells placed on very stiff surfaces have been shown to differentiate into the contractile and synthetic cell types associated with lung fibrosis 4, 44.
Lung matrix stiffness varies dramatically on a global and local scale due to regional differences in matrix configuration, and because of intrinsic differences in individual protein properties81. On a tissue level, normal lung stiffness ranges from 0.5 to 15 kPa, depending on the location in the lung and the method of stiffness measurement45, 83. On the matrix nano-level, collagen type I is at least 2 orders of magnitude stiffer than elastin29. It has been suggested that these local differences, on a nano and micro scale, of the lung parenchyma could be important in regulating the spatial distribution, differentiation, and function of cells48.
2) Matrix composition
The lungs vary regionally and temporally in ECM composition (Figure 1)17, 22. For example, collagen type I is typically found in the large bronchi, blood vessels, and irregularly placed throughout the interstitium of the alveolar septae5, 51. Collagen II is typically found only in bronchial and tracheal cartilage, whereas collagen type III is found in the large bronchi, perivascular, and interstitum of the alveolar septa5, 51. Collagen IV is primarily found in to co-distribute with collagen V in linear patterns in both the alveolar and capillary basement membranes, and collagen type VI is found co-distributed in blood vessels with collagen types I and III51. In addition to subtype, total collagen also varies regionally throughout the matrix. In a study analyzing collagen I and III distribution in rat lungs, collagen concentrations were the highest in the pulmonary artery (50 ug/ug dry tissue), followed by small airways, arteries, main intrapulmonary bronchus, and parenchyma (25 ug/ug dry tissue)39. In contrast to collagen content, elastin content is highest in the lung parenchyma, followed by blood vessels and bronchi41, 68.
Figure 1.
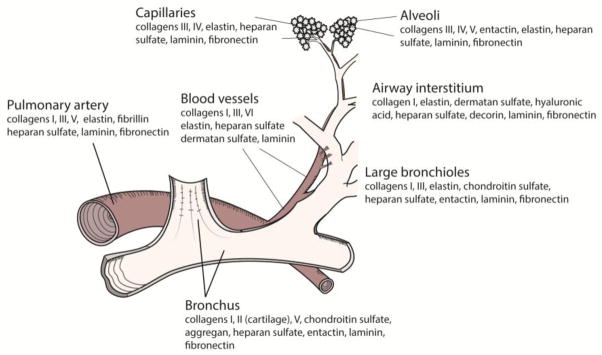
Regional ECM composition in a human lung acini. This schematic highlights some of the structural and adhesive major components and how they are distributed throughout the lung.
The composition of ECM in fetal, neonatal and adult tissue is markedly different, and has been shown to temporally regulate resident cells in terms of morphology, migration, differentiation, response to mechanical factors, growth factor production, and enhanced wound healing capacity18, 72. During embryonic development of the murine lung, all five laminin α chains are present. However, the normal adult lung tissue contains primarily laminins α3, α4, and α5.57, 59, 77. Fetal lung tissues also contain more total GAGs and PGs throughout the organ, and also more collagen I and III in the pleura and the alveolar septae than adult tissue5. Since fibronectin binds more readily to collagen type III than to collagen type I (or type IV)26, the enhanced collagen III promotes fibronectin sequestration and provides a mechanism for enhanced platelet aggregation6. Matrix components are also responsible for inducing differentiation of many cell types. Highly sulfated PGs are responsible for the spreading of cells during transdifferentiation of type II cells to mature type I cells43. The alveolar basement membrane below type I cells is also highly sulfated as compared with the matrix below type II cells 71, 89.
A wide range of matrix molecules is known to mediate cell attachment and survival in a cell type-specific manner. These include collagen (I and IV), laminin, fibronectin, and vitronectin. Each cell type has a unique integrin profile that is reflective of their matrix, function, and differentiation state28, 52. As ECM changes along the proximal or distal regions of the lung, the matrix-specific integrins also change and presumably play a role in location-specific adhesion, homing, and proliferation. For example, alveolar type II cells preferentially adhere to fibronectin, and adhere significantly less (~ 50% or greater) to alterative matrix proteins such as laminin, vitronectin, or collagens I, III, or IV15. Fibronectin alters alveolar gap junction intercellular communication2 and enhances cell migratory behavior42, suggesting that alveolar type II cells may migrate in the direction of fibronectin, adhere preferentially, and form stable cell-cell junctions. In the vasculature, endothelial cells prefer to co-localize with fibronectin over tenascin-C, collagen type I, collagen type VI, collagen type IV, decorin, or versican78. In contrast, both fibroblasts and smooth muscle cells adhere non-discriminately to collagen (types I and IV)58, although attachment to polymeric collagen has been well documented to inhibit proliferation of these cell types33, 40, 73. Interestingly, monomeric collagen has been shown to induce mesechymal proliferation40. Given that uncontrolled proliferation of fibroblasts is contributory to lung fibrosis, the maintenance of intact polymeric collagen is likely important for the construction of functional engineered lungs.
Building the Acellular Scaffold
Over the past several years, advancements have been made with regard to producing decellularized scaffolds from native lungs. Detergents that are commonly used in the decellularization process include Triton-X 100, sodium dodecyl sulfate (SDS), sodium deoxycholate (SDC), and 3-[(3-cholamindopropyl)dimethylammonio]-1-propanesulfonate (CHAPS). These detergents solubilize cell membranes, disengage cytoskeletal proteins from cells, and detach DNA and DNA remnants from proteins20. As reported by many groups, these scaffolds often retain many of the essential proteins present in the original organ32, 60, 61, 92. Ideally, this matrix would retain sufficient structural proteins within the airways and interstitium, the basement membrane, adhesive proteins required for cell engraftment, growth, and differentiation, and growth factors required for proper cell adhesion, survival and proliferation22.
In a study by White and colleagues, normal and fibrotic decellularized lung matrix was reseeded with normal healthy lung fibroblasts. Fibroblasts seeded onto decellularized tissue from fibrotic lungs demonstrated a pro-fibrotic response – these cells underwent enhanced myofibroblast differentiation and increased TGF-β1 growth factor production, whereas fibroblasts seeded onto “healthy” acellular tissue did not8. In a study examining the impact of emphysema on matrix, lung tissue from healthy and emphysetamous patients was decellularized and reseeded with a variety of cell types. Although the healthy tissue supported engraftment of all cell types for one month, the decellularized emphysetamous tissue did not92. Although decellularized lung tissue can provide a model of matrix degradation as seen in aging or lung disease8, 76, it is important to recognize that many decellularization protocols render a depleted scaffold and may not be optimized for long-term cell culture.
Factors Affecting Scaffold Quality
In the very long term, acellular lung matrices may serve as a platform for regeneration of functional lung tissue. To be functional, a regenerated lung should minimally be able to 1) maintain lung-specific cells: i.e., cells that produce surfactant, growth factors, have cilia, etc; 2) provide a barrier to separate blood from air, along with functional alveolar epithelial and microvascular endothelial cells; 3) have a hierarchical branching geometry that provides high surface area for gas exchange, 4) contain a perfusable microvasculature that is resistant to thrombosis, and 5) be sufficiently mechanically robust to allow for ventilation and physiological mechanical stresses13. To date, although significant progress has been made towards the production of such an organ7, 32, 61, 65, 69, these functional criteria have not yet been achieved. The quality of the underlying matrix scaffold will drive success or failure for many of these critical design functions.
1) Impact of tissue age on matrix characteristics
The composition and of lung tissue changes dramatically as we age34, 68. For example, studies of GAG content in rabbit lung parenchyma at different states of development have shown that fetal lungs contain a high proportion of chondroitin 4-sulfate, while older animals predominantly retain dermatan sulfate, heparan sulfate, and heparin34. Although all GAG production was low in older rabbits, there was a relatively greater synthesis of dermatan sulfate and heparin 34. In humans, the collagen content in young parenchyma, pleura, and arterial wall is higher than in older lungs. For example, collagen comprises 16% of the pulmonary artery wall in young adults, and decreases to 10% in individuals over 80 years old12, 50, 67. These differences are due to both decreases in new collagen synthesis, and to increases in collagen degradation. In the rat lung, collagen synthesis decreases with age – from 13% per day at one month of age to 1% per day at 2 years. The degradation rate of newly synthesized collagen rats increases from 28% to 62% over the same time frame (1 month to 2 years)53. In contrast to collagen, the elastin content of the lung parenchyma does not change over the same time span - less than 1% of the total body elastin pool turns over per year. The next effect is one of decreasing the collagen-to-elastin ratio68 and changing the biochemical make-up of the extracellular matrix of the lung as the animal ages. Presumably, a change in quality and content of native scaffolds from age or exposure to environmental factors will impact the matrix outcomes after decellularization. In a study examining the impact of age on ECM retention of decellularized mice lungs, tissues that were produced from older animals had reduced ECM contents76. Therefore, decellularized tissues of older animals may have diminished biologic cues that could lead to inferior recellularization and remodeling outcomes.
2) Species-Specific effects
There are intrinsic differences between species with respect to organ size, alveolar size and number, tissue density, cell numbers and composition. Taking for example differences between rat lungs and human lungs, the rat has 19.7×106 alveoli, each having diameters of ~70 μm, whereas the human has 494 × 106 alveoli, with diameters of ~ 200–400 μm19, 55, 80. Tissue composition between species is also markedly different - collagen and elastin fiber densities are 3–4 fold higher in human parenchyma than in rat parenchyma54. Additionally, the alveolar septal walls in humans are three fold thicker than in rats, and about 50% thicker than in monkeys55, and elastin fibers penetrate significantly deeper into the alveolar septal walls of human lungs than in rat lungs54. These spatial differences in matrix result in a dramatically different matrix compositions between species, and possibly different outcomes following decellularization, though investigations have not yet been published that specifically address species differences for lung decellularization.
In a study that compared the cellular composition in the alveolar regions of rats, baboons and humans, the percentage of resident cell types varied dramatically between species19. Although the total alveolar epithelial percentages did not change for type I and type 2 alveolar epithelial cells, the endothelial cell number as a percentage of total lung cells. In rat, baboon and human, endothelial cells were 46%, 36%, and 30% respectively. Although the fraction of alveolar cells remains similar across species, the total alveolar cell number increases logarithmically with body weight (larger lungs from larger species increase in total cell number)80. Given these differences in cell percentages, it is entirely possible that different methods of decellularization (perfusion of detergent via vasculature, as opposed to administering via airways) will differentially impact the matrix outcomes and amounts of cellular residuals, dependent on the species.
Although it is well established that there are intrinsic differences between species in terms of matrix and cellular composition, there is surprisingly little information regarding species-dependency on the decellularization efficiency, quality and composition of acellular lung matrices. In a study comparing decellularization efficiency on human and porcine heart valves, porcine valves had substantially more residual protein as compared to human tissue on a per gram basis, and were reportedly more difficult to decellularize than human tissues70. In a study comparing human versus porcine decellularized myocardial matrix tissue for the use of producing acellular hydrogels, porcine-derived decellularized matrix retained collagens II, V, and VI and fibulin-3, while the human decellularized tissue did not37. In contrast, human tissue retained collagen XII, fibulin-2, heparin sulfate, and periostin after decellularization, while porcine tissues were depleted of these proteins. Although these studies only investigated human and pig tissue, they highlight the need for consideration of species differences in decellularization studies.
Decellularization is a delicate balance between effective cell removal and preservation of critical matrix components. As detergents remove cellular debris, these solutions simultaneously extract and damage components of the ECM that are essential to lung function and cell adhesion, including various glycoproteins and PGs27. To date, an analysis of various decellularization protocols applied to porcine lungs has demonstrated matrix deterioration that is ranging in severity. Reported matrix damage includes loss of collagen32, 60, elastin32, 60, 61, laminins60, fibronectin60, and sulfated glycosaminoglycans (GAGs)61, 65. The functional consequences of specific protein removal can range from impaired cell engraftment due the loss of fibronectin,87 to substantial loss of tissue strength from loss of collagen type I64. Comparative studies investigating decellularization of lungs with Triton-SDC, SDS or CHAPS have found conflicting results with respect to protocol-dependent matrix loss. For example, Weiss and colleagues found comparable levels of retained collagen in Triton-SDC and SDS decelled lungs93 while CHAPS treated lungs were severely depleted, Ott and colleagues have found the most retention of collagen with SDS32 and significant loss with SDC and CHAPs treated lungs, and Vunjak-Novakovi and colleagues have found no significant differences between detergents in total collagen retention61. It should be noted that these studies were performed using mouse93, rat32, or porcine and human lungs61, and the differences in species could be a cause for the discrepancy in results.
Cell and host-Scaffold interactions
Successful decellularization should entail the removal of cell membrane epitopes, damage-associated molecular pattern (DAMP) molecules, and DNA remnants from the scaffold, since these components are known to induce inflammatory reactions47, 96. Host responses to acellular matrices include the activation of M1 (pro-inflammatory) or M2 (pro-constructive remodeling) responses3, 38. The threshold level of nuclear material that induces negative, pro-inflammatory responses has not yet been determined, and hence acceptable levels of decellularization for various organs are simply not known30, 38. As a consequence, reports in the literature of what is described as an “acellular” or “decellularized” lung matrix ranges anywhere from 75% to up to 98% donor DNA removal7, 32, 61, 65, 69. Despite the lack of clear benchmarks for what constitutes “decellularized”, it has been generally established that DNA fragments that are less than 300 bp in length will not elicit an adverse remodeling responses30. Therefore, it is possible that if residual DNA is broken into small enough fragments during the decellularization process, the negative consequences of residual DNA could be minimized. In terms of the impact of non-nuclear donor material on adverse immune responses, it remains unclear if protein sources of cell debris are problematic. Currently, there are multiple reports of decellularized tissues with detectable cytoskeletal debris, such as β-actin,124, 144, although the functional consequences of these remnents are not known. Macchiarini and colleagues reported that despite retaining some pre-existing non-nuclear intracellular elements in the cartilaginous regions of decellularized trachea, the implanted material did not incite inflammation and did not require immunosuppressive drugs. In fact, they go on to speculate that these residual elements from the donor tissue may even provide helpful signals to host cells49. Lastly, the impact of xenogeneic matrix in the field of lung transplantation has not even been addressed as yet. Though many groups are working to decellularize porcine lungs, the immunogenicity of the resulting matrix has not been reported.
Extracellular matrix breakdown is an important contributor to the progression of several lung pathologies97. Bioactive fragments of extracellular matrix, or matrikines, are known to be mediators of inflammation and immune response1. For example, collagen breakdown has been shown to promote neutrophil migration 66. Additionally, exposed native type V collagen - a “sequestered” collagen that typically does not come into direct contact with airway epithelium - is a major risk factor for bronchioloitis obliterans syndrome 36. Although it is well established in other tissues that “under-decellularizing” tissue can result in detrimental immune reactions, “over-decelluarization” can result in shattered, partially degraded or matrix devoid of components that can induce immune response. One study investigating the impact of pH on decellularized lung determined that high pH (≥10) during decellularization can induce matrix damage that stimulates an inflammatory response in vivo 86. It is very likely that degraded matrix has a larger role in immune and inflammatory response than previously anticipated and should be considered, along with adequacy of cellular removal, in efforts to minimize negative host responses after implantation.
Future directions
Currently, decellularized matrix holds great promise as means to generate lung replacements. In terms of creating readily available, patient-specific lung equivalents, the current limitation in terms of scaffold production include assessing the feasibility of xenographic sources, determining which cues from the “matrix footprint” are critical in cell adhesion and viability, and the careful characterization of host responses to matrix constituents and residual cell debris. Future studies should also include determining which pulmonary cell types are required to restore tissue functionality, how to reintroduce these cells into their proper location through seeding methods or induced migration, and how to acquire these patient-specific cells.
Acknowledgments
Acknowledgements and support
This work was supported by a grant NIH U01 HL111016-01 (to LEN). L.E.N. has a financial interest in Humacyte, Inc, a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
References
- 1.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–10. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alford AI, Rannels DE. Extracellular matrix fibronectin alters connexin43 expression by alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L680–8. doi: 10.1152/ajplung.2001.280.4.L680. [DOI] [PubMed] [Google Scholar]
- 3.Badylak SF. An Assay to Quantify Chemotactic Properties of Degradation Products from Extracellular Matrix. Methods Mol Biol. 2013 doi: 10.1007/7651_2013_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 2012;4:410–21. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 5.Bateman ED, Turner-Warwick M, Adelmann-Grill BC. Immunohistochemical study of collagen types in human foetal lung and fibrotic lung disease. Thorax. 1981;36:645–53. doi: 10.1136/thx.36.9.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bensusan HB, Koh TL, Henry KG, Murray BA, Culp LA. Evidence that fibronectin is the collagen receptor on platelet membranes. Proc Natl Acad Sci U S A. 1978;75:5864–8. doi: 10.1073/pnas.75.12.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, et al. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A. 2012;18:2437–52. doi: 10.1089/ten.tea.2011.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–76. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–16. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 10.Bottaro DP, Liebmann-Vinson A, Heidaran MA. Molecular signaling in bioengineered tissue microenvironments. Ann N Y Acad Sci. 2002;961:143–53. doi: 10.1111/j.1749-6632.2002.tb03068.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res. 2013 doi: 10.1016/j.trsl.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnett W, Yoon K, Finnigan-Bunick A, Rosenbloom J. Control of elastin synthesis. J Invest Dermatol. 1982;79 (Suppl 1):138s–45s. doi: 10.1111/1523-1747.ep12546035. [DOI] [PubMed] [Google Scholar]
- 13.Calle EA, Ghaedi M, Sundaram S, Sivarapatna A, Tseng MK, et al. Strategies for whole lung tissue engineering. IEEE Trans Biomed Eng. 2014;61:1482–96. doi: 10.1109/TBME.2014.2314261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark RA. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci. 1993;306:42–8. doi: 10.1097/00000441-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Clark RA, Mason RJ, Folkvord JM, McDonald JA. Fibronectin mediates adherence of rat alveolar type II epithelial cells via the fibroblastic cell-attachment domain. J Clin Invest. 1986;77:1831–40. doi: 10.1172/JCI112509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark RA, Nielsen LD, Welch MP, McPherson JM. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. J Cell Sci. 1995;108:1251–61. doi: 10.1242/jcs.108.3.1251. [DOI] [PubMed] [Google Scholar]
- 17.Coraux C, Delplanque A, Hinnrasky J, Peault B, Puchelle E, et al. Distribution of integrins during human fetal lung development. J Histochem Cytochem. 1998;46:803–10. doi: 10.1177/002215549804600703. [DOI] [PubMed] [Google Scholar]
- 18.Coraux C, Meneguzzi G, Rousselle P, Puchelle E, Gaillard D. Distribution of laminin 5, integrin receptors, and branching morphogenesis during human fetal lung development. Dev Dyn. 2002;225:176–85. doi: 10.1002/dvdy.10147. [DOI] [PubMed] [Google Scholar]
- 19.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;126:332–7. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- 20.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2012;32:3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, et al. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One. 2010;5:e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunsmore SE, Rannels DE. Extracellular matrix biology in the lung. Am J Physiol. 1996;270:L3–27. doi: 10.1152/ajplung.1996.270.1.L3. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak HF, Harvey VS, Estrella P, Brown LF, McDonagh J, et al. Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest. 1987;57:673–86. [PubMed] [Google Scholar]
- 24.Eisenberg JL, Safi A, Wei X, Espinosa HD, Budinger GS, et al. Substrate stiffness regulates extracellular matrix deposition by alveolar epithelial cells. Res Rep Biol. 2012;2011:1–12. doi: 10.2147/RRB.S13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 26.Engvall E, Ruoslahti E, Miller EJ. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978;147:1584–95. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faulk DM, Carruthers CA, Warner HJ, Kramer CR, Reing JE, et al. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater. 2014;10:183–93. doi: 10.1016/j.actbio.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frith JE, Mills RJ, Hudson JE, Cooper-White JJ. Tailored integrin-extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem Cells Dev. 2012;21:2442–56. doi: 10.1089/scd.2011.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. 2. Springer Verlag; New York: 1993. [Google Scholar]
- 30.Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152:135–9. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillery P, Maquart FX, Borel JP. Fibronectin dependence of the contraction of collagen lattices by human skin fibroblasts. Exp Cell Res. 1986;167:29–37. doi: 10.1016/0014-4827(86)90201-6. [DOI] [PubMed] [Google Scholar]
- 32.Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, et al. Perfusion decellularization of human and porcine lungs: Bringing the matrix to clinical scale. J Heart Lung Transplant. 2014 doi: 10.1016/j.healun.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Govignon EJ, Murphy M, Potzka J, Crews J, Billiar KL, et al. Development of a serum-free human cell derived extracellular matrix (ECM) European Tissue Repair Society; Brussels: 2000. [Google Scholar]
- 34.Horwitz AL, Crystal RC. Content and synthesis of glycosaminoglycans in the developing lung. J Clin Invest. 1975;56:1312–8. doi: 10.1172/JCI108207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989;109:317–30. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr, Chiyo M, et al. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181:5738–47. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson TD, Dequach JA, Gaetani R, Ungerleider J, Elhag D, et al. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater Sci. 2014;2014:60283D. doi: 10.1039/C3BM60283D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771–81. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 39.Kelley J, Chrin L, Coflesky JT, Evans JN. Localization of collagen in the rat lung: biochemical quantitation of types I and III collagen in small airways, vessels, and parenchyma. Lung. 1989;167:313–22. doi: 10.1007/BF02714960. [DOI] [PubMed] [Google Scholar]
- 40.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–78. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 41.Laurent GJ. Lung collagen: more than scaffolding. Thorax. 1986;41:418–28. doi: 10.1136/thx.41.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lesur O, Arsalane K, Lane D. Lung alveolar epithelial cell migration in vitro: modulators and regulation processes. Am J Physiol. 1996;270:L311–9. doi: 10.1152/ajplung.1996.270.3.L311. [DOI] [PubMed] [Google Scholar]
- 43.Li ZY, Hirayoshi K, Suzuki Y. Expression of N-deacetylase/sulfotransferase and 3-O-sulfotransferase in rat alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L292–301. doi: 10.1152/ajplung.2000.279.2.L292. [DOI] [PubMed] [Google Scholar]
- 44.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu F, Tschumperlin DJ. Micro-mechanical characterization of lung tissue using atomic force microscopy. J Vis Exp. 2011 doi: 10.3791/2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lotze MT, Deisseroth A, Rubartelli A. Damage associated molecular pattern molecules. Clin Immunol. 2007;124:1–4. doi: 10.1016/j.clim.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luque T, Melo E, Garreta E, Cortiella J, Nichols J, et al. Local micromechanical properties of decellularized lung scaffolds measured with atomic force microscopy. Acta Biomater. 2013;9:6852–9. doi: 10.1016/j.actbio.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 49.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 50.Mackay EH, Banks J, Sykes B, Lee G. Structural basis for the changing physical properties of human pulmonary vessels with age. Thorax. 1978;33:335–44. doi: 10.1136/thx.33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madri JA, Furthmayr H. Collagen polymorphism in the lung. An immunochemical study of pulmonary fibrosis. Hum Pathol. 1980;11:353–66. doi: 10.1016/s0046-8177(80)80031-1. [DOI] [PubMed] [Google Scholar]
- 52.Martin-Bermudo MD. Integrins modulate the Egfr signaling pathway to regulate tendon cell differentiation in the Drosophila embryo. Development. 2000;127:2607–15. doi: 10.1242/dev.127.12.2607. [DOI] [PubMed] [Google Scholar]
- 53.Mays PK, McAnulty RJ, Campa JS, Laurent GJ. Age-related changes in collagen synthesis and degradation in rat tissues. Importance of degradation of newly synthesized collagen in regulating collagen production. Biochem J. 1991;276 (Pt 2):307–13. doi: 10.1042/bj2760307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mercer RR, Crapo JD. Spatial distribution of collagen and elastin fibers in the lungs. J Appl Physiol (1985) 1990;69:756–65. doi: 10.1152/jappl.1990.69.2.756. [DOI] [PubMed] [Google Scholar]
- 55.Mercer RR, Russell ML, Crapo JD. Alveolar septal structure in different species. J Appl Physiol (1985) 1994;77:1060–6. doi: 10.1152/jappl.1994.77.3.1060. [DOI] [PubMed] [Google Scholar]
- 56.Mih JD, Sharif AS, Liu F, Marinkovic A, Symer MM, et al. A multiwell platform for studying stiffness-dependent cell biology. PLoS One. 6:e19929. doi: 10.1371/journal.pone.0019929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, et al. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel alpha3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray JC, Liotta L, Rennard SI, Martin GR. Adhesion characteristics of murine metastatic and nonmetastatic tumor cells in vitro. Cancer Res. 1980;40:347–51. [PubMed] [Google Scholar]
- 59.Nguyen NM, Kelley DG, Schlueter JA, Meyer MJ, Senior RM, et al. Epithelial laminin alpha5 is necessary for distal epithelial cell maturation, VEGF production, and alveolization in the developing murine lung. Dev Biol. 2005;282:111–25. doi: 10.1016/j.ydbio.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 60.Nichols JE, Niles J, Riddle M, Vargas G, Schilagard T, et al. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng Part A. 2013;19:2045–62. doi: 10.1089/ten.tea.2012.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Neill JD, Anfang R, Anandappa A, Costa J, Javidfar J, et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg. 2013;96:1046–55. doi: 10.1016/j.athoracsur.2013.04.022. discussion 55–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Otranto M, Sarrazy V, Bonte F, Hinz B, Gabbiani G, et al. The role of the myofibroblast in tumor stroma remodeling. Cell Adh Migr. 6:203–19. doi: 10.4161/cam.20377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papakonstantinou E, Karakiulakis G. The ‘sweet’ and ‘bitter’ involvement of glycosaminoglycans in lung diseases: pharmacotherapeutic relevance. Br J Pharmacol. 2009;157:1111–27. doi: 10.1111/j.1476-5381.2009.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petersen TH, Calle EA, Colehour MB, Niklason LE. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs. 2012;195:222–31. doi: 10.1159/000324896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfister RR, Haddox JL, Lam KW, Lank KM. Preliminary characterization of a polymorphonuclear leukocyte stimulant isolated from alkali-treated collagen. Invest Ophthalmol Vis Sci. 1988;29:955–62. [PubMed] [Google Scholar]
- 67.Pierce JA. Age related changes in the fibrous proteins of the lungs. Arch Environ Health. 1963;6:50–4. doi: 10.1080/00039896.1963.10663359. [DOI] [PubMed] [Google Scholar]
- 68.Pierce JA, Hocott JB. Studies on the collagen and elastin content of the human lung. J Clin Invest. 1960;39:8–14. doi: 10.1172/JCI104030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a Decellularized Lung Bioreactor System for Bioengineering the Lung: The Matrix Reloaded. Tissue Eng Part A. 2010;16:2581–91. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rieder E, Seebacher G, Kasimir MT, Eichmair E, Winter B, et al. Tissue engineering of heart valves: decellularized porcine and human valve scaffolds differ importantly in residual potential to attract monocytic cells. Circulation. 2005;111:2792–7. doi: 10.1161/CIRCULATIONAHA.104.473629. [DOI] [PubMed] [Google Scholar]
- 71.Sannes PL. Differences in basement membrane-associated microdomains of type I and type II pneumocytes in the rat and rabbit lung. J Histochem Cytochem. 1984;32:827–33. doi: 10.1177/32.8.6747274. [DOI] [PubMed] [Google Scholar]
- 72.Sawai T, Usui N, Sando K, Fukui Y, Kamata S, et al. Hyaluronic acid of wound fluid in adult and fetal rabbits. J Pediatr Surg. 1997;32:41–3. doi: 10.1016/s0022-3468(97)90089-0. [DOI] [PubMed] [Google Scholar]
- 73.Schor SL. Cell proliferation and migration on collagen substrata in vitro. J Cell Sci. 1980;41:159–75. doi: 10.1242/jcs.41.1.159. [DOI] [PubMed] [Google Scholar]
- 74.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–81. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–34. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sokocevic D, Bonenfant NR, Wagner DE, Borg ZD, Lathrop MJ, et al. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials. 2013;34:3256–69. doi: 10.1016/j.biomaterials.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sorokin LM, Pausch F, Frieser M, Kroger S, Ohage E, et al. Developmental regulation of the laminin alpha5 chain suggests a role in epithelial and endothelial cell maturation. Dev Biol. 1997;189:285–300. doi: 10.1006/dbio.1997.8668. [DOI] [PubMed] [Google Scholar]
- 78.Soucy PA, Romer LH. Endothelial cell adhesion, signaling, and morphogenesis in fibroblast-derived matrix. Matrix Biol. 2009;28:273–83. doi: 10.1016/j.matbio.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 79.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 80.Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6:235–43. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- 81.Sugihara T, Martin CJ, Hildebrandt J. Length-tension properties of alveolar wall in man. J Appl Physiol. 1971;30:874–8. doi: 10.1152/jappl.1971.30.6.874. [DOI] [PubMed] [Google Scholar]
- 82.Suki B. Assessing the functional mechanical properties of bioengineered organs with emphasis on the lung. J Cell Physiol. 2014;229:1134–40. doi: 10.1002/jcp.24600. [DOI] [PubMed] [Google Scholar]
- 83.Suki B, Stamenovic D, Hubmayr R. Lung parenchymal mechanics. Compr Physiol. 2011;1:1317–51. doi: 10.1002/cphy.c100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tee SY, Fu J, Chen CS, Janmey PA. Cell shape and substrate rigidity both regulate cell stiffness. Biophys J. 2011;100:L25–7. doi: 10.1016/j.bpj.2010.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toworfe GK, Composto RJ, Adams CS, Shapiro IM, Ducheyne P. Fibronectin adsorption on surface-activated poly(dimethylsiloxane) and its effect on cellular function. J Biomed Mater Res. 2004;71A:449–61. doi: 10.1002/jbm.a.30164. [DOI] [PubMed] [Google Scholar]
- 86.Tsuchiya T, Balestrini JL, Mendez J, Calle EA, Zhao L, et al. Influence of pH on Extracellular Matrix Preservation During Lung Decellularization. Tissue Eng Part C Methods. 2014 doi: 10.1089/ten.tec.2013.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsukahara H, Noiri E, Jiang MZ, Hiraoka M, Mayumi M. Role of nitric oxide in human pulmonary microvascular endothelial cell adhesion. Life Sci. 2000;67:1–11. doi: 10.1016/s0024-3205(00)00598-1. [DOI] [PubMed] [Google Scholar]
- 88.Tuan TL, Grinnell F. Fibronectin and fibrinolysis are not required for fibrin gel contraction by human skin fibroblasts. J Cell Physiol. 1989;140:577–83. doi: 10.1002/jcp.1041400324. [DOI] [PubMed] [Google Scholar]
- 89.van Kuppevelt TH, Cremers FP, Domen JG, van Beuningen HM, van den Brule AJ, et al. Ultrastructural localization and characterization of proteoglycans in human lung alveoli. Eur J Cell Biol. 1985;36:74–80. [PubMed] [Google Scholar]
- 90.Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29:690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 91.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–89. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wagner DE, Bonenfant NR, Parsons CS, Sokocevic D, Brooks EM, et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials. 2014;35:3281–97. doi: 10.1016/j.biomaterials.2013.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods. 2012;18:420–32. doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang HJ, Pieper J, Schotel R, van Blitterswijk CA, Lamme EN. Stimulation of skin repair is dependent on fibroblast source and presence of extracellular matrix. Tissue Eng. 2004;10:1054–64. doi: 10.1089/ten.2004.10.1054. [DOI] [PubMed] [Google Scholar]
- 95.Yuan H, Kononov S, Cavalcante FS, Lutchen KR, Ingenito EP, et al. Effects of collagenase and elastase on the mechanical properties of lung tissue strips. J Appl Physiol. 2000;89:3–14. doi: 10.1152/jappl.2000.89.1.3. [DOI] [PubMed] [Google Scholar]
- 96.Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, et al. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61–7. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 97.Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, et al. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61–7. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]


