Abstract
Accumulating evidence indicates that the immune system plays a critical role in the pathogenesis of cardiovascular diseases including hypertension. Mice lacking T lymphocytes are resistant to blood pressure elevation, suggesting a key contribution of T lymphocytes to hypertension. However, the individual T cell subsets, including CD8+, Th1, Th17, and T regulatory T cells have shown widely discrepant effects on blood pressure and target organ damage in this disorder. Moreover, the activation state of a T lymphocyte population exerts considerable influence over its role in hypertension. In turn, activated T cells regulate blood pressure through the elaboration of reactive oxygen species and vasoactive cytokines, altering the inflammatory milieu in the vascular wall and the kidney. Recent GWAS studies similarly point to a role for T lymphocytes in human hypertension.
Introduction
Hypertension is among the most prevalent chronic diseases, impacting approximately one-third of the adult population worldwide [1]. The complications of uncontrolled hypertension, including stroke, congestive heart failure, and chronic kidney disease, are associated with substantial morbidity and mortality. Despite enormous progress in hypertension research, the precise etiology of blood pressure elevation remains unknown in the vast majority of hypertensive patients. While dysfunction in cardiovascular control centers including the kidney, the vasculature, and brain can coordinately contribute to sustained hypertension, a role for immune dysregulation to potentiate hypertension has received intense scrutiny in recent years. Macrophages and T cells infiltrate in the heart, the vasculature, and the kidney during hypertension [2–4]. Enhanced expression of adhesion molecules on the blood vessels in these organs contributes to inflammatory cell accumulation by permitting increased leukocyte extravasation [4]. In turn, these infiltrating mononuclear cells secrete or induce several pro-hypertensive cytokines including IL-6, IL-17, and TNF-α [5,6]. Recently, Guzik and Harrison established a role for T lymphocytes in promoting blood pressure elevation in seminal adoptive transfer studies [7]. These experiments laid the groundwork for several advances elucidating the mechanisms through which innate and adaptive immunity participate in the pathogenesis of hypertension.
T lymphocyte subsets
Diverse subsets of T lymphocytes influence blood pressure through effects on the local cytokine milieu within cardiovascular control organs. Naive T cells originate from hematopoietic stem cells in the bone marrow and mature in the thymus before migrating to peripheral tissues. Based on the expression of key cell surface markers, T lymphocytes can be categorized into different subsets with distinct functions [8]. In simplified terms, CD4 single positive T cells are recognized as T helper cells (Th cells), CD8 single positive T cells are considered cytotoxic T cells, and CD1d positive T cells are recognized as natural killer T cells. Moreover, once an antigen is presented in the context of a major histocompatibility complex (MHC) to the T cell receptor (TCR) on the naive CD4+ T cell, the T cell polarizes toward a Th1, Th2, Th17, or T regulatory (Treg)) cell phenotype depending the local concentrations specific cytokines. For example, Th1 commitment is triggered by IL-12, the Th2 subset is induced by IL-4, Th17 differentiation requires IL-6 and IL-23, and Treg cells, characterized by unique immunosuppressive activity, emerge in response to TGF-β1 [9]. These T cell subsets then produce their own cocktails of cytokines to regulate surrounding inflammatory cells in a paracrine fashion and modulate both vascular reactivity and renal sodium handling.
T lymphocytes regulate hypertension
Prior to the emergence of sophisticated molecular methods, biopsy studies revealed that T cells figure prominently in the interstitium of the kidneys in patients with severe hypertension [3]. However, the notion that T cells contribute to hypertension gained broad acceptance when Guzik et al showed that Rag1 knockout mice lacking lymphocytes were resistant to Angiotensin II or DOCA-salt induced hypertension and vascular dysfunction and that adoptive transfer of T cells but not B cells could restore the hypertensive response [7]. Other groups including our own then confirmed this finding in severe combined immunodeficiency mice or Rag1 knockout rats, respectively, and found that functional T cells potentiate the renal sodium retention and intravascular volume expansion that underpin sustained blood pressure elevation [10,11].
Subsequent studies have described the individual roles of the different T cell subsets in hypertension. For example, our group found that mice unable to mount a Th1 response due to deficiency of the transcription factor, T-bet, had preserved hypertensive responses but were protected from renal damage during chronic angiotensin II stimulation [12]. These studies suggested that Th1 cells augment hypertensive kidney injury through blood pressure-independent mechanisms. As Th1 cells are a potent source of IFN-γ, the preserved hypertensive response in T-bet-deficient mice is consistent with the normal hypertensive response of angiotensin II-infused IFN-γ or IFN-γ receptor deficient mice [13,14].
Th17 cells secrete the proinflammatory cytokine IL-17 and have been implicated in the pathogenesis of several autoimmune diseases [15]. Madhur and colleagues found that IL-17 deficient mice had blunted hypertensive responses to chronic angiotensin II infusion, suggesting that Th17 cells promote blood pressure elevation [16]. By contrast, administration of neutralizing antibodies against IL-17 or IL-23, a cytokine that promotes and sustains Th17 differentiation, were unable to influence blood pressure in the angiotensin II infusion model [14]. Moreover, in the DOCA/salt model of hypertension, IL-17- or IL-23-deficiency led to exaggerated renal injury, pointing to a protective role for Th17 cells [17]. Collectively, these disparate findings indicate that the actions of Th17 cells in hypertension, just as in atherosclerosis [18,19], may vary depending on the characteristics of the selected model or even the stage of disease at which Th17 cells are studied.
In contrast to pro-inflammatory Th1 and Th17 cells, Treg cells act to suppress cellular immune responses. Accordingly, Treg cells suppress atherosclerosis [20], and adoptive transfer of Treg cells into hypertensive mice dramatically attenuates the extent of cardiac hypertrophy [21]. Similarly, Schiffrin and colleagues demonstrated that CD4+CD25+ Treg ameliorate vascular dysfunction and limit blood pressure elevation in response to angiotensin II or aldosterone [22,23]. IL-10 is a key effector cytokine produced by Treg cells. Consistent with an immunosuppressive function, endogenous IL-10 limits angiotensin II-mediated oxidative stress and vascular dysfunction through a blood pressure-independent mechanism [24], whereas administration of exogenous IL-10 normalized blood pressure and endothelial function in a rodent model of pregnancy-induced hypertension [25]. Thus, as in other cardiovascular diseases, T regulatory cells serve a protective function in hypertension mediated in part through the actions of IL-10.
The precise role of CD8+ T Lymphocytes in regulating hypertension still requires further elucidation. Mice lacking the transcription factor inhibitor of differentiation (Id2) have reduced natural killer cells and altered CD8+ T cell memory and were protected from angiotensin II-induced hypertension. However, bone marrow transplantation of Id2-expressing bone marrow cells into Id2-deficient mice could not restore the hypertensive response, suggesting that impaired development of CD8+ T memory cells cannot fully account for the blood pressure phenotype in this model [26]. On the other hand, CD8-deficient mice have a blunted hypertensive response, and adoptive transfer of CD8 but not CD4+ T cells into Rag1-deficient animals recapitulates a normal blood pressure increase during chronic angiotensin II infusion [27]. These latter data clearly implicate CD8+ T cells in the pathogenesis of hypertension but still allow that CD4+ T cells could influence blood pressure through effects on other inflammatory cell populations. In sum, we are just beginning to understand the divergent roles that various T lymphocyte subsets play in blood pressure regulation. Additional clarity will emerge as increasing numbers of laboratories employ complementary models to interrogate the actions of T cells in hypertension.
Activation of T lymphocytes in hypertension
While the participation of T lymphocytes in the hypertensive response would indicate that blood pressure is in part an antigen-driven, autoimmune process, the mechanisms through which T cells undergo activation in the setting of hypertension remain an intense area of investigation. Complete and specific activation of the T cell requires not only TCR ligation, referred to “Signal 1”, but also a co-stimulatory interaction between CD28 on the T cells and B7 ligands on the antigen presenting cell, defined as “Signal 2”. Vinh et al demonstrated that blocking these co-stimulatory interactions with an inhibitor or via genetic deficiency of the B7 ligands attenuated hypertension and limited perivascular infiltration of T cells [28]. Similarly, disrupting Signal 1 through the mutation of a TCR signaling molecule blunted the hypertensive response to a salt-loading diet in the Dahl salt-sensitive rat model [29]. These experiments indicate that Signals 1 and 2 in T cell activation are both necessary for the induction of hypertension.
Initial elevations of blood pressure appear to be an initiating factor for triggering T cell activation. For example, induction of hypertension draws inflammatory cells into the kidney that promote salt-sensitivity even once the hypertensive stimulus is removed [30]. More recently, Marvar found that preventing blood pressure elevation in angiotensin II-infused mice by ablating sympathetic outflow from the CNS or by treating with hydralazine also abrogated T cell activation and perivascular infiltration [31]. Activation of T cells classically requires stimulation of the TCR by an antigen presenting cell, the most potent of which is the dendritic cell. In this regard, induction of oxidative stress in these dendritic cells by highly reactive gamma-ketoaldehydes (isoketals) enhances their capacity to activate T cells, which in turn augment susceptibility to hypertension upon adoptive transfer [32]. This important discovery raises the possibility that the neo-antigen that awakens the adaptive immune response during hypertension may be an isoketal-modified self-antigen. A high salt diet may similarly contribute to the development of hypertension not only by driving intravascular volume expansion, but also by favoring Th17 differentiation through MAPK signal pathway or inducible salt-sensing kinase SGK1 [33,34]. Collectively these studies emphasize the key role of T cell activation in hypertension. Future studies will be critical to translate this understanding into precise immunomodulatory interventions that abrogate immunity’s contribution to hypertension without prohibitively impairing host defenses against invading pathogens.
Mechanisms underlying T lymphocytes’ promotion of hypertension
While global immunosuppression of T lymphocytes may be far too impractical to control hypertension, specifically targeting a few key components regulating the T cell’s contribution to blood pressure elevation still holds promise. Towards this end, several experiments have been conducted to interrogate the mechanisms through which T lymphocytes promote hypertension.
Activation of T lymphocytes potentiates hypertension through the induction of reactive oxygen species and cytokines. The NADPH oxidase subunit p47phox on the T cells contributes to angiotensin II-mediated hypertensive responses [7]. Endogenous production of angiotensin II in T cells directly regulates its production of superoxide, that in turn enhances elaboration of TNF-α [35], and TNF-deficient mice have blunted hypertensive responses to several stimuli, suggesting a critical role for this cytokine in the development of hypertension [13,36]. Of note, somatic tissues including cardiovascular control organs also synthesis pro-hypertensive cytokines, and these non-T cell-derived cytokines also have the capacity to augment blood pressure elevation [13].
T lymphocytes infiltrate several cardiovascular control organs during hypertension including the vasculature and the kidney. The adventitia of the aorta is a major site of T cell accumulation in the setting of hypertension. This T lymphocyte infiltration is associated with exaggerated aortic stiffness and endothelial dysfunction related to enhanced collagen deposition [7]. T lymphocytes also infiltrate the kidney during hypertension, particularly around the renal blood vessels [4,37]. Our group found that lymphocyte-deficient scid mice were protected from hypertension by permitting pressure-induced sodium excretion, possibly via an eNOS- and COX-2-dependent pathway [10]. More recently, Trott et al demonstrated that CD8-deficient mice are similarly resistant to acute sodium retention during chronic angiotensin II infusion [27].
Thus, T lymphocytes play a unique role in the pathogenesis of essential hypertension by integrating regulatory mechanisms across multiple cardiovascular control centers. However, determining whether infiltrating T cells cause vascular or renal injury or whether, conversely, hemodynamic injury in these organs recruits T cells has been difficult to separate. A contribution of both mechanisms seems likely as hemodynamic injury triggers inflammatory signaling cascades that, in turn, accelerate organ dysfunction.
T lymphocytes in human hypertension
In contrast to the large body of evidence supporting a role for T lymphocytes in experimental hypertension, the contribution of T cells to the pathogenesis of human hypertension requires further substantiation. However, testing causality in human studies poses a challenge, particularly given the heterogeneity of the disease. As mentioned above, early studies confirmed the infiltration of T lymphocytes into the kidneys of patients with essential hypertension [3]. In the circulation, patients with hypertension showed an increased fraction of immunosenescent CD8+ T cells and enhanced expression of the chemokine CXCR3 that recruits T cells into injured organs [38]. Hypertensive patients also have increased circulating levels cytokines secreted by T cells, including TNF-α, IL-6, IL-4, and IFN-γ-inducible protein [39–41]. Moreover, patients with active systemic lupus erythematosus but without renal impairment have an increased frequency of high blood pressure compared with patients with inactive lupus and controls, suggesting a role for inflammatory responses to promote hypertension in those patients [42]. Inversely, hypertensive patients with autoimmune diseases who received treatments that suppress lymphocyte proliferation showed improvements in blood pressure control, bolstering the notion that targeting the immune system may present a novel approach for antihypertensive therapy [43].
Large genome-wide association studies (GWAS) in humans have also demonstrated links between hypertension and variants of genes expressed in T lymphocytes. For example, one GWAS study identified a variant in CD247 that encodes the CD3ζ chain, which associated with levels of blood pressure in over 2,000 hypertensive African and European American subjects [44]. Moreover, the Global Blood Pressure Genetics consortium analyzed GWAS data from over 30,000 subjects of European ancestry, and found that the immunoreceptor signaling molecule SH2B3 (also known as lymphocyte-specific adapter protein, LNK), had a missense SNP that segregated with levels of diastolic blood pressure [45]. Finally, the most recent GWAS report revealed an association between hypertension and alleles of HLA-DQB1 and NFAT5 in a cohort of nearly 100,000 individuals [46]. These GWAS studies further support the hypothesis that T lymphocytes and the immune system contribute to the pathogenesis of human hypertension.
Summary
In summary, increasing evidence points to a prominent role for T cells in hypertension. However, several outstanding questions remain unanswered. What are the actual neo-antigens recognized by T lymphocytes in hypertension? How can novel targets in the immune system identified through experimental studies be safely translated into therapies for human patients with recalcitrant hypertension? With the scientific community confronting these questions, future studies will likely reveal a unique set of pharmacologic interventions to reduce blood pressure in patients with severe hypertension that has so far been resistant to existing therapies.
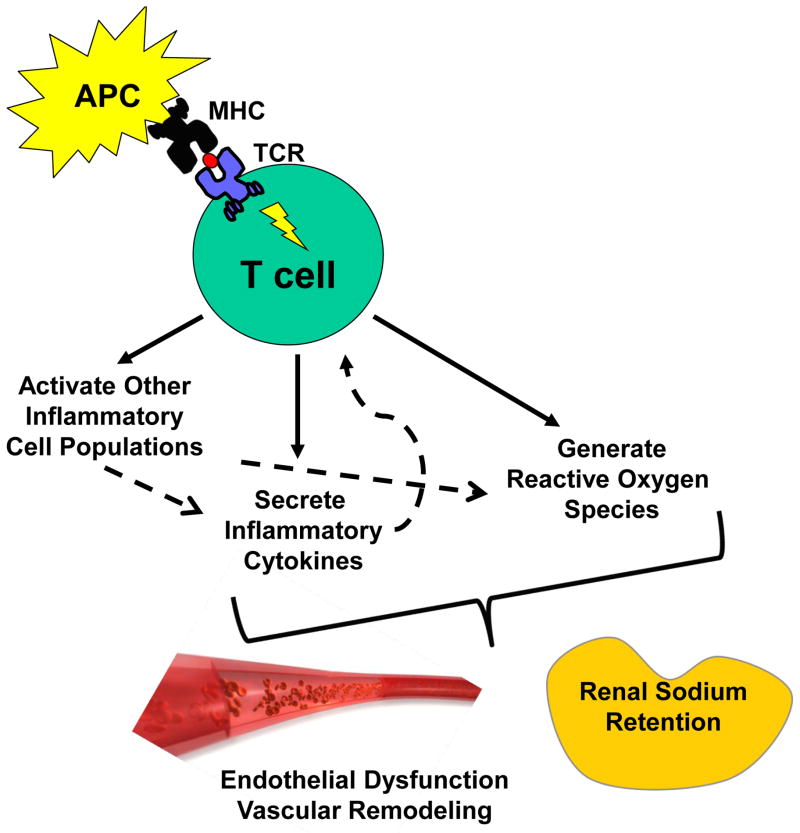
Figure 1. The role of T lymphocytes in hypertension.
A neo-antigen presented on the surface of an antigen presenting cell (APC) in the context of a major histocompatibility complex (MHC) to the T cell receptor (TCR) on the surface of the T lymphocyte triggers (a) secretion of inflammatory cytokines, (b) generation of reactive oxygen species (ROS), and (c) activation of surrounding myeloid and lymphocyte populations that generate additional ROS and make their own contribution to the cytokine milieu. These cytokines and ROS, in turn, modulate blood pressure through effects on vascular function and renal sodium handling.
Highlights.
Mice lacking functional T lymphocytes are protected from hypertension.
Th17 cells augment whereas Treg cells limit blood pressure elevation.
T cell activation by dendritic cells enhances the chronic hypertensive response.
T cells raise blood pressure by causing vascular dysfunction and sodium retention.
Acknowledgments
This work was supported by funding from (1) National Institutes of Health Grant DK087893-01, (2) the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development, Grant BX000893-01A2, (3) the Edna and Fred L. Mandel Center for Hypertension and Atherosclerosis Research, and (4) a Grant-in-Aid and Postdoctoral Fellowship from the American Heart Association (12POST11910012).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 3.Heptinstall RH. Renal biopsies in hypertension. Br Heart J. 1954;16:133–141. doi: 10.1136/hrt.16.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, et al. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 6.Ates I, Ozkayar N, Akyel F, Topcuoglu C, Akyel S, Barca AN, Dede F. The relationship between asymptomatic organ damage, and serum soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK) and Interleukin-17A (IL-17A) levels in non-diabetic hypertensive patients. BMC Nephrol. 2014;15:159. doi: 10.1186/1471-2369-15-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strom TB, Koulmanda M. Recently discovered T cell subsets cannot keep their commitments. J Am Soc Nephrol. 2009;20:1677–1680. doi: 10.1681/ASN.2008101027. [DOI] [PubMed] [Google Scholar]
- 10.Crowley SD, Song Y-S, Lin EE, Griffiths R, Kim H-S, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1089–1097. doi: 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol. 2013;304:R407–414. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JD, Patel MB, Song YS, Griffiths R, Burchette J, Ruiz P, Sparks MA, Yan M, Howell DN, Gomez JA, et al. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ Res. 2012;110:1604–1617. doi: 10.1161/CIRCRESAHA.111.261768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, et al. Tumor Necrosis Factor-alpha Produced in the Kidney Contributes to Angiotensin II-dependent Hypertension. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.03863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marko L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, et al. Interferon-gamma signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension. 2012;60:1430–1436. doi: 10.1161/HYPERTENSIONAHA.112.199265. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, O’Shea JJ. Regulation of IL-17 production in human lymphocytes. Cytokine. 2008;41:71–78. doi: 10.1016/j.cyto.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krebs CF, Lange S, Niemann G, Rosendahl A, Lehners A, Meyer-Schwesinger C, Stahl RA, Benndorf RA, Velden J, Paust HJ, et al. Deficiency of the interleukin 17/23 axis accelerates renal injury in mice with deoxycorticosterone acetate+angiotensin ii-induced hypertension. Hypertension. 2014;63:565–571. doi: 10.1161/HYPERTENSIONAHA.113.02620. [DOI] [PubMed] [Google Scholar]
- 18.Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, Kanayama M, Kurotaki D, Morimoto J, Iwakura Y, Yagita H, et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:273–280. doi: 10.1161/ATVBAHA.111.229997. [DOI] [PubMed] [Google Scholar]
- 19.Ng HP, Burris RL, Nagarajan S. Attenuated atherosclerotic lesions in apoE-Fcgamma-chain-deficient hyperlipidemic mouse model is associated with inhibition of Th17 cells and promotion of regulatory T cells. J Immunol. 2011;187:6082–6093. doi: 10.4049/jimmunol.1004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 21.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 22.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 23.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59:324–330. doi: 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]
- 24.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous Interleukin-10 Inhibits Angiotensin II-Induced Vascular Dysfunction. Hypertension. 2009;54:619–624. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R713–719. doi: 10.1152/ajpregu.00712.2009. [DOI] [PubMed] [Google Scholar]
- 26.Gratze P, Dechend R, Stocker C, Park JK, Feldt S, Shagdarsuren E, Wellner M, Gueler F, Rong S, Gross V, et al. Novel role for inhibitor of differentiation 2 in the genesis of angiotensin II-induced hypertension. Circulation. 2008;117:2645–2656. doi: 10.1161/CIRCULATIONAHA.107.760116. [DOI] [PubMed] [Google Scholar]
- 27.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, et al. Oligoclonal CD8+ T Cells Play a Critical Role in the Development of Hypertension. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL, PhysGen Knockout P. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension. 2014;63:559–564. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lombardi D, Gordon KL, Polinsky P, Suga S, Schwartz SM, Johnson RJ. Salt-sensitive hypertension develops after short-term exposure to Angiotensin II. Hypertension. 1999;33:1013–1019. doi: 10.1161/01.hyp.33.4.1013. [DOI] [PubMed] [Google Scholar]
- 31.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and Peripheral Mechanisms of T-Lymphocyte Activation and Vascular Inflammation Produced by Angiotensin II-Induced Hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208–216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sriramula S, Haque M, Majid DSA, Francis J. Involvement of Tumor Necrosis Factor-{alpha} in Angiotensin II-Mediated Effects on Salt Appetite, Hypertension, and Cardiac Hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim H-S, et al. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol. 2008;295:F515–524. doi: 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–133. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 39.Stumpf C, Auer C, Yilmaz A, Lewczuk P, Klinghammer L, Schneider M, Daniel WG, Schmieder RE, Garlichs CD. Serum levels of the Th1 chemoattractant interferon-gamma-inducible protein (IP) 10 are elevated in patients with essential hypertension. Hypertens Res. 2011;34:484–488. doi: 10.1038/hr.2010.258. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–1159. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- 41.Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: The ATTICA Study. Am J Hypertens. 2004;17:568–573. doi: 10.1016/j.amjhyper.2004.03.675. [DOI] [PubMed] [Google Scholar]
- 42.Lozovoy MA, Simao AN, Morimoto HK, Iryioda TM, Panis C, Reiche EM, Borelli SD, Oliveira SR, Cecchini R, Dichi I. Hypertension is associated with serologically active disease in patients with systemic lupus erythematosus: role of increased Th1/Th2 ratio and oxidative stress. Scand J Rheumatol. 2014;43:59–62. doi: 10.3109/03009742.2013.834963. [DOI] [PubMed] [Google Scholar]
- 43.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 44.Ehret GB, O’Connor AA, Weder A, Cooper RS, Chakravarti A. Follow-up of a major linkage peak on chromosome 1 reveals suggestive QTLs associated with essential hypertension: GenNet study. Eur J Hum Genet. 2009;17:1650–1657. doi: 10.1038/ejhg.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tragante V, Barnes MR, Ganesh SK, Lanktree MB, Guo W, Franceschini N, Smith EN, Johnson T, Holmes MV, Padmanabhan S, et al. Gene-centric meta-analysis in 87,736 individuals of European ancestry identifies multiple blood-pressure-related loci. Am J Hum Genet. 2014;94:349–360. doi: 10.1016/j.ajhg.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]