Abstract
Significant work has been done towards identifying the health-beneficial effects of the grape antioxidant resveratrol in a variety of bioassay- and disease- models, with much research being focused on its possible application to cancer management. Despite the large number of preclinical studies dealing with different aspects of the biological effects of resveratrol, it’s translation to clinics is far from reality due to a variety of challenges. In this review, we discuss the issues and questions associated with resveratrol becoming an effective in vivo anticancer drug, from basic metabolic issues to the problems faced by incomplete understanding of the mechanism(s) of action in the body. We also explore efforts taken by researchers, both public and private, to contend with some of these issues. By examining the published data and previous clinical trials, we have attempted to identify the problems and issues that hinder the clinical translation of resveratrol for cancer management.
Keywords: Resveratrol, Cancer, Clinical Trials
1. Introduction
Worldwide, cancer is one of the most frequently diagnosed diseases and is a major cause of loss of human life. Because of the intense treatment regimens and/or surgeries necessary to treat this malignancy, a diagnosis of cancer puts a major economic burden on the suffering family as well as on communities and society. Alarmingly, approximately 12.7 million cancer diagnoses and 7.6 million cancer deaths were expected to have occurred in 2008 throughout the world [1]. In the United States alone, 1,665,540 new cases of cancer and 585,720 cancer related deaths are estimated for the year 2014 [2]. Interestingly, there are areas throughout the world where certain cancers are less prominent, which could be attributed to prevailing local dietary habits and/or use of natural agents as medicines/remedies [3, 4]. In the recent past, increasing research efforts have attempted to make use of these observations and advocate the use of natural agents, alone or in combination with traditional therapeutics for cancer management. The grape antioxidant resveratrol (chemically: 3,5,4′-trihydroxystilbene) is one such agent that has been studied at large for its health-promoting effects, as evidenced from the over 6,800 publications available at present on PubMed. Almost one third of these articles have explored the link between resveratrol and cancer, indicating that this natural compound may hold tremendous promise in the field of cancer management.
Resveratrol is a naturally occurring phytoalexin, a substance synthesized de novo by plants, to counteract pathogen infections. In preclinical studies, resveratrol has been shown to enhance vascular health by reducing hypertension and counteracting against heart failure and ischemic heart disease in experimental animal models (reviewed in [5]). Further, there is ample evidence that resveratrol protects against high fat diet-induced obesity, improves insulin sensitivity, lowers serum glucose levels in several animal models, and improves diabetic kidney disease in rodents (reviewed in [5]). Similarly, resveratrol has been shown to have neuroprotective effects in experimental models of cerebral stroke [6]. Studies have also suggested that resveratrol can partially mimic the effects of a calorie restricted diet, which is known to slow the aging process and extend lifespan in diverse species ([7] and reviewed in [8, 9]). Although the exact mechanisms of the health-promoting effects of resveratrol are still being explored, the promising pharmacologic properties of resveratrol have allowed for its entry into the unregulated nutraceutical sector in the form of over the counter nutritional supplement. It is still unclear whether this is a good thing, as the clinical benefits of resveratrol are yet to be realized. Although this interesting compound seems to have potential against a variety of diseases/conditions, one of its most evident health benefits is its ability to elicit chemopreventive as well as therapeutic effects against several cancers [10]. The cancer chemopreventive properties of resveratrol were first discovered in 1997 by Jung and colleagues, when they demonstrated the anti-initiation, anti-promotion, and anti-progression activities of resveratrol in different models [10]. Building on this research, other investigators have shown that resveratrol inhibits tumor growth in vivo against several cancer types, which are dose and duration dependent (reviewed in [11]).
Although in vitro and animal experimental data are extremely promising for resveratrol’s anti-proliferative effects, there is limited development regarding its use in clinical settings. One problem with this translation is the limited bioavailability of resveratrol as it is metabolically eliminated from the body extremely fast, so much so that it is difficult to maintain a therapeutically relevant level in the bloodstream [12, 13]. Recently, we have advocated the use of other natural agents in combination with resveratrol to improve the overall therapeutic effectiveness, especially for cancer management (reviewed in [14]). One example of this is our recent hypothesis that resveratrol, when given in combination with zinc (Zn), may modulate in vivo Zn-homeostasis to enhance the cellular transport of Zn into the prostatic tissue via modulating zinc transporter proteins, thereby enhancing the therapeutic efficacy of Zn against prostate malignancy [15]. Similarly, there are considerable ongoing efforts to try to exploit resveratrol’s potential against cancer via combining it with other compounds/drugs, in order to tackle some of the limitations and to increase the overall therapeutic efficacy.
On the whole, resveratrol has been found to be effective against a number of human cancers in preclinical studies, suggesting that it could be a useful chemotherapeutic agent. A positive property of resveratrol is the fact that it is well tolerated in most patients and appears to have minimal side effects even at very high doses (reviewed in [16]). However, the immense potential that appears to be present in preclinical testing has yet to be realized in human trials. This has been explored in many reviews, including two recent ones that discuss the overall challenges of using resveratrol in humans for multiple conditions [17, 18]. In this review, we are focusing on presenting a critical discussion, including relevant clinical studies, to understand the challenges associated with bringing resveratrol into the clinical realm as an anticancer drug. As outlined in Figure 1, there are specific areas of resveratrol research that need to be extensively explored that may pave the way for efficient translation of resveratrol from the bench to the bedside.
Figure 1. Specific research areas to be prioritized for resveratrol research.
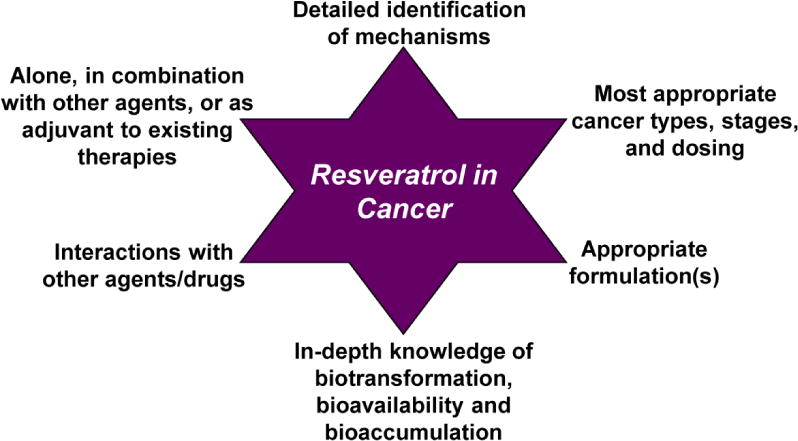
This figure outlines some of the key areas that need to be focused on in order to push resveratrol from being a success in the lab to being an ideal chemopreventive and chemotherapy agent.
2. Bioavailability, absorption and metabolism of resveratrol
The limited bioavailability of resveratrol is perceived as a major hindrance in the potential clinical use of resveratrol for cancer management. In vivo, resveratrol is absorbed through the gastrointestinal tract and is rapidly metabolized to its stable glucuronides, sulfates, and hydroxylates. In healthy humans, resveratrol has been demonstrated to be metabolized to its 3- and 4′-O-sulfate, and 3-O-glucuronide conjugates less than 2 hours after ingestion [19]. Intestinal bacteria also play a role in the metabolism of resveratrol that contributes to a variation of the fractional ratio of metabolites among individuals. Bode and colleagues have shown that resveratrol can be metabolized by human gut microbiota, resulting in dihydroresveratrol, 3,4′-dihydroxy-trans-stilbene and 3,4′-dihydroxybibenzyl [20]. Pharmacokinetic profiles of resveratrol in healthy volunteers displayed rapid and extensive metabolism to resveratrol-4′-O-glucuronide, resveratrol-3′-O-glucuronide, and resveratrol-3-O-sulfate following single or multiple oral doses of resveratrol between 0.5 to 5.0 g each [19, 21]. This does not leave much opportunity for resveratrol to impart its anticancer action, even with a large dose being delivered. Thus, there are ongoing efforts to somehow slow the metabolism of resveratrol to allow for increased tissue exposure in the body. In this direction, we recently proposed some possible scenarios to enhance resveratrol’s bioavailability, such as mechanism-based combinations with natural agents that can inhibit the in vivo metabolism of resveratrol, nanoparticle-mediated delivery, use of naturally occurring or synthetic analogues of resveratrol, and use of conjugated metabolites of resveratrol (reviewed in [22]).
The most direct way to boost the efficacy of resveratrol is to increase the amount of free resveratrol available at the target organ site. For this purpose, using other moieties, preferably naturally occurring agents, to delay the rapid metabolic elimination of resveratrol, may be useful. In this regard, in a study from our laboratory we have shown that piperine, an alkaloid derived from black pepper (Piper spp.) improves in vivo bioavailability of resveratrol in mice by inhibiting its glucuronidation [23]. At present, piperine is being considered as a bioavailability enhancer of resveratrol by several private industries. Some other studies further provide reasons to test this combination in greater detail. Huang et al recently found a synergistic effect of resveratrol and piperine combination on depressive-like behaviors in mice, which may be partly due to the potentiated stimulation of the monoaminergic system in the brain [24]. However, this study did not examine whether or not the addition of piperine affects the bioavailability of resveratrol, as they were focused on determining the mechanism of action. In another recent study, Wightman and colleagues demonstrated that piperine can increase the bioefficacy of resveratrol when co-supplemented in healthy human subjects with regard to cerebral blood flow effects [25]. In this study, co-supplementation of piperine and resveratrol were found to significantly augment cerebral blood flow during task performance without affecting cognitive function, mood or blood pressure. Interestingly, no changes were noticed in resveratrol’s bioavailability. This may be due to the metabolic differences in mice vs. humans, which may impose a big challenge in the translation of animal data to human studies.
Curcumin, a polyphenolic constituent of the popular South Asian spice turmeric, has also been shown to inhibit glucuronidation in mice by Basu and colleagues [26]. Recently, Malhotra and colleagues demonstrated a synergistic chemopreventive response of curcumin and resveratrol combination in a mouse model of lung carcinogenesis [27]. However, this study didn’t assess the effect on the bioavailability of these agents. Similarly, the polyphenol quercetin has been shown to inhibit in vivo sulfation of resveratrol [28]. Thus, a combination of resveratrol with quercetin may enhance the bioavailability of resveratrol by inhibiting both its glucuronidation and its sulfation. Interestingly, resveratrol has a natural association with quercetin as both polyphenols co-exist in red grapes, red wine and several other plants. This combination was evaluated by McAnulty and colleagues who examined it as a countermeasure against oxidative stress and inflammation in response to exercise in athletes [29]. They found that a combination of resveratrol and quercetin significantly reduced exercise-induced lipid peroxidation without associated changes in plasma antioxidant status and inflammation. However, in this study the researchers did not directly measure the serum levels of resveratrol or its metabolites. Therefore, it is difficult to determine whether quercetin inhibited the metabolism of resveratrol, or if there was an additive or combinatorial effect between the two agents. These studies open up future avenues where novel means could be identified to slow down the metabolism of resveratrol. Rapid clinical trials could be undertaken to assess the usefulness of such combinations and/or to eliminate the unsuccessful ones.
3. Resveratrol’s metabolites, derivatives, and reformulations
Because of the rapid metabolism of resveratrol into its metabolites, its undisputed effectiveness as found in several in vitro and in vivo studies leads some researchers to believe that resveratrol metabolites may have their own biological activity that is different from the activity of free resveratrol. Interestingly, there is some indication that resveratrol metabolites, especially resveratrol 3-sulfate could also afford chemopreventive effects [30]. This leads to an interesting research question as to whether resveratrol or its metabolites are the active molecule(s) in vivo. As illustrated in Figure 2, it is possible that the observed anti-proliferative effects of resveratrol may be due to a combination of actions by resveratrol and its metabolites. In fact, recent studies have demonstrated that resveratrol sulfates can provide an intracellular pool from which resveratrol can be freed through metabolic regeneration/deconjugation, ultimately inflicting its anti-proliferative effects in cancer cells [31, 32]. Patel and colleagues performed the pharmacokinetic analysis of a mixture of resveratrol-3-O-sulfate and resveratrol-4′-O-sulfate in mice, which revealed that hydrolysis of sulfate-conjugates generates free resveratrol. Further, monosulfate metabolites were also noticed to be converted to resveratrol in human colorectal cancer cells. This study suggests that resveratrol may be delivered to target tissues in a sulfate-conjugated stable form, from which the parent resveratrol could be steadily regenerated to elicit beneficial effects in vivo [31]. This provides an excellent opportunity that needs to be verified in additional studies, including in human trials. A cartoon depicting the process of regeneration of resveratrol from its conjugates, resulting in its anti-tumor effects is outlined in Figure 3.
Figure 2. An equilibrium between resveratrol and its metabolites.
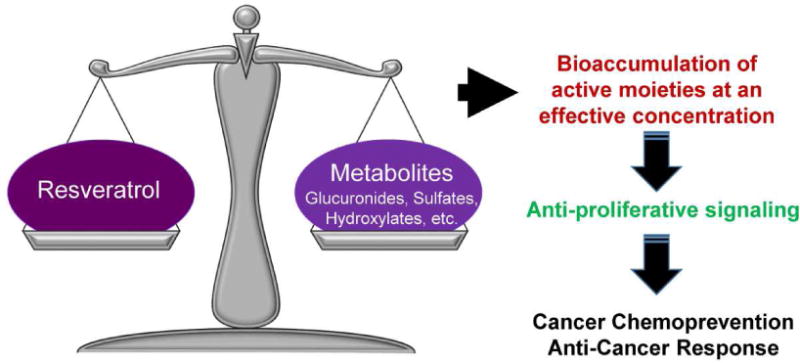
In the body, resveratrol and its metabolites naturally strive to reach a balance, at which point the active moieties are able to elicit anti-cancer responses and signaling.
Figure 3. A schematic representation of the process of regeneration of resveratrol from its conjugates, leading to their anti-proliferative effects.
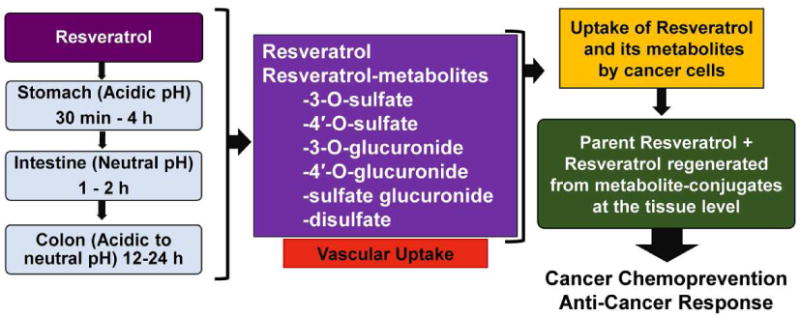
Research has shown that the body can absorb both resveratrol and its metabolites through various parts of the digestive tract. Interestingly, once these moieties reach the tissue level, some of the metabolites can be regenerated into the original resveratrol molecule. This allows both resveratrol and its metabolites better access to the tissues in order to elicit an anti-cancer response.
In the recent past, a number of laboratories have attempted to synthesize novel resveratrol derivatives or formulations [33]. However, limited efforts have been put forward to determine if these derivatives are superior to resveratrol in relevant disease models. Because of the poor bioavailability of resveratrol, it may be useful, especially for cancer chemoprevention, if resveratrol content could be increased in diets. To this end, recently multiple methods have been studied in an attempt to increase the content of resveratrol endogenously in wine products and grape juices using UV irradiation and/or ozonisation of grapes (reviewed in [34]). These types of studies are important as high-resveratrol containing diets would allow an increase in the daily resveratrol intake of individuals.
In an interesting study, Larrosa and colleagues have reformulated resveratrol to devise a set of resveratrol-prodrug forms (in this case, acyl-glucosyl derivatives) that resist absorption in the upper gastrointestinal tract and are then able to be steadily de-conjugated during gastrointestinal transit to supply an effective dose of free resveratrol to the colonic mucosa [35]. The prodrug strategy takes advantage of using a derivative of the original drug that can last longer in the body, either by rendering it temporarily inactive or less active, thereby allowing it to become active through normal metabolic processes at a later time. This can be taken further by adding other chemical groups that break down in sequential steps, resulting in a pro-prodrug. Larrosa and colleagues found that mice fed with a very low dose of pro-prodrugs of resveratrol did not develop colitis symptoms and improved the disease activity index 6-fold compared to regular resveratrol. They also noticed that these pro-prodrugs were able to i) prevent the rapid metabolism of resveratrol, thereby delivering higher quantities of resveratrol to the colon, and ii) reduce mucosal barrier imbalance and prevent diarrhea [35]. This suggests that using these resveratrol pro-prodrugs would be helpful in the management of colon cancer in the human population. Another approach to reformulating resveratrol is to attempt to change its structure or place it into a carrier that would allow it to reach the desired site in a targeted fashion before it is metabolized, thus allowing it to exert beneficial effects in localized areas. A recent study shows that the pharmacokinetics- and formulation- related limitations of resveratrol, as well as of other polyphenols, can be controlled by entrapping the molecule of interest inside unique structures such as dendrimers, which can be easily tailored for specific purposes [36, 37]. These and other changes can be explored in order to attempt to change resveratrol’s chemical properties, or targeting abilities, which leaves this area of research wide open and interesting.
4. Relevant dose of resveratrol
One of the most encouraging aspects of resveratrol for its possible development as an anti-cancer drug is that it does not appear to have debilitating or toxic side effects, especially when compared with traditional chemotherapy treatments. A wide range of resveratrol doses have been used in animal studies. For example, resveratrol has been shown to be well-tolerated in rats, without toxic effects up to 750 mg/kg bodyweight per day [38]. However, it is important for us to focus our efforts on finding the most appropriate dose and route of resveratrol administration, based on specific circumstances. For example, for cancers of the gastrointestinal tract, even lower doses may be useful because of increased exposure of the target organ with resveratrol. For other cancers that are less accessible, local delivery of resveratrol instead of systemic may be the ideal route of administration. Ginkel and colleagues noticed rapid tumor regression in a mouse xenograft model of human neuroblastoma when resveratrol was administered via peritumor injection. They also noticed significant evidence of cell death in tumor tissue with relatively no effect in adjacent normal tissues [39]. The discrimination in the cellular uptake of resveratrol between normal and tumor cells could be due to differences in the molecular status and available cellular targets in cancer cells, which may mean that resveratrol could have tumor-specific abilities. This is likely the reason that resveratrol has such low numbers of toxic side effects, and will allow researchers to be able to fine-tune doses to each disease’s specific demands. Mukherjee and collogues have reviewed the dose-dependent effects of resveratrol in relation to health benefits (both in animal and human studies), and have suggested that lower doses of resveratrol could be very useful in maintaining human health, while higher doses may be desirable to kill tumor cells via pro-apoptotic effects [40].
Several human pharmacokinetic and pharmacodynamic studies of resveratrol have been performed (reviewed in [41]). Based on these studies, resveratrol does not appear to have side effects up to a dose of 1.0 g of resveratrol in the short term. However, when patients were given 2.5 g or more per day, the observed side effects included diarrhea, vomiting, nausea and evidence of liver dysfunction in patients with non-alcoholic fatty liver disease [42]. Interestingly, no severe side effects were reported in long-term clinical trials involving administration up to 16 mg resveratrol (for up to one year) in combination with other grape polyphenols in polymedicated subjects undergoing primary and secondary prevention of cardiovascular disease [43]. In fact, resveratrol has been found to be safe and reasonably well-tolerated at up to 5 g/day, either as a single dose or as part of a multiple-day dosing regimen (reviewed in [16]). However, when using this drug in cancer or diseased patients, it is imperative to realize that these studies were done in healthy populations. For example, in one study, a proprietary formulation of resveratrol, SRT501 (developed by Sirtris, a GSK Company), given at a dose of 5 g per day (alone or in combination with the chemotherapy drug bortezomib) demonstrated renal toxicity in multiple myeloma patients [44]. However, no renal toxicity was observed when the same formulation was administered to healthy controls, type 2 diabetics, or patients with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome [44]. This suggests that choosing the right dose of resveratrol to target a specific disease may be tricky. An optimal or even relevant dose for resveratrol has still to be ascertained for different indications in carefully done human clinical studies.
5. Resveratrol’s mechanism of action as an anticancer agent
Despite the promising efficacy of resveratrol to inhibit and suppress tumor growth in several in vitro cancer studies, the exact mechanism of resveratrol’s anti-proliferative effects are still being investigated. In this direction, there are many complicating factors because the malignant cells display deregulations of multiple signaling pathways, leading to uncontrolled cell proliferation, inhibition of programed cell death, enhanced angiogenesis and enhanced uncontrolled migration of cells. The available literature suggests that in cancer, resveratrol acts through multiple mechanisms, including proapoptotic, antiproliferative, anti-inflammatory, and anti-angiogenesis mechanisms. For example, it has been suggested that one of resveratrol’s direct targets is TANK-binding kinase 1 (TBK1), an integral component in many chronic inflammatory diseases, which may lead to cancer [45]. Similarly, Nwachukwu and colleagues have suggested that the anti-inflammatory effects of resveratrol may be regulated via estrogen receptor-α [46]. In addition, it has also been suggested that in stem-like cells derived from breast cancer lines, resveratrol’s apoptosis activity comes through its ability to down-regulate fatty acid synthase (FAS) and enhance DAPK2 and BNIP3, which are both pro-apoptosis genes [47]. Although it is difficult to ascertain the cause and effect mechanism in in vivo settings, resveratrol has been shown to affect a number of molecular targets, possibly based on cancer type, resveratrol formulations, stage of disease, and dose and duration of resveratrol. It is being increasingly appreciated that the combinatorial approaches of resveratrol with other natural agents are likely to be especially useful in advanced stages of cancers because of deregulation of multiple pathways affecting cancer cell growth and oncogenic signaling (reviewed in [14]). Interestingly, naturally occurring agents tend to target multiple pathways, which makes them ideal for this use.
Our understanding of mechanisms of action are further complicated by the finding that orally delivered resveratrol is also metabolized by gut microbiota [20]. In fact, this is the only reported place in the body that the hydroxylated forms of resveratrol are produced, which have been shown to be effective cytotoxic molecules of their own. As discussed previously, this is also true of many of the other resveratrol metabolites. This complicates the issue and makes it difficult for researchers to determine if and which effects are due to resveratrol and which ones are due to its metabolites. This should not be an issue in the overall objective of using resveratrol for cancer management, as the overarching goal is to eliminate cancer cells. It is possible that in vivo, an interplay between resveratrol and its metabolites acting on either the same or different molecular targets may be accountable for the overall beneficial health effects. For example, a molecular modeling study by Calamini and colleagues [48] has shown that some of the known targets of resveratrol such as COX-1 (cyclo-oxygenase-1) and COX-2, both are potently inhibited by resveratrol as well as resveratrol 4′-O-sulfate (a resveratrol metabolite) [49]. However, when determining which doses, combinations, and routes of administrations are best for each cancer type, the details of each mechanism may become very important. The issue is further complicated by the fact that it is not clear if resveratrol’s anti-proliferative effects are due to direct effects of resveratrol on tumor cells or indirect effects on angiogenesis or as a result of binding to an extracellular target. In-depth research is needed to understand these intricacies of mechanisms of resveratrol’s action.
6. Resveratrol in the pharmaceutical industry and supplement market
The health promoting potential of resveratrol has attracted many pharmaceutical companies to try to develop this agent for the supplement market. For example, as evident from an internet search, the company Biotivia Longevity Bioceuticals has developed a micro-encapsulated formulation, Transmax TR, which was designed to protect the resveratrol from stomach acid in order to provide a steady release into the blood stream. Similarly, RevGenetics Micronized Trans-Resveratrol powder claims to be absorbed up to 220% better as compared to regular resveratrol supplements. MicroActive® Resveratrol SR is another proprietary formulation of resveratrol developed by BioActives. This compound claims to be capable of providing a sustained release over 12 hours to increase intestinal residence time. Further, in a pilot study posted on the website of the company, healthy subjects administered MicroActive® Resveratrol SR capsules after breakfast were found to have resveratrol in their blood for a longer time than a regular resveratrol formulation. However, limited information is available regarding these propriety formulations and the testing done on them in the mainstream scientific literature. In one of the few papers available, a pilot study done by Howells and colleagues has assessed the safety, pharmacokinetics, and pharmacodynamics of SRT501, a micronized form of resveratrol designed by Sirtris, a GSK Company. They found that SRT501 was tolerable, detectable in hepatic tissue and with higher resveratrol levels in plasma as compared to previously published data. The time required to reach the highest level of resveratrol was also extended in the SRT501 patients. This trial used a dose of 5 g daily for 14 days, and included patients with colorectal cancer and hepatic metastases who had been predetermined to undergo hepatectomy [50]. Similar efforts are needed from industries to come forward to join hands with researchers in undertaking well-designed clinical trials.
7. Lessons learned from clinical studies
As available on ClinicalTrials.gov, the database of public and privately supported human clinical studies, there are a total of 81 resveratrol studies. Many of these trials have been done to evaluate the safety, tolerability, pharmacokinetics and bioavailability of resveratrol. Only a small number of listed studies are focused on evaluating the efficacy of resveratrol in certain cancers. A summary of these studies is provided in Tables 1 and 2, which outline the completed and ongoing trials, respectively. These trials use various forms of resveratrol, including trans-resveratrol, extract of Polygonum cuspidatum (Japanese knotweed), SRT501 (micronized resveratrol), resveratrol rich seedless red grapes/grape juices (Muscadine grapes), and microencapsulated resveratrol. These trials are focused on several cancer types, including multiple myeloma, breast cancer, follicular lymphoma and neuroendocrine tumors, but the majority are focused on assessing the effects of resveratrol on the development of colorectal cancers. In fact, resveratrol has proven to be mildly successful in colon cancer clinical trials, possibly because of a potentially direct contact and prolonged exposure of resveratrol with colonic tissues. In addition, the intestinal epithelium seems well adapted for absorption of nutrients and active molecules from food/food components.
Table 1.
Published human studies assessing the effect of resveratrol against cancer
| Resveratrol formulation and dosages | Cancer Type | Sample size & Phase | Outcome of the study | Ref |
|---|---|---|---|---|
| Resveratrol (20 or 80 mg/day) or Grape Powder (80 or 120 g/day) for 14 days | Colon | n=8 Phase 1 |
Inhibition of Wnt signaling in normal colonic mucosa as indicated by a reduction in the expression of a panel of Wnt target genes. | [51] |
| Resveratrol (0.5 or 1.0 g) for 8 days | Colon | n=20 | Resveratrol and its metabolites were quantified in colon tissues. Higher levels were noticed in the right side of the colon. Reduction of tumor cell proliferation by 5% (Ki-67 immunostaining, P = 0.05). | [52] |
| Micronized resveratrol, (SRT501, 5.0 g) for 14 days | Colorectal with hepatic metastases | n=9 Phase 1 |
Found to be well tolerated, with higher mean plasma resveratrol level (3.6-fold), detectable resveratrol in hepatic tissue and increased the cleavage of caspase-3 by 39% in malignant hepatic tissue. | [44] |
| Micronized resveratrol, (SRT501, 5.0 g) for 20 consecutive days in a 21 day cycle for a max of 12 cycles | Multiple Myeloma | n=24 Phase 2 |
This clinical trial was designed to assess giving SRT501 with or without bortezomib to Multiple Myeloma patients. However, due to severe adverse events and minimal efficacy in patients, the study was terminated early. | [42] |
| Polygonum cuspidatum caplets (5 or 50 mg trans-resveratrol) twice daily for 12 weeks | Women at increased breast cancer risk | n=39 | RASSF-1α methylation decreased with increasing levels of serum resveratrol. This change was directly related to the change in prostaglandin (PG)E2. | [53] |
| Resveratrol (0.5, 1.0, 2.5, or 5.0 g) for 29 days | Healthy participants | n=40 Phase 1 |
Generated micromolar concentrations of resveratrol and much higher levels of glucuronide and sulfate conjugates in plasma. Decreased circulating levels of cancer biomarkers IGF-I and IGFBP-3. | [42] |
Table 2.
Ongoing clinical trials on resveratrol and cancer (listed in ClinicalTrials.gov)
| Resveratrol formulation and dosages | Cancer | NCT number/Patients/Phase | Title of the study | Objective of the study |
|---|---|---|---|---|
| Resveratrol (to be determined) for up to 8 days | Colorectal Adenocarcinoma |
NCT00433576 n=20 Phase 1 |
Resveratrol in Treating Patients With Colorectal Cancer That Can Be Removed By Surgery | Determine relationship between oral dose of resveratrol and the colon mucosal levels, and the correlative level of COX-2 and M1G in colorectal cancer tissues and WBCs. |
| Resveratrol (2.5 g) twice daily for a total of three cycles in 28 day cycles | Neuroendocrine Tumor |
NCT01476592 n=7 |
A Biological Study of Resveratrol’s Effects on Notch-1 Signaling in Subjects With Low Grade Gastrointestinal Tumors | To determine if Notch1 and other neuroendocrine marker levels are affected in patients by resveratrol and that 5 g/day will be well tolerated with minimal dose limiting toxicities. |
| Seedless red grapes (1/3, 2/3 or 1 lb/day) for up to 2 years | Colon Cancer |
NCT00578396 n=30 Phase 1 |
Phase I Biomarker Study of Dietary Grape-Derived Low Dose Resveratrol for Colon Cancer Prevention | Define the minimum dietary achievable resveratrol levels via fresh red grapes, and to see if it affects mucosa cell proliferation (Ki67 analysis of pre- and post-resveratrol biopsy specimens). |
| 100% Grape juice (495 or 660 ml) every 2nd day for 16 weeks | Follicular Lymphoma |
NCT00455416 n=45 Phase 2 |
Dietary Intervention in Follicular Lymphoma | To assess the ability of dietary factors to induce apoptosis, inhibit cell proliferation and modulate tumor cell infiltrate in Follicular Lymphoma of stage III/IV. |
7.1. Completed clinical studies
Nguyen and colleagues were the first to report their clinical trial on resveratrol in cancer [51]. They evaluated the effects of either a low dose of plant-derived resveratrol formulation or a resveratrol-containing freeze-dried grape powder (GP) on Wnt signaling in colon cancer patients, and found significant inhibition in Wnt target gene expression in normal colonic mucosa, with no change in the cancer tissue [51]. The cancer tissue and normal colonic mucosa were obtained from the same patient at the time of surgical resection and processed identically. However, patients treated with resveratrol or GP had no change in the composite Wnt target gene expression in colon cancer. In fact, several of the genes, including myc and cyclinD1, were amplified in the cancer tissue after exposure to grape powder, but no change was seen following exposure to resveratrol. However, this was a pilot study with a small number of participants for a short duration, designed to see biomarker endpoints assessing the expression of various components and target genes of the Wnt pathway. One issue with this trial was that the researchers were initially told that the plant-derived resveratrol capsules contained 20 mg of trans-resveratrol; however, the HPLC analysis revealed only 3.886 mg per capsule [51]. In addition, quercetin was also found to be present in the capsules. This trial stresses the importance of picking the right formulation/dose of resveratrol in future trials. Another issue that will be important for future trials is the issue of quality control to ensure there are no unexpected interfering effects.
Patel and colleagues reported the detection of resveratrol and its metabolites in human tissues [52]. They identified and quantitated the level of resveratrol as well as its metabolites resveratrol-3-O-glucuronide, resveratrol-4′-O-glucuronide, resveratrol-3-O-sulfate, resveratrol-4′-O-sulfate, resveratrol sulfate glucuronide, and resveratrol disulfate by HPLC/UV in colorectal resection tissue. In this study, 0.5 and 1.0 g doses of resveratrol were shown to reduce tumor cell proliferation by 5% collectively in all the colon cancer patients [52]. This is despite the high variability in resveratrol concentrations measured in the colorectal tissues of patients. The authors suggested that the doses administered are sufficient to deliver enough resveratrol to colorectal tissue to elicit chemopreventive activity. Interestingly, the levels of resveratrol and its metabolites were consistently higher in tissues originating in the right side of the colon as compared with the left. How this affects the prognosis of colorectal cancer remains to be investigated. The high variability of the resveratrol metabolites in the tissue illustrates one of the key problems found in translating this drug to clinical trials.
In a pharmacokinetic study, Howells and colleagues have shown the detection of higher levels of resveratrol in plasma (1,942 ± 1,422 ng/mL) and in hepatic tissue (1,098 ± 1,393 ng/g) following administration of 5 g of SRT501 in patients with colorectal cancer and hepatic metastases scheduled to undergo hepatectomy [50]. They found the micronized resveratrol to be better tolerated by the patients, with all adverse events being considered mild, compared to nonmicronized resveratrol. Concentrations of resveratrol achieved in hepatic metastases were able to elicit pharmacologic effect. A significant increase in cleaved caspase-3 immunoreactivity was observed in tumor tissue when compared with equivalent tissue from subjects on placebo [50]. However, no significant change was found in the other biomarkers tested, including AKT1, GSK-3, survivin, and PARP. Overall, SRT501 formulated as a suspension seems to be better tolerated and have superior bioavailability than nonmicronized resveratrol [19, 42]. The authors suggested that the doses would need to be moderately higher to achieve significant apoptosis induction.
Popat et al conducted a trial to assess the safety, pharmacokinetics and efficacy of SRT501 alone or in combination with bortezomib, one of the currently approved chemotherapy drugs for patients with multiple myeloma. In this study, the researchers noticed several unexpected adverse effects, including unexpected renal toxicity [44]. Additionally, the trial was prematurely closed because of severe adverse events like nausea, diarrhea, vomiting, fatigue, anemia, infections, and most specifically renal failure. As discussed by the authors, the observed renal failure in this trial seems to be specific to multiple myeloma patients only, as renal impairment appears to be a common and serious complication of myeloma that can occur in up to 50% patients. This trial highlights the risks of developing resveratrol (and possibly other agents) in specific disease populations, and suggests that it is necessary to consider the association of secondary complications related with each disease when designing resveratrol based clinical trials. In this case, it appears that the existing increased risk of kidney problems in multiple myeloma patients was further aggravated by the treatments. The reason for this particular interaction at a molecular level is not known. However, determining the exact and detailed mechanism of action of resveratrol in this cancer may be useful for future studies.
In another study, Zhu and colleagues have assessed the effects of resveratrol on methylation of certain breast cancer related proteins in women who were at increased risk for breast cancer. To be included in this study, women must have had i) a first degree relative with breast cancer, ii) a Gail risk of > 1.66% of developing breast cancer, iii) a breast biopsy demonstrating atypical hyperplasia in situ, or iv) invasive breast cancer (previously diagnosed but currently free of disease) [53]. The effects of either 5 or 50 mg of trans-resveratrol twice per day (for 12 weeks) was studied on methylation of certain genes, as compared to placebo. The study found decreased methylation of RASSF-1a with increasing levels of trans-resveratrol and resveratrol-glucuronide in circulation, and with decreasing prostaglandin E2 (PGE2) expression in the breast [53]. Increased levels of PGE2 as well as increased methylation of RASSF-1a have been linked with disease progression from pre-cancer to cancer in the breast [54, 55]. Different parameters and longer follow-up of this study may be relevant to decide whether short duration or continuous supplementation of resveratrol would be better for individuals with increased breast cancer risk. This also underlines the importance of determining relevant doses and treatment regimens for each type of cancer or cancer-risk group.
Brown et al have shown that the ingestion of resveratrol caused a drop in circulating insulin-like growth factor (IGF)-I and IGF-binding proteins (IGFBP)-3 in healthy volunteers [42]. This study suggested that resveratrol may affect the IGF axis, possibly by direct effects on IGF-I and/or IGFBP-3, and that these proteins may serve as potential markers of chemopreventive efficacy in human clinical trials against cancer. Studies have suggested a relationship between the levels of IGF-I and IGFBP-3 with risk of variety of human diseases including cancers [56]. These molecules critically influence many key aspects of cancer development, including apoptosis, cell differentiation, neoplastic transformation and metastasis.
Overall, these completed trials are able to give us some key hints in what needs to be considered when planning out new clinical trials, as well as what ways to steer ongoing trials to make the most effective treatment regimens.
7.2. Ongoing and future clinical trials
The ongoing human clinical trials dealing with resveratrol against cancer (according to ClinicalTrials.gov) are summarized in Table 2. It is encouraging to see that researchers have learned lessons from previous clinical trials while designing the new trials, which will hopefully lead to better results. Out of the four ongoing trials, two are focused on colorectal cancers. One of these studies (NCT00433576) is focused on determining the optimal dose of resveratrol that will result in bioactive levels in the colon mucosa. This is important because even with all the colon cancer research done with resveratrol, an optimal dose is yet to be discovered. In addition, the researchers will explore the mechanistic effects of resveratrol treatment of colorectal adenocarcinoma by determining if there are correlative levels of COX-2 and M1G (pyrimido[1,2-a]purin-10(3H)-one) adduct in cancer tissues. This may provide some useful information, as both of these molecules have been shown to be modulated in colon cancer. The other colon cancer trial that is under way (NCT00578396) is using seedless red grapes in an attempt to determine the maximum dietary levels of resveratrol that can be attained. This may be especially important for colon cancer prevention, because new dietary recommendations could easily be provided/devised for the human population.
The third study will determine the effects of resveratrol in neuroendocrine tumors (NCT01476592). This trial explores the tolerability of 5 g resveratrol/day (in two doses) in patients with low grade gastrointestinal tumors of neuroendocrine origin. This study will also examine the level of Notch1 activation by resveratrol in these patients, as Notch1 has been found to be a key molecule in this type of cancer [57, 58]. The last study (NCT00455416) is focused on follicular lymphoma, and administers resveratrol as 100% grape juice as a dietary intervention. The researchers will determine if this mode of resveratrol administration will be able to inhibit cellular proliferation and/or modulate the infiltration of tumor cells in this malignancy. This trial may provide useful information regarding a different and possibly easier mode of resveratrol administration through dietary means.
These trials should provide some useful information that may be helpful in future detailed human studies aimed at bringing resveratrol to the clinics for disease management. In addition, resveratrol containing diets may be advocated for better health and disease prevention. Although we seem to have learned from previous clinical trials, there is always room for improvement. From the analysis of the studies described above, some areas to concentrate on are, i) a systems biology approach to explore detailed global mechanisms, ii) working out the ideal formulation(s), identifying novel combination(s) of resveratrol with other agents and drugs, and iii) personalized dosing schedule. This will necessitate a step back and many more pilot studies by researchers, but will likely result in much more fruitful outcome for success in moving resveratrol from the bench to the bedside.
8. Conclusion
Resveratrol has garnered intense attention from the public as well as researchers. This interest stems from the fact that this small molecule found in grapes and red wine has shown very promising results against heart diseases, aging, metabolic disorders, and cancer in numerous preclinical studies. This is perpetuated by the media and private corporations who want to be a part of this lucrative molecule. In fact, several formulations of resveratrol are available as over the counter supplements which claim beneficial effects against various health conditions. However, only limited efforts have been undertaken to translate the preclinical success of this ‘wonder molecule’ to the clinics. There are a number of challenges that need to be overcome in order to bring resveratrol from the bench to the bedside. The single biggest problem in resveratrol’s clinical translation appears to be its rapid metabolism leading to a limited in vivo bioavailability. Pharmaceutical companies and researchers are trying to combat this by either re-deriving or reformulating resveratrol. Although the limited amount of clinical data available so far is promising, intense and concerted efforts are required from researchers, clinicians and the pharmaceutical industry to try to overcome these obstacles.
Highlights.
The main issues blocking resveratrol as a cancer treatment are discussed.
A survey of public & private research done on resveratrol forms is performed.
We suggest that the appropriate dose of resveratrol is disease-specific.
Work done to determine resveratrol’s anticancer mechanism is explored.
Completed trials are investigated to offer suggestions to plan better research.
Acknowledgments
This work was supported, in part, by funding from the NIH (R01CA176748, R21CA176867, and R21CA149560 to NA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics, CA. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014, CA. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.La Vecchia C, Bosetti C. Diet and cancer risk in Mediterranean countries: open issues. Public Health Nutr. 2006;9:1077–1082. doi: 10.1017/S1368980007668475. [DOI] [PubMed] [Google Scholar]
- 4.Zheng J, Yang B, Huang T, Yu Y, Yang J, Li D. Green tea and black tea consumption and prostate cancer risk: an exploratory meta-analysis of observational studies. Nutr Cancer. 2011;63:663–672. doi: 10.1080/01635581.2011.570895. [DOI] [PubMed] [Google Scholar]
- 5.Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, Das DK, Delmas D, Gottfried C, Lin HY, Ma QY, Mukhopadhyay P, Nalini N, Pezzuto JM, Richard T, Shukla Y, Surh YJ, Szekeres T, Szkudelski T, Walle T, Wu JM. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6:e19881. doi: 10.1371/journal.pone.0019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh N, Agrawal M, Dore S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem Neurosci. 2013;4:1151–1162. doi: 10.1021/cn400094w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam YY, Peterson CM, Ravussin E. Resveratrol vs. calorie restriction: data from rodents to humans. Exp Gerontol. 2013;48:1018–1024. doi: 10.1016/j.exger.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Dolinsky VW, Dyck JR. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim Biophys Acta. 2011;1812:1477–1489. doi: 10.1016/j.bbadis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 11.Aluyen JK, Ton QN, Tran T, Yang AE, Gottlieb HB, Bellanger RA. Resveratrol: potential as anticancer agent. J Diet Suppl. 2012;9:45–56. doi: 10.3109/19390211.2011.650842. [DOI] [PubMed] [Google Scholar]
- 12.Francioso A, Mastromarino P, Masci A, d’Erme M, Mosca L. Chemistry, stability and bioavailability of resveratrol. Med Chem. 2014;10:237–245. doi: 10.2174/15734064113096660053. [DOI] [PubMed] [Google Scholar]
- 13.Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 14.Singh CK, George J, Ahmad N. Resveratrol-based combinatorial strategies for cancer management. Ann N Y Acad Sci. 2013;1290:113–121. doi: 10.1111/nyas.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh CK, Pitschmann A, Ahmad N. Resveratrol-zinc combination for prostate cancer management. Cell Cycle. 2014;13:1867–1874. doi: 10.4161/cc.29334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- 17.Borriello A, Bencivenga D, Caldarelli I, Tramontano A, Borgia A, Zappia V, Della Ragione F. Resveratrol: from basic studies to bedside. Cancer Treat Res. 2014;159:167–184. doi: 10.1007/978-3-642-38007-5_10. [DOI] [PubMed] [Google Scholar]
- 18.Tome-Carneiro J, Larrosa M, Gonzalez-Sarrias A, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des. 2013;19:6064–6093. doi: 10.2174/13816128113199990407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 20.Bode LM, Bunzel D, Huch M, Cho GS, Ruhland D, Bunzel M, Bub A, Franz CM, Kulling SE. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutr. 2013;97:295–309. doi: 10.3945/ajcn.112.049379. [DOI] [PubMed] [Google Scholar]
- 21.Almeida L, Vaz-da-Silva M, Falcao A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha JF, Nunes T, Wright L, Soares-da-Silva P. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53(Suppl 1):S7–15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 22.Ndiaye M, Kumar R, Ahmad N. Resveratrol in cancer management: where are we and where we go from here? Ann N Y Acad Sci. 2011;1215:144–149. doi: 10.1111/j.1749-6632.2010.05851.x. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JJ, Nihal M, Siddiqui IA, Scarlett CO, Bailey HH, Mukhtar H, Ahmad N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol Nutr Food Res. 2011;55:1169–1176. doi: 10.1002/mnfr.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang W, Chen Z, Wang Q, Lin M, Wu S, Yan Q, Wu F, Yu X, Xie X, Li G, Xu Y, Pan J. Piperine potentiates the antidepressant-like effect of trans-resveratrol: involvement of monoaminergic system. Metab Brain Dis. 2013;28:585–595. doi: 10.1007/s11011-013-9426-y. [DOI] [PubMed] [Google Scholar]
- 25.Wightman EL, Reay JL, Haskell CF, Williamson G, Dew TP, Kennedy DO. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: a randomised, double-blind, placebo-controlled, crossover investigation. Br J Nutr. 2014;112:203–213. doi: 10.1017/S0007114514000737. [DOI] [PubMed] [Google Scholar]
- 26.Basu NK, Kole L, Basu M, McDonagh AF, Owens IS. Targeted inhibition of glucuronidation markedly improves drug efficacy in mice – a model. Biochem Biophys Res Commun. 2007;360:7–13. doi: 10.1016/j.bbrc.2007.05.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malhotra A, Nair P, Dhawan DK. Study to evaluate molecular mechanics behind synergistic chemo-preventive effects of curcumin and resveratrol during lung carcinogenesis. PLoS One. 2014;9:e93820. doi: 10.1371/journal.pone.0093820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Santi C, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Sulphation of resveratrol, a natural compound present in wine, and its inhibition by natural flavonoids. Xenobiotica. 2000;30:857–866. doi: 10.1080/004982500433282. [DOI] [PubMed] [Google Scholar]
- 29.McAnulty LS, Miller LE, Hosick PA, Utter AC, Quindry JC, McAnulty SR. Effect of resveratrol and quercetin supplementation on redox status and inflammation after exercise. Appl Physiol Nutr Metab. 2013;38:760–765. doi: 10.1139/apnm-2012-0455. [DOI] [PubMed] [Google Scholar]
- 30.Hoshino J, Park EJ, Kondratyuk TP, Marler L, Pezzuto JM, van Breemen RB, Mo S, Li Y, Cushman M. Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J Med Chem. 2010;53:5033–5043. doi: 10.1021/jm100274c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel KR, Andreadi C, Britton RG, Horner-Glister E, Karmokar A, Sale S, Brown VA, Brenner DE, Singh R, Steward WP, Gescher AJ, Brown K. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci Transl Med. 2013;5:205ra133. doi: 10.1126/scitranslmed.3005870. [DOI] [PubMed] [Google Scholar]
- 32.Andreadi C, Britton RG, Patel KR, Brown K. Resveratrol-sulfates provide an intracellular reservoir for generation of parent resveratrol, which induces autophagy in cancer cells. Autophagy. 2014;10:524–525. doi: 10.4161/auto.27593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pezzuto JM, Kondratyuk TP, Ogas T. Resveratrol derivatives: a patent review (2009–2012) Expert Opin Ther Pat. 2013;23:1529–1546. doi: 10.1517/13543776.2013.834888. [DOI] [PubMed] [Google Scholar]
- 34.Tříska J, Houška M. Physical methods of resveratrol induction in grapes and grape products – a review. Czech J Food Sci. 2012;30:489–502. [Google Scholar]
- 35.Larrosa M, Tome-Carneiro J, Yanez-Gascon MJ, Alcantara D, Selma MV, Beltran D, Garcia-Conesa MT, Urban C, Lucas R, Tomas-Barberan F, Morales JC, Espin JC. Preventive oral treatment with resveratrol pro-prodrugs drastically reduce colon inflammation in rodents. J Med Chem. 2010;53:7365–7376. doi: 10.1021/jm1007006. [DOI] [PubMed] [Google Scholar]
- 36.Abderrezak A, Bourassa P, Mandeville JS, Sedaghat-Herati R, Tajmir-Riahi HA. Dendrimers bind antioxidant polyphenols and cisplatin drug. PLoS One. 2012;7:e33102. doi: 10.1371/journal.pone.0033102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svenson S, Chauhan AS. Dendrimers for enhanced drug solubilization. Nanomedicine (Lond) 2008;3:679–702. doi: 10.2217/17435889.3.5.679. [DOI] [PubMed] [Google Scholar]
- 38.Williams LD, Burdock GA, Edwards JA, Beck M, Bausch J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem Toxicol. 2009;47:2170–2182. doi: 10.1016/j.fct.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 39.van Ginkel PR, Sareen D, Subramanian L, Walker Q, Darjatmoko SR, Lindstrom MJ, Kulkarni A, Albert DM, Polans AS. Resveratrol inhibits tumor growth of human neuroblastoma and mediates apoptosis by directly targeting mitochondria. Clin Cancer Res. 2007;13:5162–5169. doi: 10.1158/1078-0432.CCR-07-0347. [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee S, Dudley JI, Das DK. Dose-dependency of resveratrol in providing health benefits. Dose Response. 2010;8:478–500. doi: 10.2203/dose-response.09-015.Mukherjee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chachay VS, Kirkpatrick CM, Hickman IJ, Ferguson M, Prins JB, Martin JH. Resveratrol–pills to replace a healthy diet? Br J Clin Pharmacol. 2011;72:27–38. doi: 10.1111/j.1365-2125.2011.03966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, Brenner DE. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2013;27:37–48. doi: 10.1007/s10557-012-6427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Popat R, Plesner T, Davies F, Cook G, Cook M, Elliott P, Jacobson E, Gumbleton T, Oakervee H, Cavenagh J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br J Haematol. 2013;160:714–717. doi: 10.1111/bjh.12154. [DOI] [PubMed] [Google Scholar]
- 45.Youn HS, Lee JY, Fitzgerald KA, Young HA, Akira S, Hwang DH. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol. 2005;175:3339–3346. doi: 10.4049/jimmunol.175.5.3339. [DOI] [PubMed] [Google Scholar]
- 46.Nwachukwu JC, Srinivasan S, Bruno NE, Parent AA, Hughes TS, Pollock JA, Gjyshi O, Cavett V, Nowak J, Garcia-Ordonez RD, Houtman R, Griffin PR, Kojetin DJ, Katzenellenbogen JA, Conkright MD, Nettles KW. Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. Elife. 2014;3:e02057. doi: 10.7554/eLife.02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandey PR, Okuda H, Watabe M, Pai SK, Liu W, Kobayashi A, Xing F, Fukuda K, Hirota S, Sugai T, Wakabayashi G, Koeda K, Kashiwaba M, Suzuki K, Chiba T, Endo M, Fujioka T, Tanji S, Mo YY, Cao D, Wilber AC, Watabe K. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat. 2011;130:387–398. doi: 10.1007/s10549-010-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calamini B, Ratia K, Malkowski MG, Cuendet M, Pezzuto JM, Santarsiero BD, Mesecar AD. Pleiotropic mechanisms facilitated by resveratrol and its metabolites. Biochem J. 2010;429:273–282. doi: 10.1042/BJ20091857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zykova TA, Zhu F, Zhai X, Ma WY, Ermakova SP, Lee KW, Bode AM, Dong Z. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol Carcinog. 2008;47:797–805. doi: 10.1002/mc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward WP, Gescher AJ. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases–safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila) 2011;4:1419–1425. doi: 10.1158/1940-6207.CAPR-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen AV, Martinez M, Stamos MJ, Moyer MP, Planutis K, Hope C, Holcombe RF. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag Res. 2009;1:25–37. [PMC free article] [PubMed] [Google Scholar]
- 52.Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner DE, Steward WP, Gescher AJ, Brown K. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu W, Qin W, Zhang K, Rottinghaus GE, Chen YC, Kliethermes B, Sauter ER. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr Cancer. 2012;64:393–400. doi: 10.1080/01635581.2012.654926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu W, Qin W, Hewett JE, Sauter ER. Quantitative evaluation of DNA hypermethylation in malignant and benign breast tissue and fluids. Int J Cancer. 2010;126:474–482. doi: 10.1002/ijc.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 56.Jogie-Brahim S, Feldman D, Oh Y. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr Rev. 2009;30:417–437. doi: 10.1210/er.2008-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shan L, Aster JC, Sklar J, Sunday ME. Notch-1 regulates pulmonary neuroendocrine cell differentiation in cell lines and in transgenic mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L500–509. doi: 10.1152/ajplung.00052.2006. [DOI] [PubMed] [Google Scholar]
- 58.Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12:535–542. doi: 10.1634/theoncologist.12-5-535. [DOI] [PubMed] [Google Scholar]


