Abstract
Though cognitive testing of infant monkeys has been practiced for the past 40 years, these assessments have been limited primarily to nursery-reared infants due to the confounds of separating mother-reared infants for assessments. Here we describe a pilot study in which we developed a cognitive testing apparatus for socially housed, mother-peer-reared rhesus macaques under one year of age (Macaca mulatta) that allowed the infants to freely return to their mothers for contact comfort. Infants aged 151.2±18.3 days (mean±SEM; n=5) were trained and tested on an object detour reach task. Infants completed training in 5.0±0.2 days, and completed testing in 6.2±0.9 days. Across four days of testing, infants improved to nearly errorless performance (Friedman test: χ2=13.27, df=3, p=0.004) and learned to do the task more quickly (Friedman test: χ2=11.69, df=3, p=0.009). These are the first cognitive data in group-housed, mother-peer-reared rhesus monkeys under one year of age, and they underscore the utility of this apparatus for studying cognitive development in a normative population of infant monkeys.
Keywords: cognition, development, mother-reared, normative, rhesus macaque
INTRODUCTION
Cognitive testing in captive monkeys has been conducted since the 1960s (Davenport, Chamove, & Harlow, 1970; Golub, Germann, & Hogrefe 2004; Nealis, Carpentier, Suomi, & Harlow, 1975; Sackett, Ruppenthal, Hewitson, Simerly, & Schatten, 2006; Schrier, 1961), and in semi-free ranging populations for the past 10–15 years (Fagot & Bonté, 2010; Munakata, Santos, Spelke, Hauser, & O’Reilly, 2001; Teufel, Gutmann, Pirow, & Fischer, 2010), yet very few laboratories have gathered data on infant monkeys (under one year of age), a crucial developmental period for understanding the ontogeny of cognitive processes. The few labs that have gathered data on infant monkeys have primarily been limited to studying nursery-reared monkeys (Burbacher et al., 2013; Dettmer, Novak, Novak, Meyer, & Suomi, 2009; Ha, Kimpo, & Sackett, 1997; Mandell & Sackett, 2009; Sackett et al., 2006) owing to the stress reactions resulting from removing a mother-reared infant from its mother that would compromise the reliability of data.
A few cognitive studies have been conducted with mother-reared infant monkeys aged around six months (Gluck, Harlow & Schiltz, 1973; Gluck & Sackett, 1976; Beauchamp & Gluck, 1988), but the nuclear-family housing used in these studies is not practical for contemporary primate facilities that typically socially house infants in larger groups than those studied by Gluck and colleagues, which were composed of one male, one female, and one infant. Contemporary social housing of infants typically occurs at facilities with enough space to provide some portion of outdoor housing (Capitanio, Mendoza, Mason & Maninger, 2005; Dettmer, Novak, Suomi & Meyer, 2012), whereas indoor-housed monkeys now are usually housed in cages that do not afford the rich social experience provided by group housing owing to constraints on physical space. Thus, current facilities that test infant monkeys typically rely on nursery-reared animals.
Consequently, though a good amount is known about the cognitive development of monkeys reared by humans, we know very little about how these infants compare to monkeys reared by their mothers. One study found that nursery rearing for 48 weeks resulted in poorer cognitive performance in one-year-old rhesus monkeys (Sanchez, Hearn, Do, Rilling & Herndon, 1998), and others have speculated that rearing with animate companions facilitates the development of a cognitive style that differs from individuals reared with inanimate companions (Capitanio & Mason, 2000; Mason & Capitanio, 1988). Additionally, Anastasiou (1970) found that wild-born monkeys solved more problems, and with a greater variety of approaches, than laboratory-raised monkeys (but see Gazes, Brown, Basile & Hampton, 2013). However, for infants reared in large social groups, cognitive testing for individuals less than one year of age has not been accomplished. Filling this gap is crucial to understanding the neurodevelopmental consequences of early life experience.
Here we describe the development of a cognitive testing apparatus for socially housed, mother-peer-reared (MPR) monkeys aged 5–7 months (hereafter, infants). In this pilot study, we assessed the feasibility of employing this apparatus for routine cognitive testing of infants at this and at younger ages. We hypothesized that infants would be readily trained to participate in a cognitive task, and would show improved performance across test sessions.
METHODS
Subjects and Rearing
We studied five infant rhesus macaques (Macaca mulatta; 3 male) born at the Laboratory for Comparative Ethology (LCE) between April and August of 2013. All infants were reared following standard methods in this laboratory (Shannon, Champoux, & Suomi, 1998; Dettmer et al., 2012). Briefly, infants were born into large social groups containing 8–12 adult females, 1 adult male, and 3–5 infants housed in indoor/outdoor runs, where they lived until weaning at 7–8 months. Just before testing began, a portion of all infants born in 2013 (older infants aged 7–8 months) were relocated as a part of the LCE’s weaning procedure (Dettmer et al., 2012) and were not tested in this study, so that, at the time of testing, one run contained 12 adult females, 1 adult male, and 3 of the test infants. The other run contained 8 adult females and 3 infants, two of which were tested (the third was too young; additionally, the adult male in this run had been removed from the run a few months prior for protocol purposes, however, the infants had been reared with him in the run for several months prior to his removal). Infants began acclimation between 98–198 days (mean±SEM = 151.2±18.3 days). Table 1 displays the age of infants at the start of acclimation, and at the completion of training and testing. Subjects were trained and tested on an object detour reach (ODR) task as part of a larger study examining response inhibition and impulsivity (Lyons, Lopez, Yang, & Schatzberg, 2000).
Table 1.
Age of subjects at significant cognitive testing milestones.
| Subject | Age at Start of Acclimation | Age at Completion of Training | Age at Completion of Testing |
|---|---|---|---|
| Subject 1 | 198 | 242 | 261 |
| Subject 2 | 176 | 220 | 235 |
| Subject 3 | 163 | 179 | 195 |
| Subject 4 | 98 | 157 | 171 |
| Subject 5 | 121 | 138 | 165 |
|
| |||
| Mean ± SEM | 151.2 ± 18.3 | 187.2 ± 19.3 | 205.4 ± 18.6 |
This research complied with the protocols approved by the NICHD Animal Care and Use Committee (ACUC), and adhered to the legal requirements of the USA and to the NIH Guide for the Care and Use of Laboratory Animals.
Testing Apparatus and Acclimation
We developed a testing cage for the infants that would allow them to be trained and tested while remaining in their social groups, as the social housing environment in our laboratory, like many others, would have required daily capture and sedation of the mother to remove the infant for testing in a separate testing room/apparatus, thereby introducing confounds to the data. This cage was a 14-gauge PVC-coated wire mesh testing cage measuring 86.36cm W × 91.44cm H × 50.8cm D, with a 15.24cm W × 15.24cm H × 20.32cm D entrance tunnel that only the infant could fit through. A small opening connecting the tunnel and the main testing cage allowed the infant to enter and interact with the testing board without being restrained by its mother or other adults in the run; visual and tactile contact was available for the infant while inside the testing cage. The tunnel allowed the infant to come and go as it pleased for contact comfort with its mother (Supplemental Video 1).
Before training commenced all monkeys were given roughly 3–5 weeks of acclimation to the testing cage. For the first week of acclimation the cage was placed outside the chain link of the enclosure for approximately 1 hour each day allowing the entire social group to acclimate visually to the testing cage before it was placed inside their run. From the second week of acclimation onward, all monkeys were briefly locked into the indoor portion of the run to allow experimenters to secure the testing cage in the outdoor portion to the chain link of the enclosure with clips and zip ties. A variety of small food rewards were sprinkled inside the testing cage, and monkeys were then released and allowed indoor/outdoor access for the duration of the session. Infants were allowed to freely explore the cage, both inside and out, for about 1 hour each day. Infants were encouraged to enter the testing cage by the experimenter showing them a treat and placing it into the testing cage. Once infants were coming into the testing cage to obtain treats without prompting (usually 3–5 days), the quantity of rewards sprinkled in the cage was gradually decreased to so that infants only had access to one piece at a time. In the third week (and beyond, if necessary), infants were acclimated to reaching through the chain link to retrieve a reward from the testing platform, which was an opaque plexiglass platform the same height as the testing apparatus for the ODR task. Once infants were freely and calmly coming in and out of the testing cage to obtain rewards off the platform, training began.
The apparatus for the ODR task was a 61.7cm × 17.5cm opaque plexiglass testing board affixed with chains and clips so that it could be fastened onto the home cage. In the center of the board was a 9cm × 9cm × 9cm clear plastic box with one side open (Lyons et al., 2000; Supplemental Figure 1). The box was attached to the board so that the human experimenter could rotate the opening but the monkey could not. A removable, opaque occluder (roughly 60cm long) was also placed in front of the testing board to block the infant’s view while the experimenter set up each trial.
Object Detour Reach (ODR) Cognitive Task
We followed previously published procedures for administering this task, modified for our laboratory, which measures inhibitory control by testing monkeys’ ability to learn that they must reach around a transparent object to obtain a reward, rather than performing an impulsive line of sight reach (Lyons et al., 2000; Parker, Buckmaster, Justus, Schatzberg, & Lyons, 2005). Infants were presented with the testing apparatus and given 30sec to retrieve the reward (e.g., marshmallow) inside. For each trial, we recorded the number and direction of each reach, latency to retrieve the reward, whether the infant achieved a correct/incorrect response within 30sec, and whether the infant balked (refused to work within 30sec).
For all training and testing trials, the experimenter sat behind the center of the testing apparatus, behind the clear box, facing forward, with her eyes directed toward the center of the testing apparatus for the duration of the trial, so as to reduce the likelihood that the experimenter would cue the subject.
Training
Infants were presented with the testing apparatus for 30–90 minutes each day, between 1130–1330, Monday through Friday, prior to the afternoon feeding. Training methods were adapted from those used in Lyons et al. (2000), which began with completion of 100 straight-reach trials. Infants were presented with the testing board so that the transparent box opened directly facing the monkey; a small reward (1/2 marshmallow or 1/8 grape) was placed in the center of the box (Lyons et al., 2000). The experimenter set up the trial and lifted the occluder, then waited for an infant to enter the testing cage. Trials began when the infant’s legs entered the main portion of the testing cage and it made eye contact with the testing board. The trial ended when the infant either retrieved the reward or 30sec had elapsed. If the reward was retrieved within 30sec the trial was coded as correct (Table 2). If the reward was not retrieved within the 30sec the occluder was lowered and the trial was coded as a balk (Table 2). The hand used to retrieve the reward and the latency to retrieve the reward was also recorded, as well as any behavioral notes (e.g., cooing).
Table 2.
Response and interference codes for cognitive training and testing
| Response | Description |
|---|---|
| Correcta | Reached into the opening and retrieved the reward. |
| Bonk | Made a line of sight reach when opening was to the side |
| Incorrect Detour | Reached to the incorrect side |
| Handling Error | Reached correctly but did not retrieve the reward. |
| Balka | Did not attempt to retrieve the treat within 30 seconds |
| Interference Code | Description |
|---|---|
| 0 | No interference by another monkey |
| 1 | Other monkey(s) in the cage without interfering with testing |
| 2 | Any interference by another monkey causing a break in focus |
| 3 | Any interference by another monkey resulting in end of trial |
| 4 | Any interference by another monkey resulting in displacement from cage |
Only these codes, but no others, were employed for training.
Infants were allowed to come and go from the testing cage as desired to minimize potential stress resulting from separation from their mothers. A trial was not coded as a balk until 30sec had elapsed from the time the infant entered the cage and made eye contact with the testing board, even if the monkeys exited the testing cage as they often returned and completed the trial within 30sec. An interference scale was employed to account for interactions with other monkeys that may have had an impact on the training trial; the ID of the monkey was also noted if interference did occur (Table 2). Notably, infants seemed to self-regulate by “taking turns” (i.e., one infant would complete 10 trials and then cease to come inside the testing cage, then another infant would complete 12 trials, etc.). Interference was very low: we observed interference on only 5.6% of trials, and 60.7% of these were coded as 1 (other monkey[s] present without causing interference).
Due to the freedom the infants had to come and go, they were not presented with precisely 20 trials every day as in past studies testing monkeys in single cages (Lyons et al., 2000; Parker et al., 2005), but with as many as they were willing to complete (in this study, infants completed between 3 and 36 trials per day). A training session was concluded when the apparatus had been in the run for a maximum of 90 minutes or, if after at least 30 minutes, a trial was presented for 5min without any infant entering the cage (thus indicating no further interest in participation).
Testing
Testing methods were adapted from Lyons et al. (2000) and Parker et al. (2005). Trials were presented so that the opening of the box oriented straight, right, and left, repeatedly for the duration of the test session (Parker et al., 2005). During all trials the box was baited with a small reward (1/8 grape or 1/2 marshmallow) in the center. Infants could come and go as they pleased, which prevented the firm pattern of box orientations that the previous reports had established (Parker et al., 2005), as different infants sometimes entered from one trial to the next, but overall we observed that across the 140 testing trials the task required, each infant received roughly the same number of trials for each orientation (mean straight trials = 47, mean right trials = 50, mean left trials = 43).
Once the box was properly positioned the occluder was raised, and once an infant fully entered the main portion of the testing cage and made eye contact with the board, the trial began. Each reach attempt was scored to indicate the direction of the reach and if it was a “bonk” (a line-of-sight reach), an incorrect detour (the infant reached to the wrong side on a left or right presentation), an incorrect handling error (the infant reached correctly but did not retrieve the reward within 30sec, or reached for the top of the box), or a correct reach (Table 2). The hand used to retrieve the reward, the number of reach attempts, and the latency to retrieve the reward was also recorded, as well as any behavioral notes. The trial ended when the infant either retrieved the reward or 30sec had elapsed.
The same interference scale for training was used. If interference caused the infant to be unable to complete the trial, e.g., another infant raced in and stole the reward, that particular trial was coded as “No Data” and re-run. As with training, the infants seemed to self-regulate and very little interference occurred (we observed interference on only 8.0% of test trials, 39.3% of which were coded as a 1; see Table 2). The infants were allowed to complete as many trials as they were willing within the allotted time, attempting an average of 21.9 ± 2.2 trials per testing session per infant. Testing sessions were between 30–90min, concluding in the same manner as for training.
Data Analysis
Descriptive statistics were used to calculate the mean±SEM for start age (at acclimation), age at completion of training and testing, and number of sessions to complete training and testing. Nonparametric tests were employed for test performance data owing to the small sample size. The Friedman test examined test performance across four sessions, with the following variables as the outcomes: side error (e.g., a reach to the left when the box was opened to the right and vice versa), “bonk” (a line-of-sight reach when the box was open to either side), balk (refusal to participate in a trials within 30sec), and correct (a correct reach on straight and side orientations). All outcomes are presented as percentages across the testing day as per previously published reports (Lyons et al., 2000; Parker et al., 2005); these percentages were calculated whether or not the subject completed all 20 trials or fewer in a single day.
RESULTS
Training
On average, infants were 151.2±18.3 days old at the start of acclimation. They completed training at 187.2±19.3 days, and in 5.0±0.2 sessions. Infants completed a total average of 96±2.6 trials across the five sessions (19.2±0.5 trials per day).
Testing
Infants finished testing in 6.2±0.9 sessions (range: 4–9), at an average age of 205.4±18.6 days. Infants demonstrated fewer side errors (χ2=12.00, df=3, p=0.007), more trials correct (χ2=13.27, df=3, p=0.004), and faster response times (χ2=11.69, df=3, p=0.009) across test sessions (Figure 1). There was no change in “bonk” rate (p=0.11) or in the balk rate (p=0.94) across sessions.
Figure 1.
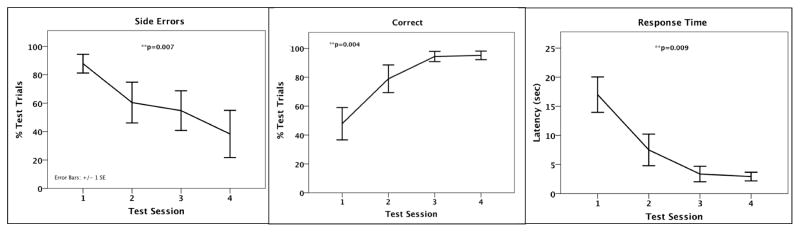
Mother-peer-reared (MPR) infants improved in test performance across trials as evidenced by fewer errors on side presentations (left), more trials correct (center), and faster response times (right).
DISCUSSION
We successfully developed and implemented a cognitive testing apparatus for use in socially housed MPR rhesus monkeys under one year old, showing that such testing is feasible in this population. Moreover, we demonstrated that MPR infants are readily trained to participate in and quickly complete a cognitive task to nearly errorless performance. This pilot study is critical in filling the gap that exists for cognitive development in socially reared young rhesus monkeys, as it is now possible to compare the cognitive development of nursery-reared monkeys, who experience an impoverished rearing environment, with the normative development of their MPR counterparts.
In support of our hypothesis, MPR infants were easily trained to participate in this cognitive task, taking five sessions (i.e., only five days) to complete training. In fact, our subjects were able to complete more trials per day, and to reach training criterion in fewer days, than has been published for juvenile squirrel monkeys (Parker et al., 2005). These findings are supported by research showing that the number of dendritic branches and spine densities in pyramidal cells of the prefrontal cortex, a brain region governing inhibitory control (especially in this task; see Roberts and Wallis, 2000), are greater in Old World vs. New World monkeys (Elston, Benavides-Piccidone, & DeFelipe, 2001). Given a suitable acclimation period to the testing cage and apparatus in their living environment, we are confident that MPR infants can be trained to perform a battery of cognitive tasks, including tasks that may be administered prior to six months of age (Ruppenthal & Sackett, 1992).
Also in support of our predictions, MPR infants improved in performance, showing greater accuracy and faster response times across test trials, as has been reported for juvenile monkeys (Parker et al., 2005). Thus, even infants with minimal exposure to experimenters experienced very little failure and frustration and learned to interact with the test apparatus quickly. By design, the test trials introduced more likelihood of failure, as the easiest straight- only trials were only presented 1/3 of the time. On these trials, infants may have initially become distracted and/or frustrated when they had to work harder for the reward; indeed, we observed infants to frequently come and go inside the testing cage on test trials – especially those oriented right or left – and interact with their mothers or peers in between entrances into the cage. It is possible that upon receiving the more difficult test trials infants left the testing cage to seek out social comfort more frequently than for the training trials, and in fact infants balked, on average, on 15.3% of the side-facing trials whereas they never balked on the straight trials. One way to address this problem could be to design a way to “trap” the infant inside the testing cage for the duration of each trial, and then release it when the trial is over. In fact, we attempted such a process prior to data collection by constructing a small door that the experimenter could manipulate which temporarily closed off the tunnel from the testing arena, but we found that this type of separation stressed the infants to such a degree that they became fearful of the testing cage and refused to participate. Future refinements to the testing cage could implement a different method for locking the infant inside during testing, and/or by acclimating infants to the separation at earlier ages.
Our findings are promising because they indicate that MPR infants learn to adapt to the rigors of testing trials by either coping with their frustrations at failure or by reducing their need for contact comfort. Indeed, by test days 3 and 4, MPR infants achieved at least 90% correct on test trials and had very low response latencies (5sec or less). We cannot exclude the possibility that, due to the repeated straight-right-left presentation on test trials, infants learned this pattern over the testing days, which accounted for at least some of the reduction in “bonks” over time. However, because each social enclosure had more than one infant being tested each day and the infants often took turns entering the testing cage, this pattern was not fixed and so the presentation was not strictly straight-right-left (see Methods). Still, randomizing the presentation of the box would enable us to tease these mechanisms apart, and we are implementing this and other randomized tasks with future cohorts.
Of note is the fact that we found no effect of dominance on cognitive performance in these infants. This is likely due in part to our small sample size, although in this cohort both high- and low-ranking infants were present in the same social group and both performed equally well. However, there is evidence that lower ranking animals tested with dominant animals perform worse than when they are tested alone (Drea & Wallen, 1999), so it is possible that with future cohorts we may see a dominance effect. On the other hand, studies with free-ranging baboons have shown an adaptation to social constraints by a low-ranking animal whereby the dominant monkey completed her trials earlier in the day and the subordinate one completed hers later in the day (Fagot & Paleressompoulle, 2009). Anecdotally, we observed a similar pattern in our infants, suggesting that dominance may not play a crucial role in the acquisition of these tasks; however, future studies are required to determine this definitively.
As Figure 1 shows, individual variation in error rate increased across testing days. Inspection of each individual subject’s results revealed that this was most certainly due to one particular infant who completed all straight-facing trials correctly, but who repeatedly made errors (or balked; data not included or shown in Figure 1) on the side-facing trials. Examining the data with this infant removed reduced the variability in this measure considerably, but not the overall pattern (not shown), underscoring the value of this type of task in revealing individual differences in inhibitory control.
Taken together, these data indicate that MPR infants under one year of age are readily trained and tested on this particular cognitive task. Future studies in our laboratory will begin training and testing of MPR cohorts at earlier ages, around 4 months of age, so as to be able to implement cognitive tasks routinely given to nursery-reared infants at these ages (e.g., object discrimination and reversal, match-to-sample, etc.; see Ruppenthal & Sackett, 1992 and Sackett et al., 2006). In this pilot study, construction and modification of the test apparatus took longer than expected and thus we were unable to administer these earlier tasks with this cohort.
Ours is the first study to demonstrate the feasibility of testing very young, socially housed, mother-reared rhesus monkeys, and they open the door to exciting studies directly comparing cognitive development in typically reared and nursery-reared infants in facilities such as ours where both populations exist in close proximity. Researchers will be able to start addressing questions regarding the underlying neurobiology guiding any observed cognitive differences owing to early life experiences, as well as influences on normative cognitive development including prenatal variables and maternal care. Further, it is now possible utilize MPR infants as models for typically developing human children, making MPR infants increasingly valuable in the search for biomarkers of cognitive development.
Supplementary Material
Acknowledgments
This research was funded by the NICHD Division of Intramural Research at NIH. We thank Ernie Davis for assistance with constructing the test cage and testing boards, and Denisse Guitarra and Nejra Isic for assistance with data collection.
References
- Anastasiou PJ. Unpublished doctoral dissertation. Tulane University; 1970. Problem solving ability of differentially reared rhesus monkeys. [Google Scholar]
- Beauchamp AJ, Gluck JP. Associative processes in differentially reared monkeys (Macaca mulatta): sensory preconditioning. Developmental Psychobiology. 1988;21:355–364. doi: 10.1002/dev.420210406. [DOI] [PubMed] [Google Scholar]
- Burbacher TM, Grant KS, Worlein J, Ha J, Curnow E, Juul S, Sackett GP. Four decades of leading-edge research in the reproductive and developmental sciences: The Infant Primate Research Laboratory at the University of Washington National Primate Research Center. American Journal of Primatology. 2013;75:1063–1083. doi: 10.1002/ajp.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mason W. Cognitive style: Problem solving by rhesus macaques (Macaca mulatta) reared with living or inanimate substitute mothers. Journal of Comparative Psychology. 2000;114:115–125. doi: 10.1037/0735-7036.114.2.115. http://dx.doi.org/10.1037/0735-7036.114.2.115. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Developmental Psychobiology. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Davenport JW, Chamove AS, Harlow HF. The semiautomatic Wisconsin general test apparatus. Behavior Research Methods & Instrumentation. 1970;2:135–138. doi: 10.3758/BF03211023. [DOI] [Google Scholar]
- Dettmer AM, Novak MF, Novak MA, Meyer JS, Suomi SJ. Hair cortisol predicts object permanence performance in infant rhesus macaques (Macaca mulatta) Developmental Psychobiology. 2009;51:706–713. doi: 10.1002/dev.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Suomi SJ, Meyer JS. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology. 2012;37:191–199. doi: 10.1016/j.psyneuen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drea CM, Wallen K. Low-status monkeys “play dumb” when learning in mixed social groups. Proceedings of the National Academy of Sciences. 1999;96:12965–12969. doi: 10.1073/pnas.96.22.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, DeFelipe J. The pyramidal cell in cognition: A comparative study in human and monkey. The Journal of Neuroscience. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-17-j0002.2001. Retrieved from www.jneurosci.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagot J, Bonté E. Automated testing of cognitive performance in monkeys: Use of a battery of computerized test systems by a troop of semi-free-ranging baboons (Papio papio) Behavior Research Methods. 2010;42:507–516. doi: 10.3758/BRM.42.2.507. [DOI] [PubMed] [Google Scholar]
- Fagot J, Paleressompoulle D. Automatic testing of cognitive performance in baboons maintained in social groups. Behavior Research Methods. 2009;41:396–404. doi: 10.3758/BRM.41.2.396. [DOI] [PubMed] [Google Scholar]
- Gazes RP, Brown EK, Basile BM, Hampton RR. Automated cognitive testing of monkeys in social groups yields results comparable to individual laboratory-based testing. Animal Cognition. 2013;16:445–458. doi: 10.1007/s10071-012-0585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck JP, Harlow HF, Schiltz KA. Differential effect of early enrichment and deprivation on learning in the rhesus monkey (Macaca mulatta) Journal of Comparative and Physiological Psychology. 1973;84:598. http://psycnet.apa.org/doi/10.1037/h0034880. [Google Scholar]
- Gluck JP, Sackett GP. Extinction deficits in socially isolated rhesus monkeys (Macaca mulatta) Developmental Psychology. 1976;12:173–174. http://dx.doi.org/10.1037/0012-1649.12.2.173. [Google Scholar]
- Golub MS, Germann SL, Hogrefe CE. Endocrine disruption and cognitive function in adolescent female rhesus monkeys. Neurotoxicology and Teratology. 2004;26:799–809. doi: 10.1016/j.ntt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Ha JC, Kimpo CL, Sackett GP. Multiple-spell, discrete-time survival analysis of developmental data: Object concept in pigtailed macaques. Developmental Psychology. 1997;33:1054. doi: 10.1037/0012-1649.33.6.1054. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Lopez JM, Yang C, Schatzberg AF. Stress-level cortisol treatment impairs inhibitory control of behavior in monkeys. The Journal of Neuroscience. 2000;20:7816–7821. doi: 10.1523/JNEUROSCI.20-20-07816.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DJ, Sackett GP. Comparability of developmental cognitive assessments between standard and computer testing methods. Developmental Psychobiology. 2009;51:1–13. doi: 10.1002/dev.20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP. Formation and expression of filial attachment in rhesus monkeys raised with living and inanimate mother substitutes. Developmental Psychobiology. 1988;21:401–430. doi: 10.1002/dev.420210502. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Santos LR, Spelke ES, Hauser MD, O’Reilly RC. Visual representation in the wild: How rhesus monkeys parse objects. Journal of Cognitive Neuroscience. 2001;13:44–58. doi: 10.1162/089892901564162. [DOI] [PubMed] [Google Scholar]
- Nealis PM, Carpentier A, Suomi SJ, Harlow HF. Dynamic stimulus display for the WGTA. Behavior Research Methods & Instrumentation. 1975;7:291–293. doi: 10.3758/BF03201451. [DOI] [Google Scholar]
- Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biological Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Wallis JD. Inhibitory control and affective processing in the prefrontal cortex: Neuropsychological studies in the common marmoset. Cerebral Cortex. 2000;10:252–262. doi: 10.1093/cercor/10.3.252. [DOI] [PubMed] [Google Scholar]
- Ruppenthal GC, Sackett GP. Research protocol and technician’s manual: A guide to the care, feeding, and evaluation of infant monkeys. 2. Seattle: University of Washington; 1992. p. 83. [Google Scholar]
- Sackett G, Ruppenthal G, Hewitson L, Simerly C, Schatten G. Neonatal behavior and infant cognitive development in rhesus macaques produced by assisted reproductive technologies. Developmental Psychobiology. 2006;48:243–265. doi: 10.1002/dev.20132. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential Rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research. 1998;812:38–49. doi: 10.1016/S0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Schrier AM. A modified version of the Wisconsin General Test Apparatus. The Journal of Psychology. 1961;52:193–200. doi: 10.1080/00223980.1961.9916519. [DOI] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. American Journal of Primatology. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Teufel C, Gutmann A, Pirow R, Fischer J. Facial expressions modulate the ontogenetic trajectory of gaze following among monkeys. Developmental Science. 2010;13:913–922. doi: 10.1111/j.1467-7687.2010.00956.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


