Abstract
Objective
To perform a randomized trial to determine if there is cardiovascular disease (CVD) risk reduction from a plant-based no added fat diet (PB) and the American Heart Association Diet (AHA) in children.
Study design
Four-week (4/20/2013-5/18/2013) prospective randomized trial in a large Midwestern hospital system’s predominantly middle class outpatient pediatric practices. Thirty children (9–18 years old) parent pairs with a last recorded child BMI >95th percentile and child cholesterol >169 mg/dL were randomized to PB or AHA with weekly 2-hour classes of nutrition education.
Results
Children on PB had nine and children on AHA had four statistically significant (P<0.05) beneficial changes from baseline (mean decreases): BMI Z-scorePB (−0.14), systolic blood pressurePB (−6.43 mm Hg), total cholesterolPB (−22.5 mg/dL), low density lipoproteinPB (−13.14 mg/dL), hsCRPPB (−2.09 mg/L), insulinPB (−5.42uU/ml), myeloperoxidasePB/AHA (−75.34/69.23 pmol/L), mid-arm circumferencePB/AHA (−2.02/−1.55 cm), weightPB/AHA (−3.05/ −1.14kg) and waist circumferenceAHA (−2.96 cm). Adults on PB and AHA had seven and two respectively statistically significant (P<0.05) beneficial changes. The significant change favoring AHA was a 1% difference in children’s waist circumference. Difficulty shopping for food for the PB was the only statistically significant acceptability barrier.
Conclusions
PB and the AHA in both children and adults demonstrated potentially beneficial changes from baseline in risk factors for CVD. Future larger, long-term randomized trials with easily accessible PB foods will further define the role of the PB in preventing CVD.
There is a need to have effective lifestyle modifications that target the growing group of obese children with dyslipidemia. The beneficial health effects of plant-based diets in adults are known. Studies have suggested that a low-fat, vegan diet (no animal products) may promote weight loss, lower body mass index (BMI), and improve lipoprotein profiles and insulin sensitivity and possibly prevent CVD.6–10 Those who follow a vegetarian diet (no animal products except for dairy and/or eggs) typically have lower cholesterol levels and a lower risk for coronary heart disease than non-vegetarians.11–13 Additionally, vegetarian diets have been shown to not only prevent but reverse heart disease in adults.15, 16
Whether the benefits of a plant-based (only plants and whole grains, limited avocado and nuts) no added fat diet (PB) extend to children is not known. We therefore conducted a four-week randomized trial comparing a PB with the American Heart Association diet (AHA)17 in children ages 9–18 with BMI >95% and total cholesterol >169mg/dL and one of their parents. Similar to the PB, the AHA encourages fruits, vegetables, whole grains and low sodium intake but permits non-whole grains, low-fat dairy, selected plant oils, and lean meat and fish in moderation.
The aim of this study was to determine if a PB and/or AHA significantly change anthropometric measurements and/or biomarkers of inflammation and CVD risk after a 4-week intervention in obese, hypercholesterolemic children age 9–18 years old and one of their parents. Our hypothesis was that both groups would show improvement in the studied outcomes and the improvements might be greater for the PB than AHA.
METHODS
This was a prospective randomized 4-week trial (from April 20, 2013, to May 18, 2013) of either a PB or AHA. It was approved by our institutional review board. We enrolled 30 children seen in a large Midwestern hospital system’s predominantly middle class pediatric practices between the ages of 9–18 years with a last recorded BMI greater than the 95th percentile for age and sex and most recent total cholesterol greater than 169 mg/dL. A parent or guardian also participated in the study and was assigned to follow the same diet that was given to his/her child to help with dietary compliance. Pregnant women were excluded from the study.
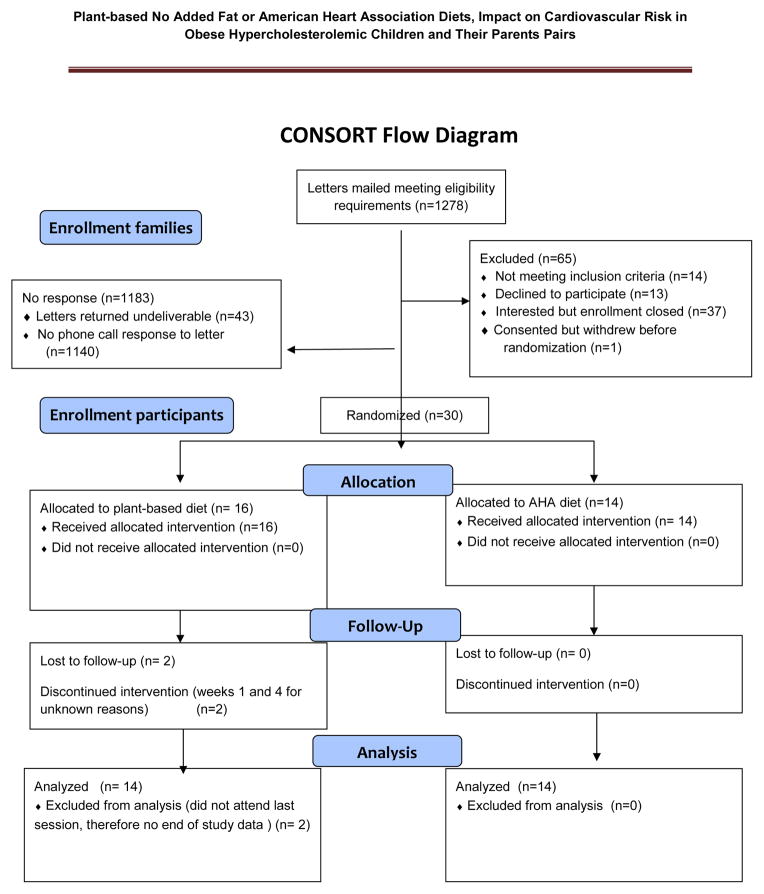
A computerized search of Cleveland Clinic medical records identified 1,278 potential participants (Figure 1; available at www.jpeds.com). Eligible patients were invited by letter to participate in the study. Those interested contacted the principal investigator (PI) and were enrolled on a first come, first served basis. Informed consent was obtained from the participants who were eighteen years of age and older. Participants younger than eighteen years provided assent with parent/guardian approval. There was a time gap between the last recorded measurements, obtaining informed consent, and the start of the study. During this time gap before the start of the study 6 previously obese (BMI >95%) children had become overweight (BMI 85%–95%), and one hypercholesterolemic (>169 mg/dL) child’s cholesterol had decreased to 169 mg/dL. Each child and parent pair received a fifty dollar stipend for each of the four weeks of the study.
Figure 1.
Consort Diagram
Participants assigned to the PB were instructed to avoid all animal products and added fat, and to limit intake of nuts and avocado.15 The AHA group was allowed 30% of calories from total fat, 7% of calories from saturated fat, less than 300 mg cholesterol and less than 1,500 mg of sodium daily.17 All participants received standardized teaching at the time of consent to learn how to record a 24-hour dietary history. Participants completed two 3-day dietary histories consisting of two weekdays and one weekend day; one before the start of the study and one during the study. During the study, participants attended a total of 4 weekly 2-hour classes specific to their assigned diet consisting of one hour of nutrition education and one hour of cooking lessons with recipes provided.
Classes were led by acknowledged study collaborators. Weeks one and two focused on reading labels, where to buy food, food preparation, and how to stay on the assigned diets when eating away from home. Weeks three and four reviewed healthy food choices, the effects of diet on health, discussions of what worked and what did not work for the study participants. At the fifth and final study session, after all laboratory samples and measurements were obtained, participants had the option to attend an introductory class on the diet they were not assigned.
At the start of the 4-week trial, fasting blood samples for biomarkers of inflammation, and CVD risk were obtained. The biomarkers of inflammation were myeloperoxidase [MPO] and high sensitivity C-reactive protein (hsCRP)— which are biomarkers for inflammation and cardiovascular risk in prepubescent obese children and adults,18, 19 as well as IL-6, ALT, and AST. The CVD risk biomarkers were total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), and included biomarkers for diabetes; HgbA1c, fasting glucose and insulin. Laboratory analysis included total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) by standard enzymatic methodology, hemoglobin A1c (HgbA1c) (in percentages %) by turbidimetric inhibition immunoassay, insulin by chemiluminescence immunoassay, high sensitivity C-reactive protein (hs-CRP) by immunoturbidometric assay and fasting plasma glucose by glucose hexokinase method.
All analyses were performed in the Preventive Research Laboratory and Lab Diagnostic Core, Cleveland Clinic.
Measurements (height, weight, mid-arm circumference, waist circumference, and blood pressure) were also obtained at the start of the trial. BMI was calculated by dividing weight in kilograms by height in meters squared. Measurements of the physical activity of the children and adolescents were self-reported using the Physical Activity Questionnaire.20 The PAQ consists of 9 questions which ask subjects to rate their physical activity for the previous 7 days, at different times of day and days of week, and how often they engaged in specific activities. All items are presented on a 5-point scale where 1 is low activity and 5 is high activity; the overall PAQ score is a mean of the 9 questions. All measurements were repeated at the completion of the study for comparison with baseline. Race/ethnicity was self-reported to help determine the comparability of the study groups. At the conclusion of the four-week trial, all participants completed a validated Food Acceptability Questionnaire,21 which subjectively rated the ease of following their assigned diets and their general like or dislike of the diet.
The sample size of 15 adults and children per group was calculated to substantially exceed, even with a 20% drop-out rate, the 6–7 patients per group required to provide a power of 90% at a significance level of 0.05 to detect the within-group changes from baseline in total cholesterol described previously (mean ± standard deviation decrease of 60±26 mg/dl)14 versus a null hypothesis mean decrease of 25±26mg/dl. We did not power our study to demonstrate statistically significant differences between two effective dietary interventions. Families were randomized to the two study groups in a 1:1 ratio in blocks of four families, stratified by the child’s age group (age strata 9–13 years vs. 14–18 years). The randomization was performed by Ms. Worley using an SAS computer program between the end of enrollment and the first weekly session.
Demographics, comorbidities, and body measurements were collected in a REDCap database,22 using double data entry. Laboratory values were provided in an Excel sheet. Diet journals were entered into and analyzed using Nutrition Data System for Research (NDSR) software.
STATISTICAL ANALYSES
Mean daily nutrients were computed for each subject within the pre-study and during-study periods. The BMI of the children was converted to age- and sex- adjusted percentiles and their corresponding z-scores; statistical analysis was performed on the z- scores. Parent and child subjects were analyzed separately because their outcomes were likely to be correlated, given genetic and environmental similarities. For the primary analysis, within- group changes from baseline to week 4 were computed and their means estimated with 95% confidence intervals; log-transformations of baseline and week 4 values of variables were performed as needed. For the secondary analysis, the PB and AHA groups were compared at the end of the trial, adjusting for baseline values, using analysis of covariance (ANCOVA) models. Where needed to meet model assumptions, both the baseline and week 4 values of variables were log-transformed. Study groups were compared on responses to each question on the food acceptability questionnaire using Fisher’s exact tests and Cochran-Armitage trend tests. Sample sizes for individual variables reflect missing data. All analyses were performed on a complete- case basis. All tests were two-tailed and performed at a significance level of 0.05. SAS 9.2 software (SAS Institute, Cary, NC) was used for all analyses and R 3.0.0 (The R Foundation for Statistical Computing) was used for plots.
RESULTS
Sixteen families were randomized to the PB and 14 families to the AHA. Two families, both in the PB group were lost to follow-up. One discontinued after the first week, and the other after the third week. Both families were excluded from the analysis because no end of study data was available. The final study cohort consisted of 28 families, 14 in each group (Figure 1). There were no significant between group differences in baseline demographic, nutrient, and clinical outcomes (Tables I–III; Tables II and III available at www.jpeds.com).
Table 1.
Demographics
| Factor | Plant-based (N=14) | AHA (N=14) |
|---|---|---|
| Child: male | 5(36) | 5(36) |
| Child: Age, years | 15.0(9.0,18.0) | 15.0(9.0,18.0) |
| Child: race | ||
| • None given | 1(7) | 0(0) |
| • White | 10(71) | 10(71) |
| • Black | 2(14) | 4(29) |
| • Asian | 1(7) | 0(0) |
| Child: Hispanic ethnicity* | 0(0) | 1(9) |
| Child: BMI at baseline | ||
| • Overweight | 4(29) | 2(14) |
| • Obese | 10(71) | 12(86) |
| Child: Blood pressure at baseline | ||
| • Normal | 8(57) | 8(57) |
| • Prehypertension | 5(36) | 4(29) |
| • Hypertension | 1(7) | 2(14) |
| Child: High cholesterol at baseline (>169) | 14(100) | 13(93) |
| Parent: male | 5(36) | 4(29) |
| Parent: Age (years) | 46.5(37.0,61.0) | 46.0(35.0,51.0) |
| Parent: race | ||
| • White | 10(71) | 9(64) |
| • Black | 3(21) | 4(29) |
| • Asian | 1(7) | 0(0) |
| • More than one race | 0(0) | 1(7) |
| Parent: Hispanic ethnicity* | 0(0) | 1(10) |
| Parent: BMI at baseline | ||
| • Normal weight | 1(7) | 3(21) |
| • Overweight | 4(29) | 3(21) |
| • Obese | 9(64) | 8(57) |
| Parent: Blood pressure at baseline | ||
| • Normal | 7(50) | 3(21) |
| • At risk | 3(21) | 8(57) |
| • High | 4(29) | 3(21) |
| Parent: High cholesterol at baseline (>199) | 8(57) | 8(57) |
Data not available for all subjects. Missing values: Child: Hispanic ethnicity = 6, Parent: Hispanic ethnicity = 8.
Values presented as Means with SDs indicated in parenthesis, Median [P25, P75], Median (min, max) or N (column %).
Table 3.
Clinical Outcomes
| Outcome | Plant-Based (N=14) Mean (SD) |
AHA (N=14) Mean (SD) |
PB - AHA Adjusted Mean Difference (95% CI) at Week 4 | PB/AHA Adjusted Mean Ratio (95% CI) at Week 4 | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Change | Baseline | Week 4 | Change | ||||
| CHILDREN | |||||||||
| BMI percentile | 96.36 (2.63) | 95.24 (2.89) | −1.12 (1.69) | 98.01 (1.81) | 97.93 (1.79) | −0.08 (0.42) | −1.17 (−2.20, −0.14) | 0.028 | |
| BMI Z score | 1.90 (0.38) | 1.77 (0.39) | −0.14 (0.16) | 2.19 (0.38) | 2.17 (0.37) | −0.03 (0.07) | −0.13 (−0.24, −0.03) | 0.017 | |
| Systolic BP (mm Hg) | 116.25 (14.10) | 109.82 (9.67) | −6.43 (8.86) | 111.50 (20.16) | 106.36 (9.87) | −5.14 (17.64) | 1.87 (−4.41, 8.15) | 0.55 | |
| Diastolic BP (mm Hg) | 68.79 (9.61) | 66.18 (8.75) | −2.61 (10.25) | 69.79 (11.56) | 65.43 (7.43) | −4.36 (14.67) | 1.01 (0.92, 1.11) | 0.83 | |
| Weight (kg) | 79.62 (23.43) | 76.57 (23.38) | −3.05 (3.27) | 87.71 (19.85) | 86.16 (19.01) | −1.55 (1.83) | −1.71 (−3.80, 0.39) | 0.11 | |
| Waist circ (cm) | 97.11 (12.05) | 95.58 (12.22) | −1.53 (3.01) | 104.89 (11.99) | 101.93 (12.53) | −2.96 (4.04) | 1.32 (−1.66, 4.30) | 0.37 | |
| Midarm circ (cm) | 33.30 (5.02) | 31.29 (4.25) | −2.02 (2.17) | 35.06 (3.64) | 33.91 (3.37) | −1.14 (1.63) | −1.25 (−2.61, 0.11) | 0.070 | |
| PAQ | 2.44 (0.63) | 2.66 (0.83) | 0.22 (0.53) | 2.36 (0.74) | 2.20 (0.63) | −0.16 (0.54) | 0.39 (−0.02, 0.80) | 0.060 | |
| CHOL (mg/dL) | 213.79 (41.97) | 191.29 (70.34) | −22.50 (36.31) | 194.07 (25.69) | 177.57 (25.53) | −16.50 (28.19) | −10.34 (−36.48, 15.80) | 0.42 | |
| TRIG (mg/dL) | 139.93 (139.03) | 114.43 (89.77) | −25.50 (95.26) | 119.29 (63.31) | 106.14 (52.58) | −13.14 (36.19) | 1.01 (0.77, 1.32) | 0.94 | |
| HDL (mg/dL) | 55.43 (19.10) | 49.50 (16.11) | −5.93 (9.37) | 43.36 (6.96) | 40.43 (6.17) | −2.93 (3.71) | 0.17 (−5.07, 5.40) | 0.95 | |
| LDL (mg/dL) | 132.21 (29.04) | 119.07 (54.22) | −13.14 (35.54) | 126.86 (19.06) | 115.86 (18.45) | −11.00 (23.31) | 0.95 (0.80, 1.13) | 0.54 | |
| GLU (mg/dL) | 98.86 (45.38) | 99.79 (48.89) | 0.93 (6.01) | 98.57 (49.50) | 97.93 (46.81) | −0.64 (5.02) | 1.01 (0.96, 1.05) | 0.81 | |
| hsCRP (mg/L) | 5.11 (8.33) | 3.03 (4.64) | −2.09 (7.10) | 4.09 (3.76) | 6.87 (8.63) | 2.78 (8.76) | 0.46 (0.21, 1.00) | 0.049 | |
| ALT (U/L) | 18.50 (6.64) | 19.29 (14.14) | 0.79 (14.35) | 25.36 (27.45) | 24.21 (28.33) | −1.14 (3.46) | 1.00 (0.76, 1.32) | 0.98 | |
| AST (U/L) | 18.43 (3.55) | 21.21 (8.40) | 2.79 (8.96) | 22.50 (13.95) | 22.50 (17.77) | 0.00 (4.69) | 1.13 (0.92, 1.40) | 0.23 | |
| IL-6 (pg/ml) | 1.72 (1.10) | 1.55 (1.34) | −0.17 (0.89) | 2.33 (2.50) | 2.14 (1.97) | −0.19 (3.14) | 0.26 (0.05, 1.37) | 0.11 | |
| MPO (pmol/L) | 287.54 (147.35) | 212.20 (138.87) | −75.34 (37.34) | 303.79 (89.92) | 234.56 (87.95) | −69.23 (72.92) | 0.95 (0.79, 1.13) | 0.52 | |
| HgbA1c | 5.77 (1.60) | 5.94 (1.63) | 0.17 (0.17) | 6.14 (1.82) | 6.34 (1.82) | 0.21 (0.12) | 0.99 (0.97, 1.01) | 0.51 | |
| Insulin (uU/ml) | 17.33 (14.12) | 11.91 (9.40) | −5.42 (5.68) | 23.19 (12.57) | 26.36 (26.25) | 3.16 (25.62) | 0.70 (0.43, 1.12) | 0.13 | |
| PARENTS | |||||||||
| BMI | 33.26 (7.69) | 31.98 (7.57) | −1.29 (1.14) | 37.08 (12.66) | 36.35 (12.20) | −0.73 (0.92) | −0.69 (−1.47, 0.09) | 0.082 | |
| Systolic BP (mm Hg) | 125.18 (16.98) | 117.21 (17.18) | −7.96 (12.28) | 121.64 (15.07) | 118.50 (11.90) | −3.14 (14.42) | 0.97 (0.89, 1.05) | 0.39 | |
| Diastolic BP (mm Hg) | 78.25 (12.06) | 74.79 (8.75) | −3.46 (11.06) | 79.29 (10.34) | 72.64 (8.58) | −6.64 (12.19) | 1.03 (0.94, 1.13) | 0.46 | |
| Weight (kg) | 93.33 (27.18) | 89.70 (26.28) | −3.64 (3.41) | 100.79 (32.16) | 98.77 (30.73) | −2.01 (2.66) | −1.95 (−4.16, 0.26) | 0.081 | |
| Waist circ (cm) | 104.41 (15.05) | 102.47 (18.20) | −1.94 (5.80) | 112.11 (24.55) | 111.62 (25.13) | −0.49 (5.73) | −1.14 (−5.75, 3.48) | 0.62 | |
| Midarm circ (cm) | 35.52 (6.61) | 34.20 (6.19) | −1.32 (2.06) | 35.89 (7.67) | 36.24 (8.61) | 0.35 (3.16) | −1.68 (−3.80, 0.44) | 0.11 | |
| CHOL (mg/dL) | 210.43 (51.63) | 176.64 (40.80) | −33.79 (30.84) | 214.43 (45.28) | 207.29 (55.66) | −7.14 (24.93) | −27.29 (−48.68, −5.90) | 0.014 | |
| TRIG (mg/dL) | 130.07 (80.18) | 136.29 (89.70) | 6.21 (41.71) | 117.21 (53.57) | 134.07 (75.74) | 16.86 (36.86) | 0.95 (0.76, 1.19) | 0.67 | |
| HDL (mg/dL) | 56.14 (17.24) | 48.00 (15.60) | −8.14 (5.40) | 59.57 (13.47) | 54.64 (15.70) | −4.93 (5.61) | 0.94 (0.87, 1.02) | 0.16 | |
| LDL (mg/dL) | 128.36 (42.00) | 101.36 (36.06) | −27.00 (26.72) | 131.36 (43.67) | 125.86 (50.02) | −5.50 (20.89) | −21.92 (−40.37, −3.46) | 0.022 | |
| GLU (mg/dL) | 101.50 (31.28) | 106.43 (53.88) | 4.93 (24.65) | 100.64 (22.69) | 95.21 (14.36) | −5.43 (12.40) | 1.06 (0.97, 1.16) | 0.20 | |
| hsCRP (mg/L) | 3.60 (4.16) | 3.36 (3.52) | −0.24 (1.36) | 5.23 (6.20) | 5.44 (5.15) | 0.21 (1.90) | 0.68 (0.44, 1.07) | 0.091 | |
| ALT (U/L) | 21.14 (8.73) | 22.00 (13.69) | 0.86 (7.01) | 20.64 (7.43) | 25.21 (13.73) | 4.57 (9.69) | 0.85 (0.67, 1.08) | 0.17 | |
| AST (U/L) | 18.93 (4.41) | 19.07 (5.59) | 0.14 (5.70) | 18.14 (5.04) | 22.57 (7.71) | 4.43 (6.73) | 0.83 (0.68, 1.03) | 0.084 | |
| IL-6 (pg/ml) | 7.86 (21.09) | 8.02 (20.83) | 0.16 (0.90) | 2.13 (1.53) | 1.94 (1.06) | −0.19 (0.85) | 1.14 (0.83, 1.57) | 0.40 | |
| MPO (pmol/L) | 297.59 (145.78) | 314.50 (236.87) | 16.91 (110.66) | 256.14 (70.52) | 257.91 (87.62) | 1.78 (45.93) | 0.93 (0.80, 1.10) | 0.39 | |
| HgbA1c | 5.89 (0.97) | 5.73 (0.88) | −0.16 (0.16) | 5.81 (0.51) | 5.94 (0.79) | 0.14 (0.49) | 0.96 (0.92, 1.00) | 0.031 | |
| Insulin (uU/ml) | 13.46 (8.25) | 10.34 (6.67) | −3.11 (8.56) | 16.33 (12.33) | 13.18 (7.96) | −3.15 (5.47) | 0.87 (0.67, 1.13) | 0.27 | |
Table 2.
Nutrients Compared Between Plant-based and American Heart Association Diets
| CHILDREN | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Energy (kcal) | 1686.32 (650.59) |
1208.02 (288.96) |
−478.30 (496.93) |
1638.16 (484.17) |
1115.90 (462.71) |
−522.26 (289.68) |
1.14 (0.93, 1.38) |
0.19 | |
| Outcome | Plant-Based (N=14) Mean (SD) |
AHA (N=14) Mean (SD) | PB - AHA Adjusted Mean Difference (95% CI) at Week 4 |
PB/AHA Adjusted Mean Ratio (95% CI) at Week 4 |
P- value |
||||
| Baseline | During study |
Change | Baseline | During study |
Change | ||||
| Total Fat (g) | 65.72 (30.97) | 26.60 (17.90) | −39.12 (26.57) | 69.66 (20.34) | 33.39 (18.31) | −36.27 (12.93) | 0.82 (0.57, 1.19) | 0.29 | |
| Total Carbohydrate (g) | 217.35 (97.86) | 215.83 (58.11) | −1.52 (74.58) | 198.09 (73.71) | 152.39 (73.06) | −45.70 (46.51) | 53.18 (15.48, 90.88) | 0.008 | |
| Total Protein (g) | 63.61 (17.76) | 39.61 (6.92) | −24.00 (16.98) | 60.06 (20.81) | 56.38 (17.83) | −3.68 (20.89) | −17.69 (−27.70, −7.68) | 0.001 | |
| Animal Protein (g) | 42.32 (13.21) | 2.24 (4.45) | −40.08 (14.35) | 38.61 (17.38) | 38.25 (15.48) | −0.36 (21.99) | −36.19 (−45.28, −27.10) | <0.001 | |
| Cholesterol (mg) | 181.23 (81.81) | 12.93 (30.63) | −168.30 (79.95) | 169.90 (84.68) | 143.70 (105.57) | −26.20 (104.27) | −134.32 (−192.59, −76.05) | <0.001 | |
| Total Saturated Fatty Acids (SFA) (g) | 21.97 (8.78) | 5.46 (4.52) | −16.51 (8.51) | 22.41 (11.32) | 10.20 (6.75) | −12.22 (7.32) | 0.49 (0.33, 0.75) | 0.002 | |
| Total Trans-Fatty Acids (TRANS) (g) | 2.11 (1.22) | 0.80 (1.03) | −1.31 (1.63) | 2.69 (1.24) | 1.05 (0.54) | −1.64 (1.28) | 0.51 (0.24, 1.08) | 0.078 | |
| Omega-3 Fatty Acids (g) | 1.33 (1.16) | 0.62 (0.51) | −0.71 (1.17) | 1.57 (0.83) | 0.87 (0.41) | −0.70 (0.81) | −0.22 (−0.58, 0.13) | 0.21 | |
| Total Dietary Fiber (g) | 13.68 (4.27) | 29.01 (7.91) | 15.32 (6.30) | 12.92 (5.15) | 13.58 (7.65) | 0.66 (3.71) | 2.22 (1.79, 2.75) | <0.001 | |
| Vitamin D (calciferol) (mcg) | 4.08 (1.88) | 1.47 (1.44) | −2.61 (1.65) | 3.78 (4.21) | 2.45 (1.66) | −1.33 (4.38) | 0.53 (0.31, 0.92) | 0.026 | |
| Vitamin B-12 (cobalamin) (mcg) | 4.75 (3.47) | 2.03 (2.55) | −2.72 (3.33) | 3.08 (1.69) | 2.96 (1.40) | −0.12 (1.74) | 0.45 (0.25, 0.82) | 0.011 | |
| Calcium (mg) | 806.89 (327.11) | 395.80 (84.63) | −411.09 (297.61) | 611.89 (315.44) | 493.82 (249.68) | −118.07 (309.23) | 0.78 (0.60, 1.02) | 0.069 | |
| Iron (mg) | 16.32 (10.20) | 15.39 (6.58) | −0.92 (11.38) | 11.72 (4.88) | 10.33 (5.13) | −1.39 (3.97) | 4.12 (−0.57, 8.82) | 0.082 | |
| Sodium (mg) | 2841.92 (962.40) | 1697.75 (401.15) | −1144.17 (1160.52) | 2842.88 (886.84) | 1699.27 (897.71) | −1143.61 (473.64) | −1.20 (−499.33, 496.93) | 0.99 | |
| % Calories from Fat | 33.48 (6.80) | 18.04 (8.56) | −15.44 (8.24) | 36.99 (6.67) | 25.38 (6.12) | −11.61 (8.50) | −6.15 (−11.98, −0.32) | 0.039 | |
| % Calories from SFA | 11.78 (4.15) | 3.59 (2.17) | −8.19 (4.39) | 11.36 (2.47) | 7.59 (2.38) | −3.77 (1.80) | 0.43 (0.31, 0.59) | <0.001 | |
| PARENTS | |||||||||
| Energy (kcal) | 1964.39 (950.71) | 1235.70 (335.76) | −728.70 (839.79) | 1660.18 (422.06) | 1142.41 (368.92) | −517.77 (460.19) | 1.07 (0.86, 1.32) | 0.54 | |
| Total Fat (g) | 84.93 (50.55) | 25.84 (14.22) | −59.09 (50.54) | 70.90 (26.34) | 33.93 (20.18) | −36.96 (29.81) | 0.75 (0.49, 1.13) | 0.16 | |
| Total Carbohydrate (g) | 218.33 (101.44) | 222.89 (67.31) | 4.56 (81.29) | 190.82 (47.77) | 158.45 (54.82) | −32.38 (48.99) | 52.30 (11.62, 92.97) | 0.014 | |
| Total Protein (g) | 83.12 (36.04) | 41.33 (11.46) | −41.79 (34.43) | 69.31 (18.91) | 57.32 (13.69) | −11.99 (19.44) | −17.71 (−27.61, −7.80) | 0.001 | |
| Animal Protein (g) | 54.22 (26.26) | 1.30 (2.27) | −52.93 (25.89) | 46.43 (16.55) | 35.40 (13.93) | −11.03 (12.93) | −35.41 (−42.90, −27.93) | <0.001 | |
| Cholesterol (mg) | 241.61 (161.29) | 7.93 (14.13) | −233.69 (155.19) | 212.75 (89.96) | 119.64 (67.33) | −93.11 (117.02) | −112.16 (−151.00, −73.33) | <0.001 | |
| Total Saturated Fatty Acids (SFA) (g) | 28.14 (17.45) | 5.03 (4.21) | −23.11 (16.49) | 22.60 (9.85) | 10.12 (5.62) | −12.48 (11.48) | 0.45 (0.29, 0.72) | 0.002 | |
| Total Trans-Fatty Acids (TRANS) (g) | 3.15 (2.21) | 0.47 (0.28) | −2.68 (2.24) | 3.07 (2.57) | 1.04 (0.49) | −2.03 (2.62) | 0.35 (0.18, 0.69) | 0.004 | |
| Omega-3 Fatty Acids (g) | 1.62 (0.62) | 0.94 (0.67) | −0.69 (0.96) | 1.63 (0.68) | 0.84 (0.72) | −0.79 (0.69) | 0.10 (−0.44, 0.64) | 0.71 | |
| Total Dietary Fiber (g) | 20.11 (9.59) | 31.99 (8.24) | 11.88 (11.32) | 17.52 (7.53) | 16.42 (6.50) | −1.10 (7.22) | 1.99 (1.55, 2.55) | <0.001 | |
| Vitamin D (calciferol) (mcg) | 2.67 (1.82) | 0.96 (1.39) | −1.72 (2.35) | 3.71 (3.32) | 2.87 (2.64) | −0.84 (3.98) | 0.45 (0.25, 0.80) | 0.008 | |
| Vitamin B-12 (cobalamin) (mcg) | 3.97 (2.35) | 1.01 (1.72) | −2.97 (2.54) | 3.37 (1.56) | 3.38 (3.04) | 0.02 (3.35) | 0.32 (0.18, 0.58) | <0.001 | |
| Calcium (mg) | 780.10 (333.35) | 387.65 (170.62) | −392.45 (350.28) | 572.29 (123.52) | 524.45 (245.01) | −47.84 (160.68) | 0.71 (0.49, 1.01) | 0.059 | |
| Iron (mg) | 14.76 (6.30) | 14.94 (13.51) | 0.19 (13.37) | 11.54 (3.01) | 11.19 (4.12) | −0.35 (3.21) | 1.10 (0.77, 1.57) | 0.58 | |
| Sodium (mg) | 3515.11 (1899.46) | 1708.01 (464.17) | −1807.11 (1748.47) | 3235.59 (1109.71) | 1754.93 (787.72) | −1480.66 (1241.75) | −78.00 (−574.67, 418.67) | 0.75 | |
| % Calories from Fat | 35.73 (7.37) | 17.40 (6.81) | −18.33 (7.69) | 36.58 (6.90) | 24.44 (7.32) | −12.14 (8.71) | −6.77 (−12.07, −1.46) | 0.015 | |
| % Calories from SFA | 11.69 (4.22) | 3.31 (1.73) | −8.38 (3.78) | 11.66 (2.91) | 7.30 (2.16) | −4.36 (4.01) | −3.99 (−5.53, −2.45) | <0.001 | |
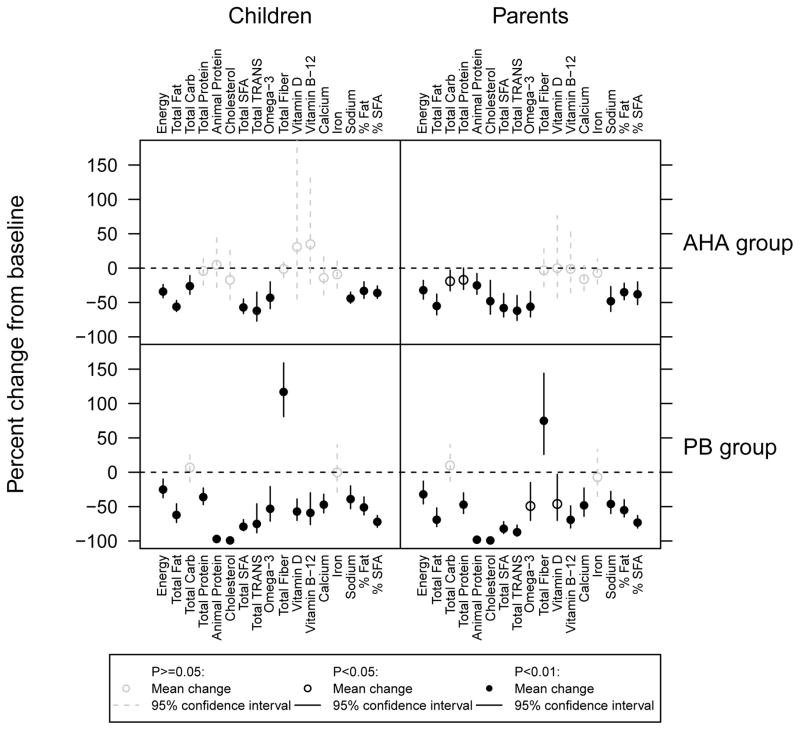
The total energy intake and the intake of almost all measured nutrients significantly decreased in children and adults in both groups, and dietary fiber intake significantly increased only in PB diet group (both children and adults) based on dietary histories completed during the study compared with those completed at baseline (Figure 2 and Table II). When comparing the PB and AHA groups during the study, children and adults in the PB group had a significantly lower intake of total protein, animal protein, cholesterol, total saturated fat, vitamin D, vitamin B12, percent of calories from fat and percent of calories from saturated fat. Children and adults in the PB group also had a significantly higher intake of total carbohydrates and dietary fiber than children in the AHA group. During the study, children and adults of both groups significantly reduced and increased intakes of the same nutrients, except for a trans-fat decrease only in adults on PB. Within-study energy intake and total fat intake were not significantly different between the two study groups, in children or adults.
Figure 2. Nutrient Outcomes Within Groups.
SFA-Saturated Fatty Acids, Trans-Trans Fats
Two goals for the children on PB were to consume no animal products and add no fat. During the study the mean (standard deviation) daily reported animal protein intake decreased from 42.32 (13.21) g to 2.24 (4.45) g (P <0.001) and the % of calories from fat and saturated fat was 18.04% (8.56%) and 3.59% (2.17%) respectively. The goals for the AHA children’s group were to consume <30% of total calories from fat, <7% of calories from saturated fat, <1,500 mg sodium, and <300 mg cholesterol. The respective mean (standard deviation) reported values during the study were 25.38% (6.12%), 7.59% (2.38%), 1,699 (897.71) mg and 144 (105.57) mg. Adults in both groups reported similar changes (Table II). These results suggest good but not perfect compliance with the assigned diets.
On The Food Acceptability Questionnaire18 using a seven-point response scale both children and parents in the PB group reported more difficulty purchasing the necessary food for their diet than the children and parents in the AHA group. Median difficulty ratings were 3 (“Slightly difficult”) in the PB group and 5 (“Fairly easy”) in the AHA group for both parent and child subject. Mean PB vs. AHA ratings were 3.7 vs. 5.1 for children and 3.5 vs. 5.1 for parents. There were no other statistically significant differences between the groups on how well they liked these foods, liked the taste, appearance appeal, how boring, ease of preparation, ease of maintaining diet at restaurants, effort to stay on diet, effect on cost of food purchases, satisfaction felt after meals, and overall satisfaction.
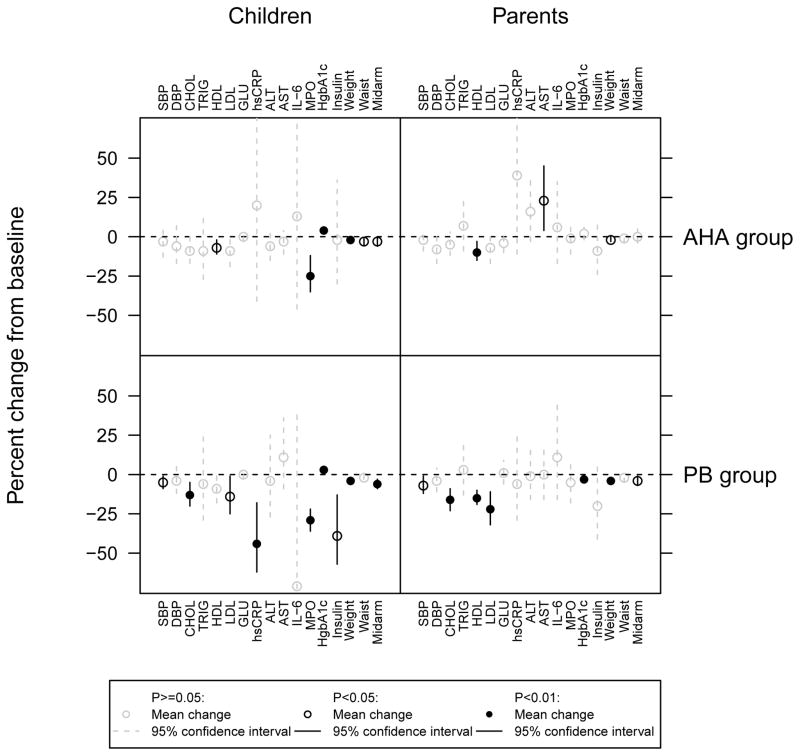
In the group of children on PB, there were statistically significant (P<0.05) mean decreases in nine measures: BMI Z-score (−0.14), systolic blood pressure (−6.43 mm Hg), weight (−3.05 kg), mid-arm circumference (−2.02 cm), total cholesterol (−22.5 mg/dL), LDL (−13.14 mg/dL), hsCRP (−2.09 mg/L), MPO (−75.34 pmol/L), and insulin (−5.42 uU/ml). In the AHA children group, there were statistically significant mean decreases in 5 measures: weight (−1.55 kg), waist circumference (−2.96 cm), mid-arm circumference (−1.14 cm), high density lipoprotein (−2.93 mg/dL), and MPO (−69.23 pmol/L). Both groups had statistically significant increases in Hgb A1c (PB +0.17%, AHA +0.21%) (Figure 3 and Table III). In the group of adults on PB, there were statistically significant (P<0.05) mean decreases in eight measures: BMI (−1.29kg/m2), systolic blood pressure (−7.96mm Hg), weight (−3.64kg), mid-arm circumference (−1.32cm), total cholesterol (−33.79mg/dL), HDL (−8.14mg/dL), LDL (−27.0mg/dL), and Hgb A1C (−0.16%). In the AHA adult group, there were statistically significant mean decreases in three measures: BMI (−0.73kg/m2), weight (−2.01kg), and HDL (−4.93mg/dL). The only statistically significant mean increase was AST (+4.43 U/L) in the AHA group (Figure 3 and Table III).
Figure 3. Clinical Outcomes Within Groups.
SBP-Systolic Blood Pressure, DBP-Diastolic Blood Pressure
The only statistically significant differences between the PB and AHA groups after the intervention were that the children in the PB group had significantly lower week 4 BMI Z-scores and hsCRP levels. Parents in the PB group had significantly lower total cholesterol, LDL, and Hgb A1C than parents in the AHA group. The only significant change favoring AHA was a 1% difference in children’s waist circumference.
The primary analysis of our study was of evaluable cases.
In this study of PB in children we were most interested in determining if adhering to a PB would improve cardiovascular risk. A secondary intent-to-treat analysis was performed including the two child/parent pairs on PB who failed to complete the study. For those pairs, we assumed that there was no change from baseline in outcomes where baseline measures were available, and for measures with unavailable baseline data (insulin, IL-6, and myeloperoxidase), we assigned the median baseline value of the measure, computed separately for all parent and all child subjects, to both the baseline and end of study values. There were no differences between the evaluable-case analysis and intent-to-treat analysis in the statistical significance of within- group changes in outcome. The only differences between the evaluable-case analysis and intent- to-treat analysis in the statistical significance of the PB and AHA group comparisons were that children in the PB group no longer had significantly lower week 4 hsCRP or vitamin D intake, and parents in the PB no longer had significantly lower total protein or percent of calories from fat (data not shown).
DISCUSSION
We believe our inability to demonstrate more than a few significant differences between our intervention groups, versus many significant differences from baseline values, most likely reflects the fact our study was powered to detect changes from baseline values in the PB group with 4 weeks intervention.14
Statistically significant differences that occurred between baseline and week 4 that might be of possible concern for increased cardiovascular risk include the elevation of Hgb A1c in both the plant-based and AHA children groups. However, the only statistically significant change in HOMA-IR in our study was in the PB children’s group with a mean ratio (95% CI) of −1.25 (−2.01, −0.99), P-value 0.004, which suggests significantly decreased insulin resistance. In the PB children’s group FIRI (fasting immunoreactive insulin) and in the adult PB group HgbA1c decreased significantly. All these values suggest, if anything, a decreased risk of diabetes in the PB group. Other studies have documented beneficial effects of PB on diabetes.6, 7, 24 The statistically significant decreases of HDL in both the adult and children AHA group and the adult PB group are also of some concern. The decrease in serum HDL cholesterol after 4 weeks was most likely associated with early weight loss.25 Vegan diets have been previously reported to be associated with decreased HDL, but vegan diets are also associated with a decreased risk of heart disease.8,15, 26 However, we are unaware of similar reports of lowering HDL on the AHA diet. There is heterogeneity described in the composition and function of HDL, and further characterization of HDL after exposure to these diets, might prove helpful in establishing the significance of these findings of decreased HDL.27, 28 The statistically significant elevation of AST in the adults on the AHA diet is of unknown significance. Mild, transient increases in ALT and AST values have been observed in weight loss studies, but usually returned to below baseline levels after substantial weight loss.29, 30
Statistically significant reported differences in the PB and AHA diet in adults and children of decreased cholesterol, fat and calorie intake and increased fiber on the PB diet most likely were of benefit to the obese hypercholesterolemic children studied.9, 24 Previous reports demonstrate that dietary records, especially in individuals with higher BMI, commonly underestimate intake by nearly 50%, so the actual intake is difficult to know for certain. The reported decrease of protein intake, especially in the context of probable under-reporting of intake, is not of concern as PB diets have been shown to provide adequate protein and most other nutrients in adults and children.31–33 The decreased intake of Vitamins B12 and D reported in the adult and child PB groups found in the current study have been noted previously. Vitamin B 12 definitely and Vitamin D probably warrant supplements for those on long-term PB diets. Intake of key nutrients is generally adequate in a balanced vegan diet, but it is still essential to monitor consumption of protein, n-3 fatty acids, iron, zinc, iodine, and calcium in long-term vegans.31–33 Dietary intake of n-3 long-chain polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid and docosahexaenoic acid, is low in vegans compared with omnivores.34 Therefore, n-3 fatty acids, particularly in a no added fat vegan diet, should be especially carefully monitored and may also require supplementation with algae-derived n-3 PUFAs. The only significant described problem in our middle class study population for diet acceptance was the difficulty purchasing food. Cost may be a barrier to a PB in lower socioeconomic populations. In another study the only identified barrier to adherence was the effort required.35 If the PB diet is to achieve ever increasing adaptation, barriers to easy, affordable access to plant-based, no added fat foods will need to be reduced.
The major limitations of our study are that this was a small study conducted for a short period of time in a select group of middle class patients with less than completely reliable measures of compliance and with no direct health outcomes measures. Also, the AHA is considered a standard of care and was used as a comparison group—there was no placebo- controlled group. There is also concern that long-term compliance with the PB could be problematic. This is especially true given the difficulties expressed by parents and children in finding food to purchase for the diet in our study, and in the effort required to maintain a PB in a previous study.21 However, there are studies describing good acceptability and compliance with a PB. 15,16,35,36
Plant-based diets are generally recognized as safe for children and adolescents as long as the intake of key nutrients is monitored and appropriate supplements are provided. The results of our study suggest that the documented benefits of PB in adults, including, but not limited to, decreased overweight and obesity and decreased cardiovascular risk, most likely would be seen in children. These benefits, especially given the known onset of CVD in childhood, could improve the lifetime health of those populations who choose to eat a PB beginning in childhood.
Acknowledgments
Supported by National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000439) and Research Program Committee and Pediatric Research Fund Grants from the Cleveland Clinic.
Alicia Thomas, MS, RD, LD (Case Western Reserve University), taught the American Heart Association Diet and contributed to the study design. Jane Esselstyn, RN, is an author and teacher who taught the Plant-Based No Added Fat Diet. Stacy Payne, BS, Megan Villarreal, BA, and Kay Stelmach, RN, helped coordinate the study and with members of their Cleveland Clinic Clinical Research Unit aided in data collection. Alan Pratt, MT (ASCP) (Preventive Research Laboratory and Lab Diagnostic Core, Cleveland Clinic), managed laboratory analysis. Amy Moore, BA (Department of Scientific Publications, Cleveland Clinic), provided medical editing. Lorelei Woody, MLIS (Cleveland Clinic Alumni Library), helped with manuscript preparation. Vaishal Shah, MD, MPH (Cleveland Clinic Children’s), did data entry and collection, and Stephanie Bernstein, BSDH, also helped with data entry. Sana Mansoor, MD, Alex Golden, MD, Asif Padiyath, MBBS, Kari Gali, MSN, RN, CPNP (all from Cleveland Clinic Children’s), performed data collection. Francette Malone provided secretarial support, and June Cassano, RN, BSN, MBA (both from Cleveland Clinic Children’s), helped with budget preparation. John Kirwan, PhD (Department of Pathobiology Cleveland Clinic and Department of Nutrition Case Western Reserve University, funded by NIH RO1 AG12834 and DK089547, and UL1 RR024989), facilitated the participation of Tammie Kong, Adam Weier, and Alicia Thomas and reviewed the initial study design.
LIST OF ABBREVIATIONS AND ACRONYMS
- CVD
Cardiovascular disease
- PB
Plant-based (only plants and whole grains, limited avocado and nuts) no added fat diet
- AHA
American Heart Association Diet (also encourages fruits, vegetables, whole grains and low sodium intake but permits non-whole grains, low-fat dairy, selected plant oils, and lean meat and fish in moderation.)
- HOMA-IR
Homeostasis Model Assessment –Insulin Resistance=FIRI (fasting immunoreactive insulin mU/l)×FPG (fasting plasma glucose mmol/l)/22.5
Footnotes
No reprints are available
The authors declare no conflicts of interest.
Registered with ClinicalTrials.gov: NCT01817491
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael Macknin, Department of General Pediatrics, Cleveland Clinic Children’s.
Tammie Kong, Department of Nutrition, Case Western Reserve University.
Adam Weier, Department of Nutrition, Case Western Reserve University; currently Nutrition and Food Service, VA Eastern Colorado Healthcare System.
Sarah Worley, Department of Quantitative Health Sciences, Cleveland Clinic.
Anne S. Tang, Department of Quantitative Health Sciences, Cleveland Clinic.
Naim Alkhouri, Department of Pediatric Gastroenterology, Cleveland Clinic Children’s.
Mladen Golubic, Center for Lifestyle Medicine, Cleveland Clinic.
References
- 1.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The bogalusa heart study. J Pediatr. 2007;150:12–17. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. National Heart, Lung, and Blood Institute. . Pediatrics. 2011;128:S213–56. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillman MW, Daniels SR. Is universal pediatric lipid screening justified? JAMA. 2012;307:259–260. doi: 10.1001/jama.2011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Psaty BM, Rivara FP. Universal screening and drug treatment of dyslipidemia in children and adolescents. JAMA. 2012;307:257–258. doi: 10.1001/jama.2011.1916. [DOI] [PubMed] [Google Scholar]
- 6.Barnard ND, Cohen J, Jenkins DJA, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29:1777–1783. doi: 10.2337/dc06-0606. [DOI] [PubMed] [Google Scholar]
- 7.Barnard ND, Scialli AR, Turner-McGrievy G, Lanou AJ, Glass J. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am J Med. 2005;118:991–997. doi: 10.1016/j.amjmed.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 8.Ferdowsian HR, Barnard ND. Effects of plant-based diets on plasma lipids. Am J Cardiol. 2009;104:947–956. doi: 10.1016/j.amjcard.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Mishra S, Xu J, Agarwal U, Gonzales J, Levin S, Barnard ND. A multicenter randomized controlled trial of a plant-based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: The GEICO study. Eur J Clin Nutr. 2013;67:718–724. doi: 10.1038/ejcn.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner-McGrievy GM, Barnard ND, Scialli AR. A two-year randomized weight loss trial comparing a vegan diet to a more moderate low-fat diet. Obesity (Silver Spring) 2007;15:2276–2281. doi: 10.1038/oby.2007.270. [DOI] [PubMed] [Google Scholar]
- 11.Fraser GE. Vegetarian diets: What do we know of their effects on common chronic diseases? Am J Clin Nutr. 2009;89:1607S–1612S. doi: 10.3945/ajcn.2009.26736K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu FB. Plant-based foods and prevention of cardiovascular disease: An overview. Am J Clin Nutr. 2003;78:544S–551S. doi: 10.1093/ajcn/78.3.544S. [DOI] [PubMed] [Google Scholar]
- 13.Tuso PJ, Ismail MH, Ha BP, Bartolotto C. Nutritional update for physicians: Plant-based diets. The Permanente journal. 2013;17:61–66. doi: 10.7812/TPP/12-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esselstyn R. The Engine 2 Diet. New York: Wellness Central Hachette Book Group; 2009. [Google Scholar]
- 15.Esselstyn CB, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD? J Fam Pract. 2014;63:356–364b. [PubMed] [Google Scholar]
- 16.Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 17.American Heart Association Nutrition Committee. Lichtenstein AH, Appel LJ, et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the american heart association nutrition committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 18.Olza J, Aguilera CM, Gil-Campos M, et al. Myeloperoxidase is an early biomarker of inflammation and cardiovascular risk in prepubertal obese children. Diabetes Care. 2012;35:2373–2376. doi: 10.2337/dc12-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pignatelli P, Loffredo L, Martino F, et al. Myeloperoxidase overexpression in children with hypercholesterolemia. Atherosclerosis. 2009;205:239–243. doi: 10.1016/j.atherosclerosis.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Janz KF, Lutuchy EM, Wenthe P, Levy SM. Measuring activity in children and adolescents using self-report: PAQ-C and PAQ-A. Med Sci Sports Exerc. 2008;40:767–772. doi: 10.1249/MSS.0b013e3181620ed1. [DOI] [PubMed] [Google Scholar]
- 21.Barnard ND, Scialli AR, Bertron P, Hurlock D, Edmonds K. Acceptability of a therapeutic low-fat, vegan diet in premenopaual women. J Nutr Educ. 2000;32:314–319. [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda Y, Suehiro T, Nakamura T, Kumon Y, Hashimoto K. Clinical significance of the insulin resistance index as assessed by homeostasis model assessment. Endocrine Journal. 2001;48:81–86. doi: 10.1507/endocrj.48.81. [DOI] [PubMed] [Google Scholar]
- 24.Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-week clinical trial. Am J Clin Nutr. 2009;89:1588S–1596S. doi: 10.3945/ajcn.2009.26736H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: A meta- analysis. Am J Clin Nutr. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 26.Kent L, Morton D, Rankin P, et al. The effect of a low-fat, plant-based lifestyle intervention (CHIP) on serum HDL levels and the implications for metabolic syndrome status - a cohort study. Nutr Metab (Lond) 2013;10:58-7075-10-58. doi: 10.1186/1743-7075-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32:2813–2820. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Didonato JA, Levison BS, et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat Med. 2014;20:193–203. doi: 10.1038/nm.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasteyger C, Larsen TM, Vercruysse F, Astrup A. Effect of a dietary-induced weight loss on liver enzymes in obese subjects. Am J Clin Nutr. 2008;87:1141–1147. doi: 10.1093/ajcn/87.5.1141. [DOI] [PubMed] [Google Scholar]
- 30.Jhaveri MA, Anderson JW. Sequential changes of serum aminotransferase levels in severely obese patients after losing weight through enrollment in a behavioral weight loss program. Postgrad Med. 2010;122:206–212. doi: 10.3810/pgm.2010.07.2188. [DOI] [PubMed] [Google Scholar]
- 31.Amit M. Vegetarian diets in children and adolescents. Paediatr Child Health. 2010;15:303–314. [PMC free article] [PubMed] [Google Scholar]
- 32.Craig WJ, Mangels AR American Dietetic Association. Position of the american dietetic association: Vegetarian diets. J Am Diet Assoc. 2009;109:1266–1282. doi: 10.1016/j.jada.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Dewell A, Weidner G, Sumner MD, Chi CS, Ornish D. A very-low-fat vegan diet increases intake of protective dietary factors and decreases intake of pathogenic dietary factors. J Am Diet Assoc. 2008;108:347–356. doi: 10.1016/j.jada.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 34.Welch AA, Shakya-Shrestha S, Lentjes MAH, Wareham NJ, Khaw K. Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat eaters, vegetarians, and vegans and the precursor-product ratio of a-linolenic acid to long-chain n-3 polyunsaturated fatty acids: Results from the EPIC-norfolk cohort. (american journal of clinical nutrition (2010) 92, (1040–51)) Am J Clin Nutr. 2011;93:676. doi: 10.3945/ajcn.2010.29457. [DOI] [PubMed] [Google Scholar]
- 35.Barnard ND, Scialli AR, Turner-McGrievy G, Lanou AJ. Acceptability of a very low-fat, vegan diet compares favorably to a more moderat low-fat diet in a randomized, controlled trial. J Cardiopulm Rehabil. 2004;24:229–235. doi: 10.1097/00008483-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Barnard ND, Gloede L, Cohen J, et al. A low-fat vegan diet elicits greater macronutrient changes, but is comparable in adherence and acceptability, compared with a more conventional diabetes diet among individuals with type 2 diabetes. J Am Diet Assoc. 2009;109:263–272. doi: 10.1016/j.jada.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]