Abstract
To investigate the association between analyses of sub-maximal treadmill exercise test (TMT) and long-term myocardial ischemia (Mis) and silent Mis in community-dwelling older adults, 898 Rancho Bernardo Study participants (mean age 55) without coronary heart disease underwent TMT and were followed up to 27 years. The main outcome measures are incidence of Mis and silent Mis. During follow up, 97 Mis and 103 silent Mis events occurred. We measured ST change, inability to achieve target heart rate (iTHR), abnormal heart rate recovery (HRR), and chronotropic incompetence (ChI). Each parameter was a significant predictor for Mis and silent Mis. An integrated scoring model was based on these 4 parameters and defined as sum of numbers of abnormal parameters. After multiple adjustments, an integrated scoring model independently predicted Mis and silent Mis. The incidence rates of abnormalities of parameters are 36.5% for 1 abnormality, 9.1% for 2 abnormalities, and 2.0% for 3 or 4 abnormalities. Compared to those with normal results, participants with 1 or 2 abnormalities had significantly increased risk for Mis (HR 1.79 or 2.34) and silent Mis (HR 1.80 or 2.64), respectively. Participants with 3 or more positive findings showed an even higher risk for Mis (HR 7.96, [3.02-21.00]) and silent Mis (HR 3.22, [0.76-13.60]). In conclusion, ST change, ChI, abnormal HRR, iTHR, and integrated scoring model of TMT were independent predictors of long-term Mis and silent Mis in an asymptomatic middle-aged population. Management of ChI or abnormal HRR in an asymptomatic population may prevent future ischemic heart disease and thus improve the quality of life.
Keywords: Chronotropic incompetence, Heart Rate Recovery, Myocardial ischemia, ST change, Target Heart Rate, Treadmill Exercise Test
Introduction
It is well known that chronotropic incompetence (ChI) and abnormal heart rate recovery (HRR) are independent predictors of major adverse cardiovascular events and overall mortality (1-4). However, the independent value of the treadmill exercise test (TMT) used as a screening tool in asymptomatic adults to predict future coronary artery disease, and especially to predict silent ischemia, is not yet known (5, 6). The present study was designed to assess ST change, ChI, inability to achieve target heart rate (iTHR), abnormal HRR, and integrated analysis of these parameters as predictors of myocardial ischemia (Mis) and silent Mis in community-dwelling asymptomatic older adults followed up to 27 years.
Methods
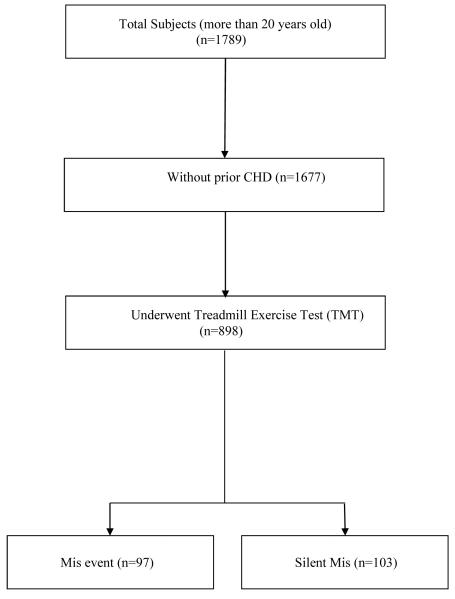
The Rancho Bernardo Study is a prospective population-based study of older adults residing in a suburban southern California community. The cohort of residents enrolled was quite homogeneous—they were almost entirely Caucasian and most were white-collar workers. Between 1972 and 1974, a total of 1789 community-dwelling adults participated in a heart disease risk factor survey, which served as the baseline visit for the present study. Participants with a history of CHD (myocardial infarction, angina, or coronary artery bypass surgery) were excluded from the TMT. The data of 898 participants who underwent TMT at baseline are used for this analysis (Figure 1). The study protocol was approved by the Human Research Protection Program at the University of California, San Diego; all participants gave written informed consent prior to participation. Participants were followed by annual mailed questionnaires, and they returned for research clinic visits approximately every four years through 1999, up to 27 years.
Figure 1.
Summary of study population (CHD—coronary heart disease; Mis— myocardial ischemia).
A sub-maximal TMT was administered to participants (7, 8); exclusions included aortic stenosis, congestive heart failure, severe hypertension, R-on-T type premature ventricular contractions, ventricular tachycardia, parasystolic focus, atrial flutter, congenital heart disease, second reschedule required others. The exercise test was terminated for any of the following reasons; 1) subjective response: the subject was unwilling or unable to continue exercise; 2) development of potential hazards to the subject; 3) attainment of near-maximal exercise--exercise was stopped if the subject attained age-predicted target heart rate (THR) and maintained it for one minute, if the subject maintained THR until the end of the ongoing exercise stage, or if subject’s heart rate exceeded target heart rate by 8 beats/min, whichever occurred first (8, 9). A test was considered to be positive if 1) ST depression or elevation of 1 mm or more was recorded by the visual coders, 2) the ST integral fell by at least 10 diV-sec from its resting value to a value of 10 gV-sec or less, or 3) the ST integral rose by at least 10 gV-sec from its resting value.
Three non-electrocardiographic measures were defined as: 1) an abnormal HRR–a decrease of <22 bpm after 2 min of recovery(10); 2) ChI--the inability to achieve 80% of heart rate reserve, using a standard equation to define the percentage heart rate reserve [(maximal heart rate − resting heart rate)/ (174-0.54 × age) − (resting heart rate) × 100] (11); 3) THR was considered achieved when 90% of maximal heart rate predicted for subject’s age was attained(2).
The primary outcomes were Mis and silent Mis. Myocardial ischemia, determined by using standard epidemiologic methods (such as annual mailed questionnaires and interviews at regular clinic visits), consisted of a history of myocardial infarction, angina pectoris, coronary revascularization, or coronary artery bypass graft history.
Silent Mis was defined as ≥1 ischemic resting ECG abnormalities, newly revealed at a follow-up visit with no history of myocardial infarction, angina pectoris, or chest pain not meeting the Rose algorithm.
i) “ECG coronary probable”--major Q or QS wave [Minnesota Code 1.1, 1.2]; complete left bundle branch block [Minnesota Code 7.1.1]
ii) “ECG coronary possible”--small Q or QS wave [Minnesota Code 1.3]; ST depression [Minnesota Code 4.1 – 4.3]; T wave items [Minnesota Code 5.1 – 5.3](12).
No Evidence of Cardiovascular Disease was defined as: no ECG changes and no history of myocardial infarction, angina pectoris, or chest pain (≥ 30 min). Data on vital status was collected on all participants. More than 99% of this cohort was followed for vital status by annual mailer through 1999.
Death certificates were obtained for all decedents and coded for cause of death by a certified nosologist using the 9th revision of the “International Classification of Diseases, Adapted” (ICDA-9). Deaths due to coronary heart disease included coronary death, myocardial infarction, coronary insufficiency, and angina (ICD-9 codes 410.00-414.00). We classified deaths due to coronary heart disease as apparent myocardial ischemia.
Categorical variables are reported as numbers (percentages), and continuous variables are presented as means (standard deviation). Cox proportional hazards regression analyses were performed to obtain multivariate-adjusted hazard ratios of Mis and silent Mis of those who had abnormal test results during the TMT versus those with normal test results. Hazard ratios were adjusted for age by decade, sex, cholesterol level, history of diabetes, and smoking. We performed the supremum test for proportional hazards assumption with 1000 replications in Cox regression model. Although TMT had a marginal significance in the test of proportionality, we used the time-dependent Cox regression model because the other exposure variables fit the proportionality assumption. We restricted study subjects who had performed TMT in our analyses, and there were no missing in exposure variables such as TMT and target HR. There was 1 missing in the variables of HRR and ChI. Also our main exposure variables such as ST change, THR, HRR, ChI were binomial scales (achievement vs. no-achievement; positive vs. negative etc.), not continuous scales, and so there were no outliers. There was no interaction effect between the main exposure variable and the other confounders in our multivariate models. A two-tailed p<0.05 was considered statistically significant. Data were analyzed using the SAS statistical package (SAS institute, Chicago, Illinois).
Results
The baseline characteristics of participants are provided in Table 1; 898 Rancho Bernardo Study participants underwent TMT and were followed for up to 27 years (mean age at baseline 55.04±14.85, 481 were women); 218 (24.3%) were current smokers, 366 (40.8%) were daily drinkers, 180 (20.0%) had metabolic syndrome, and 38 (4.2%) had diabetes mellitus.
Table 1.
Baseline characteristics of study population.
| Variable | Total cohort (n=898) | Myocardial Ischemia |
|
|---|---|---|---|
| Apparent (n=97) | Silent (n=103) | ||
| Age (years) | 55.04±14.85 | 65.65± 10.46 | 59.5±98.33 |
| BMI (Kg/m2) | 24.96 ±3.46 | 24.77 ± 3.37 | 24.97 ± 3.54 |
| Total Cholesterol (mg/dL) | 228.54 ± 43.58 | 238.05 ± 40.58 | 230.53 ± 43.14 |
| Triglycerides (mg/dL) | 141.87 ± 101.92 | 127.05 ± 71.18 | 141.00 ± 87.49 |
| HDL (mg/dL) | 58.20 ± 18.76 | 58.71 ± 18.41 | 60.32 ± 18.65 |
| LDL (mg/dL) | 152.51 ± 40.53 | 164.46 ± 37.89 | 153.72 ± 42.66 |
| SBP (mmHg) | 147.57 ± 18.78 | 158.02 ± 20.89 | 151.42 ± 17.08 |
| DBP (mmHg) | 99.23 ± 9.47 | 100.20 ± 10.03 | 100.00 ± 9.31 |
| HR (beats/min) | 84.24 ± 13.14 | 81.53 ± 11.64 | 81.69 ± 13.43 |
| Fasting plasma glucose (mg/dL) | 99.30 ± 18.23 | 103.71 ± 27.62 | 98.58 ± 13.36 |
| Current Smoker | 218(24.3%) | 25(26.3%) | 19(18.4%) |
| Daily Alcohol Drinker | 366(40.8%) | 46(48.4%) | 43(41.7%) |
| Regular Exercise (3+ times per week) | 109(88.6%) | 9(90.0%) | 10(90.9%) |
| Family History of CVD | 147(16.6%) | 18(19.4%) | 25(24.3%) |
| Diabetes mellitus | 38(4.2%) | 8(8.4%) | 3(2.9%) |
| Metabolic Syndrome (Modified WHO) | 180(20.0%) | 20(21.1%) | 22(21.4%) |
| Lipid-modifying agent | 68(7.6%) | 25(26.3%) | 14(13.6%) |
| Anti-Diabetes mellitus agent | 15(1.7%) | 4(4.2%) | 1(1.0%) |
| Anti-Hypertension | 99(11.0%) | 18(18.9%) | 13(12.6%) |
| Diuretics | 44(5.1%) | 6(6.4%) | 7(6.8%) |
| Anti-Arrhythmia | 6(0.7%) | 0 | 1(1.0%) |
Categorical variables are reported as number (percentages) and continuous variables as mean (standard deviation). CVD—cardiovascular disease.
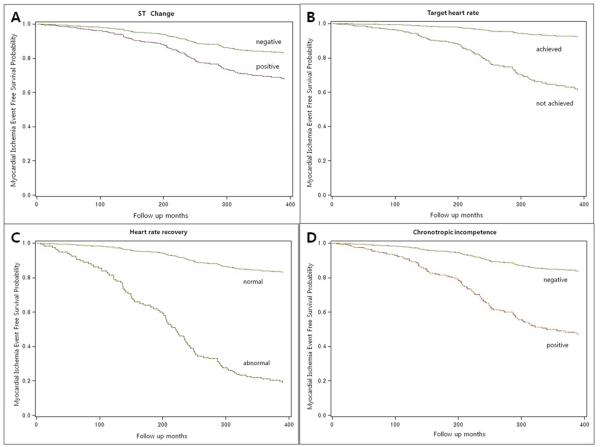
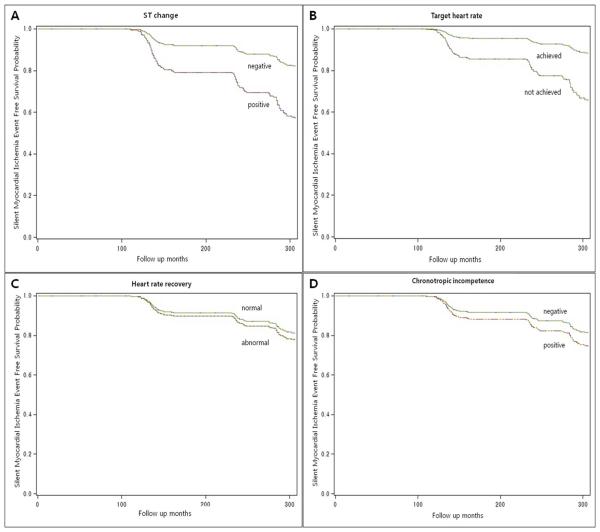
Fifty-three (5.9%) participants showed positive TMT (ST change). Overall, 418 participants (46.5%) were unable to achieve their THR. 22 participants (2.5%) had abnormal HRR, and chronotropic incompetence (ChI) was detected in 56 participants (6.2%). In Cox proportional hazards models, after adjusting for age, sex, cholesterol level, diabetes, and smoking history, positive TMT was independently associated with Mis (HR 1.72, 95% CI 0.83-3.59) and silent Mis (HR 2.16, 95% CI 1.16-4.19); iTHR was associated with Mis (adjusted HR 2.11, 95% CI 1.25-3.57) and silent Mis (HR 2.16, 95% CI 1.33-3.50) regardless of causes for stopping TMT (Table 2, Figure 2 and 3). Abnormal HRR was also independently associated with Mis (adjusted HR 5.30, 95% CI 2.14-13.15) and silent Mis (HR 1.29, 95% CI 1.18-9.37). And ChI was associated with Mis (HR 1.92, 95% CI 1.01-3.65) but not silent Mis (adjusted HR 0.99, 95% CI 0.40-2.47) (Table 2, Figures 2 and 3).
Table 2.
Risk for myocardial ischemia (Mis) and silent myocardial ischemia by result of each test.
| Test Result | N of total cohort |
N of Mis events |
HR (95% CI) c | HR (95% CI) d | N of total cohort |
N of Silent Mis events |
HR (95% CI) c | HR (95% CI) d |
|---|---|---|---|---|---|---|---|---|
| Treadmill Exercise Test | ||||||||
| Negative | 845 | 89 | 1.00 | 1.00 | 845 | 93 | 1.00 | 1.00 |
| Positive | 53 | 8 | 1.72(0.83-3.55) | 1.72(0.83-3.59) | 53 | 10 | 2.26(1.17-4.36) | 2.16(1.16-4.19) |
| Target Heart Rate | ||||||||
| Achieved | 480 | 26 | 1.00 | 1.00 | 480 | 40 | 1.00 | 1.00 |
| Not achieved | 418 | 71 | 2.24(1.32-3.81) | 2.11(1.25-3.57) | 418 | 63 | 2.10(1.30-3.39) | 2.16(1.33-3.50) |
| Due to heart-related symptoms | 273 | 46 | 2.06(1.17-3.61) | 1.94(1.11-3.37) | 273 | 50 | 2.53(1.53-4.17) | 2.60(1.57-4.31) |
| Due to leg pain and weakness | 90 | 14 | 2.52(1.25-5.05) | 2.41(1.20-4.84) | 90 | 9 | 1.55(0.72-3.32) | 1.56(0.73-3.36) |
| Miscellaneous | 55 | 11 | 2.86(1.32-6.18) | 2.69(1.28-5.79) | 55 | 4 | 1.00(0.34-2.89) | 1.06(0.36-3.07) |
| Heart rate recoverya | ||||||||
| Normal | 875 | 91 | 1.00 | 1.00 | 875 | 102 | 1.00 | 1.00 |
| Abnormal | 22 | 6 | 5.67(2.34-13.75) | 5.30(2.14-13.15) | 22 | 1 | 1.17(0.16-8.47) | 1.29(1.18-9.37) |
| Chronotropic Incompetenceb | ||||||||
| Negative | 841 | 85 | 1.00 | 1.00 | 841 | 98 | 1.00 | 1.00 |
| Positive | 56 | 12 | 1.91(1.01-3.61) | 1.92(1.01-3.65) | 56 | 5 | 0.97(0.39-2.42) | 0.99(0.40-2.47) |
Abnormal heart rate recovery was defined as a decrease of <22bpm after 2 min of recovery.
Chronotropic incompetence was defined as the inability to achieve 80% of heart rate reserve, using the regression equation [(maximal heart rate - resting heart rate)/(174-0.54 × age)-(resting heart rate) x100].
Adjusted for age and sex.
Adjusted for age, sex, cholesterol level, diabetes history, and smoking history.
Figure 2.
Myocardial ischemia event free survival probability per (A) ST change, (B) inability to achieve target heart rate, (C) abnormal heart rate recovery, and (D) chronotropic incompetence.
Figure 3.
Silent myocardial ischemia event free survival probability per (A) ST change, (B) inability to achieve target heart rate, (C) abnormal heart rate recovery, and (D) chronotropic incompetence.
Even in the sub-analysis excluding ST segment abnormalities, iTHR was persistently associated with higher Mis (adjusted HR 2.10, 95% CI 1.22-3.61) and silent Mis (adjusted HR 1.74, 95% CI 1.05-2.90), and abnormal HRR remained a predictor of Mis (adjusted HR 3.94, 95% CI 1.34-11.63) (Table 3).
Table 3.
Risk for myocardial ischemia (Mis) and silent myocardial ischemia by result of each test in subjects with negative treadmill exercise test.
| Test Result | N of total cohort |
N of Mis events |
HR (95% CI) c | HR (95% CI) d | N of total cohort |
N of Silent Mis events |
HR (95% CI) c | HR (95% CI) d |
|---|---|---|---|---|---|---|---|---|
| Target Heart Rate | ||||||||
| Achieved | 470 | 26 | 1.00 | 1.00 | 470 | 40 | 1.00 | 1.00 |
| Not achieved | 375 | 63 | 2.16(1.24-3.75) | 2.10(1.22-3.61) | 375 | 53 | 1.71(1.03-2.83) | 1.74(1.05-2.90) |
| Due to heart related symptoms | 241 | 43 | 2.11(1.18-3.78) | 2.01(1.13-3.56) | 241 | 41 | 2.00(1.18-3.40) | 2.06(1.21-3.52) |
| Due to leg pain and weakness | 81 | 10 | 2.02(0.92-4.40) | 2.09(0.95-4.58) | 81 | 8 | 1.39(0.62-3.12) | 1.38(0.61-3.09) |
| Miscellaneous | 53 | 10 | 2.64(1.19-5.87) | 2.59(1.17-5.72) | 53 | 4 | 0.91(0.31-2.65) | 0.94(0.32-2.73) |
| Heart Rate Recoverya | ||||||||
| Normal | 824 | 85 | 1.00 | 1.00 | 824 | 92 | 1.00 | 1.00 |
| Abnormal | 20 | 4 | 4.48(1.55-12.92) | 3.94(1.34-11.63) | 20 | 1 | 1.29(0.17-8.97) | 1.34(0.18-9.76) |
| Chronotropic Incompetenceb | ||||||||
| Negative | 792 | 79 | 1.00 | 1.00 | 792 | 89 | 1.00 | 1.00 |
| Positive | 52 | 10 | 1.86(0.92-3.73) | 1.77(0.87-3.59) | 52 | 4 | 0.86(0.31-2.38) | 0.89(0.32-2.49) |
Abnormal heart rate recovery was defined as a decrease of <22bpm after 2 min of recovery.
Chronotropic incompetence was defined as the inability to achieve 80% of heart rate reserve, using the regression equation [(maximal heart rate - resting heart rate)/(174-0.54 × age)-(resting heart rate) x100].
Adjusted for age and sex.
Adjusted for age, sex, cholesterol level, diabetes history, and smoking history.
The number of positive findings among these 4 measures (positive TMT, iTHR, abnormal HRR, and ChI) was closely associated with higher Mis and silent Mis. The incidence rates of abnormalities of parameters are 36.5% for 1 abnormality, 9.1% for 2 abnormalities, and 2.0% for 3 or 4 abnormalities. Compared with normal findings, any one abnormal finding predicted a 1.79-fold higher risk for Mis and 1.80-fold higher risk for silent Mis. Two and three or more positive findings were associated with a 2.34- and 7.96-fold higher risk for Mis and 2.64- and 3.22-fold higher risk for silent Mis, respectively (Table 4).
Table 4.
Hazard ratios (95% confidence intervals) for myocardial ischemia (Mis) and silent myocardial ischemia of treadmill exercise test-related scoring system.
| Treadmill Exercise Test resultsa | N of total cohort |
N of Mis events |
HR (95% CI)b | HR (95% CI)c | N of total cohort |
N of silent Mis events |
HR (95% CI)b | HR (95% CI)c |
|---|---|---|---|---|---|---|---|---|
| Incidence of Mis or Silent Mis | ||||||||
| All normal results | 468 | 26 | 1.00 | 468 | 40 | 1.00 | ||
| One abnormal result | 328 | 52 | 1.85(1.07-3.22) | 1.79(1.03-3.09) | 328 | 49 | 1.77(1.07-2.93) | 1.80(1.09-2.99) |
| Two abnormal results | 82 | 13 | 2.53(1.23-5.19) | 2.34(1.14-4.82) | 82 | 12 | 2.56(1.27-5.14) | 2.64(1.30-5.36) |
| Three or four abnormal results | 18 | 6 | 8.37(3.21-21.79) | 7.96(3.02-21.00) | 18 | 2 | 3.23(0.77-13.64) | 3.22(0.76-13.60) |
| p-trend | 0.0001 | 0.0001 | 0.027 | 0.025 |
Abnormal Graded Exercise Test results (treadmill exercise test positive, abnormal heart rate recovery, inability to achieve target heart rate during treadmill exercise test, chronotropic incompetence).
Adjusted for age and sex.
Adjusted for age, sex, cholesterol level, diabetes history, and smoking history.
Discussion
Silent Mis is defined as objective documentation of Mis in the absence of angina or angina equivalents. Its clinical significance is now well established, but there are few prognostic studies of silent ischemia in the general population or in truly asymptomatic populations (13-15). Silent Mis is usually diagnosed when there is asymptomatic ST depression during TMT or ambulatory ECG monitoring; however, whether ChI, iTHR, or abnormal HRR can predict future silent Mis in a community-dwelling population had not been evaluated.
Chronotropic incompetence (ChI), broadly defined as the inability of the heart to increase its rate commensurate with increased activity or demand, is common in patients with cardiovascular disease, produces exercise intolerance that impairs quality of life, and is predictive of increased mortality and coronary heart disease risk, independent of various confounding factors, including age, gender, physical fitness, traditional cardiovascular risk factors, and ST change during exercise (2, 16, 17). Our study showed that ChI was associated with Mis (HR 1.92, 95% CI 1.01-3.65) but not silent Mis (adjusted HR 0.99, 95% CI 0.40-2.47)
Traditionally, the ability to reach THR was used as a signal of sufficient cardiac loading during the TMT; iTHR is also considered an impaired chronotropic response. In this cohort study, iTHR was associated with 2.11- and 2.16-fold increased risk for Mis and silent Mis, respectively. And it was persistently associated with risk for Mis and silent Mis in only GXT-negative subjects.
Abnormal HRR after exertion also has been associated with increased all-cause mortality risk in a variety of asymptomatic and diseased populations (18), even after adjusting for severity of cardiovascular disease, left ventricle (LV) function, and exercise capacity (19). In alignment with earlier reports, our study confirmed that abnormal HRR was a strong predictor of future Mis including silent Mis (Table 3).
ChI, iTHR, and abnormal HRR have a similar pathophysiologic mechanism, failure of heart rate control. The mechanisms that have been proposed to explain ChI and iTHR are 1) underlying autonomic nervous system imbalance; 2) reduced myocardial viability; and 3) attenuated protective response to permit greater myocardial perfusion in the presence of narrowed coronary arteries (20). The ability of HRR following exercise is related to the capacity of the cardiovascular system to reverse autonomic nervous system and baroreceptor adaptations that occur during exercise, often termed vagal (21, 22). We investigated whether integration of these parameters can show an additive value of prediction for future ischemic heart disease including silent Mis.
Strengths of this study include the well-characterized, population-based TMT of community-dwelling older adults, and the long-term follow up. There are also limitations. First, Mis and silent Mis were not confirmed by coronary angiogram or imaging studies, which may raise questions on validity of our data. Second, these results may not be applied to the general population because the cohort of residents was quite homogeneous--almost entirely Caucasian. Third, TMT protocol is submaximal, which would make it difficult to assess the prognostic importance of exercise capacity.
Our study demonstrates that an integrated analysis was useful to predict Mis and silent Mis in the Rancho Bernardo cohort. The higher number of abnormal findings was well correlated with increased risk for Mis and silent Mis. Participants with three or more abnormal findings had more than a 7-fold increased risk for Mis compared to those without abnormal findings, which clearly shows that these parameters provide further information to predict future Mis and silent Mis.
To our knowledge, this is the first paper to show the predictive value of integrated analysis of sub-maximal TMT for Mis and, to a lesser degree, silent Mis, in healthy, community-dwelling older adults followed for up to 27 years. Acknowledgments: The Rancho Bernardo Study received funding support from NIH [National Institutes of Health/National Institute on Aging grants AG07181 and AG028507 and the National Institute of Diabetes and Digestive and Kidney Diseases, grant DK31801]. Dr. So-Young Shin had no relationship with industry when this paper was written; she is now employed by Bayer HealthCare Pharmaceuticals. This financial support does not represent a conflict of interest.
Acknowledgments
Funding: Dr. Barrett-Connor has received grant support for the Rancho Bernardo Study from NIH (National Institutes of Health/National Institute on Aging grants AG07181 and AG028507 and the National Institute of Diabetes and Digestive and Kidney Diseases, grant DK31801).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest to declare. Dr. So-Young Shin had no relationship with industry when this paper was written; she is now employed by Bayer HealthCare Pharmaceuticals but this financial support does not represent a conflict of interest.
References
- 1.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 2.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the framingham heart study. Circulation. 1996;93:1520–1526. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
- 3.Savonen KP, Lakka TA, Laukkanen JA, Halonen PM, Rauramaa TH, Salonen JT, Rauramaa R. Heart rate response during exercise test and cardiovascular mortality in middle-aged men. Eur Heart J. 2006;27:582–588. doi: 10.1093/eurheartj/ehi708. [DOI] [PubMed] [Google Scholar]
- 4.Savonen KP, Kiviniemi V, Laukkanen JA, Lakka TA, Rauramaa TH, Salonen JT, Rauramaa R. Chronotropic incompetence and mortality in middle-aged men with known or suspected coronary heart disease. Eur Heart J. 2008;29:1896–1902. doi: 10.1093/eurheartj/ehn269. [DOI] [PubMed] [Google Scholar]
- 5.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, et al. Acc/aha 2002 guideline update for exercise testing: Summary article: A report of the american college of cardiology/american heart association task force on practice guidelines (committee to update the 1997 exercise testing guidelines) Circulation. 2002;106:1883–1892. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 6.Screening for coronary heart disease: Recommendation statement. Ann Intern Med. 2004;140:569–572. doi: 10.7326/0003-4819-140-7-200404060-00001. [DOI] [PubMed] [Google Scholar]
- 7.Kansal S, Roitman D, Sheffield LT. Stress testing with st-segment depression at rest. An angiographic correlation. Circulation. 1976;54:636–639. doi: 10.1161/01.cir.54.4.636. [DOI] [PubMed] [Google Scholar]
- 8.Sheffield LT, Roitman D. Stress testing methodology. Prog Cardiovasc Dis. 1976;19:33–49. doi: 10.1016/0033-0620(76)90007-4. [DOI] [PubMed] [Google Scholar]
- 9.Sheffield LT. Holter monitoring--state of the art. Ala J Med Sci. 1982;19:426–431. [PubMed] [Google Scholar]
- 10.Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M, Do D, Myers J. Heart rate recovery: Validation and methodologic issues. J Am Coll Cardiol. 2001;38:1980–1987. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]
- 11.Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehabil. 2007;14:215–221. doi: 10.1097/HJR.0b013e328088cb92. [DOI] [PubMed] [Google Scholar]
- 12.Reid DD, Brett GZ, Hamilton PJ, Jarrett RJ, Keen H, Rose G. Cardiorespiratory disease and diabetes among middle-aged male civil servants. A study of screening and intervention. Lancet. 1974;1:469–473. doi: 10.1016/s0140-6736(74)92783-4. [DOI] [PubMed] [Google Scholar]
- 13.Fleg JL, Kennedy HL. Long-term prognostic significance of ambulatory electrocardiographic findings in apparently healthy subjects greater than or equal to 60 years of age. Am J Cardiol. 1992;70:748–751. doi: 10.1016/0002-9149(92)90553-b. [DOI] [PubMed] [Google Scholar]
- 14.Cohn PF, Fox KM, Daly C. Silent myocardial ischemia. Circulation. 2003;108:1263–1277. doi: 10.1161/01.CIR.0000088001.59265.EE. [DOI] [PubMed] [Google Scholar]
- 15.Conti CR, Bavry AA, Petersen JW. Silent ischemia: Clinical relevance. J Am Coll Cardiol. 2012;59:435–441. doi: 10.1016/j.jacc.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Gulati M, Shaw LJ, Thisted RA, Black HR, Bairey Merz CN, Arnsdorf MF. Heart rate response to exercise stress testing in asymptomatic women: The st. James women take heart project. Circulation. 122:130–137. doi: 10.1161/CIRCULATIONAHA.110.939249. [DOI] [PubMed] [Google Scholar]
- 17.Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA. 1999;281:524–529. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- 18.Lauer MS. Autonomic function and prognosis. Cleve Clin J Med. 2009;76(Suppl 2):S18–22. doi: 10.3949/ccjm.76.s2.04. [DOI] [PubMed] [Google Scholar]
- 19.Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003;42:831–838. doi: 10.1016/s0735-1097(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 20.Hammond HK, Kelly TL, Froelicher V. Radionuclide imaging correlatives of heart rate impairment during maximal exercise testing. J Am Coll Cardiol. 1983;2:826–833. doi: 10.1016/s0735-1097(83)80228-9. [DOI] [PubMed] [Google Scholar]
- 21.Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 22.Rosenwinkel ET, Bloomfield DM, Arwady MA, Goldsmith RL. Exercise and autonomic function in health and cardiovascular disease. Cardiol Clin. 2001;19:369–387. doi: 10.1016/s0733-8651(05)70223-x. [DOI] [PubMed] [Google Scholar]