Abstract
Background
Gastrointestinal (GI) dysfunction is a major cause of morbidity in AIDS. HIV-1-induced neuropathogenesis is significantly enhanced by opiate abuse, which increases proinflammatory chemokine/cytokine release, the production of reactive species, glial reactivity, and neuronal injury in the CNS. Despite marked interactions in the gut, little is known about the effects of HIV-1 in combination with opiate use on the enteric nervous system (ENS).
Methods
To explore HIV-opiate interactions in myenteric neurons, effects of Tat ± morphine (0.03 μM, 0.3 μM, 3 μM) were examined in isolated neurons from doxycycline- (DOX-) inducible HIV-1 Tat1-86 transgenic mice or following in vitro Tat 100 nM exposure (> 6 h).
Key Results
Current clamp recordings demonstrated increased neuronal excitability in neurons of inducible Tat(+) mice (Tat+/DOX) compared to control Tat−/DOX. In neurons from Tat+/DOX, but not from Tat−/DOX mice, 0.03 μM morphine significantly reduced neuronal excitability, fast transient and late long-lasting sodium currents. There was a significant leftward shift in V0.5 of inactivation following exposure to 0.03 μM morphine, with a 50% decrease in availability of sodium channels at −100 mV. Similar effects were noted with in vitro Tat exposure in the presence of 0.3 μM morphine. Additionally, GI motility was significantly more sensitive to morphine in Tat(+) mice than Tat(−) mice.
Conclusions & Inferences
Overall, these data suggest that the sensitivity of enteric neurons to morphine is enhanced in the presence of Tat. Opiates and HIV-1 may uniquely interact to exacerbate the deleterious effects of HIV-1-infection and opiate exposure on GI function.
Keywords: HIV-1 Tat, opioid drug abuse, gut, ileum, enteric neurons, sodium currents, excitability, MOR-1 expression
Introduction
It is well known that effects of human immunodeficiency virus type 1 (HIV-1) on the central nervous system (CNS) are exacerbated by drug use (1-4) but not much is known about the effects of HIV-1 in combination with drug use on the enteric nervous system (ENS). The ENS regulates gastrointestinal (GI) motility, secretion and digestion and plays a major role in HIV-1 enteropathy, a syndrome characterized by a chronic, diarrheal illness in HIV-1-infected patients (5, 6). Additionally, opioids such as morphine can directly affect the ENS, causing severe constipation through reduced peristalsis, increased water and electrolyte absorption, and antisecretory actions (7). The increases in HIV-1 pathogenesis caused by opioid abuse have largely been attributed to opioid suppression of immune function (8-10). Additionally, interactions between HIV-1 proteins, such as Tat or gp120, and morphine in the CNS appear to be orchestrated by glial cells (11-14) and likely involve synergistic upregulation of proinflammatory chemokine/cytokine release and production of reactive species (12, 15-18).
ENS bacterial infections, including those that cause infection in the healthy host as well as those that are more opportunistic, occur very commonly among HIV-1 infected patients (19). Bacterial infections are a direct result of the severe cellular immune defects found in HIV-1 patients and epidemiologic factors, such as intravenous drug use and stage of disease progression, play important roles in the disease progression (19).
Although viral loads are suppressed with combined antiretroviral therapy (cART), HIV-1 enteropathy still exists despite cART (20, 21) possibly due to the sustained production of HIV-1 Tat (22). The presence of viral proteins in the gut suggests that HIV itself may be a direct diarrheal pathogen (23, 24). Tat released from infected T-cells and macrophages is intrinsically enterotoxic and may disrupt enteric homeostasis through bystander effects in enterocytes and/or enteric neurons resulting in HIV-1-associated diarrhea (25). We have recently shown that Tat enhances enteric neuronal excitability and elicits an inflammatory response due to the upregulation of pro-inflammatory cytokines in the mouse ileum (26). Acute morphine has been shown to decrease neurotransmitter release via both pre- and post-synaptic mechanisms (27-30) and to decrease the excitability of myenteric neurons (31-33). The decreases in neuronal activity in mouse isolated enteric neurons are due to an inhibition of sodium currents that results from a 50% decrease in availability of channels at hyperpolarized potentials (33).
The present study investigated the effects of acute morphine on the biophysical properties of sodium channels in the presence of Tat. Primary adult mouse myenteric neurons from the ileum were cultured and either treated with 100 nM Tat (> 6 h) or isolated from HIV-Tat1-86 transgenic mice. Results demonstrated that Tat exposure significantly increases the sensitivity of enteric neurons to morphine-mediated decrease in neuronal excitability. The increased neuronal sensitivity was accompanied by a decrease in the availability of sodium channels. Tat-exposure also enhanced the effects of morphine on GI motility in vivo.
Material and Methods
Experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All procedures were reviewed and approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee (VCU IACUC).
Primary neuron culture
Primary myenteric neuronal cultures were prepared from adult mouse as previously described (33, 34). Male Swiss Webster mice (25-30g, Harlan Sprague Dawley, Inc.) or female doxycycline (DOX)-inducible, HIV-Tat1–86 transgenic mice (20-25g, 10-14 days) were used. The HIV-Tat1–86 transgenic mouse model was developed on a C57BL/6J hybrid background and is described in detail elsewhere (35-37). Tat expression, which is under the control of a tetracycline responsive, glial fibrillary acidic protein (GFAP)-selective promoter, was induced with a specially formulated chow containing 6 mg/g DOX (Harlan, Product#: TD.09282, Indianapolis, IN), fed to both the Tat− controls (Tat−/DOX) and the inducible Tat+ mice (Tat+/DOX). Mice were sacrificed by cervical dislocation. The ileum was immediately removed and placed in ice-cold Krebs solution (118 mM NaCl, 4.6 mM KCl, 1.3 mM NaH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 11 mM glucose and 2.5 mM CaCl2) bubbled with carbogen (95 % O2 / 5 % CO2) and divided into short segments which were then threaded longitudinally on a plastic rod through the lumen. Strips from the longitudinal muscle containing the myenteric plexus (LMMP) were gently obtained using a cotton-tipped applicator and LMMP strips were rinsed three times in 1 ml Krebs and gathered by centrifugation (350 × g, 30 s). LMMP strips were then minced with scissors and digested in 1.3 mg/ml collagenase type II (Worthington) and 0.3 mg/ml bovine serum albumin in bubbled Krebs (37 °C) for 1 h, followed by 0.05% trypsin for 7 min. Following each digestion, cells were triturated and collected by centrifuge (1300 × g for 8 min). Cells were then plated on laminin (BD Biosciences) and poly-D-lysine coated coverslips in Neurobasal A media containing B-27 supplement, 1% fetal bovine serum, 10 ng/ml glial cell line-derived neurotrophic factor (GDNF, Neuromics, Edina, MN), and penicillin/streptomycin. Half of the cell media was replaced every 2-3 days with fresh complete neuron media. Cell culture reagents were purchased from Gibco (Grand Island, NY), whereas all other chemicals and reagents were obtained from Sigma Aldrich (St Louis, MO), except were mentioned differently.
Chemicals and treatments
Treatments included HIV-1 Tat1–86 (100 nM; 6 - 16 h; ImmunoDiagnostics; clade B) and morphine sulfate (0.03 μM – 3 μM; acute perfusion ~ 10-20 min; National Institute of Drug Abuse, Bethesda, MD). A Tat concentration of 100 nM was used to elicit functional deficits in glia and neurons that are similar to those occurring with HIV-1 infection, and are considered to reflect levels seen pathophysiologically (12, 18, 38-41). Morphine concentrations were based on our previous findings (33) and preliminary experiments and were chosen to maximally stimulate μ-opioid receptors (MOR). Reagent stocks were stored at −80 °C for less than one month.
Whole cell patch clamp
Neurons were studied from 1-3 days in culture when cells were still rounded and had not flattened out. Coverslips containing cells were placed in an experimental chamber and perfused (1-2 ml/min) with an external physiological solution containing (in mM): 135 NaCl, 5.4 KCl, 0.3 NaH2PO4, 5 HEPES, 1 MgCl2, 2 CaCl2, and 5 glucose. Patch electrodes (2-4 MΩ) were pulled from borosilicate glass capillaries (Sutter Instruments, CA) and filled with internal solution containing (in mM): 100 K-aspartic acid, 30 KCl, 4.5 ATP, 1 MgCl2, 10 HEPES, and 0.1 EGTA. In sodium channel experiments, K-aspartic acid was replaced by cesium (Cs+)-aspartate to block any outward potassium currents. Whole-cell patch-clamp recordings were made with an Axopatch 200A amplifier (Molecular Devices, CA) at room temperature and pulse generation and data acquisition were achieved with Clampex and Clampfit 10.2 software (Molecular Devices, CA). Neuronal excitability was determined in current clamp mode with current provided in 13 sweeps of 0.5 s duration ranging from –0.03 nA to 0.09 nA in 0.01 nA increments. Current-voltage relationships were determined in voltage clamp mode, from 16 or 32 sweeps (0.5 s/sweep) beginning at –100 mV and increasing in 10 mV or 5 mV intervals, respectively, to +50 mV. Current amplitudes were normalized to cell capacitance (pF) to determine current density. The peak amplitude of inward currents and the end of pulse current were measured. In order to determine the effects on the voltage dependence of steady-state activation/inactivation of sodium channels, a double-pulse protocol was used in which a variable conditioning pulse was applied from −100 mV to +50 mV in 10 mV ramps for 300 ms followed by a test pulse. The test potential was 0 mV. The voltage-dependence of steady-state inactivation and activation were determined and fit via Boltzmann's distribution as described previously (42).
Real time PCR
Quantitative real-time PCR assays of μ-opioid receptor 1 (MOR-1) expression levels were performed using a MiniOpticon Real-Time PCR System (Bio-Rad, Hercules, CA, USA). β-actin was used as the reference gene control. Total RNA was extracted by the total RNA Purification System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. cDNA synthesis and subsequent polymerization was performed in a one-step using the iTaq Universal SYBR Green One-Step Kit (Bio-Rad, Hercules, CA, USA). The reaction mixture (20 μl) contained 200 nM forward primer, 200 nM reverse primer, 1x iTaq universal SyBR Green reaction mix, 1x iScript reverse transcriptase, and 200 ng total RNA. Reverse transcription was performed for 10 min at 50 °C with an enzyme activation step for 1 min at 95 °C. The PCR protocol consisted of 40 cycles of denaturation (15 s at 95 °C), annealing and extension (60 s at 60 °C). The cycle threshold value, Ct count, for each sample was normalized by subtracting it from the Ct value of the reference gene β-actin to establish ΔCt (ΔCt = MOR-1 Ct – β-actin Ct) for each sample. All primers were designed using the Vector NTI software (Invitrogen, Carlsbad, CA, USA), and the sequences in this study used were: 5′-CTGCCAAGCTGGCCTTCCCCGGAT-3′ (forward) and 5′-AGCAGTTCCAGAGGGTTGCAAGG-3′ (reverse) for MOR-1, 5′-TGTTACCAACTGGGACGA-3′ (forward) and 5′-GGGGTGTTGAAGGTCTCAAA-3′ (reverse) for β-actin.
Gastrointestinal (GI) motility study
Overall GI transit was determined by counting the fecal pellet output as described previously (26). Briefly, age and weight matched tat transgenic mice were fed DOX chow for one week to activate the tat transgene. The DOX chow was then substituted for regular chow for two weeks to avoid the interference of the DOX diet with the microbiota of the GI tract. A previous PCR study confirmed the continued expression of the tat gene in the mouse ileum after a similar experimental design (26). On testing days, mice were administered either saline or morphine with animals being repeatedly tested every other day for the different doses of morphine (0.03, 0.1, 0.3, or 1.0 mg/kg morphine, i.p., n = 5). 20 min after injection animals were placed in clean cages and the number of fecal pellets was counted after 1 hour (43).
Statistical analyses
Data were analyzed using analysis of variance (ANOVA) (SYSTAT 11.0 for Windows, SYSTAT) followed by Student's t-test or post hoc tests, using Bonferroni's correction or Dunnett's test, when necessary. Percent inhibition data of the peak response at −10 mV were also analyzed using a one-sample t-test. An alpha level of p < 0.05 was considered significant for all statistical tests used. Data are expressed as the mean ± standard error of the mean (SEM) from 5 to 7 neurons.
Results
Effects of morphine on enteric neuronal excitability are enhanced in neurons from Tat+/DOX mice
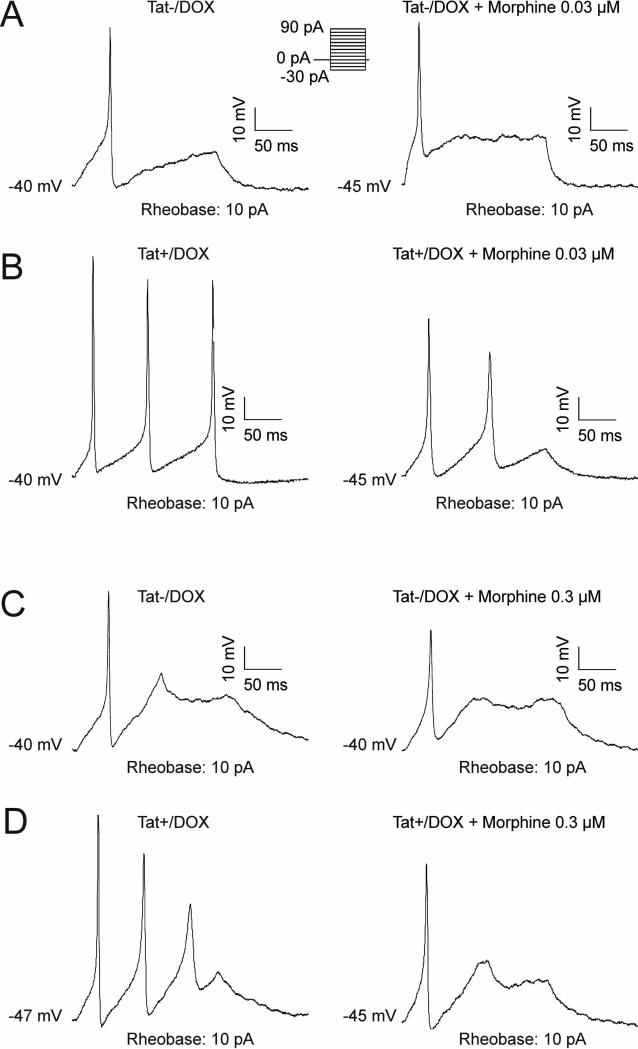
Whole cell patch clamp recordings were performed in current clamp mode to assess action potentials and determine neuronal excitability (Figure 1). Neurons obtained from Tat(+)/DOX were significantly more excitable than those from Tat(−)/DOX (Figure 1A and B, left). Neurons isolated from Tat+/DOX mice, fired multiple action potentials (4 out of 7 neurons) than those isolated from Tat−/DOX (0 out of 7 neurons) similar to our previous report (26). Neuronal excitability was significantly reduced by perfusion with 0.03 μM morphine in Tat+/DOX, while the same concentration of morphine had no effect on Tat−/DOX neurons (Figure 1A and B, right). Current clamp recordings of a representative neuron exposed to Tat−/DOX showed that in the absence of morphine, the neuron elicited single action potentials at a threshold of 0.01 nA, and higher current stimuli (Figure 1A, left). The addition of 0.03 μM morphine to neurons from Tat−/DOX mice had no effect on the number of action potentials elicited, their threshold for firing, or the amplitude of the action potential (Figure 1A, right). In contrast, in neurons from Tat+/DOX mice, multiple action potentials were present at the same rheobase of 10 pA (Figure 1B, left), indicating increased excitability in the presence of Tat. Interestingly, the addition of 0.03 μM morphine significantly reduced the number of action potentials as well as the action potential height (Figure 1B, right). The reduction of excitability with 0.03 μM morphine for neurons of Tat+/DOX but not Tat−/DOX mice suggests there is increased neuronal sensitivity to morphine in the presence of Tat. Importantly, at the higher 0.3 μM concentration of morphine, neurons from both Tat−/DOX and Tat (+)/DOX mice responded with decreased excitability (Figure 1C and D), indicating that neurons from Tat(−)/DOX are responsive to morphine but are less sensitive in the absence of Tat.
Figure 1. Effect of morphine on enteric neuronal excitability is enhanced in neurons from Tat+/DOX but not Tat−/DOX mice.
Neuronal excitability of enteric neurons isolated from adult mice ileum was assessed by whole-cell patch-clamp studies in current clamp mode in neurons from Tat−/DOX (A & C) and Tat+/DOX mice (B & D). (A & C) Neurons isolated from Tat−/DOX elicit single action potentials at a rheobase of 10 pA, with (A) no effect of a low 0.03 μM concentration of morphine on excitability, but (C) a significant reduction in neuronal excitability with a high 0.3 μM concentration of morphine. (B & D) In contrast, neurons from Tat+/DOX elicit multiple action potentials at the same rheobase, with (B) the low 0.03 μM concentration and (D) the high 0.3 μM concentration of morphine significantly reducing neuronal excitability. Thus, neurons from Tat+/DOX demonstrate an increased neuronal sensitivity to morphine.
The effect of morphine on sodium current density is enhanced in neurons from Tat+/DOX mice and following exposure to 100 nM Tat in vitro
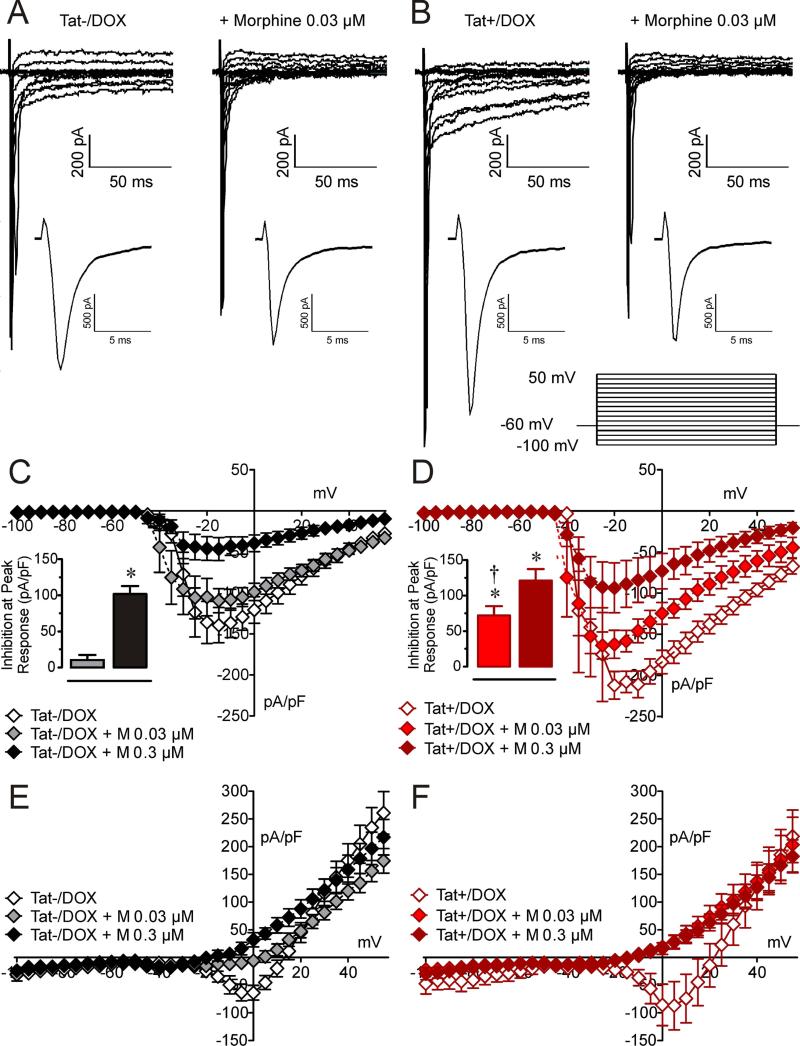
We have previously shown that morphine reduces neuronal excitability by inhibition of sodium channel currents (33). We therefore examined whether the difference in sensitivity to morphine between neurons from the ilea of Tat(+)/DOX and Tat (−)/DOX mice resulted from inhibition in sodium currents. Sodium currents were measured in voltage clamp studies using a Cs+-containing internal solution to block outward potassium currents as reported previously (26, 33). In the presence of Cs+, fast and slow inward-sodium currents were observed in response to depolarizing steps in voltage clamp mode (Figure 2A and B). The sodium current amplitude was significantly greater in neurons from Tat(+)/DOX. As shown in Figure 2, the fast inward sodium currents of neurons from Tat−/DOX mice were not affect by 0.03 μM concentration of morphine (Figure 2A) but were reduced in neurons from Tat(+)/DOX mice (Figure 2B). The current-voltage relationship for the peak amplitude of the fast sodium currents shows a significant inhibition (p < 0.05; insets in Figure 2C and D) at the low (0.03 μM) morphine concentration in enteric neurons from Tat(+)/DOX but not for the Tat (−)/DOX mice. Note that sodium current density was significantly increased at −10 mV in neurons from Tat+/DOX (−210.9 ± 16.03 pA/pF) compared to Tat−/DOX (136.6 ± 17.74 pA/pF, p < 0.001) mice as has been reported previously (26). When looking at the average data of the delayed inward sodium current density it is noted that morphine also reduces this long-lasting inward sodium current density, specifically at the higher 0.3 μM morphine concentration in neurons from Tat−/DOX mice, and for both morphine concentrations in neurons from Tat+/DOX mice (Figure 2E and F). Overall, these results suggest that the effects of morphine on the transient and the long-lasting inward sodium current densities are enhanced in neurons from Tat+/DOX mice.
Figure 2. Effect of morphine on sodium current densities is significantly enhanced in neurons from Tat+/DOX mice.
Sodium current density was assessed by voltage clamp recordings with Cs+ in the internal solution. (A & B) Raw traces of sodium channel currents of neurons from (A) Tat−/DOX mice show no effect for a low 0.03 μM concentration of morphine on sodium currents, whereas (B) Tat+/DOX show a significant reduction in the presence of 0.03 μM morphine. (C & D) A current density-voltage relationship shows that for the fast transient inward sodium currents (C) in neurons from Tat−/DOX, 0.3 μM inhibits sodium flow but not 0.03 μM morphine, whereas (D) in neurons from Tat+/DOX a significant reduction is noticed for 0.3 μM and 0.03 μM morphine. Insets indicate inhibition at peak responses with morphine that was significantly enhanced for morphine 0.03 μM in Tat+/DOX compared to Tat−/DOX. (E & F) A current density-voltage relationship shows that for the late component of the inward sodium currents (E) in Tat−/DOX morphine reduces the long-lasting inward sodium current density, specifically for the high 0.3 μM morphine concentration, whereas (F) in Tat+/DOX a significant reduction is noticed similarly for 0.3 μM and 0.03 μM morphine. Data are expressed as mean ± SEM. Bar graphs: one-sample t-test, *p < 0.05 vs 0 % inhibition, †p < 0.05 vs. Tat−/DOX + Morphine 0.03 μM, M = morphine.
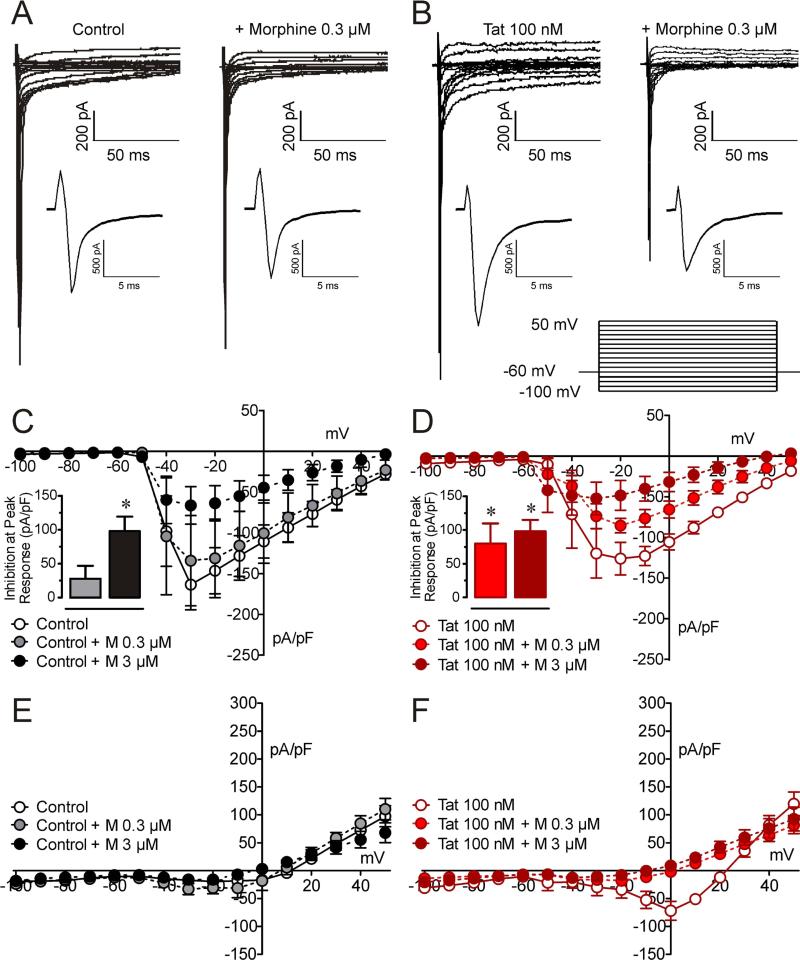
To further examine whether these differences in sensitivity are Tat dependent, the effects of morphine on sodium currents were determined in neurons exposed to Tat 100 nM in vitro (Figure 3). Interestingly, neurons isolated from Swiss Webster mice were less sensitive to morphine compared to C57BL/6J mice, which is the background of the Tat transgenic mice. Thus, we increased morphine concentrations to 0.3 μM and 3 μM. Figure 3 shows representative raw traces of sodium currents obtained from neurons treated with Tat (100 nM) for > 6 h. In control cells, 0.3 μM morphine had minimal effect (Figure 3A) whereas in Tat-treated cells, sodium currents were noticeably decreased by 0.3 μM (Figure 3B). The current-voltage relationship for the peak amplitude of the fast inward currents (Figure 3C and D) and the end of pulse current (Figure 3E and F) show significant inhibition at peak responses in the presence of 0.3 μM morphine in Tat treated neurons (p < 0.05) but only at 3 μM morphine for control neurons. No effect was noted for the lower 0.3 μM concentration of morphine in control cells (Figure 3C). In contrast, when neurons were exposed to 100 nM Tat for more than 6 h, a significant inhibition of the peak current occurred with exposure to 0.3 μM or 3 μM morphine (p < 0.05, Figure 3D). The delayed inward currents were also significantly more sensitive to lower concentration of morphine in the Tat-treated neurons, compared to the untreated neurons (Figure 3E and F). Thus, similar to the Tat transgenic data, in vitro Tat exposure also enhances the ability of morphine to inhibit sodium currents.
Figure 3. Effect of morphine on sodium current densities is significantly enhanced in the presence of in vitro Tat 100 nM.
As seen for neurons of Tat transgenic mice, similar effects were noted for in vitro Tat exposure on sodium current density. (A & B) Raw traces of sodium channel currents of (A) control neurons show no effect of a low 0.03 μM concentration of morphine on sodium currents, whereas (B) with in vitro Tat exposure a significant reduction in the presence of 0.03 μM morphine is noted. (C & D) A current density-voltage relationship shows that for the fast inward Na+ currents (C) in control neurons 0.3 μM inhibits sodium flow but not 0.03 μM morphine, whereas (D) in the presence of Tat a significant reduction is noticed for 0.3 μM and 0.03 μM morphine, thus demonstrating an enhanced inhibition to 0.3 μM morphine in the presence of Tat. (E & F) A current density-voltage relationship shows that for the late component of the inward Na+ currents (E) in control neurons no effect was noted for either concentration of morphine, whereas (F) in the presence of Tat morphine significantly decreases the late inward sodium current density similarly for both morphine concentrations. Data are expressed as mean ± SEM. Bar graphs: one-sample t-test, *p < 0.05 vs 0 % inhibition, M = morphine.
Effects of Tat on sodium channel kinetics
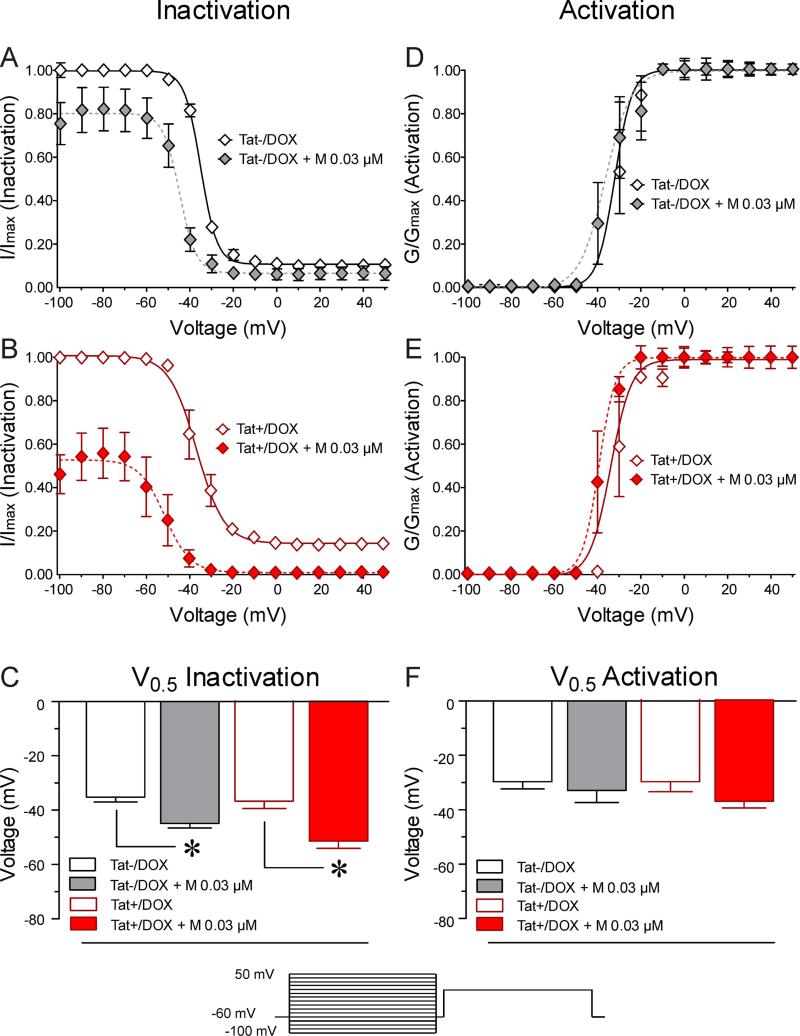
The voltage dependence of steady-state activation and inactivation kinetics and the effects of Tat and morphine on activation and inactivation kinetics were studied using a double-pulse protocol. For a conventional time- and voltage-dependent Hodgkin-Huxley conductance, the steady-state activation and inactivation curves describe the relative number of available sodium channels as a function of voltage. The resultant sigmoidal curves were fit to a Boltzmann distribution (Figures 4 & 5).
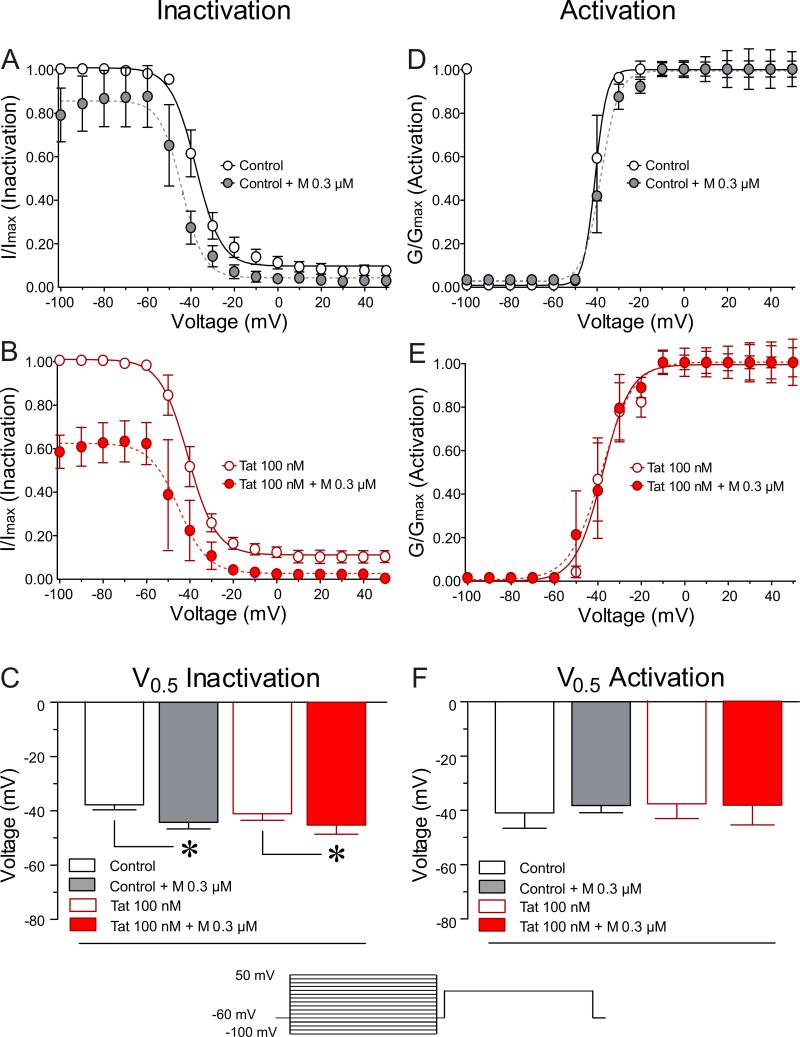
Figure 4. Neurons from Tat+/DOX mice show increased sensitivity to morphine on the inactivation kinetics of sodium channels.
Neurons from Tat transgenic mice in combination with morphine 0.03 μM were tested on the voltage-dependence of steady-state activation/inactivation of sodium channels. (A-C) Boltzmann curve analyses of inactivation kinetic of sodium channels. (A, B) In contrast to neurons from Tat−/DOX, Tat+/DOX indicate a significant downward shift of the inactivation curve for 0.03 μM morphine with the fraction of available channels being approximately 55 % decreased. (C) For both, neurons of Tat−/DOX and Tat+/DOX, a leftward shift for the inactivation curve in response to morphine is noted, indicated by significant differences in V0.5 inactivation values. (D-F) No effects are noted on the activation kinetics of sodium channels. Data are expressed as mean ± SEM. *p < 0.05.
Figure 5. In vitro Tat 100 nM increases the sensitivity to morphine on the inactivation kinetics of sodium channels.
Similarly, effects of Tat (100nM) in combination with morphine 0.3 μM were tested on the voltage-dependence of steady-state activation/inactivation of sodium channels. (A-C) Boltzmann curve analyses of inactivation kinetic of sodium channels. (A, B) In contrast to control neurons, neurons exposed to 100 nM Tat indicate a significant downward shift of the inactivation curve for 0.3 μM morphine concentration, similar to the neurons of Tat+/DOX, with the fraction of available channels being approximately 40 % decreased. (C) For both, control neurons and neurons exposed to 100 nM Tat, a leftward shift for the inactivation curve in response to morphine is noted, indicated by a significant difference in V0.5 inactivation values. (D-F) No effects are noted on the activation kinetics of sodium channels. Data are expressed as mean ± SEM. *p < 0.05.
Inactivation kinetics
For neurons isolated from Tat transgenic C57BL/6J mice, V0.5 of inactivation, the voltage at which half the channels were inactivated, was −35.1 ± 1.88 mV for Tat−/DOX neurons (Figure 4A and C). A small fraction of sodium channels remained available at more positive potentials (Figure 4A). The presence of 0.03 μM morphine shifted the V0.5 to more negative potentials (Figure 4A and C) with a 9.8 mV decrease in the V0.5 (p < 0.05; Figure 4C). At more positive potentials, almost all channels were inactivated in neurons from Tat−/DOX mice after morphine treatment. In neurons from Tat+/DOX mice, two significant effects were noted. First, as seen for neurons from Tat−/DOX, 0.03 μM morphine significantly reduced the V0.5 by 14.6 mV (Figure 4C). At more positive potentials, almost all sodium channels were inactivated in neurons from Tat+/DOX mice after morphine treatment. Second, there was a significant decrease in the availability of the channels with 0.03 μM morphine (Figure 4B). At the potential of −100 mV, the fraction of available channels in neurons from Tat+/DOX mice was significantly decreased from 100 ± 1.49% to 46.3 ± 8.96% (p < 0.05) in the presence of 0.03 μM morphine. This effect was not observed in neurons from Tat−/DOX mice (Figure 4A). Similarly, morphine's effects on V0.5 of inactivation of neurons exposed to Tat 100 nM in vitro were significantly altered (Figure 5). V0.5 of inactivation was −37.8 ± 1.82 mV for control neurons (Figure 5A and C) with a small fraction of sodium channels remaining available at more positive potentials. 0.3 μM morphine significantly shifted the V0.5 by 6.6 mV (p < 0.05) (Figure 5C). In neurons exposed to Tat 100 nM, 0.3 μM morphine significantly shifted the V0.5 by 4.7 mV (Figure 5C). Further, as seen in neurons from Tat+/DOX mice, in neurons exposed to Tat 100 nM there was a significant decrease in the availability of the channels for 0.3 μM morphine (Figure 5B). At the potential of −100 mV, the fraction of available channels was decreased from 100 ± 1.88 % to 58.0 ± 7.62 % (p < 0.05) in the presence of 0.3 μM morphine.
Overall, these results suggest that the effects of morphine on sodium current density were enhanced in neurons of Tat+/DOX mice and in vitro Tat exposure.
Activation kinetics
No effects were noted for Tat and/or morphine on the activation kinetics of the sodium channels and the, V0.5, the voltage at which half the channels are activated (Figures 4D-F and 5D-F).
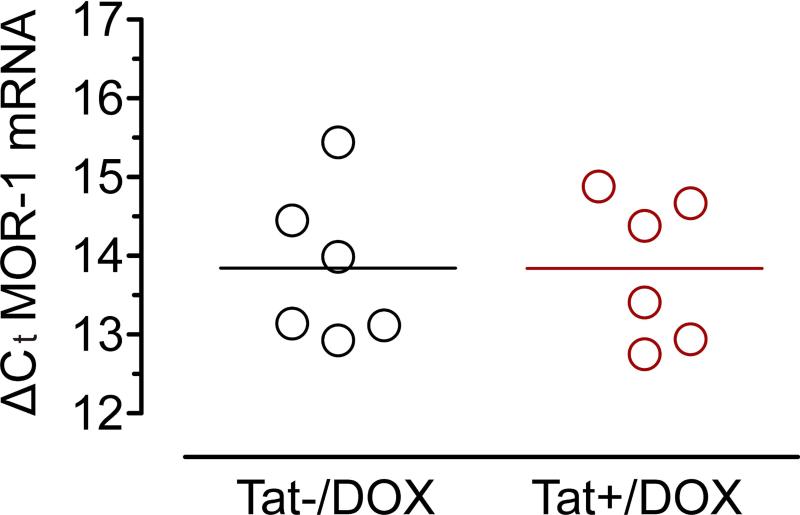
MOR-1 mRNA expression in ileum tissue of Tat transgenic mice showed no significant difference
To determine if Tat increased neuronal sensitivity to morphine by modulating MOR-1 transcription real time PCR with internal primers for the variant MOR-1 was performed. No significant differences were noted in ileal tissue of Tat+/DOX compared to Tat−/DOX mice (Figure 6).
Figure 6. MOR-1 mRNA expression is not significantly different in ileum tissue of Tat+/DOX compared to Tat−/DOX mice.
Real time PCR with a unique primer for the splice variant MOR-1 shows no significant difference in the relative abundance of MOR-1 in ileal tissue of Tat+/DOX and Tat−/DOX mice. The mean ΔCt value from ileal tissue preparations was calculated and plotted as a histogram (3 independent experiments).
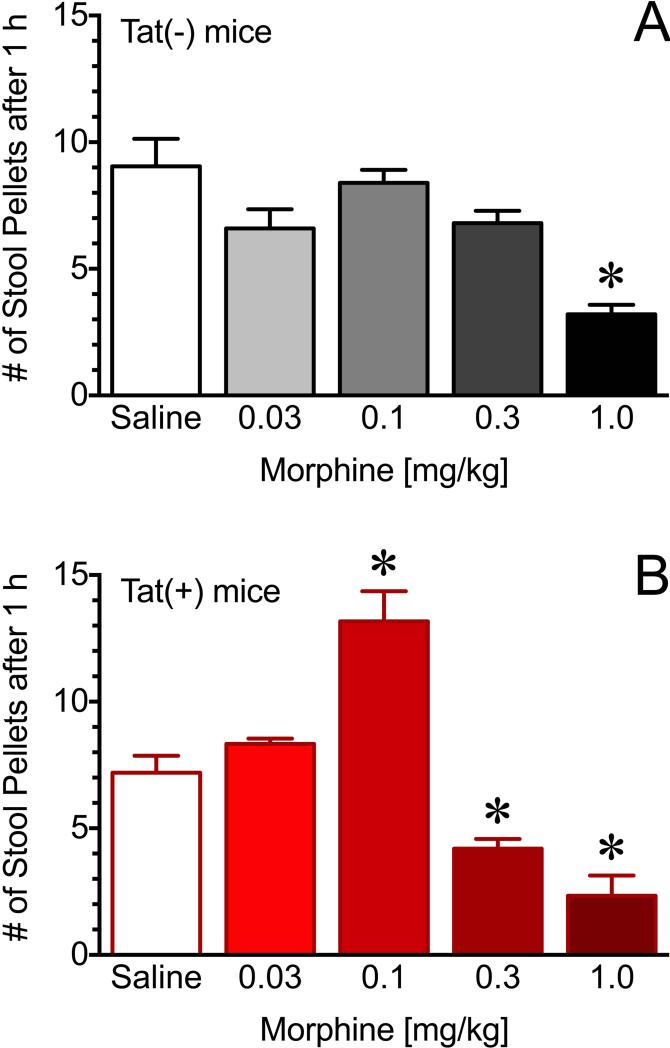
Effects of morphine on GI motility are significantly altered in Tat+ mice
To determine the effects of Tat and morphine on GI motility, fecal pellets expelled within one hour following treatment with morphine were measured in Tat(+) and Tat(−) mice. There was no significant difference in the rate of fecal pellet expulsion in saline treated Tat(+) or Tat(−) mice; however, a stimulatory effect of morphine was observed in the Tat(+) mice at 0.1mg/kg and significant reduction in fecal pellets at 0.3 mg/kg. Neither of these doses of morphine affected fecal expulsion in the Tat(−) mice (p < 0.05) (Figure 7). These concentrations of morphine are considerably lower than those required to produce antinociceptive effects.
Figure 7. Effects of morphine on GI motility are significantly altered in Tat(+) mice.
Analysis of the fecal pellet output in Tat(−) mice (upper panel) and Tat(+) mice (bottom panel). Fecal pellets were counted for 1hr following saline/morphine (i.p.) at doses indicated. Morphine treatment increased stool output at 0.1 mg/kg, whereas it reduced 0.3 mg/kg in Tat(+) mice but not Tat(−). Data are expressed as mean ± SEM. *p < 0.05 (n=5 for each group).
Discussion
Primary mouse enteric neurons from the ileum were cultured for 1-3 days and exposed to Tat either by Tat protein application (> 6 h) or by inducing the tat transgene in our inducible Tat transgenic mouse model using DOX administration in vivo for more than 10 days. In both cases, Tat exposure resulted in an enhanced excitability and an increase in sensitivity to morphine. In Tat treated neurons, either via transgene expression or direct exposure, morphine reduced excitability at lower drug concentrations. Furthermore, in the presence of Tat, acute administration to low concentrations of morphine demonstrated (i) an increased reduction in the fast transient and late long-lasting sodium current densities, and (ii) a significant downregulation of available sodium channels at hyperpolarized voltages compared to when Tat was absent. Additionally, GI motility showed higher sensitivity to morphine in the presence of Tat.
It is well known that about half of all treated patients with HIV-1 have HIV-1-associated neurocognitive disorders (HAND), despite combined antiretroviral therapy (cART) (44), including symptoms affecting their attention, memory, language, problem solving and decision making skills. Synaptodendritic damage and injury are the best predictors of HIV-associated neurocognitive deficits (HAND) (45-48). Tat likely contributes to synaptodendritic injury (39, 49-52). Recent studies have demonstrated that the transgenic Tat mice (Tat+/DOX) used in the present study show reduced spine density, dendritic pathology, and synaptic injury in the striatum and hippocampus (53, 54) and also display neurobehavioral impairments corresponding with a HAND-associated phenotype (36, 53, 55, 56). Interestingly, in addition to markedly reduced spine density in the striatum by Tat itself, morphine amplified the effect of higher levels of Tat on spines, and also potentiated Tat-mediated dendritic pathology (54).
In comparison to the effects of Tat ± morphine on CNS neurons, where morphine enhanced Tat-induced cytotoxicity of neurons our findings report significant interactions of HIV/Tat and opioids in the gut. As chronic pain is frequently reported by HIV-1-infected patients (57, 58) it is important to study HIV/Tat and opioid interaction. The predominant side effects of opioid is constipation, which occurs through reduced peristalsis, increased water and electrolyte absorption, and antisecretory actions (7). It has been shown in early studies that morphine sulfate can be used as an antidiarrheal agent to treat cryptosporidial diarrhea in acquired immunodeficiency syndrome (AIDS) patients (59). Recent reports refer to morphine and other opioid treatments as nonspecific but standard medications that are available for management of AIDS-related diarrhea with no identifiable pathogen (60, 61). However, it should be noted that that opiate/morphine sensitivity could be significantly altered in HIV-1-infected patients. The altered sensitivity may be due to variable drug absorption in patients with advanced HIV-1 infection, as well as an increased metabolic rates, which have been reported (62).
Alternatively, HIV and morphine may interact to alter other aspects of ENS function. It has been proposed that opioids affect the neuropathogenesis of HIV-1 in the CNS through direct actions of opiate drugs on opioid receptor-expressing neurons and glia within the CNS (11, 63-65). For Tat neurotoxicity, morphine-dependent exacerbation has also been attributed to direct actions on glia (17). The direct crosstalk between opiate drug and Tat appears to exacerbate critical proinflammatory and excitotoxic events (12, 15, 16, 66, 67). The increase in cytokine release may contribute to an increased neuronal sensitivity to morphine. Our recent findings also indicate that several cytokines are released in the ileum of Tat+/DOX transgenic mice (26).
A result of early HIV infection is a substantial depletion of CD4 T cells causing mucosal immune dysfunction, increased permeability of the gut and ultimately translocation of bacterial products (68). A recent study has shown that the gut bacterial profiles are associated with immune status even after starting cART (69). Higher proportions of Lactobacillales in the distal gut of recently HIV-infected individuals on cART were associated with higher CD4 counts, less microbial translocation, less systemic immune activation, and less gut T lymphocyte proliferation (69). In addition, it is known that morphine compromises gut barrier function by lowering host defenses to enteric bacteria, probably in a Toll-like receptor-dependent manner (70). This has implications specifically for HIV-1-infected patients that are on morphine medication or are heroin abusers, as the gut barrier function is not only compromised by HIV-1, but also drug use. A recent study showed that the only predictor for premature mortality among young injection drug users was HIV infection (71, 72). Whether, HIV infection might be a relevant factor associated to deaths for overdose in drug abusers needs to be studied in more detail.
The increased sensitivity to morphine does not appear to be due to increased expression of the MOR-1. Future experiments may be necessary to examine differences in MOR-1 internalization, phosphorylation and/or second messenger signaling molecules. A possible mechanism for the interaction between Tat and morphine may be due to the cross-talk between MOR and NMDA receptors (73, 74). NMDAR activation has been shown to induce opioid release in enteric neurons by triggering MOR internalization via glutamate-, glycine B-, and ion channel- mediated sites (75). The increase in endogenous opioid release by NMDAR activation could explain the increased neuronal sensitivity to morphine in the presence of Tat.
GI dysmotilities and diarrhea are persistent problems in the cART era (76, 77). We recently showed that gastrointestinal transit was markedly enhanced in the Tat(+) transgenic mice with selective increases in Nav1.8 and Nav1.7 expression concomitant with increased IL-6, Il-10, IL-1β and RANTES release (26). The mechanisms underlying Tat-mediated increases in pro-inflammatory cytokine release in the gut are not yet well understood. In the CNS, it has been shown that crosstalk between opiate drugs and Tat appear to exacerbate critical proinflammatory and excitotoxic events (12, 15, 16, 66, 67). The increase in cytokine release may also be contributing to and/or augmenting the rise in neuronal excitability observed in Tat exposed neurons. Thus, increased GI motility is suggested to correlate with enhanced neuronal excitability and increased pro-inflammatory cytokine levels, which may contribute to the diarrhea observed in many GI disorders as well as in HIV-1-infected patients.
Recently we showed that the fecal pellet output was greater in the Tat(+) mice compared to Tat(−) following recolonization of the gut (26). This contrasts with our present finding of similar rates of fecal pellet outputs in Tat(+) and Tat(−) transgenic mice. One possible explanation of the difference between the two studies may be due to additional handling of the mice due to injection of saline/morphine, which may have induced some stress and accompanying alterations in GI function. Notwithstanding the lack of difference in saline controls, the Tat(+) mice were more sensitive to morphine compared to Tat(−) mice. A U-shaped dose response was observed for morphine in the Tat(+) mice with a low concentration (0.1 mg/kg) inducing a stimulatory effect while 0.3 mg/kg significantly reduced fecal pellet expulsion. It is unclear as to why there is an enhanced effect of low doses in the Tat(+) mice; however, it is noteworthy that these doses are significantly lower than doses of morphine necessary to reduce colonic motility or antinociception (78).
Taken together, the present study demonstrates that Tat (i) significantly increases neuronal sensitivity to morphine in enteric neurons from the ileum by modulating sodium channel availability, and (ii) significantly increases GI sensitivity to morphine. In the setting of HIV-1 infection, this sensitization needs to be considered in patients receiving opioid therapy.
Key Message.
The present study demonstrates that HIV-1 Tat significantly increases neuronal sensitivity to morphine in enteric neurons by modulating sodium channel availability. Additionally, the effects of morphine on GI motility are significantly enhanced in the presence of Tat in vivo. In the setting of HIV-1 infection, this sensitization needs to be considered for opioid therapy in these patients.
The goal of the study was to investigate how opiates and HIV-1 interact to exacerbate the deleterious effects of HIV-1-infection and opiate exposure on gastrointestinal function.
To explore HIV-opiate interactions in the myenteric neurons, effects of Tat ± morphine (0.03 μM, 0.3 μM, 3 μM) were examined in isolated neurons from doxycycline- (DOX−) inducible HIV-1 Tat1-86 transgenic mice or following in vitro Tat 100 nM exposure (> 6 h). Whole-cell patch-clamp studies were conducted in current clamp mode to determine neuronal excitability, and in voltage clamp mode to study the biophysical properties of sodium channels.
HIV-1 Tat exposure resulted in an enhanced sensitivity to morphine. In the presence of Tat, acute administration to low concentrations of morphine (i) reduced neuronal excitability, (ii) reduced the fast transient and late long-lasting sodium current densities, and (iii) significantly reduced the available sodium channels at hyperpolarized voltages compared to when Tat was absent. Additionally, the effects of morphine on GI motility were significantly enhanced in Tat(+) compared to Tat(−) mice in vivo.
Acknowledgements
We gratefully acknowledge support from the National Institute on Drug Abuse grants R01 DA018633 (KFH) ; K02 DA027374 (KFH), R01 DK046367 (HIA), R01 DA024009 (HIA/WLD), K99 DA033878 (SF), F31 NS087952 (JN).
Footnotes
Author contributions: SF, PEK, KFH, HIA designed the research study; SF and JN performed the research; MK conducted the PCR experiment and analyzed the PCR data, DLS, FAK, JN conducted the GI motility experiment, SF analyzed other data and SF, KFH, HIA interpreted the data; SF and HIA wrote the paper, PEK WLD made important intellectual contributions and contributed to writing the paper.
No competing interests declared.
Reference List
- 1.Anthony IC, Arango JC, Stephens B, Simmonds P, Bell JE. The effects of illicit drugs on the HIV infected brain. Front Biosci. 2008;13:1294–1307. doi: 10.2741/2762. [DOI] [PubMed] [Google Scholar]
- 2.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Does drug abuse alter microglial phenotype and cell turnover in the context of advancing HIV infection? Neuropathol Appl Neurobiol. 2005;31:325–338. doi: 10.1111/j.1365-2990.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 3.Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121(Pt 11):2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- 4.Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–191. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox CM, Rabeneck L, Friedman S. AGA technical review: malnutrition and cachexia, chronic diarrhea, and hepatobiliary disease in patients with human immunodeficiency virus infection. Gastroenterology. 1996;111:1724–1752. doi: 10.1016/s0016-5085(96)70040-9. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin B, Wormser GP, Rosenthal WS, et al. Gastrointestinal manifestations of the acquired immunodeficiency syndrome: a review of 22 cases. Am J Gastroenterol. 1985;80:774–778. [PubMed] [Google Scholar]
- 7.De Luca A, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol Ther. 1996;69:103–115. doi: 10.1016/0163-7258(95)02053-5. [DOI] [PubMed] [Google Scholar]
- 8.Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63–69. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- 9.Carr DJ, Serou M. Exogenous and endogenous opioids as biological response modifiers. Immunopharmacology. 1995;31:59–71. doi: 10.1016/0162-3109(95)00033-6. [DOI] [PubMed] [Google Scholar]
- 10.Adler MW, Geller EB, Rogers TJ, Henderson EE, Eisenstein TK. Opioids, receptors, and immunity. Adv Exp Med Biol. 1993;335:13–20. doi: 10.1007/978-1-4615-2980-4_3. [DOI] [PubMed] [Google Scholar]
- 11.Gurwell JA, Nath A, Sun Q, et al. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine potentiates HIV-1 gp120-induced neuronal apoptosis. J Infect Dis. 2005;191:886–889. doi: 10.1086/427830. [DOI] [PubMed] [Google Scholar]
- 14.Turchan-Cholewo J, Liu Y, Gartner S, et al. Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and L-deprenyl. Neurobiol Dis. 2006;23:109–119. doi: 10.1016/j.nbd.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Hauser KF, El-Hage N, Stiene-Martin A, et al. HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. J Neurochem. 2007;100:567–586. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Hage N, Wu G, Wang J, et al. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53:132–146. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou S, Fitting S, Hahn YK, et al. Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at μ-opioid receptor-expressing glia. Brain. 2011;134:3613–3628. doi: 10.1093/brain/awr281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Hage N, Bruce-Keller AJ, Yakovleva T, et al. Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca2+]i, NF-κB trafficking and transcription. PLoS One. 2008;3:e4093. doi: 10.1371/journal.pone.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fish DN, Danziger LH. Neglected pathogens: bacterial infections in persons with human immunodeficiency virus infection. A review of the literature (1). Pharmacotherapy. 1993;13:415–439. [PubMed] [Google Scholar]
- 20.MacArthur RD, DuPont HL. Etiology and pharmacologic management of noninfectious diarrhea in HIV-infected individuals in the highly active antiretroviral therapy era. Clin Infect Dis. 2012;55:860–867. doi: 10.1093/cid/cis544. [DOI] [PubMed] [Google Scholar]
- 21.Foudraine NA, Weverling GJ, van Gool T, et al. Improvement of chronic diarrhoea in patients with advanced HIV-1 infection during potent antiretroviral therapy. AIDS. 1998;12:35–41. doi: 10.1097/00002030-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Johnson TP, Patel K, Johnson KR, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A. 2013;110:13588–13593. doi: 10.1073/pnas.1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotler DP, Gaetz HP, Lange M, Klein EB, Holt PR. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:421–428. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- 24.Ullrich R, Zeitz M, Heise W, L'Age M, Hoffken G, Riecken EO. Small intestinal structure and function in patients infected with human immunodeficiency virus (HIV): evidence for HIV-induced enteropathy. Ann Intern Med. 1989;111:15–21. doi: 10.7326/0003-4819-111-1-15. [DOI] [PubMed] [Google Scholar]
- 25.Buccigrossi V, Laudiero G, Nicastro E, Miele E, Esposito F, Guarino A. The HIV-1 transactivator factor (Tat) induces enterocyte apoptosis through a redox-mediated mechanism. PLoS One. 2011;6:e29436. doi: 10.1371/journal.pone.0029436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngwainmbi J, De DD, Smith TH, et al. Effects of HIV-1 Tat on Enteric Neuropathogenesis. J Neurosci. 2014;34:14243–14251. doi: 10.1523/JNEUROSCI.2283-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaumann W. The paralysing action of morphine on the guinea-pig ileum. Br J Pharmacol Chemother. 1955;10:456–461. doi: 10.1111/j.1476-5381.1955.tb00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paton WD. The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br J Pharmacol Chemother. 1957;12:119–127. doi: 10.1111/j.1476-5381.1957.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karras PJ, North RA. Inhibition of neuronal firing by opiates: evidence against the involvement of cyclic nucleotides. Br J Pharmacol. 1979;65:647–652. doi: 10.1111/j.1476-5381.1979.tb07877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collier HO, Cuthbert NJ, Francis DL. Model of opiate dependence in the guinea-pig isolated ileum. Br J Pharmacol. 1981;73:921–932. doi: 10.1111/j.1476-5381.1981.tb08747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.North RA, Williams JT. Extracellular recording from the guinea-pig myenteric plexus and the action of morphine. Eur J Pharmacol. 1977;45:23–33. doi: 10.1016/0014-2999(77)90054-1. [DOI] [PubMed] [Google Scholar]
- 32.Karras PJ, North RA. Acute and chronic effects of opiates on single neurons of the myenteric plexus. J Pharmacol Exp Ther. 1981;217:70–80. [PubMed] [Google Scholar]
- 33.Smith TH, Grider JR, Dewey WL, Akbarali HI. Morphine decreases enteric neuron excitability via inhibition of sodium channels. PLoS One. 2012;7:e45251. doi: 10.1371/journal.pone.0045251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith TH, Ngwainmbi J, Grider JR, Dewey WL, Akbarali HI. An in-vitro preparation of isolated enteric neurons and glia from the myenteric plexus of the adult mouse. J Vis Exp. 2013 doi: 10.3791/50688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, et al. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahn YK, Podhaizer EM, Farris SP, Miles MF, Hauser KF, Knapp PE. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0676-6. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hahn YK, Vo P, Fitting S, Block ML, Hauser KF, Knapp PE. beta-Chemokine production by neural and glial progenitor cells is enhanced by HIV-1 Tat: effects on microglial migration. J Neurochem. 2010;114:97–109. doi: 10.1111/j.1471-4159.2010.06744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry SW, Barbieri J, Tong N, et al. Human immunodeficiency virus-1 Tat activates calpain proteases via the ryanodine receptor to enhance surface dopamine transporter levels and increase transporter-specific uptake and Vmax. J Neurosci. 2010;30:14153–14164. doi: 10.1523/JNEUROSCI.1042-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruman, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- 40.Nath A, Booze RM, Hauser KF, et al. Critical questions for neuroscientists in interactions of drugs of abuse and HIV infection. NeuroAIDS. 1999;2:1–12. [Google Scholar]
- 41.Singh IN, Goody RJ, Dean C, et al. Apoptotic death of striatal neurons induced by human immunodeficiency virus-1 Tat and gp120: differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10:141–151. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akbarali HI, Giles WR. Ca2+ and Ca(2+)-activated Cl− currents in rabbit oesophageal smooth muscle. J Physiol. 1993;460:117–133. doi: 10.1113/jphysiol.1993.sp019462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. 2012;143:1006–1016. e1004. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. The Lancet Infectious diseases. 2013;13:976–986. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masliah E, Heaton RK, Marcotte TD, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 46.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 48.Everall IP, Heaton RK, Marcotte TD, et al. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein tat. J Neurochem. 1999;73:1363–1374. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- 50.Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 51.Bertrand SJ, Aksenova MV, Mactutus CF, Booze RM. HIV-1 Tat protein variants: critical role for the cysteine region in synaptodendritic injury. Exp Neurol. 2013;248:228–235. doi: 10.1016/j.expneurol.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertrand SJ, Mactutus CF, Aksenova MV, Espensen-Sturges TD, Booze RM. Synaptodendritic recovery following HIV Tat exposure: neurorestoration by phytoestrogens. J Neurochem. 2014;128:140–151. doi: 10.1111/jnc.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fitting S, Ignatowska-Jankowska BM, Bull C, et al. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry. 2013;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitting S, Xu R, Bull C, et al. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010;177:1397–1410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paris JJ, Carey AN, Shay CF, Gomes SM, He JJ, McLaughlin JP. Effects of conditional central expression of HIV-1 tat protein to potentiate cocaine-mediated psychostimulation and reward among male mice. Neuropsychopharmacology. 2014;39:380–388. doi: 10.1038/npp.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirsattari SM, Power C, Nath A. Primary headaches in HIV-infected patients. Headache. 1999;39:3–10. doi: 10.1046/j.1526-4610.1999.3901003.x. [DOI] [PubMed] [Google Scholar]
- 58.Zhang SX, Underwood M, Landfield A, Huang FF, Gison S, Geddes JW. Cytoskeletal disruption following contusion injury to the rat spinal cord. J Neuropathol Exp Neurol. 2000;59:287–296. doi: 10.1093/jnen/59.4.287. [DOI] [PubMed] [Google Scholar]
- 59.Connolly GM, Dryden MS, Shanson DC, Gazzard BG. Cryptosporidial diarrhoea in AIDS and its treatment. Gut. 1988;29:593–597. doi: 10.1136/gut.29.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cello JP. AIDS-related diarrhea: A need for effective control. Medscape. 1996 http://www.medscape.org/viewarticle/439683_439685.
- 61.Smart T. Diarrhoea in children with HIV: a clinical review. nam aidsmap. 2010 http://www.aidsmap.com/Diarrhoea-in-children-with-HIV-a-clinical-review/page/1405880/
- 62.Lefkowitz M, Grant I. Pain management in HIV infection. In: Gendelman HE, Grant I, Everall IP, Lipton SA, Swindells S, editors. The neurology of AIDS. Second Edition edn. Oxford University Press; Oxford: 2005. pp. 811–819. [Google Scholar]
- 63.Hauser KF, El-Hage N, Buch S, et al. Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res. 2005;8:63–80. doi: 10.1007/BF03033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nath A, Anderson C, Jones M, et al. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- 65.Nath A, Hauser KF, Wojna V, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- 66.Turchan-Cholewo J, Dimayuga FO, Ding Q, et al. Cell-specific actions of HIV-Tat and morphine on opioid receptor expression in glia. J Neurosci Res. 2008;86:2100–2110. doi: 10.1002/jnr.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bellizzi MJ, Lu SM, Gelbard HA. Protecting the synapse: evidence for a rational strategy to treat HIV-1 associated neurologic disease. J Neuroimmune Pharmacol. 2006;1:20–31. doi: 10.1007/s11481-005-9006-y. [DOI] [PubMed] [Google Scholar]
- 68.Paiardini M, Frank I, Pandrea I, Apetrei C, Silvestri G. Mucosal immune dysfunction in AIDS pathogenesis. AIDS reviews. 2008;10:36–46. [PubMed] [Google Scholar]
- 69.Perez-Santiago J, Gianella S, Massanella M, et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921–1931. doi: 10.1097/qad.0b013e3283611816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meng J, Yu H, Ma J, et al. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One. 2013;8:e54040. doi: 10.1371/journal.pone.0054040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller CL, Kerr T, Strathdee SA, Li K, Wood E. Factors associated with premature mortality among young injection drug users in Vancouver. Harm reduction journal. 2007;4:1. doi: 10.1186/1477-7517-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roy E, Haley N, Leclerc P, Sochanski B, Boudreau JF, Boivin JF. Mortality in a cohort of street youth in Montreal. JAMA. 2004;292:569–574. doi: 10.1001/jama.292.5.569. [DOI] [PubMed] [Google Scholar]
- 73.Celerier E, Laulin J, Larcher A, Le Moal M, Simonnet G. Evidence for opiate-activated NMDA processes masking opiate analgesia in rats. Brain Res. 1999;847:18–25. doi: 10.1016/s0006-8993(99)01998-8. [DOI] [PubMed] [Google Scholar]
- 74.Garzon J, Rodriguez-Munoz M, Sanchez-Blazquez P. Direct association of Mu-opioid and NMDA glutamate receptors supports their cross-regulation: molecular implications for opioid tolerance. Curr Drug Abuse Rev. 2012;5:199–226. doi: 10.2174/1874473711205030199. [DOI] [PubMed] [Google Scholar]
- 75.Patierno S, Zellalem W, Ho A, et al. N-methyl-D-aspartate receptors mediate endogenous opioid release in enteric neurons after abdominal surgery. Gastroenterology. 2005;128:2009–2019. doi: 10.1053/j.gastro.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 76.Mathur MK, Verma AK, Makwana GE, Sinha M. Study of opportunistic intestinal parasitic infections in human immunodeficiency virus/acquired immunodeficiency syndrome patients. Journal of global infectious diseases. 2013;5:164–167. doi: 10.4103/0974-777X.122012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitra AK, Hernandez CD, Hernandez CA, Siddiq Z. Management of diarrhoea in HIV-infected patients. International journal of STD & AIDS. 2001;12:630–639. doi: 10.1258/0956462011923840. [DOI] [PubMed] [Google Scholar]
- 78.Ross GR, Gabra BH, Dewey WL, Akbarali HI. Morphine tolerance in the mouse ileum and colon. J Pharmacol Exp Ther. 2008;327:561–572. doi: 10.1124/jpet.108.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]