Abstract
Previous studies have shown that the pharmacologic effects of GABAergic drugs and the postsynaptic phasic GABAAergic inhibitory responses in the anterior part of the rat substantia nigra pars reticulata (SNRA) are age- and sex-specific. Here, we investigate whether there are age- and sex-related differences in the expression of the δ GABAA receptor (GABAAR) subunit and GABAAR mediated tonic currents. We have used δ-specific immunochemistry and whole cell patch clamp to study GABAAR mediated tonic currents in the SNRA of male and female postnatal day (PN) PN5-9, PN11-16, and PN25-32 rats. We observed age-related decline, but no sex-specific changes, in bicuculline (BIM) sensitive GABAAR tonic current density, which correlated with the decline in δ subunit in the SNRA between PN15 and 30. Furthermore, we show that the GABAAR tonic currents can be modified by muscimol (GABAAR agonist; partial GABACR agonist), THIP (4,5,6,7-tetrahydroisoxazolo (5,4-c)pyridin-3-ol: α4β3δ GABAARs agonist and GABACR antagonist), and zolpidem (α1-subunit selective GABAAR agonist) in age-and sex-dependent manner specific for each drug. We propose that the emergence of the GABAAR-sensitive anticonvulsant effects of the rat SNRA during development may depend upon the developmental decline in tonic GABAergic inhibition of the activity of rat SNRA neurons, although other sex-specific factors are also involved.
Keywords: Substantia nigra pars reticulata, Patch clamp, Tonic inhibition, Development, Sex differences, GABA agonists
Introduction
The substantia nigra reticulata (SNR) is a midbrain structure closely involved in regulation of movement and seizure control [1–5]. Its role in seizure modulation depends on age, sex and is also different in the anterior (SNRA) versus the posterior SNR region [2, 6]. Specifically, bilateral infusions of muscimol in the SNRA of PN21 or younger rats have proconvulsant effects in males but have no effect in female rats in the flurothyl model of generalized clonic seizures [7]. During maturation, a muscimol-sensitive anticonvulsant region emerges in the SNRA of both sexes, but this functional shift occurs earlier in females (first seen at PN25) than in males (first seen at PN30) [2, 6, 7].
We previously described that the properties of spontaneous inhibitory postsynaptic GABAA receptor (GABAAR) mediated currents (sIPSCs) in GABAergic neurons of the SNRA are age- and sex-dependent, in part explained by different types of α GABAAR subunits [8–11].
To further elucidate the molecular and electrophysiological mechanisms underlying the developmental functional changes in the role of GABAergic SNRA neurons in seizure control, we studied the expression of the δGABAAR subunit, a component of extrasynaptic GABAARs that mediate tonic GABAAR inhibition, as well as the age and sex differences of GABAAR tonic currents in GABAergic SNRA neurons, using immunohistochemistry and whole cell patch clamp [12–18]. We found that the bicuculline (BIM)-sensitive GABAAR-mediated tonic currents decline between PN15 to PN30 in parallel with the age-related decrease in δ subunit expression, in both sexes. Furthermore, we show that the GABAAR tonic currents can be modified by muscimol (GABAAR agonist; partial GABACR agonist), THIP (4,5,6,7-Tetrahydroisoxazolo (5,4-c)pyridin-3-ol (gaboxadol): α4β3δ GABAARs agonist and GABACR antagonist), and zolpidem (α1-subunit selective GABAAR agonist) in age- and sex-dependent manner.
Materials and Methods
Animals
Male and female Sprague–Dawley rats (Taconic Farms, New York, USA) were divided into 3 different age groups PN5-9, PN11-16 and PN25-32, with the date of birth taken as PN0. Rats were kept at constant temperature (21–23 °C), relative humidity (40–60 %) and a 12 h dark/ 12 h light cycle (lights on at 7:00 a.m.) with food and water ad libitum in our animal facility accredited by the American Association for the Accreditation of Laboratory Animal Care. Rats younger than 21 days were kept with a dam. After weaning, rats were kept in cages of 3–4 same sex rats with water and food ad libitum. All procedures and experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Institute Committee of our institution.
Immunohistochemistry
For immunochemical detection of the δ subunit immunoreactive cells (δ-ir), PN5, PN15, and PN30 male and female rats were euthanized and transcardially perfused with saline and 10 % neutral buffered formalin (SIGMA-ALDRICH, St Louis, MO), cryoprotected in 30 % sucrose in phosphate buffered saline and stored frozen at −80 °C till further use. Sagittal 40 µm brain sections containing the SNR were immunostained as described previously [8]. We used two sources of rabbit anti-δ antibodies to confirm that the observed expression changes are independent of the δ subunit specific antibody used. Because the results regarding the developmental changes in δ subunit expression were comparable, the data were combined. The first antibody was developed by Dr. Gunther Sperk (1:300; Innsbruck Medical University, Austria) and recognized an epitope consisting of the 44 amino-terminal amino acids of the rat GABAAR δ subunit [19]. The second was a commercial polyclonal rabbit anti-δ antibody, also recognizing the amino-terminus of the rat GABAAR δ subunit (Catalog number AB9752, 1:300; Millipore, Billerica Massachusetts). Cellular densitometry of δ-ir SNR sections was performed as described previously [8, 20]. A mean value for the cellular δ-ir signal density was obtained per rat and was used in the statistics. In the cell count experiments, the number of total δ-ir SNRA neurons was counted from 1 section per brain in sagittal SNR sections at the level of the subthalamic nucleus. Similar cell counts were done on adjacent Nissl-stained SNRA sections. Statistics were performed on the “total numbers of δ-ir SNRA neurons per section” as well as the percent of δ-ir neurons among the total Nissl-stained SNRA neurons expressed as “[(total numbers of δ-ir SNRA neurons)/(total numbers of Nissl-stained SNRA neurons) * 100]”.
Drugs
BIM, SR 95531 hydrobromide (gabazine), TTX, THIP, muscimol, and D-AP5 were dissolved in distilled water whereas CNQX and zolpidem were dissolved in DMSO (final dilution 1:1,000). Bicuculline (and its water soluble preparations such as bicuculline methobromide - BIM) is a competitive GABAARs antagonist. Gabazine is a selective high-affinity antagonist binding at low-affinity GABAARs [21, 22]. TTX is a highly selective neuronal Na+ channel blocker [23], which completely inhibits firing action potentials [24]. D-AP5 blocks glutamatergic NMDA receptors-mediated currents whereas CNQX inhibits AMPA receptors [25]. Muscimol is a GABAAR agonist and partial GABACR agonist. THIP is an agonist for α4δcontaining GABAARs and GABACR antagonist. All drugs were diluted to the desired concentration after bath applied in aCSF and washed in the recording chamber at a flow rate of 4 ml/min. BIM, and zolpidem were purchased from Sigma-Aldrich, St. Louis, MO; gabazine, TTX, THIP, muscimol, D-AP5 and CNQX from Tocris Bioscience, Ellisville, MO.
Slice Preparation
Sagittal slices containing SNR were prepared fromanimals at PN5-9, 11–16 and 25–32. Rats were deeply anesthetized with isoflurane and decapitated. The brain was quickly removed and placed in oxygenated (95O2, 5 %CO2) ice-cold sucrose slicing solution containing (in mM): 187 sucrose, 3 KCl, 2 CaCl2, 1.9MgCl2, 1.2 NaH2PO4, 26 NaHCO3 and 20 d-glucose, pH 7.4, 300–310 mOsm. 300 µM thick sagittal slices were cut using a vibratome (Leica, VT1000S). Slices were transferred into oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM): 124 NaCl; 2.5 KCl; 1 NaH2PO4; 26 NaHCO3; 2 CaCl2; 1.3MgSO4 and 20 glucose, pH7.3–7.4, 290–300 mOsm, and allowed to recover at room temperature for at least 1 h before recording.
Electrophysiology
Cells were visualized with an upright Eclipse E600-FN microscope (Nikon) in the SNRA [7, 8]. Whole-cell patch clamp recordings were made from electrophysiologically identified GABAergic neurons using an Axopatch 200B amplifier (Molecular Devices, Union City, CA). Patch pipettes were pulled using Flaming/Brown micropipette puller (Sutter Instruments Co, Novato, CA) from thin-wall borosilicate glass tubing (1.5 mm OD; World Precision Instruments, Sarasota, FL) and had open tip resistance 2–3 MX when filled with an intracellular solution containing (in mM): 140 CsCl, 4 NaCl, 1 MgCl2, 10 HEPES, 10 EGTA, 2 Mg-ATP, 290 mOsm, pH 7.3 adjusted with CsOH. No correction was made for the liquid junction potential of +4.3 mV. Slices were continuously perfused at a rate of 4 ml/min with oxygenated aCSF solution. All recordings were performed at room temperature.
Neurons were voltage-clamped at a holding potential of −70 mV, therefore all GABAergic events were observed as inward currents. Series resistance was estimated by measuring the transient current in response to either −1 or −5 mV, 200 ms-long hyperpolarizing voltage steps. Cells were accepted for further analysis provided that the series resistance after 40–60 % compensation did not exceed 15 MX and/or did not change by more than 15 % during data acquisition. The input resistance could not be exactly measured due to the high intracellular Cs+ concentration, which blocks K+ channels [26]. Synaptic currents were recorded in the presence of glutamate antagonists D-AP5 (50 µM) and CNQX (10 µM) to block excitatory amino acid-mediated transmission. Recorded data were filtered at 2 kHz (low-pass Bessel filter) and sampled at 10 kHz. The bandwidth was sufficient enough to include all fast frequencies of interest [8]. All data were recorded with pClamp 8 analysis software (Molecular Devices Co, Sunnyvale, CA) through a Digidata 1322A digitizer (Molecular Devices Co, Sunnyvale, CA).
Baseline and post-drug holding currents (Ihold) were measured by averaging the Ihold from 20 epochs (50–100 ms each), 1 epoch per second, over a 20 s period. For baseline Ihold, the 20 s period immediately prior to the time of drug application was used. For post-drug Iholds, 20 s periods during the time of peak or trough drug responses were used, which was usually 80–100 s from the time of the drug administration. Gaussian all-point histograms were constructed from these epochs using 0.5 pA bins. The data-points not contaminated by IPSCs were fitted according to the Levenberg–Marquardt method to obtain the mean Ihold amplitudes. The difference between the baseline and postdrug Iholds expressed the magnitude of the tonic current. In order to eliminate the cell size as a confounding factor in measurements, all drug-induced changes in Ihold were related to the cell capacitance and expressed as a tonic current density (pA/pF) and this value was eventually used for definite comparison of age- and sex-related differences. The cell capacitance was calculated from current transients recorded in response to 5 mV hyperpolarizing voltage steps.
In the first set of experiments, bicuculline methobromide (BIM, 100 µM) was used to reveal the tonic current measured as the change in the Ihold. TTX (1 µM) and gabazine (500 nM) were used to eliminate IPSCs prior to BIM application to determine baseline Ihold as used in other papers to separate synaptic and extrasynaptic responses [15, 27].
In the remaining pharmacological studies (muscimol, THIP, zolpidem), TTX and gabazine were not used to simulate our previous in vivo studies [2, 6, 7]. In order to obtain the mean Ihold and tonic current density changes, only all point histograms of episodes uncontaminated by IPSCs were used.
Statistics
Two-way ANOVA followed by Fisher’s post hoc t test was used to compare age and sex differences in tonic current changes. Because the sensitivity of the two-way ANOVA comparisons decreases as the number of inter-group comparisons increases, we utilized unpaired t test to explore whether significant differences in the studied variables existed in specific same age groups that demonstrated visible gender-related differences. All values are expressed as least square mean values ± SE. F values for each variable are given as Fvariable (degrees of freedom, residuals).
Results
δ Subunit Expression
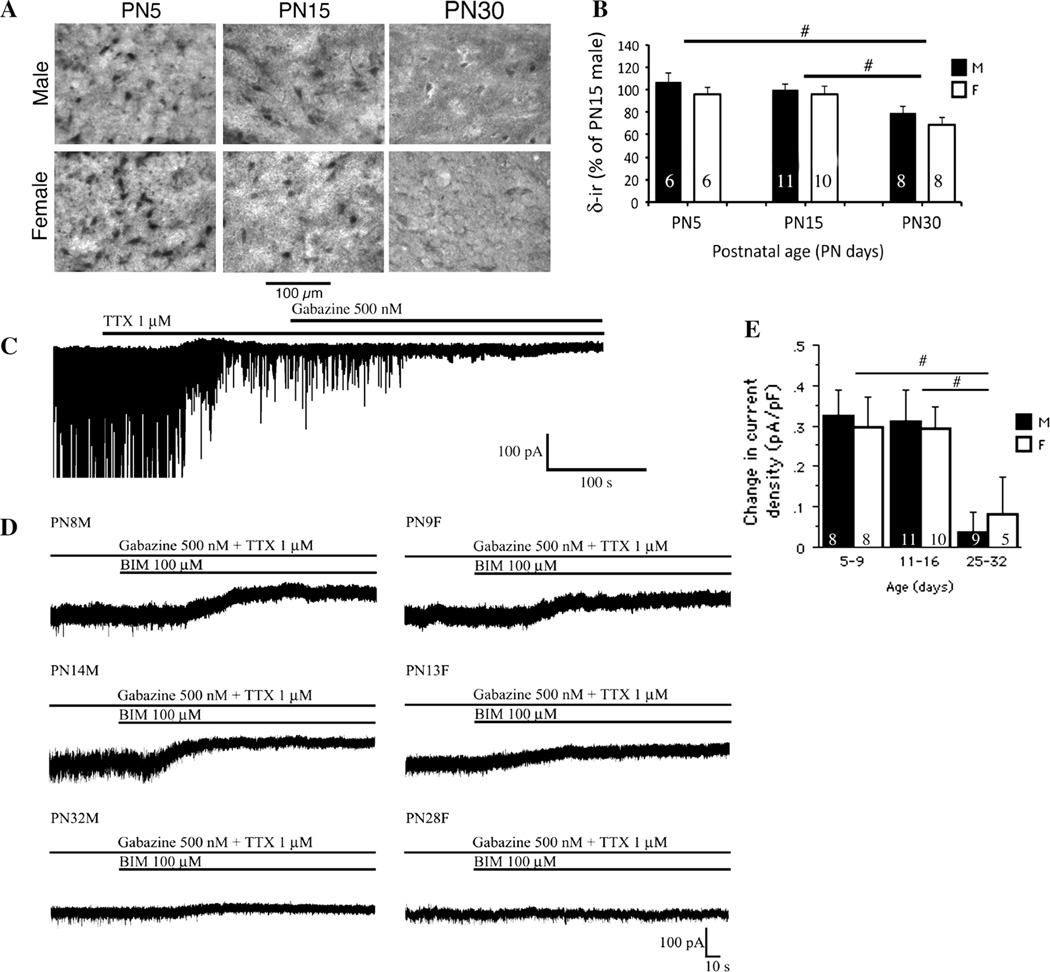
To study the age- and sex-specific differences in δ GABAAR subunit immunoreactivity (δ-ir) in SNRA neurons we used immunohistochemistry, because it allows comparisons in protein expression at the cellular level and avoids contamination of readouts by heterogeneous cell populations. The perisomatic δ-ir in the SNRA changed as a function of age [Fage(2,43) = 15.45; P > 0.0001) but not sex (Fsex(1,43) = 0.4; P > 0.05]. The δ-ir was high at PN5 and PN15 male and female rats and declined significantly at PN30 (Fig. 1a, b). In parallel, a greater than 50 % decrease in the total number of δ-ir SNRA neurons occurred between PN15 and PN30 [Fage(1,19) = 21.99, P = 0.0002], without any sex differences [Fsex(1, 19) = 0.049, P = 0.8) (n = 5 rats per group]. The percentage of SNRA neurons expressing δ-ir declined from 79.9 ± 9.8 %atPN15 to only 35.9 ± 4.3 % at PN30 [Fage(1,11) = 13.67, P < 0.0061, n = 6 rats/age group].
Fig. 1.
Developmental changes in δ-ir and BIM-sensitive tonic currents in rat SNRA neurons. a Representative photographs from SNRA sections from male and female rats show strong δ-ir expression at PN5 and PN15 and significant reduction at PN30, without any sex differences. Significant reduction in the numbers of δ-ir SNR neurons was also seen at PN30 compared to PN15 SNR sections, as discussed in the results section. b Schematic depiction of cellular δ-ir densitometric results obtained from SNRA sections confirms the decrease in cellular δ-ir expression between PN5 and PN30 as well as between PN15 and PN30, without sex differences. Results are expressed as “ % of δ-ir in PN15 males”, which were included as a reference group in each set of immunochemistry. This approach helped minimize interassay variability and allowed comparisons across the different sets of immunochemistry assays. Please note that the PN30 δ-ir reflects the mean intensity of the few remaining δ-ir cells, which were already significantly reduced in number (see Fig. 1a). The pound keys (hash) indicate significant differences (P < 0.05, post hoc Fisher’s test) between linked age groups. No sex differences were noted. c A representative recording in the presence of glutamatergic antagonists D-AP5 and CNQX shows an outward shift of Ihold following TTX 1 µM application indicating reduction of tonic inward current. At the same time, action potential dependent IPSCs are blocked. No further Ihold shifts were observed after gabazine 500 nM was washed in while all residual miniature IPSCs disappeared. d Representative recordings from GABAergic nigral neurons demonstrate the GABAARs-mediated tonic current as an outward shift of baseline Ihold after BIM 100 µM application. All IPSCs were previously eliminated by means of TTX 1 µM and gabazine 500 nM. e The BIM-induced changes in tonic current density significantly decrease with age while no sex differences were noted (Fage(2, 45) = 6.77, P < 0.05; Fsex(1, 45) = 0.0003, P > 0.05; Fage*sex(2, 45) = 0.12, P > 0.05, two-way ANOVA). The current density was smaller between PN25-32 and PN5-9 as well as PN25-32 and PN11-16 while no differences were observed between PN5-9 and PN11-16 groups (#P < 0.05, post hoc Fisher’s test). The numbers in the column bars indicate the number of cells. All values are expressed as least square mean values ± SE. M male, F female. Number of animals per group: males PN5-9 n = 5, PN11-16 n = 6, PN28-32 n = 7; females PN5-9 n = 6, PN11-16 n = 7, PN28-32 n = 5
BIM-Sensitive GABAAR Tonic Current
Since δ subunit mediates extrasynaptic tonic GABAAR responses, we investigated whether the observed age but not sex-dependent changes in δ-ir functionally correlate with similar changes in BIM-sensitive GABAAR-mediated tonic currents also occurs in SNRA neurons. In order to separate synaptic and tonic currents, whole cell patch clamp recordings were performed using TTX and gabazine, applied prior to BIM. In the presence of glutamatergic inhibitors CNQX and D-AP5, the TTX -induced outward shift of the baseline Ihold in all groups indicated that action potentials contribute to a certain degree to the tonic inward current (Fig. 1c). We found no age or sex differences in TTX-induced tonic current [Fage(2, 45) = 2.84, Fsex(1, 45) = 1.09, Fage*sex(2, 45) = 1.69, P > 0.05, two-way ANOVA]. These findings suggest that action potential dependent neurotransmitter spillover from the synaptic cleft or depolarizing shifts caused by ionic concentration disturbances due to fast-firing post-synaptic sodium channel activation may contribute to tonic currents [28, 29]. Further addition of gabazine (500 nM) completely blocked residual miniature IPSCs (mIPSCs), but did not alter the Ihold indicating that solely synaptic receptors were blocked (Fig. 1c).
Bath application of BIM (100 µM) revealed significant age- but not sex-specific changes in Ihold (Fig. 1d). When expressed as a tonic current density, the results show that these were not attributable to developmental changes in cell size [Fage(2, 45) = 6.77, P < 0.05; Fsex(1, 45) = 0.0003, P > 0.05; Fage*sex(2, 45) = 0.12, P > 0.05, two-way ANOVA] (Fig. 1e). The tonic current density was similar in PN5-9 and PN11-16 groups, without sex differences, but almost disappeared in PN25-32 neurons. The percentage of cells generating tonic current: males PN5-9 88 %, PN11-16 82 %, PN25-32 33 %; females PN5-9 88 %, PN11-16 100 %, PN25-32 20 %, paired t test, P < 0.05. The developmental changes in δ subunit expression and the concurrent differences in the tonic current density unmasked by BIM suggest that the reduction in δ-ir between PN15 and PN30 may underlie the decline in tonic current density in SNRA GABAergic neurons.
THIP Induced Changes in Tonic Current Density
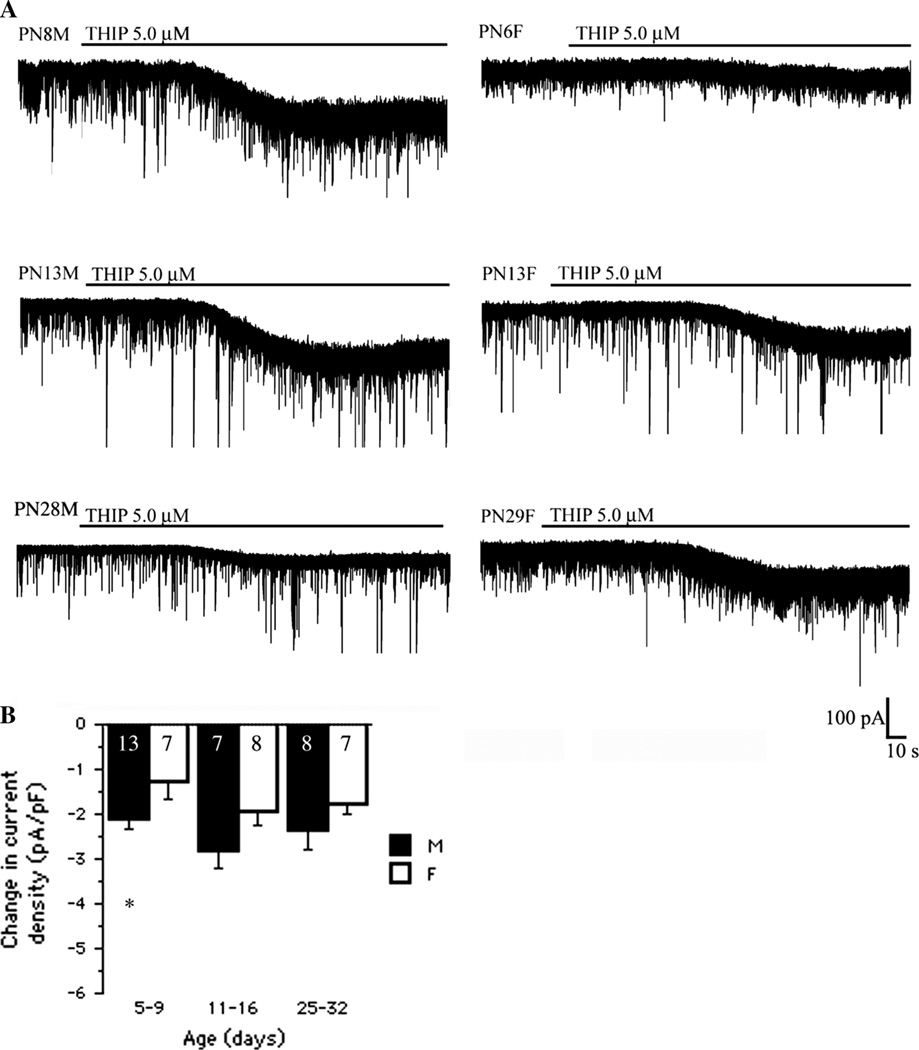
We then tested whether the decline in δ GABAAR subunit also parallels the developmental changes in tonic currents induced by THIP, an α4β3δ GABAARs agonist and GABACR antagonist. THIP (5 µM) application resulted in inward tonic current shifts in all age groups [Fage(2, 44) = 3.34, Fsex(1, 44) = 11.85, P < 0.05; Fage*sex(2, 44) = 0.17, P > 0.05; two-way ANOVA] (Fig. 2a). However, THIP induced changes in current density did not follow the same age-specific patterns as the δ-ir and BIM data. Consequently, no age-related differences were found in either sex (P > 0.05, unpaired-t test). There was an early trend for males to respond with greater THIP-induced tonic current density than females, which was significant in PN5- 9 SNRA (P < 0.05, unpaired-t test). No other sex differences were observed, although the statistical significance was almost reached in the PN11-16 groups (P = 0.055, unpaired-t test). The dissociation between the effects of THIP and BIM on tonic current density suggests that there are sex-, but not age-, related differences in unoccupied THIP-sensitive receptors which are not due to differences in δ subunit expression in SNRA neurons.
Fig. 2.
Sex-specific changes in THIP-induced tonic current in rat SNRA neurons. a Representative recordings demonstrating THIP (5 µM) induced changes in the tonic current in SNRA GABAergic neurons in the presence of D-AP5 50 µM and CNQX 10 µM. b When the cell size was taken into consideration, the THIP-induced changes in current density measurements demonstrate significant age- and sex-related differences (Fage(2, 44) = 3.34, Fsex(1, 44) = 11.85, P < 0.05, Fage*sex(2, 44) = 0.17, P > 0.05, two-way ANOVA). However, intergroup comparisons showed bigger current density shifts in PN5-9 males than same age females (*P < 0.05, unpaired t test) and borderline in PN11-16 group (P = 0.055, unpaired t test). No age-related differences between same-sex groups were found. The numbers in the column bars indicate the number of cells. All values are expressed as least square mean values ± SE. M male, F female. Number of animals per group: males PN5-9 n = 11, PN11-16 n = 6, PN28- 32 n = 6; females PN5-9 n = 5, PN11-16 n = 6, PN28- 32 n = 7
Muscimol-Induced Tonic Current Density
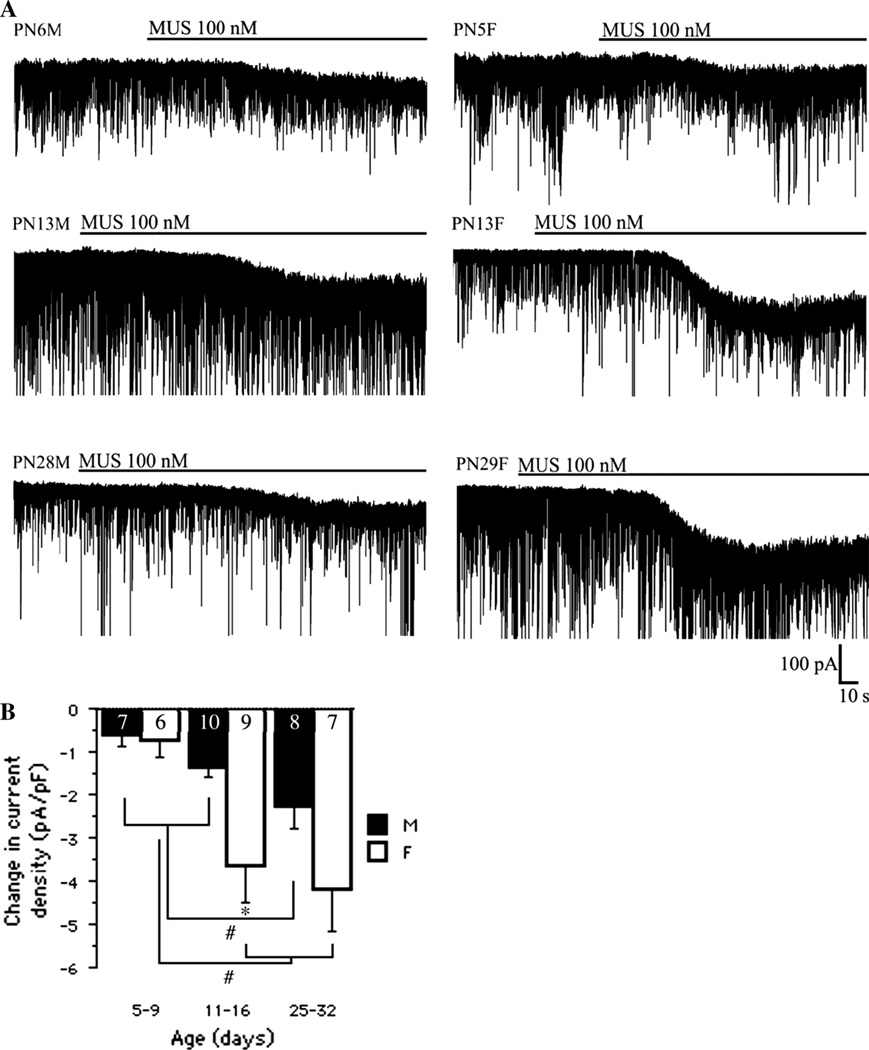
Because our prior studies indicated sex- and age-related differences in α1 subunit expression in rat SNRA [8, 9, 11, 30], we investigated whether the direct α1 agonist muscimol augments inward tonic currents in SNRA neurons in the similar sex-specific pattern. Muscimol 100 nM induced large changes in current density in all age groups (Fig. 3a) with age- and sex-specific differences [Fage(2, 41) = 9.89, P < 0.05; Fsex(1, 41) = 9.78, P < 0.05; Fage*sex(2, 41) = 1.99, P > 0.05, two-way ANOVA] (Fig. 3b). Muscimol- enhanced current density increased with age in both sexes (PN25-32 and PN11-16 > PN5-9), and was significantly greater in PN11-16 females than in same age males (P < 0.05, unpaired t test). These findings indicate a developmental increase in muscimol-induced tonic current in the SNRA, irrespective of the developmental changes in cell size, and this increase was more pronounced and appeared earlier in females.
Fig. 3.
Sex and age-specific changes in muscimol-induced tonic currents in rat SNRA. a Representative traces demonstrating that muscimol 100 nM induced an inward current in all groups. Recordings were performed in the presence of D-AP5 50 µM and CNQX 10 µM. Note the larger deflection in PN13 and PN29 female cells compared with their male counterparts. b The current density shifts induced by muscimol changed as a function of age and sex (Fage(2, 41) = 9.89, P < 0.05; Fsex(1, 41) = 9.78, P < 0.05; Fage*sex(2, 41) = 1.99, P > 0.05, two-way ANOVA). Significant sex differences were found only in the PN11-16 group (*P < 0.05, unpaired t test). In males, the current density in PN25-32 was significantly higher than in the two younger age groups (#P < 0.05, one-way ANOVA). In females, the current density in PN5-9 group was significantly lower than in PN11-16 and PN25-32 groups (#P < 0.05, one-way ANOVA). The numbers in the column bars indicate the number of cells. All values are expressed as least square mean values ± SE. M male, F female. Number of animals per group: males PN5-9 n = 3, PN11-16 n = 3, PN28- 32 n = 5; females PN5-9 n = 3, PN11-16 n = 4, PN28- 32 n = 5
Zolpidem Effects
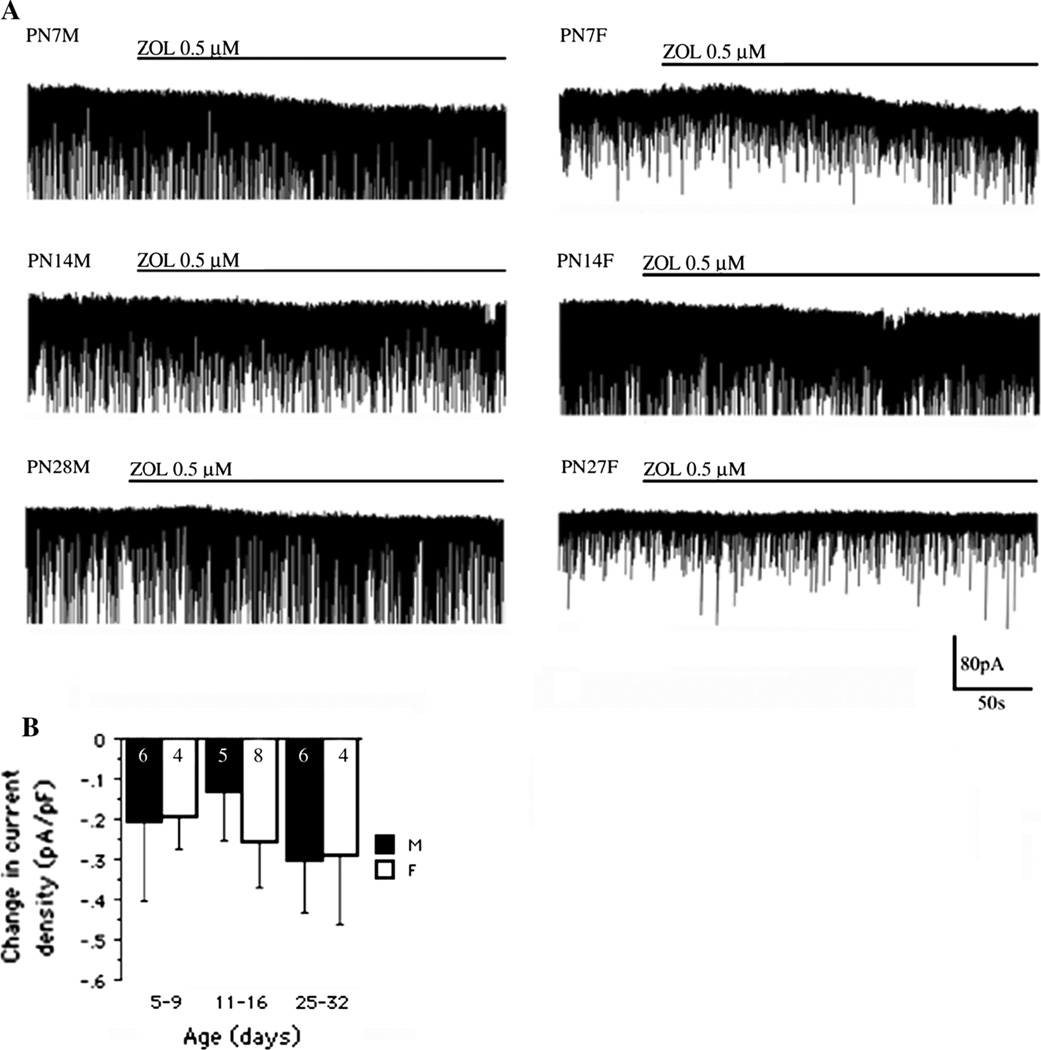
To determine whether age- and sex-related changes in α1- containing GABAAR mediated tonic responses may explain the age and sex differences in muscimol effects, we examined the effects of zolpidem, a selective agonist of α1 subunit containing GABAARs [31]. Zolpidem (0.5 µM) induced a small inward current in more than 70 % of all tested cells (Fig. 4a). There were no significant age and sex differences observed in zolpidem-induced current densities [Fage(2, 27) = 0.3; Fsex(1, 27) = 0.09, Fage*sex(2, 33) = 0.167, P > 0.05, two-way ANOVA] (Fig. 4c).
Fig. 4.
Zolpidem-induced tonic responses in rat SNRA neurons. a Representative traces show that zolpidem 0.5 µM gave rise to a small inward current in SNRA neurons across all age groups in the presence of D-AP5 50 µM and CNQX 10 µM. b The zolpidem-induced changes in current density measurements did not, however, demonstrate any significant sex- or age-dependent differences (Fage(2, 27) = 0.3; Fsex(1, 27) = 0.09, Fage*sex(2, 33) = 0.167, P > 0.05, two-way ANOVA). The numbers in the column bars indicate the number of cells. All values are expressed as least square mean values ± SE. M male, F female. Number of animals per group: males PN5-9 n = 5, PN11-16 n = 2, PN28- 32 n = 3; females PN5-9 n = 4, PN11-16 n = 3, PN28- 32 n = 4
The dissociation in the magnitude of zolpidem and muscimol sensitive tonic currents may therefore reflect direct agonistic effects of muscimol on α1-subunit containing GABAARs (extra- or post-synaptic).
Discussion
Our study demonstrates that PN5-16 GABAergic SNRA neurons are under the influence of a pronounced BIM-sensitive tonic GABAAR-mediated current, which disappears by PN32, in both sexes. The parallel developmental decline in δ GABAAR subunit expression suggests that the age-related reduction in BIM-sensitive tonic current density could be due to decrease in extrasynaptic δ receptors. In contrast, the pharmacologically-induced changes in GABAAR-mediated tonic current density follow drug-, age- and sex-specific patterns that cannot be fully explained by the extrasynaptic δ GABAARs and probably reflect changes in THIP, muscimol or zolpidem sensitive GABAARs and/or GABA availability (synaptic GABA release and uptake).
Our findings demonstrate a pronounced BIM-sensitive GABAAR-mediated tonic current during the first two postnatal weeks when GABAAR responses are depolarizing in SNRA neurons [32] and a significant decrease till PN32, probably due to the parallel reduction in the expression of the δ subunit-containing extrasynaptic GABAARs in both sexes. GABAAR tonic currents have been proposed to enhance shunting-mediated inhibition, which prevents neuronal excitation [33]. It is therefore possible that the increased GABAAR tonic conductance in PN5-16 SNRA neurons may protect against the appearance of excitatory effects, by augmenting shunting inhibition. Similar age-related decline in δ subunit has been shown in CA1 pyramidal neurons [34] but not in cerebellar and cortical neurons [35, 36]. Although the exact subcellular localization of the δ subunit was not explored in this study, the extrasynaptic localization of δ-containing GABAARs has been well documented [12, 13]. The age-dependent decline in the tonic current density mediated by δ-containing GABAARs is compensated for by an increase of the α1 subunit expression and synaptic GABAAR inhibition [8]. One can hypothesize that, early in development, activation of tonic GABAARs participates in cell differentiation and maturation, filtering out excessive neuronal activation. In contrast, in older ages, subsequent to the establishment of synaptic connectivity, GABAAR-mediated tonic inhibition subsides, yielding to the faster synaptic GABAAR-mediated inhibition that mediates specific functional processes that depend upon inter-neuronal communications [37, 38].
In the presence of glutamatergic inhibitors, TTX inhibited action potential-dependent IPSCs and reduced a tonic inward current shown as an outward shift in the baseline Ihold, without significant age and sex differences. The presence of TTX-sensitive tonic currents suggests that activity-dependent presynaptic neurotransmitter release contributes to the generation of tonic currents controlling GABAergic SNR neurons [39–41].
Interestingly, THIP induced significantly greater tonic responses in PN5-9 males than in females, with no definite age-related differences, when results were adjusted for cell size. Although the α4/δ combination may partially mediate the THIP currents [27, 42–44], the sex-specific and absence of significant age-specific THIP responses do not agree with the δ subunit expression patterns in the SNRA. The α4 mRNA was detected in PN15 and PN30 SNR by RT-PCR at very low levels, but it is not known if it demonstrates sex-specific expression patterns in the SNRA (A.S. Galanopoulou, unpublished observations). The dissociation in the age-and sex-related tonic current shifts induced by THIP (also GABACR antagonist) and muscimol (GABACR agonist) raises the possibility that GABACRs may contribute to these age and sex-related tonic currents. In preliminary studies we have found very low mRNA levels of the ρ1 subunit of GABACR in the PN30 SNR (Galanopoulou AS, unpublished). In addition, a developmental increase in ρ2 subunit and decrease in ρ3 mRNA has been reported in other brain regions [45] Further studies will be useful to identify the specific GABACR or GABAAR subunit combinations that underlie the observed sex dependent, THIP-induced changes in tonic current in SNRA neurons.
In contrast, muscimol-induced GABAAR-mediated responses were more pronounced, in general, in older age groups and in females. Muscimol is a GABAAR agonist which avidly binds at the high affinity site located at the α1 subunit [30, 46], but it also binds to α4 [47], α5 [48], γ or δ subunit-containing GABAARs [49, 50]. Muscimol also acts as a partial agonist of ρ1 GABAC receptors {Chang, 2000 #732; Wang, 1994 #733} and is a weak inhibitor of GABA uptake [51]. The changes in muscimol-induced tonic currents reported here correlate with the higher expression of α1 subunit mRNA in PN15 females than in males and in PN30 SNRA [9] and the developmental increase in high affinity muscimol binding sites between PN16 and adult rat SNR [30]. However, muscimol responses are dissociated from the zolpidem-induced changes in GABAAR tonic current density. The discordance between the developmental and sex specific patterns between muscimol and zolpidem emphasizes that other mechanisms of action are involved in the generation of these tonic GABAARs mediated responses. The much stronger tonic currents elicited by muscimol are most likely due to a direct agonistic effect, as muscimol is a potent direct GABAAR agonist, and—unlike zolpidem—does not depend upon GABA availability. Also, the washout of ambient GABA, under the in vitro recording conditions, may diminish the zolpidem effect. Muscimol-induced activation of α5 GABAARs or of GABACRs would also be worth testing in the future as to their role in underlying the age- and sex-specific muscimol effects in the SNRA [52, 53].
We did not observe any age- or sex-related differences in zolpidem induced tonic responses, despite the significantly higher perisomatic α1 subunit protein expression in PN25-32 than in PN5-9 and PN11-16 groups [8]. This can be due to lower levels of ambient GABA in PN25-32 SNRA than in younger groups, possibly due to enhanced GABA reuptake by GABA transporting mechanisms or washout of external GABA. This would limit the action of zolpidem which solely increases the affinity for GABA. Moreover, zolpidem is not a pure α1 modulator but can also bind to α2 and α3 subunits. The increased expression of α2 and α3 subunits in the PN5-9 and PN11-16 SNRA neurons compared to PN25-32 neurons could therefore compensate for the lower expression of α1 subunits, masking any anticipated age differences in zolpidem- induced tonic currents [8].
Possible Implications for the SNRA Mediated Seizure Control
Previous studies from our laboratory have shown age- and sex-specific effects of bilateral SNRA infusions of GABAAR agonists and antagonists in the flurothylmodel of seizures [2, 6, 7, 54]. Specifically, intra-SNRA infusions of muscimol elicit proconvulsant effects in PN15 males and have no effect in PN15 females, whereas the muscimol-sensitive anticonvulsant SNRA function develops earlier in females (starting at PN25) than in males (starting at PN30). Based on the developmental profile of phasic and tonic GABAAR responses of SNRA neurons ([8] and this study), there appears to be a developmental shift from an “enhanced tonic GABAAR-mediated SNRA control” state early in development to a “predominant phasic GABAAR-mediated regulation of SNRA” in older ages, which is accelerated in females, due to the earlier rise in α1 subunit [8].We propose that under conditions with prominent δ-dependent tonic GABAAR activity (i.e. in PN5-16 rats), sustained activation of GABAARs leads to inhibition of SNRA neuronal firing due to shunting inhibition [55, 56] thereby reducing the GABA outflow to downstream target regions (e.g., thalamus, superior colliculus), which are then tonically disinhibited, and precipitating seizures. Of note, silencing of SNRA neuronal firing by tonic GABAAR currents (i.e. with muscimol) has been shown in immature SNRA neurons whether they have depolarizing or hyperpolarizing GABAARs [56]. The presence of increased GABAAR-mediated tonic current in PN5-9 SNRA neurons, known to have depolarizing GABAAR responses, could generate greater shunting inhibition, potentiating this effect [32, 33]. In contrast, during development, the incremental control of GABAergic SNRA neurons by phasic (i.e. α1-containing)GABAARs, which have faster inactivation kinetics, and the gradual disappearance of tonic GABAAR-mediated control may result in an intermittent, i.e. less persistent, inhibition of the firing of SNRA neurons. However, nigral neurons can still provide sufficient GABA outflow to the downstream output regions to influence seizure expression.
In support of the differential involvement of tonic and phasic GABAARs in SNR-mediated seizure control, previous studies in PN15 male rats reported proconvulsant responses following intranigral infusions of GABAergic agonists that elicit prominent tonic GABA responses (i.e. muscimol, THIP) but not with drugs that preferentially activate phasic GABAARs (i.e. zolpidem) [2, 57–59]. However, both the in vitro and in vivo studies support that additional sex-specific factors may modify the effects of these GABAergic agonists on the activity of SNRA neurons and in seizure control during development. These may include the age- and sex-specific developmental profiles of the shift from depolarizing to hyperpolarizing GABAAR signaling in rat SNRA [32, 56], potentially complicated by distinct EGABA maturational patterns in extrasynaptic and postsynaptic GABAARs [60], age and sex differences in the synaptic organization of the basal ganglia, as well as the complex pharmacological effects of administered GAB-Aergic drugs as shown in this study.
Acknowledgments
The authors acknowledge the Grant support by NIH NINDS Grants NS020253, NS045243, NS058303, NS062947, NS078333, grants from the International Rett Syndrome Foundation, PACE, Heffer Family Foundation, Autism Speaks, Citizens United for Research in Epilepsy (CURE), Department of Defense, and GAČR 309/08/H079. We are also grateful to the Segal Family Foundation and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/ Dan Levitz families for providing research funding. SLM is the Charles Frost Chair in Neurosurgery and Neurology.
Abbreviations
- SNR
Substantia nigra pars reticulata
- SNRA
Anterior part of the substantia nigra pars reticulata
- sIPSCs
Spontaneous inhibitory postsynaptic currents
- PN
Postnatal days
- -ir
Immunoreactivity
- GABAARs
GABAA receptors
- GABACRs
GABAC receptors
- aCSF
Artificial cerebrospinal fluid
- D-AP5
d-(-)-2-Amino-5-phosphonopentanoic acid
- CNQX
6-Cyano-2, 3-dihydroxy-7-nitro-quinoxaline
- BIM
Bicuculline methobromide
- DMSO
Dimethyl sulfoxide
- TBS
Tris based saline
- THIP
4, 5, 6, 7-Tetrahydroisoxazolo (5, 4-c)pyridin-3-ol
- TTX
Tetrodotoxin
- gabazine
SR 95531 hydrobromide
- RT
Room temperature
- NGS
Normal goat serum
- SE
Standard error
- Rs
Series resistance
Footnotes
Conflict of interest The authors declare that they have no conflict of interest
Contributor Information
O. Chudomel, Email: ondrej.chudomel@gmail.com, Saul R. Korey Department of Neurology, Laboratory of Developmental Epilepsy Bronx, Albert Einstein College of Medicine, 1410 Pelham Pkwy South, Kennedy Rm 306, Bronx, NY 10461, USA; Department of Neurology, 2nd Faculty of Medicine, Charles University in Prague and Motol University Hospital, Prague, The Czech Republic.
H. Hasson, Saul R. Korey Department of Neurology, Laboratory of Developmental Epilepsy Bronx, Albert Einstein College of Medicine, 1410 Pelham Pkwy South, Kennedy Rm 306, Bronx, NY 10461, USA
M. Bojar, Department of Neurology, 2nd Faculty of Medicine, Charles University in Prague and Motol University Hospital, Prague, The Czech Republic
S. L. Moshé, Saul R. Korey Department of Neurology, Laboratory of Developmental Epilepsy Bronx, Albert Einstein College of Medicine, 1410 Pelham Pkwy South, Kennedy Rm 306, Bronx, NY 10461, USA Dominick P. Purpura Department of Neuroscience, Albert Einstein College of Medicine, 1410 Pelham Pkwy South, Kennedy Rm 306, Bronx, NY 10461, USA; Department of Pediatrics, Albert Einstein College of Medicine, 1410 Pelham Parkway South, Kennedy Center, New York City, NY, USA.
A. S. Galanopoulou, Saul R. Korey Department of Neurology, Laboratory of Developmental Epilepsy Bronx, Albert Einstein College of Medicine, 1410 Pelham Pkwy South, Kennedy Rm 306, Bronx, NY 10461, USA Dominick P. Purpura Department of Neuroscience, Albert Einstein College of Medicine, 1410 Pelham Pkwy South, Kennedy Rm 306, Bronx, NY 10461, USA.
References
- 1.Iadarola MJ, Gale K. Substantia nigra: site of anticonvulsant activity mediated by gamma-aminobutyric acid. Science. 1982;218(4578):1237–1240. doi: 10.1126/science.7146907. [DOI] [PubMed] [Google Scholar]
- 2.Moshe SL, Albala BJ. Nigral muscimol infusions facilitate the development of seizures in immature rats. Brain Res. 1984;315(2):305–308. doi: 10.1016/0165-3806(84)90165-2. [DOI] [PubMed] [Google Scholar]
- 3.Gale K. Mechanisms of seizure control mediated by gamma-aminobutyric acid: role of the substantia nigra. Fed Proc. 1985;44(8):2414–2424. [PubMed] [Google Scholar]
- 4.Veliskova J, Moshe SL. Update on the role of substantia nigra pars reticulata in the regulation of seizures. Epilepsy Curr. 2006;6(3):83–87. doi: 10.1111/j.1535-7511.2006.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deransart C, Vercueil L, Marescaux C, Depaulis A. The role of basal ganglia in the control of generalized absence seizures. Epilepsy Res. 1998;32(1–2):213–223. doi: 10.1016/s0920-1211(98)00053-9. [DOI] [PubMed] [Google Scholar]
- 6.Garant DS, Xu SG, Sperber EF, Moshe SL. Age-related differences in the effects of GABAA agonists microinjected into rat substantia nigra: pro- and anticonvulsant actions. Epilepsia. 1995;36(10):960–965. doi: 10.1111/j.1528-1157.1995.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 7.Veliskova J, Moshe SL. Sexual dimorphism and developmental regulation of substantia nigra function. Ann Neurol. 2001;50(5):596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- 8.Chudomel O, Herman H, Nair K, Moshe SL, Galanopoulou AS. Age- and gender-related differences in GABA(A) receptor-mediated postsynaptic currents in GABAergic neurons of the substantia nigra reticulata in the rat. Neuroscience. 2009;163(1):155–167. doi: 10.1016/j.neuroscience.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravizza T, Friedman LK, Moshe SL, Veliskova J. Sex differences in GABA(A)ergic system in rat substantia nigra pars reticulata. Int J Dev Neurosci. 2003;21(5):245–254. doi: 10.1016/s0736-5748(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 10.Moshe SL, Brown LL, Kubova H, Veliskova J, Zukin RS, Sperber EF. Maturation and segregation of brain networks that modify seizures. Brain Res. 1994;665(1):141–146. doi: 10.1016/0006-8993(94)91164-9. [DOI] [PubMed] [Google Scholar]
- 11.Veliskova J, Kubova H, Friedman LK, Wu R, Sperber EF, Zukin RS, Moshe SL. The expression of GABA(A) receptor subunits in the substantia nigra is developmentally regulated and region-specific. Ital J Neurol Sci. 1998;19(4):205–210. doi: 10.1007/BF02427602. [DOI] [PubMed] [Google Scholar]
- 12.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18(5):1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23(33):10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci. 1994;14(11 Pt 2):7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59(4):814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- 16.Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87(5):2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- 17.Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci. 2002;22(10):RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27(5):262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101(4):815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 20.Galanopoulou AS. Sex- and cell-type-specific patterns of GABAA receptor and estradiol-mediated signaling in the immature rat substantia nigra. Eur J Neurosci. 2006;23(9):2423–2430. doi: 10.1111/j.1460-9568.2006.04778.x. [DOI] [PubMed] [Google Scholar]
- 21.McCabe RT, Wamsley JK, Yezuita JP, Olsen RW. A novel GABAA antagonist [3H]SR 95531: microscopic analysis of binding in the rat brain and allosteric modulation by several benzodiazepine and barbiturate receptor ligands. Synapse. 1988;2(2):163–173. doi: 10.1002/syn.890020208. [DOI] [PubMed] [Google Scholar]
- 22.Heaulme M, Chambon JP, Leyris R, Wermuth CG, Biziere K. Characterization of the binding of [3H]SR 95531, a GABAA antagonist, to rat brain membranes. J Neurochem. 1987;48(6):1677–1686. doi: 10.1111/j.1471-4159.1987.tb05723.x. [DOI] [PubMed] [Google Scholar]
- 23.Catterall WA, Goldin AL, Waxman SG. International union of pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57(4):397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 24.Atherton JF, Bevan MD. Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro. J Neurosci. 2005;25(36):8272–8281. doi: 10.1523/JNEUROSCI.1475-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King AE, Lopez-Garcia JA. Excitatory amino acid receptor-mediated neurotransmission from cutaneous afferents in rat dorsal horn in vitro. J Physiol. 1993;472:443–457. doi: 10.1113/jphysiol.1993.sp019955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao LR, Dudek FE. Changes in mIPSCs and sIPSCs after kainate treatment: evidence for loss of inhibitory input to dentate granule cells and possible compensatory responses. J Neurophysiol. 2005;94(2):952–960. doi: 10.1152/jn.01342.2004. [DOI] [PubMed] [Google Scholar]
- 27.Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther. 2004;310(3):1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- 28.Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol. 2003;90(4):2690–2701. doi: 10.1152/jn.00240.2003. [DOI] [PubMed] [Google Scholar]
- 29.Leao RM, Mellor JR, Randall AD. Tonic benzodiazepine-sensitive GABAergic inhibition in cultured rodent cerebellar granule cells. Neuropharmacology. 2000;39(6):990–1003. doi: 10.1016/s0028-3908(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 30.Wurpel JN, Tempel A, Sperber EF, Moshe SL. Age-related changes of muscimol binding in the substantia nigra. Brain Res. 1988;471(2):305–308. doi: 10.1016/0165-3806(88)90108-3. [DOI] [PubMed] [Google Scholar]
- 31.Pritchett DB, Luddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245(4924):1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- 32.Kyrozis A, Chudomel O, Moshe SL, Galanopoulou AS. Sex-dependent maturation of GABAA receptor-mediated synaptic events in rat substantia nigra reticulata. Neurosci Lett. 2006;398(1–2):1–5. doi: 10.1016/j.neulet.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Song I, Savtchenko L, Semyanov A. Tonic excitation or inhibition is set by GABA(A) conductance in hippocampal interneurons. Nat Commun. 2011;2:376. doi: 10.1038/ncomms1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for alpha4betadelta GABAA receptors in shaping learning deficits at puberty in mice. Science. 2010;327(5972):1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurie DJ, Wisden W, Seeburg PHMS. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12(11):4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peden DR, Petitjean CM, Herd MB, Durakoglugil MS, Rosahl TW, Wafford K, Homanics GE, Belelli D, Fritschy JM, Lambert JJ. Developmental maturation of synaptic and extrasynaptic GABAA receptors in mouse thalamic ventrobasal neurones. J Physiol. 2008;586(4):965–987. doi: 10.1113/jphysiol.2007.145375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305(2):187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 39.Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol. 2003;548(Pt 1):97–110. doi: 10.1113/jphysiol.2002.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007;582(Pt 3):1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497(Pt 3):753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8(6):797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 43.Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA. 2006;103(41):15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136(7):965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogurusu T, Yanagi K, Watanabe M, Fukaya M, Shingai R. Localization of GABA receptor rho 2 and rho 3 subunits in rat brain and functional expression of homooligomeric rho 3 receptors and heterooligomeric rho 2 rho 3 receptors. Recept Channels. 1999;6(6):463–475. [PubMed] [Google Scholar]
- 46.Baur R, Sigel E. On high- and low-affinity agonist sites in GABAA receptors. J Neurochem. 2003;87(2):325–332. doi: 10.1046/j.1471-4159.2003.01982.x. [DOI] [PubMed] [Google Scholar]
- 47.Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of alpha4 and delta subunits of the gamma-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56(1):110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- 48.Sur C, Quirk K, Dewar D, Atack J, McKernan R. Rat and human hippocampal alpha5 subunit-containing gamma-aminobutyric acidA receptors have alpha5 beta3 gamma2 pharmacological characteristics. Mol Pharmacol. 1998;54(5):928–933. doi: 10.1124/mol.54.5.928. [DOI] [PubMed] [Google Scholar]
- 49.Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96(22):12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Storustovu SI, Ebert B. Pharmacological characterization of agonists at delta-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of gamma2. J Pharmacol Exp Ther. 2006;316(3):1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- 51.Corey JL, Guastella J, Davidson N, Lester HA. GABA uptake and release by a mammalian cell line stably expressing a cloned rat brain GABA transporter. Mol Membr Biol. 1994;11(1):23–30. doi: 10.3109/09687689409161026. [DOI] [PubMed] [Google Scholar]
- 52.Chang Y, Covey DF, Weiss DS. Correlation of the apparent affinities and efficacies of gamma-aminobutyric acid(C) receptor agonists. Mol Pharmacol. 2000;58(6):1375–1380. doi: 10.1124/mol.58.6.1375. [DOI] [PubMed] [Google Scholar]
- 53.Wang TL, Guggino WB, Cutting GR. A novel gamma-aminobutyric acid receptor subunit (rho 2) cloned from human retina forms bicuculline-insensitive homooligomeric receptors in Xenopus oocytes. J Neurosci. 1994;14(11 Pt 1):6524–6531. doi: 10.1523/JNEUROSCI.14-11-06524.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sperber EF, Moshe SL. Age-related differences in seizure susceptibility to flurothyl. Brain Res. 1988;467(2):295–297. doi: 10.1016/0165-3806(88)90033-8. [DOI] [PubMed] [Google Scholar]
- 55.Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol. 1992;68(1):197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- 56.Galanopoulou AS, Kyrozis A, Claudio OI, Stanton PK, Moshe SL. Sex-specific KCC2 expression and GABA(A) receptor function in rat substantia nigra. Exp Neurol. 2003;183(2):628–637. doi: 10.1016/s0014-4886(03)00213-9. [DOI] [PubMed] [Google Scholar]
- 57.Sperber EF, Wong BY, Wurpel JN, Moshe SL. Nigral infusions of muscimol or bicuculline facilitate seizures in developing rats. Brain Res. 1987;465(1–2):243–250. doi: 10.1016/0165-3806(87)90245-8. [DOI] [PubMed] [Google Scholar]
- 58.Xu SG, Garant DS, Sperber EF, Moshe SL. The proconvulsant effect of nigral infusions of THIP on flurothyl-induced seizures in rat pups. Brain Res Dev Brain Res. 1992;68(2):275–277. doi: 10.1016/0165-3806(92)90070-d. [DOI] [PubMed] [Google Scholar]
- 59.Veliskova J, Loscher W, Moshe SL. Regional and age specific effects of zolpidem microinfusions in the substantia nigra on seizures. Epilepsy Res. 1998;30(2):107–114. doi: 10.1016/s0920-1211(97)00096-x. [DOI] [PubMed] [Google Scholar]
- 60.Romo-Parra H, Trevino M, Heinemann U, Gutierrez R. GABA actions in hippocampal area CA3 during postnatal development: differential shift from depolarizing to hyperpolarizing in somatic and dendritic compartments. J Neurophysiol. 2008;99(3):1523–1534. doi: 10.1152/jn.01074.2007. [DOI] [PubMed] [Google Scholar]