Abstract
Magnetic resonance imaging (MRI) evaluation of the developing brain has dramatically increased over the last decade. Faster acquisitions and the development of advanced MRI sequences such as magnetic resonance spectroscopy (MRS), diffusion tensor imaging (DTI), perfusion imaging, functional MR imaging (fMRI), and susceptibility weighted imaging (SWI), as well as the use of higher magnetic field strengths has made MRI an invaluable tool for detailed evaluation of the developing brain. This article will provide an overview of the use and challenges associated with 1.5T and 3T static magnetic fields for evaluation of the developing brain. This review will also summarize the advantages, clinical challenges and safety concerns specifically related to MRI in the fetus and newborn, including the implications of increased magnetic field strength, logistics related to transporting and monitoring of neonates during scanning, sedation considerations and a discussion of current technologies such as MRI-conditional neonatal incubators and dedicated small-foot print neonatal intensive care unit (NICU) scanners.
Introduction
The use of magnetic resonance imaging (MRI) in evaluation of the developing brain is well established. MRI has proven itself as a beneficial modality in the evaluation of fetal and neonatal neurological conditions due to its unsurpassed sensitivity and excellent tissue contrast.(1-6) Fetal MRI, which was first introduced in the 1980’s, was not widely accepted until nearly a decade later due to long imaging times and limited availability. Even with long imaging times, MRI demonstrated improved anatomic detail, better sensitivity for white matter lesions and consistently detected abnormalities which were not identified on prenatal ultrasound, such as cortical malformations, heterotopias and posterior fossa abnormalities.(6-11) (Fig 1) MRI has become an important adjunct to transcranial ultrasound in the evaluation of neonates, especially in preterm and very low birth weight infants. The development of faster imaging acquisitions has made imaging of the moving fetus and neonate more feasible.(12-16) MRI conditional neonatal incubators, specially designed neonatal head coils and dedicated neonatal intensive care (NICU) MRI magnets have increased the accessibility and feasibility of MRI in the neonatal population.(16-26) Advanced imaging techniques such as magnetic resonance spectroscopy (MRS), diffusion tensor imaging (DTI), perfusion imaging, functional magnetic resonance imaging (fMRI) and susceptibility weighted imaging (SWI), combined with higher clinically applicable static magnetic field strengths have provided new insights into brain development and increased sensitivity for a wider variety of pathology in the developing brain.(16,24,27-30)
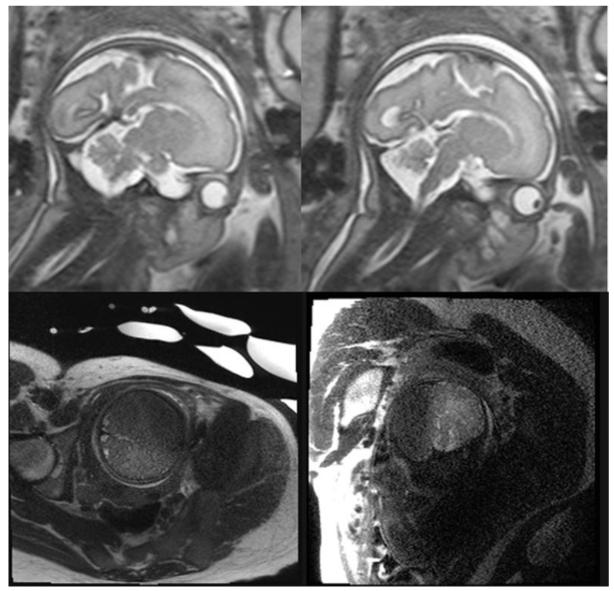
Fig 1. Fetal 3T Imaging and Artifacts.
The top row shows two sagittal single-shot fast-spin echo (SSFSE) T2-weighted images obtained on a 3T magnet, demonstrating high resolution anatomic detail of the midline structures in the fetal brain. The bottom row shows two coronal SSFSE images of the fetus demonstrating motion artifact on the left and dielectric artifact on the right, which are both more conspicuous at 3T field strengths and can degrade images of the brain.
Improvements and Implications of Increased Magnetic Field Strength (1.5T vs. 3T)
Effects of Increased Field Strength
The utilization of 3 Tesla (3T) static magnetic fields for clinical imaging in pediatrics has increased in recent years. Higher magnetic field strengths provide increased signal to noise which can be used to improve temporal and/or spatial resolution. However, 3T MRI also increases artifacts, such as susceptibility and chemical shift can be used in advanced imaging such as fMRI, SWI and MRS.(16,22,28,31-37)
Increased Signal to Noise Ratio
The signal to noise ratio (SNR) refers to the amount of useful information (signal) relative to the amount of signal degradation (noise), inherent in a given image acquisition. The SNR is theoretically linearly related to changes in magnetic field strength, resulting in twice the SNR at 3T compared to 1.5T field strengths, which is one of the advantages of 3T imaging.(28,35,36,38-41) Initially, the actual improvement in the SNR was only 1.7 to 1.8 times the SNR at 1.5T due to numerous factors including increased T1 relaxation time, increased chemical shift, receiver coil design, and increased field inhomogeneity.(31-33,39,42) However, with hardware improvements such as high density phased array receive coils and appropriate pulse sequence modification, SNR increases approach the theoretical value of 2.(43,44)
Variation in the SNR at higher magnetic field strengths is related to the performance and design of the radiofrequency (RF) coils. For example, in fetal imaging, a surface coil, placed over the mother’s anterior abdomen, can increase available signal and improve image quality.(33) Increases in the SNR can also be used to improve acquisition speed and/or spatial resolution in any imaging sequence, including magnetic resonance spectroscopy (MRS), diffusion weighted imaging (DWI), diffusion tensor imaging (DTI) and functional MRI (fMRI).(32,45) Phased array coils, which incorporate multiple coil elements to provide a larger field of view (FOV) without losing signal, can be used for parallel image acquisition.(31,33,46-49) Parallel imaging decreases image acquisition time by under-sampling the available data. A variety of reconstruction algorithms are then applied to the under-sampled data to fill in the missing information and produce the final image.(49-51) At both 1.5T and 3T field strengths, phased-array coils and parallel imaging techniques can be used to improve the image quality (32,33,52) and decrease acquisition time, which has proven useful in both fetal and neonatal evaluations.(33,46,47,49,52) The cost of parallel imaging, is a decrease in SNR which is uniquely adequately compensated for by increased signal availability at higher magnetic field strengths, making parallel imaging more practical at 3T.(32-34,37,46,49)
Improved Temporal and Spatial Resolution
Imaging at higher magnetic field strengths improves SNR which can be used to increase temporal and/or spatial resolution.(31,33,35,53,54) With respect to temporal resolution, the increased SNR at higher magnetic field strengths shortens acquisition time without compromising image quality. Shorter acquisition times, make it possible to obtain additional imaging sequences, such as MRS, DTI and three-dimensional (3-D) images (32), which can improve diagnostic accuracy.
With regards to spatial resolution, increased SNR at 3T compared to 1.5T field strengths can be used to improve spatial resolution. The enhanced spatial resolution results in better visualization of small parts and decreased volume averaging, which are invaluable when evaluating the developing brain, especially in cases of subtle brain injury and/or malformations. The higher spatial resolution can also be used to evaluate other parts of the nervous system such as the brachial plexus following birth trauma, as well as the inner ear structures and the cranial nerves, although these applications are beyond the scope of this review.(32)
Changes in T1Relaxation
Longitudinal (T1) relaxation time is an inherent tissue property describing the rate of recovery of longitudinal magnetization as it returns to the equilibrium state after excitation by an RF pulse.(31) T1 relaxation times are increased at higher magnetic field strengths, resulting in a change in the T1 tissue contrast at a fixed repetition time (TR) compared to imaging at 1.5T.(32-35,37,55-57) TR refers to the interval between the applications of successive RF pulses to the same slice of tissue. Increasing the TR to compensate for the longer T1 relaxation time, results in increased acquisition time, which can lead to unwanted motion artifact in both fetal and neonatal imaging.(31,32,53) Individual tissue types are affected to varying degrees by increasing the magnetic field strength, resulting in negligible changes in the T1 relaxation of CSF and 42-62% increases in the T1 relaxation of white and gray matter respectively.(31,32,45,55,58) Despite its effect on the tissue contrast, longer T1 relaxation times can be used to improve MRA (34,59), post-contrast imaging (56,60) and arterial spin labeling (ASL) imaging.(61).
Changes in T2 Relaxation and T2* Effects
Transverse (T2) relaxation is another inherent tissue property describing the rate of decay of signal coherence due to spin-spin relaxation following the application of an RF pulse. T2* represents the effective T2 value of a tissue, which is the result of the intrinsic T2 value combined with the effects of local field inhomogeneity.(33) T2* effects are most pronounced whenever there are adjacent tissues or structures with significant variations in magnetic field susceptibility, such as at tissue interfaces between air, bone and soft tissue, resulting in signal loss, heterogeneous fat saturation, and geometric distortion.(36)
The T2 relaxation time decreases by up to 10% when imaging at 3T versus 1.5T.(35,55,57,58) The resulting change in T2 tissue contrast however, is not as apparent as the changes in T1 tissue contrast.(32,58) T2* decay, on the other hand, is considerably shorter at 3T due to more pronounced local magnetic field inhomogeneities, which improve T2* contrast but also results in unwanted artifacts or increased diagnostic sensitivity, depending on the pulse sequence.(32,58) The presence of T2* effects is proportional to the magnetic field strength, resulting in rather substantial changes at 3T (62); and while the presence of variations in tissue T2* values can produce deleterious artifacts in an image, it can also be used to produce clinically relevant information in contrast enhanced perfusion imaging, fMRI and SWI.(31-33,35,54,63)
Increased Chemical Shift Artifacts
Chemical shift artifacts also increase at higher magnetic field strengths. The magnitude of the chemical shift artifacts double when the magnetic field strength is increased from 1.5T to 3T.(28,34,35,41,58,64) Chemical shift artifacts cause the spatial misregistration of fat and water on the image when they occur in a single voxel producing a dark band at one side of the fat-soft tissue interface and a whiter band on the opposite border of that same fat-soft tissue interface. (32)
Challenges of Imaging the Brain at 3T and Possible Solutions
Altered T1 Contrast and Artifacts
At 3T, at the TR values typically utilized, the signal intensities of various tissues tend to become more uniform, resulting in less contrast between adjacent tissues on the final T1-weighted (T1W) image.(41,57) In order to optimize T1 tissue contrast and avoid homogenization of adjacent tissues, modifications need to be made to the standard imaging protocols and pulse sequences used at 1.5T.(31,32,59) Modifications include increasing the TR and the use of inversion recovery sequences. The utilization or non-utilization of parallel imaging can improve scan time and SNR, without significantly affecting the T1 tissue values or tissue specific signal intensities throughout the image.(32)
Increased susceptibility at 3T leads to image distortion at tissue interfaces and in regions of local field inhomogeneity.(32,35,42) Possible pulse sequence modifications to minimize, but not completely eliminate, the effect of susceptibility include changing the direction of the phase and frequency encoding gradients, removing the source of field inhomogeneity (e.g. metallic implants), decreasing voxel size, decreasing echo time (TE), increasing receiver bandwidth, using parallel imaging techniques, and shimming.(31-34,53)
Increased chemical shift artifacts at 3T produce areas of signal loss at the interface between fat and soft tissue. Modifications to compensate for increased chemical shift include increasing the readout or receiver bandwidth, altering (TE) and using fat saturation techniques. Increasing the bandwidth decreases the SNR with the tradeoff being the ability to acquire more slices per TR (e.g. echo planar imaging), which can shorten overall imaging time. Fat saturation can be used without a significant decreases in the SNR although the accompanying decrease in slices per TR may increase total scan time.(32,58)
Field Inhomogeneities
The main magnetic field created by an MRI unit is designated B0. MR image generation depends on the application of a second magnetic field, called B1, in the form of a RF pulse, oriented at 90 degrees to B0. Variable application of the B1 RF pulse will excite protons in the desired image slice, and provide a variable amount of signal for image generation. Field inhomogeneities, from both the B0 and B1 magnetic fields, are harder to manage at higher field strengths, resulting in more artifacts at 3T.(33,35,58) The increased presence of these artifacts can pose a challenge in evaluation of the fetus, and is one reason fetal 3T imaging has not gained wide acceptance in clinical practice.(33,35) Modifications to decrease the effects of B0 field inhomogeneity, include the use of shimming to move the foci of field inhomogeneity out of the region of interest, and the application of dielectric pads to change the geometry of the maternal abdomen in the case of fetal imaging.(32,33,65,66) In addition, some of the newest MRI scanners incorporate special pulses, called adiabatic pulses, which are insensitive to the effect of B1-inhomogeneity.(32,67)
Inhomogeneities in the B1 magnetic field result in regional signal reduction in the center of an imaged object, producing standing wave artifacts when a discrepancy exists between the object size and the radiofrequency wavelength. The presence of B1 field inhomogeneities leads to artifacts in an unpredictable manner, which is in part dependent on the patient’s body habitus and the size of the organ being imaged. This is of particular concern in fetal imaging as the size and body habitus of the pregnant female can adversely affect the image quality.(32,33)
Advanced Magnetic Resonance Imaging (MRI) Pulse Sequences: How Can We Exploit 3T?
Advanced MRI techniques and sequences, including magnetic resonance angiography (MRA), diffusion weighted imaging (DWI), diffusion tensor imaging (DTI), diffusion tensor tractography (DTT), magnetic resonance spectroscopy (MRS), perfusion imaging, functional MRI (fMRI), 3-D imaging, and motion correction sequences (e.g. PROPELLER) can be applied in both fetal and neonatal MRI to answer specific clinical questions and provide a more comprehensive diagnostic evaluation in the appropriate clinical setting.
Magnetic Resonance Angiography (MRA)
Magnetic resonance angiography (MRA) can provide non-contrast or contrast enhanced images of the cervical and intracranial vasculature. Longer T1 relaxation times, produced at higher magnetic field strengths, result in improved background suppression, creating larger signal differences between the blood vessels and adjacent tissue in both contrast and non-contrast enhanced time of flight acquisitions.(34,37,41,59) In contrast enhanced imaging, the signal difference between the intravascular contrast and the adjacent unenhanced tissue is further increased because the relaxivity of paramagnetic contrast agents (e.g. gadolinium) is only slightly lower at 3T compared to 1.5T.(40,59) The improved contrast resolution can be used to reduce the gadolinium dose without significantly degrading diagnostic information.(31,41,56,60) Unfortunately, evaluation of intravascular signal can be degraded at 3T in unenhanced MRA due to the effects of increased susceptibility, which are further complicated by the accentuation of normal susceptibility and hypointensity in the vasculature related to deoxyhemoglobin.(33)
Diffusion Weighted Imaging (DWI), Diffusion Tensor Imaging (DTI) and Diffusion Tensor Tractography (DTT)
Increased signal at 3T makes it possible to image in a greater number of planes, use thinner slices and implement higher B-values, all of which contribute to better diffusion weighted images. B-values, in the setting of diffusion, refer to the sensitivity of a sequence to diffusion, and their selection determines the strength and duration of the diffusion gradients used for imaging.(68) The minimum detectable diffusion is determined by the maximum selectable B-value.(28,34,37,64,69) Increased magnetic susceptibility at higher field strengths, especially in echo planar imaging (EPI), may produce unwanted artifacts on diffusion weighted images at 3T, which can be partially compensated for, but not completely eliminated as previously discussed.(32,34,64) Diffusion weighted sequences are also very sensitive to motion, which can make the use of diffusion weighted imaging of the fetus and neonate difficult at times.(29,64)
Diffusion Weighted Imaging (DWI)
The signal in diffusion weighted imaging (DWI) is based on the microscopic movement of water molecules in a tissue and can be used to calculate the apparent diffusion coefficient (ADC) of that tissue. The ADC value is a representation of the overall magnitude of diffusion within a tissue.(70) DWI is performed as a single-shot spin-echo echo-planar pulse sequence using pulsed diffusion gradients with variable B-values. The pulsed diffusion gradients provide quantitative information about the motion of water and tissue microstructure, making it possible to identify areas of focal injury and abnormal brain development.(16) Increased signal at 3T allows the selection of higher B-values, thinner slices, and the possibility of anisotropic mapping, which is discussed below.(28,34,37)
Diffusion Tensor Imaging (DTI)
Diffusion tensor imaging (DTI) provides clinical information based on the diffusion properties of a tissue, and provides an effective way of characterizing normal and abnormal patterns of white matter development.(71-80) DTI does this by emphasizing directional information in the information acquired in DWI/ADC, allowing calculation of diffusion anisotropy, fractional anisotropy, and mean diffusivity. These calculations can provide insight into pathologic processes and microstructural abnormalities during development.(16,70,74-83) For example, diffusion anisotropy can be detected before myelination is complete, making it possible to use DTI to detect premyelinating elements.(16,72,76,77,84)
The qualitative parameters of mean diffusivity and fractional anisotropy are independent of patient head position, making them useful when imaging a fetus in multiple orientations.(32) The quantitative parameters obtained with DTI correspond to sequences of myelination in the developing brain, making it possible to determine if there is derangement in the normal patterns of myelination.(76,77,79,80,82,85-88) Directional information corresponding to white matter fiber tract orientation within collimated bundles can be displayed in two or three dimensions, illustrating the structural and functional connectivity of the maturing brain.(32,89-91) DTI is more sensitive, than DWI, to patient motion, which can be challenging in neonatal and fetal imaging; however, at 3T field strengths shorter scan times and wider field coverage with adequate SNR may increase its feasibility.(6,32,73,75)
The need for detailed delineation of neuronal fiber tracts has led to the development of multi-band diffusion tensor imaging (MB-DTI), which may have utility in this application. Multiband acquisitions use multi-slice single-shot EPI pulses, which increase the number of slices obtained during a single acquisition and therefore significantly reduces the TR.(92,93) Unfortunately, multiband pulses have the potential to increase the power deposition in the patient, relative to the number of slices that are excited during each pulse, and in order to comply with specific absorption rate (SAR) limits (discussed below), sequence modifications such as increasing the TR or reducing the number of slices must be made.(94)
Diffusion Tensor Tractography
Diffusion tensor tractography (DTT) uses information obtained for DTI to map white matter tracts using the knowledge that water movement across fibers can be impeded by white matter elements.(95-97) Tractography can be used to evaluate selected neural pathways using two different fiber tracking algorithms (deterministic and probabilistic), which have advantages and disadvantages depending on the clinical situation. Data obtained in tractography produces a specific color and brightness for each voxel, which corresponds to the orientation and density of the fibers passing through that voxel.(16,91) 3-D representations of the tractography output can be generated, providing a global view of the brain’s interconnectivity.(96,97)
Magnetic Resonance Spectroscopy (MRS)
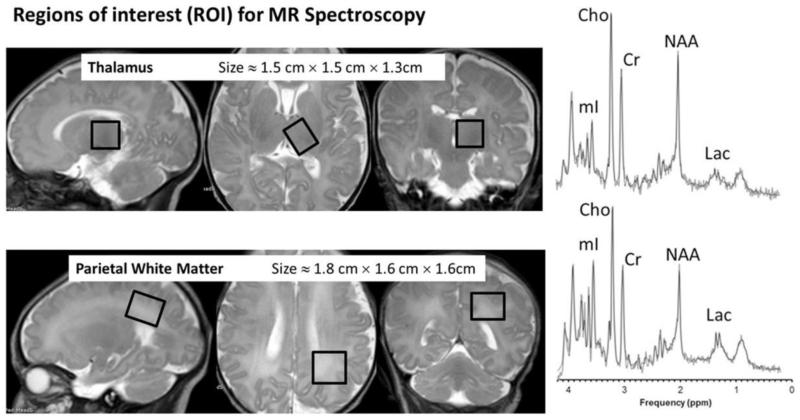
MRS provides a non-invasive way to evaluate the biochemistry of the brain (25,29,70,98) by generating a spectrum of metabolite peaks with different radiofrequencies resulting from proton (hydrogen) nuclei in different chemical environments.(16,99) The generation of an MRS spectrum takes advantage of intrinsic differences in chemical shift among the various chemicals in the body/brain.(34,41) The area under the curve of each peak is proportional to the number of contributing protons, and is increased at higher magnetic field strengths. The peaks of greatest clinical significance in MRS include N-acetyl-aspartate (NAA), creatine, choline, myoinositol, glutamate, glutamine, lipid and lactate.(16,98-100) Each peak aids in evaluation of a particular cell line or metabolic pathway within the brain. For example NAA is considered a marker for neurons and axons, choline-containing compounds are a marker for cell membranes and myelination, creatine is used as a marker for energy metabolism and lactate is used as a marker for anaerobic metabolism during hypoxic-ischemic injury.(29,70,100-102) At 3T the amount the signal generated by each metabolite is increased, making the peaks larger and easier to select out from the background noise.(38,58,103-105) Metabolite peaks and the appearance of the spectrum vary with changes in the echo time (TE) and consideration must be given to the selection of the TE used in diagnostic examinations.
The use of variable TE values can illustrate changes in the MRS output to highlight different metabolic derangements. The greatest gain in SNR occurs with a short TE, making detection of metabolites with short T2 relaxation times, such as myoinositol, glutamate, glutamine and lipids easier.(16,105) Short TE MRS at 3T however, produces overlap between the lipid and lactate peaks and causes distortions of the baseline related to local electrical currents in the patient. Intermediate TE values suffer from variable lactate inversion, which can complicate evaluation of hypoxic injury. Long TE values essentially eliminate any signal benefit gained at 3T and create a smooth baseline, which may obscure small metabolite peaks. As a result of the variations in the spectrum at each TE, clinically applicable spectra are usually obtained using two different TE values.(32,41,106) For example, short and long TE MRS can be used to detect NAA and lactate levels in cases of hypoxic injury.(16)
Increased chemical shift at higher magnetic field strengths can improve MRS by increasing metabolite resolution and the size of the individual metabolite peaks. The intrinsic increase in SNR at 3T can also decrease the amount of time needed to generate the equivalent MRS spectrum.(32,58,105) Long imaging times for each voxel and limitations of voxel size relative to the size of the fetal brain currently limit the use of MRS in the fetus; although the use of 3T magnets may decrease imaging time enough to make fetal MRS feasible, especially during the third trimester when the fetal head is engaged in the maternal pelvis.(6,107-109) Increased signal and decreased acquisition time at 3T also make MRS more feasible in neonates who may not tolerate long scan times.(58) Newer parallel imaging techniques such as localized adiabatic selective refocusing (LASER),and other novel imaging sequences currently used in research applications hold promise for increasing the quality and feasibility of fetal MRS.(110) This is important as multiple investigations have demonstrated that MRS can have potentially substantial benefits in brain imaging, especially in cases of white matter injury.(22,24,26,111)
Another benefit of performing MRS at 3T is the ability to select smaller voxels resulting in a reduced likelihood of contamination from adjacent fat, especially in peripherally located lesions.(32,107) Unfortunately, contamination cannot be completely avoided and, in cases where misregistration occurs within the sampled voxel, there may be poor inversion of the lactate peak, which can degrade analysis and confound clinical diagnosis.(32,106)
In addition to measuring metabolite concentrations, MRS can be used to measure the absolute brain temperature using the temperature-dependent water chemical shift and comparing it to other metabolites such as NAA, creatine, and choline.(112,113) A study performed by Wu et al. in 2014, using MRS measurements of brain temperature in 18 neonates, demonstrated higher brain temperature and brain-rectal temperature gradients in neonates with severe HIE compared to those with moderate HIE both during and after therapeutic hypothermia.(114)
Perfusion Imaging, Functional MRI (fMRI) and Susceptibility Weighted Imaging (SWI)
Perfusion Imaging and functional MRI (fMRI) provide non-invasive methods of evaluating cerebral blood flow and the functional development of the brain. The most commonly used methods of measuring brain perfusion in pediatric MRI include intravascular contrast and arterial spin labeling (ASL).(63,115-117) Each of these techniques utilizes a specific cerebral perfusion state to generate signal, and each best highlights a particular perfusion parameter.(16,118-122)
Contrast enhanced perfusion MRI uses paramagnetic intravascular contrast agents to generate signal intensity versus time activity curves, which can be used to calculate the relative cerebral blood volume, relative cerebral blood flow and mean transit time.(16,63,117) ASL provides perfusion information without contrast administration and is useful in evaluating cerebral blood flow in neonates.(16,63,119-123) (Fig 3) At higher magnetic field strengths, the contrast created using ASL of blood lasts longer, due to the longer T1 relaxation times, producing a potential 3-fold increase in SNR.(32,61,119,124) The effects of higher magnetic field strength on T2 relaxation, although small, may produce additional gains in the SNR in ASL imaging.(119) This is especially useful, in fetal and neonatal brain imaging, due to higher blood flow rates, which allow the label to pass further into the vascular bed without any loss in signal intensity.(32,119,120)
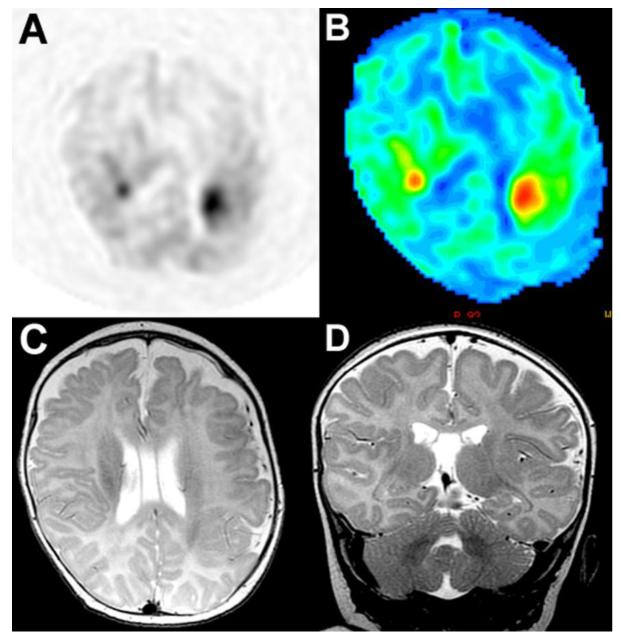
Fig 3. ASL perfusion in the newborn brain, patient with recurrent seizures.
(A) Gray-scale ASL perfusion image showing two areas of increased perfusion in the region of the peri-Sylvian gyri bilaterally. (B) Corresponding color ASL perfusion image re-demonstrating two foci of increased perfusion in the region of the peri-Sylvian gyri bilaterally. (C) Axial T2-weighted image through the level of the peri-Sylvian gyri corresponding to the region of increased ASL perfusion. (D) Coronal T2-weighted image through the peri-Sylvian gyri corresponding to the region of increased ASL perfusion. Increased perfusion in this case may represent epileptogenic foci in the brain and/or areas of subtle cortical dysplasia.
Functional MRI (fMRI) uses blood oxygenation level-dependent (BOLD) signal, to detect regional hemodynamic changes within the brain parenchyma that correlate with response to various visual, auditory and sensorimotor stimuli.(29,70,125-128) BOLD fMRI is significantly improved at 3T, as a result of the higher susceptibility contrast sensitivity and higher SNR, resulting in up to a 40% increase in detected activation on BOLD images compared to 1.5T.(34,40,45,129-131) In fMRI of the developing brain, this has been used to establish normal and abnormal patterns of brain activation.(132) Unfortunately, BOLD images can be degraded by signal loss due to low-frequency fluctuations in brain oxygenation.(121,122)
Functional MRI has also been used to evaluate the activity of the brain in its resting state; also known as resting state functional MRI (rs-fMRI). Resting state fMRI is well-suited for evaluation of neonates as it allows assessment of the brain during sleep.(133-135) Resting state fMRI uses functional MRI data collected during rest, analyzes the data by detecting low-frequency (< 0.1 Hz) fluctuations in the brain which represent specific spatial structures, and produces a representation of the brain’s spontaneous fluctuations in the absence of external stimuli.(126,127,133-139) Resting state fMRI has identified many distinct brain networks, which are involved in both sensory and cognitive functioning (94,126,134,140-142), and multiple studies have demonstrated these networks in both term and preterm infants.(133,134,141-145) When rs-FMRI is combined with tractography it can provide a non-invasive map of both the functional and structural interconnectivity of the brain, or “the human brain connectome.”(88,145-149) The ability to successfully identify resting-state networks in healthy infants, provides a comparison for cases of brain injury where altered resting-state connectivity may be present.(126)
The quality of both perfusion imaging and fMRI is dependent on the presence of susceptibility artifacts and on patient motion.(6,150-153) The increased conspicuity of susceptibility artifacts at 3T can be advantageous in both SWI and fMRI. In SWI the presence of susceptibility artifacts increases sensitivity for blood products and mineralization, which can be used clinically in the detection of non-accidental trauma, birth trauma and diffuse axonal injury. In fMRI the increased susceptibility improves sensitivity for the presence of deoxyhemoglobin resulting in improved BOLD signal.(32,33,37) Unfortunately, not all susceptibility sensitivity is good, and in fetal fMRI, images can be degraded by susceptibility produced by the adjacent maternal organs when the fetal head is in the pelvis.(6,33,150-153) Functional MRI is also sensitive to patient motion and may require the application of complex motion correction algorithms, the use of motion insensitive sequences, or the use of patient sedation. The use of sedation in fetal scanning is not commonly employed in the United States, and because it is desirable to minimize neonatal exposure to sedation this can provide a significant impediment to high quality fMRI acquisition.(6,32,154-158) More recently, a new pipeline has been developed to help with correcting resting state BOLD data with respect to motion, bias field and spin history correction to improve the recovery of non-corrupted datasets.(159)
Three-Dimensional (3-D) and Volumetric Imaging
Volumetric imaging techniques use two-dimensional (2-D) and three-dimensional (3-D) images to quantitatively characterize brain development.(70) Dynamic 3-D imaging can be used, in the appropriate clinical setting, to obtain sub-second time sequential 3-D images, which is especially useful in post-contrast perfusion imaging.(32,160,161) With higher magnetic field strengths and the application of parallel imaging techniques, gradient echo T1 and turbo spin-echo T2 images can be obtained in an isotropic volumetric manner in one plane, and reconstructed in any other reformatted plane, allowing faster multi-planar imaging. Volumetric evaluation of the brain allows for post-acquisition segmentation, measurement and detailed evaluation of subsets of the brain based on differences in signal intensity on multiple sequences.(16,70,160-163) 3-D and volumetric imaging are sensitive to motion and their use is limited in fetal MRI for this reason. Post-processing methods for motion correction have been developed and can be applied to fetal data to produce a single geometrically consistent 3-D image of the moving fetus (160-162,164,165), making 3-D imaging of the fetus more feasible.
Motion Correction Sequences
Patient motion presents imagers with a significant challenge, in terms of image quality, especially when imaging during the fetal and neonatal period. This has led to the development of motion correction sequences, which compensate for rotational and/or translational head motion to produce a smoother final image. Motion correction sequences work by correcting for the spatial inconsistencies in position and rotation of the head between imaging strips and produce an averaging effect at low spatial frequencies resulting in less motion artifact on the final image.(16,37,166,167) Motion correction sequences are available on most new MRI systems and can be referred to by various names including, PROPELLER (GE) (167-171), BLADE (Siemens) (172-174), MULTIVANE (Phillips) (175). Multiple studies have shown that the application of corrective sequences can reduce the extent of motion artifact on brain imaging in moving patients, improving image quality, increasing lesion conspicuity and improving parenchymal contrast (167,168,171-175), even when compared to fast spin-echo sequences.(168,171,173) Motion correction techniques have been applied to both conventional and advanced MRI sequences such as DWI (170), FLAIR (173,174) and fMRI (169).
Safety Concerns Associated with Imaging the Developing Brain
As the role of MRI has increased in the diagnosis and management of fetal and neonatal neurologic conditions, concerns about its safety have become an important consideration. Since evaluation of the risks and safety to neonates and the fetus are difficult to obtain directly, the safety of imaging these patients has been inferred from a number of studies evaluating general MRI safety. According to the June 2014 FDA guidelines, MRI carries a “non-significant risk” in neonates (infants less than 1 month old) at static magnetic field strengths below 4 Tesla. This designation is based on over 30 years of use accompanied by no reports of deleterious short or long-term effects when MR scanners were operate within set regulatory limits.(1) In fetal MRI, current data and research studies have failed to demonstrate any reproducible harmful effects on pregnant females at magnetic field strengths of 3T or less. Based on this and recent practice parameters it seems reasonable to assume that imaging the fetus at 3T field strengths would not pose an increased risk to the mother or fetus.(176) The ACR guidelines further reiterate this, stating that the “present data have not conclusively documented any deleterious effects of MR imaging exposure of the developing fetus. Therefore no special consideration is recommended for the first, versus any other, trimester in pregnancy.”(177,178)
Despite the available data supporting the safety of MRI use in both fetal and neonatal imaging at 1.5 and 3 Tesla, there are still some safety parameters which should be considered when performing MRI exams, including (1) static magnetic field exposure, (2) specific absorption rate (SAR), (3) gradient field switching, and (4) sound pressure levels (SPL).
Static Magnetic Field Exposure
Static magnetic field exposure refers to the effects of the magnetic field on any person placed in close proximity to the MRI magnetic. The primary concerns associated with static magnetic fields are two-fold and include, the hazards of ferromagnetic objects becoming projectiles and the potential effects of the static magnetic field on biological systems. The first issue has been addressed with the implementation of careful screening and safety measures in MRI areas, put in place to prevent the accidental placement of a ferromagnetic object in areas with a strong static magnetic field.(177-181)
In terms of the effects on biological systems, numerous studies have been performed, none of which demonstrate any direct evidence of deleterious effects with short term exposure to high static magnetic field strengths; however, extrapolating data from the available animal models to establish the true effect on the fetus or neonate is challenging.(33,182-194) Despite the lack of data to support potential harm from the static magnetic field strengths currently used for clinical imaging, the FDA has set limits on magnetic field exposure of 8T for patients greater than 1 month of age and 4T for patients less than one month of age.(1)
Specific Absorption Rate (SAR)
The specific absorption rate (SAR) is a measure of the rate of energy deposition within a given mass of tissue (units = watts/kilogram = W/kg).(32,33,35,182) Deposition of energy created by radiofrequency (RF) pulses is dissipated as heat in the surrounding tissue. This poses a safety concern as it can cause serious tissue burns and undesirable increases in body temperature in the imaged subject, which might produce adverse outcomes in the fetus or neonate.(32,33,177,178,180,181,195,196) Regulations, in both Europe and the United States, have been put in place, which define the maximum local and global SAR values in various parts of the human body per unit time.(1,32,182,197) The SAR limits, none of which can be exceeded during any MR examination, defined by the FDA in 2014 (1) state that exposure should be:
Less than 4 W/kg averaged over 15 minutes in the whole body; and
Less than 3.2 W/kg average over 10 minutes in the head
As a result of the guidelines, MR scanners used for clinical exams have failsafe mechanisms in place to halt scanning when the SAR regulatory limits have been reached that are independent of the magnetic field strength used for imaging.(182)
The rate of energy deposition in a mass of tissue depends on the applied RF fields, which increase with increasing magnetic field strength. It is important, however, to remember that an equivalent quantity of absorbed energy, independent of the magnetic field strength, will produce an identical increase in temperature in the imaged subject.(182) The SAR is proportional to the square of the static magnetic field strength (B02) leading to four times the rate of energy deposition at 3T compared to 1.5T.(22,28,32,36,45,53) SAR is also dependent on other factors including flip angle and TR (39), which need to be considered when utilizing fast contrast enhanced 3-D angiographic sequences and fully rephrase gradient echo techniques, which use high flip angles and short TR to yield optimal signal.(31,32,34,53)
Given regulatory limits on SAR and the increased rate of energy deposition at higher magnetic field strengths, it is important to consider sequence selection and timing when performing examinations at 3 Tesla. SAR is increased to a greater degree in sequences using larger flip angles, as discussed above. Increased SAR is also a concern with fast spin-echo (FSE) sequences and fluid attenuation inversion recovery (FLAIR) sequences. In FSE sequences, SAR is increased due to the use of multiple refocusing pulses, each of which deposit energy in the imaged subject. In FLAIR imaging, the amount of radiofrequency energy required to generate the signal used in image production is higher than in normal spin-echo sequences.(41) SAR is also increased in sequences that utilize short TR gradient echo pulses such as true-FISP and FIESTA, due to the rapid sequential application of the RF pulses.(32)
Reductions in SAR can be achieved by using special radiofrequency (RF) coil designs such as multichannel transmit phased-array coils, reducing the number of slices, increasing the repetition time (TR), decreasing the flip angle, using parallel imaging or shortening the echo train length (ETL).(31-34,51) It is important to note that the echo train length is increased in fast spin-echo (FSE) sequences, such as single-shot fast spin echo (SSFSE), which are the workhorse of fetal imaging.(33,198) Reducing the flip-angle can also decrease the SAR, but causes alterations in tissue contrast, which has led to the development of special RF pulses that use variable flip angles through the sequence (e.g. hyperechoes, TRAPS). The application of these specialized sequences can reduce the SAR four-fold but at the expense of image contrast and/or SNR.(32,35,199)
Gradient Field Switching
Gradient fields are magnetic fields created by passing currents through combinations of three spatially co-registered orthogonal gradient coils during imaging to encode spatial position information in the imaged portion of the body. Further, inverting gradient fields create useable signals or echoes, the timing of which produces the ultimately detected tissue signal at the desired TE. Gradient field switching results in a local magnetic field incident on the patient resulting from pulsing the gradient on and off during scanning. The rate of magnetic field switching is referred to as the slew rate. The slew rate is dependent upon the selected imaging parameters and the capabilities of the gradient equipment available in that MR scanner. While it is not in and of itself field strength dependent the more expensive 3T imaging systems are frequently accompanied by more capable gradient sub-systems. Gradient field switching poses the potential hazards of unwanted neurostimulation by Faraday’s Law as well as the production of acoustic noise (which will be discussed in the next section). The main biologic effects we are concerned with are peripheral nerve stimulation and the potential for fetal distress during scanning as a result of increased noise exposure and neurostimulation.(33,182)
The sequential application of gradient pulses produces changes in the magnetic field experienced by the imaged subject during each pulse. According to Faraday’s Law, exposure to rapidly changing magnetic field, such as those produced during gradient switching in echo-planar imaging (EPI), can result in involuntary peripheral nerve stimulation during scanning. In practice, this may result in a mild tingling or tapping sensation when gradients are turned on and off.(33,182) Due to the potentially unwanted effects of gradient switching, the FDA has created limits on the exposure to changing magnetic fields, which are independent of magnetic field strength.(33) The 2014 FDA guidelines state that gradient field switching should be limited “any time [the] rate of change of gradient fields (dB/dt) [is] sufficient to produce severe discomfort or painful nerve stimulation.”(1) As a result, MR scanners have been installed with programming that is able to monitor the rate of gradient switching and maintain dB/dt values within acceptable ranges.(33,200)
Acoustic Noise and Sound Pressure Levels (SPL)
Acoustic noise is produced by gradient field switching when the energy produced by the gradient coil is in the audible frequency range. In general, the acoustic noise produced during MR image acquisition is increased at higher magnetic field strengths as a result of more expensive and capable gradient systems, which are able to achieve higher dB/dt values, longer duty cycles and faster slew rates.(34,35,182,201,202) Noise exposure in MRI can exceed levels that cause the patient pain. The actual noise level at which pain occurs is patient dependent and occurs over a range of values, sometimes as low as 102 to 105 dBA.(203-205) This is especially important in high-speed sequences such as echo planar imaging (EPI), where sequential gradients are rapidly applied.(202,206,207)
FDA regulations require that noise exposure from MRI (in adults) not exceed peak unweighted sound pressure levels of 140 dB (1), and while no similar regulatory guidelines exist for neonates, ad hoc limits have been suggested (e.g. neonatal noise exposure limits should not exceed 60 dB for inter-institutional transport).(207-209) The FDA also requires that the “A-weighted root mean square (rms) sound pressure level [not exceed] 99 dBA with hearing protection in place.”(1,177,178) As a result of these limits, and despite the fact that there are no specific guidelines about the use and type of hearing protection applied during MRI, passive hearing protection is used on all neonates undergoing MRI examination.(210) In the case of the fetus, multiple studies have demonstrated that the gravid uterus provides an acceptable barrier for sound attenuation, and that sound generated by MRI during in utero imaging would not be harmful to the fetal ear.(211-216)
Fetal Imaging
Introduction
The first fetal brain MRI performed in the early 1980’s demonstrated improved detection capabilities compared to prenatal sonography, allowing imagers to see lesions which were not apparent on ultrasound.(6,9,10) However, it was not until the 1990’s when single-shot rapid acquisitions, which essentially freeze fetal motion, were developed that fetal MRI became feasible clinically.(11-15) Increased speed of acquisition and other technical improvements have resulted in the practical performance of in vivo exanimation of fetal anatomy at both 1.5T and 3T. The unparalleled soft tissue detail and insensitivity to a number of technical factors associated with ultrasound (US) have resulted in more frequent MRI examinations. As a result, MRI has gained increased recognition as a proven modality for evaluating fetal brain anomalies in cases where ultrasound is either not adequate or where a high familial risk exists, making fetal brain MRI at 1.5T field strengths part of routine clinical practice.(6,47,176,182,198,217-221) MRI has also provided a means by which to determine whether the fetal brain is following normal patterns of maturation and development.(222-228)
While US remains the primary modality for imaging the fetus, in special and complex cases, such as those with equivocal findings or known pathologic abnormalities, MRI has become an important addition to complete imaging evaluation.(6,12,14,217-220,229-231) Fetal brain MRI improves detection and visualization of potentially life altering abnormalities in cases where there are high pre-test risks or when early detection of abnormalities could help in timely and informed patient management decisions.(6,33,232) MRI offers superior soft tissue contrast, which in addition to advanced techniques, such as DWI, MRS and fMRI, improves the diagnostic accuracy and identification of subtle fetal central nervous system (CNS) abnormalities.(6,107-109,220,221,227,228,233) The most common indications for fetal brain MRI include: ventriculomegaly, anomalies of the corpus callosum, cortical malformations, complications of monochorionic twinning, and posterior fossa abnormalities. MRI has also been used to image the fetus before and after in utero interventions, for parental counseling, delivery planning and postnatal therapy.(6,11-13,176,198,217-219,230-232,234)
Unlike US, MRI is not limited by factors such as fetal lie, oligohydramnios, overlying maternal structures, calvarial development or maternal obesity. MRI also has the capability of imaging the fetus in multiple planes, which provides a better morphologic assessment of the intracranial contents. MRI also acquires images using a larger field of view (FOV), which can include the placenta.(6,12,47,217,230-232,235,236) Large field of view, multi-planar evaluation of the fetus can be instrumental in planning and implementation of multidisciplinary care when approaching complex cases, which may require detailed delivery planning, fetal or postnatal surgical planning, and family counseling about current pregnancy risk and the risk of recurrence in future pregnancies.(6,229,230) Fetal MRI is limited in some cases when patients are extremely obese and exceed the MRI table weights or are too large to fit within the bore of a traditional MRI scanner (35), although the introduction of large bore MR scanners has helped to accommodate larger maternal size/girths. The development of fast acquisition sequences, such as single-shot fast spin-echo (SSFSE), half-Fourier acquisitions, and single-shot turbo spin echo have made imaging the fetus in multiple planes clinically feasible.(6,12,33) Additionally sequences such as fast multi-planar spoiled gradient-recalled sequences, have been used to generate T1-weighted images that are able to detect fat, hemorrhage and calcifications.(6)
The use of advanced MRI techniques, such as MRS, diffusion imaging, fMRI and parallel imaging have improved the diagnostic accuracy and evaluation of the fetal brain.(6,77,107-109,219-221,228,233) MRS, for example, has been used in fetal imaging to detect in utero insults to the brain resulting in earlier diagnosis and better estimates of long-term prognosis for affected infants. MRS has also been used to examine the normal physiologic changes that occur during brain development, providing a basis of comparison in abnormal studies in both the fetus and neonate.(78,80,85,86,100,103,107-109,228,233) DTI has been successful applied in the fetal brain to calculate normative mean diffusivity values in the second and third trimesters, which can be used to evaluate cerebral maturation and provide a quantitative marker of in utero brain development.(6,29,222,223,225,226) Functional MRI has been performed by multiple groups during the third trimester to assess the response to auditory and visual stimuli placed over the maternal abdomen. Unfortunately, many of the studies were limited by fetal motion and susceptibility artifacts produced by the adjacent maternal organs.(150-152,237-239) Additionally, in two studies, low magnetic field strengths (0.5T) were used, resulting in a low SNR, decreased BOLD signal and low-resolution anatomic images for fMRI localization.(150,238) Thomason et al. used BOLD fMRI techniques to demonstrate cross-hemispheric functional connectivity in the fetus between the 24th and 39th weeks of gestation, confirming callosal development and inter-hemispheric communication in the developing brain.(240)
In the following sections, we will discuss some of the specifics related to fetal imaging at 3T versus 1.5T field strengths. We will discuss the advantages and challenges as they related to fetal imaging specifically, including the timing of fetal MRI scanning and how to optimize diagnostic yield. Finally, we will address safety issues related to MRI scanning and how they pertain to imaging of the fetus.
Specifics Related to Fetal Imaging at 1.5T versus 3T
Fetal imaging at higher magnetic field strengths can provide us with improved tissue contrast and better anatomical delineation of the fetus. Unfortunately, increased magnetic field strength does not simply equate to better images, and a number of factors need to be considered when transitioning to a higher magnetic field strength. Improved image quality must be balanced with a need for fast imaging, due to the rapidly moving fetus, and with safety concerns related to higher energy deposition and noise production at 3T. As a result single-shot fast spin-echo (SSFSE) sequences have become the workhorse of fetal MRI.(33)
One major hurdle encountered with 3T imaging is the need for protocol modification resulting from changes in factors such as T1, T2 and T2*. Numerous modifications exist to overcome the associated changes in tissue contrast and increased conspicuity of artifacts at higher magnetic field strengths. One of the most significant modifications, particularly in fetal imaging, is increasing the TR to improve T1 tissue contrast at the expense of longer scan times and possible motion artifact from the moving fetus. As a result of competing interests, a balance must be struck between optimal imaging time and optimal tissue contrast. While T2 relaxation is not significantly affected by the increase in magnetic field strength, the T2* effects become significantly more pronounced at 3T leading to greater susceptibility artifact production, which can be used when performing SWI, increasing the sensitivity for blood products and calcifications within the fetal brain.(32,33)
Phased-array coils and surface coils specifically designed for use at 3T can be created with more elements than those used at 1.5T.(33,47,48) The use of these specifically designed coils improves the SNR and can compensate for some of the losses resulting from decreased T1 relaxation.(33,47) The use of parallel imaging with phased-array coils at 3T magnetic field strengths allows faster acquisition times as a result of faster k-space filling.(31,46) Phased-array coils, with parallel imaging capabilities, can be applied in fetal imaging to acquire high quality images in the setting of poor maternal breath holding and a constantly moving fetus.(33,47)
In many institutions the increased radiofrequency energy deposition/SAR at 3T has limited its use in fetal imaging (35), however, a study performed by Krishnamurthy et al. in 2014 directly comparing 1.5T examinations to 3.0T examinations in the same patients, showed that SAR limits were not violated during fetal MRI and that 3T imaging of the fetal brain could be performed safely while reaping the benefits of improved tissue contrast and lesions conspicuity compared to 1.5T examinations.(39) Despite its apparent safety, sequence modification is needed when transition from 1.5T to 3T in order to comply with FDA guidelines regarding specific absorption rates (SAR) and energy deposition.(182) Decreased flip angles (at the expense of the very SNR advantage that 3T holds over 1.5T) and optimization of other scan parameters need to be considered to maintain SAR below the regulatory limits, while maintaining diagnostic image quality.(32,53)
Advantages of Fetal MRI Scanning at 3T
There are numerous advantages to fetal MRI scanning, and although US remains the primary imaging modality in evaluation of the fetus, MRI has proven itself to be an important adjunct in complex cases and cases where there is a high index of suspicion for potential anomalies. MRI at higher magnetic field strengths, especially for evaluation of the brain, also has the advantage of improved SNR, better anatomic delineation and increased beneficial artifact production, which can be used to highlight various pathologic and non-pathologic processes.
The improved signal strength at 3T, results in an increased SNR of approximately 1.7 to 1.8 in most cases (31-33,39,42), however, new hardware improvements may result in SNR increases that approach 2.(43,44) Despite a less than two-fold increase, the increased signal we do gain can be used to improve image quality or can be traded for increased spatial and/or temporal resolution. Increased signal allows more streamlined implementation of parallel imaging with multichannel coils which decreases the acquisition time for single-shot sequences, reduces echo time (TE) in long echo train sequences, reduces susceptibility and decreases radiofrequency heating by minimizing the number of pulses required to fill k-space and generate an image (33) leading to more efficient imaging evaluation of the fetus.
Compared to US, the mainstay of fetal imaging, there is improved anatomic delineation even with MRI performed at low magnetic field strengths. The anatomic detail provided by MRI is one of the primary reasons it has become an important adjunct to US in complex cases. Higher magnetic field strengths, such as 3T, further improve anatomic delineation due to increases in the amount of available signal. The increased spatial resolution results in an ability to highlight small differences in adjacent tissues when the imaging protocols are optimized, providing more detailed anatomic information and improved visualization of existing abnormalities in the fetus. The improved diagnostic quality of the images produced at 3T allows better informed decisions about intervention, management and outcomes in fetal medicine.(6,33)
Challenges of Fetal MRI Scanning at 3T
Artifacts
Artifacts including magnetic field inhomogeneity, standing wave artifacts, susceptibility artifacts, and chemical shift artifacts can degrade fetal images, and each will be discussed below.
Magnetic Field Inhomogeneity and Standing Wave Artifacts
Magnetic field inhomogeneity (B1) in fetal imaging can lead to standing wave or dielectric resonance artifacts that result in degradation of echoplanar, spin-echo, steady state free precession (SSFP) and single-shot fast spin-echo sequences (SSFSE), the latter of which is the mainstay in fetal imaging.(32,33,37) (Fig 1) Higher magnetic field strength results in increased frequency and decreased wavelength of the magnetic field radiofrequency. The radiofrequency wavelength in amniotic fluid may approximates the field of view (FOV), especially at 3T where the wavelength is decreased, producing an interference pattern that results in heterogeneous signal and areas of hypointensity or blackout mixed with areas of brightening. This artifact is more apparent when the discrepancy between the FOV and wavelength increases, and results in more pronounced artifactual bands in obese and pregnant patients.(31,33,35,42,53)
In the maternal abdomen, standing wave artifacts result from both the increased abdominal circumference and the presence of amniotic fluid, resulting in areas of blackout near the center of the field of view, which is unfortunately where the fetus is positioned. To reduce the appearance of standing wave artifacts, dielectric pads or radiofrequency cushions can be used to alter the magnetic field inhomogeneities by changing the geometry of the imaged subject.(31-35,65,66) The use of dielectric pads, which are placed on the anterior abdominal wall of the pregnant patient, can be uncomfortable, especially in the case of gel pads that incorporate gadolinium or manganese-based mediums. This has led to the development of lighter saline filled pads.(33,34,42,66) Other methods of decreasing radiofrequency inhomogeneity include multichannel transmission body coils and active RF shimming combined with parallel imaging, however, these methods are not always available on commercial MR scanners (35,48,52,241,242) and will not be discussed in any further detail in this review.
The effects of field inhomogeneity in steady state free precession (SSFP) sequences result in off-resonance effects that can potentially exacerbate the appearance of banding artifacts, alternating dark and light bands, especially at the edge of the field of view. (31,33) To compensate for this increased artifact, the orientation of the scanning field of view or frequency encoding gradient can be changed, or modifications can be made to the applied RF frequency or bandwidth selection to displace the artifact away from the area of interest, while not eliminating the artifact entirely.(33)
Magnetic Susceptibility Artifacts
Magnetic susceptibility artifacts are increased at higher magnetic fields strengths, and are therefore of concern when transitioning to 3T imaging.(32,33) Magnetic susceptibility occurs, for example, at the interface between the uterus and adjacent gas-filled colon, and is of particular concern, in the vertex-positioned fetus, where it can degrade brain imaging. The presence of susceptibility artifacts can be minimized by changing the readout direction of the acquisition to alter the location of the artifact away from the primary FOV. It can also be decreased by implementing parallel imaging techniques and using shorter echo times (TE). In brain imaging, in particular, the use of shorter TE at higher field strengths is often necessary in order to correct for geometric distortion of the head and accentuation of the intracranial vasculature on echo planar images.(33) Susceptibility induced signal loss from maternal organs can also degrade fMRI acquisitions, especially late in pregnancy when the fetal head is engaged in the pelvis.(6,150-153)
Chemical Shift Artifacts
The magnitude of the chemical shift artifact is linearly more prominent at 3T than it is at 1.5T. Fortunately, the effects of chemical shift are not a significant concern in fetal imaging; due to the fact that the two sources of fetal fat (subcutaneous and intra-abdominal) are not present until late in pregnancy and do not appreciably contribute to artifact formation during fetal imaging.(33)
Circulating Electrical Current Field Created by Amniotic Fluid
Circulating electrical currents are created in amniotic fluid secondary to rapidly changing magnetic fields. The circulating current in the amniotic fluids acts like an electromagnet, which opposes the fluctuating magnetic field and leads to a reduction in the amplitude and dissipation of the radiofrequency field. This effect is also known as radiofrequency shielding artifact.(31,33,35) The production of radiofrequency shielding artifact results in hypointense areas in regions of radiofrequency inhomogeneity.(31) In fetal imaging the combination of radiofrequency shielding artifacts and an enlarged abdomen is associated with magnetic field inhomogeneity and standing wave artifacts, which produce areas of blackout in the center of the field of view where the fetus is located.(33) As described above, these tend to be more pronounced at 3T than they are at 1.5T due to the shorter RF wavelengths inherent to 3T MRI.
Fetal Motion
Motion from both fetal and maternal sources can produce artifacts, which confounds already difficult to interpret images and is exacerbated at 3T field strengths. (Fig 1) Fast scanning sequences, such as fast spin-echo and turbo spin-echo, are limited and must be optimized to decrease scanning time and motion artifact, while preserving T1 and T2 signal differences in a given acquisition.(217,232) The use of a surface phased-array coil and parallel imaging to decrease acquisition time, by filling k-space faster, can increase image resolution and decrease motion artifact, with the added bonus of a lower specific absorption rate (SAR).(6,12,15,33) Parallel imaging is more practical at higher magnetic field strengths, as a result of the increased signal, which compensates for the inherent decrease in SNR when using parallel imaging.(33) Fetal motion can make it difficult to apply advanced sequences such as DTI and MRS at 1.5T, however, increased speed of acquisition at 3T may make these sequences more feasible.(6,38) Functional MRI is also sensitive to motion artifact, and is usually performed late in pregnancy when the fetal head is engaged in the pelvis, however, if earlier evaluation is desired, the presence of significant motion may require the application of complex correction algorithms in order to produce diagnostically acceptable image output.(150-153,238) Maternal comfort measures, such as positioning on the left side, as well as keeping patients NPO prior to examinations can be used to decrease fetal and maternal motion during exams.(11,12)
Other
Additional factors that present unique challenges in fetal imaging include the small size of the structures being imaged and the distance between the receiver coil and the fetus. While the development of new and better coils may partially mitigate these factors, their presence makes imaging prior to 22 weeks suboptimal.(6,15)
Optimal Timing of Fetal MRI
As important as all the technical factors associated with the MR scanner itself are, the selection of optimal imaging time is also important and depends on the clinical question being answered.(217,232) Prior to 16-18 weeks of life, the value of fetal MRI is limited due to the small size of the fetus, increased fetal motion and the fact that some anomalies, such as cortical dysplasia, have not developed yet.(14,232) Between 18-22 weeks of life, the use of MRI can be helpful in further evaluating or confirming findings identified on prenatal US, which may impact pregnancy and delivery planning.(232) Imaging at this point in gestation can help decrease fetal motion, especially when the fetal head is engaged in the pelvis, making it easier to obtain true midline sagittal images of the brain for evaluation of the posterior fossa and corpus callosum.(6) MRI is further improved in the third trimester, and examination during this time frame is optimal for assessment of cortical anomalies, because of improved spatial resolution and decreased fetal motion.(232) Unfortunately, studies this late in pregnancy carry the risk of identifying anomalies too late for optimal intervention, and in cases where the fetus is at high risk for abnormalities or in cases requiring complex delivery planning, such as neck masses, MR studies should be performed during the second trimester with potential follow-up in the third trimester.(229,232)
Safety Issues
Safety is always a concern when we implement diagnostic testing, even when those tests are non-invasive. For every diagnostic test, the benefit of performing the examination must outweigh the potential risks to the patient, whether or not adverse effects have been identified.(180,181,243) While no current evidence exists to show that fetal MRI is harmful to either the mother or fetus, there is a lack of consensus as to whether any true risk exists. Current evidence is limited and inconclusive due to small sample size, data variability and potential confounders, such as a lack of studies evaluating field strengths above 1.5 Tesla. To that effect, there are no long-term studies available regarding 3T magnetic field strengths and the long-term safety effects related to RF field exposure and loud acoustic environments, both of which are still being evaluated.(177,178,193,197,212,214,232,244-246) Based on the currently available data, MRI during the second and third trimester appears to pose minimal risk to the fetus (12), although concern still exists about potential auditory safety in late trimester imaging at 3T (relative to 1.5T) and further evaluation is this area is needed.
Currently the FDA has no specific safety guidelines related to fetal MRI at any magnetic field strength as a result of the limited data availability and need for larger sample sizes even at 1.5T field strengths. As a result of this lack of data at 1.5T, there has been a hesitance to shift the performance of fetal MRI at 3T.(182) However, due to the unknown effects of higher magnetic field strengths and potentially prolonged imaging times it is generally recommended that MRI be performed after 18 weeks.(232) The current FDA recommendation, as referenced in MRI in Practice by Westbrook, et al., states that “if non-ionizing imaging is suboptimal, or if the information to be gained by MR would have required more invasive testing, MRI is acceptable” and goes on to explain that “in light of the high risk potential for pregnant patients in general, many facilities prefer to delay any examination of pregnant patients until after the first trimester.”(247) Guidelines from the FDA also indicate that the safety of MRI with respect to the fetuses and embryos “has not been completely established”.(248) The American College of Radiology (ACR) guidelines state that “pregnant patient can be accepted to undergo MR scan at any stage of pregnancy if, in the determination of a level 2 MR personnel-designated attending radiologist, the risk-benefit ratio to the patient warrants that the study be performed” and requires documentation that the “study cannot be acquired via nonionizing means (e.g. ultrasonography),” “the data are need to potentially affect the care of the patient or fetus during the pregnancy,” and “the referring physician does not feel it is prudent to wait until the patient is no longer pregnant to obtain these data.”(177,178) The 2004 International Non-Ionizing Radiation Protection (ICNIRP) guidelines recommend MR procedures in pregnant patients should only be performed “after critical risk/benefit analysis, in particular in the first trimester, to investigate important clinical problems or to manage potential complications for the patient or fetus.”(180,181)
Safety issues directly related to imaging the fetus at any magnetic field strength include possible teratogenic effects and the use of contrast agents. Also, as we increase the magnetic field strength from 1.5T to 3T, we must consider the effects of static magnetic field exposure, radiofrequency (RF) power deposition, specific absorption rate (SAR), gradient field switching, and acoustic noise/sound pressure levels.(1,33,182,197)
Teratogenic Effects
Teratogenic effects of MRI are of greatest concern when examinations are performed early in pregnancy, especially during the first trimester.(12) These effects are related to heat generated by transmitted RF power, as well as direct non-thermal interactions with the electromagnetic field. The teratogenic effects of heating have not been directly studied in humans; however, animal studies have shown that such effects do exist. Unfortunately, due to the study design in animal models, it is difficult to extrapolate reliable data about the effects on humans.(192,193,246,249-253) The effects of SAR and energy deposition on the fetus are also not well established and require further study.(196,254,255) Kanal et al (256) evaluated 280 pregnant MR workers and demonstrated no statistically significant negative effects on the fetus from work-related exposure to the static magnetic fields (up to 1.5T) used for diagnostic MRI.
Contrast Agents
The use of gadolinium has not been adequately studied in pregnant human subjects and is not used during pregnancy unless absolutely clinically necessary, especially during organogenesis.(232) The main concern with gadolinium is its ability to cross the placenta and circulate for long periods of time in the amniotic fluid, prolonging the exposure time for the fetus, and increasing the potential for toxicity related to disassociation of the gadolinium from its chelate.(12,176-178,257,258) At doses several times higher than those used for human diagnostic MRI examinations, and in cases of repeated dosing, animal studies have demonstrated some teratogenic effects, including growth retardation, visual problems and structural anomalies.(257,259) However, to date there are no animal models demonstrating carcinogenic or mutagenic effects related to gadolinium use, and of the few clinical studies evaluating contrast administration in humans, all have failed to demonstrate adverse effects to the fetus or neonate, even when contrast was administered during the first trimester.(232,260-264)
The ACR-SPR guidelines recommend against the routine administration of gadolinium to pregnant patients, and at this time gadolinium is a class C drug, indicating that safety in humans has not be proven.(176) The ACR also states that “MR contrast agents should not be routinely provided to pregnant patients.”(177,178) The 2004 ICNIRP guidelines state that contrast agents “should only be used during pregnancy if the potential benefit justifies the risk to the fetus,” which is based on data demonstrating that administration of large doses of gadolinium-based contrasts agents have been shown to cause numerous adverse effects including “post-implantation fetal loss, retarded development, increased locomotive activity and skeletal and visceral abnormalities” in animal models.(180,181)
Safety Considerations Related to Higher Static Magnetic Field Strength
Static Magnetic Field Exposure
Static field exposure risks are two-fold, including the risk of ferromagnetic objects becoming projectiles and the potential negative effects on biological systems that are exposed to strong magnetic fields. Numerous studies have evaluated these effects on growth and development in biological systems, and while a small number of reports suggest potential harm, there are currently no long-term harmful effects firmly established as scientific fact. (179,182-194,246,249-253,265-274) Harmful effects have not, to date, been demonstrated at the magnetic field strengths used for diagnostic imaging and in cases where the collected data was obtained at higher field strengths (e.g. 16T), it is difficult to extrapolate the possible effects on the human fetus.(33,186,193,246,269-274) Myers et al. studied 74 women who underwent five serial MRI scans at 0.5T between 20 weeks and 40 weeks gestation and demonstrated that MRI did not significantly affect intrauterine fetal growth when compared to matched controls.(244) Additional follow-up studies evaluating the development of children exposed to MRI during pregnancy have failed to demonstrate any significant long-term developmental deficits related to their exposure.(214,245)
Radiofrequency (RF) Power Deposition and Specific Absorption Rate (SAR)
Radiofrequency power deposition can create temperature changes in the imaged subject, which may produce undesired outcomes in the fetus.(195) Radiofrequency field inhomogeneity and dielectric/standing wave effects may complicate the effects of heating by creating focal hot spots where the SAR is higher, and these focal hotspots can be projected around or within the fetus producing localized unwanted temperature changes.(31,33,35) Because of this and the increased field inhomogeneity at 3 Tesla, the SAR needs to be more closely monitored during 3T examinations.(232) Despite the field inhomogeneities and focal hotspot creation at 3T, the current literature, which was reliant primarily on electromagnetic simulations, showed no significant safety concerns for 3T compared to 1.5T in fetal imaging as long as the scanner was operated in the normal-level SAR mode.(182)
The theoretical effects of RF energy deposition and tissue heating in the fetus have relied on data extrapolation from animal models, mathematical models, phantom simulations and electromagnetic simulations of the fields created by RF excitation.(177,178,182,196,254,275-283) At 3T the effects of RF induced heating are increased, especially in pregnant patients during the second and third trimester, when the volume of conductive amniotic fluid is larger and the abdominal circumference has increased.(182) In 2006, Hand et al. used a simplified model of the pregnant woman to study the effects on the fetus related to SAR, and demonstrated that the maximum local SAR is always located in the mother and not directly in the fetus, and that fetal safety is not compromised as long as the established SAR limits are not exceeded.(280) A follow-up study performed by the same group in 2010, using an enhanced model of the gravid female, demonstrated that if scan parameters were compliant with the International Electrochemical Commission (IEC) normal-level SAR mode conditions, the temperature increase in the mother did not exceed the IEC limit of 0.5°C and the temperature of the fetus remained below 38°C.(254) Numerous additional studies have been performed, further supporting that there is no reason not to perform fetal MR on a 3T system, although caution is suggested when performing scans without using normal-level SAR mode, as the maximum local SAR value can be violated and may fall in the body of the fetus.(177,178,182,281-283)
Despite the lack of evidence supporting any actual harm to the fetus, there is continued concern about the potentially unknown risks of specific absorption rate and radiofrequency energy deposition. As a result of these continued concerns, the FDA has imposed limits (1) for RF exposure of 4 W/kg for maternal whole body exposure, independent of the magnetic field strength, and scanner failsafe mechanisms have been put in place to ensure these exposure levels are not exceeded. The ICNIRP 2004 guidelines state that the body temperature of the pregnant patient should not rise more than 0.5°C and the temperature of the fetus should not exceed 38°C.(180,181) Victoria et al. demonstrated that at both 1.5T and 3T field strengths, using the typical fetal MR pulse sequences (T1, EPI, SSFSE, SSFP), that the SAR was kept well below the whole-body exposure limit of 4 W/kg.(33) Also, modeling by Hand et al., showed that care must be taken to limit the amount of time the fetus is imaged, because the maximum temperature predicted in the fetus can exceed 38°C following continuous exposures lasting longer than 7.5 minutes.(254) Hand et al. (280) demonstrated that the maximum local SAR occurs within the mother, and the maximum local SAR experienced by fetus is approximately 40-70% of the maximum local SAR experienced by the mother, and based on these observations, suggested that when safety guidelines related to local, rather than whole body, SAR limits are adhered to that fetal SAR exposure would remain within regulatory limits. Kikuchi et al. (282) showed that if the scanner calculated maternal whole-body SAR was less than 2 W/kg, that it would take approximately 47 minutes to increase the fetal body temperature 0.5°C and exceed the safety recommendations for acceptable temperature change. The amount of safe scanning time decreased significantly when the whole-body averaged SAR was increased to 4 W/kg. These findings suggest that care should be taken to monitor both the local and whole-body SAR in pregnant patients and suggest that if careful monitoring is performed scanning of the fetus can be performed safely at both 1.5T and 3T.
Gradient Field Switching
Gradient fields are used in MRI to encode spatial information during image generation. The major safety concerns associated with gradient field switching are the potential for peripheral nerve stimulation and fetal distress during scanning as a result of increased noise exposure and neurostimulation (sound production is also a concern, and will be discussed on the next section).(182) A study performed by Rodegerdts et al., showed that there were no effects on cell proliferation when fetal human fibroblasts were exposed to varying gradient fields, even in cases of prolonged exposure of up to 24 hours, further suggesting a lack of teratogenicity related to magnetic field exposure and gradient field switching.(284) There is also little support in the current literature to suggest that peripheral nerve stimulation poses an increased risk to the fetus when transitioning from 1.5T to 3T. The effects of peripheral nerve stimulation, independent of the magnetic field strength, have a smaller effect on the region of the image towards the geometric centers of the gradient fields used, which is where the fetus is located.(33,182)
Acoustic Noise and Sound Pressure Level (SPL)
Concern has been raised related to the potential for acoustic damage to the fetal ear as a result of MRI scanning. Gradient field switching produces acoustic noise, manifested as a loud tapping or buzzing noise when coils are exposed to rapidly changing gradients, which may be detrimental to fetal auditory development.(182,232,246) Some studies report evidence of high frequency hearing loss, shortened gestation and low birth weight in cases where the fetus was exposed to excessive noise in utero; however the noise in these cases was a result of chronic maternal exposure to loud noise, and did not reflect the acute noise levels experienced during fetal MRI examinations.(285,286)
A 1994 Study performed by Baker et al. followed 20 children who had undergone in utero MRI evaluation, with echo-planar imaging, and concluded that there was no demonstrable increase in disease, disability or hearing loss in the studied children.(212) A 1995 study performed by Glover et al. suggested that attenuation of the sound by the gravid uterus was sufficient to prevent harmful effects on the fetal ear.(211) Additional studies performed by Kok et al. and Reeves et al. also support these findings, reinforcing that there is no significant increased risk of hearing loss secondary to fetal acoustic noise exposure from MRI.(213,214) Of note, all of the above referenced studies were performed at 1.5T and there are currently no similar outcome studies performed following 3T exposure.(182)
Despite the lack of concrete data supporting a true risk of acoustic damage to the fetus, some safety measures have been put in place to decrease sound further than the natural barrier of the uterus, amniotic fluid and overlying soft tissues. Acoustic foam, placed on the scanner table, can be used to further decrease sound transmission to the fetus. Engaging vendors, to determine the peak and root mean square sound pressure levels for various sequences used in fetal imaging, can also reduce the risk of noise exposure to the fetus. Precautions such as acoustic foam should be applied at both 1.5T and 3T field strengths to insure adequate protection independent of the imaging parameters selected.(182) Recently, Siemens and GE introduced new advanced noise reduction technology referred to as “Quiet Suite” (Siemens) and “Silent Scan” (GE), which addresses fast gradient switches and can provide acoustic noise reduction during MRI scanning.(287,288)
Neonatal Imaging
Introduction
Cranial ultrasound has been used to image the neonatal brain since the late 1970’s and remains the most commonly used imaging modality in the evaluation of the neonatal brain, as a result of its versatility, portability and safety.(2,3,7,8,258,289-295) Despite the wide availability of ultrasound, and in much the same way that fetal MRI has become a routine part of clinical practice, post-natal MRI, in both term and pre-term neonates has become integral in the management of the sickest and most vulnerable newborns, although cranial ultrasound is still regularly used in clinical practice.(7,258) Unlike fetal MRI, which is still primarily performed at 1.5T field strengths, neonatal MRI has gained wide acceptance at 3T, where phased-array coils and parallel imaging have improved image quality and decreased examination times.(52,182) MRI provides valuable information about brain injury during the neonatal period through early detection and evaluation of metabolic and physiological derangements, in addition to quantitative assessment of the injured brain parenchyma. MRI evaluation of the neonate can provide important information about prognosis and neurodevelopmental outcomes, which combined with the use of advanced imaging sequences allows for more targeted examinations that directly assess clinical concerns and patient risk factors.(2,4,5,16,27,99,295-300). Myelination and cortical in-folding occur in predictable patterns, which can be can be used as markers of cerebral maturation and are well assessed with MRI.(2,82)
Advanced sequences, such as MRS, DWI, DTI and fMRI, can improve diagnostic assessment and supplement the information obtained in standard imaging sequences. MRS improves assessment of brain maturation and increases sensitivity for detecting brain injury from hypoxic, metabolic and neurodegenerative conditions.(24,26,32,85-87,99,101-103,111,300-311) Vigneron et al. demonstrated the benefit of multi-voxel MRS in assessing metabolic differences between anatomic regions of the brain in preterm infants.(312) Diffusion weighted imaging (DWI) and diffusion tensor imaging (DTI) can be used to better characterize brain injury from hypoxic insults.(2,5,24,32,76,87,99,298,300,311,313-316) For example, DWI has been successful used in the assessment of multiple pathologic processes including, but not limited to, hypoxic-ischemic encephalopathy (HIE), stroke, traumatic brain injury, diffuse excessive high signal intensity (DEHSI) of the white matter, and metabolic disorders (32,70,99,311,313-315,317-320), although some reports suggest that the extent of injury may be underestimated on diffusion images alone.(311,313) In the case of isolated DEHSI, multiple studies have shown that in the absence of other lesions, isolated DEHSI does not correlate with neurodevelopmental disability.(70,319,320) Finally, functional MRI has been used to evaluate patterns of normal and abnormal functional activation in the brain.(321)
Exploiting alterations in T1 and T2 weighting, combined with MRS and DWI, can better define whether perinatal white matter injury is focal or diffuse, which can have a profound impact on clinical outcomes.(4,18,24,76,99,294,300,314,317,318,322-326) Optimal timing of examinations is an important consideration when performing and ordering brain MRI in preterm infants. For example, early MRI allows timely counseling, implementation of rehabilitation strategies and evaluation of early biomarkers for brain injury, while scanning at term equivalent age tends to predict long term prognosis more accurately.(4,327,328) DWI is especially sensitive to abnormalities during the acute phase of a hypoxic insult, such as HIE, which makes prompt image acquisition in suspected cases of HIE imperative to achieving the best diagnosis.(2,16,316)
The early detection of brain injury in premature infants is important as many of the infants who survive early life experience some degree of neurodevelopmental disability. Preterm infants are at increased risk for germinal matrix hemorrhage, interventricular hemorrhage, parenchymal infarction, periventricular leukomalacia (PVL) and diffuse white matter injury which increases their risk for motor, cognitive and other neurological impairments.(7,8,326,329) This is of particular concern since the number of preterm births has increased and the survival of very low birth weight infants has improved, without a concomitant improvement in their associated neurodevelopmental outcomes, resulting in an increased number of children with long term neurological deficits such as cerebral palsy, cognitive deficits and sensory impairment, all of which are much less commonly seen in term infants.(2,7,8,4,297,325,329-341) In 2001, Roelants-van Rijn et al. showed that early and late MRI examinations were helpful at predicting neurologic outcomes in cases where ultrasound was abnormal, however, their utility was decreased in cases with serially normal ultrasound.(8) In 2004, Mirmiran et al. showed that MRI was superior to ultrasound as a predictor of poor outcomes, such as cerebral palsy, in preterm and near term infants, but that both imaging modalities, when positive, had a high specificity for adverse outcomes.(342) In 2006, Woodward et al. studied 167 preterm infants, and showed that abnormal findings on MRI at term equivalent in these patients was a strong predictor of poor neurodevelopmental outcomes and suggested that MRI performed at term equivalent age could be used in risk stratification of these infants.(343) On the other hand, a normal MRI examination carries with it a high negative predictive value for poor outcomes.(70)
Indications for MRI in neonates include prematurity (gestational age less than 30 weeks), hypoxic ischemic encephalopathy or neurologic signs of encephalopathy, ultrasound demonstration of significant brain injury or abnormality, traumatic delivery, suspicion of posterior fossa abnormality or cerebral convexity abnormality, severe and/or symptomatic hypoglycemia, suspected metabolic disease, suspicion of meningitis, encephalitis or brain abscess, congenital malformations with possible brain involvement and equivocal findings on cranial ultrasound.(2,3,344,345) While there are many clinical indications for the use of MRI in neonates, the medical risks and logistics associated with transporting sick neonates to the radiology department has led to the development of dedicated NICU magnets and MRI conditional neonatal incubators.(17,18,20-23,49,321,258,289,346,347)
In the following sections we will discuss specifics related to imaging neonates at 3T field strengths vs. 1.5T. We will discuss special monitoring and imaging equipment, such as neonatal coils, MRI conditional incubators and small footprint NICU MRI scanners that are specially designed to improve neonatal imaging and make imaging even the sickest babies possible. Finally, we will discuss some of the advantages, challenges, and safety concerns as they specifically relate to neonatal imaging.
Specifics Related to Neonatal Imaging at 1.5T versus 3T
Increased magnetic field strength increases SNR and allows thinner slices with better anatomic evaluation of small structures. Unfortunately, in neonates, the higher water content of the brain, especially in the white matter, makes the delineation of gray and white matter difficult due to its effect on T1 and T2 relaxation.(32,315) This is further complicated at 3T because of the longer T1 relaxation times, which already decrease T1 tissue contrast. The decreased gray-white matter contrast at 3T can be improved by adjusting scan sequence parameters such as TR, TE and flip angles and through the use of T1-fluid attenuation inversion recovery (T1-FLAIR) sequences.(32)
The ability to apply advanced imaging sequences such as diffusion tensor imaging (DTI) at 3T is more feasible and may provide the ability to assess neural tracts such as the brachial plexus in the setting of post-delivery brachial nerve palsy and define aberrant connectivity in cases of structural brain malformations.(32) Many of the other advanced imaging sequences, including fMRI, MRA, BOLD, ASL, DTI, MRS, and T2* sequences for blood are improved at 3T.(28,40,41,58,347) Improved SWI is particularly important given the increased frequency of hemorrhagic and ischemic events in preterm neonates.(289)
Equipment Considerations and MR Conditional Devices Used in Neonatal Imaging
The tenuous nature of neonates has led to the development of highly specialized equipment for monitoring and imaging neonates such as MR conditional incubators and dedicated neonatal coils. Additionally the risk of transport and the demands on staff required to bring sick neonates to the radiology department for scanning has led to the development of dedicated small-footprint magnets, which can be placed directly in the NICU.
Monitoring Equipment
One of the most important challenges in neonatal imaging is providing adequate support to a thermally unstable neonate throughout the entirety of an MRI examination whether the exam is performed on a department MRI scanner or in a dedicated NICU scanner. Neonates, especially pre-term neonates, are vulnerable to hypothermia, hemodynamic instability and often require respiratory support.(17,258,346) These physiological demands have led to the development of both MR conditional incubators, specialized neonatal head and body coils and to the installation of magnets directly in the NICU decreasing both handling and transport of these small babies.(20-23,49,289,346) Additionally, when scanning at any field strength, especially 3T, the use of specifically designed and tested monitoring equipment must be used to ensure patient safety related to equipment malfunction and inappropriate heating.(49,99,177,178,258,348)
MR Conditional Incubators
The lack of available dedicated NICU magnets combined with vulnerability of neonates has necessitated the development of MR conditional incubators, which are designed to decrease the risks of transporting neonates between the NICU and the radiology department. Most commercially available incubators are embedded with dedicated neonatal head and body coils, and can be used in both 1.5T and 3T systems. Dedicated MRI incubators allow the transfer of the neonate into the incubator in the controlled environment of the NICU, maintaining continuity of monitoring and, hopefully, patient stability. The entire incubator is transported from the NICU to the radiology department and once in the department the entire incubator is moved from the transport cart to the MRI table without physically have to manipulate the infant.(17-21,49,99,154) (Fig 4) Incubators designed with integrated RF coils are commercially available for adult-sized MRI systems, at both 1.5T and 3T field strengths; however, despite the presence of an incubator, the logistics of transportation and stability of the neonate can in some cases preclude scanning of the smallest, sickest children.(2,17,20,21,25,26,289,321,344,349)
Fig 4. Neonatal Incubator.
The above image shows a second generation SREE MRI conditional, neonate imaging sub-system (NISS-MR). The incubator allows a seamless way to safely bundle, transport and image high-risk infants. Patented, custom built neonatal coils have been integrated into the incubator design allowing high-quality scanning of the infant without removal from the incubator. The NISS-MR consists of an MRI transport incubator, MRI trolley and back-up power supply box (2 hour capacity), which are classified as MR conditional. The incubator is designed to safely accommodate infants up to one month of age, weighing less than 4.5kg or with a whole body length of less than 55cm. The MRI system offers air temperature and humidity regulation as well as the ability to monitor patient skin temperature. The incubator is also designed to accommodate a transport ventilator and wireless pulse-oximeter. (incubator images courtesy of Ravi Srinivasan)
The FDA has approved MR conditional incubators and monitoring systems to ensure that the systems being used are able to maintain consistent, reliable and effective monitoring, as well as respiratory and cardiovascular support throughout the duration of the exam.(16) Incubators provide the ability to maintain airflow, humidity, temperature regulation, monitoring and respiratory support in systems with integrated RF coils, by integrating gas supply, pulse oximetry, MRI conditional ventilators, infusion pumps, and suction devices, creating a safe and controlled environment for imaging neonates.(16-19,21) Specially designed coils are integrated into the incubators eliminating some of the risks and limitations associated with using adult coils for neonatal MRI.(17-19,21) Bluml et al. published a report on the safety and efficacy of neonatal MR incubator use in clinical practice, which found that the diagnostic quality of the acquired images was superior to images taken with standard MR equipment.(21) Rona et al. demonstrated that the use of a neonatal MR conditional incubator made imaging younger, smaller and more clinically unstable infants feasible, with improved image quality and decreased mean imaging time.(18) Additional studies by Whitby et al. (20) and Erberich et al. (321) demonstrated the safe use of a neonatal incubator in obtaining high quality images of the neonatal brain. These studies illustrate the advantage of using an MR conditional incubator and its benefits on the acquired images.
While dedicated incubators have increased the feasibility of neonatal MR exams, they are not without their limitations. One important limitation is the maximum patient size that can be accommodated by the incubator. Lane et al. employed an incubator that was suitable for patients weighing up to approximately 4500g, with a head circumference up to approximately 40cm.(17) The incubator used by Bluml et al. (21) and Erberich et al. (321) was designed for temporary used during scanning and could accommodate an infant weighing up to 4.5 kg, or with a body length of 55cm. (Fig 4) These size limitations may make evaluation of critically ill, full-term neonates difficult or impossible. Additional challenges are associated with the timing and logistics of using the incubator itself. For example, in order to perform an exam using the incubator, the NICU requires advanced notice to pre-heat the unit before transferring the baby into the incubator. Also, the displays on the incubators can be difficult to read from the MRI control room due to small display screens and room configuration, making constant monitoring in the radiology department difficult.(17)
Neonatal Coils
For many years neonates were transported to the main radiology department and imaged using adult MRI coils. The increased use of MRI in neonatal evaluation and clinical management has led to the development of dedicated neonatal coils. The main disadvantage with using adult radiofrequency coils is the size mismatch between the neonate and the coils, which negatively impacts image quality because of decreased SNR and decreased spatial resolution.(22,49) The use of RF head coils, designed specifically for newborns (Fig 5), improves the quality of MR images by increasing the signal to noise and improving image contrast to noise. In addition to the development of multichannel arrays, the improved SNR from dedicated coils has also resulted in decreased scan time while maintaining high quality diagnostic image output.(16,21,49,321) Bluml et al. showed that there was no increased risk of energy deposition or focal hot spot creation in the infant when a specially designed neonatal coil was employed.(21) As discussed in the previous section, specialized, appropriately sized, neonatal coils have been integrated into MR conditional incubators resulting in better image quality and the ability to perform high-quality advanced imaging sequences such as fMRI.(18-21,49,321)
Fig 5. Neonatal Coils.
Images of three custom neonatal and infant sized head coils, which can be used to improve signal to noise ratio on examination. (A) Infant Cocoon, which can be used to image infants from 0-6 months of age. (B) Infant Head Spine Array, which can be used to image infants 0-6 months of age. (C) Neonatal Head Coil, which can be used in infants 0-1 months of age. The above coils can all be integrated with the neonatal incubator show in Fig 4. (coil images courtesy of Ravi Srinivasan)
Other design characteristics that need to be considered when developing standalone and incubator integrated neonatal coils include the integration of monitoring and ventilation equipment, the ease of access to and visualization of the infant by the support staff, and the space allowance for placement of oxygen masks and other support equipment on the infant in cases where this is necessary. Ideally, a well-designed coil would provide both high quality diagnostic images and easy access and monitoring of the infant being scanned. In the case of standalone coils, many coil designs have been created for easy removal, in cases of emergent airway or cardiovascular compromise.(49) However, there are sometimes issues with removing and accessing the baby to provide support when the coils are integrated with an incubator.(20)
Dedicated Neonatal Intensive Care Unit (NICU) Magnets
In many institutions, neonates undergoing MRI examination are still transported from the NICU to the main radiology department. This process has inherent risks, both medical and logistical, which have led to research and development into MRI scanners that can be placed directly in the NICU.(22,49,154,289,344,349) A few MRI systems have been developed and installed in NICUs around the world, with a range spanning low magnetic field strengths up to 3T.(49) These dedicated NICU MRI systems eliminate some of the medical risks and many of the logistical challenges of transporting the smallest and sickest patients in the hospital to and from the radiology department.(22,23,49,289) Dedicated NICU scanners also create more flexible schedules for scanning that can better accommodate the feed and sleep patterns and ongoing needs of the infant.(49)
In 2012 a 1.5T small footprint dedicated NICU scanner was developed and tested by Tkach et al. at Cincinnati Children’s Hospital Medical Center (Cincinnati, OH, USA). The scanner used in their study was a modified OPTIMA™ MR430s (GE Healthcare, Waukesha, WI) musculoskeletal scanner.(Fig 6) Modifications were made to the scanner to better facilitate its use in the NICU, which included changing the orientation and height of the magnet and development of a custom built MRI patient table. Due to the inherent small size of the OPTIMA magnet there were overall changes to the bore diameter, alteration of the 5-gauss line, decreased magnet weight, decreased cryogen requirements, and improved gradient performance (secondary to smaller gradient coils). In addition, control electronics and the radiofrequency system from a state-of-the-art 1.5T GE scanner were integrated with the basic OPTIMA™ MR430s system to give the system the capabilities of a normal department MRI scanner, including advanced imaging capabilities, such as MRS, DTI, fMRI, ASL and phase-contrast angiography. Radiofrequency shielding of the room, air temperature and humidity requirements remained the same as an adult-size MRI system.(22)
Fig 6. Modified OPTIMA™ MR430s (GE Healthcare, Waukesha, WI) musculoskeletal scanner used at Cincinnati Children’s Hospital Medical Center (CCHMC).
Above is an image of the modified OPTIMA™ MR430s (aka ONI) small-footprint MRI scanner installed at CCHMC. Modifications to the scanner included changing the orientation and height of the magnet, and development of a custom built MRI patient table. The scanner inherently has a smaller, changes to the bore diameter, alteration of the 5-gauss line, decreased magnet weight, decreased cryogen requirements, and improved gradient performance (secondary to smaller gradient RF coils). In addition, control electronics and the radiofrequency system from a state-of-the-art 1.5T GE scanner were integrated with the basic OPTIMA™ MR430s system to give the system the capabilities of a normal department MRI scanner, including advanced imaging techniques, such as MRS, DTI, fMRI, ASL and phase-contrast angiography. The modifications result in feasible placement of a scanner in the NICU and acquisition of high quality diagnostic images.
In their examination of the initial feasibility of a dedicated NICU magnet, Tkach et al. showed that the cost associated with exams could be decreased when using a dedicated NICU scanner due to the smaller footprint, smaller fringe field, reduced weight and lower cryogen consumption of the system. They also demonstrated that exams could be completed with acceptable scan times for brain MRI with visualization of gray-white matter differentiation and areas of early myelination compatible with immature white matter tracts.(22,23) Tkach et al. also demonstrated that lower noise levels produced by NICU magnets were less disruptive to the neonates sleep, which is better developmentally, and increases the likelihood that an exam can be completed without the use of sedation.(210) One limitation of NICU scanners is the inability to accommodate larger patients, as demonstrated by the magnet used by Tkach et al. which was able to accommodate neonates weighting 4.5kg or less. These size restrictions may limit the use of a NICU magnet in critically ill full-term infants. At the time of publication in 2014, Tkach et al. reported that they were in the process of developing a head coils with a patient bore diameter or 20cm accommodating infants up to 6.25kg in their NICU MRI scanner.(23)
In addition to the size mismatch between the neonate and the adult-sized equipment, there are issues related to access and monitoring of the neonate when they are placed in the long-bore of an adult MRI scanner and the potential safety risks associated with non-MRI conditional support and monitoring equipment being placed in the scan room. This is especially true at 3T where there is limited availability of monitoring equipment that is cleared at that field strength.(22,347,348) Vital sign monitoring on the dedicated NICU system, used by Tkach et al, was done using MRI conditional equipment, similar to that used on adult-sized 1.5T magnets.(22) Also, due to the shorter bore and better room design there was better visualization of and access to the neonate throughout scanning.(22,23,289)
There are numerous advantages of a dedicated NICU magnet, including lower purchasing and operating costs, improved ease of access, lower weight, smaller size, smaller fringe field, lower noise output, improved gradient performance, and most importantly reduced medical risk to the neonate.(22,23,210,289) In examinations by Tkach et al. they were able to obtain high-quality images with good spatial resolution, signal-to-noise and tissue contrast using 30 minute scan times with standard clinical protocols on a 1.5T small-footprint scanner. In their study, the SNR was slightly better for the NICU magnet compared to an adult-sized 1.5T magnet.(22) Also, the sound pressure levels (SPL) experienced by the neonate were lower on the NICU scanner for all six MR acquisitions (spin-echo, gradient-echo, fast RF spoiled gradient-echo, echoplanar, fully balanced SSFP and DWI).(22,210) Dedicated NICU MRI scanners demonstrate improved gradient performance compared with department MRI scanners. Superior gradient performance was achieved with a smaller field of view and high spatial resolution using a NICU scanner without compromising scanning parameters such as TR and TE.(22) The gradient performance of the NICU magnet is roughly twice the performance of a conventional whole-body adult system.(22,210)
While there are numerous advantages to implementing NICU scanners, some limitations still exist that deserve discussion. In general, 3T scanners are typically preferred for neonatal brain imaging; however, 3T scanners are less well established for neonatal cardiac, abdominal and musculoskeletal evaluation. As a result, 3T scanners have not been tested or implemented as dedicated NICU magnets at this point in time. The same safety concerns associated with 3T scanning previously discussed (including increased susceptibility artifacts, increased risk of tissue heating and the limited number of available MRI conditional support systems) would apply to a small-footprint 3T NICU MRI system.(22) Despite these issues, as better 3T sequences are developed consideration may be given to placing 3T MRI units in the NICU, although further studies would be required to ensure their safe use and feasibility.
Advantages of Neonatal MRI Scanning
There are many advantages to neonatal MRI including early diagnosis and better clinically management of neonates without the use of ionizing radiation, which makes repeated long-term imaging follow-up feasible.(22) Increased magnetic field strengths and the increased availability of 3T magnets have shortened acquisition times making neonatal exams more feasible. Improved SNR at 3T allows acquisition of thinner slices and improved delineation of smaller structures (32), making it possible to demonstrate the site and extent of white matter abnormalities, intracranial hemorrhage, infarction, posterior fossa abnormalities and maturational changes in the brain.(289,292,293,350,351) As a result of the improved spatial resolution, serial MRI examinations are able to better delineate the rapidly occurring changes in the developing brain, especially when performed at higher magnetic field strengths.(32)
MRI is especially important in the evaluation of pre-term and extremely low birth weight infants, because it allows early detection and diagnosis of patterns of white matter injury that can help predict clinical outcomes and guide potential interventions.(87,290-292,313,323,331,350-353) In a study performed in 2001 by Childs et al., MRI was shown to be more sensitive than cranial ultrasound for the detection of non-cystic focal white matter disease.(291) In 2003, Debillon et al. demonstrated that MRI was able to better delineate less severe forms of white matter damage, and found that early MRI findings were more predictive of late MRI findings, when compared to findings from early ultrasound evaluation.(351) In general, MRI has become important, either as a replacement for or in addition to transcranial ultrasound, due to the relatively poor sensitivity of ultrasound in detecting diffuse and focal, non-cystic white matter lesions.(3,7,290-295,314,318,324,351,352,354) Traditional T1 and T2-weighted MRI sequences may miss cases of diffuse white matter injury, and the addition of advanced sequences such as DWI/ADC and DTI with mean diffusivity and fractional anisotropy calculations can prove invaluable in making accurate diagnoses.(16) This is clinically relevant as infants with cerebral white matter injury are at increased risk for motor, cognitive and behavioral deficits if they survive beyond the neonatal period.(355)
Advanced MRI techniques, which are more feasible at 3T, can be used on serial examinations to evaluate a patients developing brain and compare it with normal developmental controls.(310) Techniques such as 3-D volumetric MRI can be used to measure cortical folding and determine volumes of various anatomical regions in the brain. DWI and DTI can provide insight into developing white matter structure and cerebral interconnectivity.(5,350)
Challenges of Neonatal MRI Scanning
Challenges associated with MRI scanning in neonates are related to the logistics and medical risks of scanning pre-term and very low birth weight patients, temperature and vital sign monitoring, patient motion and the possible necessity for sedation, which are all addressed below.
Preterm and Very-Low Birth Weight Infants
Preterm and very-low birth weight infants (weight < 1500 g) are at increased risk for hypothermia and vital sign instability, in addition to the fact that many of these babies require respiratory support and intravenous infusions to support homeostasis. As a result, minimal handling and transport of newborn infants is highly desirable, and cranial ultrasound is the initial imaging exam of choice.(17,258,346) Studies have shown that MRI can be performed on preterm infants without physiologically significant upset in vital signs, including heart rate, oxygen saturation, ventilator settings, blood pressure and temperature.(356-358) A 2009 study performed by Merchant et al. (347) used a feed and swaddle technique with vacuum bag immobilizer to safely and successful perform neuroimaging on very low birth weight infants at 3T without any major complications related to thermoregulatory or vital sign instability. Of note is the fact that of 70 infants included in this study, none were receiving positive pressure ventilation and all were able to maintain normal oxygen saturation with nasal continuous positive airway pressure (CPAP), nasal cannula or without assistance. Plasier et al., on the other hand, demonstrated minor adverse effects in 50% of its study group of 52 infants at term-equivalent 30 weeks gestational age, including respiratory instability and hypothermia, which in three cases required the termination of the exam.(359) MRI conditional incubators and dedicated NICU MRI scanners aid in mitigating these issues; however, in situations where there is no access to this equipment; multidisciplinary coordination and intensive monitoring can lead to safe and successful scanning in the radiology department, and the lack of MRI conditional incubators and NICU scanners should not limit the use of MRI in this population.(17,22,23,49,154,344,346,349)
Temperature and Vital Sign Regulation
The effects of radiofrequency energy deposition and specific absorption rate can create problems with temperature control in neonates, necessitating accurate temperature monitoring during scanning. Energy deposition can lead to inappropriate increases in body temperature in the neonate who is often already swaddled during scanning. Monitoring, using MRI conditional equipment via skin or rectal temperature probes, is useful in detecting even small changes in body temperature during scanning.(32) These changes in temperature can be confounded by the use of an MR conditional incubator, as poor dissipation of heat and higher baseline temperatures in infants requiring incubators may necessitate the use of stricter guidelines for energy deposition and require very close monitoring of infants to avoid unwanted physiological effects from prolonged hyperthermia.(21,197)
Consideration must also be given to vital sign regulation during scanning of both term and preterm neonates. In 1996, Philbin et al. (358) showed that rapid changes in blood pressure, heart rate and oxygen saturation occurred in term infants undergoing MRI scanning, but suggested that these changes were unlikely to be dangerous in healthy term infants and were likely not related to transport, sedation or magnetic field exposure. In a follow-up study performed by the same group in 1998, Taber et al. (357) demonstrated that physiologic fluctuations in heart rate occurred during scanning in almost all infants, and suggested that these changes were unlikely to pose a threat to healthy term newborns. In both studies, however, there were concerns raised that fluctuations in both blood pressure and heart rate occurring in preterm infants with immature cerebral autoregulation could potentially increase risk for worsening intracranial hemorrhage, and that carefully monitoring of vital signs should be in place in these cases. A study performed by Battin et al. (356) evaluating the presence of physiological disturbances in preterm infants undergoing MRI, including changes in heart rate, oxygen saturation, blood pressure and temperature, showed that even in infants requiring intensive care during scanning, that MRI could be performed without any major physiologic upset. These findings were confirmed in a study performed by Benavente-Fernandez et.al in 2010 (346), further supporting the safety and feasibility of MRI scanning even in the smallest patients with little risk when a coordinated team approach and intensive monitoring were employed.
Patient Motion
Patient motion is a potential source of image degradation in all MRI exams, but it is especially important in neonatal imaging when trying to evaluate small structures. Motion artifact degrades images and may reduce the diagnostic accuracy of a study. In some cases motion artifact can be so significant that sedation is required to complete the imaging.(22,32,154) Unfortunately, sedation is not free of its own risks and attempts are made in all cases to avoid sedation if at all possible. The development of new sequences to correct for patient motion (e.g. PROPELLER, BLADE and MULTIVANE), are being used clinically as an alternative method to correct and compensate for patient motion.(16,49)
Sedation
Sedation may be required for MRI examinations in neonates, although its use is avoided whenever possible due to potential risks to the patient, timing, cost and the specialized staffing requirements associated with the actual sedation administration.(32,49,154-158,258) Some of the potential risks associated with the medications used for sedation include brain apoptosis, neurodegeneration, apnea, bradycardia and oxygen desaturations, creating an emphasis on obtaining neonatal MR images without the use of sedation.(22,157,360-365) A population-based cohort study of 5,357 children from Rochester, Minnesota performed in 2009 (157), showed that there was a higher risk of learning disabilities in patients exposed to longer duration (> 120 minutes) and multiple administrations (2 or more) of anesthesia prior to the age of four, but that there was no associated increased risk of learning disability in cases with only a single exposure. Given the data suggesting potential deleterious effects on neurodevelopment related to sedation and anesthesia, avoidance of its use is desirable if at all possible.
The desire to decrease neonatal exposure to sedation agents has resulted in the development of feed and sleep or “feed and bundle” strategies. Additional methods to decrease anesthesia use have also been developed including the use of bean bags, timing scans with feed and sleep cycles and education of staff and parents.(158) In 2012, Windram et al. (366) described a successful strategy, in patients younger than 6 months of age, where an infant was fasted for 4 hours prior to scanning, and then fed, swaddled and placed in a vacuum-bag immobilizer just prior to imaging. In 2010, Haney et al. successful completed 94% of their attempted MRI examinations on NICU patients, without the use of sedation, using a vacuum bag immobilizer combined with a coordinated team approach to the examination and coordinated transport to and from the radiology department.(367) These strategies may prove even more useful when used in conjunction with dedicated NICU scanners as the sound production from these machines is much lower than traditional department MRI scanners.(210) Also, shorter scan times increase the success of completing imaging without the use of sedation, making the increased temporal resolution at 3T advantageous in these cases.(32)
Most premature infants can be scanned during the first few weeks of life without the use of sedation using feed and swaddle techniques and gentle head immobilization with vacuum-pack bags and bean bags.(2,258) In general, sedation is usually not required prior to 3 months of age during which time feed and sleep techniques can be used. Beyond age 3 months sedation is almost always required to achieve a good quality motion free study.(258,315) The most commonly used substance for sedation in this population is chloral hydrate, either orally or as a suppository, and the use of general anesthesia is rarely required in patients less than 2 years of age.(315)
Safety
To date there are no short or long term harmful effects associated with MRI performed in a neonate at the magnetic field strengths and scan durations used clinically.(182) The safety and feasibility of scanning newborns at 3T field strengths has been demonstrated in both sedated and non-sedated patients, using both conventional and advanced MRI techniques.(69,71,347) In cases where dedicated NICU scanners are not available, the transportation logistics and medical risks of removing the baby from the NICU for scanning must be weighed against the benefits of performing the scan in the radiology department.(154,344,349,358) Irrespective of the type of scanner and scanner location, consideration must always be given to safety concerns associated with the effects of static magnetic field exposure, radiofrequency (RF) power deposition, specific absorption rate (SAR), gradient field switching and acoustic noise/sound pressure levels.
Static Magnetic Field Exposure
According to the FDA, MRI carries that status of “non-significant risk” in neonates (infants less than 1 month old) when they are scanned in static magnetic fields of 4T or less. This designation is based on 30 years of use accompanied by no reports of deleterious short or long-term effects when MR scanners are operated within set guidelines.(1,182)
Radiofrequency (RF) Power Deposition and Specific Absorption Rate (SAR)
The radiofrequency energy deposition and specific absorption rate considerations for neonatal imaging are similar to those for the fetus and often require adjustment to scan parameters when transition from 1.5T to 3T.(32) Heating as a result of radiofrequency energy deposition at higher magnetic field strengths may lead to temperature instability in the neonate during scanning resulting in a need for close temperature monitoring.(22,154) An additional concern in neonatal imaging is the safety of monitoring equipment which, if not safety tested at the field strength used for imaging, could lead to tissue heating and focally elevated surface temperature. This necessitates specially designed equipment that is specifically designed and tested at the static magnetic field strength (and therefore specific radiofrequency) being used.(49,154,348) The ICNIRP 2004 guidelines state that the body temperature of the infant should not rise more than 0.5°C.(180,181) Also, as previously noted, close monitoring of patient temperature in cases employing an MRI conditional incubator is warranted as elevated SAR at 3T field strengths can lead to unwanted increases in patient body temperature and potentially harm to the infant.(21)
Gradient Field Switching and Acoustic Noise and Sound Pressure Level (SPL)
The safety concerns associated with gradient field switching include peripheral nerve stimulation and the production of acoustic noise, both of which can be decreased when using dedicated NICU MRI scanners. In two separate studies, performed in 2012 and 2014, Tkach et al. showed that sound pressure levels were lower on dedicated NICU scanners for all six MR acquisitions, despite concomitant gains in gradient performance.(22,210) In 2014, Tkach et al. demonstrated that NICU scanners were quieter than conventional MRI scanners, increasing the safety for the neonate being scanned. The decreased sound production allowed placement of a magnet which did not exceed sound pressure levels above 65dBA directly in the NICU without disrupting other patients. In the same study, it was shown that on average the NICU scanner was 14.2 dB (11 dBA) quieter than a comparable adult-sized 1.5T scanner, when identical imaging sequences and acquisition parameters were selected. These decreases in sound production were attributed to the use of smaller gradient coils.(210) A study performed by Battin et al. (356) using a departmental MRI scanner with an ambient noise level of 67-72 dBA, demonstrated no significant physiological disturbance in any of the 23 preterm infants scanned.
Neonates are very sensitive to the presences of sensory stimuli, and sensory stimuli in the form of acoustic noise can elicit autonomic instability in both term and pre-term neonates.(2,22,202,358) Despite the lack of reports demonstrating serious acoustic noise related injury, various forms of hearing protection have been employed to decrease the exposure to acoustic noise while undergoing MRI scanning.(2,22,358) Exposure to inappropriate and excessive sounds is thought to have both direct and indirect deleterious effect on growth and neurological development in term and pre-term infants.(368,369) This is especially apparent in pre-term infants where hearing impairment, sleep disturbances, undesired somatic effects, impaired auditory perception and impaired emotional development have been demonstrated.(209) The Committee on Environmental Health recommends monitored sound in the NICU, maintenance of noise levels below 45 dB and using simple strategies to reduce noise exposure, such as no tapping on incubators.(286) As a result, consideration to both the type and level of sound exposure in the NICU and during early development is critical.
As a result of studies demonstrating risks associated with auditory noise, multiple forms of passive ear protection, including earmuffs, earplugs, dental putty, and acoustic hoods, have been implemented.(202,207,209) Soft shell earmuffs (e.g. MiniMuffs™, Natus Medical Inc., San Carlos, CA) are able to provide additional sound attenuation of up to 7-12 dB in the neonatal population.(209) Foam earplugs (e.g. E.A.R Classic™, 3M, St. Paul, MN) have also been used in conjunction with the soft-shell earmuffs to shield against noise. The combination of earmuffs and earplugs has been demonstrated to be better than either device individually, however, the improvement in sound protection is generally less than the combined rating of the two methods individually.(370-372) In the case of the acoustic hood, used by Nordell et al., there was a potential reduction in the peak sound pressure of approximately 16-22 dBA depending on the pulse sequence selected.(207) In evaluation of sound production in a dedicated NICU scanner, Tkach et al suggested that with modifications to patient position and the use of passive devices to attenuate sound (earplugs and soft-shell earmuffs), that a reduction of 29 dBA could be achieved. They also demonstrated that high-performance diagnostic exams could be performed near or below acceptable levels when hearing protection was used.(22,210)
Conclusion
In conclusion, MRI has become an important addition in the imaging evaluation of the developing brain. The use of 3 Tesla magnets in both neonatal and fetal brain MRI has increased the diagnostic quality of scans, improved the availability and quality of advanced imaging sequences, and allowed for better anatomic delineation of the brain, as a result of its superior signal-to-noise ratio when compared to 1.5 Tesla. To date, no studies, have demonstrated any definite risk to the fetus, mother, or neonate when MR scanners are operated within the regulatory guidelines set forth by the FDA and other regulatory agencies. In addition, the development of MR conditional neonatal incubators, specialized neonatal coils and dedicated NICU magnets has increased the safety and feasibility of performing exams on the smallest and sickest babies, has resulted in timely diagnosis of metabolic and ischemic insults, and improved predictive value of MRI for long-term outcome in high-risk fetal and neonatal populations.
Fig 2. Short echo MR spectroscopy in Newborn Brain.
T2-weighted sagittal, axial, and coronal MR images of the newborn brain are shown above. The boxes indicate the region of interest (ROI) from where MR spectra will be acquired, with their approximate dimensions provided. The top row of images shows sagittal, axial and coronal images through the left thalamus, with an ROI in the thalamus. At the end of the first row, the spectrum appears normal with no associated abnormality. The bottom row of images shows sagittal, axial and coronal images through the left parietal lobe, with an ROI in the parietal white matter. The spectrum at the end of the row shows an increase in the lactate peak, compatible with hypoxic ischemic injury (HIE). Note that the both ROIs are drawn slightly oblique in order to maximize the sampled volume in the area of interest and avoid partial volume effects. (spectra courtesy of Dr. Stefan Bluml)
Acknowledgements
The authors would like to thank Dr. Stephan Bluml (Children’s Los Angeles) for contributing the MRS spectra and voxel images, Mr. Ravi Srinivasan for contributing information and images relating to neonatal coils and incubators and Mr. Vincent Kyu Lee for his help in creating and formatting the reference figures. The authors also thank Dr. Ellen Grant (Boston Children’s) for helpful comments and advice on the final draft.
Acknowledge of grant support – This work was supported by funding from, NINDS K23063371, The Children’s Heart Foundation (Chicago), the Ian Harrison Family Neonatal Brain Injury Fund, The Twenty Five Club Preterm Brain Injury Fund, The Lemieux Foundation and the Pittsburgh Children’s Hospital Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Shannon Tocchio – no disclosures
Beth Kline-Fath – no disclosures
Emanuel Kanal – no disclosures
Vincent Schmithorst – no disclosures
Ashok Panigrahy – no disclosures
References
- 1.US Department of Health and Human Services. Food and Drug Administration. Center for Devices and Radiological Health Criteria for Significant Risk Investigations of Magnetic Resonance Diagnostic Devices - Guidance for Industry and Food and Drug Administration Staff [Internet] 2014 [cited 2014 Sep 3]. Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm072686.htm.
- 2.Arthur R. Magnetic resonance imaging in preterm infants. Pediatr Radiol. 2006 Jul;36(7):593–607. doi: 10.1007/s00247-006-0154-x. [DOI] [PubMed] [Google Scholar]
- 3.Van Wezel-Meijler G, Steggerda SJ, Leijser LM. Cranial ultrasonography in neonates: role and limitations. Semin Perinatol. 2010 Feb;34(1):28–38. doi: 10.1053/j.semperi.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005 Nov;147(5):609–16. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Mathur AM, Neil JJ, Inder TE. Understanding brain injury and neurodevelopmental disabilities in the preterm infant: the evolving role of advanced magnetic resonance imaging. Semin Perinatol. 2010 Feb;34(1):57–66. doi: 10.1053/j.semperi.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glenn OA. MR imaging of the fetal brain. Pediatr Radiol. 2010 Jan;40(1):68–81. doi: 10.1007/s00247-009-1459-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maalouf EF, Duggan PJ, Counsell SJ, Rutherford MA, Cowan F, Azzopardi D, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001 Apr;107(4):719–27. doi: 10.1542/peds.107.4.719. [DOI] [PubMed] [Google Scholar]
- 8.Roelants-van Rijn AM, Groenendaal F, Beek FJ, Eken P, van Haastert IC, de Vries LS. Parenchymal brain injury in the preterm infant: comparison of cranial ultrasound, MRI and neurodevelopmental outcome. Neuropediatrics. 2001 Apr;32(2):80–9. doi: 10.1055/s-2001-13875. [DOI] [PubMed] [Google Scholar]
- 9.Smith FW, Adam AH, Phillips WD. NMR imaging in pregnancy. Lancet. 1983 Jan 1;1(8314-5):61–2. doi: 10.1016/s0140-6736(83)91588-x. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy SM, Filly RA, Stark DD, Hricak H, Brant-Zawadzki MN, Callen PW, et al. Obstetrical magnetic resonance imaging: fetal anatomy. Radiology. 1985 Feb;154(2):427–32. doi: 10.1148/radiology.154.2.3966129. [DOI] [PubMed] [Google Scholar]
- 11.Glenn OA. Fetal central nervous system MR imaging. Neuroimaging Clin N Am. 2006 Feb;16(1):1–17. vii. doi: 10.1016/j.nic.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Coakley FV, Glenn OA, Qayyum A, Barkovich AJ, Goldstein R, Filly RA. Fetal MRI: a developing technique for the developing patient. AJR Am J Roentgenol. 2004 Jan;182(1):243–52. doi: 10.2214/ajr.182.1.1820243. [DOI] [PubMed] [Google Scholar]
- 13.Ertl-Wagner B, Lienemann A, Strauss A, Reiser MF. Fetal magnetic resonance imaging: indications, technique, anatomical considerations and a review of fetal abnormalities. Eur Radiol. 2002 Aug;12(8):1931–40. doi: 10.1007/s00330-002-1383-5. [DOI] [PubMed] [Google Scholar]
- 14.Huisman TAGM, Martin E, Kubik-Huch R, Marincek B. Fetal magnetic resonance imaging of the brain: technical considerations and normal brain development. Eur Radiol. 2002 Aug;12(8):1941–51. doi: 10.1007/s00330-001-1209-x. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Zhang X. Advanced MR Imaging Technologies in Fetuses. OMICS J Radiol. 2012 Sep;1(4):e113. doi: 10.4172/2167-7964.1000e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panigrahy A, Borzage M, Blüml S. Basic principles and concepts underlying recent advances in magnetic resonance imaging of the developing brain. Semin Perinatol. 2010 Feb;34(1):3–19. doi: 10.1053/j.semperi.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane A, Chuk L-MR, Colditz PB, Coulthard A. The MRI-compatible neonatal incubator in practice. J Paediatr Child Health. 2013 Sep;49(9):E377–80. doi: 10.1111/jpc.12222. [DOI] [PubMed] [Google Scholar]
- 18.Rona Z, Klebermass K, Cardona F, Czaba CD, Brugger PC, Weninger M, et al. Comparison of neonatal MRI examinations with and without an MR-compatible incubator: advantages in examination feasibility and clinical decision-making. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc. 2010 Sep;14(5):410–7. doi: 10.1016/j.ejpn.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Dumoulin CL, Rohling KW, Piel JE, Rossi CJ, Giaquinto RO, Watkins RD, et al. Magnetic resonance imaging compatible neonate incubator. Concepts Magn Reson. 2002 Jun 1;15(2):117–28. [Google Scholar]
- 20.Whitby EH, Griffiths PD, Lonneker-Lammers T, Srinivasan R, Connolly DJA, Capener D, et al. Ultrafast magnetic resonance imaging of the neonate in a magnetic resonance-compatible incubator with a built-in coil. Pediatrics. 2004 Feb;113(2):e150–2. doi: 10.1542/peds.113.2.e150. [DOI] [PubMed] [Google Scholar]
- 21.Blüml S, Friedlich P, Erberich S, Wood JC, Seri I, Nelson MD. MR imaging of newborns by using an MR-compatible incubator with integrated radiofrequency coils: initial experience. Radiology. 2004 May;231(2):594–601. doi: 10.1148/radiol.2312030166. [DOI] [PubMed] [Google Scholar]
- 22.Tkach JA, Hillman NH, Jobe AH, Loew W, Pratt RG, Daniels BR, et al. An MRI system for imaging neonates in the NICU: initial feasibility study. Pediatr Radiol. 2012 Nov;42(11):1347–56. doi: 10.1007/s00247-012-2444-9. [DOI] [PubMed] [Google Scholar]
- 23.Tkach JA, Merhar SL, Kline-Fath BM, Pratt RG, Loew WM, Daniels BR, et al. MRI in the neonatal ICU: initial experience using a small-footprint 1.5-T system. AJR Am J Roentgenol. 2014 Jan;202(1):W95–105. doi: 10.2214/AJR.13.10613. [DOI] [PubMed] [Google Scholar]
- 24.Panigrahy A, Blüml S. Advances in magnetic resonance neuroimaging techniques in the evaluation of neonatal encephalopathy. Top Magn Reson Imaging TMRI. 2007 Feb;18(1):3–29. doi: 10.1097/RMR.0b013e318093e6c7. [DOI] [PubMed] [Google Scholar]
- 25.Rutherford M. MRI of the neonatal brain. W.B. Saunders; Philadelphia: 2002. [Google Scholar]
- 26.Barkovich AJ. MR imaging of the neonatal brain. Neuroimaging Clin N Am. 2006 Feb;16(1):117–35. viii–ix. doi: 10.1016/j.nic.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Limperopoulos C. Advanced neuroimaging techniques: their role in the development of future fetal and neonatal neuroprotection. Semin Perinatol. 2010 Feb;34(1):93–101. doi: 10.1053/j.semperi.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez-Linera J. 3T MRI: advances in brain imaging. Eur J Radiol. 2008 Sep;67(3):415–26. doi: 10.1016/j.ejrad.2008.02.045. [DOI] [PubMed] [Google Scholar]
- 29.Garel C. New advances in fetal MR neuroimaging. Pediatr Radiol. 2006 Jul;36(7):621–5. doi: 10.1007/s00247-006-0200-8. [DOI] [PubMed] [Google Scholar]
- 30.Panigrahy A. Advanced pediatric neuroimaging techniques: clinical and research applications. Pediatr Radiol. 2010 Jan;40(1):1–2. doi: 10.1007/s00247-009-1463-7. [DOI] [PubMed] [Google Scholar]
- 31.Soher BJ, Dale BM, Merkle EM. A review of MR physics: 3T versus 1.5T. Magn Reson Imaging Clin N Am. 2007 Aug;15(3):277–90. v. doi: 10.1016/j.mric.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Dagia C, Ditchfield M. 3T MRI in paediatrics: challenges and clinical applications. Eur J Radiol. 2008 Nov;68(2):309–19. doi: 10.1016/j.ejrad.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Victoria T, Jaramillo D, Roberts TPL, Zarnow D, Johnson AM, Delgado J, et al. Fetal magnetic resonance imaging: jumping from 1.5 to 3 tesla (preliminary experience) Pediatr Radiol. 2014 Apr;44(4):376–86. doi: 10.1007/s00247-013-2857-0. quiz 373-5. [DOI] [PubMed] [Google Scholar]
- 34.Tanenbaum LN. Clinical 3T MR imaging: mastering the challenges. Magn Reson Imaging Clin N Am. 2006 Feb;14(1):1–15. doi: 10.1016/j.mric.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Prayer D, Baert AL. Fetal MRI. 2011 edition Springer; Berlin, Heidelberg: 2011. p. 528. [Google Scholar]
- 36.Ting Ging Ma H. Local experience of MR imaging with 3T (TRIO) equipped with TIM. Biomed Imag Interv J. 2007;3(1):12–39. [Google Scholar]
- 37.DeLano MC, Fisher C. 3T MR imaging of the brain. Magn Reson Imaging Clin N Am. 2006 Feb;14(1):77–88. doi: 10.1016/j.mric.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Gonen O, Gruber S, Li BS, Mlynárik V, Moser E. Multivoxel 3D proton spectroscopy in the brain at 1.5 versus 3.0 T: signal-to-noise ratio and resolution comparison. AJNR Am J Neuroradiol. 2001 Oct;22(9):1727–31. [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnamurthy U, Neelavalli J, Mody S, Yeo L, Jella PK, Saleem S, et al. MR imaging of the fetal brain at 1.5T and 3.0T field strengths: comparing specific absorption rate (SAR) and image quality. J Perinat Med. 2014 Oct 17; doi: 10.1515/jpm-2014-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willinek WA, Kuhl CK. 3.0 T neuroimaging: technical considerations and clinical applications. Neuroimaging Clin N Am. 2006 May;16(2):217–28. ix. doi: 10.1016/j.nic.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 41.High-Field MRI: Is It Time for 3T? [Internet] Imaging Economics; [cited 2014 Sep 7]. Available from: http://www.imagingeconomics.com/2004/02/high-field-mri-is-it-time-for-3t/ [Google Scholar]
- 42.Merkle EM, Dale BM. Abdominal MRI at 3.0 T: The Basics Revisited. Am J Roentgenol. 2006 Jun 1;186(6):1524–32. doi: 10.2214/AJR.05.0932. [DOI] [PubMed] [Google Scholar]
- 43.Keil B, Alagappan V, Mareyam A, McNab JA, Fujimoto K, Tountcheva V, et al. Size-optimized 32-channel brain arrays for 3 T pediatric imaging. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2011 Dec;66(6):1777–87. doi: 10.1002/mrm.22961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lattanzi R, Grant AK, Polimeni JR, Ohliger MA, Wiggins GC, Wald LL, et al. Performance evaluation of a 32-element head array with respect to the ultimate intrinsic SNR. NMR Biomed. 2010 Feb;23(2):142–51. doi: 10.1002/nbm.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welsh RC, Nemec U, Thomason ME. Fetal magnetic resonance imaging at 3.0 T. Top Magn Reson Imaging TMRI. 2011 Jun;22(3):119–31. doi: 10.1097/RMR.0b013e318267f932. [DOI] [PubMed] [Google Scholar]
- 46.Glockner JF, Hu HH, Stanley DW, Angelos L, King K. Parallel MR imaging: a user’s guide. Radiogr Rev Publ Radiol Soc N Am Inc. 2005 Oct;25(5):1279–97. doi: 10.1148/rg.255045202. [DOI] [PubMed] [Google Scholar]
- 47.Serai SD, Merrow AC, Kline-Fath BM. Fetal MRI on a multi-element digital coil platform. Pediatr Radiol. 2013 Sep;43(9):1213–7. doi: 10.1007/s00247-013-2695-0. [DOI] [PubMed] [Google Scholar]
- 48.Vernickel P, Röschmann P, Findeklee C, Lüdeke K-M, Leussler C, Overweg J, et al. Eight-channel transmit/receive body MRI coil at 3T. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2007 Aug;58(2):381–9. doi: 10.1002/mrm.21294. [DOI] [PubMed] [Google Scholar]
- 49.Hillenbrand CM, Reykowski A. MR Imaging of the Newborn: a technical perspective. Magn Reson Imaging Clin N Am. 2012 Feb;20(1):63–79. doi: 10.1016/j.mric.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Deshmane A, Gulani V, Griswold MA, Seiberlich N. Parallel MR imaging. J Magn Reson Imaging JMRI. 2012 Jul;36(1):55–72. doi: 10.1002/jmri.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heidemann RM, Seiberlich N, Griswold MA, Wohlfarth K, Krueger G, Jakob PM. Perspectives and limitations of parallel MR imaging at high field strengths. Neuroimaging Clin N Am. 2006 May;16(2):311–20. xi. doi: 10.1016/j.nic.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Willinek WA, Gieseke J, Kukuk GM, Nelles M, König R, Morakkabati-Spitz N, et al. Dual-source parallel radiofrequency excitation body MR imaging compared with standard MR imaging at 3.0 T: initial clinical experience. Radiology. 2010 Sep;256(3):966–75. doi: 10.1148/radiol.10092127. [DOI] [PubMed] [Google Scholar]
- 53.Barth MM, Smith MP, Pedrosa I, Lenkinski RE, Rofsky NM. Body MR imaging at 3.0 T: understanding the opportunities and challenges. Radiogr Rev Publ Radiol Soc N Am Inc. 2007 Oct;27(5):1445–62. doi: 10.1148/rg.275065204. discussion 1462-4. [DOI] [PubMed] [Google Scholar]
- 54.Zimmerman RA, Bilaniuk LT, Pollock AN, Feygin T, Zarnow D, Schwartz ES, et al. 3.0 T versus 1.5 T pediatric brain imaging. Neuroimaging Clin N Am. 2006 May;16(2):229–39. ix. doi: 10.1016/j.nic.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2005 Sep;54(3):507–12. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- 56.Bernstein MA, Huston J, Ward HA. Imaging artifacts at 3.0T. J Magn Reson Imaging JMRI. 2006 Oct;24(4):735–46. doi: 10.1002/jmri.20698. [DOI] [PubMed] [Google Scholar]
- 57.De Bazelaire CMJ, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology. 2004 Mar;230(3):652–9. doi: 10.1148/radiol.2303021331. [DOI] [PubMed] [Google Scholar]
- 58.Schick F. Whole-body MRI at high field: technical limits and clinical potential. Eur Radiol. 2005 May;15(5):946–59. doi: 10.1007/s00330-005-2678-0. [DOI] [PubMed] [Google Scholar]
- 59.Bernstein MA, Huston J, Lin C, Gibbs GF, Felmlee JP. High-resolution intracranial and cervical MRA at 3.0T: technical considerations and initial experience. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2001 Nov;46(5):955–62. doi: 10.1002/mrm.1282. [DOI] [PubMed] [Google Scholar]
- 60.Krautmacher C, Willinek WA, Tschampa HJ, Born M, Träber F, Gieseke J, et al. Brain tumors: full- and half-dose contrast-enhanced MR imaging at 3.0 T compared with 1.5 T--Initial Experience. Radiology. 2005 Dec;237(3):1014–9. doi: 10.1148/radiol.2373041672. [DOI] [PubMed] [Google Scholar]
- 61.Martirosian P, Klose U, Mader I, Schick F. FAIR true-FISP perfusion imaging of the kidneys. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2004 Feb;51(2):353–61. doi: 10.1002/mrm.10709. [DOI] [PubMed] [Google Scholar]
- 62.Moseley ME, Liu C, Rodriguez S, Brosnan T. Advances in Magnetic Resonance Neuroimaging. Neurol Clin. 2009 Feb;27(1):1–xiii. doi: 10.1016/j.ncl.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huisman TAGM, Sorensen AG. Perfusion-weighted magnetic resonance imaging of the brain: techniques and application in children. Eur Radiol. 2004 Jan;14(1):59–72. doi: 10.1007/s00330-003-1972-y. [DOI] [PubMed] [Google Scholar]
- 64.Le Bihan D, Poupon C, Amadon A, Lethimonnier F. Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging JMRI. 2006 Sep;24(3):478–88. doi: 10.1002/jmri.20683. [DOI] [PubMed] [Google Scholar]
- 65.Franklin KM, Dale BM, Merkle EM. Improvement in B1-inhomogeneity artifacts in the abdomen at 3T MR imaging using a radiofrequency cushion. J Magn Reson Imaging JMRI. 2008 Jun;27(6):1443–7. doi: 10.1002/jmri.21164. [DOI] [PubMed] [Google Scholar]
- 66.Kataoka M, Isoda H, Maetani Y, Nakamoto Y, Koyama T, Umeoka S, et al. MR imaging of the female pelvis at 3 Tesla: evaluation of image homogeneity using different dielectric pads. J Magn Reson Imaging JMRI. 2007 Dec;26(6):1572–7. doi: 10.1002/jmri.21173. [DOI] [PubMed] [Google Scholar]
- 67.De Graaf RA, Nicolay K. Adiabatic rf pulses: Applications to in vivo NMR. Concepts Magn Reson. 1997 Jan 1;9(4):247–68. [Google Scholar]
- 68.Graessner J. Frequently Asked Questions: Diffusion-Weighted Imaging (DWI) [Internet] 2011 [cited 2014 Sep 29]. Available from: http://www.healthcare.siemens.com/siemens_hwem-hwem_ssxa_websites-context-root/wcm/idc/siemens_hwem-hwem_ssxa_websites-context-root/wcm/idc/groups/public/@global/@imaging/@mri/documents/download/mdaw/mdey/~edisp/frequently_asked_questions_diffusion-weighted_imaging-00012754.pdf.
- 69.Rutherford M, Malamateniou C, Zeka J, Counsell S. MR imaging of the neonatal brain at 3 Tesla. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc. 2004;8(6):281–9. doi: 10.1016/j.ejpn.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Kwon SH, Vasung L, Ment LR, Huppi PS. The role of neuroimaging in predicting neurodevelopmental outcomes of preterm neonates. Clin Perinatol. 2014 Mar;41(1):257–83. doi: 10.1016/j.clp.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Gilmore JH, Zhai G, Wilber K, Smith JK, Lin W, Gerig G. 3 Tesla magnetic resonance imaging of the brain in newborns. Psychiatry Res. 2004 Nov 15;132(1):81–5. doi: 10.1016/j.pscychresns.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Wimberger DM, Roberts TP, Barkovich AJ, Prayer LM, Moseley ME, Kucharczyk J. Identification of “premyelination” by diffusion-weighted MRI. J Comput Assist Tomogr. 1995 Feb;19(1):28–33. doi: 10.1097/00004728-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002 Dec;15(7-8):456–67. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- 74.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging JMRI. 2001 Apr;13(4):534–46. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 75.Yoshida S, Oishi K, Faria AV, Mori S. Diffusion tensor imaging of normal brain development. Pediatr Radiol. 2013 Jan;43(1):15–27. doi: 10.1007/s00247-012-2496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hüppi PS, Murphy B, Maier SE, Zientara GP, Inder TE, Barnes PD, et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001 Mar;107(3):455–60. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- 77.Hüppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998 Oct;44(4):584–90. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 78.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007 Nov 8;357(19):1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 79.Miller JH, McKinstry RC, Philip JV, Mukherjee P, Neil JJ. Diffusion-tensor MR imaging of normal brain maturation: a guide to structural development and myelination. AJR Am J Roentgenol. 2003 Mar;180(3):851–9. doi: 10.2214/ajr.180.3.1800851. [DOI] [PubMed] [Google Scholar]
- 80.Bartha AI, Yap KRL, Miller SP, Jeremy RJ, Nishimoto M, Vigneron DB, et al. The normal neonatal brain: MR imaging, diffusion tensor imaging, and 3D MR spectroscopy in healthy term neonates. AJNR Am J Neuroradiol. 2007 Jul;28(6):1015–21. doi: 10.3174/ajnr.A0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002 Dec;15(7-8):435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 82.Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998 Oct;209(1):57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- 83.Shimony JS, McKinstry RC, Akbudak E, Aronovitz JA, Snyder AZ, Lori NF, et al. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology. 1999 Sep;212(3):770–84. doi: 10.1148/radiology.212.3.r99au51770. [DOI] [PubMed] [Google Scholar]
- 84.Prayer D, Barkovich AJ, Kirschner DA, Prayer LM, Roberts TP, Kucharczyk J, et al. Visualization of nonstructural changes in early white matter development on diffusion-weighted MR images: evidence supporting premyelination anisotropy. AJNR Am J Neuroradiol. 2001 Sep;22(8):1572–6. [PMC free article] [PubMed] [Google Scholar]
- 85.Evans AC, Brain Development Cooperative Group The NIH MRI study of normal brain development. NeuroImage. 2006 Mar;30(1):184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 86.Almli CR, Rivkin MJ, McKinstry RC, Brain Development Cooperative Group The NIH MRI study of normal brain development (Objective-2): newborns, infants, toddlers, and preschoolers. NeuroImage. 2007 Mar;35(1):308–25. doi: 10.1016/j.neuroimage.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 87.Barkovich AJ, Miller SP, Bartha A, Newton N, Hamrick SEG, Mukherjee P, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006 Mar;27(3):533–47. [PMC free article] [PubMed] [Google Scholar]
- 88.Vasung L, Fischi-Gomez E, Hüppi PS. Multimodality evaluation of the pediatric brain: DTI and its competitors. Pediatr Radiol. 2013 Jan;43(1):60–8. doi: 10.1007/s00247-012-2515-y. [DOI] [PubMed] [Google Scholar]
- 89.Kasprian G, Brugger PC, Weber M, Krssák M, Krampl E, Herold C, et al. In utero tractography of fetal white matter development. NeuroImage. 2008 Nov 1;43(2):213–24. doi: 10.1016/j.neuroimage.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 90.Jiang S, Xue H, Counsell S, Anjari M, Allsop J, Rutherford M, et al. Diffusion tensor imaging (DTI) of the brain in moving subjects: application to in-utero fetal and ex-utero studies. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2009 Sep;62(3):645–55. doi: 10.1002/mrm.22032. [DOI] [PubMed] [Google Scholar]
- 91.Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am. 2006 Feb;16(1):19–43. vii. doi: 10.1016/j.nic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 92.Setsompop K, Cohen-Adad J, Gagoski BA, Raij T, Yendiki A, Keil B, et al. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. NeuroImage. 2012 Oct 15;63(1):569–80. doi: 10.1016/j.neuroimage.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Poot DHJ, Jeurissen B, Bastiaensen Y, Veraart J, Van Hecke W, Parizel PM, et al. Super-resolution for multislice diffusion tensor imaging. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2013 Jan;69(1):103–13. doi: 10.1002/mrm.24233. [DOI] [PubMed] [Google Scholar]
- 94.Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, et al. Multiplexed Echo Planar Imaging for Sub-Second Whole Brain FMRI and Fast Diffusion Imaging. PLoS ONE. 2010 Dec 20;5(12):e15710. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999 Aug 31;96(18):10422–7. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999 Feb;45(2):265–9. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 97.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2000 Oct;44(4):625–32. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 98.Kreis R, Ernst T, Ross BD. Absolute Quantitation of Water and Metabolites in the Human Brain. II. Metabolite Concentrations. J Magn Reson B. 1993 Aug;102(1):9–19. [Google Scholar]
- 99.Prager A, Roychowdhury S. Magnetic resonance imaging of the neonatal brain. Indian J Pediatr. 2007 Feb;74(2):173–84. doi: 10.1007/s12098-007-0012-3. [DOI] [PubMed] [Google Scholar]
- 100.Brighina E, Bresolin N, Pardi G, Rango M. Human fetal brain chemistry as detected by proton magnetic resonance spectroscopy. Pediatr Neurol. 2009 May;40(5):327–42. doi: 10.1016/j.pediatrneurol.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 101.Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Hüppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2002 Dec;48(6):949–58. doi: 10.1002/mrm.10304. [DOI] [PubMed] [Google Scholar]
- 102.Leth H, Toft PB, Peitersen B, Lou HC, Henriksen O. Use of brain lactate levels to predict outcome after perinatal asphyxia. Acta Paediatr Oslo Nor 1992. 1996 Jul;85(7):859–64. doi: 10.1111/j.1651-2227.1996.tb14168.x. [DOI] [PubMed] [Google Scholar]
- 103.Vigneron DB. Magnetic resonance spectroscopic imaging of human brain development. Neuroimaging Clin N Am. 2006 Feb;16(1):75–85. viii. doi: 10.1016/j.nic.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 104.Dydak U, Schär M. MR spectroscopy and spectroscopic imaging: comparing 3.0 T versus 1.5 T. Neuroimaging Clin N Am. 2006 May;16(2):269–83. x. doi: 10.1016/j.nic.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 105.Barker PB, Hearshen DO, Boska MD. Single-voxel proton MRS of the human brain at 1.5T and 3.0T. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2001 May;45(5):765–9. doi: 10.1002/mrm.1104. [DOI] [PubMed] [Google Scholar]
- 106.Kim J, Chang K-H, Na DG, Song IC, Kwon BJ, Han MH, et al. 3T 1H-MR spectroscopy in grading of cerebral gliomas: comparison of short and intermediate echo time sequences. AJNR Am J Neuroradiol. 2006 Aug;27(7):1412–8. [PMC free article] [PubMed] [Google Scholar]
- 107.Heerschap A, Kok RD, van den Berg PP. Antenatal proton MR spectroscopy of the human brain in vivo. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2003 Aug;19(7-8):418–21. doi: 10.1007/s00381-003-0774-5. [DOI] [PubMed] [Google Scholar]
- 108.Fenton BW, Lin CS, Macedonia C, Schellinger D, Ascher S. The fetus at term: in utero volume-selected proton MR spectroscopy with a breath-hold technique--a feasibility study. Radiology. 2001 May;219(2):563–6. doi: 10.1148/radiology.219.2.r01ma29563. [DOI] [PubMed] [Google Scholar]
- 109.Kok RD, van den Bergh AJ, Heerschap A, Nijland R, van den Berg PP. Metabolic information from the human fetal brain obtained with proton magnetic resonance spectroscopy. Am J Obstet Gynecol. 2001 Nov;185(5):1011–5. doi: 10.1067/mob.2001.117677. [DOI] [PubMed] [Google Scholar]
- 110.Bogner W, Hess AT, Gagoski B, Tisdall MD, van der Kouwe AJW, Trattnig S, et al. Real-time motion- and B0-correction for LASER-localized spiral-accelerated 3D-MRSI of the brain at 3T. NeuroImage. 2013 Nov 5;88C:22–31. doi: 10.1016/j.neuroimage.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hüppi PS, Lazeyras F. Proton magnetic resonance spectroscopy ((1)H-MRS) in neonatal brain injury. Pediatr Res. 2001 Mar;49(3):317–20. doi: 10.1203/00006450-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 112.Bizzi A, Stefano ND. Clinical MR Spectroscopy: Techniques and Applications. Cambridge University Press; 2009. p. 275. [Google Scholar]
- 113.Bainbridge A, Kendall GS, Vita ED, Hagmann C, Kapetanakis A, Cady EB, et al. Regional neonatal brain absolute thermometry by (1) H MRS. NMR Biomed. 2013 Apr;26(4):416–23. doi: 10.1002/nbm.2879. [DOI] [PubMed] [Google Scholar]
- 114.Wu T-W, McLean C, Friedlich P, Wisnowski J, Grimm J, Panigrahy A, et al. Brain Temperature in Neonates with Hypoxic-Ischemic Encephalopathy during Therapeutic Hypothermia. J Pediatr. 2014 Aug 20; doi: 10.1016/j.jpeds.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 115.Ball WS, Holland SK. Perfusion imaging in the pediatric patient. Magn Reson Imaging Clin N Am. 2001 Feb;9(1):207–30. ix. [PubMed] [Google Scholar]
- 116.Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R. Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1999 Jul;19(7):701–35. doi: 10.1097/00004647-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 117.Tanner SF, Cornette L, Ramenghi LA, Miall LS, Ridgway JP, Smith MA, et al. Cerebral perfusion in infants and neonates: preliminary results obtained using dynamic susceptibility contrast enhanced magnetic resonance imaging. Arch Dis Child Fetal Neonatal Ed. 2003 Nov;88(6):F525–30. doi: 10.1136/fn.88.6.F525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):212–6. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang J, Licht DJ, Jahng G-H, Liu C-S, Rubin JT, Haselgrove J, et al. Pediatric perfusion imaging using pulsed arterial spin labeling. J Magn Reson Imaging JMRI. 2003 Oct;18(4):404–13. doi: 10.1002/jmri.10372. [DOI] [PubMed] [Google Scholar]
- 120.Wang J, Licht DJ. Pediatric perfusion MR imaging using arterial spin labeling. Neuroimaging Clin N Am. 2006 Feb;16(1):149–67. ix. doi: 10.1016/j.nic.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 121.Liu TT, Brown GG. Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. J Int Neuropsychol Soc JINS. 2007 May;13(3):517–25. doi: 10.1017/S1355617707070646. [DOI] [PubMed] [Google Scholar]
- 122.Brown GG, Clark C, Liu TT. Measurement of cerebral perfusion with arterial spin labeling: Part 2. Applications. J Int Neuropsychol Soc JINS. 2007 May;13(3):526–38. doi: 10.1017/S1355617707070634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miranda MJ, Olofsson K, Sidaros K. Noninvasive measurements of regional cerebral perfusion in preterm and term neonates by magnetic resonance arterial spin labeling. Pediatr Res. 2006 Sep;60(3):359–63. doi: 10.1203/01.pdr.0000232785.00965.b3. [DOI] [PubMed] [Google Scholar]
- 124.Golay X, Petersen ET. Arterial spin labeling: benefits and pitfalls of high magnetic field. Neuroimaging Clin N Am. 2006 May;16(2):259–68. x. doi: 10.1016/j.nic.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 125.Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993 Mar;64(3):803–12. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seghier ML, Hüppi PS. The role of functional magnetic resonance imaging in the study of brain development, injury, and recovery in the newborn. Semin Perinatol. 2010 Feb;34(1):79–86. doi: 10.1053/j.semperi.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 127.Shimony JS, Zhang D, Johnston JM, Fox MD, Roy A, Leuthardt EC. Resting State Spontaneous Fluctuations in Brain Activity: A New Paradigm for Presurgical Planning using fMRI. Acad Radiol. 2009 May;16(5):578. doi: 10.1016/j.acra.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krüger G, Kastrup A, Glover GH. Neuroimaging at 1.5 T and 3.0 T: comparison of oxygenation-sensitive magnetic resonance imaging. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2001 Apr;45(4):595–604. doi: 10.1002/mrm.1081. [DOI] [PubMed] [Google Scholar]
- 130.Voss HU, Zevin JD, McCandliss BD. Functional MR imaging at 3.0 T versus 1.5 T: a practical review. Neuroimaging Clin N Am. 2006 May;16(2):285–97. x. doi: 10.1016/j.nic.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 131.Thulborn KR. Clinical rationale for very-high-field (3.0 Tesla) functional magnetic resonance imaging. Top Magn Reson Imaging TMRI. 1999 Feb;10(1):37–50. doi: 10.1097/00002142-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 132.Seghier ML, Lazeyras F, Huppi PS. Functional MRI of the newborn. Semin Fetal Neonatal Med. 2006 Dec;11(6):479–88. doi: 10.1016/j.siny.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 133.Fransson P, Skiöld B, Horsch S, Nordell A, Blennow M, Lagercrantz H, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci. 2007 Sep 25;104(39):15531–6. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fransson P, Skiöld B, Engström M, Hallberg B, Mosskin M, Aden U, et al. Spontaneous brain activity in the newborn brain during natural sleep--an fMRI study in infants born at full term. Pediatr Res. 2009 Sep;66(3):301–5. doi: 10.1203/PDR.0b013e3181b1bd84. [DOI] [PubMed] [Google Scholar]
- 135.Smyser CD, Snyder AZ, Neil JJ. Functional connectivity MRI in infants: Exploration of the functional organization of the developing brain. NeuroImage. 2011 Jun 1;56(3):1437–52. doi: 10.1016/j.neuroimage.2011.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fransson P, Metsäranta M, Blennow M, Åden U, Lagercrantz H, Vanhatalo S. Early Development of Spatial Patterns of Power-Law Frequency Scaling in fMRI Resting-State and EEG Data in the Newborn Brain. Cereb Cortex. 2013 Mar 1;23(3):638–46. doi: 10.1093/cercor/bhs047. [DOI] [PubMed] [Google Scholar]
- 137.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007 Sep;8(9):700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 138.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007 Oct 1;37(4):1083–90. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097-9. [DOI] [PubMed] [Google Scholar]
- 139.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 1995 Oct;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 140.Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006 Sep 12;103(37):13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, et al. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci. 2010 Nov 16;107(46):20015–20. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lin W, Zhu Q, Gao W, Chen Y, Toh C-H, Styner M, et al. Functional Connectivity MR Imaging Reveals Cortical Functional Connectivity in the Developing Brain. AJNR Am J Neuroradiol. 2008 Nov;29(10):1883–9. doi: 10.3174/ajnr.A1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Damaraju E, Phillips JR, Lowe JR, Ohls R, Calhoun VD, Caprihan A. Resting-State Functional Connectivity Differences in Premature Children. Front Syst Neurosci [Internet] 2010 Jun 17;:4. doi: 10.3389/fnsys.2010.00023. [cited 2014 Oct 27] Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2923563/ [DOI] [PMC free article] [PubMed]
- 144.Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, et al. Longitudinal Analysis of Neural Network Development in Preterm Infants. Cereb Cortex N Y NY. 2010 Dec;20(12):2852–62. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fransson P, Åden U, Blennow M, Lagercrantz H. The Functional Architecture of the Infant Brain as Revealed by Resting-State fMRI. Cereb Cortex. 2011 Jan 1;21(1):145–54. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- 146.Sporns O, Tononi G, Kötter R. The Human Connectome: A Structural Description of the Human Brain. PLoS Comput Biol. 2005 Sep 30;1(4):e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hüppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006 Dec;11(6):489–97. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 148.Uğurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, et al. Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. NeuroImage. 2013 Oct 15;80:80–104. doi: 10.1016/j.neuroimage.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Seghier ML, Lazeyras F, Zimine S, Maier SE, Hanquinet S, Delavelle J, et al. Combination of event-related fMRI and diffusion tensor imaging in an infant with perinatal stroke. NeuroImage. 2004 Jan;21(1):463–72. doi: 10.1016/j.neuroimage.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 150.Moore RJ, Vadeyar S, Fulford J, Tyler DJ, Gribben C, Baker PN, et al. Antenatal determination of fetal brain activity in response to an acoustic stimulus using functional magnetic resonance imaging. Hum Brain Mapp. 2001 Feb;12(2):94–9. doi: 10.1002/1097-0193(200102)12:2<94::AID-HBM1006>3.0.CO;2-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fulford J, Vadeyar SH, Dodampahala SH, Ong S, Moore RJ, Baker PN, et al. Fetal brain activity and hemodynamic response to a vibroacoustic stimulus. Hum Brain Mapp. 2004 Jun;22(2):116–21. doi: 10.1002/hbm.20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jardri R, Pins D, Houfflin-Debarge V, Chaffiotte C, Rocourt N, Pruvo J-P, et al. Fetal cortical activation to sound at 33 weeks of gestation: a functional MRI study. NeuroImage. 2008 Aug 1;42(1):10–8. doi: 10.1016/j.neuroimage.2008.04.247. [DOI] [PubMed] [Google Scholar]
- 153.Gowland P, Fulford J. Initial experiences of performing fetal fMRI. Exp Neurol. 2004 Nov;190(Suppl 1):S22–7. doi: 10.1016/j.expneurol.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 154.Stokowski LA. Ensuring safety for infants undergoing magnetic resonance imaging. Adv Neonatal Care Off J Natl Assoc Neonatal Nurses. 2005 Feb;5(1):14–27. doi: 10.1016/j.adnc.2004.07.006. quiz 52-4. [DOI] [PubMed] [Google Scholar]
- 155.Dearlove O, Corcoran JP. Sedation of children undergoing magnetic resonance imaging. Br J Anaesth. 2007 Apr;98(4):548–9. doi: 10.1093/bja/aem015. [DOI] [PubMed] [Google Scholar]
- 156.Beauve B, Dearlove O. Sedation of children under 4 weeks of age for MRI examination. Paediatr Anaesth. 2008 Sep;18(9):892–3. doi: 10.1111/j.1460-9592.2008.02580.x. [DOI] [PubMed] [Google Scholar]
- 157.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, et al. Early Exposure to Anesthesia and Learning Disabilities in a Population-Based Birth Cohort. Anesthesiology. 2009 Apr;110(4):796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Etzel-Hardman D, Kapsin K, Jones S, Churilla H. Sedation reduction in a pediatric radiology department. J Healthc Qual Off Publ Natl Assoc Healthc Qual. 2009 Aug;31(4):34–9. doi: 10.1111/j.1945-1474.2009.00035.x. [DOI] [PubMed] [Google Scholar]
- 159.Ferrazzi G, Kuklisova Murgasova M, Arichi T, Malamateniou C, Fox MJ, Makropoulos A, et al. Resting State fMRI in the moving fetus: a robust framework for motion, bias field and spin history correction. NeuroImage. 2014 Nov 1;101:555–68. doi: 10.1016/j.neuroimage.2014.06.074. [DOI] [PubMed] [Google Scholar]
- 160.Jiang S, Xue H, Glover A, Rutherford M, Rueckert D, Hajnal JV. MRI of moving subjects using multislice snapshot images with volume reconstruction (SVR): application to fetal, neonatal, and adult brain studies. IEEE Trans Med Imaging. 2007 Jul;26(7):967–80. doi: 10.1109/TMI.2007.895456. [DOI] [PubMed] [Google Scholar]
- 161.Kim K, Habas PA, Rousseau F, Glenn OA, Barkovich AJ, Studholme C. Intersection based motion correction of multislice MRI for 3-D in utero fetal brain image formation. IEEE Trans Med Imaging. 2010 Jan;29(1):146–58. doi: 10.1109/TMI.2009.2030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rousseau F, Glenn OA, Iordanova B, Rodriguez-Carranza C, Vigneron DB, Barkovich JA, et al. Registration-based approach for reconstruction of high-resolution in utero fetal MR brain images. Acad Radiol. 2006 Sep;13(9):1072–81. doi: 10.1016/j.acra.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 163.Srinivasan L, Dutta R, Counsell SJ, Allsop JM, Boardman JP, Rutherford MA, et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics. 2007 Apr;119(4):759–65. doi: 10.1542/peds.2006-2508. [DOI] [PubMed] [Google Scholar]
- 164.Rousseau F, Glenn O, Iordanova B, Rodriguez-Carranza C, Vigneron D, Barkovich J, et al. A novel approach to high resolution fetal brain MR imaging. Med Image Comput Comput-Assist Interv MICCAI Int Conf Med Image Comput Comput-Assist Interv. 2005;8(Pt 1):548–55. doi: 10.1007/11566465_68. [DOI] [PubMed] [Google Scholar]
- 165.Kim K, Hansen M, Habas P, Rousseau F, Glenn O, Barkovich AJ, et al. Intersection based registration of slice stacks to form 3D images of the human fetal brain; 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, 2008 ISBI 2008; 2008.pp. 1167–70. [Google Scholar]
- 166.Lin W, Huang F, Duensing GR, Reykowski A. High temporal resolution retrospective motion correction with radial parallel imaging. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2012 Apr;67(4):1097–105. doi: 10.1002/mrm.23092. [DOI] [PubMed] [Google Scholar]
- 167.Forbes KP, Pipe JG, Bird CR, Heiserman JE. PROPELLER MRI: clinical testing of a novel technique for quantification and compensation of head motion. J Magn Reson Imaging JMRI. 2001 Sep;14(3):215–22. doi: 10.1002/jmri.1176. [DOI] [PubMed] [Google Scholar]
- 168.Forbes KP, Pipe JG, Karis JP, Farthing V, Heiserman JE. Brain imaging in the unsedated pediatric patient: comparison of periodically rotated overlapping parallel lines with enhanced reconstruction and single-shot fast spin-echo sequences. AJNR Am J Neuroradiol. 2003 May;24(5):794–8. [PMC free article] [PubMed] [Google Scholar]
- 169.Krämer M, Jochimsen TH, Reichenbach JR. Functional magnetic resonance imaging using PROPELLER-EPI. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2012 Jul;68(1):140–51. doi: 10.1002/mrm.23220. [DOI] [PubMed] [Google Scholar]
- 170.Li Z, Pipe JG, Aboussouan E, Karis JP, Huo D. A parallel imaging technique using mutual calibration for split-blade diffusion-weighted PROPELLER. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2011 Mar;65(3):638–44. doi: 10.1002/mrm.22646. [DOI] [PubMed] [Google Scholar]
- 171.Vertinsky AT, Rubesova E, Krasnokutsky MV, Bammer S, Rosenberg J, White A, et al. Performance of PROPELLER relative to standard FSE T2-weighted imaging in pediatric brain MRI. Pediatr Radiol. 2009 Oct;39(10):1038–47. doi: 10.1007/s00247-009-1292-8. [DOI] [PubMed] [Google Scholar]
- 172.Nyberg E, Sandhu GS, Jesberger J, Blackham KA, Hsu DP, Griswold MA, et al. Comparison of brain MR images at 1.5T using BLADE and rectilinear techniques for patients who move during data acquisition. AJNR Am J Neuroradiol. 2012 Jan;33(1):77–82. doi: 10.3174/ajnr.A2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lavdas E, Mavroidis P, Kostopoulos S, Glotsos D, Roka V, Topalzikis T, et al. Improvement of image quality using BLADE sequences in brain MR imaging. Magn Reson Imaging. 2013 Feb;31(2):189–200. doi: 10.1016/j.mri.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 174.Tachibana Y, Niwa T, Kwee TC, Takahara T, Kusagiri K, Nagaoka T, et al. Effective performance of T(1)-weighted FLAIR Imaging with BLADE in pediatric brains. Magn Reson Med Sci MRMS Off J Jpn Soc Magn Reson Med. 2012;11(1):17–26. doi: 10.2463/mrms.11.17. [DOI] [PubMed] [Google Scholar]
- 175.Ohshita T. [Basic examination of an imagecharacteristic in Multivane] Nihon Hoshasen Gijutsu Gakkai Zasshi. 2011;67(10):1298–303. doi: 10.6009/jjrt.67.1298. [DOI] [PubMed] [Google Scholar]
- 176.American College of Radiolgy and Society for Pediatric Radiology . ACR-SPR Practice Parameter for the Safe and Optimal Performance of Fetal Magnetic Resonance Imaging (MRI), Resolution (39) [Internet] American College of Radiology; 2014. [cited 2014 Nov 16]. Available from: http://www.acr.org/~/media/CB384A65345F402083639E6756CE513F.pdf. [Google Scholar]
- 177.Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG, Froelich JW, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007 Jun;188(6):1447–74. doi: 10.2214/AJR.06.1616. [DOI] [PubMed] [Google Scholar]
- 178.Expert Panel on MR Safety. Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging JMRI. 2013 Mar;37(3):501–30. doi: 10.1002/jmri.24011. [DOI] [PubMed] [Google Scholar]
- 179.Schenck JF. Physical interactions of static magnetic fields with living tissues. Prog Biophys Mol Biol. 2005 Apr;87(2-3):185–204. doi: 10.1016/j.pbiomolbio.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 180.International Non-Ionizing Radiation Protection (ICNIRP) Statement of Medical Magnetic Resonance Procedures: Protection of Patients. Health Phys. 2004;87(2):197–216. doi: 10.1097/00004032-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 181.International Commission on Non-Ionizing Radiation Protection Amendment to the ICNIRP “Statement on medical magnetic resonance (MR) procedures: protection of patients.”. Health Phys. 2009 Sep;97(3):259–61. doi: 10.1097/HP.0b013e3181aff9eb. [DOI] [PubMed] [Google Scholar]
- 182.Grant PE, Wald LL, Adalsteinsson E. Potential Risks of Fetal Magnetic Resonance Imaging: 3 Tesla Compared to 1.5 Tesla. 2014 In Press. [Google Scholar]
- 183.De Vocht F, van Drooge H, Engels H, Kromhout H. Exposure, health complaints and cognitive performance among employees of an MRI scanners manufacturing department. J Magn Reson Imaging JMRI. 2006 Feb;23(2):197–204. doi: 10.1002/jmri.20485. [DOI] [PubMed] [Google Scholar]
- 184.De Vocht F, Stevens T, van Wendel-de-Joode B, Engels H, Kromhout H. Acute neurobehavioral effects of exposure to static magnetic fields: analyses of exposure-response relations. J Magn Reson Imaging JMRI. 2006 Mar;23(3):291–7. doi: 10.1002/jmri.20510. [DOI] [PubMed] [Google Scholar]
- 185.Toyomaki A, Yamamoto T. Observation of changes in neural activity due to the static magnetic field of an MRI scanner. J Magn Reson Imaging JMRI. 2007 Nov;26(5):1216–21. doi: 10.1002/jmri.21151. [DOI] [PubMed] [Google Scholar]
- 186.Chakeres DW, de Vocht F. Static magnetic field effects on human subjects related to magnetic resonance imaging systems. Prog Biophys Mol Biol. 2005 Apr;87(2-3):255–65. doi: 10.1016/j.pbiomolbio.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 187.Vogl TJ, Paulus W, Fuchs A, Krafczyk S, Lissner J. Influence of magnetic resonance imaging on evoked potentials and nerve conduction velocities in humans. Invest Radiol. 1991 May;26(5):432–7. doi: 10.1097/00004424-199105000-00010. [DOI] [PubMed] [Google Scholar]
- 188.Hong CZ, Shellock FG. Short-term exposure to a 1.5 tesla static magnetic field does not affect somato-sensory-evoked potentials in man. Magn Reson Imaging. 1990;8(1):65–9. doi: 10.1016/0730-725x(90)90214-m. [DOI] [PubMed] [Google Scholar]
- 189.Wiskirchen J, Grönewäller EF, Heinzelmann F, Kehlbach R, Rodegerdts E, Wittau M, et al. Human fetal lung fibroblasts: in vitro study of repetitive magnetic field exposure at 0.2, 1.0, and 1.5 T. Radiology. 2000 Jun;215(3):858–62. doi: 10.1148/radiology.215.3.r00jn11858. [DOI] [PubMed] [Google Scholar]
- 190.Schwartz JL, Crooks LE. NMR imaging produces no observable mutations or cytotoxicity in mammalian cells. AJR Am J Roentgenol. 1982 Sep;139(3):583–5. doi: 10.2214/ajr.139.3.583. [DOI] [PubMed] [Google Scholar]
- 191.Kay HH, Herfkens RJ, Kay BK. Effect of magnetic resonance imaging on Xenopus laevis embryogenesis. Magn Reson Imaging. 1988 Oct;6(5):501–6. doi: 10.1016/0730-725x(88)90124-5. [DOI] [PubMed] [Google Scholar]
- 192.Tyndall DA, Sulik KK. Effects of magnetic resonance imaging on eye development in the C57BL/6J mouse. Teratology. 1991 Mar;43(3):263–75. doi: 10.1002/tera.1420430310. [DOI] [PubMed] [Google Scholar]
- 193.Saunders R. Static magnetic fields: animal studies. Prog Biophys Mol Biol. 2005 Apr;87(2-3):225–39. doi: 10.1016/j.pbiomolbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 194.Schenck JF. Safety of strong, static magnetic fields. J Magn Reson Imaging JMRI. 2000 Jul;12(1):2–19. doi: 10.1002/1522-2586(200007)12:1<2::aid-jmri2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 195.Edwards MJ, Saunders RD, Shiota K. Effects of heat on embryos and foetuses. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. 2003 Jun;19(3):295–324. doi: 10.1080/0265673021000039628. [DOI] [PubMed] [Google Scholar]
- 196.Levine D, Zuo C, Faro CB, Chen Q. Potential heating effect in the gravid uterus during MR HASTE imaging. J Magn Reson Imaging JMRI. 2001 Jun;13(6):856–61. doi: 10.1002/jmri.1122. [DOI] [PubMed] [Google Scholar]
- 197.International Commission on Non-Ionizing Radiation Protection Medical magnetic resonance (MR) procedures: protection of patients. Health Phys. 2004 Aug;87(2):197–216. doi: 10.1097/00004032-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 198.Bahado-Singh RO, Goncalves LF. Techniques, terminology, and indications for MRI in pregnancy. Semin Perinatol. 2013 Oct;37(5):334–9. doi: 10.1053/j.semperi.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 199.Hennig J, Scheffler K. Hyperechoes. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2001 Jul;46(1):6–12. doi: 10.1002/mrm.1153. [DOI] [PubMed] [Google Scholar]
- 200.Hebrank FX, Gebhardt M. SAFE-Model - A New Method for Predicting Peripheral Nerve Stimulations in MRI [Internet] Siemans Medical Systems, MR Division; 2000. [cited 2014 Sep 19]. Available from: http://cds.ismrm.org/ismrm-2000/PDF7/2007.PDF. [Google Scholar]
- 201.Price DL, De Wilde JP, Papadaki AM, Curran JS, Kitney RI. Investigation of acoustic noise on 15 MRI scanners from 0.2 T to 3 T. J Magn Reson Imaging JMRI. 2001 Feb;13(2):288–93. doi: 10.1002/1522-2586(200102)13:2<288::aid-jmri1041>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 202.McJury M, Shellock FG. Auditory noise associated with MR procedures: a review. J Magn Reson Imaging JMRI. 2000 Jul;12(1):37–45. doi: 10.1002/1522-2586(200007)12:1<37::aid-jmri5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 203.CDC - NIOSH Publications and Products - Preventing Occupational Hearing Loss - A Practical Guide (96-110) [Internet] [cited 2014 Sep 19]. Available from: http://www.cdc.gov/niosh/docs/96-110/pdfs/96-110.pdf.
- 204.Morris BH, Philbin MK, Bose C. Physiological effects of sound on the newborn. J Perinatol Off J Calif Perinat Assoc. 2000 Dec;20(8 Pt 2):S55–60. doi: 10.1038/sj.jp.7200451. [DOI] [PubMed] [Google Scholar]
- 205.Boyle Y, Bentley DE, Watson A, Jones AKP. Acoustic noise in functional magnetic resonance imaging reduces pain unpleasantness ratings. NeuroImage. 2006 Jul 1;31(3):1278–83. doi: 10.1016/j.neuroimage.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 206.Amaro E, Williams SCR, Shergill SS, Fu CHY, MacSweeney M, Picchioni MM, et al. Acoustic noise and functional magnetic resonance imaging: current strategies and future prospects. J Magn Reson Imaging JMRI. 2002 Nov;16(5):497–510. doi: 10.1002/jmri.10186. [DOI] [PubMed] [Google Scholar]
- 207.Nordell A, Lundh M, Horsch S, Hallberg B, Aden U, Nordell B, et al. The acoustic hood: a patient-independent device improving acoustic noise protection during neonatal magnetic resonance imaging. Acta Paediatr Oslo Nor 1992. 2009 Aug;98(8):1278–83. doi: 10.1111/j.1651-2227.2009.01339.x. [DOI] [PubMed] [Google Scholar]
- 208.Macnab A, Chen Y, Gagnon F, Bora B, Laszlo C. Vibration and noise in pediatric emergency transport vehicles: a potential cause of morbidity? Aviat Space Environ Med. 1995 Mar;66(3):212–9. [PubMed] [Google Scholar]
- 209.Watts C, Trim E, Metherall J, Lightfoot E. Neonatal transport - the comfort zone. Infant. 2008;4(1):27–30. [Google Scholar]
- 210.Tkach JA, Li Y, Pratt RG, Baroch KA, Loew W, Daniels BR, et al. Characterization of acoustic noise in a neonatal intensive care unit MRI system. Pediatr Radiol. 2014 Aug;44(8):1011–9. doi: 10.1007/s00247-014-2909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Glover P, Hykin J, Gowland P, Wright J, Johnson I, Mansfield P. An assessment of the intrauterine sound intensity level during obstetric echo-planar magnetic resonance imaging. Br J Radiol. 1995 Oct;68(814):1090–4. doi: 10.1259/0007-1285-68-814-1090. [DOI] [PubMed] [Google Scholar]
- 212.Baker PN, Johnson IR, Harvey PR, Gowland PA, Mansfield P. A three-year follow-up of children imaged in utero with echo-planar magnetic resonance. Am J Obstet Gynecol. 1994 Jan;170(1 Pt 1):32–3. doi: 10.1016/s0002-9378(94)70379-5. [DOI] [PubMed] [Google Scholar]
- 213.Reeves MJ, Brandreth M, Whitby EH, Hart AR, Paley MNJ, Griffiths PD, et al. Neonatal cochlear function: measurement after exposure to acoustic noise during in utero MR imaging. Radiology. 2010 Dec;257(3):802–9. doi: 10.1148/radiol.10092366. [DOI] [PubMed] [Google Scholar]
- 214.Kok RD, de Vries MM, Heerschap A, van den Berg PP. Absence of harmful effects of magnetic resonance exposure at 1.5 T in utero during the third trimester of pregnancy: a follow-up study. Magn Reson Imaging. 2004 Jul;22(6):851–4. doi: 10.1016/j.mri.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 215.Eskandar O, Eckford S, Watkinson T. Safety of diagnostic imaging in pregnancy. Part 2: magnetic resonance imaging, ultrasound scanning and Doppler assessment. Obstet Gynaecol. 2010 Jul 1;12(3):171–7. [Google Scholar]
- 216.Poutamo J, Partanen K, Vanninen R, Vainio P, Kirkinen P. MRI does not change fetal cardiotocographic parameters. Prenat Diagn. 1998 Nov;18(11):1149–54. doi: 10.1002/(sici)1097-0223(199811)18:11<1149::aid-pd421>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 217.Garel C, Brisse H, Sebag G, Elmaleh M, Oury JF, Hassan M. Magnetic resonance imaging of the fetus. Pediatr Radiol. 1998 Apr;28(4):201–11. doi: 10.1007/s002470050334. [DOI] [PubMed] [Google Scholar]
- 218.Glenn OA, Goldstein RB, Li KC, Young SJ, Norton ME, Busse RF, et al. Fetal magnetic resonance imaging in the evaluation of fetuses referred for sonographically suspected abnormalities of the corpus callosum. J Ultrasound Med Off J Am Inst Ultrasound Med. 2005 Jun;24(6):791–804. doi: 10.7863/jum.2005.24.6.791. [DOI] [PubMed] [Google Scholar]
- 219.Benacerraf BR, Shipp TD, Bromley B, Levine D. What does magnetic resonance imaging add to the prenatal sonographic diagnosis of ventriculomegaly? J Ultrasound Med Off J Am Inst Ultrasound Med. 2007 Nov;26(11):1513–22. doi: 10.7863/jum.2007.26.11.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Levine D. Obstetric MRI. J Magn Reson Imaging JMRI. 2006 Jul;24(1):1–15. doi: 10.1002/jmri.20608. [DOI] [PubMed] [Google Scholar]
- 221.Garel C. Fetal MRI: what is the future? Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2008 Feb;31(2):123–8. doi: 10.1002/uog.5249. [DOI] [PubMed] [Google Scholar]
- 222.Righini A, Bianchini E, Parazzini C, Gementi P, Ramenghi L, Baldoli C, et al. Apparent diffusion coefficient determination in normal fetal brain: a prenatal MR imaging study. AJNR Am J Neuroradiol. 2003 May;24(5):799–804. [PMC free article] [PubMed] [Google Scholar]
- 223.Prayer D, Brugger PC, Mittermayer C, Nasel CJO, Hoche B, Schindler E. Diffusion-weighted imaging in intrauterine fetal brain development. Abstr Am Soc Neuroradiol Meet Wash. 2003 [Google Scholar]
- 224.Manganaro L, Perrone A, Savelli S, Di Maurizio M, Maggi C, Ballesio L, et al. Evaluation of normal brain development by prenatal MR imaging. Radiol Med (Torino) 2007 Apr;112(3):444–55. doi: 10.1007/s11547-007-0153-5. [DOI] [PubMed] [Google Scholar]
- 225.Schneider JF, Confort-Gouny S, Le Fur Y, Viout P, Bennathan M, Chapon F, et al. Diffusion-weighted imaging in normal fetal brain maturation. Eur Radiol. 2007 Sep;17(9):2422–9. doi: 10.1007/s00330-007-0634-x. [DOI] [PubMed] [Google Scholar]
- 226.Schneider MM, Berman JI, Baumer FM, Glass HC, Jeng S, Jeremy RJ, et al. Normative apparent diffusion coefficient values in the developing fetal brain. AJNR Am J Neuroradiol. 2009 Oct;30(9):1799–803. doi: 10.3174/ajnr.A1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Clouchoux C, Limperopoulos C. Novel applications of quantitative MRI for the fetal brain. Pediatr Radiol. 2012 Jan;42(Suppl 1):S24–32. doi: 10.1007/s00247-011-2178-0. [DOI] [PubMed] [Google Scholar]
- 228.Girard N, Fogliarini C, Viola A, Confort-Gouny S, Fur YL, Viout P, et al. MRS of normal and impaired fetal brain development. Eur J Radiol. 2006 Feb;57(2):217–25. doi: 10.1016/j.ejrad.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 229.Levine D. Timing of MRI in pregnancy, repeat exams, access, and physician qualifications. Semin Perinatol. 2013 Oct;37(5):340–4. doi: 10.1053/j.semperi.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 230.Reddy UM, Filly RA, Copel JA, Pregnancy and Perinatology Branch. Eunice Kennedy Shriver National Institute of Child Health and Human Development. Department of Health and Human Services. NIH Prenatal imaging: ultrasonography and magnetic resonance imaging. Obstet Gynecol. 2008 Jul;112(1):145–57. doi: 10.1097/01.AOG.0000318871.95090.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Garel C. Imaging the fetus: when does MRI really help? Pediatr Radiol. 2008 Jun;38(Suppl 3):S467–70. doi: 10.1007/s00247-008-0837-6. [DOI] [PubMed] [Google Scholar]
- 232.Bulas D, Egloff A. Benefits and risks of MRI in pregnancy. Semin Perinatol. 2013 Oct;37(5):301–4. doi: 10.1053/j.semperi.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 233.Kok RD, van den Berg PP, van den Bergh AJ, Nijland R, Heerschap A. Maturation of the human fetal brain as observed by 1H MR spectroscopy. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2002 Oct;48(4):611–6. doi: 10.1002/mrm.10264. [DOI] [PubMed] [Google Scholar]
- 234.Tang PH, Bartha AI, Norton ME, Barkovich AJ, Sherr EH, Glenn OA. Agenesis of the corpus callosum: an MR imaging analysis of associated abnormalities in the fetus. AJNR Am J Neuroradiol. 2009 Feb;30(2):257–63. doi: 10.3174/ajnr.A1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Switzer PJ, James CA, Frettag MA. Value and Limitations of Obstetrical Ultrasound: Uncovering abnormalities at earlier stages. Can Fam Physician Médecin Fam Can. 1992 Jan;38:121–8. [PMC free article] [PubMed] [Google Scholar]
- 236.Dashe JS, McIntire DD, Twickler DM. Maternal obesity limits the ultrasound evaluation of fetal anatomy. J Ultrasound Med Off J Am Inst Ultrasound Med. 2009 Aug;28(8):1025–30. doi: 10.7863/jum.2009.28.8.1025. [DOI] [PubMed] [Google Scholar]
- 237.Hykin J, Moore R, Duncan K, Clare S, Baker P, Johnson I, et al. Fetal brain activity demonstrated by functional magnetic resonance imaging. Lancet. 1999 Aug 21;354(9179):645–6. doi: 10.1016/S0140-6736(99)02901-3. [DOI] [PubMed] [Google Scholar]
- 238.Fulford J, Vadeyar SH, Dodampahala SH, Moore RJ, Young P, Baker PN, et al. Fetal brain activity in response to a visual stimulus. Hum Brain Mapp. 2003 Dec;20(4):239–45. doi: 10.1002/hbm.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fulford J, Gowland PA. The emerging role of functional MRI for evaluating fetal brain activity. Semin Perinatol. 2009 Aug;33(4):281–8. doi: 10.1053/j.semperi.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 240.Thomason M, Dassanayake M, Shen S, Katkuri Y, Alexis M, Anderson A, et al. Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med. 2013 Feb 20;5(173):173ra24. doi: 10.1126/scitranslmed.3004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ullmann P, Junge S, Wick M, Seifert F, Ruhm W, Hennig J. Experimental analysis of parallel excitation using dedicated coil setups and simultaneous RF transmission on multiple channels. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2005 Oct;54(4):994–1001. doi: 10.1002/mrm.20646. [DOI] [PubMed] [Google Scholar]
- 242.Duensing R, Fitzsimmons J. 3.0 T versus 1.5 T: coil design similarities and differences. Neuroimaging Clin N Am. 2006 May;16(2):249–57. x. doi: 10.1016/j.nic.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 243.Wieseler KM, Bhargava P, Kanal KM, Vaidya S, Stewart BK, Dighe MK. Imaging in pregnant patients: examination appropriateness. Radiogr Rev Publ Radiol Soc N Am Inc. 2010 Sep;30(5):1215–29. doi: 10.1148/rg.305105034. discussion 1230-3. [DOI] [PubMed] [Google Scholar]
- 244.Myers C, Duncan KR, Gowland PA, Johnson IR, Baker PN. Failure to detect intrauterine growth restriction following in utero exposure to MRI. Br J Radiol. 1998 May;71(845):549–51. doi: 10.1259/bjr.71.845.9691901. [DOI] [PubMed] [Google Scholar]
- 245.Clements H, Duncan KR, Fielding K, Gowland PA, Johnson IR, Baker PN. Infants exposed to MRI in utero have a normal paediatric assessment at 9 months of age. Br J Radiol. 2000 Feb;73(866):190–4. doi: 10.1259/bjr.73.866.10884733. [DOI] [PubMed] [Google Scholar]
- 246.Magin RL, Lee JK, Klintsova A, Carnes KI, Dunn F. Biological effects of long-duration, high-field (4 T) MRI on growth and development in the mouse. J Magn Reson Imaging JMRI. 2000 Jul;12(1):140–9. doi: 10.1002/1522-2586(200007)12:1<140::aid-jmri15>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 247.Westbrook C, Roth CK. MRI in Practice. John Wiley & Sons; 2011. p. 497. [Google Scholar]
- 248.Health C for D and R. Guidance Documents (Medical Devices and Radiation-Emitting Products) - Guidance for Industry: Guidance for the Submission Of Premarket Notifications for Magnetic Resonance Diagnostic Devices [Internet] [cited 2014 Dec 1]. Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm073817.htm.
- 249.Yip YP, Capriotti C, Talagala SL, Yip JW. Effects of MR exposure at 1.5 T on early embryonic development of the chick. J Magn Reson Imaging JMRI. 1994 Oct;4(5):742–8. doi: 10.1002/jmri.1880040518. [DOI] [PubMed] [Google Scholar]
- 250.Yip YP, Capriotti C, Yip JW. Effects of MR exposure on axonal outgrowth in the sympathetic nervous system of the chick. J Magn Reson Imaging JMRI. 1995 Aug;5(4):457–62. doi: 10.1002/jmri.1880050416. [DOI] [PubMed] [Google Scholar]
- 251.Mevissen M, Buntenkötter S, Löscher W. Effects of static and time-varying (50-Hz) magnetic fields on reproduction and fetal development in rats. Teratology. 1994 Sep;50(3):229–37. doi: 10.1002/tera.1420500308. [DOI] [PubMed] [Google Scholar]
- 252.Yip YP, Capriotti C, Norbash SG, Talagala SL, Yip JW. Effects of MR exposure on cell proliferation and migration of chick motoneurons. J Magn Reson Imaging JMRI. 1994 Dec;4(6):799–804. doi: 10.1002/jmri.1880040610. [DOI] [PubMed] [Google Scholar]
- 253.Heinrichs WL, Fong P, Flannery M, Heinrichs SC, Crooks LE, Spindle A, et al. Midgestational exposure of pregnant BALB/c mice to magnetic resonance imaging conditions. Magn Reson Imaging. 1988 Jun;6(3):305–13. doi: 10.1016/0730-725x(88)90407-9. [DOI] [PubMed] [Google Scholar]
- 254.Hand JW, Li Y, Hajnal JV. Numerical study of RF exposure and the resulting temperature rise in the foetus during a magnetic resonance procedure. Phys Med Biol. 2010 Feb 21;55(4):913–30. doi: 10.1088/0031-9155/55/4/001. [DOI] [PubMed] [Google Scholar]
- 255.Dimbylow P. Development of pregnant female, hybrid voxel-mathematical models and their application to the dosimetry of applied magnetic and electric fields at 50 Hz. Phys Med Biol. 2006 May 21;51(10):2383–94. doi: 10.1088/0031-9155/51/10/003. [DOI] [PubMed] [Google Scholar]
- 256.Kanal E, Gillen J, Evans JA, Savitz DA, Shellock FG. Survey of reproductive health among female MR workers. Radiology. 1993 May;187(2):395–9. doi: 10.1148/radiology.187.2.8475280. [DOI] [PubMed] [Google Scholar]
- 257.Sundgren PC, Leander P. Is administration of gadolinium-based contrast media to pregnant women and small children justified? J Magn Reson Imaging JMRI. 2011 Oct;34(4):750–7. doi: 10.1002/jmri.22413. [DOI] [PubMed] [Google Scholar]
- 258.Barkovich A. Pediatric Neuroimaging. 4th ed. Lippincott; Philadelphia, PA: 2005. [Google Scholar]
- 259.Okuda Y, Sagami F, Tirone P, Morisetti A, Bussi S, Masters RE. [Reproductive and developmental toxicity study of gadobenate dimeglumine formulation (E7155) (3)--Study of embryo-fetal toxicity in rabbits by intravenous administration] J Toxicol Sci. 1999 Nov;24(Suppl 1):79–87. doi: 10.2131/jts.24.supplementi_79. [DOI] [PubMed] [Google Scholar]
- 260.Rofsky NM, Pizzarello DJ, Weinreb JC, Ambrosino MM, Rosenberg C. Effect on fetal mouse development of exposure to MR imaging and gadopentetate dimeglumine. J Magn Reson Imaging JMRI. 1994 Dec;4(6):805–7. doi: 10.1002/jmri.1880040611. [DOI] [PubMed] [Google Scholar]
- 261.Rofsky NM, Pizzarello DJ, Duhaney MO, Falick AK, Prendergast N, Weinreb JC. Effect of magnetic resonance exposure combined with gadopentetate dimeglumine on chromosomes in animal specimens. Acad Radiol. 1995 Jun;2(6):492–6. doi: 10.1016/s1076-6332(05)80405-2. [DOI] [PubMed] [Google Scholar]
- 262.Morisetti A, Bussi S, Tirone P, de Haën C. Toxicological safety evaluation of gadobenate dimeglumine 0.5 M solution for injection (MultiHance), a new magnetic resonance imaging contrast medium. J Comput Assist Tomogr. 1999 Nov;23(Suppl 1):S207–17. doi: 10.1097/00004728-199911001-00025. [DOI] [PubMed] [Google Scholar]
- 263.Soltys RA. Summary of preclinical safety evaluation of gadoteridol injection. Invest Radiol. 1992 Aug;27(Suppl 1):S7–11. [PubMed] [Google Scholar]
- 264.Mühler MR, Clément O, Salomon LJ, Balvay D, Autret G, Vayssettes C, et al. Maternofetal pharmacokinetics of a gadolinium chelate contrast agent in mice. Radiology. 2011 Feb;258(2):455–60. doi: 10.1148/radiol.10100652. [DOI] [PubMed] [Google Scholar]
- 265.McRobbie D, Foster MA. Pulsed magnetic field exposure during pregnancy and implications for NMR foetal imaging: a study with mice. Magn Reson Imaging. 1985;3(3):231–4. doi: 10.1016/0730-725x(85)90351-0. [DOI] [PubMed] [Google Scholar]
- 266.Von Klitzing L. Do static magnetic fields of NMR influence biological signals? Clin Phys Physiol Meas Off J Hosp Phys Assoc Dtsch Ges Für Med Phys Eur Fed Organ Med Phys. 1986 May;7(2):157–60. doi: 10.1088/0143-0815/7/2/006. [DOI] [PubMed] [Google Scholar]
- 267.Innis NK, Ossenkopp KP, Prato FS, Sestini E. Behavioral effects of exposure to nuclear magnetic resonance imaging: II. Spatial memory tests. Magn Reson Imaging. 1986;4(4):281–4. doi: 10.1016/0730-725x(86)91037-4. [DOI] [PubMed] [Google Scholar]
- 268.Ossenkopp KP, Innis NK, Prato FS, Sestini E. Behavioral effects of exposure to nuclear magnetic resonance imaging: I. Open-field behavior and passive avoidance learning in rats. Magn Reson Imaging. 1986;4(4):275–80. doi: 10.1016/0730-725x(86)91036-2. [DOI] [PubMed] [Google Scholar]
- 269.Atkinson IC, Renteria L, Burd H, Pliskin NH, Thulborn KR. Safety of human MRI at static fields above the FDA 8 T guideline: sodium imaging at 9.4 T does not affect vital signs or cognitive ability. J Magn Reson Imaging JMRI. 2007 Nov;26(5):1222–7. doi: 10.1002/jmri.21150. [DOI] [PubMed] [Google Scholar]
- 270.Prasad N, Wright DA, Ford JJ, Thornby JI. Safety of 4-T MR imaging: study of effects on developing frog embryos. Radiology. 1990 Jan;174(1):251–3. doi: 10.1148/radiology.174.1.2294557. [DOI] [PubMed] [Google Scholar]
- 271.High WB, Sikora J, Ugurbil K, Garwood M. Subchronic in vivo effects of a high static magnetic field (9.4 T) in rats. J Magn Reson Imaging JMRI. 2000 Jul;12(1):122–39. doi: 10.1002/1522-2586(200007)12:1<122::aid-jmri14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 272.Valiron O, Peris L, Rikken G, Schweitzer A, Saoudi Y, Remy C, et al. Cellular disorders induced by high magnetic fields. J Magn Reson Imaging JMRI. 2005 Sep;22(3):334–40. doi: 10.1002/jmri.20398. [DOI] [PubMed] [Google Scholar]
- 273.Denegre JM, Valles JM, Lin K, Jordan WB, Mowry KL. Cleavage planes in frog eggs are altered by strong magnetic fields. Proc Natl Acad Sci U S A. 1998 Dec 8;95(25):14729–32. doi: 10.1073/pnas.95.25.14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Short WO, Goodwill L, Taylor CW, Job C, Arthur ME, Cress AE. Alteration of human tumor cell adhesion by high-strength static magnetic fields. Invest Radiol. 1992 Oct;27(10):836–40. doi: 10.1097/00004424-199210000-00014. [DOI] [PubMed] [Google Scholar]
- 275.Collins CM, Li S, Smith MB. SAR and B1 field distributions in a heterogeneous human head model within a birdcage coil. Specific energy absorption rate. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 1998 Dec;40(6):847–56. doi: 10.1002/mrm.1910400610. [DOI] [PubMed] [Google Scholar]
- 276.Collins CM, Liu W, Wang J, Gruetter R, Vaughan JT, Ugurbil K, et al. Temperature and SAR calculations for a human head within volume and surface coils at 64 and 300 MHz. J Magn Reson Imaging JMRI. 2004 May;19(5):650–6. doi: 10.1002/jmri.20041. [DOI] [PubMed] [Google Scholar]
- 277.Collins CM, Smith MB. Calculations of B(1) distribution, SNR, and SAR for a surface coil adjacent to an anatomically-accurate human body model. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2001 Apr;45(4):692–9. doi: 10.1002/mrm.1092. [DOI] [PubMed] [Google Scholar]
- 278.Turro NJ. Influence of nuclear spin on chemical reactions: Magnetic isotope and magnetic field effects (A Review) Proc Natl Acad Sci U S A. 1983 Jan;80(2):609–21. doi: 10.1073/pnas.80.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Dimbylow P. SAR in the mother and foetus for RF plane wave irradiation. Phys Med Biol. 2007 Jul 7;52(13):3791–802. doi: 10.1088/0031-9155/52/13/009. [DOI] [PubMed] [Google Scholar]
- 280.Hand JW, Li Y, Thomas EL, Rutherford MA, Hajnal JV. Prediction of specific absorption rate in mother and fetus associated with MRI examinations during pregnancy. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2006 Apr;55(4):883–93. doi: 10.1002/mrm.20824. [DOI] [PubMed] [Google Scholar]
- 281.Gowland PA, De Wilde J. Temperature increase in the fetus due to radio frequency exposure during magnetic resonance scanning. Phys Med Biol. 2008 Nov 7;53(21):L15–8. doi: 10.1088/0031-9155/53/21/L01. [DOI] [PubMed] [Google Scholar]
- 282.Kikuchi S, Saito K, Takahashi M, Ito K. Temperature elevation in the fetus from electromagnetic exposure during magnetic resonance imaging. Phys Med Biol. 2010 Apr 21;55(8):2411–26. doi: 10.1088/0031-9155/55/8/018. [DOI] [PubMed] [Google Scholar]
- 283.Dagang Wu, Shamsi S, Chen J, Kainz W. Evaluations of Specific Absorption Rate and Temperature Increase Within Pregnant Female Models in Magnetic Resonance Imaging Birdcage Coils. IEEE Trans Microw Theory Tech. 2006 Dec;54(12):4472–8. [Google Scholar]
- 284.Rodegerdts EA, Grönewäller EF, Kehlbach R, Roth P, Wiskirchen J, Gebert R, et al. In vitro evaluation of teratogenic effects by time-varying MR gradient fields on fetal human fibroblasts. J Magn Reson Imaging JMRI. 2000 Jul;12(1):150–6. doi: 10.1002/1522-2586(200007)12:1<150::aid-jmri16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 285.Brezinka C, Lechner T, Stephan K. [The fetus and noise] Gynäkol-Geburtshilfliche Rundsch. 1997;37(3):119–29. doi: 10.1159/000272841. [DOI] [PubMed] [Google Scholar]
- 286.American Academy of Pediatrics. Committee on Environmental Health Noise: a hazard for the fetus and newborn. Pediatrics. 1997 Oct;100(4):724–7. [PubMed] [Google Scholar]
- 287.Alibek S, Vogel M, Sun W, Winkler D, Baker CA, Burke M, et al. Acoustic noise reduction in MRI using Silent Scan: an initial experience. Diagn Interv Radiol Ank Turk. 2014 Aug;20(4):360–3. doi: 10.5152/dir.2014.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Katsunuma A, Takamori H, Sakakura Y, Hamamura Y, Ogo Y, Katayama R. Quiet MRI with novel acoustic noise reduction. Magma N Y N. 2002 Jan;13(3):139–44. doi: 10.1007/BF02678588. [DOI] [PubMed] [Google Scholar]
- 289.Whitby EH, Paley MN, Smith MF, Sprigg A, Woodhouse N, Griffiths PD. Low field strength magnetic resonance imaging of the neonatal brain. Arch Dis Child Fetal Neonatal Ed. 2003 May;88(3):F203–8. doi: 10.1136/fn.88.3.F203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Rademaker KJ, Uiterwaal CSPM, Beek FJA, van Haastert IC, Lieftink AF, Groenendaal F, et al. Neonatal cranial ultrasound versus MRI and neurodevelopmental outcome at school age in children born preterm. Arch Dis Child Fetal Neonatal Ed. 2005 Nov;90(6):F489–93. doi: 10.1136/adc.2005.073908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Childs AM, Cornette L, Ramenghi LA, Tanner SF, Arthur RJ, Martinez D, et al. Magnetic resonance and cranial ultrasound characteristics of periventricular white matter abnormalities in newborn infants. Clin Radiol. 2001 Aug;56(8):647–55. doi: 10.1053/crad.2001.0754. [DOI] [PubMed] [Google Scholar]
- 292.Leijser LM, Liauw L, Veen S, de Boer IP, Walther FJ, van Wezel-Meijler G. Comparing brain white matter on sequential cranial ultrasound and MRI in very preterm infants. Neuroradiology. 2008 Sep;50(9):799–811. doi: 10.1007/s00234-008-0408-4. [DOI] [PubMed] [Google Scholar]
- 293.O’Shea TM, Counsell SJ, Bartels DB, Dammann O. Magnetic resonance and ultrasound brain imaging in preterm infants. Early Hum Dev. 2005 Mar;81(3):263–71. doi: 10.1016/j.earlhumdev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 294.Miller SP, Cozzio CC, Goldstein RB, Ferriero DM, Partridge JC, Vigneron DB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR Am J Neuroradiol. 2003 Sep;24(8):1661–9. [PMC free article] [PubMed] [Google Scholar]
- 295.Hintz SR, O’Shea M. Neuroimaging and neurodevelopmental outcomes in preterm infants. Semin Perinatol. 2008 Feb;32(1):11–9. doi: 10.1053/j.semperi.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 296.Levene MI, Chervenak FA. Fetal and Neonatal Neurology and Neurosurgery. Elsevier Health Sciences; 2009. p. 940. [Google Scholar]
- 297.Mathur A, Inder T. Magnetic resonance imaging--insights into brain injury and outcomes in premature infants. J Commun Disord. 2009 Aug;42(4):248–55. doi: 10.1016/j.jcomdis.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Counsell SJ, Tranter SL, Rutherford MA. Magnetic resonance imaging of brain injury in the high-risk term infant. Semin Perinatol. 2010 Feb;34(1):67–78. doi: 10.1053/j.semperi.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 299.Glass HC, Bonifacio SL, Sullivan J, Rogers E, Ferriero DM, Goldstein R, et al. Magnetic resonance imaging and ultrasound injury in preterm infants with seizures. J Child Neurol. 2009 Sep;24(9):1105–11. doi: 10.1177/0883073809338328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Simbrunner J, Riccabona M. Imaging of the neonatal CNS. Eur J Radiol. 2006 Nov;60(2):133–51. doi: 10.1016/j.ejrad.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 301.Penrice J, Cady EB, Lorek A, Wylezinska M, Amess PN, Aldridge RF, et al. Proton magnetic resonance spectroscopy of the brain in normal preterm and term infants, and early changes after perinatal hypoxia-ischemia. Pediatr Res. 1996 Jul;40(1):6–14. doi: 10.1203/00006450-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 302.Hanrahan JD, Sargentoni J, Azzopardi D, Manji K, Cowan FM, Rutherford MA, et al. Cerebral metabolism within 18 hours of birth asphyxia: a proton magnetic resonance spectroscopy study. Pediatr Res. 1996 Apr;39(4 Pt 1):584–90. doi: 10.1203/00006450-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 303.Holshouser BA, Ashwal S, Luh GY, Shu S, Kahlon S, Auld KL, et al. Proton MR spectroscopy after acute central nervous system injury: outcome prediction in neonates, infants, and children. Radiology. 1997 Feb;202(2):487–96. doi: 10.1148/radiology.202.2.9015079. [DOI] [PubMed] [Google Scholar]
- 304.Groenendaal F, Veenhoven RH, van der Grond J, Jansen GH, Witkamp TD, de Vries LS. Cerebral lactate and N-acetyl-aspartate/choline ratios in asphyxiated full-term neonates demonstrated in vivo using proton magnetic resonance spectroscopy. Pediatr Res. 1994 Feb;35(2):148–51. doi: 10.1203/00006450-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 305.Hüppi PS, Posse S, Lazeyras F, Burri R, Bossi E, Herschkowitz N. Magnetic resonance in preterm and term newborns: 1H-spectroscopy in developing human brain. Pediatr Res. 1991 Dec;30(6):574–8. doi: 10.1203/00006450-199112000-00017. [DOI] [PubMed] [Google Scholar]
- 306.Cheong JLY, Cady EB, Penrice J, Wyatt JS, Cox IJ, Robertson NJ. Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury: metabolite peak-area ratios, relaxation times, and absolute concentrations. AJNR Am J Neuroradiol. 2006 Aug;27(7):1546–54. [PMC free article] [PubMed] [Google Scholar]
- 307.Moorcraft J, Bolas NM, Ives NK, Sutton P, Blackledge MJ, Rajagopalan B, et al. Spatially localized magnetic resonance spectroscopy of the brains of normal and asphyxiated newborns. Pediatrics. 1991 Mar;87(3):273–82. [PubMed] [Google Scholar]
- 308.Peden CJ, Cowan FM, Bryant DJ, Sargentoni J, Cox IJ, Menon DK, et al. Proton MR spectroscopy of the brain in infants. J Comput Assist Tomogr. 1990 Dec;14(6):886–94. doi: 10.1097/00004728-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 309.Barkovich AJ, Baranski K, Vigneron D, Partridge JC, Hallam DK, Hajnal BL, et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol. 1999 Sep;20(8):1399–405. [PMC free article] [PubMed] [Google Scholar]
- 310.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 1993 Oct;30(4):424–37. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 311.Barkovich AJ, Westmark KD, Bedi HS, Partridge JC, Ferriero DM, Vigneron DB. Proton spectroscopy and diffusion imaging on the first day of life after perinatal asphyxia: preliminary report. AJNR Am J Neuroradiol. 2001 Oct;22(9):1786–94. [PMC free article] [PubMed] [Google Scholar]
- 312.Vigneron DB, Barkovich AJ, Noworolski SM, von dem Bussche M, Henry RG, Lu Y, et al. Three-dimensional proton MR spectroscopic imaging of premature and term neonates. AJNR Am J Neuroradiol. 2001 Aug;22(7):1424–33. [PMC free article] [PubMed] [Google Scholar]
- 313.Grant PE, Yu D. Acute injury to the immature brain with hypoxia with or without hypoperfusion. Radiol Clin North Am. 2006 Jan;44(1):63–77. viii. doi: 10.1016/j.rcl.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 314.Counsell SJ, Allsop JM, Harrison MC, Larkman DJ, Kennea NL, Kapellou O, et al. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003 Jul;112(1 Pt 1):1–7. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- 315.Counsell SJ, Rutherford MA. Magnetic resonance imaging of the newborn brain. Curr Paediatr. 2002 Oct;12(5):401–13. [Google Scholar]
- 316.Rutherford M, Srinivasan L, Dyet L, Ward P, Allsop J, Counsell S, et al. Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol. 2006 Jul;36(7):582–92. doi: 10.1007/s00247-006-0164-8. [DOI] [PubMed] [Google Scholar]
- 317.Sagar P, Grant PE. Diffusion-weighted MR imaging: pediatric clinical applications. Neuroimaging Clin N Am. 2006 Feb;16(1):45–74. viii. doi: 10.1016/j.nic.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 318.Volpe JJ. Neurology of the Newborn. 5 edition Saunders; 2008. p. 1120. [Google Scholar]
- 319.Hart A, Whitby E, Wilkinson S, Alladi S, Paley M, Smith M. Neuro-developmental outcome at 18 months in premature infants with diffuse excessive high signal intensity on MR imaging of the brain. Pediatr Radiol. 2011 Oct;41(10):1284–92. doi: 10.1007/s00247-011-2155-7. [DOI] [PubMed] [Google Scholar]
- 320.Jeon TY, Kim JH, Yoo S-Y, Eo H, Kwon J-Y, Lee J, et al. Neurodevelopmental outcomes in preterm infants: comparison of infants with and without diffuse excessive high signal intensity on MR images at near-term-equivalent age. Radiology. 2012 May;263(2):518–26. doi: 10.1148/radiol.12111615. [DOI] [PubMed] [Google Scholar]
- 321.Erberich SG, Friedlich P, Seri I, Nelson MD, Blüml S. Functional MRI in neonates using neonatal head coil and MR compatible incubator. NeuroImage. 2003 Oct;20(2):683–92. doi: 10.1016/S1053-8119(03)00370-7. [DOI] [PubMed] [Google Scholar]
- 322.Shu SK, Ashwal S, Holshouser BA, Nystrom G, Hinshaw DB. Prognostic value of 1H-MRS in perinatal CNS insults. Pediatr Neurol. 1997 Nov;17(4):309–18. doi: 10.1016/s0887-8994(97)00140-9. [DOI] [PubMed] [Google Scholar]
- 323.Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003 Jul;112(1 Pt 1):176–80. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 324.Inder T, Huppi PS, Zientara GP, Maier SE, Jolesz FA, di Salvo D, et al. Early detection of periventricular leukomalacia by diffusion-weighted magnetic resonance imaging techniques. J Pediatr. 1999 May;134(5):631–4. doi: 10.1016/s0022-3476(99)70251-9. [DOI] [PubMed] [Google Scholar]
- 325.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003 Aug;143(2):171–9. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 326.Maalouf EF, Duggan PJ, Rutherford MA, Counsell SJ, Fletcher AM, Battin M, et al. Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J Pediatr. 1999 Sep;135(3):351–7. doi: 10.1016/s0022-3476(99)70133-2. [DOI] [PubMed] [Google Scholar]
- 327.Plaisier A, Govaert P, Lequin MH, Dudink J. Optimal timing of cerebral MRI in preterm infants to predict long-term neurodevelopmental outcome: a systematic review. AJNR Am J Neuroradiol. 2014 May;35(5):841–7. doi: 10.3174/ajnr.A3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dyet LE, Kennea N, Counsell SJ, Maalouf EF, Ajayi-Obe M, Duggan PJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006 Aug;118(2):536–48. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 329.Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes . In: Preterm Birth: Causes, Consequences, and Prevention [Internet] Behrman RE, Butler AS, editors. National Academies Press (US); Washington (DC): 2007. [cited 2014 Nov 5]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK11362/ [PubMed] [Google Scholar]
- 330.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005 Apr;115(4):997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 331.Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol. 2008 Apr;21(2):123–8. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- 332.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009 Jan;8(1):110–24. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR, EPICure Study Group Neurologic and developmental disability after extremely preterm birth. N Engl J Med. 2000 Aug 10;343(6):378–84. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 334.Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol. 2006 Apr;30(2):81–8. doi: 10.1053/j.semperi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 335.Banker BQ, Larroche JC. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962 Nov;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- 336.Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12(2):129–40. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 337.Hack M, Taylor H. Perinatal brain injury in preterm infants and later neurobehavioral function. JAMA. 2000 Oct 18;284(15):1973–4. doi: 10.1001/jama.284.15.1973. [DOI] [PubMed] [Google Scholar]
- 338.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990’s. Early Hum Dev. 1999 Jan;53(3):193–218. doi: 10.1016/s0378-3782(98)00052-8. [DOI] [PubMed] [Google Scholar]
- 339.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol SN. 2000 May;5(2):89–106. doi: 10.1053/siny.1999.0001. [DOI] [PubMed] [Google Scholar]
- 340.Uggetti C, Egitto MG, Fazzi E, Bianchi PE, Bergamaschi R, Zappoli F, et al. Cerebral visual impairment in periventricular leukomalacia: MR correlation. AJNR Am J Neuroradiol. 1996 May;17(5):979–85. [PMC free article] [PubMed] [Google Scholar]
- 341.Jacobson L, Ek U, Fernell E, Flodmark O, Broberger U. Visual impairment in preterm children with periventricular leukomalacia--visual, cognitive and neuropaediatric characteristics related to cerebral imaging. Dev Med Child Neurol. 1996 Aug;38(8):724–35. doi: 10.1111/j.1469-8749.1996.tb12142.x. [DOI] [PubMed] [Google Scholar]
- 342.Mirmiran M, Barnes PD, Keller K, Constantinou JC, Fleisher BE, Hintz SR, et al. Neonatal brain magnetic resonance imaging before discharge is better than serial cranial ultrasound in predicting cerebral palsy in very low birth weight preterm infants. Pediatrics. 2004 Oct;114(4):992–8. doi: 10.1542/peds.2003-0772-L. [DOI] [PubMed] [Google Scholar]
- 343.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006 Aug 17;355(7):685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 344.Van Wezel-Meijler G, Leijser LM, de Bruïne FT, Steggerda SJ, van der Grond J, Walther FJ. Magnetic resonance imaging of the brain in newborn infants: practical aspects. Early Hum Dev. 2009 Feb;85(2):85–92. doi: 10.1016/j.earlhumdev.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 345.American College of Radiolgy. American Society of Neuroradiology and Society for Pediatric Radiology . ACR-ASNR-SPR Practice Parameter for the Performance and Interpretation of Magnetic Resonance Imaging (MRI) of the Brain, Resolution (39) [Internet] American College of Radiology; 2014. [cited 2014 Sep 18]. Available from: http://www.acr.org/~/media/E712C7C597AE44D8B1765D5ED5086086.pdf. [Google Scholar]
- 346.Benavente-Fernández I, Lubián-López PS, Zuazo-Ojeda MA, Jiménez-Gómez G, Lechuga-Sancho AM. Safety of magnetic resonance imaging in preterm infants. Acta Paediatr Oslo Nor 1992. 2010 Jun;99(6):850–3. doi: 10.1111/j.1651-2227.2010.01708.x. [DOI] [PubMed] [Google Scholar]
- 347.Merchant N, Groves A, Larkman DJ, Counsell SJ, Thomson MA, Doria V, et al. A patient care system for early 3.0 Tesla magnetic resonance imaging of very low birth weight infants. Early Hum Dev. 2009 Dec;85(12):779–83. doi: 10.1016/j.earlhumdev.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 348.Groenendaal F, Leusink C, Nijenhuis M, Janssen MJH. Neonatal life support during magnetic resonance imaging. J Med Eng Technol. 2002 Apr;26(2):71–4. doi: 10.1080/03091900210127915. [DOI] [PubMed] [Google Scholar]
- 349.Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008 Mar;38(3):260–4. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- 350.Counsell SJ, Rutherford MA, Cowan FM, Edwards AD. Magnetic resonance imaging of preterm brain injury. Arch Dis Child Fetal Neonatal Ed. 2003 Jul;88(4):F269–74. doi: 10.1136/fn.88.4.F269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Debillon T, N’Guyen S, Muet A, Quere MP, Moussaly F, Roze JC. Limitations of ultrasonography for diagnosing white matter damage in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2003 Jul;88(4):F275–9. doi: 10.1136/fn.88.4.F275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Nongena P, Ederies A, Azzopardi DV, Edwards AD. Confidence in the prediction of neurodevelopmental outcome by cranial ultrasound and MRI in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2010 Nov;95(6):F388–90. doi: 10.1136/adc.2009.168997. [DOI] [PubMed] [Google Scholar]
- 353.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004 Nov 4;351(19):1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 354.Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol. 2003 May;24(5):805–9. [PMC free article] [PubMed] [Google Scholar]
- 355.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008 Mar;93(2):F153–61. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Battin M, Maalouf EF, Counsell S, Herlihy A, Hall A, Azzopardi D, et al. Physiological stability of preterm infants during magnetic resonance imaging. Early Hum Dev. 1998 Sep;52(2):101–10. doi: 10.1016/s0378-3782(98)00024-3. [DOI] [PubMed] [Google Scholar]
- 357.Taber KH, Hayman LA, Northrup SR, Maturi L. Vital sign changes during infant magnetic resonance examinations. J Magn Reson Imaging JMRI. 1998 Dec;8(6):1252–6. doi: 10.1002/jmri.1880080612. [DOI] [PubMed] [Google Scholar]
- 358.Philbin MK, Taber KH, Hayman LA. Preliminary report: changes in vital signs of term newborns during MR. AJNR Am J Neuroradiol. 1996 Jul;17(6):1033–6. [PMC free article] [PubMed] [Google Scholar]
- 359.Plaisier A, Raets MMA, van der Starre C, Feijen-Roon M, Govaert P, Lequin MH, et al. Safety of routine early MRI in preterm infants. Pediatr Radiol. 2012 Oct;42(10):1205–11. doi: 10.1007/s00247-012-2426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci Off J Soc Neurosci. 2003 Feb 1;23(3):876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Allegaert K, Naulaers G. Procedural sedation of neonates with chloral hydrate: a sedation procedure does not end at the end of the acquisition of the images. Paediatr Anaesth. 2008 Dec;18(12):1270–1. doi: 10.1111/j.1460-9592.2008.02792.x. [DOI] [PubMed] [Google Scholar]
- 362.Coté CJ. Safety after chloral hydrate sedation of former preterm and term infants for magnetic resonance imaging: are the data clear? Anesth Analg. 2010 Mar 1;110(3):671–3. doi: 10.1213/ANE.0b013e3181cd4428. [DOI] [PubMed] [Google Scholar]
- 363.Litman RS, Soin K, Salam A. Chloral hydrate sedation in term and preterm infants: an analysis of efficacy and complications. Anesth Analg. 2010 Mar 1;110(3):739–46. doi: 10.1213/ANE.0b013e3181ca12a8. [DOI] [PubMed] [Google Scholar]
- 364.Allegaert K, Daniels H, Naulaers G, Tibboel D, Devlieger H. Pharmacodynamics of chloral hydrate in former preterm infants. Eur J Pediatr. 2005 Jul;164(7):403–7. doi: 10.1007/s00431-005-1648-5. [DOI] [PubMed] [Google Scholar]
- 365.Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007 Mar;104(3):509–20. doi: 10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- 366.Windram J, Grosse-Wortmann L, Shariat M, Greer M-L, Crawford MW, Yoo S-J. Cardiovascular MRI without sedation or general anesthesia using a feed-and-sleep technique in neonates and infants. Pediatr Radiol. 2012 Feb;42(2):183–7. doi: 10.1007/s00247-011-2219-8. [DOI] [PubMed] [Google Scholar]
- 367.Haney B, Reavey D, Atchison L, Poull J, Dryer L, Anderson B, et al. Magnetic resonance imaging studies without sedation in the neonatal intensive care unit: safe and efficient. J Perinat Neonatal Nurs. 2010 Sep;24(3):256–66. doi: 10.1097/JPN.0b013e3181e8d566. [DOI] [PubMed] [Google Scholar]
- 368.Graven SN, Browne JV. Sensory Development in the Fetus, Neonate, and Infant: Introduction and Overview. Newborn Infant Nurs Rev. 2008 Dec;8(4):169–72. [Google Scholar]
- 369.Graven SN, Browne JV. Auditory Development in the Fetus and Infant. Newborn Infant Nurs Rev. 2008 Dec;8(4):187–93. [Google Scholar]
- 370.Berger EH. Attenuation of earplugs worn in combination with earmuffs. Occup Health Saf Waco Tex. 1984 May;:72–3. [PubMed] [Google Scholar]
- 371.Witt B, Bacou-Dalloz Hearing Safety Group Sound Source Dual Protection [Internet] 2007 [cited 2014 Sep 20]. Available from: http://www.howardleight.com/images/pdf/0000/0270/Sound_Source_11a_Dual_Protection.pdf.
- 372.Berger EH. Laboratory Attenuation of Earmuffs and Earplugs Both Singly and in Combination. Am Ind Hyg Assoc J. 1983 May 1;44(5):321–9. [Google Scholar]