Abstract
The human prefrontal cortex, important for executive functions, loses gray matter throughout the adolescent period. In rats, our laboratory demonstrated that a loss of neurons between adolescence and adulthood partially underlies the loss of volume, and this loss is greater in females than males. Here, we examine whether being deprived of gonadal hormones before puberty through adulthood influences the number of neurons in the medial prefrontal cortex (mPFC). Prior to puberty, the testes or ovaries were removed in male and female rats. In adulthood, the number of neurons and glia in the mPFC were quantified using unbiased stereology, and the volume of the frontal white matter was measured. Prepubertal ovariectomy resulted in a higher number of neurons and glia and a larger volume of white matter compared to sham control littermates. Castrated males were not different from sham males on any measure. Thus ovarian hormones secreted after puberty influence the cellular composition of the medial prefrontal cortex.
Keywords: puberty, prefrontal cortex, adolescence, ovariectomy, gonadectomy
Human structural magnetic resonance imaging (MRI) studies indicate that the prefrontal cortex is not fully developed until early adulthood. MRI studies indicate that the volume and thickness of this area increases throughout childhood until adolescence, after which time the volume steadily declines until approximately 20 years of age (Jernigan, Trauner, Hesselink, & Tallal, 1991; Lenroot & Giedd, 2006). These data also indicate a sex difference in which the peak in volume is later in boys than girls (Lenroot & Giedd, 2006). Previous work from our laboratory demonstrates a similar pattern of development in the rat medial prefrontal cortex (mPFC) (Markham, Morris, & Juraska, 2007), a structure homologous to the human PFC (Uylings, Groenewegen, & Kolb, 2003). Here, a significant loss of volume occurs between postnatal day (P) 35 (around puberty) and P90 (adulthood). A decrease in the number of neurons, which is greater in females than males, is a major contributor to the decrease in volume between these ages (Markham et al., 2007). These studies suggest a potential role for postpubertal hormones in the maturation of the adolescent prefrontal cortex.
Studies in other areas of the cortex indicate a role for ovarian hormones after puberty. In the visual cortex, prepubertal loss of ovarian hormones results in a greater number of neurons in adulthood in females, while loss of the testes before puberty is without effect (Nuñez, Sodhi, & Juraska, 2002). This suggests that the presence of ovarian hormones at or after puberty eliminates neurons in the visual cortex through apoptosis, comparable to the effects of estrogen, from aromatized testosterone, in the early development of many subcortical structures (Forger, 2009; MacLusky, Walters, Clark, & Toran-Allerand, 1994).
Ovarian hormones secreted after puberty are implicated in the shaping of other neural structures and neuroanatomical measures. The pruning of dendritic spines during adolescence in the visual cortex is influenced by ovarian hormones in female rats (Munoz-Cueto, Garcia-Segura, & Ruiz-Marcos, 1990), as is the number of axons that are myelinated in the splenium of the corpus callosum (Yates & Juraska, 2008). In the hypothalamic anteroventral periventricular nucleus (AVPV), where females have more neurons than males, the addition of new cells seen in adolescence is reliant on ovarian hormones (Ahmed et al., 2008). However, not all hormone-dependent anatomical changes occurring after puberty are due to interactions with the ovarian hormones. Dendritic losses in the adolescent male hippocampus are eliminated following castration of males before puberty (Meyer, Ferres-Torres, & Mas, 1978), as are increases of cell proliferation in the medial amygdala (Ahmed et al., 2008). Likewise in the medial amygdala, the pre-pubertal removal of testes alters the volume and the number of neurons in the Syrian hamster (De Lorme, Schulz, Salas-Ramirez, & Sisk, 2012) and number of dendritic spines in the rat (Cooke & Woolley, 2009).
The present study investigated the potential role of the hormones that are secreted starting at puberty on the number of neurons and glia in adulthood in the rat mPFC. We hypothesized that the mPFC is similar to the visual cortex where the loss of ovarian hormones before puberty results in a lower number of neurons in adulthood, while the loss of testicular hormones has no effect. This hypothesis is tested by the removal of the gonads before puberty in male and female rats and comparing the number of glia and neurons, as well as the volume of the frontal white matter, with sham-operated littermates of the same sex in adulthood.
Methods
Subjects
Subjects were the offspring of Long-Evans rats obtained from Harlan (Indianapolis, IN) and bred in our vivarium. They were maintained on a 12:12-h light-dark cycle with free access to food and water. Surgeries were performed on animals at P20-22, with P0 being the day of birth. Gonadectomies and sham surgeries were performed on litter-mate pairs of same-sex animals, resulting in 9 castrated males, 9 sham-operated males, 12 ovariectomized females and 12 sham-operated females. Each pair of same-sex animals was from the same litter, but the male and female animals were not all from the same litters. Subjects came from two cohorts of rats, born 2 months apart, from different breeding pairs. Weaning occurred at P24 and rats were pair housed with their same-sex littermate. All animal procedures were in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Urbana-Champaign.
Gonadectomy
Surgeries were performed similar to those done previously in our laboratory (Nuñez et al., 2002; Yates & Juraska, 2008). On P20-22 each litter was separated from their mother and temporarily housed in a separate cage. Using 2.75% isoflurane gas anesthesia, each gonadectomy and sham surgery was completed with an average duration period of 45 minutes. Both ovariectomies and castrations were performed with a ventral incision in the lower abdominal area through the skin and then another through the muscle wall, after which testes or ovaries were located on each side of the rat, tied off with absorbable surgical thread and removed. The muscle incision and skin incision were closed using sutures and animals were then placed in a separate cage until fully recovered from anesthesia before being placed back with their mothers. A skin incision was also performed on sham animals during which the duration of isoflurane exposure was equal for each sex- and litter-matched pair. During surgeries, all pups were removed from their mothers for a maximum of 6 hours. Post-surgical checks were performed daily for 7 days, which involved a small amount of handling. Sutures were removed 7 days after surgery; however most sutures fell out freely.
Puberty Markers
Starting at P28, rats were handled, weighed and examined for the appearance of puberty markers daily until the markers were apparent. The puberty marker used in females was vaginal opening, which has been demonstrated to coincide with increased estrogen levels and the beginning of LH surges from the pituitary (Castellano et al., 2011). In males, preputial separation was used as a marker of puberty because it has been correlated with the beginning of significant androgen secretion (Korenbrot, Huhtaniemi, & Weiner, 1977). Vaginal opening and preputial separation never occurred in ovariectomized (OVX) females and castrated (CST) males. In sham-operated animals, vaginal opening occurred between P33 and P39 and preputial separation occurred between P40 and P45 except for one male where puberty onset was at P51.
Histology
At P90-91, rats were weighed, injected (i.p.) with 100 mg/kg sodium pentobarbital and perfused intracardially with 0.1 M phosphate buffer saline (PBS) followed by 4% paraformaldehyde in PBS. Brains were removed, weighed and stored in 4% paraformaldehyde solution overnight, then transferred to a 30% sucrose solution for 4 days until sectioning. Using a freezing microtome, 60 micron sections were cut, and every 4th section was mounted on slides then stained with Methylene Blue/Azure II the following day.
Cell number quantification
Parcellations of the ventral mPFC (infralimbic and prelimbic regions) were performed based on differences in cytoarchitecture as previously described (Koss, Sadowski, Sherrill, Gulley, & Juraska, 2012; Markham et al., 2007) (Fig.1a). The boundaries of the mPFC were parcellated on every 4th section beginning at the most anterior mounted section containing frontal white matter and ending at the appearance of the genu of the corpus callosum. This resulted in the analysis of 4–6 sections per brain. Within each section, dorsal and ventral boundaries were drawn as well as boundaries for cortical layers 2/3 and layers 5/6 (the rat mPFC does not have a layer 4). Layer 1 was not included because it contains very few neurons. Volumes were calculated using the Cavalieri method, as the product of the areas of each section, the post-shrinkage tissue thickness and the number of sections between each saved section. Due to dissimilarities in the amount of cellular loss between the sexes in the different layers of the mPFC found by Markham et al. (2007), all measurements were calculated separately for the whole mPFC as well as for the division into the upper layers (layers 2/3) and lower layers (layers 5/6).
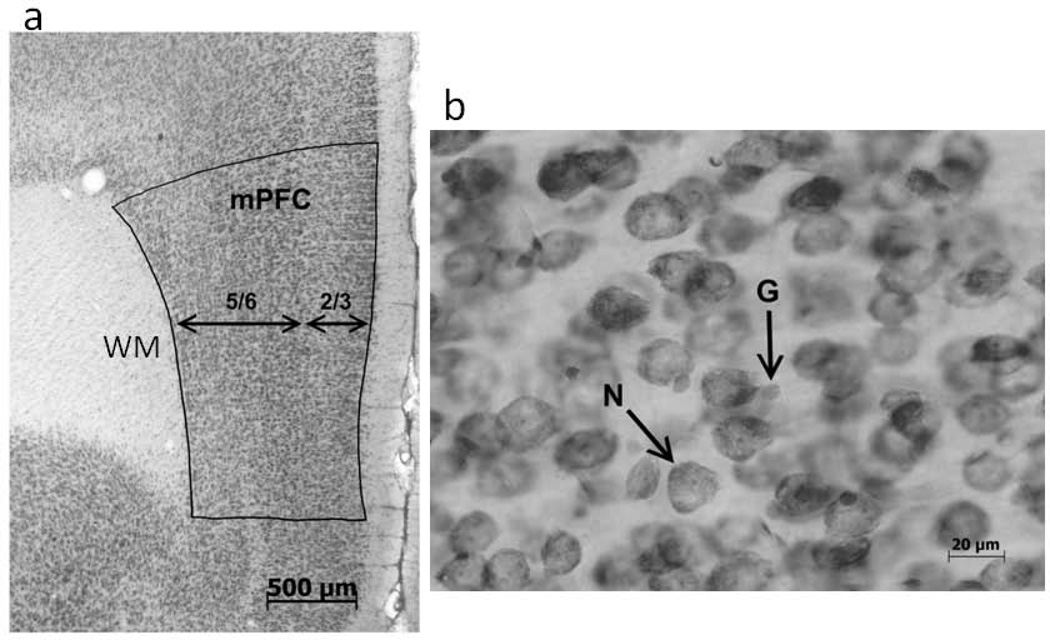
Figure 1.
The anatomical methods for counting cells. (a) A photograph illustrating the parcellation of the mPFC and the division into layers which is necessary for the calculation of volume. The white matter (WM) under the cortex is also labeled. (b) A photograph showing a neuron (N) and glial cell (G) under high magnification used for counting. (From Koss et al., 2012).
The density of neurons and glia was measured using the optical disector with the Stereoinvestigator program (Microbrightfield, Williston, VT), as previously described (Koss et al., 2012; Markham et al., 2007). Cell counts were taken from both hemispheres throughout the entire anterior-posterior extent of the mPFC. The counting frame was 35µm × 35µm × 15µm (length × width × height) with 1.5µm guard zones. Neurons and glia were separately counted and were distinguished based on morphology, size, and color as in previous work in our laboratory (e.g., Koss et al., 2012; Rubinow & Juraska, 2009) and in others (Pelvig, Pakkenberg, Stark & Pakkenberg, 2008) (Fig. 1b). At least 200 neurons and 100 glia were counted from each of the upper and lower layers for each subject. These numbers were divided by the volume of the counting frames to determine density and then multiplied by the volume of the structure to determine the total number of neurons and glia for each animal.
Volume of the Frontal White Matter
The volume of the frontal white matter was quantified as an indirect indictor of myelination. The white matter was traced with a camera lucida in every 4th section beginning at the most anterior mounted section containing frontal white matter and ending at the appearance of the genu of the corpus callosum. As in the cortical parcellation described above, volume was calculated using the Cavalieri method, as the product of the areas of each section, the post-shrinkage tissue thickness and the number of sections between each saved section.
Statistical Analyses
Pre-planned paired t-tests were performed on littermate pairs of sham control and gonadectomy (GDX) animals of the same sex. Opposite sex animals, often from different litters, were not compared because this was not the focus of the study.
Results
Body and Brain Weights
Body weight was altered by gonadectomy in both sexes. Control males weighed more than castrated males [t(10) = 15.02, p<0.0001], and control females weighed less than ovariectomized female rats [t(11) = 15.07, p<0.0001] (Table 1). These effects have been previously reported from our laboratory (Nuñez et al., 2002; Sherrill, Koss, Foreman, & Gulley, 2011; Yates & Juraska, 2008) and others (Slob & Van der Werff Ten Bosch, 1975; Wade, 1975).
Table 1.
Body and Brain Weights
| Male Sham | Male GDX | Female Sham | Females GDX | |
|---|---|---|---|---|
| Body Weight (mean grams±SEM) | 456.2 ± 10. 9 | 374.6 ± 7. 5* | 251.9 ± 4. 8 | 344.3 ± 6. 1* |
| Brain Weight (mean grams±SEM) | 1.90 ± 0. 03 | 1.91 ± 0. 02 | 1.74 ± 0. 03 | 1.86 ± 0. 03* |
significantly different from the sham control of the same sex
There were no differences in brain weight between the male groups, while control females had lower brain weights (6%) than OVX [t(11) = 5.803, p<0.05] (Table 1). This difference had appeared in prior studies from our laboratory but had not reached significance (Nuñez et al., 2002; Yates & Juraska, 2008).
Cell Number
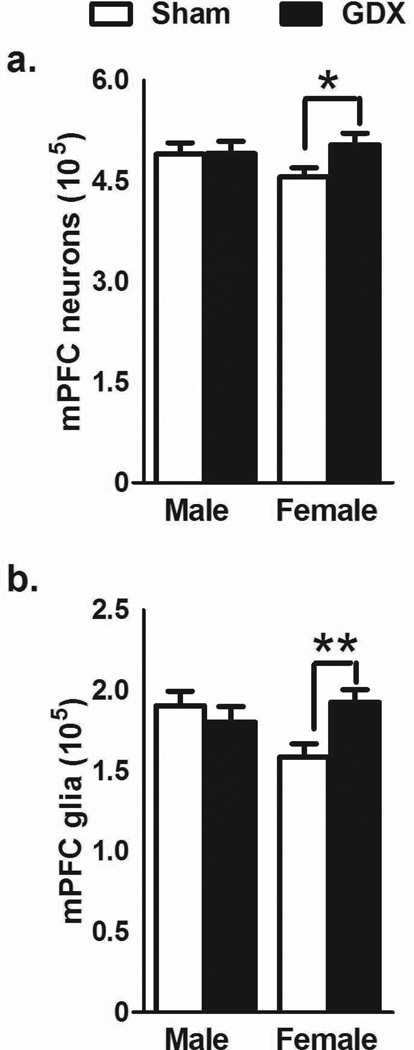
In the whole mPFC (all cellular layers), OVX females had a larger number of neurons [t(11) = 2.238, p<0.05; Fig. 2a] and more glia [t(11) = 4.035, p<0.01; Fig. 2b] than control females. Castrated males were not significantly different than control males on any measure.
Figure 2.
The number of neurons (a) and glia (b) (mean±SEM) in the mPFC. Significant differences were found between OVX females and sham control females in the number of both neurons and glia. No differences were found between GDX males and control males. *p<.05; **p<.01
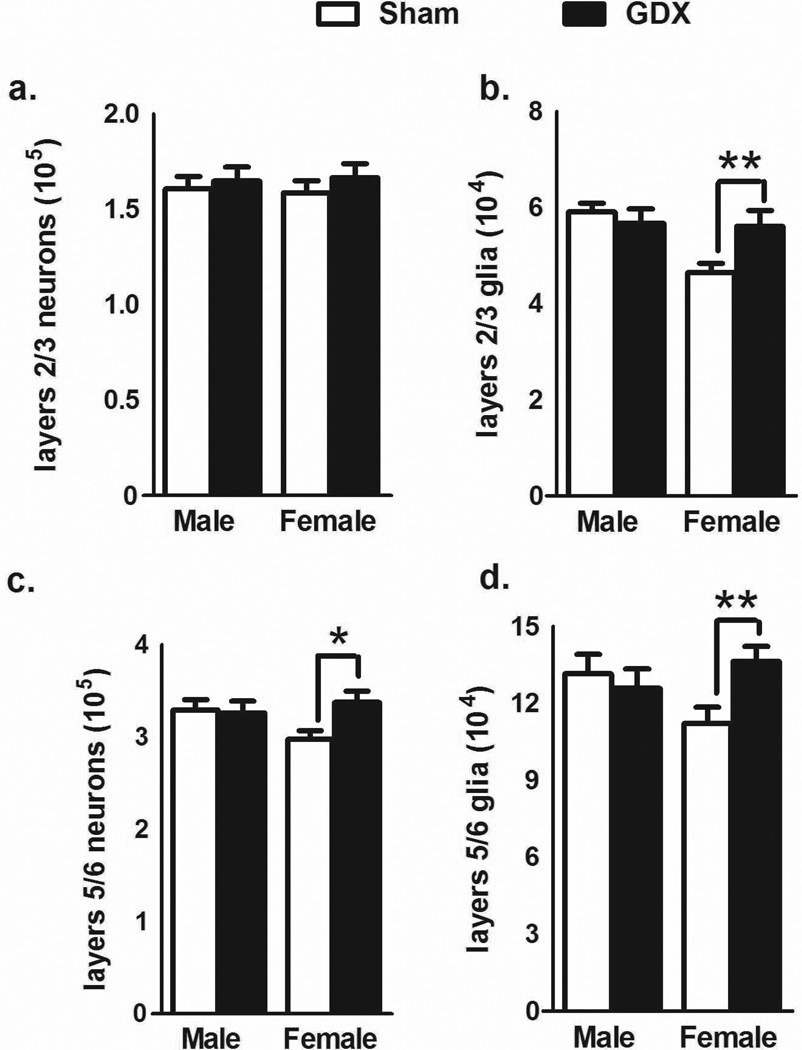
The upper (2/3) and lower (5/6) layers were also analyzed separately. GDX did not significantly alter the number of neurons in either sex in layers 2/3 (Fig. 3a). However, there was a significant effect of OVX on the number of glia in the females[t(11) = 3.913, p<0.003] while there were no differences due to GDX in males (Fig. 3b). In layers 5/6, the number of neurons [t(11) = 2.583, p<0.03; Fig. 3c] and glia [t(11) = 3.423, p<0.006; Fig. 3d] were significantly higher in OVX females than in control females. There were no differences in males.
Figure 3.
The number of neurons and glia (mean±SEM) in the upper and lower layers of the mPFC. In the upper layers, no change in the number of neurons was found after male or female gonadectomy (a), but there was a significant difference in the number of glia between OVX and sham control females (b). In the lower layers, the number of both neurons (c) and glia (d) were significantly different between OVX and sham control females. GDX males were not affected by castration. *p<.05; **p<.01
Volume of the Frontal White Matter
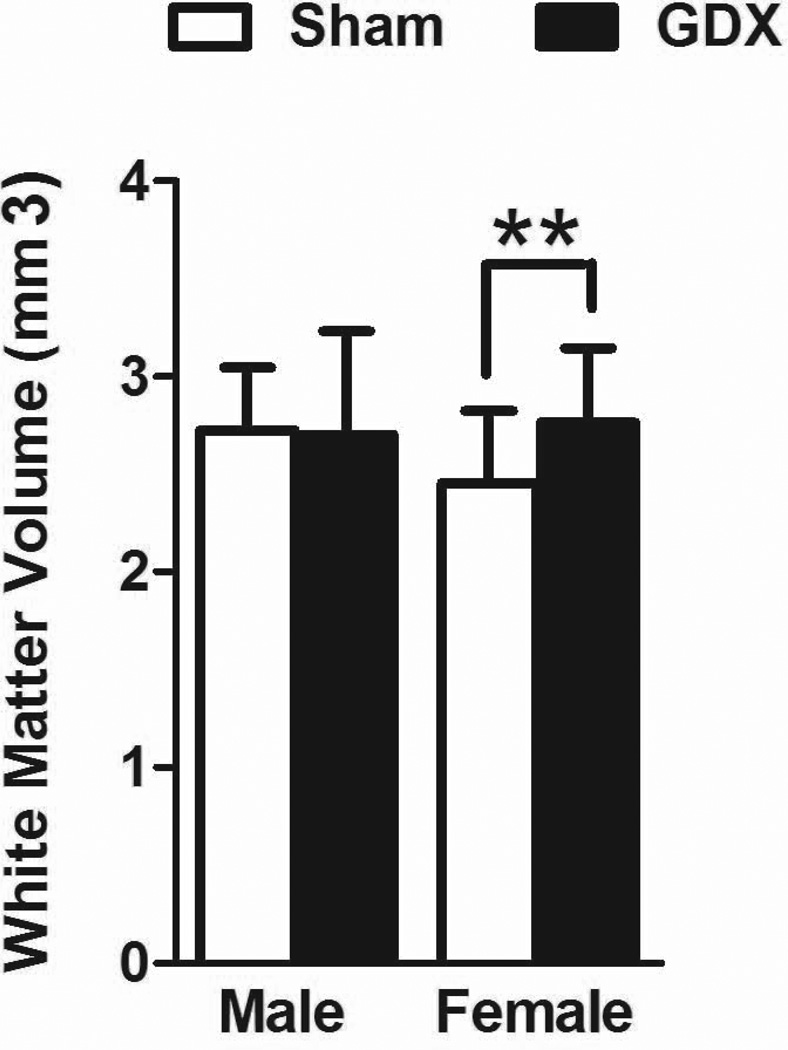
The volume of the frontal white matter was significantly larger in OVX, compared to control females [t(11) = 3.408, p = .006; Fig 4]. No differences were seen between castrated and control males.
Figure 4.
The volume (mean±SEM) of the frontal white matter. OVX females had a larger volume than control females. No significant differences were found between castrated and control males. **p<.01
Discussion
In the present study, females that had their ovaries removed before puberty had more neurons in the mPFC compared to controls. In contrast, no anatomical differences were found between prepubertal castrated and control males. These results support a major role for ovarian hormones secreted after puberty in the structure of the female mPFC. This is further supported by preliminary data from our laboratory that there is a significant drop in the number of neurons in the adolescent mPFC between P35 and P45 in intact, cycling female rats. The number of neurons then does not change between P45 and P60 and P90 (Willing, Kim, Brodsky, Cortes & Juraska, 2014). The most parsimonious explanation is that the loss of neurons that occurs in intact females at some time after puberty did not occur, or was decreased in magnitude, in the ovariectomized females. These results are similar to the role of ovarian hormones in the visual cortex, where removal of the ovaries, but not the testes, before puberty results in more neurons in the adult visual cortex (Nuñez et al., 2002).
One question that we did not directly addressed here is whether the addition of ovarian steroids to the ovariectomized females at a later time, such as adulthood, would result in a decreased number of neurons in the mPFC. In other words, the specificity of the effect for the pubertal period was not proven. The present data in combination with the loss of neurons between P35 and 45 in Willing et al. (2014) indicates that neurons can be lost during puberty but whether the cortex retains this property beyond adolescence is unknown. We do know the ovariectomies at middle age (12–13 mo.) did not result in a change in the number of neurons in the mPFC regardless of subsequent ovarian hormone replacement status (Chisholm, Packard, Koss & Juraska, 2012); however, these female rats presumably would have undergone a loss of neurons earlier when they were intact.
The number of glia was also higher following prepubertal ovariectomy, the same pattern as neurons. This is consistent with Markham et al. (2007) in which the lack of a sex difference in glial number in the mPFC at the beginning of puberty changed to adult females having fewer glia than males in adulthood. However unlike cortical neurons (Ehninger & Kempermann, 200), both astrocytes and oligodendrocytes continue to proliferate at high rates after development (Lee, Mayer-Proschel, & Rao, 2000). Thus it is not known whether ovarian hormones are attenuating proliferation or inducing apoptosis in glia. Whether one or several types of glia (e.g., astrocytes, microglia) are affected also remains to be investigated.
The volume of the white matter underlying the frontal cortex was also found to be sensitive to ovarian hormones: the volume was larger in the ovariectomized females. This is consistent with a previous electron microscopic study from our laboratory in a different portion of the cortical while matter. Yates and Juraska (2008) found that the number of myelinated axons in the splenium (posterior) of the corpus callosum was higher in adult females that had been ovariectomized before puberty, even while the total number of axons was unchanged. Although myelinated axons were not directly measured in the current study, it is plausible that ovarian hormones are slowing myelination in the frontal white matter.
Which particular ovarian hormone causes the loss of neurons and glia in the mPFC is unknown. The potential role of progesterone in producing cell death, alone or in concert with estrogen, has not been studied in adolescence or adulthood. In fact, progesterone and its metabolites are associated with neuroprotection in development and adulthood (Deutsch et al., 2013; Irwin & Brinton, 2014; Mellon, 2007). However, the ovariectomies here occurred after progesterone receptors have peaked in the cortex at P14 when receptors are falling to low levels by P27 and adulthood (Lopez & Wagner, 2009), making it unlikely that the changes are progesterone driven. There is, however, considerable evidence for estrogen-induced apoptosis. In early rat development, estrogen, from aromatized testosterone, induces apoptosis in some neural regions while concurrently saving cells in other nuclei (Arai, Sekine, & Murakami, 1996; Forger, 2009). The mechanism behind estrogen-induced death in early development has been linked to estrogen acting on cellular proteins in the apoptotic pathway (Arai et al., 1996; Forger, 2009), which could also occur after puberty.
The lack of a detectable effect of prepubertal ovariectomy on the neurons in the upper layers of the cortex is consistent with our prior findings where neuron loss between adolescence and adulthood is restricted to females in the lower layers while both sexes lose neurons in the upper layers of the mPFC (Markham et al., 2007). This indicates that cell elimination in the upper layers may be due to a different mechanism that is not influenced by pubertal hormones. Causes of such lamina-specific development are unknown, but estrogen receptors (ER), especially ERβ, are localized to the deep layers of the cortex (Shughrue, Lane, & Merchenthaler, 1997) suggesting a possible mechanism for hormone-dependent laminar differences. Laminar differences also occur in the growth and pruning of the dendritic tree between adolescence and adulthood (Koss, Belden, Hristov, & Juraska, 2014; Markham, Mullins, & Koenig, 2012). However, glia were affected in all layers of the cortex, which could suggest that ovarian hormones may be having a different effect on glia. One dissimilarity is that both astrocytes and oligodendrocytes continue to proliferate at high rates after early development in the cortex, unlike neurons (Ehninger & Kempermann, 2003; Lee, Mayer-Proschel, & Rao, 2000). Thus, the lack of ovarian hormones could be augmenting glia proliferation as well decreasing glial elimination. Moreover, our work has demonstrated glia to be more sensitive to insults during adolescence than are neurons (Koss et al., 2012; Wise, Sadowski, Kim, Schantz & Juraska, 2014).
The functional implications of the loss of neurons, potentially during adolescence in females, are currently unknown. We have found that adolescent rats of both sexes perseverated more than adults in a delayed alternation task and this preservation was more prominent for females (Koss, Franklin & Juraska, 2011). Similarly adolescents, especially females, do not perform as well as adults on a delayed discounting task (Hammerslag & Gulley, 2014). Also, sex differences in activity and maze learning do not appear until after puberty (Kanit et al, 2000; Krasnoff and Weston, 1976). Studies comparing the cognitive behavior of rats that were gonadectomized after early development but before puberty are needed.
In conclusion, the ovarian hormones, rather than testicular hormones, secreted after puberty influence the number of both neurons and glia in the mPFC. These effects of ovarian may contribute to the vulnerability to psychiatric disorders, and drug addiction that often start during adolescence (Kessler et al., 2007). They also indicate possible vulnerability to endocrine disruptors after puberty. More research is needed to understand how hormones secreted after puberty influence the structure of the cerebral cortex.
Acknowledgements
We thank the staff in Microscopy Suite at the Beckman Institute and Daniel Kougias for assistance. We thank Dr. Jari Willing for comments on the manuscript. Supported by NIH grant MH099625. R.N.S. was supported by NIEHS T32 ES007326.
Contributor Information
Wendy A. Koss, Email: WKoss@childrensnational.org.
Madeline M. Lloyd, Email: madeline_lloyd@yahoo.com.
Renee N. Sadowski, Email: rnhaag@illinois.edu.
Leslie M. Wise, Email: lwise2@illinois.edu.
References
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11(9):995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai Y, Sekine Y, Murakami S. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neuroscience Research. 1996;25(4):403–407. doi: 10.1016/0168-0102(96)01070-x. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Bentsen AH, Sanchez-Garrido MA, Ruiz-Pino F, Romero M, Garcia-Galiano D, Tena-Sempere M. Early metabolic programming of puberty onset: Impact of changes in postnatal feeding and rearing conditions on the timing of puberty and development of the hypothalamic kisspeptin system. Endocrinology. 2011;152(9):3396–3408. doi: 10.1210/en.2010-1415. [DOI] [PubMed] [Google Scholar]
- Chisholm NC, Packard AR, Koss WA, Juraska JM. The effects of long-term treatment with estradiol and medroxyprogesterone acetate on tyrosine hydroxylase fibers and neuron number in the medial prefrontal cortex of aged female rats. Endocrinology. 2012;153(10):4872–82. doi: 10.1210/en.2012-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Effects of prepubertal gonadectomy on a male-typical behavior and excitatory synaptic transmission in the amygdala. Dev Neurobiol. 2009;69(2–3):141–152. doi: 10.1002/dneu.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorme KC, Schulz KM, Salas-Ramirez KY, Sisk CL. Pubertal testosterone organizes regional volume and neuronal number within the medial amygdala of adult male Syrian hamsters. Brain Res. 2012;1460:33–40. doi: 10.1016/j.brainres.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch ER, Espinoza TR, Atif F, Woodall E, Kaylor J, Wright DW. Progesterone's role in neuroprotection, a review of the evidence. Brain Res. 2013;1530:82–105. doi: 10.1016/j.brainres.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cerebral Cortex. 2003;13:845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- Forger NG. The organizational hypothesis and final common pathways: Sexual differentiation of the spinal cord and peripheral nervous system. Hormones and Behavior. 2009;55(5):605–610. doi: 10.1016/j.yhbeh.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerslag LR, Gulley JM. Age and sex differences in reward behavior in adolescent and adult rats. Dev Psychobiol. 2014;56(4):611–621. doi: 10.1002/dev.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RW, Brinton RD. Allopregnanolone as regenerative therapeutic for Alzheimer's disease: Translational development and clinical promise. Prog Neurobiol. 2014;113:40–55. doi: 10.1016/j.pneurobio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain : A Journal of Neurology. 1991;114(5):2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Kanit L, Taskiran D, Yilmaz OA, Balkan B, Demirgoren S, Furedy JJ, Pogun S. Sexually dimorphic cognitive style in rats emerges after puberty. Brain Res Bull. 2000;52(4):243–248. doi: 10.1016/s0361-9230(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: A review of recent literature. Current Opinion in Psychiatry. 2007;20(4):359–364. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biology of Reproduction. 1977;17(2):298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68(2):61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- Koss WA, Franklin AD, Juraska JM. Delayed alternation in adolescent and adult male and female rats. Dev Psychobio. 2011;53:724–731. doi: 10.1002/dev.20543. [DOI] [PubMed] [Google Scholar]
- Koss WA, Sadowski RN, Sherrill LK, Gulley JM, Juraska JM. Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Res. 2012;1466:24–32. doi: 10.1016/j.brainres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnoff A, Weston LM. Puberal status and sex differences: Activity and maze behavior in rats. Dev Psychobiol. 1976;9(3):261–269. doi: 10.1002/dev.420090310. [DOI] [PubMed] [Google Scholar]
- Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000;30(2):105–121. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- López V, Wagner CK. Progestin receptor is transiently expressed perinatally in neurons of the rat isocortex. J Comp Neurol. 2009;512(1):124–139. doi: 10.1002/cne.21883. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Walters MJ, Clark AS, Toran-Allerand CD. Aromatase in the cerebral cortex, hippocampus, and mid-brain: Ontogeny and developmental implications. Molecular and Cellular Neurosciences. 1994;5(6):691–698. doi: 10.1006/mcne.1994.1083. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144(3):961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Markham JA, Mullins SE, Koenig JI. Peri-adolescent maturation of the prefrontal cortex is sex-specific and disrupted by prenatal stress. J Comp Neurol. 2012;521(8):1828–1843. doi: 10.1002/cne.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacology & Therapeutics. 2007;116(1):107–124. doi: 10.1016/j.pharmthera.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Ferres-Torres R, Mas M. The effects of puberty and castration on hippocampal dendritic spines of mice. A Golgi study. Brain Res. 1978;155(1):108–112. doi: 10.1016/0006-8993(78)90309-8. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cueto JA, Garcia-Segura LM, Ruiz-Marcos A. Developmental sex differences and effect of ovariectomy on the number of cortical pyramidal cell dendritic spines. Brain Res. 1990;515(1–2):64–68. doi: 10.1016/0006-8993(90)90577-x. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce n Reid euron number in the rat primary visual cortex. Journal of Neurobiology. 2002;52:312–321. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brain. Neurobiol Aging. 2008;29:1754–1762. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Rubinow M, Juraska JM. Neuron and glia numbers in the in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: A stereological study. J Comp Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill LK, Koss WA, Foreman ES, Gulley JM. The effects of pre-pubertal gonadectomy and binge-like ethanol exposure during adolescence on ethanol drinking in adult male and female rats. Behavioural Brain Research. 2011;216(2):569–575. doi: 10.1016/j.bbr.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Slob AK, Van der Werff Ten Bosch JJ. Sex differences in body growth in the rat. Physiology & Behavior. 1975;14(3):353–361. doi: 10.1016/0031-9384(75)90044-x. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural Brain Research. 2003;146(1–2):3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. Journal of Comparative and Physiological Psychology. 1975;88(1):183–193. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- Willing J, Kim T, Brodsky JM, Cortes LR, Juraska J. The timing of neuroanatomical changes across adolescence in the male and female rat medial prefrontal cortex. Program No. 210.22/A66 Neuroscience 2014 Abstracts. Washington, DC: Society for Neuroscience. 2014 Online. [Google Scholar]
- Wise LM, Sadowski RN, Kim T, Schantz SL, Juraska JM. Long-term effects of adolescent exposure to bisphenol A on neuron and glia number in the rat prefrontal cortex differs between the sexes. Program No. 210.11/A55 Neuroscience 2014 Abstracts. Washington, DC: Society for Neuroscience. 2014 Online. [Google Scholar]
- Yates MA, Juraska JM. Pubertal ovarian hormone exposure reduces the number of myelinated axons in the splenium of the rat corpus callosum. Exp Neurol. 2008;209(1):284–287. doi: 10.1016/j.expneurol.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]