Abstract
Cells of the innate immune system have a dual role in cancer development in both tumor initiation and progression. Innate immune cells can, on the one hand, aid malignant transformation and tumor outgrowth and, on the other hand, prevent tumor progression. The innate immune system has the ability to tune the inflammatory response and is a key player in cancer-related inflammation, which can precede development of malignancy or be induced by oncogenic changes promoting a pro-tumor inflammatory milieu. In this review we discuss the emerging cellular and molecular mechanisms of the innate immune system and inflammation in tumor initiation and progression and point to the outstanding questions that remain.
Keywords: Innate immune cells, inflammation, cancer development
Inflammation: a hallmark of cancer
The inflammatory microenvironment in a malignant tumor, regarded to be a critical aspect of cancer, influences multiple hallmarks of cancer (e.g. cell proliferation, cell death, angiogenesis, invasion and metastasis)[1, 2]. Cancer development can be triggered, promoted and prevented by innate immune cells, which, accordingly, will affect the fate of tumors and the clinical outcome of cancer patients. For a tumor to thrive it has to gain genetic and epigenetic alterations that modulate the inflammatory repertoire to promote tumor growth. Accumulating evidence now indicates that the majority of cancers are linked to chronic inflammation, influencing cancer development from inception of tumor formation and throughout malignant progression. The characteristics of chronic inflammation, i.e., infiltration of inflammatory cells, influence of inflammatory mediators, tissue remodeling and angiogenesis, can, however, be found in tumors for which a causal relationship to inflammation has not been found[3].
There are many triggers of chronic inflammation[4]. Up to 20% of all cancers worldwide are related to microbial-induced chronic inflammation[5]. Chronic infection with human papilloma virus (HPV) causes 90-100% of all cervical cancers, the third most commonly diagnosed cancer in women. Hepatitis B virus (HBV) and HCV increase the risk of hepatocellular carcinoma (HCC) and chronic bacterial infection with Helicobacter pylori is a major cause of gastric cancers and is associated with mucosa-associated lymphoid tissue (MALT) lymphoma[5-7]. Chronic inflammation can also be caused by autoimmunity and immune deregulation. Indeed, inflammatory bowel disease (IBD) gives an elevated risk for colorectal cancer (CRC) and prostatic inflammation has been linked to prostate cancer[8, 9]. Emerging data now indicate that tobacco and obesity, which together account for 50% of all cancers, trigger low-grade inflammation[10-12]. Hence, it is becoming evident that the majority of cancers are associated with a tissue repair response that has gone awry, i.e., chronic inflammation.
Innate immune cells including macrophages, neutrophils, dendritic cells (DCs) and innate lymphoid cells (ILCs), are involved in the initial response to tissue perturbation and can control or prevent tumor initiation and progression but also facilitate cellular transformation and malignant development. Understanding how the innate immune system influences cancer development will therefore be crucial in fighting cancer. Here we focus on recent advances further clarifying the involvement of the innate immune system in tumor initiation and progression and illustrate the complexity of differentiating the friend from the foe. Our goal is not to give an in depth overview but to highlight the emerged complexity of the innate immune system in cancer development.
Innate immune system and tumor initiation
Tumor development is characterized by progressive changes on the genetic, epigenetic and cellular levels. Chronic inflammation can create a mutagenic microenvironment capable of either initiating malignant transformation by inducing DNA damage, impinging on DNA repair pathways, and thereby cause genomic instability accompanied by genetic mutations, or by accelerating the genetic mutation rate and enhancing proliferation of existing mutated cells (Figure 1)[4].
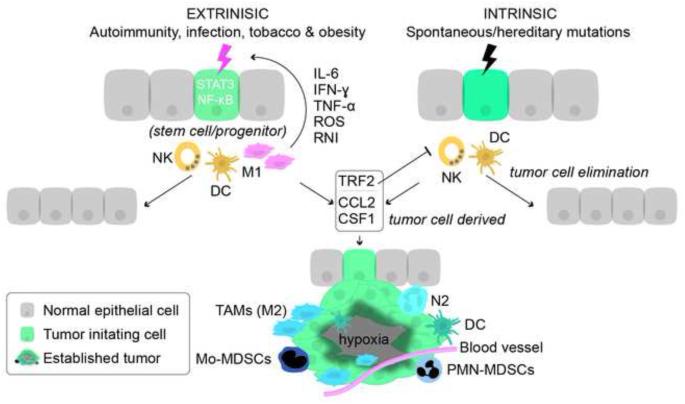
Figure 1. Innate immune cells in tumor initiation and progression.
Tumor initiation can either be induced by chronic inflammation caused by extrinsic factors such as infection, autoimmunity, tobacco and obesity or by intrinsic factors e.g. spontaneous or hereditary mutations. The extrinsic factors are dependent on innate immune cells to initiate tumor formation (compared to intrinsic tumor formation) and emerging data indicate that cancer initiated by chronic inflammation might originate from tissue stem cell/progenitors. Pro-inflammatory M1 macrophages and to some extent DCs are responsible for creating a mutagenic microenvironment. Pro-inflammatory mediators (IL-6, IFN-γ, TNF-α, ROS and RNI) activate STAT3 and NF-κB and induce genetic instability. Premalignant cells acquire traits, such as upregulation of TRF2 that inhibit tumoricidal NK cells, and upregulation of CSF1/M-CSF and CCL2 that recruit immature myeloid cells from the bone marrow and convert them into pro-tumor myeloid cells, i.e., TAMs, N2, DCs, PMN-MDSCs and Mo-MDSCs that are pro-angiogenic and promote development of malignancy.
Abbreviations: DC, dendritic cells; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; TRF2, telomeric repeat-binding factor 2, ROS, reactive oxygen species, RNI, reactive nitrogen intermediates, NK, natural killer cells; CSF1/M-CSF, macrophage colony stimulating factor 1; CCL2, chemokine (C-C motif) ligand 2; TAMs, tumor-associated macrophages, N2, pro-tumor neutrophil; PMN-MDSCs, tumor-associated T cell-suppressive neutrophils; Mo-MDSCs, monocyte/macrophages.
Innate immune cells are capable of creating a mutagenic microenvironment. DCs are terminally differentiated myeloid cells[13]. Differentiated DCs reside in tissues and actively take up tissue and tumor antigens. Langerhans cells, which are tissue resident DCs in the epidermis, metabolically convert chemical carcinogens into an activated mutagenic state that facilitates epithelial DNA damage and thereby induces squamous cell carcinoma[14]. Macrophages are also terminally differentiated myeloid cells that are capable of directly producing mutagenic mediators. The macrophages initially recruited to the sites of tissue perturbation are pro-inflammatory. Simplified, macrophages can be divided into either pro-inflammatory M1 macrophages or anti-inflammatory M2 macrophages. However, macrophages have functional plasticity and make up a continuum of different phenotypes[15, 16]. Even though pro-inflammatory M1 macrophages exhibit a tumoricidal effect in established tumors they also account for the mutagenic microenvironment initiating tumor formation in chronic inflammation[7]. Obesity, for example, can induce chronic, low-grade inflammation due to a phenotypic change in adipose tissue macrophages. Adipose tissue macrophages normally have a M2 phenotype and produce anti-inflammatory interleukin-10 (IL-10) that protects tissue against pro-inflammatory mediators. However, progressive obesity may recruit pro-inflammatory M1 macrophages that will overwhelm the protective effects of M2 macrophages and induce low-grade inflammation through their production of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α)[17]. Obesity further induces elevated production of both IL-6 and TNF-α, which activates STAT3 in hepatocytes promoting hepatic inflammation that eventually causes HCC development[11]. The tumor-protective role of M2 macrophages and the contribution of M1 macrophages in tumor initiation have also been reported in colon cancer. Genetic ablation of the anti-inflammatory transcription factor Stat3 in macrophages provokes an inflammatory response mediated by a marked increase of pro-inflammatory macrophages in the colon that ultimately induce colon cancer[18]. Importantly, the macrophages in the Stat3 knockout mice overproduced pro-inflammatory cytokines, including TNF-α and IL-6, which, like interferon-γ (IFN-γ), have an important role in promoting tumor initiation and progression (reviewed in [19, 20]).
Intriguingly evidence indicates that STAT3 and other inflammatory pathways enhance the tumor-initiating, i.e., the tumorigenic, capacity of tumor cells[21-24]. Cancer stem cells are characterized by their self-renewal and tumorigenic capacity and, indeed, a link between the innate immune system and cancer stem cells is starting to emerge[25]. Macrophages can, for example, produce IL-6, epidermal growth factor (EGF) and milk-fat globule epidermal factor VIII, which enhance the tumorigenic capacity of lung, colon, breast and pancreatic cancer cells[26-30]. Moreover, depletion of macrophages in pancreatic cancer mediates a reduction of cancer stem cells, while infiltration of macrophages, evokes tumor-initiating characteristics in pancreatic cells and elevates the frequency of cancer stem cells in a pancreatic cancer model[31, 32]. Interestingly, MMP7 is a key mediator of STAT3 activation in pancreatic ductal adenocarcinoma development[33].
A question to be clarified in the cancer-inflammation context is whether cancers caused by inflammation originate from tissue stem cells/progenitors, since accumulating data imply that activation of inflammatory pathways in normal tissue stem cell/progenitors is linked to tumor initiation[34]. The transcription factor SRY (sex determining region Y)-box 2 (Sox2), required for self-renewal of stem cells, cooperates with activated STAT3 to increase cell proliferation and malignant transformation of foregut basal progenitors and activation of STAT3 in urothelial stem cells induces development of bladder cancer[35, 36]. Moreover, elevated NF-κB signaling can both dedifferentiate intestinal epithelial cells that eventually acquire stem cell-like properties and tumor-initiating capacity, and facilitate Wnt-driven proliferation of intestinal stem cells, which ultimately induce colorectal cancer[37, 38]. Hence inflammatory pathways are not only intertwined with enhanced tumorigenic capacity in various cancers but also initiate tumor formation in tissue stem cell/progenitors.
Aberrant cell proliferation and innate immune system
Oncogenic mutations can constitutively activate RAS, PI3K and MAPK pathways that not only promote cell growth and proliferation but also induce activation of the tumor suppressor p53, whose function is to inhibit cell-cycle progression, induce senescence or programmed cell death. Accumulating evidence implies that there is a fundamental link between control of cell proliferation and the capability of innate immune cells to detect incipient cellular transformation[34]. NK cells, a subset of the more recently characterized ILC population, can not only prevent tumor outgrowth by inducing senescence in tumor cells (through the production and cooperation of IFN-γ and TNF-α), but they can also eliminate senescent tumor cells expressing p53[39, 40]. Expression of p53 in HCC cells mediates the production of various chemokines, including IL-6, IL-12, IL-15 and chemokine (C-C motif) ligand 2 (CCL2), known to recruit NK cells. Antibodies against CCL2 dramatically reduce the infiltration of NK cells in tumors and delay elimination of senescent tumor cells. The elimination of HCC cells by NK cells depends on the recognition between retinoic acid early inducible-1 (RAE-1) ligands on senescent cancer cells and NKG2D receptors on NK cells[40]. NKG2D belongs to the fixed repertoire of germline receptors on innate immune cells that differentiate normal cells from tumor cells. Intriguingly the NKG2D ligand, RAE-1, is upregulated upon activation of RAS and PI3K pathways and by transcription factor E2F in response to proliferation-related signals, pointing to an intricate connection between cell proliferation and the ability of the innate immune system to recognize proliferating cells[41-44]. Aberrant cell proliferation is a hallmark of cancer and it is tempting to speculate that other hallmarks of cancer might induce expression of similar ligands that can be recognized by innate immune cells.
Balancing innate immune system in tumor progression
Tumor-associated macrophages
Innate immune cells, initially involved in incipient tumor formation, are pro-inflammatory[7]. However, due to genetic instability tumor cells acquire features that render them resistant to pro-inflammatory immune attack, and differentiate immune cells into becoming pro-tumor. Malignant tumors are frequently infiltrated with pro-tumor innate immune cells, and tumor-associated macrophages (TAMs) account for the major component of innate leukocyte infiltration within a tumor. TAMs have many characteristics in common with M2 macrophages involved in tissue repair. They are anti-inflammatory, enhance cell proliferation and motility, produce matrix-remodeling proteins and promote angiogenesis[16]. M2-like TAMs can, however, be re-educated back to a tumoricidal phenotype. NK cells reprogram macrophages into becoming tumoricidal through the production of IFN-γ, and the overexpression of miR-155 in TAMs repolarizes them into a tumoricidal M1 phenotype[45, 46]. Activation of Toll-like receptor 3 (TLR3) coupled with Toll–IL-1 receptor domain-containing adaptor molecule 1 (TICAM-1) induces TNF-α production and thereby converts M2-like TAMs in the tumor microenvironment of Lewis lung carcinoma into tumoricidal M1 macrophages[47]. Moreover, treatment with a CSF-1R inhibitor in a glioblastoma mouse model re-educates M2 macrophages, which, importantly, promotes regression of established high-grade glioma[48].
TAMs are not a homogenous group of macrophages; instead there are functionally distinct macrophage populations within a tumor (Figure 2)[15, 49]. Tie-2+ macrophages derived from the bone marrow promote tumor angiogenesis, facilitate tumor growth and progression and are often aligned along the outside of blood vessels where they bind to angiopoietin-2 (ANGPT2) displayed by endothelial cells[50]. Accumulating evidence suggests that tumor-derived exosomes have an important role in immunomodulation (reviewed in [51]); indeed, melanoma-derived exosomes can reprogram bone marrow derived cells toward a Tie-2+ pro-metastatic phenotype[52]. Tie-2+ macrophages produce Wnt7b that stimulates the production of pro-angiogenic vascular endothelial growth factor (VEGF), which also directly recruits macrophages under the influence of IL-4[53, 54]. Targeting Tie-2 or ANGPT2 inhibits angiogenesis and thereby impairs tumor outgrowth and metastasis[55]. The Tie-2 macrophage population is not the only pro-angiogenic macrophage subpopulation found in a tumor. Colony stimulating factor 1 (CSF1), also known as macrophage colony stimulating factor (M-CSF), a well known tumor-derived chemoattractant for TAMs recruits monocytes from the bone marrow and differentiates them into pro-angiogenic macrophages and further expands the population of Tie2+ macrophages[56]. CSF1-regulated macrophages (CSF1R-macrophages) attract tumor cells to blood vessels through a paracrine loop. Tumor cells produce CSF1, while CSF1R-macrophages produce EGF, instigating the migration of tumor cells toward blood vessels[57]. Yet another population of TAMs, the monocytes displaying the C-C chemokine receptor type 2 (CCR2), are preferentially recruited to the metastatic site by CCL2, which can be derived from either tumor or stromal cells[58, 59]. CCL2 can further activate CCR2+ endothelium to increase vascular permeability and recruit monocytes that will facilitate extravasation and metastatic seeding of tumor cells[60]. The interaction between tumor, endothelial and myeloid cells creates a niche that favors tumor outgrowth. Indeed, the niche has been hypothesized to be a crucial determinant for cancer growth[61]. Accordingly, direct contact between a macrophage, an endothelial cell and a tumor cell in the tumor microenvironment is predictive of the metastatic potential in primary human breast cancer, hence further highlighting the unfavorable clinical impact of interaction between macrophages, endothelium and tumor cells[62].
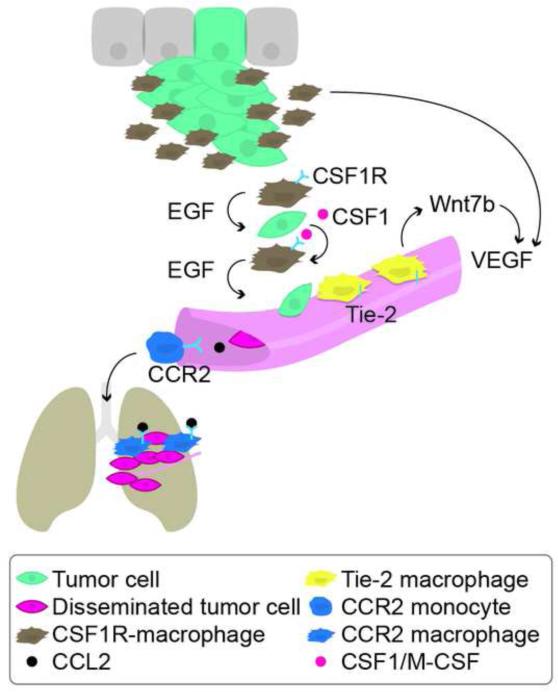
Figure 2. Subpopulations of tumor-associated macrophages promote malignant progression.
TAMs are recruited to the primary tumor and to metastatic sites by chemokines derived from tumor cells. There are distinct subpopulations of macrophages within a tumor. CSF1R-macrophages attract tumor cells to blood vessels through a paracrine loop. Tumor cells produce CSF1/M-CSF, while CSF1R-macrophages produce EGF, instigating the migration of tumor cells toward blood vessels. Tie2-macrophages align blood vessels and produce pro-angiogenic mediators, e.g., Wnt7b and VEGF. Tumor cells produce CCL2 that recruit CCR2+ monocytes to the metastatic site where they facilitate extravasation of tumor cells and promote tumor outgrowth.
Abbreviations: TAMs, tumor-associated macrophages; CSF1/M-CSF, macrophage colony stimulating factor 1; CCL2, chemokine (C-C motif) ligand 2; CCR2, C-C chemokine receptor type 2; CSF1R, colony stimulating factor 1 receptor; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor.
Tumor-associated neutrophils
The role of tumor-associated neutrophils (TANs) in disease progression has only recently emerged, although an association between increased circulating neutrophils and metastasis in human cancer has been known for decades[63]. While there are relatively little data on neutrophil infiltration in human cancer, an increase in the presence of TANs correlates with advanced disease and poor outcome in patients in several types of human cancer [64]. Several recent reviews have extensively summarized the recent advances in neutrophil biology in cancer[65-68]. TANs induce the angiogenic switch during early tumor progression [69], and continue to promote tumor cell growth and invasion by remodeling the extracellular matrix and modulating tumor cell biology in later stages[65-68]. Neutrophils, like macrophages, polarize in the tumor microenvironment to a pro-tumor (N2) phenotype [70]. Neutrophil-derived MMP-9, oncostatin M, CXCL8 and Bv8 have been shown to promote angiogenesis [71] and are associated with the N2 phenotype of TANs [66]. While neutrophils appear to play a mostly pro-tumor role in tumor progression, the N2 phenotype of TANs can be reversed to an anti-tumor N1 phenotype with TGFβ blockade[70] and IFN-β can instruct neutrophils to have an anti-tumor phenotype [72], illustrating their ability to be pro- or anti-tumor depending on the microenvironment.
Innate lymphoid cells and dendritic cells
ILCs are major producers of cytokines in response to tissue damage and are able to tune the inflammatory response[73, 74]. Their role in cellular transformation and malignant progression is however largely unknown. Hence unraveling the role of ILCs in cancer development and the interplay between ILCs and other immune cells would significantly contribute to the understanding of how the innate immune system tunes the inflammatory response in cancer.
NK cells are an important subset of ILCs. In contrast to TAMs and TANs, which can exert either pro- or anti-tumor roles in tumor progression, NK cells are devoted anti-tumor contenders. Tumor cells, however, adopt traits that allow them to inhibit the function of NK cells. Neuroblastoma tumor cells produce TGF-β1 that affects the repertoire of chemokine receptors (upregulates CXCR3 and CXCR4) on NK cells, thereby stimulating homing of NK cells to the bone marrow and preventing their recruitment to the tumor[75]. Tumor cells can further evade NK immunity by upregulation of telomeric repeat-binding factor 2 (TRF2). TRF2 is a key factor involved in telomere protection and found to be increased in various human cancers; however, the mechanism, by which TRF2 controls cancer formation was unknown until a recent study that found that TRF2 inhibits recruitment of NK cells and prevents tumor cells from NK-mediated elimination[76]. NK cells also target tumor-initiating cells in colon cancer and melanoma[77, 78]. IL-15 activated NK cells are capable of eradicating large established tumors through a perforin-dependent elimination and IL-15-activated DCs are able to induce apoptotic cell death in tumor cells (predominantly by granzyme B)[79, 80]. Moreover, colorectal cancer patients with deletion at the IL-15 locus have a higher risk of recurrence, which further points to the importance of IL-15 induced activation of DCs and NK cells in combating cancer[81].
DCs can control malignant development of CAC through the production of IL-22BP, which neutralizes the effect of IL-22[82]. IL-22 initially protects the intestine in the early phase of tissue damage, caused by bacterial infection, via induction of antimicrobial peptides and via tissue repair proliferation of intestinal stem cells. Exposure of IL-22 for a long period (due to, for example, chronic bacterial stimulation) will, however, induce hyper-proliferation of intestinal epithelial cells and chronic inflammation[83]. IL-17+IL-22+ colonic ILCs produce IL-22 and their depletion in mice occludes development of colon cancer[84].
Even though DCs can have tumoricidal activity and activate anti-tumor immunity (mediated by type I IFN-α/β) they frequently become pro-tumor in a cancer context[85, 86]. The acquired pro-tumor function of DC can be caused by impaired IFN-α production or upregulation of transcription factor Forkhead box O3 (Foxo3)[87, 88]. Foxo3 has a critical role in inhibiting anti-tumor function of DCs. Silencing Foxo3 partially restores the anti-tumor function of DCs, however it is yet to be determined what induces upregulation of Foxo3 in intratumoral DCs[88]. Yet another way by which DCs lose their anti-tumor function is due to hypoxia[13]. Hypoxia further reduces the tumoricidal capacity of NK cells and attracts T cell-suppressive myeloid cells[89].
Tumor-induced T cell-suppressive myeloid cells
Accumulation of T cell-suppressive myeloid cells in peripheral tissues in cancer is well documented, along with their pro-tumor role in tumor progression[90]. Two heterogeneous subsets of myeloid cells have been characterized that share the unique ability to suppress T cell function in cancer, which is not found in their healthy or naïve myeloid counterparts. The monocytic subset (Mo-MDSC) is CD33+CD14+HLADR− (humans) and CD11b+Ly6ChiLy6Gneg (mice), while the granulocytic/neutrophil subset (G-MDSC, PMN-MDSC) is CD33+CD15+HLA-DR− (humans) and CD11b+Ly6CintLy6G+ (mice). In mice, the monocytic Ly6ChiLy6Gneg (same as Gr1-int) population contains a more heterogeneous population of cells that includes not only monocytes, but also myeloid precursors and progenitors [91, 92]. MDSCs also are involved in tumor initiation. CXCR2+ MDSCs are recruited to inflamed colon where they promote chronic inflammation and contribute to tumor initiation and development of colitis-associated cancer [93].
In most solid tumor cancers (human and mice), T cell-suppressive granulocytes/neutrophils appear to be the predominant subtype [94, 95]. Of note, the markers utilized to define T cell-suppressive myeloid cells in human cancer, defined as CD3−CD14−CD19−CD57−CD11b+CD33+, are shared by circulating neutrophils, and, similarly, these cells are also highest in patients with extensive metastatic tumor burden [96]. It is becoming increasingly clear that while TANs and T cell-suppressive granulocytes/neutrophils differ significantly from naïve neutrophils [97, 98], there is significant functional overlap between these populations, and most likely they are generated by a similar mechanism in cancer [65, 68].
While originally thought to be a homogeneous population of pro-inflammatory cells, neutrophils are now known to be essential during both pro- and anti-inflammatory responses and emerging identification of neutrophil subsets suggest these cells are more heterogeneous [99]. In response to VEGF-A, a subset of CD11b+Gr1+CXCR4hi neutrophils with increased MMP9 activity were found to promote angiogenesis and reintegration of transplanted hypoxic tissue, which was not recruited in response to MIP-2 (CXCL2)[100]. This VEGF-recruited neutrophil population shares characteristics with TANs and it is interesting to speculate that immune suppression in the context of wound repair is beneficial for the host, though detrimental in cancer. Understanding the molecular mechanisms that generate subsets of neutrophils that provide pro- or anti-inflammatory responses could provide therapeutic targets.
Concluding remarks
Innate immune cells have the ability to tune the inflammatory response and have a key role in cancer-related inflammation. An important question, among others (Box 1), is to elucidate whether the innate immune system, while trying to prevent an overt inflammatory response will respond by tuning the inflammatory response and consequently inducing a pro-tumor microenvironment. Ultimately the balance of innate immune cells will dictate the fate of cancer development and understanding what distinguishes pro-tumor innate immune cells from their anti-tumor counterpart and being able to therapeutically tune the inflammatory response will be crucial in the fight against cancer. However, the tumor context, i.e., ‘tumor initiation’ or ‘established tumor’ will have to be taken into account when differentiating the friend (anti-tumor) from the foe (pro-tumor)(Figure 3).
Box 1. Outstanding questions that remain.
Why do all chronic inflammatory disorders not give an elevated risk of cancer?
Do cancers caused by inflammation originate from tissue stem cells/progenitors?
Do certain oncogenes induce certain inflammatory responses?
Besides aberrant cell proliferation, what other hallmarks of cancer induce expression of ligands that can be recognized by innate immune cells?
Tumor cells acquire features that enable them to convert the pro-inflammatory response into anti-inflammatory, but can the innate immune system itself try to prevent an overt inflammatory response and thus, responds by tuning the inflammatory response, which results in a pro-tumor environment?
What is the role of different innate lymphoid cell populations in tumor initiation and progression?
Do certain combinations of chemokines/cytokines interconnect in malignant development or does a single cytokine/chemokine always act autonomously?
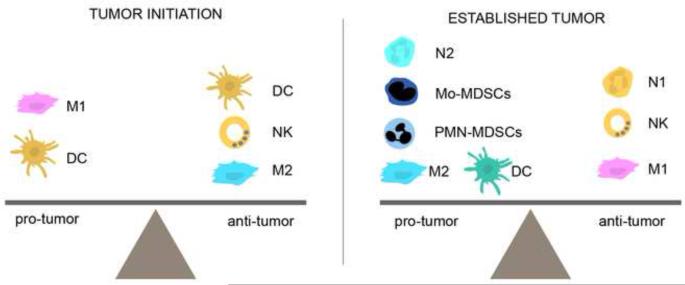
Figure 3. Balancing of the innate immune system.
The pro-tumor versus anti-tumor role of different innate immune cells can differ depending on the tumor context, i.e., ‘tumor initiation’ or ‘established tumor’, making it difficult to differentiate friend from foe. The balance of innate immune cells will ultimately dictate the fate of cancer development
Highlights.
The outcome of innate immune system is determined by the tumor context.
The balance of innate immune cells dictates the fate of cancer development.
Innate lymphoid cells are important in regulating tumorigenesis
Emerging role of neutrophil subsets in tumor progression
Acknowledgments
This work was supported by funds from the National Cancer Institute (R01 CA057621) and the Tegger Foundation. The authors thank Carl Hagerling for helping with the drawing of the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Elinav E, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev. Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, et al. Cancer statistics, 2010. CA: Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Bosch FX, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Path. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivennikov SI, et al. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J oGastroenterol. 2008;14:3937–3947. doi: 10.3748/wjg.14.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkahwaji JE. The role of inflammatory mediators in the development of prostatic hyperplasia and prostate cancer. Res Rep Urol. 2012;5:1–10. doi: 10.2147/RRU.S23386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coussens LM, et al. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park EJ, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi H, et al. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, et al. Coordinated regulation of myeloid cells by tumours. Nat Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modi BG, et al. Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science. 2012;335:104–108. doi: 10.1126/science.1211600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumeng CN, et al. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng L, et al. A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am J Path. 2010;176:952–967. doi: 10.2353/ajpath.2010.090622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Wynn TA, et al. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jinushi M, et al. Regulation of cancer stem cell activities by tumor-associated macrophages. Am J Canc Res. 2012;2:529–539. [PMC free article] [PubMed] [Google Scholar]
- 22.Kendellen MF, et al. Canonical and non-canonical NF-kappaB signaling promotes breast cancer tumor-initiating cells. Oncogene. 2014;33:1297–1305. doi: 10.1038/onc.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajasekhar VK, et al. Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-kappaB signalling. Nat Commun. 2011;2:162. doi: 10.1038/ncomms1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford JW, Baldwin AS. IKK/nuclear factor-kappaB and oncogenesis: roles in tumor-initiating cells and in the tumor microenvironment. Adv Cancer Res. 2014;121:125–145. doi: 10.1016/B978-0-12-800249-0.00003-2. [DOI] [PubMed] [Google Scholar]
- 25.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jinushi M, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, et al. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2013;31:248–258. doi: 10.1002/stem.1281. [DOI] [PubMed] [Google Scholar]
- 28.Marotta LL, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121:2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesina M, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Mitchem JB, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panni RZ, et al. Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol Immunother. 2014;63:513–528. doi: 10.1007/s00262-014-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda A, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 35.Liu K, et al. Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell. 2013;12:304–315. doi: 10.1016/j.stem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho PL, et al. Stat3 activation in urothelial stem cells leads to direct progression to invasive bladder cancer. Cancer Res. 2012;72:3135–3142. doi: 10.1158/0008-5472.CAN-11-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myant KB, et al. ROS production and NF-kappaB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 2013;12:761–773. doi: 10.1016/j.stem.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwitalla S, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Braumuller H, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 40.Iannello A, et al. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung H, et al. RAE-1 ligands for the NKG2D receptor are regulated by E2F transcription factors, which control cell cycle entry. J Exp Med. 2012;209:2409–2422. doi: 10.1084/jem.20120565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcus A, et al. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol. 2014;122:91–128. doi: 10.1016/B978-0-12-800267-4.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu XV, et al. Ras activation induces expression of Raet1 family NK receptor ligands. J Immunol. 2012;189:1826–1834. doi: 10.4049/jimmunol.1200965. [DOI] [PubMed] [Google Scholar]
- 44.Tokuyama M, et al. Expression of the RAE-1 family of stimulatory NK-cell ligands requires activation of the PI3K pathway during viral infection and transformation. PLoS Path. 2011;7:e1002265. doi: 10.1371/journal.ppat.1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Sullivan T, et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med. 2012;209:1869–1882. doi: 10.1084/jem.20112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai X, et al. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol. 2012;4:341–343. doi: 10.1093/jmcb/mjs044. [DOI] [PubMed] [Google Scholar]
- 47.Shime H, et al. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc Natll Acad Sci U S A. 2012;109:2066–2071. doi: 10.1073/pnas.1113099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Movahedi K, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 50.De Palma M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Altevogt P, et al. Novel insights into exosome-induced, tumor-associated inflammation and immunomodulation. Semin Cancer Biol. 2014;28C:51–57. doi: 10.1016/j.semcancer.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeo EJ, et al. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. 2014;74:2962–2973. doi: 10.1158/0008-5472.CAN-13-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linde N, et al. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol. 2012;227:17–28. doi: 10.1002/path.3989. [DOI] [PubMed] [Google Scholar]
- 55.Mazzieri R, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Forget MA, et al. Macrophage colony-stimulating factor augments Tie2-expressing monocyte differentiation, angiogenic function, and recruitment in a mouse model of breast cancer. PloS One. 2014;9:e98623. doi: 10.1371/journal.pone.0098623. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ren G, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFalpha. Cell Stem Cell. 2012;11:812–824. doi: 10.1016/j.stem.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf MJ, et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell. 2012;22:91–105. doi: 10.1016/j.ccr.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 61.Barcellos-Hoff MH, et al. The evolution of the cancer niche during multistage carcinogenesis. Nat Rev Cancer. 2013;13:511–518. doi: 10.1038/nrc3536. [DOI] [PubMed] [Google Scholar]
- 62.Rohan TE, et al. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shoenfeld Y, et al. Leukocytosis in non hematological malignancies--a possible tumor-associated marker. J Cancer Res Clin Oncol. 1986;111:54–58. doi: 10.1007/BF00402777. [DOI] [PubMed] [Google Scholar]
- 64.Tazzyman S, et al. Neutrophil-mediated tumour angiogenesis: subversion of immune responses to promote tumour growth. Semin Cancer Biol. 2013;23:149–158. doi: 10.1016/j.semcancer.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Pillay J, et al. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 67.Dumitru CA, et al. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23:141–148. doi: 10.1016/j.semcancer.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Brandau S, et al. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: cousins, siblings or twins? Semin Cancer Biol. 2013;23:171–182. doi: 10.1016/j.semcancer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 69.Nozawa H, et al. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 72.Jablonska J, et al. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nowarski R, et al. Innate immune cells in inflammation and cancer. Cancer Immunol Res. 2013;1:77–84. doi: 10.1158/2326-6066.CIR-13-0081. [DOI] [PubMed] [Google Scholar]
- 74.Walker JA, et al. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 75.Castriconi R, et al. Neuroblastoma-derived TGF-beta1 modulates the chemokine receptor repertoire of human resting NK cells. J Immunol. 2013;190:5321–5328. doi: 10.4049/jimmunol.1202693. [DOI] [PubMed] [Google Scholar]
- 76.Biroccio A, et al. TRF2 inhibits a cell-extrinsic pathway through which natural killer cells eliminate cancer cells. Nat Cell Biol. 2013;15:818–828. doi: 10.1038/ncb2774. [DOI] [PubMed] [Google Scholar]
- 77.Pietra G, et al. Natural killer cells kill human melanoma cells with characteristics of cancer stem cells. Internat Immunol. 2009;21:793–801. doi: 10.1093/intimm/dxp047. [DOI] [PubMed] [Google Scholar]
- 78.Tallerico R, et al. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190:2381–2390. doi: 10.4049/jimmunol.1201542. [DOI] [PubMed] [Google Scholar]
- 79.Liu RB, et al. Densely granulated murine NK cells eradicate large solid tumors. Cancer Res. 2012;72:1964–1974. doi: 10.1158/0008-5472.CAN-11-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anguille S, et al. Interleukin-15-induced CD56(+) myeloid dendritic cells combine potent tumor antigen presentation with direct tumoricidal potential. PloS One. 2012;7:e51851. doi: 10.1371/journal.pone.0051851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mlecnik B, et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci Transl Med. 2014;6:228ra237. doi: 10.1126/scitranslmed.3007240. [DOI] [PubMed] [Google Scholar]
- 82.Huber S, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sonnenberg GF, et al. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 84.Kirchberger S, et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med. 2013;210:917–931. doi: 10.1084/jem.20122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diamond MS, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fuertes MB, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sisirak V, et al. Impaired IFN-alpha production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012;72:5188–5197. doi: 10.1158/0008-5472.CAN-11-3468. [DOI] [PubMed] [Google Scholar]
- 88.Watkins SK, et al. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121:1361–1372. doi: 10.1172/JCI44325. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Sceneay J, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–3911. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 90.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ueda Y, et al. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hestdal K, et al. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- 93.Katoh H, et al. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Youn JI, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Serafini P, et al. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 96.Diaz-Montero CM, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Youn JI, et al. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukocyte Biol. 2012;91:167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fridlender ZG, et al. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS One. 2012;7:e31524. doi: 10.1371/journal.pone.0031524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 100.Christoffersson G, et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120:4653–4662. doi: 10.1182/blood-2012-04-421040. [DOI] [PMC free article] [PubMed] [Google Scholar]