Abstract
In most situations, the angiotensin AT2-receptor (AT2R) mediates physiological actions opposing those mediated by the AT1-receptor (AT1R), including a vasorelaxant effect. Nevertheless, experimental evidence vastly supports that systemic application of AT2R-agonists is blood pressure neutral. However, stimulation of AT2R locally within the brain or the kidney apparently elicits a systemic blood pressure lowering effect. A systemic effect of AT2R stimulation on blood pressure can also be achieved, when the prevailing effect of continuous background AT1R-stimulation is attenuated by low-dose AT1R blockade. Despite a lack of effect on blood pressure, AT2R stimulation still protects from hypertensive end-organ damage. Current data and evidence therefore suggest that AT2R agonists will not be suitable as future anti-hypertensive drugs, but that they may well be useful for end-organ protection in combination with established anti-hypertensives.
Introduction
It is now generally accepted that the renin angiotensin system (RAS) has many more facets than solely the well-known effects of angiotensin II (Ang II) acting on the AT1-receptor (AT1R). In fact, the RAS harbours several other receptors and hormones (Ang II metabolites) which elicit actions opposing those of the AT1R, resulting in tissue protective effects. Currently known components of the so called “Protective arm of the RAS” are the hormones angiotensin-(1-7) [1] and alamandine [2], angiotensin converting enzyme 2 (ACE2) [the enzyme responsible for Ang-(1-7) synthesis] [1], and the receptors Mas [for angiotensin-(1-7)], Mas-related G-protein coupled receptor D (MrgD; for alamandine) and the AT2-receptor (AT2R; binding Ang II) [3,4].
In case of the AT2R, research in recent years has been facilitated and fostered by the availability of a specific and selective non-peptide AT2R agonist, Compound 21 (C21), which for the first time has allowed stimulation of the AT2R in long-term preclinical studies [5]. Using this new research tool, the role of the AT2R in blood pressure (BP) regulation and hypertensive end-organ damage has been reassessed and studied by several groups [6].
The following article will review studies that have addressed the effects of AT2R stimulation in the periphery, in the brain or the kidney on BP regulation and hypertensive end-organ damage with a focus on findings published during the last two years.
Effects of systemic AT2R stimulation on blood pressure control
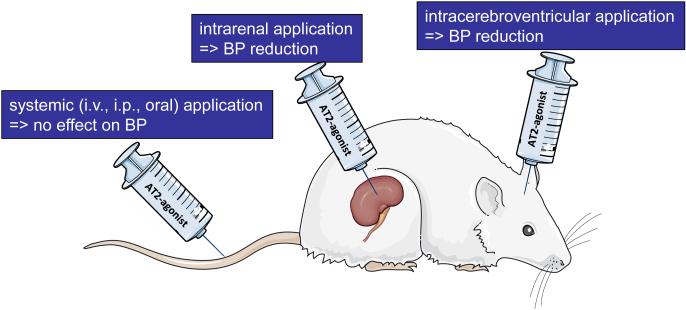
Since the AT2R is known to counteract actions of the AT1R by direct (through dimerisation) [7] or indirect (through dephosphorylation) interference with AT1R-coupled signalling [8], it has been assumed that AT2R-stimulation would result in a lowering of BP. This conclusion is supported by multiple studies showing a weak, but consistent vasorelaxant effect of AT2R-stimulation ex vivo in isolated vessels originating from various vascular beds such as mesenteric, renal, coronary, cerebral, cutaneous, and uterine arteries [reviewed in 6]. Moreover, AT2R-stimulation has been shown to elicit a strong natriuretic effect [9–11]. Nevertheless, the vast majority of studies looking at short- or long-term effects of AT2R-stimulation did not observe any anti-hypertensive effect. This holds true for models of genetic hypertension (spontaneously hypertensive rats, SHR; stroke-prone SHR, SHR-SP) [12–16], hypertension induced by inhibition of NO synthesis [17], by Na+- or volume-overload [11], for renal hypertension [18] and for lean or obese normotensive animals [10,19,20]. Since in some of these models the activity of the RAS (plasma Ang II levels) is suppressed (Na+- and volume overload), while in others it is rather unchanged (genetic hypertension) or even activated (renal hypertension), the state of activity of the RAS seems to play no role with regard to the efficacy of AT2R-agonists in lowering blood pressure.
However, there are few exceptions, which are as follows (Fig. 1):
- AT2R-stimulation in the CNS seems to have a BP lowering effect as discussed in more detail later in this review.
- The BP lowering effect of AT2R-agonists administered peripherally appears to be unmasked when co-administered with a low dose of an AT1R-blocker (ARB), which by itself has no or only a marginal BP lowering effect. This phenomenon has been shown by the groups of Robert Widdop and Robert Carey using peptide or non-peptide AT2R-agonists [16,21–23]. These data can be interpreted in a way that a constant angiotensinergic tone acting via the AT1R normally dominates over the vasodilatory effect of the AT2R. When an ARB is applied at a high dose, there is no additive effect of AT1R-blockade and AT2R-stimulation on BP.
- The group of Robert Carey recently reported that in various animal models (volume expansion in rats; Na+-loaded male and female rats; normal C57BL/6 and AT2R-KO mice) systemic infusion of the AT2R-agonist C21 did not alter BP despite a strong natriuretic and diuretic effect [11]. However, in female rats chronically (7 days) and systemically infused with Ang II, the resulting elevated BP was markedly reduced when C21 (60 ng/kg/min) was concomitantly infused intrarenally, supporting the existence of an independent, functional, intrarenal RAS [24]. Since in this latter experimental setup several parameters were changed in comparison to the experiments, in which C21 had no effect on BP, it became not entirely clear from this study, what the actual cause for this rather unexpected BP-lowering effect of C21 was. Potential causes could be a) the model of Ang II induced hypertension, b) the much longer duration of C21 application (7 days versus 3 × 30 minutes), c) the intrarenal route of application, d) the fact that these experiments were performed in female rats, or a combination of some of these parameters. The assumption that female sex is essential is supported by a series of experiments performed by Kate Denton’s group, which showed that adult females express more AT2Rs than males, which leads to lower baseline levels of MAP, but also to a leftward shift of chronic pressure-natriuresis compared to males [12,25].
- C21 may have an impact on BP, depending upon whether blood pressure measurements are made in conscious or anaesthetised rats, because there are at least two examples of AT2R-mediated reductions in blood pressure in anaesthetised rats of strains/models (SHR, obese Zucker rats), in which in the conscious state blood pressure was not affected by AT2R-stimulation [13,16], while under anaesthesia it was [5,26].
Figure 1. The effect of AT2-receptor stimulation on blood pressure is dependent on the way of application.
While systemic application (i.v., i.p. or oral) of AT2-receptor agonists is blood pressure neutral (unless the effect is unmasked by low-dose AT1R blockade), application into the kidney or the brain has a blood pressure lowering effect. The figure was created using Servier Medical Art (http://www.servier.com/Powerpoint-image-bank).
Central nervous system effects of AT2R stimulation on blood pressure control
The overriding view of blood pressure regulation via the RAS within the central nervous system (CNS) begins and ends with pressor and hypertensive effects of Ang II mediated by AT1R [27–29]. This is perhaps not surprising as, according to traditional receptor binding and autoradiography techniques, CNS cardiovascular control areas such as the paraventricular nucleus of the hypothalamus (PVN), rostral ventrolateral medulla (RVLM) and solitary tract nucleus (NTS) within the brainstem are rich in AT1R but are either devoid of or express only low levels of AT2R [30,31]. Nonetheless, a limited number of functional studies have implicated or suggested that AT2R in the brain influence cardiovascular regulation. For example, AT2R knockout mice display elevated basal blood pressure [32], and increased susceptibility to develop DOCA-salt hypertension [33]. Electrophysiological studies examining the CNS cardiovascular control centres of AT1Ra knockout mice suggest that AT2R in the RVLM play an antagonistic role against AT1R-mediated actions of Ang II within this nucleus [34]. Furthermore, virally-mediated over expression of AT2R within the RVLM [35] or NTS [36] elicits respective decreases in mean arterial blood pressure of normotensive rats and in 2 kidney-1 clip hypertensive rats. In the latter study, it was also demonstrated that AT2R over expression in the NTS restores baroreflex sensitivity [36]. The recent availability of C21, has enabled Gao et al to demonstrate that infusion of this agent into the cerebroventricles lowers blood pressure in normotensive rats [37,38], and more recently to show that similar infusions into rats with heart failure suppresses sympathetic outflow by improving baroreflex sensitivity [39]. While these collective studies have indicated blood pressure lowering and anti-hypertensive actions of AT2R, the mechanisms underlying these effects were, for the most part, unknown due to an inability to localize AT2R to specific cell types within the brain.
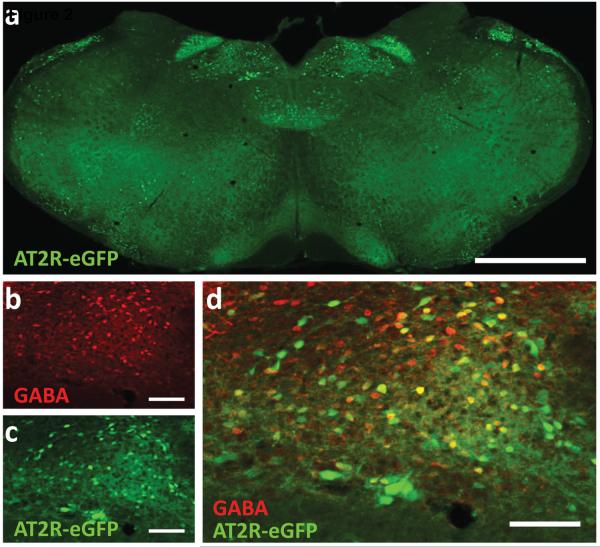
The recent development of a novel transgenic model (Agtr2-eGFP BAC reporter mouse) that provides higher sensitivity for localizing AT2R, has allowed for identification of the discrete cellular localization of AT2R in the brain [40], and may explain the results of the functional studies referenced above. This approach has revealed that AT2R reside or are in close proximity to CNS cardiovascular control centres, and are associated with neuronal phenotypes that are known to influence blood pressure. For example, there is a major concentration of AT2R containing neurons within the intermediate region of the NTS (intNTS), a nucleus that is essential for blood pressure control and baroreflex regulation. Further, these AT2R in the intNTS are primarily localized to GABA neurons [40], as illustrated by the fluorescence micrographs in Figure 2.
Figure 2. Co-localization of AT2R-eGFP and GABA immunoreactivity in NTS of an AT2R-eGFP reporter mouse.
Mice were generated and brains processed for immunostaining as described recently [39]. (a) Low magnification (2.5×) image of a coronal section through the NTS of an AT2R-eGFP mouse. High magnification (10×) images through the NTS depicting (b) eGFP immunoreactivity in green, (c) GABA immunoreactivity in red, and (d) the merged image. Scale bars = 1 mm (a) and 100 µm (b - d)
When considering that GABA acts within the intNTS to exert a powerful pressor action [41,42], an effect that is exacerbated in hypertension [43–45], the localization of AT2R on GABA neurons in the intNTS raises the possibility that they can exert depressor effects by influencing GABA activity. In contrast to the intNTS, localization of AT2R within the PVN and the RVLM, areas important in controlling sympathetic outflow, is restricted to neuronal fibres and terminals but not cell bodies [40]. Within the PVN, these AT2R-containing neuron fibres and terminals appear to synapse onto pre-autonomic neuron cell bodies [40], raising the possibility that AT2R can influence sympathetic outflow and blood pressure through these connections.
In summary, the role of CNS AT2R in the control of blood pressure is not well established. However, recent functional studies in conjunction with data that indicate localization of AT2R within brain cardiovascular control centres, strongly suggest that AT2R contribute to the neural control of the circulation. The therapeutic relevance of this contribution, such as whether selective activation of CNS AT2R can efficaciously reduce blood pressure and offset hypertensive mechanisms, including AT1R activation, and alleviate end organ damage, can only be determined by investigating whether specific neuronal circuits that regulate cardiovascular function are indeed influenced by AT2R activation.
AT2R stimulation and hypertensive end organ damage
Stimulation of peripheral AT2R has been tested in models of hypertension-induced kidney damage [14], cerebral haemorrhage [14], and vascular remodelling [15,17].
Remarkably, in all of these studies performed in hypertensive animals, systemic AT2R-stimulation had no anti-hypertensive effect, i.e. all protective effects of AT2R-stimulation as determined on molecular level or in terms of organ function were not secondary to BP-lowering effects, but independent of BP levels.
The first evidence for a protective effect of AT2R-stimulation with C21 in a model of hypertensive end-organ damage came from a study by Luigi Sironi’s group performed in SHR-SP on a high-salt diet [14]. This study investigated both renal and cerebral hypertension-induced pathology. With respect to the kidneys, oral administration of C21 ameliorated hypertensive nephropathy as shown by an inhibition of renal inflammation, vimentin overexpression and collagen accumulation. Brain pathology was assessed by MRI and by analysing survival rates, since these rats usually die of stroke. Survival as well as time until occurrence of brain pathology was extended in SHR-SP treated with C21. These effects of C21 could be inhibited by the AT2R-antagonist PD123319 indicating that they were AT2R-specific. The protective effect of C21 became only apparent at the highest dose used, which was 10 mg/kg/day p.o. This dose is much higher than that normally used in rats, which can be explained by the fact that in this study C21 was applied in 0.5% sodium carboxymethylcellulose as vehicle, which is known to delay drug release.
So far, the study reviewed above is the only one that has investigated application of an AT2R-agonist in hypertension-induced hemorrhagic stroke. However, there are a number of publications reporting reduced infarct size and improved neurological outcome by AT2R-stimulation in ischemic stroke (the main risk factor for which is hypertension), no matter whether treatment was started prior to or up to 6 hours after stroke, and no matter whether the AT2R-agonist was applied peripherally or into the cerebroventricles [46–51].
In 2012, the group of Ernesto Schiffrin and our group published two studies, which both investigated the effect of AT2R-stimulation by C21 on vascular remodelling [15,17]. While the Schiffrin group used SHR-SP (fed a diet with normal salt content) for their study, our study was performed in Wistar rats treated with the inhibitor of nitric oxide synthase, N-Nitro-L-Arginine-Methyl Ester (L-NAME). In both studies, over a 6 week period rats gradually developed hypertension of up to 190 mmHg systolic blood pressure (SBP). AT2R-stimulation had no effect on SBP, while an AT1R-blocker (ARB), which was used in both studies for comparison (losartan in the Schiffrin study, Olmesartan in our study), completely prevented the gradual rise in BP. Both studies were in agreement that despite the lack of BP reduction, C21 significantly reduced collagen accumulation within the vascular wall to a similar extent as the ARB, which normalised BP. Using different techniques, i.e. measurement of stress/strain relationship in mesenteric arteries ex vivo or pulse wave velocity in vivo, respectively, both studies further revealed a reduction in vascular stiffness by treatment with C21. They also conform in terms of an additive effect of AT2R-stimulation and AT1R-blockade on the prevention of vascular collagen accumulation – again despite a lack of an additive BP lowering effect.
Conclusions
In conclusion, current evidence indicates that systemic application (intravenous, intraperitoneal or oral) of AT2R-agonists has no BP-lowering effect in vivo, no matter whether they are applied to normotensive or hypertensive animals, despite a vasodilatory effect ex vivo. However, a blood pressure lowering effect of AT2R-stimulation in vivo can be unmasked through dampening the prevailing angiotensinergic tone (mediated via AT1R) by low-dose AT1R-blockade. It may also be unmasked by anaesthesia through unknown mechanisms, whereas the state of activation of the RAS seems to play no role.
In contrast to systemic application, application of AT2R-agonists into the brain and possibly also into the kidney does exert a blood pressure lowering effect in normotensive animals, as does overexpression of AT2R within CNS cardiovascular control centres of hypertensive rats.
Despite the lack of an antihypertensive effect in most instances, AT2R-stimulation is still able to attenuate hypertensive end-organ damage in kidneys, vasculature and the brain.
According to current knowledge, AT2R-stimulation will most probably not become a future therapeutic approach for the treatment of hypertension – at least as long as AT2R-agonists that are able to cross the blood-brain-barrier and have central effects after peripheral application are unavailable. Nevertheless, AT2R-agonism may be therapeutically effective in preventing hypertensive end-organ damage when used in combination with established anti-hypertensive drugs.
Highlights.
- The AT2-receptor (AT2R) mediates vasodilation
- Systemic application of AT2R-agonists does not lower blood pressure
- Low-dose blockade of AT1-receptors unmasks the BP lowering effects of AT2Rs
- Intracerebroventricular or intrarenal application of AT2R-agonists reduces BP.
- AT2R-stimulation protects from hypertensive end-organ damage without BP lowering
Acknowledgments
This work was supported by NIH grants HL-076803 (CS), HL-093186 (CS), HL-096830 (EGK), T32-HL-083810 (ADdK) and F32-HL-116074 (ADdK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
U. Muscha Steckelings received modest research support from Vicore Pharma. The other authors have no conflicts to declare.
References
- 1.Santos RAS, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J. Endocrinol. 2013;216:R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 2.Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, Jankowski J, Jankowski V, Sousa F, Alzamora A, et al. Discovery and characterization of alamandine: a novel component of the renin-angiotensin system. Circ. Res. 2013;112:1104–1111. doi: 10.1161/CIRCRESAHA.113.301077. [DOI] [PubMed] [Google Scholar]
- 3.Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol. Ther. 2008;120:292–316. doi: 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steckelings UM, Kaschina E, Unger T. The AT2 receptor--a matter of love and hate. Peptides. 2005;26:1401–1409. doi: 10.1016/j.peptides.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlén A, et al. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J. Med. Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- 6.Danyel LA, Schmerler P, Paulis L, Unger T, Steckelings UM. Impact of AT2-receptor stimulation on vascular biology, kidney function, and blood pressure. Integr. Blood Press. Control. 2013;6:153–161. doi: 10.2147/IBPC.S34425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J. Biol. Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- 8.Akazawa H, Yano M, Yabumoto C, Kudo-Sakamoto Y, Komuro I. Angiotensin II type 1 and type 2 receptor-induced cell signaling. Curr. Pharm. Des. 2013;19:2988–2995. doi: 10.2174/1381612811319170003. [DOI] [PubMed] [Google Scholar]
- 9.Hilliard LM, Jones ES, Steckelings UM, Unger T, Widdop RE, Denton KM. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: a novel therapeutic target for hypertension. Hypertension. 2012;59:409–414. doi: 10.1161/HYPERTENSIONAHA.111.184986. A study reporting a natriuretic effect and an increase in renal blood flow in response to AT2R-stimulation in normotensive animals with no major difference between male and female animals. [DOI] [PubMed] [Google Scholar]
- 10.Ali Q, Hussain T. AT2 receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2012;35:654–660. doi: 10.1038/hr.2012.13. A study reporting that AT2R stimulation promotes natriuresis by targeting the proximal tubules and that this effect is linked to the nitric oxide/cyclic guanosine monophosphate pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. AT2 receptor activation induces natriuresis and lowers blood pressure. Circ. Res. 2014;115:388–399. doi: 10.1161/CIRCRESAHA.115.304110. A study showing a natriuretic effect initiated by AT2R activation, which is associated with a translocation of the AT2R to the renal proximal tubule cell apical membrane, and the bradykinin-nitric oxide-cGMP-dependent internalization of Na(+)-H(+) exchanger-3 and Na(+)/K(+)ATPase. The authors further report that intrarenal infusion of C21 lowers blood pressure in Ang II dependent hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilliard LM, Chow CLE, Mirabito KM, Steckelings UM, Unger T, Widdop RE, Denton KM. Angiotensin type 2 receptor stimulation increases renal function in female, but not male, spontaneously hypertensive rats. Hypertension. 2014;64:378–383. doi: 10.1161/HYPERTENSIONAHA.113.02809. A study showing that acute AT2R stimulation in female, but not in male rats leads to renal vasodilatation and sodium excretion without alterations in glomerular filtration rate or blood pressure. [DOI] [PubMed] [Google Scholar]
- 13.Brouwers S, Smolders I, Massie A, Dupont AG. Angiotensin II type 2 receptor-mediated and nitric oxide-dependent renal vasodilator response to compound 21 unmasked by angiotensin-converting enzyme inhibition in spontaneously hypertensive rats in vivo. Hypertension. 2013;62:920–926. doi: 10.1161/HYPERTENSIONAHA.112.00762. A study reporting unmasking of a renal vasodilator effect to AT2R-stimulation by angiotensin-converting enzyme inhibition in the absence of additive blood pressure lowering. [DOI] [PubMed] [Google Scholar]
- 14.Gelosa P, Pignieri A, Fändriks L, de Gasparo M, Hallberg A, Banfi C, Castiglioni L, Turolo L, Guerrini U, Tremoli E, et al. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J. Hypertens. 2009;27:2444–2451. doi: 10.1097/HJH.0b013e3283311ba1. [DOI] [PubMed] [Google Scholar]
- 15.Rehman A, Leibowitz A, Yamamoto N, Rautureau Y, Paradis P, Schiffrin EL. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2012;59:291–299. doi: 10.1161/HYPERTENSIONAHA.111.180158. A study showing that AT2R-stimulation attenuates vascular collagen-accumulation and vascular stiffness in SHR-SP, although blood pressure was not lowered. [DOI] [PubMed] [Google Scholar]
- 16.Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br. J. Pharmacol. 2010;159:709–716. doi: 10.1111/j.1476-5381.2009.00575.x. A study showing that AT2R-stimulation by C21 lowers blood pressure only when combined with low-dose AT1R-blockade, and that very high doses of C21 increase blood pressure through AT1R-activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulis L, Becker STR, Lucht K, Schwengel K, Slavic S, Kaschina E, Thöne-Reineke C, Dahlöf B, Baulmann J, Unger T, et al. Direct angiotensin II type 2 receptor stimulation in Nω-nitro-L-arginine-methyl ester-induced hypertension: the effect on pulse wave velocity and aortic remodeling. Hypertension. 2012;59:485–492. doi: 10.1161/HYPERTENSIONAHA.111.185496. A study showing that AT2R-stimulation attenuates hypertension-induced vascular collagen-accumulation and reduces pulse wave velocity, although blood pressure was not lowered. [DOI] [PubMed] [Google Scholar]
- 18.Matavelli LC, Huang J, Siragy HM. Angiotensin AT2 receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57:308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202. A study reporting a lack of blood pressure reduction, but an anti-inflammatory effect by AT2R-stimulation in a 2-kidney-1-clip model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, et al. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation. 2008;118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- 20.Steckelings UM, Rompe F, Kaschina E, Namsolleck P, Grzesiak A, Funke-Kaiser H, Bader M, Unger T. The past, present and future of angiotensin II type 2 receptor stimulation. J. Renin-Angiotensin-Aldosterone Syst. JRAAS. 2010;11:67–73. doi: 10.1177/1470320309347791. [DOI] [PubMed] [Google Scholar]
- 21.Barber MN, Sampey DB, Widdop RE. AT(2) receptor stimulation enhances antihypertensive effect of AT(1) receptor antagonist in hypertensive rats. Hypertension. 1999;34:1112–1116. doi: 10.1161/01.hyp.34.5.1112. [DOI] [PubMed] [Google Scholar]
- 22.Carey RM, Howell NL, Jin XH, Siragy HM. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension. 2001;38:1272–1277. doi: 10.1161/hy1201.096576. [DOI] [PubMed] [Google Scholar]
- 23.Jones ES, Del Borgo MP, Kirsch JF, Clayton D, Bosnyak S, Welungoda I, Hausler N, Unabia S, Perlmutter P, Thomas WG, et al. A single beta-amino acid substitution to angiotensin II confers AT2 receptor selectivity and vascular function. Hypertension. 2011;57:570–576. doi: 10.1161/HYPERTENSIONAHA.110.164301. [DOI] [PubMed] [Google Scholar]
- 24.Carey RM. The intrarenal renin-angiotensin and dopaminergic systems: control of renal sodium excretion and blood pressure. Hypertension. 2013;61:673–680. doi: 10.1161/HYPERTENSIONAHA.111.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, Moritz KM, Evans RG, Denton KM. Sex- and age-related differences in the chronic pressure-natriuresis relationship: role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol. 2014;307:F901–907. doi: 10.1152/ajprenal.00288.2014. A study demonstrating that the chronic pressure-natriuresis relationship is modulated by the angiotensin type 2 receptor in an age- and sex-dependent manner. [DOI] [PubMed] [Google Scholar]
- 26.Ali Q, Wu Y, Hussain T. Chronic AT2 receptor activation increases renal ACE2 activity, attenuates AT1 receptor function and blood pressure in obese Zucker rats. Kidney Int. 2013;84:931–939. doi: 10.1038/ki.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold AC, Okamoto LE, Gamboa A, Shibao C, Raj SR, Robertson D, Biaggioni I. Angiotensin II, independent of plasma renin activity, contributes to the hypertension of autonomic failure. Hypertension. 2013;61:701–706. doi: 10.1161/HYPERTENSIONAHA.111.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, Pickel VM, Davisson RL, Iadecola C. Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin II hypertension. J. Neurosci. Off. J. Soc. Neurosci. 2012;32:4878–4886. doi: 10.1523/JNEUROSCI.6262-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Callaghan EL, Choong Y-T, Jancovski N, Allen AM. Central angiotensinergic mechanisms associated with hypertension. Auton. Neurosci. Basic Clin. 2013;175:85–92. doi: 10.1016/j.autneu.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front. Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- 31.Millan MA, Jacobowitz DM, Aguilera G, Catt KJ. Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proc. Natl. Acad. Sci. U. S. A. 1991;88:11440–11444. doi: 10.1073/pnas.88.24.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 33.Gross V, Milia AF, Plehm R, Inagami T, Luft FC. Long-term blood pressure telemetry in AT2 receptor-disrupted mice. J. Hypertens. 2000;18:955–961. doi: 10.1097/00004872-200018070-00018. [DOI] [PubMed] [Google Scholar]
- 34.Matsuura T, Kumagai H, Onimaru H, Kawai A, Iigaya K, Onami T, Sakata K, Oshima N, Sugaya T, Saruta T. Electrophysiological properties of rostral ventrolateral medulla neurons in angiotensin II 1a receptor knockout mice. Hypertension. 2005;46:349–354. doi: 10.1161/01.HYP.0000173421.97463.ac. [DOI] [PubMed] [Google Scholar]
- 35.Gao L, Wang W, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal rats. Hypertension. 2008;51:521–527. doi: 10.1161/HYPERTENSIONAHA.107.101717. [DOI] [PubMed] [Google Scholar]
- 36.Blanch GT, Freiria-Oliveira AH, Speretta GFF, Carrera EJ, Li H, Speth RC, Colombari E, Sumners C, Colombari DSA. Increased expression of angiotensin II type 2 receptors in the solitary-vagal complex blunts renovascular hypertension. Hypertension. 2014;64:777–783. doi: 10.1161/HYPERTENSIONAHA.114.03188. This study demonstrated that viral mediated overexpression of AT2R in the NTS exerted powerful effects to lower blood pressure and restore baroreflex function in renovascular hypertensive rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao J, Zhang H, Le KD, Chao J, Gao L. Activation of central angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am. J. Hypertens. 2011;24:724–730. doi: 10.1038/ajh.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao L, Zucker IH. AT2 receptor signaling and sympathetic regulation. Curr. Opin. Pharmacol. 2011;11:124–130. doi: 10.1016/j.coph.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao J, Zucker IH, Gao L. Activation of central angiotensin type 2 receptors by compound 21 improves arterial baroreflex sensitivity in rats with heart failure. Am. J. Hypertens. 2014;27:1248–1256. doi: 10.1093/ajh/hpu044. A study in rats with heart failure showing a blood pressure lowering effect of C21 when applied into the cerebroventricles plus an improvement of baroreflex sensitivity and a reduction in sympathetic outflow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Kloet A, Wang L, Ludin JA, Smith JA, Pioquinto DJ, Hiller H, Steckelings UM, Scheuer DA, Sumners C, Krause EG. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct. Funct. 2014 Nov 27; doi: 10.1007/s00429-014-0943-1. [Epub ahead of print] This study was the first to show discrete cellular and regional localization of AT2R in the mouse brain, and to demonstrate that in normal mice brain AT2R are localized on neurons, but not on glia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sved AF, Sved JC. Endogenous GABA acts on GABAB receptors in nucleus tractus solitarius to increase blood pressure. Brain Res. 1990;526:235–240. doi: 10.1016/0006-8993(90)91227-8. [DOI] [PubMed] [Google Scholar]
- 42.Takenaka K, Sasaki S, Nakamura K, Uchida A, Fujita H, Itoh H, Nakata T, Takeda K, Nakagawa M. Hypothalamic and medullary GABAA and GABAB-ergic systems differently regulate sympathetic and cardiovascular systems. Clin. Exp. Pharmacol. Physiol. Suppl. 1995;22:S48–50. doi: 10.1111/j.1440-1681.1995.tb02966.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsukamoto K, Sved AF. Enhanced gamma-aminobutyric acid-mediated responses in nucleus tractus solitarius of hypertensive rats. Hypertension. 1993;22:819–825. doi: 10.1161/01.hyp.22.6.819. [DOI] [PubMed] [Google Scholar]
- 44.Durgam VR, Vitela M, Mifflin SW. Enhanced gamma-aminobutyric acid-B receptor agonist responses and mRNA within the nucleus of the solitary tract in hypertension. Hypertension. 1999;33:530–536. doi: 10.1161/01.hyp.33.1.530. [DOI] [PubMed] [Google Scholar]
- 45.Vitela M, Mifflin SW. gamma-Aminobutyric acid(B) receptor-mediated responses in the nucleus tractus solitarius are altered in acute and chronic hypertension. Hypertension. 2001;37:619–622. doi: 10.1161/01.hyp.37.2.619. [DOI] [PubMed] [Google Scholar]
- 46.Alhusban A, Fouda AY, Bindu Pillai, Ishrat T, Soliman S, Fagan SC. Compound 21 is pro-angiogenic in the brain and results in sustained recovery after ischemic stroke. J. Hypertens. 2014 doi: 10.1097/HJH.0000000000000364. doi:10.1097/HJH.0000000000000364. A study showing a reduction in infarct size, neurological deficits and apoptosis and an induction of angiogenesis in C21 treated rats after transient middle, cerebral occlusion. [DOI] [PubMed] [Google Scholar]
- 47.Joseph JP, Mecca AP, Regenhardt RW, Bennion DM, Rodríguez V, Desland F, Patel NA, Pioquinto DJ, Unger T, Katovich MJ, et al. The angiotensin type 2 receptor agonist Compound 21 elicits cerebroprotection in endothelin-1 induced ischemic stroke. Neuropharmacology. 2014;81:134–141. doi: 10.1016/j.neuropharm.2014.01.044. This study showed that systemic treatment with C21 starting 4 hours after ischemic stroke in rats (and other treatment protocols) reduced intracerebral infarct size and neurological deficits, effects associated with anti-chemotactic/anti-inflammatory mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarthy CA, Vinh A, Broughton BRS, Sobey CG, Callaway JK, Widdop RE. Angiotensin II type 2 receptor stimulation initiated after stroke causes neuroprotection in conscious rats. Hypertension. 2012;60:1531–1537. doi: 10.1161/HYPERTENSIONAHA.112.199646. A study in a model of transient middle cerebral artery occlusion in spontaneously hypertensive rats showing for the first time that post-stroke AT2R-stimulation (by intracerebroventricular application of the peptide agonist CGP42112A started 6 hours after stroke) still reduces infarct size and improves motor function. [DOI] [PubMed] [Google Scholar]
- 49.McCarthy CA, Vinh A, Callaway JK, Widdop RE. Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke J. Cereb. Circ. 2009;40:1482–1489. doi: 10.1161/STROKEAHA.108.531509. [DOI] [PubMed] [Google Scholar]
- 50.McCarthy CA, Vinh A, Miller AA, Hallberg A, Alterman M, Callaway JK, Widdop RE. Direct angiotensin AT2 receptor stimulation using a novel AT2 receptor agonist, compound 21, evokes neuroprotection in conscious hypertensive rats. PloS One. 2014;9:e95762. doi: 10.1371/journal.pone.0095762. A study showing that in a model of transient middle cerebral artery occlusion in spontaneously hypertensive rats, intracerebroventricular application of C21 started 6 hours after stroke conferred neuroprotection and reduced infarct size. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Min L-J, Mogi M, Tsukuda K, Jing F, Ohshima K, Nakaoka H, Kan-No H, Wang X-L, Chisaka T, Bai H-Y, et al. Direct stimulation of angiotensin II type 2 receptor initiated after stroke ameliorates ischemic brain damage. Am. J. Hypertens. 2014;27:1036–1044. doi: 10.1093/ajh/hpu015. A study in a model of permanent middle cerebral occlusion in mice showing that systemic treatment with the AT2R-agonist C21 initiated immediately after cerebral ischemia resulted in reduced infarct size and improved neurological outcome. [DOI] [PubMed] [Google Scholar]