Abstract
Since the revitalization of “the Warburg effect”, there has been great interest in mitochondrial oxidative metabolism, not only from the cancer perspective but also from the general biomedical science field. As the center of oxidative metabolism, mitochondria and their metabolic activity are tightly controlled to meet cellular energy requirements under different physiological conditions. One such mechanism is through the inducible transcriptional co-regulators PGC1α and NCOR1, which respond to various internal or external stimuli to modulate mitochondrial function. However, the activity of such co-regulators depends on their interaction with transcriptional factors that directly bind to and control downstream target genes. The nuclear receptors PPARs and ERRs have been shown to be key transcriptional factors in regulating mitochondrial oxidative metabolism and executing the inducible effects of PGC1α and NCOR1. In this review, we summarize recent gain- and loss-of-function studies of PPARs and ERRs in metabolic tissues and discuss their unique roles in regulating different aspects of mitochondrial oxidative metabolism.
Introduction
Energy is vital to all living organisms. In humans and other mammals, the vast majority of energy is produced by oxidative metabolism in mitochondria [1]. As a cellular organelle, mitochondria are under tight control of the nucleus. Although the majority of mitochondrial proteins are encoded by nuclear DNA (nDNA) and their expression regulated by the nucleus, mitochondria retain their own genome, mitochondrial DNA (mtDNA), encoding 13 polypeptides of the electron transport chain (ETC) in mammals. However, all proteins required for mtDNA replication, transcription, and translation, as well as factors regulating such activities, are encoded by the nucleus [2].
The cellular demand for energy varies in different cells under different physiological conditions. Accordingly, the quantity and activity of mitochondria are differentially controlled by a transcriptional regulatory network in both the basal and induced states. A number of components of this network have been identified, including members of the nuclear receptor superfamily, the peroxisome proliferator-activated receptors (PPARs) and the estrogen-related receptors (ERRs) [3–5].
The Yin-Yang Co-Regulators
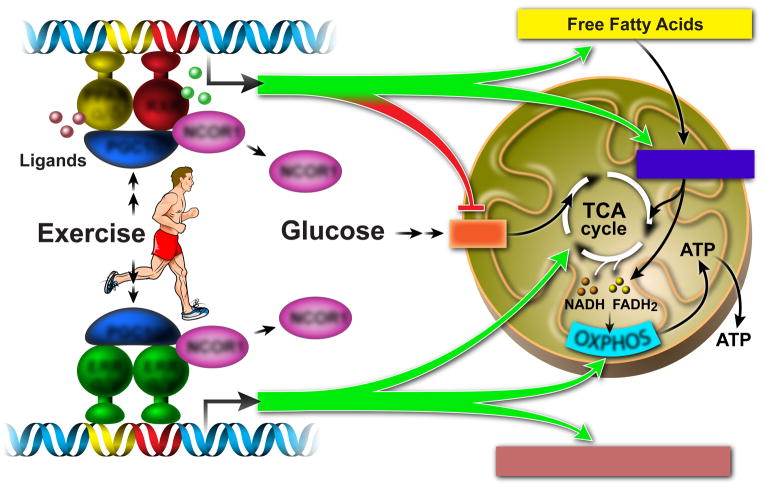
A well-known inducer of mitochondrial oxidative metabolism is the peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α) [6], a nuclear cofactor which is abundantly expressed in high energy demand tissues such as heart, skeletal muscle, and brown adipose tissue (BAT) [7]. Induction by cold-exposure, fasting, and exercise allows PGC1α to regulate mitochondrial oxidative metabolism by activating genes involved in the tricarboxylic acid cycle (TCA cycle), beta-oxidation, oxidative phosphorylation (OXPHOS), as well as mitochondrial biogenesis [6,8](Figure 1).
Figure 1. PPARs and ERRs are major executors of PGC1α-induced regulation of oxidative metabolism.
Physiological stress such as exercise induces both the expression and activity of PGC1α, which stimulates energy production by activating downstream genes involved in fatty acid and glucose metabolism, TCA cycle, β-oxidation, OXPHOS, and mitochondrial biogenesis. The transcriptional activity of PGC1α relies on its interactions with transcriptional factors such as PPARs (for controlling fatty acid metabolism) and ERRs (for regulating mitochondrial OXPHOS).
The effect of PGC1α on mitochondrial regulation is antagonized by transcriptional corepressors such as the nuclear receptor corepressor 1 (NCOR1) [9,10]. In contrast to PGC1α, the expression of NCOR1 is suppressed in conditions where PGC1α is induced such as during fasting, high-fat-diet challenge, and exercise [9,11]. Moreover, the knockout of NCOR1 phenotypically mimics PGC1α overexpression in regulating mitochondrial oxidative metabolism [9]. Therefore, coactivators and corepressors collectively regulate mitochondrial metabolism in a Yin-Yang fashion.
However, both PGC1α and NCOR1 lack DNA binding activity and rather act via their interaction with transcription factors that direct the regulatory program. Therefore the transcriptional factors that partner with PGC1α and NCOR1 mediate the molecular signaling cascades and execute their inducible effects on mitochondrial regulation.
PPARs: Master Executors Controlling Fatty Acid Oxidation
Both PGC1α and NCOR1 are co-factors for the peroxisome proliferator-activated receptors (PPARα, γ, and δ) [7,11–13]. It is now clear that all three PPARs play essential roles in lipid and fatty acid metabolism by directly binding to and modulating genes involved in fat metabolism [13–19]. While PPARγ is known as a master regulator for adipocyte differentiation and does not seem to be involved with oxidative metabolism [14,20], both PPARα and PPARδ are essential regulators of fatty acid oxidation (FAO) [3,13,15,19,21] (Figure 1).
PPARα was first cloned as the molecular target of fibrates, a class of cholesterol-lowering compounds that increase hepatic FAO [22]. The importance of PPARα in regulating FAO is indicated in its expression pattern which is restricted to tissues with high capacity of FAO such as heart, liver, BAT, and oxidative muscle [23]. On the other hand, PPARδ is ubiquitously expressed with higher levels in the digestive tract, heart, and BAT [24]. In the past 15 years, extensive studies using gain- and loss-of-function models have clearly demonstrated PPARα and PPARδ as the major drivers of FAO in a wide variety of tissues.
Heart
The adult heart relies heavily on FAO as the energy source. PPARα plays an important role in regulating cardiac FAO. However, it only activates genes in FA metabolic pathways such as fatty acid uptake and beta-oxidation, rather than in the TCA cycle or OXPHOS [15]. Notably, PPARα activation suppresses mitochondrial OXPHOS genes that are regulated by PGC1α and ERRs in cardiomyocytes [25]. When ectopically expressed in the heart, PPARα induces cellular FA uptake and beta-oxidation while reducing glucose import and glycolysis [15]. However, the increased FA uptake seems to exceed the burning capacity of mitochondrial OXPHOS, resulting in myocardial lipid accumulation and cardiac hypertrophy [15]. Knockout of PPARα leads to reduced FA uptake and beta-oxidation [26], further confirming its importance in regulating FAO. PPARδ shares certain similarities with PPARα in regulating FAO in the heart [16]. When overexpressed, PPARδ also induces FAO by up-regulating genes in mitochondrial FA transport and beta-oxidation. But unlike PPARα, overexpression of PPARδ does not cause lipid accumulation or cardiac dysfunction, likely due to increased glucose utilization [19]. The importance of PPARδ in regulating cardiac FAO has been further demonstrated in a loss-of-function study, where heart-specific deletion of PPARδ causes down-regulation of key FAO genes and reduces FAO, resulting in myocardial lipid accumulation and cardiac hypertrophy [27].
Skeletal Muscle
Skeletal muscle also burns fatty acids for energy production. There are generally two types of muscle fibers: type I oxidative fibers that are rich in mitochondria and predominantly powered by oxidation of glucose and FA; and type II glycolytic fibers that contain less mitochondria and heavily rely on glycolysis for energy [28].
Both PPARα and PPARδ play important roles in regulating muscle FAO. Overexpression of either in muscle induces FAO by up-regulating genes involved in fatty acid utilization and beta-oxidation [17,18,29,30]. However, PPARα overexpression also promotes a fiber-type transition towards more glycolytic fibers which have lower mitochondrial OXPHOS activity. This induces lipid accumulation and drives the development of glucose intolerance and insulin resistance [18,30]. PPARα deletion, on the other hand, increases the number of oxidative fibers and improves glucose homeostasis, despite the reduction in muscle FAO [18,30].
In contrast to PPARα, overexpression of PPARδ does not cause lipid accumulation or glucose intolerance [17,29]. In addition to induced FAO, PPARδ also increases the proportion of oxidative fibers that are rich in mitochondria, thus dramatically boosting mitochondrial oxidative metabolism. This is associated with ~90% increase in endurance capacity and resistance against diet-induced diabetes [17,30]. Conversely, muscle-specific PPARδ depletion leads to an oxidative-to-glycolytic fiber-type switch with reduced FAO and OXPHOS. The mutant mice gain more weight on high fat diet and are more susceptible to insulin resistance [31].
The ability of PPARα and PPARδ to transform fiber types in opposite directions seem to be mediated by two microRNAs, miR-208b and miR-499 [30], which directly activate the oxidative and repress the glycolytic myofibril gene program [32].
Liver
In the liver PPARα is the predominant PPAR isoform [24]. It is essential in regulating hepatic FA uptake, beta-oxidation, and ketogenesis, especially during fasting. PPARα knockout suppresses the expression of genes involved in FA uptake and FAO, resulting in decreased basal-state hepatic FA uptake and beta-oxidation [33]. In addition, fasting-induced hepatic responses, including elevated FA oxidation, gluconeogenesis, and ketogenesis, are all impaired in PPARα-null mice. As a result, the fasted mutant mice develop hypoketogenesis, hypoglycemia, and liver steatosis [34] [26].
PPARδ seems to play a different role in regulating hepatic energy metabolism. Unlike PPARα, PPARδ deletion reduces the expression of genes involved in lipogenesis and glucose utilization instead of FA metabolism. Moreover, fasted PPARδ-null mice have a normal ketogenic response, increased serum glucose levels and no sign of liver steatosis [35]. When overexpressed in the liver, PPARδ increases hepatic glycogen and lipid storage as a result of up-regulation of genes in glucose utilization and lipogenesis [36]. In addition, hepatic PPARδ activates muscle FA oxidation through a lipid molecule PC(18:0/18:1), which is produced by a PPARδ-dependent lipogenic pathway [37].
Fat
PPARα is highly expressed in BAT but not in white adipose tissue (WAT) [24]. Its primary function in BAT seems to be the regulation of PGC1α and UCP1 (a mitochondrial uncoupling protein) expression. PPARα deletion reduces expression of PGC1α and UCP1 under both basal and cold-exposure conditions [21,38]. However, unlike in other tissues, FA metabolism in BAT is not affected by PPARα knockout, suggesting the contribution of other PPARs [39]. When activated in human and mouse adipocytes, PPARα induces the expression of FAO genes and increases energy expenditure [40] [41].
PPARδ is expressed in both BAT and WAT. While its function in WAT is unknown, it is clear that PPARδ plays an essential role in regulating FA oxidation and thermogenesis in BAT [13,42,43]. When ectopically expressed in adipose tissue, PPARδ dramatically induces the expression of genes in FAO, OXPHOS, and thermogenesis, which results in increased FAO in BAT, reduced systemic adiposity and improved serum lipid profiles [13,44]. Conversely, deletion of PPARδ in BAT reduces the expression of FAO and thermogenic genes, which impairs in vivo thermogenesis [13,43].
ERRS: Master Executors Controlling Mitochondrial OXPHOS
ERRs are essential regulators of mitochondrial energy metabolism [4]. ERRα is ubiquitously expressed but particularly abundant in tissues with high energy demands such as brain, heart, muscle, and BAT. ERRβ and ERRγ have similar expression patterns, both are selectively expressed in highly oxidative tissues including brain, heart, and oxidative muscle [45]. Instead of endogenous ligands, the transcriptional activity of ERRs is primarily regulated by co-factors such as PGC1αand NCOR1 [4,46] (Figure 1).
Of the three ERRs, ERRβ is the least studied and its role in regulating mitochondrial function is unclear [4,47]. In contrast, when PGC1α is induced, ERRα is the master regulator of the mitochondrial biogenic gene network. As ERRα binds to its own promoter, PGC1α can also induce an autoregulatory loop to enhance overall ERRα activity [48]. Without ERRα, the ability of PGC1α to induce the expression of mitochondrial genes is severely impaired. However, the basal-state levels of mitochondrial target genes are not affected by ERRα deletion, suggesting induced mitochondrial biogenesis is a transient process and that other transcriptional factors such as ERRγ may be important maintaining baseline mitochondrial OXPHOS [41–43]. Consistent with this idea, ERRγ(which is active even when PGC1α is not induced) shares many target genes with ERRα[49,50].
Genome-wide analysis of ERRα and ERRγ has confirmed their direct and overlapping binding in promoter regions of a large number of mitochondrial genes, many of which are PGC1α targets [8,49]. These genes cover many aspects of mitochondrial oxidative metabolism, ranging from glucose utilization, FA oxidation, the TCA cycle, and OXPHOS. About a quarter of the binding sites are shared by both ERRs, indicating their cooperative regulation of those genes. Recently several gain- and loss-of-function studies have revealed a better understanding of their in vivo roles [44–50].
Heart
Both ERRα and ERRγ are highly expressed in the heart [45]. Knockout of ERRα causes down-regulation of a number of genes in mitochondrial oxidative metabolism, some of which are direct ERRα targets. Interestingly, another set of genes in mitochondrial oxidative metabolism, including PGC1α and ERRγ, are up-regulated by ERRα deletion, indicating a compensatory effect of ERRγ [49]. The ERRα-null heart only shows minimal defects with normal mitochondrial function in the basal state. However, when challenged with pressure overload (a common method to induce cardiac hypertrophy), the knockout mice exhibit more severe phenotypes with dilated hypertrophy and early heart failure, possibly due to a defect in energy reserve, indicating the requirement of ERRαduring stressed conditions [51].
Similar to ERRα, ERRγ deletion also causes both down- and up-regulation of genes in mitochondrial oxidative metabolism, as well as up-regulated PGC1α and ERRα. The altered expression of OXPHOS genes causes severe mitochondrial defects: increased mtDNA copy number, reduced ETC complex I activity, and increased complex IV activity. As a result, most ERRγ-null mice die within the first week of their life due to heart failure [50].
Skeletal Muscle
ERRα is uniformly expressed in both oxidative and glycolytic muscles [52]. The knockout of ERRα in skeletal muscle causes no phenotypic change in the basal state [53] [54]. However, in a cardiotoxin induced injury model, its deficiency reduces mitochondrial activity and impairs muscle regeneration [54].
Unlike ERRα, ERRγ is selectively expressed in oxidative muscle [52]. When ectopically expressed in glycolytic muscle, ERRγ drives a fiber-type switch from glycolytic to oxidative fibers, with dramatically induced mitochondrial biogenesis and vascularization. This is accompanied by the induction of genes in pathways such as FA oxidation, TCA cycle, and OXPHOS, many of which are direct ERR targets [49,52,55]. However, whether ERRγ is required for basal-sate mitochondrial function in oxidative muscle remains unclear.
Fat
Both ERRα and ERRγ are expressed higher in BAT than in WAT [45]. While the role of ERRγ in fat is still under investigation, ERRα seems to be required in regulating stress-induced metabolism in both WAT and BAT. Whole-body knockout of ERRα causes reduced fat mass which becomes more striking when challenged with a high-fat diet. The ERRα-null WAT has altered expression of metabolic genes including direct ERR targets [56]. When challenged with cold-exposure, the mutant mice display impaired thermogenesis, accompanied by decreased mitochondrial biogenesis and increased lipid accumulation in BAT. Gene expression analysis reveals repression of mitochondrial oxidative metabolism genes, many of which are direct ERR targets [57].
Therefore, ERRα and ERRγ cooperatively regulate oxidative metabolism by directly controlling the expression of genes in this pathway. Despite their shared target genes, each appears to have its own unique function, with ERRα more involved in stress-induced responses and ERRγ required for maintaining the baseline mitochondrial integrity.
Conclusion and Perspectives
Taken together, recent studies have clearly demonstrated the essential roles of PPARs and ERRs in regulating mitochondrial oxidative metabolism and executing the inducible effects of PGC1α (Figure 1). Both PPARα and PPARδ are key regulators for FA oxidation. While the function of PPARα seems more restricted in FA uptake, beta-oxidation, and ketogenesis, PPARδ plays a broader role in controlling oxidative metabolism and fuel preference, with its target genes involved in FA oxidation, mitochondrial OXPHOS, and glucose utilization. However, it is still not clear how much redundancy exists between PPARα and PPARδ, a question which may require the generation of a double knockout model. In addition, more effort is needed to fully understand how PPARα and PPARδ control their target genes in response to environmental changes.
Likewise, ERRα and ERRγ have been shown to be key regulators of mitochondrial OXPHOS. Knockout studies of ERRαsuggest it to be the principal executor of PGC1αinduced up-regulation of mitochondrial genes, though its role in exercise-dependent changes in skeletal muscle needs further investigation. Transgenic models have demonstrated ERRγ’s powerful induction of mitochondrial biogenesis and its ability to act in a PGC1α-independent manner. However, it remains to be elucidated whether ERRγ is sufficient for basal-state mitochondrial function in general, and whether ERRα can compensate for its function.
Acknowledgments
We thank C. Brondos for administrative support, R. Yu and M. Downes for discussion and comments. R.M.E. is an HHMI Investigator at the Salk Institute for Biological Studies and March of Dimes Chair. W.F. and R.M.E. are supported by NIH grants (DK057978, DK090962, HL088093, HL105278 and ES010337), the Glenn Foundation for Medical Research, the Leona M. and Harry B. Helmsley Charitable Trust, Ipsen/Biomeasure, CIRM, and The Ellison Medical Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23:459–466. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poulsen L, Siersbaek M, Mandrup S. PPARs: fatty acid sensors controlling metabolism. Semin Cell Dev Biol. 2012;23:631–639. doi: 10.1016/j.semcdb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Eichner LJ, Giguere V. Estrogen related receptors (ERRs): a new dawn in transcriptional control of mitochondrial gene networks. Mitochondrion. 2011;11:544–552. doi: 10.1016/j.mito.2011.03.121. [DOI] [PubMed] [Google Scholar]
- 5.Fan W, Downes M, Atkins A, Yu R, Evans RM. Nuclear receptors and AMPK: resetting metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:17–22. doi: 10.1101/sqb.2012.76.010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 7.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 8.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto H, Williams EG, Mouchiroud L, Canto C, Fan WW, Downes M, Heligon C, Barish GD, Desvergne B, Evans RM, et al. NCoR1 Is a Conserved Physiological Modulator of Muscle Mass and Oxidative Function. Cell. 2011;147:827–839. doi: 10.1016/j.cell.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Fan W, Xu J, Lu M, Yamamoto H, Auwerx J, Sears DD, Talukdar S, Oh D, Chen A, et al. Adipocyte NCoR knockout decreases PPARgamma phosphorylation and enhances PPARgamma activity and insulin sensitivity. Cell. 2011;147:815–826. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Schindler J, Summermatter S, Salatino S, Zorzato F, Beer M, Balwierz PJ, van Nimwegen E, Feige JN, Auwerx J, Handschin C. The corepressor NCoR1 antagonizes PGC-1alpha and estrogen-related receptor alpha in the regulation of skeletal muscle function and oxidative metabolism. Molecular and Cellular Biology. 2012;32:4913–4924. doi: 10.1128/MCB.00877-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 14.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 15.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilde AJ, van der Lee KA, Willemsen PH, Chinetti G, van der Leij FR, van der Vusse GJ, Staels B, van Bilsen M. Peroxisome proliferator-activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ Res. 2003;92:518–524. doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- 17.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere LL, Holloszy JO, Semenkovich CF, Kelly DP. A potential link between muscle peroxisome proliferator-activated receptor-alpha signaling and obesity-related diabetes. Cell Metab. 2005;1:133–144. doi: 10.1016/j.cmet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest. 2007;117:3930–3939. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Mullican SE, DiSpirito JR, Peed LC, Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARgamma. Proc Natl Acad Sci U S A. 2013;110:18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbera MJ, Schluter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem. 2001;276:1486–1493. doi: 10.1074/jbc.M006246200. [DOI] [PubMed] [Google Scholar]
- 22.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 23.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 24.Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, Desvergne B. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology. 2001;142:4195–4202. doi: 10.1210/endo.142.10.8458. [DOI] [PubMed] [Google Scholar]
- *25.Oka S, Alcendor R, Zhai P, Park JY, Shao D, Cho J, Yamamoto T, Tian B, Sadoshima J. PPARalpha-Sirt1 complex mediates cardiac hypertrophy and failure through suppression of the ERR transcriptional pathway. Cell Metab. 2011;14:598–611. doi: 10.1016/j.cmet.2011.10.001. Demonstrates the negative impact of Pparα on cardiac mitochondrial function by directly recruiting Sirt1 to ERR-response elements in mitochondrial genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPAR alpha) in the cellular fasting response: The PPAR alpha-null mouse as a model of fatty acid oxidation disorders. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 28.Fan W, Atkins AR, Yu RT, Downes M, Evans RM. Road to exercise mimetics: targeting nuclear receptors in skeletal muscle. J Mol Endocrinol. 2013;51:T87–T100. doi: 10.1530/JME-13-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Gan Z, Burkart-Hartman EM, Han DH, Finck B, Leone TC, Smith EY, Ayala JE, Holloszy J, Kelly DP. The nuclear receptor PPARbeta/delta programs muscle glucose metabolism in cooperation with AMPK and MEF2. Genes Dev. 2011;25:2619–2630. doi: 10.1101/gad.178434.111. Using muscle-specific transgenic mouse models, this study illustrates that Pparδ but not Pparα positively regulates muscle glucose oxidation by directly activating lactate dehydrogenase b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **30.Gan Z, Rumsey J, Hazen BC, Lai L, Leone TC, Vega RB, Xie H, Conley KE, Auwerx J, Smith SR, et al. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J Clin Invest. 2013;123:2564–2575. doi: 10.1172/JCI67652. Interesting study demonstrates the opposing roles of Pparδ and Pparα in regulating muscle fiber type composition through an Errγ-dependent micro-RNA network that targets Myh7 and Myh7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, Desvergne B, Wahli W, Chambon P, Metzger D. PGC1 alpha expression is controlled in skeletal muscles by PPAR beta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metabolism. 2006;4:407–414. doi: 10.1016/j.cmet.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 32.van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ, Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17:662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 34.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanderson LM, Boekschoten MV, Desvergne B, Muller M, Kersten S. Transcriptional profiling reveals divergent roles of PPARalpha and PPARbeta/delta in regulation of gene expression in mouse liver. Physiol Genomics. 2010;41:42–52. doi: 10.1152/physiolgenomics.00127.2009. [DOI] [PubMed] [Google Scholar]
- *36.Liu S, Hatano B, Zhao M, Yen CC, Kang K, Reilly SM, Gangl MR, Gorgun C, Balschi JA, Ntambi JM, et al. Role of peroxisome proliferator-activated receptor {delta}/{beta} in hepatic metabolic regulation. J Biol Chem. 2011;286:1237–1247. doi: 10.1074/jbc.M110.138115. A liver-restricted PPARδ overexpression study demonstrates the novel role of Pparδ in liver that enhances hepatic glucose utilization and lipogenesis which improves whole body glucose homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **37.Liu S, Brown JD, Stanya KJ, Homan E, Leidl M, Inouye K, Bhargava P, Gangl MR, Dai L, Hatano B, et al. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature. 2013;502:550–554. doi: 10.1038/nature12710. A follow-up study of #36 that identifies a circulating lipid molecule that is regulated by hepatic Pparδ activity and activates muscle lipid oxidation through Pparα. This is the first report of a liver-to-muscle metabolic crosstalk mediated by a lipid molecule. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *38.Hondares E, Rosell M, Diaz-Delfin J, Olmos Y, Monsalve M, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor alpha (PPARalpha) induces PPARgamma coactivator 1alpha (PGC-1alpha) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. J Biol Chem. 2011;286:43112–43122. doi: 10.1074/jbc.M111.252775. Demonstrates the requirement of Pparα in thermogenic activation in brown fat through the regulation of Pgc1α and Prdm16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goetzman ES, Tian L, Wood PA. Differential induction of genes in liver and brown adipose tissue regulated by peroxisome proliferator-activated receptor-alpha during fasting and cold exposure in acyl-CoA dehydrogenase-deficient mice. Mol Genet Metab. 2005;84:39–47. doi: 10.1016/j.ymgme.2004.09.010. [DOI] [PubMed] [Google Scholar]
- *40.Lee JY, Hashizaki H, Goto T, Sakamoto T, Takahashi N, Kawada T. Activation of peroxisome proliferator-activated receptor-alpha enhances fatty acid oxidation in human adipocytes. Biochem Biophys Res Commun. 2011;407:818–822. doi: 10.1016/j.bbrc.2011.03.106. [DOI] [PubMed] [Google Scholar]
- *41.Goto T, Lee JY, Teraminami A, Kim YI, Hirai S, Uemura T, Inoue H, Takahashi N, Kawada T. Activation of peroxisome proliferator-activated receptor-alpha stimulates both differentiation and fatty acid oxidation in adipocytes. J Lipid Res. 2011;52:873–884. doi: 10.1194/jlr.M011320. These two studies elucidate the positive impact of Pparα in activating the FAO program in cultured adipocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci U S A. 2002;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan D, Fujimoto M, Lopes A, Wang YX. Twist-1 is a PPARdelta-inducible, negative-feedback regulator of PGC-1alpha in brown fat metabolism. Cell. 2009;137:73–86. doi: 10.1016/j.cell.2009.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts LD, Murray AJ, Menassa D, Ashmore T, Nicholls AW, Griffin JL. The contrasting roles of PPARdelta and PPARgamma in regulating the metabolic switch between oxidation and storage of fats in white adipose tissue. Genome Biol. 2011;12:R75. doi: 10.1186/gb-2011-12-8-r75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- 47.Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguere V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature. 1997;388:778–782. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- 48.Laganiere J, Tremblay GB, Dufour CR, Giroux S, Rousseau F, Giguere V. A polymorphic autoregulatory hormone response element in the human estrogen-related receptor alpha (ERRalpha) promoter dictates peroxisome proliferator-activated receptor gamma coactivator-1alpha control of ERRalpha expression. J Biol Chem. 2004;279:18504–18510. doi: 10.1074/jbc.M313543200. [DOI] [PubMed] [Google Scholar]
- 49.Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab. 2007;5:345–356. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, et al. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6:13–24. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguere V, Murphy E, Kelly DP. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- **52.Narkar VA, Fan W, Downes M, Yu RT, Jonker JW, Alaynick WA, Banayo E, Karunasiri MS, Lorca S, Evans RM. Exercise and PGC-1alpha-independent synchronization of type I muscle metabolism and vasculature by ERRgamma. Cell Metabolism. 2011;13:283–293. doi: 10.1016/j.cmet.2011.01.019. This in vivo study nicely demonstrates that the overexpression of Errγ in skeletal muscle induces oxidative fiber-type transformation by upregulating mitochondrial and angiogenic genes without the induction of Pgc1α. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huss JM, Torra IP, Staels B, Giguere V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol. 2004;24:9079–9091. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LaBarge S, McDonald M, Smith-Powell L, Auwerx J, Huss JM. Estrogen-related receptor-alpha (ERRalpha) deficiency in skeletal muscle impairs regeneration in response to injury. FASEB J. 2014;28:1082–1097. doi: 10.1096/fj.13-229211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *55.Rangwala SM, Wang XM, Calvo JA, Lindsley L, Zhang YY, Deyneko G, Beaulieu V, Gao JP, Turner G, Markovits J. Estrogen-related Receptor gamma Is a Key Regulator of Muscle Mitochondrial Activity and Oxidative Capacity. Journal of Biological Chemistry. 2010;285:22619–22629. doi: 10.1074/jbc.M110.125401. This study shows that the overexpression of Errγ in skeletal muscle induces oxidative fiber-type transformation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguere V. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor alpha. Mol Cell Biol. 2003;23:7947–7956. doi: 10.1128/MCB.23.22.7947-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villena JA, Hock MB, Chang WY, Barcas JE, Giguere V, Kralli A. Orphan nuclear receptor estrogen-related receptor alpha is essential for adaptive thermogenesis. Proc Natl Acad Sci U S A. 2007;104:1418–1423. doi: 10.1073/pnas.0607696104. [DOI] [PMC free article] [PubMed] [Google Scholar]