Abstract
The octocoral Swiftia exserta has been utilized extensively in our laboratory to study innate immune reactions in Cnidaria such as wound healing, auto- and allo-graft reactions, and for some classical “foreign body” phagocytosis experiments. All of these reactions occur in the coenenchyme of the animal, the colonial tissue surrounding the axial skeleton in which the polyps are embedded, and do not rely on nematocysts or directly involve the polyps. In order to better understand some of the cellular reactions occurring in the coenenchyme, the present study employed several cytochemical methods (periodic acid–Schiff reaction, Mallory’s aniline blue collagen stain, and Gomori’s trichrome stain) and correlated the observed structures with electron microscopy (both scanning and transmission). Eight types of cells were apparent in the coenenchyme of S. exserta, exclusive of gastrodermal tissue: (i) epithelial ectoderm cells, (ii) oblong granular cells, (iii) granular amoebocytes, (iv) morula-like cells, (v) mesogleal cells, (vi) sclerocytes, (vii) axial epithelial cells, and (viii) cnidocytes with mostly atrichous isorhiza nematocysts. Several novel organizational features are now apparent from transmission electron micrographs: the ectoderm consists of a single layer of flat epithelial cells, the cell types of the mesoglea extend from beneath the thin ectoderm throughout the mesogleal cell cords, the organization of the solenia gastroderm consists of a single layer of cells, and two nematocyst types have been found. A new interpretation of the cellular architecture of S. exserta, and more broadly, octocoral biology is now possible.
Keywords: Cnidaria, Gorgonians, Octocoral biology, Coenenchyme, Nematocysts
1. Introduction
Swiftia exserta (Cnidaria, Anthozoa, Octocorallia, Alcyonacea, Holaxonia, Plexauridae) forms branching colonies composed of a supporting hollow axis of gorgonin, an iodinated fibrous protein (Szmant-Froehlich, 1974), surrounded by coenenchyme (colonial tissue) with embedded polyps (Fig. 1a, b, and schematic diagram in Fig. 2). This species was initially described by Ellis and Solander (1786) as Gorgonia exserta and animals in this order are commonly referred to as gorgonians. We will continue to use ‘gorgonian’. The genus was renamed Swiftia and S. exserta designated the type species of the genus Swiftia described by Duchassaing and Michelotti in 1860.
Fig. 1.
(a) A complete small colony (approximately 25 cm tall) of Swiftia exserta in a holding aquarium. (b) Branchlet removed from a Swiftia exserta colony in artificial seawater. Scale in millimeters.
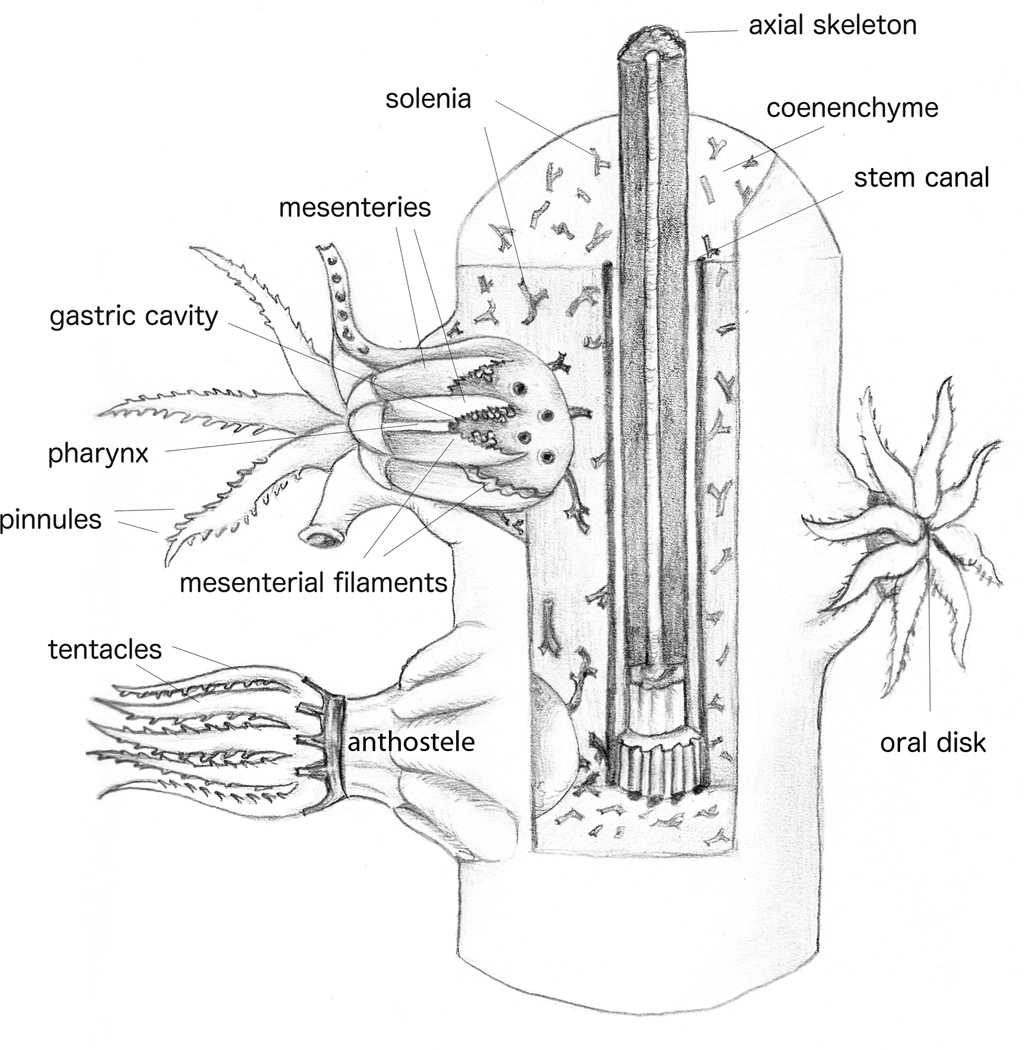
Fig. 2.
Schematic representation of an octocoral. Diagram redrawn from Bayer et al. (1983) by Ellen Bigger Streeter.
Initial characterizations of alcyonarians (Gorgonidae; Koch, 1887) described triploblastic tissue organization. Currently, Cnidaria are considered diploblastic, with an outer ectoderm separated from the endo-/gastroderm by a fibrous or gelatinous mesoglea layer. The thickness and cellular infiltration of the mesoglea varies by class among the Cnidaria (Chapman, 1974): Hydra spp. representing the Hydrozoa have a very thin acellular mesoglea (Davis and Haynes, 1968), while both the Cubozoa and Scyphozoa in the medusa stage have a thick, mostly acellular, mesoglea (Chapman, 1953). The anthozoan mesoglea is generally laced with individual cells (Tucker et al., 2011) or cords of cells (Bayer, 1974; Silveira and van’t Hof, 1977).
The polyps of octocorals, and thus gorgonians, are composed of eight pinnate (feathered) tentacles that unite at the oral disk (illustrated nicely in Koch, 1887 and Hickson, 1895; see Fig. 2 for a schematic diagram of octocorals). Tentacles are histologically simple structures composed of an outer ectoderm cell layer, a thin acellular mesoglea, and an inner endo-/gastroderm layer (Nutting, 1889; Chester, 1913). ‘Aboral’ and ‘oral’ are used to differentiate the surfaces of the hollow tentacles (Fautin and Mariscal, 1991), with the oral ectoderm facing the oral disk. The inside of the tentacles is composed of a layer of endo-/gastroderm. The tentacles unite at the oral disk, which leads into the coelenteron, yet the tentacles retain their separation internally by sheets of fibrous mesoglea. These eight dividers in the gastric cavity, termed mesenteries, are lined with musculo-epithelial cells, muscle bundles, and gastroderm (Koch, 1887; Hickson, 1895). The oral ectoderm extends into the upper gastric cavity in an area described variously as a pharynx or stomodeum. At the elongated ends of the polyp mouth is a heavily ciliated groove, the siphonoglyph (Hickson, 1883). Gonads, when present, are located along the mesenteries (Bayer et al., 1983).
In gorgonians, the gastrovascular cavities of the individual polyps are interconnected by ciliated tubes (solenia) (Murdock, 1978) and larger axis-parallel canals (Bayer, 1956, 1961; Bayer et al., 1983) (Fig. 2). Solenia are lined with endo-/gastroderm (Bayer, 1956, 1961, 1974) and are embedded in the mesogleal matrix. Solenia have been shown to circulate nutrients throughout the coenenchyme and between anthozoan polyps (Murdock, 1978; Gladfelter, 1983; Harmata et al., 2013). Similarly embedded in the mesogleal matrix are the sclerites that are characteristic of a given species and are thus used to delineate genera and species (often regardless of outward morphological differences or similarities) (Nutting, 1889; Bayer, 1974; Bayer et al., 1983; Goldberg, 2001).
Covering the mesoglea is a cellular layer of ectoderm. In various cnidarian classes the ectoderm ranges from a single columnar layer (Hickson, 1895; Chester, 1913; Bayer, 1974) to complex layers of cells (Kawaguti, 1966). In Koch’s 1887 description, the ectoderm consists of: (i) polygonal, mostly flat to cylindrical cells with fine hairs (“wimpern”) covering their exterior surface. These cells overlay a mix of other ectoderm cell types, including (ii) epithelio-muscular cells; (iii) round, undifferentiated cells; (iv) thin sensory cells with thicker projections upwards and thinner ones downward to (v) interconnected ganglion cells; and interspersed between the taller, upper, cells are (vi) cnidocytes of various sizes and shapes.
A small handful of published reports describe the histology of zooxanthellate gorgonians: Pseudoplexaura crassa Wright and Studer [RS1][now Pseudaoplexaura porosa] (Chester, 1913), Plexaura homomalla (Bayer, 1974), and Plexaura flexuosa (Silveira and van’t Hof, 1977). Kawaguti described some ultrastructural aspects of the polyps in (a) Dendrophyllia cribrosa (Kawaguti and Yokoyama, 1966); and (b) Heteroxenia elisabethae (Kawaguti, 1969). Various parts of the zooxanthellate octocoral Leptogorgia virgulata have been described in a number of reports: the spicules (sclerites) (Kingsley and Watabe, 1982a), the axial skeleton (Kingsley and Watabe, 1982b, 1983), and the polyp ectoderm (Mariscal and Bigger, 1977).
In these reports, cell types are described based on morphology, while function is inferred from structural similarity to known (mammalian) cell types. Classifying cnidarian cell types solely on morphology has limitations, however, as functional roles cannot be assigned with certainty. Only a few reports describe the presence of specific enzymes within Hydra spp. tissues (Lentz and Barrnett, 1961; Hand, 1976) and in the mesenteries of the anthozoan Pachycerianthus fimbriatus (Tiffon and Hugon, 1977) in attempts to assign some function to cell types. However, any described cell type may serve several functions, either concurrently or in developmental succession (Bigger and Hildemann, 1982).
In the present report on S. exserta we will illustrate for the first time the structure, basic cell types, and tissue organization of an azooxanthellate gorgonian. This is part of a longterm organized study of the cellular/immunological defense responses of S. exserta, a model organism from a phylum that diverged prior to the deuterostome–protostome split, including histological studies of the auto- and allo-graft reactions and phagocytic cells (e.g., Olano, 1993; Bigger and Olano, 1994; Olano and Bigger, 2000; Bigger et al., 2000), on-going studies at the protein and cellular level (e.g., Menzel and Bigger, 2013a, b; Menzel, 2013), and molecular studies to discover an ancient homolog to the complement protein C3 (Dishaw et al., 2005), which plays a pivotal role in activating the immune system. To understand the cellular responses, such as tissue fusion or rejection, response to injury, and foreign body responses, it is truly necessary to first understand the animal’s normal anatomical and cellular condition and structure.
2. Materials and methods
2.1. Maintenance of animals
Colonies of the gorgonian S. exserta were obtained from Dr. Henry Feddern (Tavernier, FL, USA), who collected them off the coast of Southeast Florida by scuba diving at depths of approximately 20–30 m. Both whole colonies and experimental tissues were maintained in 113 or 2080 l closed-system aquaria with artificial sea salts (e.g., Instant Ocean, Red Sea, or Reef Crystals) adjusted to 37 ppt salinity. Water quality was minimally conditioned via sub-gravel filters, activated charcoal filters, and protein skimmers. Care was taken to avoid contact between the colonies. The animals were fed 1–2-day old Artemia nauplii (San Francisco Bay Brand, Newark, CA, USA) three times a week. Water temperatures ranged from 19 to 23 °C.
2.2. Microscopy
2.2.1. Light microscopy
Tissues were fixed in Helley’s fixative (Clark, 1981), rinsed in 0.22 micron filtered sea water (FSW), decalcified in 2% ascorbic acid, dehydrated in a graded series of ethanol, and embedded in Paraplast (Thermo Fisher Scientific, Waltham, MA, USA) at 56 °C. Sectioning was performed on a Reichert-Jung 2800 Frigocut N cryostat at −20 °C, using a metal knife to obtain 5–6 µm sections. Tissues were stained with Harris’ hematoxylin and eosin Y (H&E), H&E/orange G; periodic acid–Schiff’s reagent/Alcian blue; toluidine blue; Mallory’s aniline blue collagen stain (without iodine); and Gomori’s trichrome stain following Clark (1981).
2.2.2. Electron microscopy
For transmission electron microscopy (TEM), short branches (4–6 cm) were cut from the larger colonies, transported to the laboratory in FSW and the polyps allowed to expand. Expanded polyps were anesthetized by adding 2 M magnesium chloride and gently fixed by the addition of paraformaldehyde (Ted Pella Inc., Redding, CA, USA) and glutaraldehyde (Ted Pella Inc.), to 4% and 2% final concentration, respectively, overnight at room temperature. Fixed branchlets were washed several times with FSW before polyps, individual tentacles, or coenenchyme pieces were dissected out and post-fixed in 1% osmium tetroxide for 4 h at room temperature. Following dehydration with a graded ethanol series and propylene oxide (Electron Microscopy Sciences, Hatfield, PA, USA), the tissue pieces were slowly infiltrated with EmBed812 (Electron Microscopy Sciences) over two days, oriented in flat embedding molds (Ted Pella), and polymerized at 60 °C for 48 h. Trimmed blocks were sectioned with a diamond knife (DuPont, Wilmington, DE, USA) on a Porter-Blum MT-2B microtome (Sorvall, Norwalk, CT, USA). Sections were double-stained with aqueous uranyl acetate for 15 min at 60 °C and Reynold’s lead citrate (Reynolds, 1963) for 2.5 min at room temperature, and viewed with a JEM1010 (JEOL, Peabody, MA, USA).
For scanning electron microscopy (SEM), branchlets were collected, relaxed, anesthetized, fixed, washed, and post-fixed as above. Short (1–2 cm) branch pieces were dehydrated through a graded ethanol series, individually mounted on SEM stubs with sugar-free chewing gum (Walter Goldberg, pers. comm.), critical-point dried with a Samdri-PVT-3D (Tousimis Research Corp., Rockville, MD, USA), coated with approximately 30 nm gold with an SPI-Module sputter coater (Structure Probe, Inc, West Chester, PA, USA), and viewed on a JSM 5910LV (JEOL).
2.3. Cell counts
Cell counts were performed by randomly selecting 25 micrographs and counting all membrane-delimited cells (electron microscopy). Since cells are not evenly distributed throughout the coenenchyme we combined all cell counts to arrive at a better approximation of cell type prevalence. Counts were converted to percentages by dividing the observed number of a given cell type (in all fields of view) by the total number of cells counted (again in all fields of view); the resultant ratio was multiplied by 100 percent to derive the relative cell population percentages.
3. Results
3.1. Coenenchyme tissue morphology
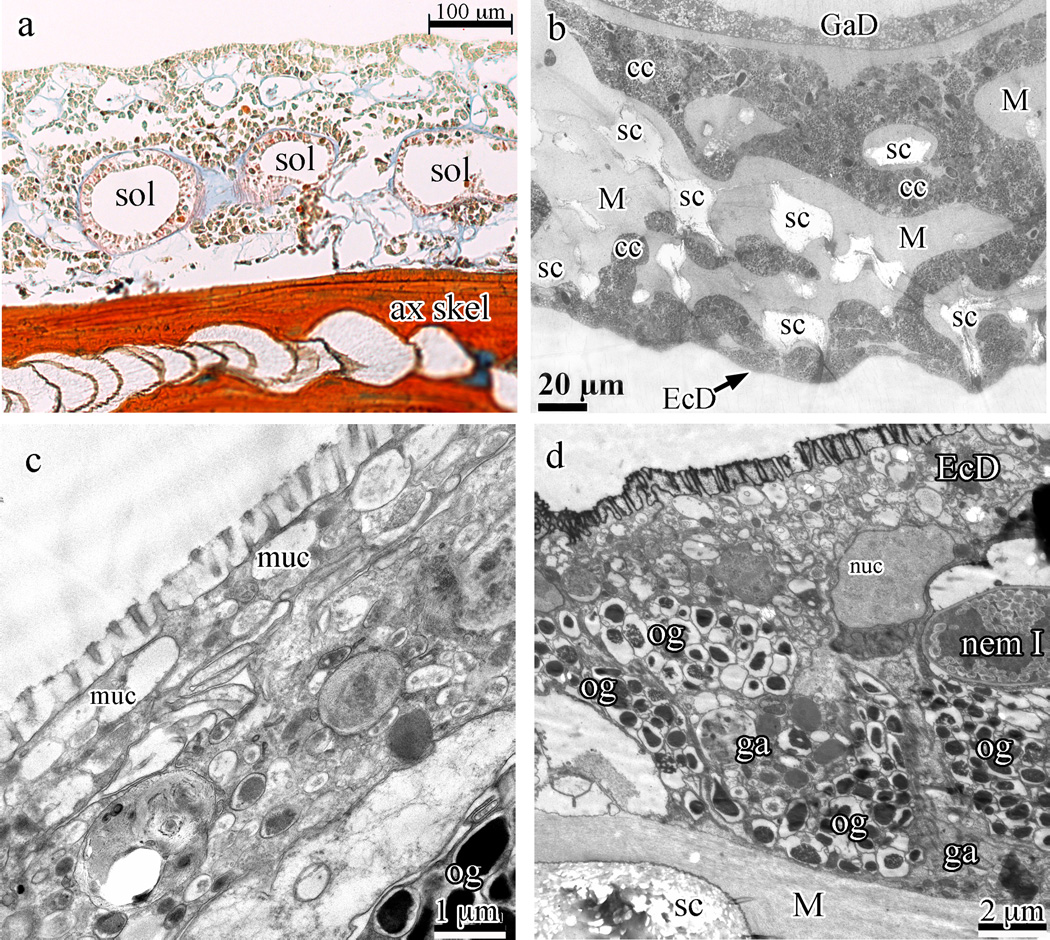
The typical anthozoan three-layered body plan (ectoderm, mesoglea, and endoderm) is present in S. exserta (Fig. 3a). These tissues surround a hollow axial skeleton as in other members of the Holaxonia (Fig. 3a). The outer layer of the coenenchyme rind is composed of a single layer of squamous epithelial cells covered with microvilli (Fig. 3b–d) overlaying oval, heavily granulated cells (Figs. 3d, 4a and b). Sclerites are embedded in a matrix of mesoglea surrounded by sclerocytes (arrows in Fig. 4a, arrowhead in Fig. 4c) and can, at times, extend to just below the surface such that only the flat ectoderm epithelium covers them.
Fig. 3.
Swiftia exserta coenenchyme and ectoderm. (a) Longitudinal paraffin section stained with Mallory’s aniline blue (connective tissue stain) showing the hollow axial skeleton (ax skel) at the bottom, several circular solenia (sol) in the mesoglea between the skeleton and the outer rind of cells, and the blue mesoglea. (b) A cross-section of the coenenchyme by TEM shows light areas within the coenenchyme that hold remnants of sclerites (sc) which do not stain with electron-dense dyes. Ectoderm (EcD) is found at the bottom of the image and gastroderm (GaD) at the top. Cell cords (cc) can be seen permeating the fibrous mesoglea (M). (c and d) High magnification TEM shows the microvilli in section and the thin, flattened ectoderm cells filled with electron-lucent granules (panel c) while panel (d) gives a survey of the stratified rind of the coenenchyme, with thin ectoderm cells (EcD) at the top covering oblong granular cells with electron-dense granules and amorphously shaped granular amoebocytes. At center-right is a type I nematocyst (nem I). Oblong granular cells (og), granular amoebocytes (ga), fibrous mesoglea (M), and sclerites (sc) are indicated in the panels.
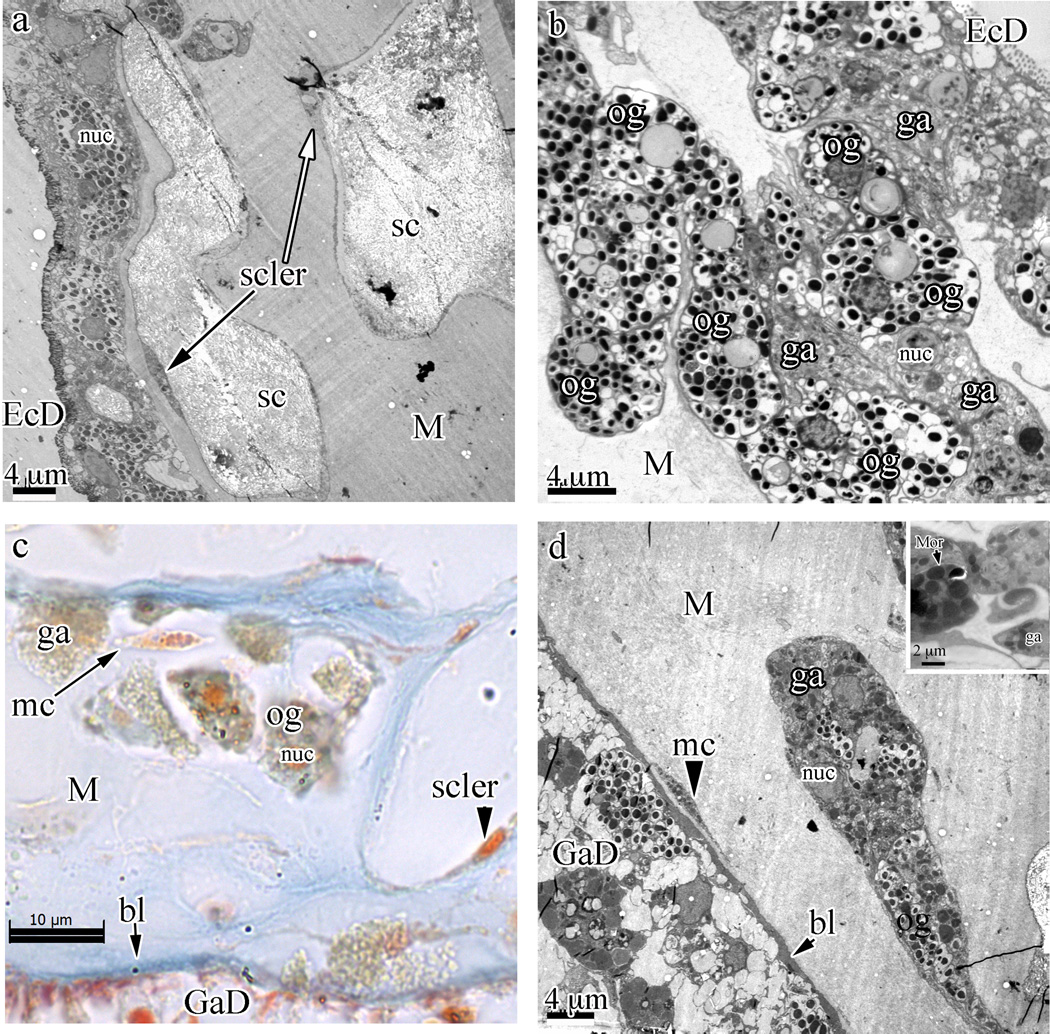
Fig. 4.
Cell cords in the coenenchyme of Swiftia exserta with embedded sclerites (sc). (a) TEM overview showing the cellular architecture of the coenenchyme from the ectoderm (EcD) into the fibrous mesoglea (M). (b) A closer view of the dense packing and the irregular shape of the cell cords filled with oblong granular cells (og, with electron-dense granules) and granular amoebocytes (ga, of indeterminate shape and with granules staining less intensely and of different sizes) shows the continuity of cells from beneath the thin ectodermal cells into the cell cords. (c) Paraffin section stained with Mallory’s aniline blue showing the acellular nature of the mesogleal matrix. The orange nucleus of a sclerocyte is visible towards the bottom right of the sclerite space (small arrowhead). Blue staining of the fibrous mesoglea matrix indicates a collagen-like stain affinity. An isolated, single mesogleal cell (mc) is indicated by a long arrow. The basal lamina (bl) is indicated by a short arrow. (d) An area of the mesoglea with an isolated cell cord embedded within the fibrous mesogleal matrix shows a single amoebocytic mesogleal cell (arrowhead to mc) and the basal lamina (short arrow to bl) that separates the gastroderm cells (GaD) from the mesoglea (M). The insert at the top right shows a morula-like cell (Mor).
Underlying the ectoderm is the thick, richly cellular mesoglea, permeated with solenia (Fig. 3a) and studded with sclerites (Fig. 4a and c). Cells in the mesoglea are generally concentrated under the thin ectoderm, loosely arranged into ubiquitous cell cords, or surround the sclerites with thin cellular extensions (Fig. 3a). The fibrous matrix of the mesoglea conforms to published reports on cnidarian mesoglea in general (Figs. 4a, d and 6c) (Davis and Haynes, 1968; Bouillon and Coppois, 1977). However, ‘granular amoebocytes’ do not exist individually in the fibrous mesogleal matrix, but are restricted to the mesogleal cell cords.
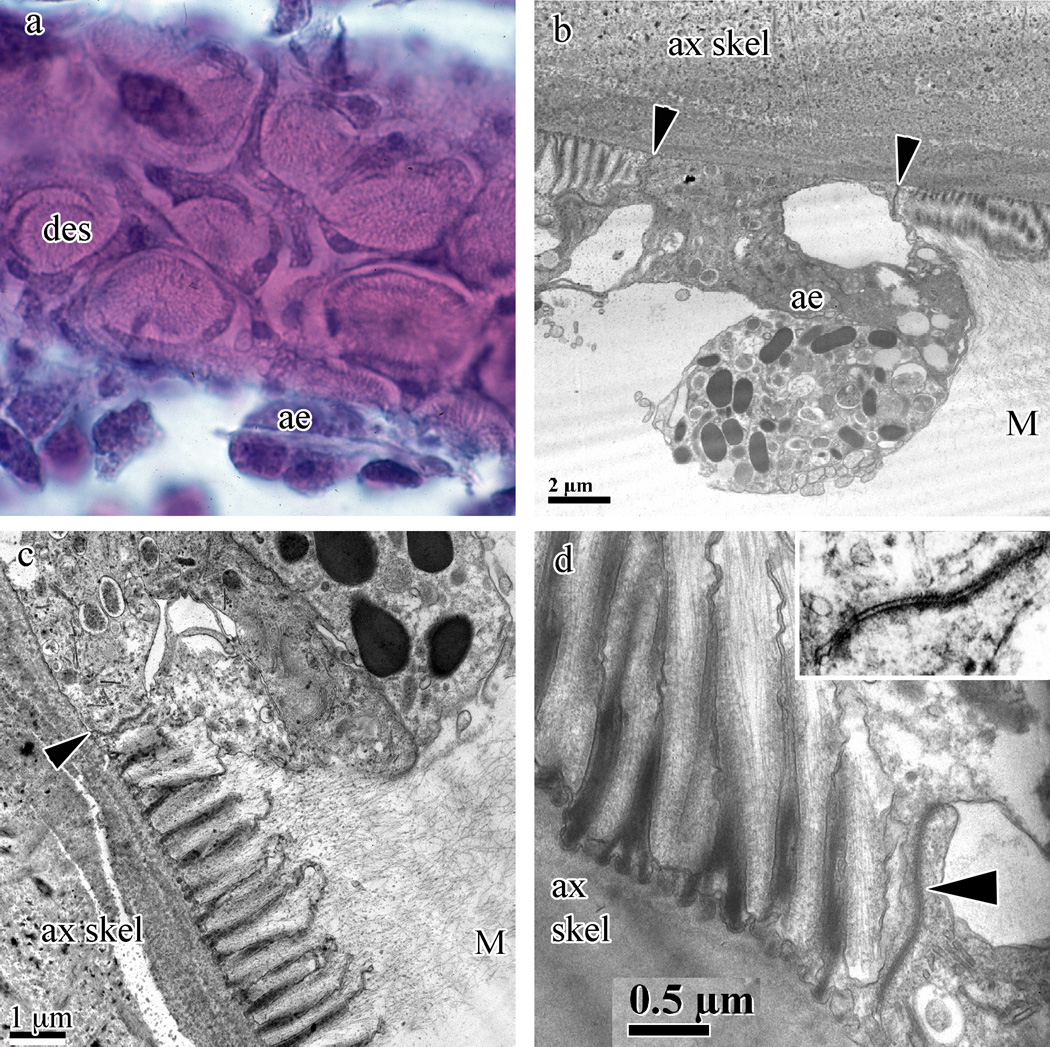
Fig. 6.
Swiftia exserta desmocytes and axial epithelia. (a) Desmocyte plaques (des) and axial epithelia (ae) with H&E staining from paraffin sections. (b–d) TEM images demonstrate the elevation of the axial epithelial cells (ae) above the plane of the desmocytes (panel b) as well as an active axial epithelial cell capable of secreting gorgonin. Panels (c) and (d) show the fine structure of the desmocyte anchors into the mesoglea and the many secretory vesicles and golgi bodies found in the axial epithelial cells (panel c). Insert in (d) shows the septae found in these desmocyte-to-axial epithelium junctions. Junctions between the desmocyte and axial epithelium cell are indicated by arrowheads in panels (b–d). The axial skeleton (ax skel) and mesoglea (M) are indicated in panels (b–d).
The gastroderm of the solenia in S. exserta coenenchyme is shown histologically in Fig. 5a, and by TEM in Fig. 5b and c, respectively. Several secretory cell types (Fig. 5b and c) including a cell type that resembles the zymogen cells found in Hydra spp. (thick arrowhead in Fig. 5b), cells with phagocytic vesicles (thin arrows in Fig. 5c), and flagellated cells (arrowhead in Fig. 5d) are easily distinguished.
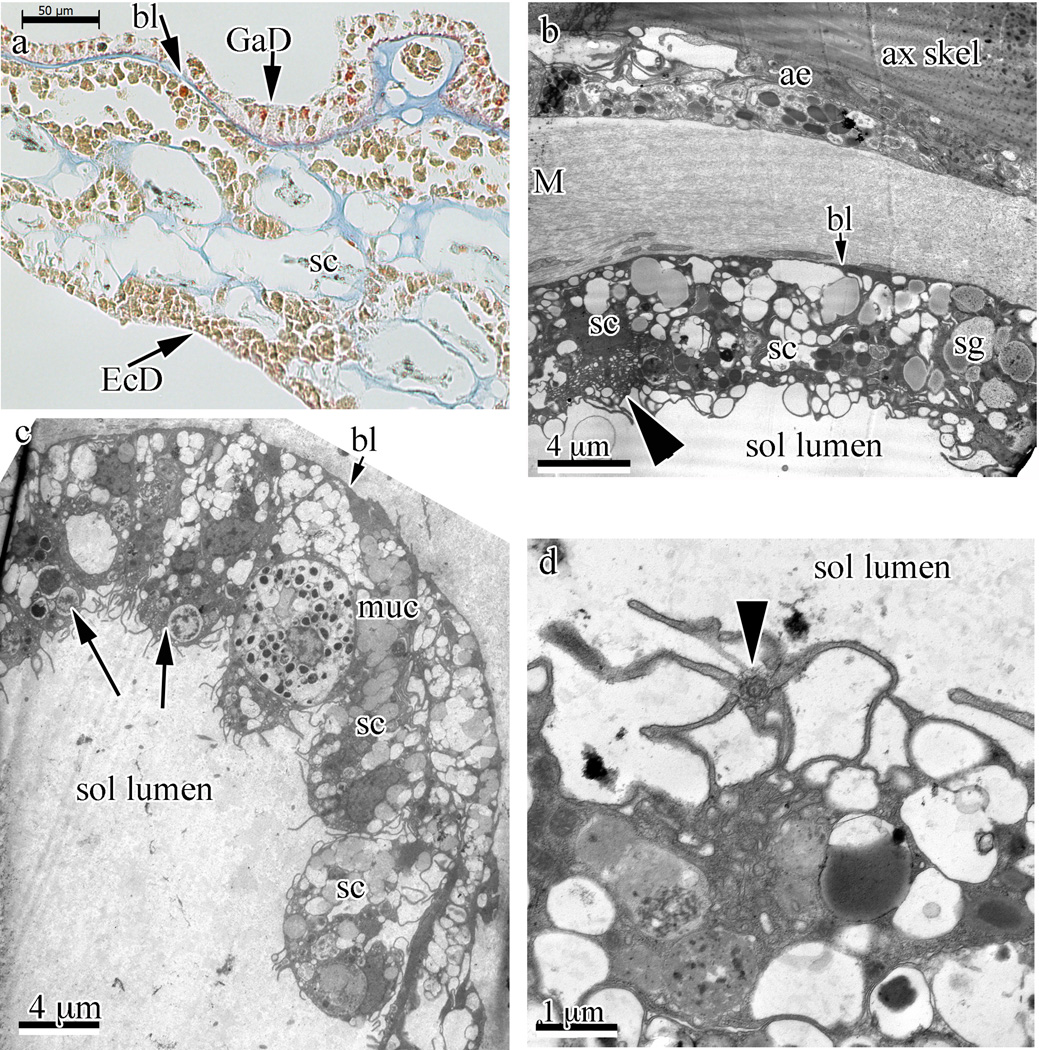
Fig. 5.
Solenia-type endo-/gastroderm in Swiftia exserta. (a) Solenia-type gastroderm (GaD) lining is visible at the upper part in a paraffin section stained with Mallory’s stain. The staining indicates that one of the digestive cell types is acidophilic (bright orange-red granules in cytoplasm). A basal lamina (bl) structure isolates the gastroderm from the mesoglea. (b–d) Transmission electron micrographs of solenia show different aspects. A solenium near the axial skeleton (ax skel) and axial epithelia (ae) is seen. This solenium is separated from the fibrous mesoglea (M) by a basal lamina-like layer (short arrow to bl). This basal lamina layer is faintly visible in panels (b) and (c), and clearly visible in panel (a) (arrow and bl). The arrowhead indicates a zymogen-like cell. Several secretory cells (sc) and a secretory granule (sg) are indicated. (c) Low magnification TEM of a section through a solenia (sol) showing several types of digestive cells. Long arrows indicate phagocytic vesicles. Short arrow indicates the basal lamina-like structure. A mucus-secreting cell’s granules are indicated (muc), as are several secretory cells (sc). Panels (b) and (c) show secretory cells (sc) packed with granules (sg) and phagocytic cells with membrane ruffles. To move liquid through the solenia (sol lumen), a flagellated cell with flagellum, seen in panel (d) with a section perpendicular to the flagellum (arrowhead) and base, is used.
Desmocytes, the attachment foci of the coenenchyme mesoglea, and thus tissue, are present throughout the Cnidaria. The desmocytes of S. exserta are arranged in groups called plaques, as seen in Fig. 6a. Longitudinal sections of the attachment foci are shown in Fig. 6b–d where they are anchored to the axial skeleton with rounded, ‘T’-shaped extensions at the base of the wedge-shaped desmocyte structures. These attachment foci merge into mesogleal fibers at the opposing end away from the axial skeleton. Desmocytes are joined to their neighboring axial epithelia cells by septate junctions (Fig. 6d and insert). Compartments within the axial skeleton that define the group Holaxonia, as described in Pterogorgia bipinnata (Fig. 112 in Kükenthal, 1919), are clearly visible in S. exserta (Fig. 3a).
3.2. Cell types of the coenenchyme
The gastrodermal cells of S. exserta’s solenia resemble anthozoan gastroderm cells described histologically by Chester (1913, Plate 2, Fig. 6) for Pseudoplexaura porosa and by Bayer (1974, Fig. 13) for Plexaura homomalla. The gastrodermal cells of the solenia are separated from the mesoglea (matrix and cells) by a basal lamina (bl, thick arrow in Figs. 4c and 5a, thin arrow in Figs. 4d and 5b). A detailed description of the observed cell types in the ectoderm and the mesoglea follows in Sections 3.2.1–3.2.8.
3.2.1. Squamous epithelial ectoderm cells
These cells of the ectoderm are thin, flattened and cover the entire animal colony. Outer surfaces of the epithelial cells are covered with microvilli (Fig. 3b–d), while the cytoplasm is finely granular and contains a few large mucus granules and small granules (highly variable, see Fig. 3c and d). The nucleus is flattened, ovoid, and finely granular with a prominent nucleolus. The outer surface of the epithelial cells stains strongly with Alcian blue, indicating a large amount of acidic mucopolysaccharides, mostly strongly sulphated acidic mucopolysaccharides (Parker and Diboll, 1966). Intense staining of the ectoderm–environment interface is seen with imidazole-buffered osmium tetroxide (Fig. 3d), indicating that this outer surface is covered with a thin layer of polyunsatured lipids (Angermüller and Fahimi, 1982). The morphology of the zonula adherens between these cells resembles the adherent junctions found in metazoan lineages.
3.2.2. Oblong granular cells
These cells, found just underneath the flat epithelial layer (e.g. in Figs. 3c and d, and 4b–d), are generally oblong or oval in shape (sometimes more elongate) and constitute approximately 63% of the cells in the cell cords. Their shape is mainly determined by contact with adjacent cells. Average dimensions are 8 × 6 µm with a small (2 µm), round and dense nucleus. The cytoplasm is completely filled with highly refractile, small (0.5–1 µm), uniformly sized granules. Although in some preparations these cells stain weakly with hematoxylin or eosin, they are generally neutrophilic, staining a light yellow-brown color. As visualized by TEM, these granules are intensely osmiophilic and completely fill the cytoplasm (Fig. 4b). There are occasional very electron-lucent granules, likely containing unsaturated lipids washed out by processing, interspersed between the many intensely osmiophilic granules. These cells are also located throughout the cell cords in the coenenchyme and are the most abundant cell type in the coenenchyme.
3.2.3. Granular amoebocytes
Approximately 36% of the cells in cell cords are granular amoebocytes. Granular amoebocytes (ga in Figs. 3d and 4b–d) do not represent a homogeneous population of cells, but include various sub-populations and/or developmental stages (Bigger and Hildemann, 1982). Consequently, granule size and content vary greatly in these cells. Often elongate, with sizes ranging in length from 6 to 20 µm and in width from 4 to 10 µm, these cells are all amoeboid in shape with nuclei irregularly round or ovoid and 2–3 µm in diameter. H&E usually stains the nuclei basophilic (on occasion moderately acidophilic) and the cytoplasm weakly basophilic. Small to large granular amoebocytes have an assortment of granules of different sizes (0.5–2 µm), staining properties (acidophilic, basophilic, and neutrophilic), and relative quantities. Distinct subpopulations of granular amoebocytes are difficult to classify by microscopy alone.
3.2.4. Morula-like cells
These globular cells have average dimensions of 9 × 7 µm with a very small (2 µm), dense nucleus. The cytoplasm is completely filled with spherical, uniformly sized granules (1–2 µm) (not shown). With H&E staining, the nucleus is strongly basophilic and the granules are strongly acidophilic. The granules also demonstrated an acidophilic nature with Chromotrope 2R (Gomori’s stain) and stained weakly with PAS. These cells were not very numerous, representing only approximately 0.5% of the cells in mesogleal cell cords and were scattered throughout the coenenchyme (see insert in Fig. 4d).
3.2.5. Sclerocytes
The sclerocytes (responsible for secreting sclerites) are located either directly on the sclerites (Fig. 4a) or in the mesoglea surrounding the sclerite cavity (arrows in Fig. 4a, arrowhead in Fig. 4c). Occasionally, more than one sclerocyte is found in association with a sclerite. These cells are elongate or very thin and flattened, measuring up to 35 µm in length and 0.5–2 µm in thickness. The finely granular, ovoid nucleus is equally flattened, measuring up to 6 µm at its largest diameter and 1 µm in thickness. The cytoplasm contains many small granules and small vacuoles measuring between 0.5 and 2 µm. With H&E staining, the cytoplasm is very weakly basophilic. PAS-positive granules are most often found in the sclerocytes directly on the sclerites.
3.2.6. Mesogleal cells
The mesogleal cells are embedded directly within the gelatinous mesogleal matrix and are relatively uncommon. These cells are very small and amoeboid, with one or more elongated and branched processes extending into the mesoglea. In one case a mesogleal cell with branching processes measured 51 µm in length. The cell body is usually less than 9 µm in length and very thin (as thin as 1.5 µm). The nucleus occupies a large portion of the cell body and is ovoid, finely granular, and 2–5 µm in diameter. Often the cytoplasm contains granules that stain intensely with both osmium and aniline blue (arrow in Fig. 4c, arrowhead in Fig. 4d).
3.2.7. Axial epithelial cells and desmocytes
The axial skeleton possesses striations atop the skeletal material, normal to the surface of the axis. The striated zones (desmocyte plaques) have an irregularly oval outline and are areas of axis formation (Szmant-Froehlich, 1974; Kingsley and Watabe, 1982b). Descriptions of the desmocytes of Leptogorgia virgulata (Fig. 2 in Kingsley and Watabe, 1982b) are very similar, showing cells filled with secretory vesicles surrounding the striated desmocytes. While the structure of the desmocyte attachments is very similar across octocorals, they do not resemble Chapman’s report on desmocytes in the scyphozoan Aurelia aurita or the hydrozoan Clava multicornis (Chapman, 1969). The nuclei of S. exserta axial epithelium cells are finely granular, approximately 3 µm in diameter and, along with the cytoplasm, basophilic with H&E staining. The cytoplasm contains many small granules and vacuoles, likely for secretion (Fig. 6b). Each desmocyte plaque is individually surrounded by one axial epithelial cell that curves around the group of striations. Individual desmocytes are attached to each other via septate junctions, binding them to neighboring desmocytes (Fig. 6b–d; insert in Fig. 6d shows septate morphology) within a desmocyte plaque and to axial epithelia cells. Unfortunately, no desmocyte developmental stages were observed. For a schematic of desmocyte development in Hydrozoa, Scyphozoa, and Anthozoa see Tidball (1982a).
3.2.8. Cnidocytes
S. exserta cnidocytes produce two types of nematocysts in the coenenchyme: small basophilic nematocysts (Type I – atrichous isorhiza) (at center right of Fig. 3d) and larger acidophilic nematocysts (Type II – macrobasic amastigophores). Nematocyst types were identified by characteristics published in 1934 by Robert Weill.
Type I nematocysts (atrichous isorhiza) are mainly found in the coenenchyme away from the anthostele (coenenchyme-to-polyp tissue protrusion into which the polyp retracts) and occasionally in groups of up to three. These shorter nematocysts have kidney-shaped capsules 6–8 µm in length and 4–5 µm in width. The nematocyst thread stains green with Gomori’s trichrome stain. These nematocysts do not stain distinctively with PAS or Alcian blue. The cigar-shaped Type II nematocysts, approximately 8–10 µm in length and 2–2.5 µm in width, are found primarily in the polyp and the anthostele. Both types of nematocysts will be documented in detail in a future report.
4. Discussion
While the gross morphology of S. exserta conformed to reports on other holaxonian gorgonians (Chester, 1913; Bayer, 1974), many differences were observed at the cellular level.
Histological descriptions of gorgonian tissues and cell types are limited. The most appropriate descriptions are those of Pseudoplaxaura crassa (properly known as Pseudoplexaura porosa) by Chester (1913), of Plexaura homomalla by Bayer (1974), and of Plexaura flexuosa in a study on regeneration by Silveira and van’t Hof (1977). Even the ultrastructural studies by Kawaguti (1966, 1969) only discuss polyps, while the reports involving Leptogorgia virgulata (Kingsley and Watabe, 1982a,b, 1983) focus on the axial skeleton or sclerite formation and composition. However, none of these studies examined the coenenchyme in detail, nor was electron microscopy utilized to study the coenenchyme organization.
The coenenchymal epithelial cells of S. exserta are unlike those of most cnidarians in that they are not columnar (Bourne, 1895; Hickson, 1895); instead, they are thin and flattened, forming a squamous epithelium over the entire coenenchyme of the colony. Some incidental views of squamous epithelia (from the coenenchyme of Leptogorgia virgulata) can be seen in Kingsley and Watabe (1982a, Fig. 9), Fautin and Mariscal (1991, Figs. 3 and 13–15), and Satterlie and Case (1978). Chester (1913), Bayer (1974), and Silveira and van’t Hof (1977) all describe columnar ectoderm epithelia. The thin ectoderm cells of S. exserta are connected to each other, by a zonula occludens and adherent junctions (Staehelin, 1974; Magie and Martindale, 2008), to form an epithelium and isolate the interior animal from the external milieu (Tyler, 2003).
Due to the similarity of the cells underlying the ectoderm epithelium and their continuity with the cells in the mesogleal cell cords, we do not consider these cells to be part of the ectoderm. Only the thin layer of flattened cells should be referred to as the ectoderm. The oblong granular cells of S. exserta are pigmented and thus responsible for giving the colony its orange color (Olano, 1993; W. Goldberg, pers. comm. for TEM evidence). This is unlike the coloration of Leptogorgia virgulata, where pigments are found surrounding the sclerites (Adams, 1979). Pigment cells similar to those of S. exserta have been found in the ahermatypic soft coral Dendrophillia cribrosa (Kawaguti and Yokoyama, 1966); they contain 1 µm granules that are composed at least partially of carotenoids. The absence of oblong granular cells in Plexaura homomalla and Pseudoplexaura porosa was not surprising because the color in these colonies is attributable to their sclerites (Bayer, 1961). However, the oblong granular cells described for Plexaura flexuosa closely resemble those of S. exserta, yet in P. flexuosa they are not responsible for the color of the colony (Bayer, 1961); the color is also found in the sclerites.
Pseudoplexaura porosa lacks the oblong granular cells of S. exserta; however, the cells of its coenenchyme appear the most similar to those of S. exserta. The P. porosa cells that Chester (1913) identified as interstitial cells and granular interstitial cells appear to be the same as the granular amoebocytes of Swiftia (see Bigger and Hildemann, 1982 for a discussion of the interstitial cell/amoebocyte issue). The mesogleal cells of both species are likewise identical. Chester (1913) suggested that the interstitial cells, the interstitial cells with few granules, the interstitial cells with large granules, and the mesogleal cells were a developmental series. The mesogleal cells of S. exserta are amoebocytic as well, but are classified separately in the present study because they are a distinctly different type of amoebocyte found isolated within the mesogleal matrix fibers. Both the interstitial cells and the mesogleal cells described for P. flexuosa are very different from those of S. exserta. S. exserta also lacks the frothy cells (cells from the coenenchymal cell cords) and the blood-corpuscle-like cells described for Plexaura homomalla (Bayer, 1974).
Morula-like cells have been described for a number of invertebrates (Ratcliffe and Rowley, 1981) where this cell type has been implicated in immune reactions (Ballarin et al., 2001; Ballarin, 2008). The relative abundance of morula-like cells in S. exserta is within the same range as that of mammalian basophils (0.1–0.3%) (Stone et al., 2010). Accordingly, even though there are low numbers of morula-like cells in S. exserta coenenchyme, their potential role in an immune response should not be discounted at this time.
In S. exserta the gastroderm lining the solenia appears to differ from other reported anthozoan solenia gastroderm cell types and arrangements (Chester, 1913; Bayer, 1974). Morphologically, the solenia gastroderm resembles the endoderm of Hydra ssp., including zymogen cells (arrowhead in Fig. 5b) (Haynes and Davis, 1969), several types of secretory cells (Fig. 5b and c), and flagellated cells (Fig. 5d) (Fautin and Mariscal, 1991). The many flagella present in the solenia are likely used for liquid and nutrient movement between polyps and from polyps to the cells in the coenenchyme (Murdock, 1978; Gladfelter, 1983; Harmata et al., 2013).
The study of Goldberg and Benayahu (1987) with Pseudoplexaura flagellosa demonstrated both primary and secondary scleroblasts. The sclerocytes observed in the tissues of S. exserta seem to be engaged in the extracellular calcification of sclerites (secondary sclerocytes). Primary sclerocytes, cells with internal calcification vesicles, were not found. Electron micrographs occasionally capture a nucleus, but rarely have cell boundaries been seen around sclerites/sclerite vacuoles (Fig. 4a), indicating a possible syncytial nature of sclerite formation (Goldberg and Benayahu, 1987).
The axial epithelium of S. exserta is not columnar, unlike that of Leptogorgia virgulata (Tidball, 1981, 1982a,b), but was found to form a thin layer covering the axis, more similar to (although somewhat thinner than) that of P. homomalla (Bayer, 1974). Sections of the attachment foci (Fig. 6b and c) parallel to the skeleton axis are most similar to those found in the octocorallians Leptogorgia virgulata (Tidball, 1982a), Dendronephthya hemprichi (Barneah et al., 2002), Cornularia cornucopia (Benke and Hündgen, 1984), and to desmocytes found in the hexacorallian Stylophora psitillata (Muscatine et al., 1997).
Tissue homeostasis, i.e. the exclusion of microbes, in S. exserta is maintained at the external epithelia (ectoderm and axial epithelia/desmocytes) by intercellular junctions. In the zonula adherens of the ectoderm, S. exserta shows typical invertebrate adherent junctions (sensu Tyler, 2003). The axial epithelium-to-desmocyte plaque junctions, however, are morphologically and structurally different (arrowhead in Fig 6d) – these junctions, sealing off the innermost tissues of the animal, are septate junctions (sensu Staehelin, 1974 and references therein). The use of septate junctions at the axial epithelium-to-desmocyte boundaries may indicate that the relative mechanical stress levels are higher here than between the ectodermal cell junctions.
A true understanding of the microanatomy of experimental animals is an important prerequisite before attempting to investigate biological processes, e.g. immunological responses. This is particularly important in comparative situations, such as the present study, where only a few species within a phylum have been examined before. Thus, the present report helps to lay a strong foundation for future investigations and focuses attention on what appears to be a biologically important but research-neglected anatomical area, the colonial tissues between the polyps that are found in many Anthozoa.
Acknowledgements
L.P.M. thanks Tom Beasley and the Florida Center for Advanced Electron Microscopy (FCAEM) at Florida International University for assistance and for training on the use of the JEOL JSM 5910LV SEM. Figure 2 has been redrawn from Bayer et al., 1983 by Ellen Bigger Streeter. We gratefully thank the anonymous reviewers for their help in improving this manuscript. Partial support was provided by NIH (RR08205) and NIGMS (GM08205) grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RO. Results of crossbreeding color varieties of the gorgonian, Leptogorgia virgulata (Cnidaria, Octocorallia) Am. Zool. 1979;19:985. [Google Scholar]
- Angermüller S, Fahimi D. Imidazole-buffered osmium tetroxide: an excellent stain for visualization of lipids in transmission electron microscopy. Histochem. J. 1982;14:823–835. doi: 10.1007/BF01033631. [DOI] [PubMed] [Google Scholar]
- Ballarin L. Immunobiology of compound ascidians with particular reference to Botryllus schlosseri: state of art. Inv. Surv. J. 2008;5:54–74. [Google Scholar]
- Ballarin L, Franchini A, Ottaviani E, Sabbadin A. Morula cells as the major immunomodulatory hemocytes in ascidians: evidences from the colonial species Botryllus schlosseri. Biol. Bull. 2001;201:59–64. doi: 10.2307/1543526. [DOI] [PubMed] [Google Scholar]
- Barneah O, Malik Z, Benayahu Y. Attachment to the substrate by soft coral fragments: desmocyte development, structure, and function. Invert. Biol. 2002;121:81–90. [Google Scholar]
- Bayer FM. M.S. Thesis. Washington, D.C.: George Washington University; 1956. The Microanatomy of Five Holaxonian Corals. [Google Scholar]
- Bayer FM. Studies on the Fauna of Curacao and other Caribbean Islands No. 12. The Hague: M. Nijhoff; 1961. The shallow-water octocorallia of the West Indian region. [Google Scholar]
- Bayer FM. Studies on the anatomy and histology of Plexaura homomalla in Florida. In: Bayer FM, Weinheimer AJ, editors. Prostaglandins from Plexaura homomalla: Ecology, Utilization and Conservation of a Major Medical Marine Resource. A Symposium. Coral Gables, FL: University of Miami Press; 1974. pp. 62–100. [Google Scholar]
- Bayer FM, Grasshoff M, Versefeldt J. Illustrated Tri-lingual Glossary of Morphological and Anatomical Terms Applied to Octocorallia. Leiden: E.J. Brill; 1983. [Google Scholar]
- Benke H, Hündgen M. Mophologie und Struktur der Koralle Cornularia cornucopiae (Anthozoa, Octocorallia) Helgoländer Meeresunters. 1984;38:149–170. [Google Scholar]
- Bigger CH, Hildemann WH. Cellular defense systems of the Coelenterata. In: Cohen N, Sigel M, editors. The Reticuloendothelial System. New York: Plenum Press; 1982. pp. 59–87. [Google Scholar]
- Bigger CH, Olano CT. Alloimmune responses of the gorgonian coral Swiftia exserta. Dev. Comp. Imm. 1994;18:S1. [Google Scholar]
- Bigger CH, Dishaw LJ, Olano CT. Immune defenses of the gorgonian coral Swiftia exserta simple or complex? Dev. Comp. Imm. 2000;24:S43. [Google Scholar]
- Bourne GC. On the structure and affinities of Heliopora coerulea (Pallas), with some observations on the structure of Xenia and Heteroxenia. Proc. R. Soc. Lond. B. 1895;186:455–483. [Google Scholar]
- Bouillon J, Coppois G. Étude Comparative de la Mésoglé des Cnidaires. Cahiers Biol. Mar. 1977;18:339–368. [Google Scholar]
- Chapman DM. The nature of Cnidaria desmocytes. Tiss. Cell. 1969;1:619–632. doi: 10.1016/s0040-8166(69)80036-4. [DOI] [PubMed] [Google Scholar]
- Chapman DM. Cnidarian histology. In: Muscatine L, Lenhoff HM, editors. Coelenterate Biology. Reviews and New Perspectives. New York, San Francisco, London: Academic Press; 1974. pp. 2–92. [Google Scholar]
- Chapman G. Studies of the mesoglea of the coelenterates. I. Histology and chemical properties. Quart. J. Micr. Sci. 1953;94:155–176. [Google Scholar]
- Chester WM. The structure of the gorgonian coral Pseudoplexaura crassa Wright and Studer. Proc. Am. Acad. Arts Sci. 1913;48:737–774. [Google Scholar]
- Clark G. Staining Procedures Used by the Biological Stain Commission. Baltimore, MD: Williams & Wilkins; 1981. [Google Scholar]
- Davis LE, Haynes JF. An ultrastructural examination of the mesoglea of Hydra. Z. Zellforsch. Mikr. Anat. Histochem. 1968;92:149–158. doi: 10.1007/BF00335643. [DOI] [PubMed] [Google Scholar]
- Dishaw LJ, Smith SL, Bigger CH. Characterization of a C3-like cDNA in a coral: phylogenetic implications. Immunogenetics. 2005;57:535–548. doi: 10.1007/s00251-005-0005-1. [DOI] [PubMed] [Google Scholar]
- Duchassaing P, Michelotti G. Memoire sur les Coralliaires des Antilles. Turin: L’Imprimerie Royale; 1860. [Google Scholar]
- Ellis J, Solander D. The Natural History of Many Curious and Uncommon Zoophytes: Collected From Various Parts of the Globe. London: Benjamin White & Son; 1786. [Google Scholar]
- Fautin DG, Mariscal RN. Cnidaria: Anthozoa. In: Harrison FW, Westfall JA, editors. Microscopic Anatomy of Invertebrates, vol. 2. Placozoa, Porifera, Cnidaria, and Ctenophora. New York: Wiley–Liss; 1991. pp. 267–358. [Google Scholar]
- Gladfelter EH. Circulation of fluids in the gastrovascular system of the reef coral Acropora cervicornis. Biol. Bull. 1983;165:619–636. doi: 10.2307/1541469. [DOI] [PubMed] [Google Scholar]
- Goldberg WM. The sclerites and geographical distribution of the gorgonian Swiftia exserta. Bull. Biol. Soc. Wash. 2001;10:100–109. [Google Scholar]
- Goldberg WM, Benayahu Y. Spicule formation in the gorgonian coral Pseudoplexaura flagellosa 1. Demonstration of intracellular and extracellular growth and the effect of ruthenium red during decalcification. Bull. Mar. Sci. 1987;40:287–303. [Google Scholar]
- Hand AR. Ultrastructural localization of catalase and L-alpha-hydroxy acid oxidase in microperoxisomes of Hydra. J. Histochem. Cytochem. 1976;24:915–925. doi: 10.1177/24.8.956644. [DOI] [PubMed] [Google Scholar]
- Harmata KL, Parrin AP, Morrison PR, McConnell KK, Bross LS, Blackstone NW. Quantitative measure of gastrovascular flow in octocorals and hydroids: toward a comparative biology of transport systems in cnidarians. Invert. Biol. 2013;132:291–304. [Google Scholar]
- Haynes JF, Davis LE. The ultrastructure of the zymogen cells in Hydra viridis. Z. Zellforsch. 1969;100:316–324. doi: 10.1007/BF00343886. [DOI] [PubMed] [Google Scholar]
- Hickson SJ. On the ciliated groove (siphonoglyphe) in the stomodeum of the alcyonarians. Proc. R. Soc. London. 1883;174:693–705. [Google Scholar]
- Hickson SJ. The anatomy of Alcyonium digitatum Quart. J. Micr. Sci. N.S. 1895;37:343–388. [Google Scholar]
- Kawaguti S. Electron microscopy on the fluorescent green of reef corals with a note on mucous cells. Biol. J. Okayama Univ. 1966;12:11–21. [Google Scholar]
- Kawaguti S. Electron microscopy on a soft coral, Heteroxenia elisabethae Kölliker. Biol. J. Okayama Univ. 1969;15:25–35. [Google Scholar]
- Kawaguti S, Yokoyama T. Electron microscopy on polyp and pigment granules of an ahermatypic coral, Dendrophylla cribosa. Biol. J. Okayama Univ. 1966;12:69–80. [Google Scholar]
- Kingsley RJ, Watabe N. Ultrastructural investigation of spicule formation in the gorgonian Leptogorgia virgulata (Lamarck) (Coelenterata, Gorgonacea) Cell Tiss. Res. 1982a;223:325–334. doi: 10.1007/BF01258493. [DOI] [PubMed] [Google Scholar]
- Kingsley RJ, Watabe N. Ultrastructure of the axial region in Leptogorgia virgulata (Cnidaria, Gorgonacea) Trans. Am. Micr. Soc. 1982b;101:325–339. [Google Scholar]
- Kingsley RJ, Watabe N. Analysis of proteinaceous components of the organic matrices of spicules from the gorgonian Leptogorgia virgulata. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 1983;76:443–447. [Google Scholar]
- Koch G von. Mittheilungen der Zoologischen Station zu Neapel. Berlin: Friedländer & Sohn; 1887. Fauna und Flora des Golfes von Neapel und der angrenzenden Meeresabschnitte. XV. Monographie: Die Gorgoniden. [Google Scholar]
- Kükenthal W. Gorgonaria, Allgemeiner Teil. In: Chun C Hrsg, editor. Wissenschaftliche Ergebnisse der Deutschen Tiefsee-Expedition auf dem Dampfer “Valdivia” 1898–1899, 13. Band. Jena: Gustav Fischer Verlag; 1919. [Google Scholar]
- Lentz JL, Barrnett RJ. Enzyme histochemistry of Hydra. J. Exp. Zool. 1961;147:125–149. doi: 10.1002/jez.1401470204. [DOI] [PubMed] [Google Scholar]
- Magie CR, Martindale MQ. Cell–cell adhesion in the cnidaria: insights into the evolution of tissue morphogenesis. Biol. Bull. 2008;214:218–232. doi: 10.2307/25470665. [DOI] [PubMed] [Google Scholar]
- Mariscal RN, Bigger CH. Proceedings of the Third International Symposium on Coral Reefs. University of Miami Press; 1977. Possible ecological significance of octocoral epithelial ultrastructure; pp. 127–133. [Google Scholar]
- Menzel LP. Ph.D. Thesis. Miami, FL: Florida International University; 2013. Aspects of the innate immune system in the Caribbean octocoral Swiftia exserta. [Google Scholar]
- Menzel LP, Bigger CH. Antibacterial peptides from the Carribean octocoral Swiftia exserta. Integr. Comp. Biol. 2013a;53(Suppl. 1):e144. [Google Scholar]
- Menzel LP, Bigger CH. Can enzyme histochemistry identify the immune cells of the octocoral Swiftia exserta? Integr. Comp. Biol. 2013b;53(Suppl. 1):e333. [Google Scholar]
- Murdock GR. Circulation and digestion of food in the gastrovascular system of gorgonian octocorals (Cnidaria; Anthozoa) Bull. Mar. Sci. 1978;28:363–370. [Google Scholar]
- Muscatine L, Tambutté E, Allemand D. Morphology of coral desmocytes, cells that anchor the calicoblastic epithelium to the skeleton. Coral Reefs. 1997;16:205–213. [Google Scholar]
- Nutting CC. Contribution to the anatomy of Gorgonidae. Univ. Iowa Lab. Nat. Hist. Bull. 1889;1:97–160. [Google Scholar]
- Olano CT. M.S. Thesis. Miami, FL: Florida International University; 1993. Cellular aspects of alloimmunity and other responses in the gorgonian Swiftia exserta. [Google Scholar]
- Olano CT, Bigger CH. Phagocytic activities of the gorgonian coral Swiftia exserta. J. Invert. Path. 2000;76:176–184. doi: 10.1006/jipa.2000.4974. [DOI] [PubMed] [Google Scholar]
- Parker BC, Diboll AG. Alcian stains for histochemical localization of acid and sulfated polysaccharides in algae. Phycologia. 1966;6:37–46. [Google Scholar]
- Ratcliffe NA, Rowley AF. Invertebrate Blood Cells, vols. 1 and 2. London: Academic Press; 1981. [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterlie RA, Case JF. Neurobiology of the gorgonian coelenterates, Muricea californica and Lophogorgia chilensis II. Morphology. Cell Tiss. Res. 1978;187:379–396. doi: 10.1007/BF00229604. [DOI] [PubMed] [Google Scholar]
- Silveira FL, van’t Hof T. Regeneration in the gorgonian Plezaura flexuosa (Cnidaria, Octocorallia) Bijdr. Dierkd. 1977;47:98–108. [Google Scholar]
- Staehelin LA. Structure and function of intercellular junctions. Int. Rev. Cyt. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmant-Froehlich A. Structure, iodination and growth of the axial skeletons of Muricea californica and M. fruticosa (Coelenterata: Gorgonacea) Mar. Biol. 1974;27:299–306. [Google Scholar]
- Tidball JG. Lipoprotein secretion during the formation of the axial skeleton in Leptogorgia virgulata (Cnidaria: Gorgonacea) Cell Tissue Res. 1981;219:327–338. doi: 10.1007/BF00210152. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Fine structural aspects of anthozoan desmocyte development (phylum Cnidaria) Tissue & Cell. 1982a;14:85–96. doi: 10.1016/0040-8166(82)90009-x. [DOI] [PubMed] [Google Scholar]
- Tidball JG. An ultrastructural and cytochemical analysis of the cellular basis for tyrosine-derived collagen crosslinks in Leptogorgia virgulata (Cnidaria: Gorgonacea) Cell Tissue Res. 1982b;222:635–645. doi: 10.1007/BF00213861. [DOI] [PubMed] [Google Scholar]
- Tiffon Y, Hugon JS. Localisation ultrastructurale de la phosphatase acide et de la phosphatase alkaline dans le cloisons septales steriles de l’anthozoaire Pachycerianthus fimbriatus. Histochem. 1977;54:289–297. doi: 10.1007/BF00508272. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Shibata B, Blankenship TN. Ultrastructure of the mesoglea of the sea anemone Nematostella vectensis (Edwadsiidae) Invert. Biol. 2011;130:11–24. [Google Scholar]
- Tyler S. Epithelium – the primary building block for metazoan complexity. Integr. Comp. Biol. 2003;43:55–63. doi: 10.1093/icb/43.1.55. [DOI] [PubMed] [Google Scholar]
- Weill R. Contribution a l’Étude des Cnidaires et de leurs Nématocystes. I. Recherches sur les Nématocystes (Morphologie, Physiologie, Développement) Travaux de la Station Zoologique de Wimeraux, vol. X, Bordeaux. 1934:1–347. [Google Scholar]