Abstract
According to the World Health Organization, diabetes mellitus (DM) in the year 2030 will be ranked the seventh leading cause of death in the world. DM impacts all systems of the body with oxidant stress controlling cell fate through endoplasmic reticulum stress, mitochondrial dysfunction, alterations in uncoupling proteins, and the induction of apoptosis and autophagy. Multiple treatment approaches are being entertained for DM with Wnt1 inducible signaling pathway protein 1 (WISP1), mechanistic target of rapamycin (mTOR), and silent mating type information regulation 2 homolog) 1 (S. cerevisiae) (SIRT1) generating significant interest as target pathways that can address maintenance of glucose homeostasis as well as prevention of cellular pathology by controlling insulin resistance, stem cell proliferation, and the programmed cell death pathways of apoptosis and autophagy. WISP1, mTOR, and SIRT1 can rely upon similar pathways such as AMP activated protein kinase as well as govern cellular metabolism through cytokines such as EPO and oral hypoglycemics such as metformin. Yet, these pathways require precise biological control to exclude potentially detrimental clinical outcomes. Further elucidation of the ability to translate the roles of WISP1, mTOR, and SIRT1 into effective clinical avenues offers compelling prospects for new therapies against DM that can benefit hundreds of millions of individuals throughout the globe.
Keywords: AMPK, apoptosis, autophagy, cardiac, CCN, diabetes mellitus, erythropoietin, forkhead, FoxO, metformin, nervous system, oxidative stress, mTOR, sirtuins, SIRT1, stem cells, vascular, WISP1, Wnt
1. Introduction
Each year, metabolic disease impacts a significantly greater portion of the global population. Such observations appear to be in contrast to the high expenditures provided for healthcare in developed nations. For example, according to the Centers for Medicare and Medicaid Services (CMS) (1), the United States in the year 2012 spent 2.8 trillion on healthcare that was equal to $8, 915 per person and 17.2 percent of the Gross Domestic Product (GDP). Hospital care costs were increased 4.9 percent from the prior year to equal $882.3 billion and spending on physician and clinical services increased 4.6 percent to $565 billion. In addition, out of pocket spending per individual on healthcare in the year 2012 was estimated to have grown 4.1 percent to $320.2 billion. By the year 2022, healthcare spending is projected to be 19.9 percent of the GDP.
Contributing to these costs and the growing prevalence of metabolic disorders such as diabetes mellitus (DM) is the increased incidence of obesity in the population (2–6). Obesity leads to a number of metabolic disorders that includes cellular oxidative stress and insulin resistance (7, 8), lipid –induced dysfunction of pancreatic β cells (9), cellular inflammation (10), altered trophic factor release (11–14), and impairment in protein tyrosine phosphatase signaling (6, 15). Yet, it is the duration of obese-years rather than the body mass index (BMI) that corresponds to a strong risk for developing DM (16).
Given the increased presence and progressive contribution of risk factors such as obesity for metabolic disorders, DM also is growing at an exponential rate. The World Health Organization predicts that DM will be the seventh leading cause of death by the year 2030 (17). For the year 2013, 347 million individuals are believed to have DM and more than one million of these individuals are dying from the disease. In the United States, it is estimated that DM costs employers $69 billion in reduced productivity and another $176 billion for direct medical costs. At least 21 million individuals are diagnosed with DM in the US and another 8 million individuals are estimated to be undiagnosed with DM (18). A strong case can be made for clinical programs that assist with the early diagnosis of DM given that a significant portion of the population currently remains undiagnosed with DM (10, 19). Furthermore, the incidence of impaired glucose tolerance in the young also raises additional concerns (3). Individuals with impaired glucose tolerance have more than twice the risk for the onset of diabetic complications than individuals with normal glucose tolerance (20).
DM is considered to be either non-insulin dependent (Type 1) or insulin dependent (Type 2) (21). Type 1 DM occurs in approximately 5–10% of DM patients. It is an autoimmune disorder with the presence of alleles of the human leukocyte antigen (HLA) class II genes within the major histocompatibility complex (MHC) (22). Destruction of pancreatic β-cells with inflammatory infiltration of the islets of Langerhans leads to the loss of insulin production and regulation. Activation of T-cell clones that are capable of recognizing and destroying β-cells can result in severe insulin deficiency. T-cell clones escape thymus control that yields high affinity for MHC molecules with T-cell receptors, but these clones have incorrect low affinity for self-peptides. Once released into the body, these T-cell clones can become activated to destroy self-antigens. Almost 90% of patients with Type 1 DM have increased titers of autoantibodies (Type 1A DM). The remaining 10% of Type 1 DM individuals do not have serum autoantibodies. These individuals are considered to have maturity-onset diabetes of the young (MODY) that can be a result of β-cell dysfunction with autosomal-dominant inheritance (Type IB DM).
Type 2 DM occurs in approximately 90% of individuals most notably in individuals over the age of 40 and has a progressive deterioration of glucose tolerance with early β-cell compensation (23). Initial cell hyperplasia is followed by a decrease in pancreatic β-cell mass with subsequent insulin resistance and impairments in insulin secretion occurring. Defective insulin secretion can result from impaired β-cell function, chronic exposure to free fatty acids and hyperglycemia, as well as the absence of inhibitory feedback through plasma glucagon levels. Interestingly, Type 1 and Type 2 DM may have common links since approximately 10% of individuals with Type 2 DM may have elevated serum autoantibodies similar to Type 1 DM and insulin resistance also may be a component of Type 1 DM in some patients.
2. DM, Involvement of Multiple Organ Systems, and Oxidative Stress
DM can injure multiple organ systems throughout the body. As a result, DM has been reported to lead to vascular disease (24–30), cardiac disorders (31–39), renal disease (2, 40–43), hepatic disorders (44–49), and immune cell impairment (38, 50–54). In the neurovascular arena, DM can contribute to cognitive loss through acute stroke onset (55). During chronic neurodegenerative disorders that involve Alzheimer’s disease with DM (22, 56), insulin resistance has been reported in patients with Alzheimer’s disease that links impaired cellular metabolism with cognitive loss (57–59). DM also leads to neuropsychiatric disorders (60, 61), peripheral nerve disorders (27), and retinal disease (62–64). At the vascular cell level, elevated glucose levels reflective of those that occur during DM can result in endothelial cell senescence (25), impaired mobilization of endothelial progenitor cells from the bone marrow (65), neuroglialvascular unit compromise (62), inhibition of angiogenesis (26), and loss of endothelial cells (14, 30, 66–70).
Oxidative stress is an important determinant of cell injury in DM (4, 21, 71–76). Oxidant stress that results in the generation of reactive oxygen species (ROS) can significantly affect cellular metabolism and lead to cell injury during DM (77, 78) and contribute to disability that involves impaired cognitive function (79–81), cerebral ischemia (77, 82), and epigenetic linked disease (83–87). ROS are formed through superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide (NO), and peroxynitrite that lead to mitochondrial dysfunction, loss of DNA integrity, cellular dysfunction, and protein misfolding (21, 76, 88–91). Endogenous antioxidant systems can limit the generation of ROS and include catalase, superoxide dismutase, glutathione peroxidase, and vitamins C, D, E, and K (23, 75, 85, 90, 92–100).
Yet, excessive production of ROS or impairments in the endogenous antioxidant system such as those that can occur during DM can ultimately lead to oxidative stress and cell death (8, 22, 23, 27, 29, 63, 74, 76, 101). In murine animal models of Type 2 DM, oxidative stress results in elevated glutathione levels and increased lipid peroxidation (33). Advanced glycation end products (AGEs), entities that promote complications in DM (32, 36), also result in the release of reactive oxygen species (ROS) and caspase activation (74). In experimental cell models, exposure to elevated glucose levels foster oxidant stress mechanisms that lead to cell injury in cardiomyocytes (35, 39, 102), neurons (55, 63, 69, 103, 104), and endothelial cells (62, 65–68, 105). Elevations in serum glucose also can increase antioxidant enzyme levels in human endothelial cells, suggesting that some cells can attempt a reparative process during oxidative stress exposure (106). In clinical studies, patients with Type 2 DM display serum markers of oxidative stress with ischemia-modified albumin (107). However, chronic hyperglycemia during DM is not necessary to lead to oxidative stress injury, since even brief periods of hyperglycemia generate ROS (108). Clinical correlates show that both acute glucose swings as well as chronic hyperglycemia can trigger oxidative stress mechanisms during Type 2 DM (109).
Oxidative stress during DM also leads to mitochondrial dysfunction, endoplasmic reticulum stress, and alterations in uncoupling proteins (UCPs) (72, 110). ROS exposure during DM can result in the opening of the mitochondrial membrane permeability transition pore, reduce mitochondrial NAD+ stores, activate cytochrome c release, and initiate caspase activity (29, 66, 68, 70, 105, 111, 112). Exposure of glucolipotoxicity caused by elevated plasma glucose and lipid levels to pancreatic β-cells promotes oxidative stress with cytochrome c release, caspase activation, and apoptosis (111). High fat diets (113) as well as free fatty acid release that occurs during DM have been shown to release ROS, lead to mitochondrial DNA damage, and impair pancreatic β-cell function (114). Subsequently, mitochondrial dysfunction and cell death leads to apoptosis and autophagy (63). In patients with Type 2 DM, skeletal muscle mitochondria have been reported to be smaller than those in control subjects (115). A decrease in the levels of mitochondrial proteins and mitochondrial DNA in adipocytes also has been associated with the development of Type 2 DM (116). “Highly-oxidized glycated” low density lipoproteins that can occur in DM also result in oxidative and endoplasmic reticulum stress in human retinal capillary pericytes (63).
In addition to the role that mitochondria play during oxidative stress and DM, UCPs are a significant component in modulating cell survival in DM (72, 110, 117). UCPs are a family of carrier proteins found in the inner membrane of mitochondria and consist of the mammalian members UCP-1, 2,3,4,5 (118). UCPs uncouple oxygen consumption through the respiratory chain from ATP synthesis and can lead to the generation of ROS. UCPs disperse a proton electrochemical potential gradient across the mitochondrial inner membrane resulting in the activation of substrate oxidation and dissipation of oxidation energy as heat instead of ATP (72, 110). In addition, UCP family members also can influence insulin sensitivity. Uncoupling of respiration by UCPs plays a role in regulating ATP synthesis, fatty acid release, and glucose oxidation. For example, UCP1 may have beneficial effects during DM. Muscle-specific overexpression of UCP for skeletal muscle can increase energy expenditure and enhance insulin sensitivity to protect in animal models from high-fat diet induced insulin resistance (119). Skeletal muscle respiratory uncoupling also can enhance insulin sensitivity in obesity (120). Yet, other UCPs such as UCP2 may have detrimental effects. Overexpression of UCP2 in isolated pancreatic islets results in decreased ATP levels and blunted glucose-stimulated insulin secretion. Deletion UCP2 improves insulin secretion and decreases hyperglycemia in leptin-deficient mice (121). In relation to other UCP members during DM, UCP3 can stimulate insulin uptake (122) and may function to facilitate fatty acid oxidation and minimize ROS production (123).
3. DM, Apoptosis, and Autophagy
The programed cell death pathways of apoptosis (4, 23, 27, 70, 75, 124) as well as autophagy (2, 21, 125, 126) play significant roles during DM and oxidative stress (127). Necroptosis, another pathway involved in programmed cell death, does not presently appear to contribute significantly to cell survival in DM (128), but further published work may change these observations. Recent studies in murine models of Type 1 DM suggest that necroptosis may have less than an essential role in cell death during DM (129).
Apoptosis can oversee tissue development and remodeling during early stages of development, but in mature cells and tissues, the induction of apoptosis can lead to cell death during DM (4, 125). Apoptosis consists of both an early phase that involves the loss of plasma membrane lipid phosphatidylserine (PS) asymmetry and a later phase that leads to genomic DNA degradation (79, 130–132). Since the early phase of apoptosis with membrane PS externalization alerts inflammatory cells to engulf and remove injured cells, prevention of membrane PS externalization is vital to block the loss of functional cells that may become temporarily disabled (21). In DM, apoptosis can lead to neuronal injury (60, 103, 104, 133), cardiomyocyte destruction (34, 35, 38), pancreatic β-cell loss (134–136), endothelial cell injury (25, 26, 67, 105, 137), and renal cell dysfunction (138–140).
In regards to autophagy during DM, this pathway of programmed cell death may have variable outcomes (141). Autophagy recycles cytoplasmic components and discards defective organelles for tissue remodeling (125, 142). During DM, the sub classification of macroautophagy plays a principal role and involves the sequestration of cytoplasmic proteins into autophagosomes that fuse with lysosomes for degradation and recycling for future cellular processes (2, 29). Autophagy may be cytoprotective during DM. Recent work suggests that loss of autophagy may foster the progression from obesity to DM, since autophagy haploinsufficiency in murine animal models of obesity can lead to increased insulin resistance with elevated lipids and inflammation (143). Autophagy also may be required to eliminate misfolded proteins and non-functioning mitochondria to avert β-cell dysfunction and the onset of DM (144). Exercise in mice also has been shown to initiate autophagy and regulate glucose homeostasis (145). These results may be associated with observations that autophagy has been reported to improve insulin sensitivity during high fat diets in mice (8). Pathways of autophagy and apoptosis also may complement one another to control cell survival. For example, induction of autophagy may protect cardiomyocytes from apoptotic cell death during DM (34).
However, it should be noted that autophagy might not be consistently beneficial (128, 141, 146). Under some conditions, autophagy may be less of a prominent modulator of cell survival than apoptosis in some experimental models (147). In addition, during elevated glucose exposure, autophagy has been shown to impair endothelial progenitor cells, lead to mitochondrial oxidative and endoplasmic reticulum stress (148), and prevent the formation of new blood vessels (29). Increased activity of autophagy also has been associated with significant loss of cardiac and liver tissue in diabetic rats during attempts to achieve glycemic control through diet modification (149). During periods of elevated glucose, AGEs have been shown to lead to the induction of autophagy and vascular smooth muscle proliferation that can result in atherosclerosis (28) as well as cardiomyopathy (102).
4. WISP1 and DM
Multiple pathways can result in cellular injury through oxidative stress mechanisms during DM. As a result, recent investigations have concentrated upon pathways that involve anti-oxidant therapies (3, 23, 27, 30, 53, 71, 75), mammalian forkhead transcription factors (9, 37, 47, 150, 151), protein tyrosine phosphatases (6, 15, 152), and growth factors (11–13, 50, 65, 70, 104). In addition, new therapeutic strategies are now focusing upon the role of extracellular matrix associated proteins such as the CCN family of proteins (153, 154). The CCN family of proteins consists of six secreted extracellular matrix associated proteins. This family is defined by the first three members of the family that include Cysteine-rich protein 61, Connective tissue growth factor, and Nephroblastoma over-expressed gene (155). The CCN family members contain four cysteine-rich modular domains that include insulin-like growth factor-binding domain, thrombospondin domain, von Willebrand factor type C module, and C-terminal cysteine knot-like domain.
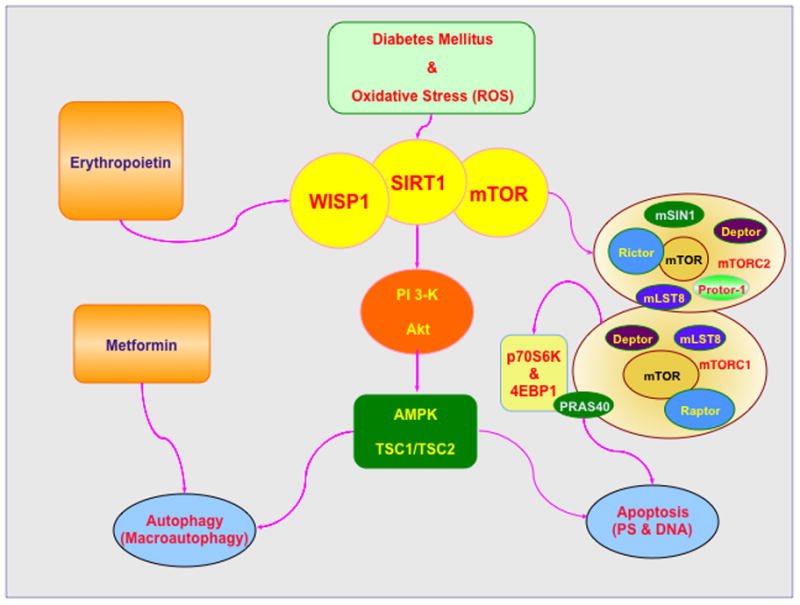
Of the CCN family members, Wnt1 inducible signaling pathway protein 1 (WISP1) is increasingly being recognized as a potential target for the complications tied to DM (Fig. 1). The WISP1 gene was identified in a mouse mammary epithelial cell line (156) and later shown to modulate gastric tumor growth (157). WISP1 is a target of the wingless pathway Wnt1, a cysteine-rich glycosylated protein that can modulate neuronal development, angiogenesis, bone growth, immune cell modulation, tumorigenesis, programmed cell death, and stem cell proliferation (158–170). WISP1 is present in a multiple sites throughout the body including the epithelium, heart, kidney, lung, pancreas, placenta, ovaries, small intestine, spleen, and brain (125). WISP1 is a matricellular protein that alters the signaling of other pathways to impact processes such as programmed cell death, extracellular matrix production, cellular migration, and mitosis (171). Early work highlighted that WISP1 can block p53 mediated DNA damage and prevent the induction of apoptosis (172). Yet, WISP1 also can control other pathways of programmed cell death such as autophagy (125, 147) as well as apoptosis (172–175) and caspase activation (173, 174, 176).
Figure 1.
Topical Highlights for WISP1, mTOR, and SIRT1 in Diabetes Mellitus
WISP1 drives cellular proliferation and survival through several pathways that involve phosphoinositide 3 –kinase (PI 3-K), protein kinase B (Akt), sirtuins, and the mechanistic target of rapamycin (mTOR) (21, 153, 177). WISP1 can up-regulate PI 3-K and Akt during oxidative stress (147, 174, 176), DNA damage (172), fibroblast proliferation in airway remodeling (178), cardiomyocyte injury (173), vascular smooth muscle proliferation (179), and toxic β-amyloid (Aβ) exposure (180). Through Akt activation, WISP1 leads to the inhibitory phosphorylation of glycogen synthase kinase -3β (GSK-3β) (147, 173, 176, 178) that maintains the integrity of β-catenin and allows translocation of this protein to the cell nucleus to block apoptotic cell death (159, 164, 181–185).
WISP1 cellular protection also relies upon sirtuin and mTOR mediated pathways. Sirtuins are histone deacetylases that transfer acetyl groups from ε-N-acetyl lysine amino acids on the histones of DNA to regulate transcription (10, 131, 186–190). In regards to silent mating type information regulation 2 homolog) 1 (S. cerevisiae) (SIRT1), a member of the sirtuin family that can modulate cellular metabolism during DM (44, 54, 191, 192), WISP1 increases SIRT1 activity and promotes SIRT1 nuclear translocation (174) that results in the blockade of apoptotic injury (105, 193, 194). WISP1 controls the mammalian forkhead transcription factor FoxO3a, a mediator of cellular metabolism as well as caspase activity (9, 37, 47, 150, 151) to maintain the integrity of SIRT1 during oxidative stress (174).
With mTOR, WISP1 can activate this pathway and phosphorylate the mTOR down-stream components of p70 ribosomal S6 kinase (p70S6K) and the eukaryotic initiation factor 4E-binding protein 1 (4EBP1) (32, 195). In addition, WISP1 increases mTOR activity by blocking the inhibitory actions of the mTOR component proline rich Akt substrate 40 kDa (PRAS40) (196). WISP1 also oversees the post-translational phosphorylation of AMP activated protein kinase (AMPK) that is involved in glucose homeostasis (197–200) to control the activity of this protein as well as mTOR. AMPK regulates the activity of the hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex that is an inhibitor of the mTOR complex mTOR Complex 1 (mTORC1) (201). When active, AMPK phosphorylates TSC2 as well as Raptor to block the activity of mTOR and the complex mTORC1 during energy stress (202). WISP1 modulates AMPK activation by differentially decreasing phosphorylation of TSC2 at Ser1387, a target of AMPK, and increasing phosphorylation of TSC2 at Thr1462, a target of Akt (180). As a result, WISP1 increases TSC2 activation with concurrent limits placed upon AMPK activation. For proper cellular function and survival with WISP1, a minimal level of TSC2 and AMPK activity is necessary (180). The ability of WISP1 to modulate AMPK activity is critical for proper cellular metabolism during DM (200). In some cases, AMPK activity can lead to a reduction in insulin resistance and diminished oxidative stress mediated through activation of autophagy (8). In addition, AMPK may limit myocardial ischemia in experimental models of DM (203), promote proper metabolic function of cells (204), and block adipocyte differentiation, lipid accumulation, and obesity (205). However, the level of AMPK activity may be an important consideration in DM since in some experimental models of Type 2 DM, AMPK activation can lead to apoptosis in pancreatic islet cells (206).
The reparative processes of WISP1 that involve DM also may be linked to stem cell proliferation, migration, and differentiation. Expression of WISP1 is increased during stem cell migration (207). WISP1 can influence induced pluripotent stem cell reprogramming (208, 209). In relation to cellular metabolism, WISP1 is differentially regulated during human embryonic stem cell and adipose-derived stem cell differentiation. WISP1 is up-regulated during human adipocyte differentiation (154), in human embryonic stem cells, and is repressed in adipose-derived stem cells during hepatic differentiation (210). Furthermore, in studies that examine pancreatic regeneration, WISP1 is one of several genes that are over-expressed during this process, suggesting that WISP1 may control a protective process during DM (211). WISP1 may be critical for the development of therapeutic strategies against vascular complications of DM. WISP1 expression is selectively up-regulated and may support vascular repair and regeneration during saphenous vein crush injury (212). WISP1 also promotes vascular smooth muscle proliferation that may be important for tissue repair during injury or affect restenosis following vascular grafting (179, 213). Importantly, WISP1 can lead to cellular senescence (214) and does not appear to foster excessive cellular proliferation in aging vascular cells (215) that may result in the development of atherosclerosis. As a potential endogenous reparative response to injury, WISP1 expression is affected by weight change in humans and increases during insulin resistance and inflammation in glucose-tolerant individuals (154).
5. mTOR and DM
As noted with some of the metabolic pathways linked to WISP1, mTOR is one of the principal pathways necessary for the control of aging and cellular metabolism during DM (4, 199, 216) (Fig. 1). Also known as the mammalian target of rapamycin and FK506-binding protein 12-rapamycin complex-associated protein 1, mTOR is a 289-kDa serine/threonine protein kinase that is encoded by a single gene FRAP1 (217–219). mTOR is a component of the protein complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) (197, 199). mTORC1 is composed of Raptor (Regulatory-Associated Protein of mTOR), the proline rich Akt substrate 40 kDa (PRAS40), Deptor (DEP domain-containing mTOR interacting protein), and mLST8/GβL (mammalian lethal with Sec13 protein 8, termed mLST8). In contrast, mTORC2 is composed of Rictor (Rapamycin-Insensitive Companion of mTOR), Deptor, mLST8, the mammalian stress-activated protein kinase interacting protein (mSIN1), and the protein observed with Rictor-1 (Protor-1) (199, 220).
mTOR can influence both apoptotic and autophagic pathways of programmed cell death (221). In relation to cellular metabolism and DM, mTOR activation through glucagon-like peptide-1 agonists can protect pancreatic β-cells from cholesterol mediated apoptotic cell injury (222), promote pancreatic β-cell proliferation (136), and block neural apoptotic cell loss during DM through the epidermal growth factor receptor (133). mTOR can prevent apoptosis and limit insulin resistance as well as vascular thrombosis in patients with metabolic syndrome (223). Through mTOR activation, pathways associated with apoptosis and atherosclerosis also can be blocked (224).
Furthermore, control of mTOR is seen as a vital component to other strategies that may be effective to treat DM and its complications. For example, erythropoietin (EPO), a cytokine and an investigational therapeutic strategy for DM (12, 225), targets multiple cellular signal transduction pathways in the body (226, 227) and relies upon mTOR for cytoprotection (2, 32, 42, 199, 228). EPO uses mTOR to increase cell survival during oxygen-glucose deprivation (195, 229), prevent cell injury during β-amyloid exposure (230), modulate bone homeostasis (231), improve cognitive function sepsis-associated encephalopathy (232), promote retinal progenitor cell survival during oxidant stress (233), prevent retinal degeneration in models of polycystic kidney disease (62), and foster the neuronal phenotype of adult neuronal precursor cells (234). During abnormalities in cellular metabolism, EPO facilitates wound healing during DM (50), attenuates AGE-induced toxicity (235), protects endothelial cell survival during experimental models of DM (66, 67), maintains cellular mitochondrial function and energy metabolism (70), limits high glucose-induced oxidative stress in renal tubular cells (138), and reduces the detrimental effects of obesity in animal models (14).
Agents that are effective in controlling DM rely also upon mTOR and the modulation of autophagy to offer cytoprotection. Metformin, a drug used to control hyperglycemia in DM, blocks mTOR activity and promotes autophagy. Metformin protects against endothelial cell senescence (25), limits androgen up-regulation during prostate cancer through mTOR inhibition (236), and protects against neuronal apoptotic cell death (237). Metformin through pathways that activate AMPK limits cell loss during hypoxia through increased AMPK activity (238), prevents cardiomyopathy in experimental models of DM (239), enhances cardiomyocyte cell survival (34), and reduces cortical infarction during cerebral ischemia (240). Although AMPK under some conditions may provide cellular protection by limiting oxidative stress that can lead to vascular hypertension (95), increasing cell survival during hypoxia (238), and promoting autophagy that may resolve memory impairment (241), AMPK activity as previously noted also may have detrimental effects. In some studies examining cell survival, AMPK activity may foster neuroinflammation (242), lead to aberrant Aβ stress (243) and Aβ toxicity (180), and result in cardiac dysfunction (31) and cardiac tissue hypertrophy (244).
6. SIRT1 and DM
SIRT1, also known as NAD-dependent deacetylase sirtuin-1, has become a key component for the development of therapies directed against DM (4, 10, 186, 245) (Fig. 1). In addition to pathways previously described for WISP1, SIRT1 also employs signal transduction pathways of mTOR to govern cellular survival and metabolism. SIRT1, a histone deacetylase, is one of seven mammalian homologues of the yeast silent information regulator-2 (Sir2) that also oversee post-translational changes of proteins involved with cellular proliferation, survival, and senescence (25, 105, 193, 245). SIRT1 is dependent upon NAD+ as a substrate (54, 186, 246, 247). Through the salvage pathway of NAD+ synthesis, nicotinamide phosphoribosyltransferase (NAMPT) catalyzes the conversion of nicotinamide to nicotinamide mononucleotide (53). Nicotinamide mononucleotide is converted to NAD+ by the nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT) enzyme family (248). NAMPT activity not only increases cellular NAD levels, but also increases the activity of SIRT1 transcription.
SIRT1 activity also is overseen by NMNAT, mammalian forkhead transcription factors, and AMPK (186, 249–251). NMNAT modulates the deacetylating activity of SIRT1. Mammalian forkhead transcription factors bind to the SIRT1 promoter region that contains a cluster of five putative core binding repeat motifs (IRS-1) and a forkhead-like consensus-binding site (FKHD-L) (127). This allows forkhead transcription factors, such as FoxO1, to control SIRT1 transcription and increase SIRT1 expression (252). AMPK that phosphorylates TSC2 and inhibits mTORC1 activity (21, 199) can increase the cellular NAD+/NADH ratio leading to the deacetylation of downstream SIRT1 targets that include the peroxisome proliferator-activated receptor-gamma coactivator 1 (PGC-1α), FoxO1 (37), and FoxO3a (253). AMPK also can increase NAMPT during glucose restriction that results in increased NAD+ and decreased levels of nicotinamide (254), an inhibitor of SIRT1 (3). SIRT1 activators, such as resveratrol, also can activate AMPK through SIRT1 dependent and independent mechanisms (253, 255). Importantly, the level of SIRT1 activity can yield significant consequences for cellular protection. Insufficient SIRT1 activity can be detrimental for vascular cell survival (105, 193, 256), protection against cardiovascular disease (85), and prevention of neuronal injury (174, 257, 258). However, a reduction in SIRT1 activity also may be required to promote cellular survival in systems involving trophic factors such as as insulin growth factor-1 (259).
Cellular survival through SIRT1 is closely regulated through apoptotic and autophagic pathways. SIRT1 can control the early phases of apoptotic cell death by preventing the externalization of membrane PS exposure (105, 131, 193, 260). In the presence of tumor necrosis factor-α (TNF-α), SIRT1 can protect endothelial progenitor cells (261) and enhance skeletal myoblast survival (262). SIRT1 also can limit neuronal apoptosis in models of traumatic brain injury (263). Loss of SIRT1 in mouse cochlear neurons and in the auditory cortex is associated with hearing loss (264) and loss of SIRT1 activity in human mesenchymal stem cells results in a reduced proliferation rate with increased apoptosis (265). In addition, decreased levels of SIRT1 can occur in smokers and chronic obstructive disease patients that leads to endothelial progenitor cell dysfunction with apoptotic cell death (266). Decreased levels of SIRT1 activity can be the result of apoptotic pathways associated with p38 (267) and c-Jun N-terminal kinase -1 (JNK1) (46) as well as caspase degradation of SIRT1 (268) that can then lead to further activation of caspases (268, 269). As previously described, pathways such as WISP1 prevent SIRT1 degradation and block caspase activation that would otherwise lead to the degradation of SIRT1 (174, 270–272).
SIRT1 also is dependent upon the induction of autophagy to foster cellular survival. For example, SIRT1 activity that promotes autophagy is necessary for the protection of chondrocytes during oxidative stress, since knockdown of the forkhead transcription factors FoxO1 and FoxO3 lead to loss of SIRT1 activity, reduced autophagic related proteins, and subsequent cell death (92). In models of cognitive loss that employ chronic intermittent hypoxia hypercapnia exposure, SIRT1 activation is able to limit apoptotic cell injury and improve cognition through the induction of autophagy (273). During pathways that are associated with cellular metabolism, SIRT1 promotes autophagy in mitochondria (274) that may be required to maintain a healthy mitochondrial pool (275). SIRT1 up-regulation in conjunction with AMPK activation leads to autophagy that is necessary for cellular protection in endothelial cells exposed to oxidized low density lipoproteins that can lead to atherosclerosis (251). These studies that support a protective role for SIRT1 with autophagy and AMPK activation suggest an inverse relationship with mTOR (4). SIRT1 blocks mTOR activity and promotes autophagy to preserve the integrity of embryonic stem cells during oxidant stress (190). SIRT1 also inhibits mTOR signaling to promote neuronal growth (276) and assist with mesangial cell proliferation during high glucose exposure (277). Yet, it should be noted that not all cases of cytoprotection with SIRT1 required induction of autophagy as well as potential mTOR inhibition. In pulmonary models of oxidative stress such as the exposure to cigarette smoke in bronchial epithelial cells, SIRT1 blocks cell injury through the inhibition of of autophagy (187, 278).
Activation of SIRT1 in mature and differentiated cells during DM is in most instances cytoprotective and can avert insulin resistance. SIRT1 activation activation has been shown to increase lifespan in higher organisms such as Drosophila and protect cells from oxidative stress (279, 280). The presence of SIRT1 appears vital for the prevention of insulin resistance. Loss of SIRT1 can result in insulin resistance and excessive hepatic lipid accumulation (44). Gene deletion or inhibition of SIRT1 can alter insulin signaling by interfering with insulin stimulated insulin receptor phosphorylation and glycogen synthase (281). Over-expression of SIRT1 can decrease hepatic steatosis and improve insulin sensitivity that leads to improved glucose homeostasis (48). In addition, SIRT1 also is utilized by the cytokine EPO to block cell injury during DM. EPO can increase endogenous cellular SIRT1 activity and foster the subcellular trafficking of SIRT1 to the nucleus to result in endothelial cell protection during oxidative stress (193). SIRT1 also is one component that allows EPO to maintain adipose cell energy homeostasis and protect against metabolic disorders such as DM (192).
SIRT1 may avert insulin resistance through a number of mechanisms that involve fat mobilization (44), mTOR signaling (282), as well as modulation of inflammation (101). SIRT1 also can increase insulin signaling in insulin-sensitive organs through pathways that involve Akt and PI 3-K (105, 131, 193, 258, 283, 284) as well as stimulate glucose-dependent insulin secretion from pancreatic β cells by repressing UCP2 (285). Regulation of insulin sensitivity by SIRT1 may require AMPK. Endothelial cell protection from oxidized low-density lipoproteins has been shown to involve SIRT1 as well as AMPK activation (205, 251). Interestingly, SIRT1 activation with AMPK also may be necessary to protect against spatial memory impairment in combined experimental models of DM and Alzheimer’s disease, since these studies demonstrate a loss of SIRT1 and AMPK activities that lead to cognitive loss, oxidative stress, and neuronal cell apoptosis (286).
In addition to mature and differentiated cells, SIRT1 also prevents cell injury in stem cells that may be important for treatments related to DM. Recent studies have suggested that stem cell strategies may be effective for at least treating and maintaining glucose homeostasis during DM in animal models (287, 288). SIRT1 has been shown to be necessary to modulate autophagic flux (289) and for the transition of muscle stem cells from a quiescence state to an active state through the induction of autophagy (290). SIRT1 blocks apoptotic cell injury during oxidative stress through the induction of autophagy in endothelial progenitor cells (291). In the cardiovascular system, increased SIRT1 expression enhances the survival of cardiomyoblasts (292). SIRT1 prevents senescence and impaired differentiation in endothelial progenitor cells (293). Mesenchymal stem cells that are subjected to SIRT1 over-expression show increased blood vessel density in the area of cardiac infarcts, reduced cardiac remodeling, and improved cardiac performance in rodent models (294). SIRT1 also may assist aged stem cells that are senescent to foster repair. Aged mesenchymal stem cells that were subjected to pre-conditioning with glucose depletion demonstrated increased expression of SIRT1 in addition to other proliferative entities such as growth factors to lead to improved cardiac performance (295). Other work demonstrates that SIRT1 is necessary for endothelial progenitor cell mobilization and vascular repair during DM in mice (191). In rodent models of DM, SIRT1 can preserve angiogenesis derived from bone marrow-derived early outgrowth cells (296). In addition, patients with Type 2 DM show a down-regulation of endothelial progenitor cells that has been associated with decreased SIRT1 protein levels (297).
However, SIRT1 activation may require a level of modulation since in some systems of the body, decreased SIRT1 activity is necessary for proper stem cell development. In the nervous system, loss of SIRT1 expression with the induction of heat shock protein -70 (HSP70) is required to promote neural differentiation, maturation of embryonic cortical neurons (298), and the differentiation of human embryonic stem cells into motoneurons (299). SIRT1 also is considered a negative regulator of subventricular zone and hippocampal neural precursors in murine animal models, since knockdown of SIRT1 does not eliminate neural precursor numbers but increases the production of neurons in the subventricular zone and the hippocampus (300). In mouse neural stem cells, neuronal differentiation can be driven through the microRNA miR-34a that leads to decreased SIRT1 expression and DNA-binding of p53 (301). Yet, a level of SIRT1 activity appears to be required for different cell types, since in studies with neuronal differentiation, increased expression of SIRT1 enhanced the astrocytic subpopulation of cells that are necessary to support neuronal cell populations (301).
7. Future Considerations
DM affects a significantly greater portion of the world’s population each year with many additional individuals remaining undiagnosed. Risk factors such as obesity and concurrent disorders with DM that can involve the cardiovascular systems, renal system, and the nervous system ultimately lead to significant death and disability with staggering healthcare costs consuming large portions of the GDP for many countries. Current therapies for both achieving glucose homeostasis during DM and averting the complications of DM are limited and require the development of novel strategies that can address oxidant stress pathways to regulate programmed cell death through both apoptosis and autophagy. WISP1, a CCN family member, drives cellular proliferation and survival through mechanisms that oversee PI 3-K, Akt, SIRT1, and mTOR that ultimately can limit insulin resistance, lead to stem cell regeneration of injured tissues, and enhance cellular protection through modulation of apoptosis and autophagy during DM (Fig. 2). Independently, mTOR can block apoptotic pathways to enhance pancreatic β-cell proliferation, resolve insulin resistance, inhibit pathways tied to atherosclerosis, and avert oxidative stress mediated cellular injury through agents that involve cytokines such as EPO and oral hypoglycemics such as metformin. In some pathways with mTOR, it is the inhibition of mTOR that is required for cytoprotective pathways of autophagy to proceed. Although SIRT1 employs “anti-apoptotic” mechanisms to increase cell survival and preserve insulin signaling during oxidant stress exposure, SIRT1 also appears at times to have an inverse relationship with mTOR to block mTOR activation and foster autophagy for the preservation of cellular energy organelles involving mitochondria, the promotion of stem cell proliferation, and the prevention of apoptosis. Pathways linking WISP1, mTOR, and SIRT1 with apoptosis and autophagy involve AMPK, a pathway intimately tied to glucose homeostasis that can prevent tissue ischemia, insulin resistance, cognitive loss, and cell death. Targeting WISP1, mTOR, and SIRT1 for the treatment of glucose control in DM as well as the complications of this disease opens exciting prospects to eventually limit the devastating and growing impact DM has on the world’s population. Yet, it is imperative that future work addresses the fine biological control WISP1, mTOR, and SIRT1 hold over metabolism to precisely modulate cellular signaling, since these pathways under specific conditions can yield unwanted clinical outcomes that involve induction of fibrotic tissue injury (161), tumorigenesis (302–305), inflammation (242), progression of neurodegenerative disorders (180, 243), cardiac dysfunction (31), loss of neuronal embryonic stem cells that may limit reparative processes in the nervous system (298, 300), and apoptosis in pancreatic islet cells (206).
Figure 2. Apoptosis and Autophagy Pathways for WISP1, mTOR, and SIRT1 During Oxidative Stress and Diabetes Mellitus.
Oxidative stress is an important determinant of cell injury in diabetes mellitus (DM) and leads to the generation of reactive oxygen species (ROS) that can significantly affect cellular metabolism. Ultimately, DM through oxidative stress can lead to apoptotic cell injury that consists of an early phase involving the loss of plasma membrane lipid phosphatidylserine (PS) asymmetry and a later phase that leads to genomic DNA degradation. During autophagy in DM, macroautophagy plays a principal role and involves the sequestration of cytoplasmic proteins into autophagosomes that fuse with lysosomes for degradation and recycling for future cellular processes. WISP1, mTOR, and SIRT1 through shared as well as independent pathways can oversee phosphoinositide 3 –kinase (PI 3-K), protein kinase B (Akt), AMP activated protein kinase (AMPK), and the the hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex to control multiple biological outcomes that include insulin resistance, stem cell proliferation, glucose homeostasis, and cell survival. The cytokine erythropoietin (EPO) uses wingless pathways of Wnt1, SIRT1, and mTOR to help maintain mitochondrial function and vascular survival during DM. Metformin, a hypoglycemic agent, limits mTOR activity and promotes autophagy to not only regulate serum glucose, but also limit cellular injury during DM. mTOR is an essential component of the protein complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2). Activation of mTOR leads to down stream signaling with the cytoprotective pathways of p70 ribosomal S6 kinase (p70S6K) and the eukaryotic initiation factor 4E-binding protein 1 (4EBP1). In contrast, mTOR activity can be blocked by the proline rich Akt substrate 40 kDa (PRAS40) as well as by AMPK through the TSC1/TSC2 complex.
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
Conflict of Interests: The authors declare no conflict of interests regarding the publication of this paper.
References
- 1.Centers for Medicare and Medicaid Services. National Health Expenditure Projections 2012–2022. 2013 www.cms.gov.
- 2.Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR. Invited Review: Over-nutrition, mTOR Signaling and Cardiovascular Diseases. Am J Physiol Regul Integr Comp Physiol. 2014 Sep 24; doi: 10.1152/ajpregu.00262.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maiese K, Chong ZZ, Shang YC, Hou J. Novel Avenues of Drug Discovery and Biomarkers for Diabetes Mellitus. Journal of clinical pharmacology. 2011 Mar 10;51(2):128–52. doi: 10.1177/0091270010362904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert opinion on drug discovery. 2013 Jan;8(1):35–48. doi: 10.1517/17460441.2013.736485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutter MK, Massaro JM, Hoffmann U, O’Donnell CJ, Fox CS. Fasting Glucose, Obesity, and Coronary Artery Calcification in Community-Based People Without Diabetes. Diabetes Care. 2012 Jul 6; doi: 10.2337/dc11-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu E, Schwab M, Marette A. Role of protein tyrosine phosphatases in the modulation of insulin signaling and their implication in the pathogenesis of obesity-linked insulin resistance. Rev Endocr Metab Disord. 2014 Mar;15(1):79–97. doi: 10.1007/s11154-013-9282-4. [DOI] [PubMed] [Google Scholar]
- 7.Himmetoglu S, Teksoz S, Zengin K, Yesim T, Taskin M, Dincer Y. Serum levels of fetuin A and 8-hydroxydeoxyguanosine in morbidly obese subjects. Exp Clin Endocrinol Diabetes. 2013 Aug;121(8):505–8. doi: 10.1055/s-0033-1345162. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high fat diet feeding in mice. Diabetes. 2014 Jul 28;64(1):36–48. doi: 10.2337/db14-0267. [DOI] [PubMed] [Google Scholar]
- 9.Shao S, Yang Y, Yuan G, Zhang M, Yu X. Signaling molecules involved in lipid-induced pancreatic Beta-cell dysfunction. Dna Cell Biol. 2013 Feb;32(2):41–9. doi: 10.1089/dna.2012.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Essser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert opinion on investigational drugs. 2014 Oct;25:1–25. doi: 10.1517/13543784.2015.974804. [DOI] [PubMed] [Google Scholar]
- 11.Maiese K, Chong ZZ, Shang YC, Wang S. Erythropoietin: new directions for the nervous system. International journal of molecular sciences. 2012;13(9):11102–29. doi: 10.3390/ijms130911102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Jama. 2005 Jan 5;293(1):90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White MF. IRS2 integrates insulin/IGF1 signalling with metabolism, neurodegeneration and longevity. Diabetes Obes Metab. 2014 Sep;16( Suppl 1):4–15. doi: 10.1111/dom.12347. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Wang L, Dey S, Alnaeeli M, Suresh S, Rogers H, et al. Erythropoietin action in stress response, tissue maintenance and metabolism. International journal of molecular sciences. 2014;15(6):10296–333. doi: 10.3390/ijms150610296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007 Nov;22(11):1251–67. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdullah A, Wolfe R, Mannan H, Stoelwinder JU, Stevenson C, Peeters A. Epidemiologic Merit of Obese-Years, the Combination of Degree and Duration of Obesity. Am J Epidemiol. 2012 Jun 28; doi: 10.1093/aje/kwr522. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Global status report on noncommunicable diseases 2010. Geneva: 2011. [Google Scholar]
- 18.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. US Department of Health and Human Services; Atlanta, GA: 2014. [Google Scholar]
- 19.Maiese K. Diabetic stress: new triumphs and challenges to maintain vascular longevity. Expert Rev Cardiovasc Ther. 2008 Mar;6(3):281–4. doi: 10.1586/14779072.6.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 2000 Jul-Aug;16(4):230–6. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr122>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant Stress and Signal Transduction in the Nervous System with the PI 3-K, Akt, and mTOR Cascade. International journal of molecular sciences. 2013;13(11):13830–66. doi: 10.3390/ijms131113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14(16):1729–38. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiese K, Shang YC, Chong ZZ, Hou J. Diabetes mellitus: channeling care through cellular discovery. Curr Neurovasc Res. 2010 Feb 1;7(1):59–64. doi: 10.2174/156720210790820217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexandru N, Popov D, Georgescu A. Platelet dysfunction in vascular pathologies and how can it be treated. Thromb Res. 2012 Feb;129(2):116–26. doi: 10.1016/j.thromres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014 Jan;171(2):523–35. doi: 10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JX, Tuo Q, Liao DF, Zeng H. Inhibition of Protein Tyrosine Phosphatase Improves Angiogenesis via Enhancing Ang-1/Tie-2 Signaling in Diabetes. Exp Diabetes Res. 2012;2012:836759. doi: 10.1155/2012/836759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes MB, Negrato CA. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetology & metabolic syndrome. 2014;6(1):80. doi: 10.1186/1758-5996-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu P, Lai D, Lu P, Gao J, He H. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med. 2012 Apr;29(4):613–8. doi: 10.3892/ijmm.2012.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KA, Shin YJ, Akram M, Kim ES, Choi KW, Suh H, et al. High glucose condition induces autophagy in endothelial progenitor cells contributing to angiogenic impairment. Biol Pharm Bull. 2014;37(7):1248–52. doi: 10.1248/bpb.b14-00172. [DOI] [PubMed] [Google Scholar]
- 30.Schaffer SW, Jong CJ, Mozaffari M. Role of oxidative stress in diabetes-mediated vascular dysfunction: unifying hypothesis of diabetes revisited. Vascul Pharmacol. 2012 Nov-Dec;57(5–6):139–49. doi: 10.1016/j.vph.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Aragno M, Mastrocola R, Ghe C, Arnoletti E, Bassino E, Alloatti G, et al. Obestatin induced recovery of myocardial dysfunction in type 1 diabetic rats: underlying mechanisms. Cardiovasc Diabetol. 2012;11:129. doi: 10.1186/1475-2840-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chong ZZ, Maiese K. Mammalian Target of Rapamycin Signaling in Diabetic Cardiovascular Disease. Cardiovasc Diabetol. 2012 Apr 30;11(1):45. doi: 10.1186/1475-2840-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das A, Durrant D, Koka S, Salloum FN, Xi L, Kukreja RC. Mammalian Target of Rapamycin (mTOR) Inhibition with Rapamycin Improves Cardiac Function in Type 2 Diabetic Mice: POTENTIAL ROLE OF ATTENUATED OXIDATIVE STRESS AND ALTERED CONTRACTILE PROTEIN EXPRESSION. J Biol Chem. 2014 Feb 14;289(7):4145–60. doi: 10.1074/jbc.M113.521062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013 Apr;62(4):1270–81. doi: 10.2337/db12-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling S, Birnbaum Y, Nanhwan MK, Thomas B, Bajaj M, Li Y, et al. Dickkopf-1 (DKK1) phosphatase and tensin homolog on chromosome 10 (PTEN) crosstalk via microRNA interference in the diabetic heart. Basic Res Cardiol. 2013;108(3):352. doi: 10.1007/s00395-013-0352-2. [DOI] [PubMed] [Google Scholar]
- 36.Maiese K. Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008 Apr-May;62(4):218–32. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond) 2009 Feb;116(3):191–203. doi: 10.1042/CS20080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Portbury AL, Ronnebaum SM, Zungu M, Patterson C, Willis MS. Back to your heart: ubiquitin proteasome system-regulated signal transduction. J Mol Cell Cardiol. 2012 Mar;52(3):526–37. doi: 10.1016/j.yjmcc.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, Zhang L, Chen S, Feng B, Lu X, Bai Y, et al. The prevention of diabetic cardiomyopathy by non-mitogenic acidic fibroblast growth factor is probably mediated by the suppression of oxidative stress and damage. PLoS One. 2013;8(12):e82287. doi: 10.1371/journal.pone.0082287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012 May 28;172(10):761–9. doi: 10.1001/archinternmed.2011.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao J, Li F, Liu W, Liu Q, Liu S, Li H, et al. Phosphorylation of PRAS40-Thr246 Involves in Renal Lipid Accumulation of Diabetes. J Cell Physiol. 2013 Dec 18; doi: 10.1002/jcp.24533. [DOI] [PubMed] [Google Scholar]
- 42.Hao J, Zhu L, Li F, Liu Q, Zhao X, Liu S, et al. Phospho-mTOR: a novel target in regulation of renal lipid metabolism abnormality of diabetes. Exp Cell Res. 2013 Aug 15;319(14):2296–306. doi: 10.1016/j.yexcr.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Gallardo RV, Noriega-Cisneros R, Esquivel-Gutierrez E, Calderon-Cortes E, Cortes-Rojo C, Manzo-Avalos S, et al. Effects of diabetes on oxidative and nitrosative stress in kidney mitochondria from aged rats. Journal of bioenergetics and biomembranes. 2014 Nov 26; doi: 10.1007/s10863-014-9594-4. [DOI] [PubMed] [Google Scholar]
- 44.Caron AZ, He X, Mottawea W, Seifert EL, Jardine K, Dewar-Darch D, et al. The SIRT1 deacetylase protects mice against the symptoms of metabolic syndrome. FASEB J. 2014 Mar;28(3):1306–16. doi: 10.1096/fj.13-243568. [DOI] [PubMed] [Google Scholar]
- 45.Castano D, Larequi E, Belza I, Astudillo AM, Martinez-Anso E, Balsinde J, et al. Cardiotrophin-1 eliminates hepatic steatosis in obese mice by mechanisms involving AMPK activation. Journal of hepatology. 2014 May;60(5):1017–25. doi: 10.1016/j.jhep.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Gao Z, Zhang J, Kheterpal I, Kennedy N, Davis RJ, Ye J. Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity. J Biol Chem. 2011 Jun 24;286(25):22227–34. doi: 10.1074/jbc.M111.228874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone. 2012 Feb;50(2):437–43. doi: 10.1016/j.bone.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. Faseb J. 2011 Feb 14; doi: 10.1096/fj.10-173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh A, Singh N, Akanksha, Jayendra, Maurya R, Srivastava A. Coagulanolide modulates hepatic glucose metabolism in C57BL/KsJ-db/db mice. Human & experimental toxicology. 2012 Oct;31(10):1056–65. doi: 10.1177/0960327112438289. [DOI] [PubMed] [Google Scholar]
- 50.Hamed S, Bennett CL, Demiot C, Ullmann Y, Teot L, Desmouliere A. Erythropoietin, a novel repurposed drug: an innovative treatment for wound healing in patients with diabetes mellitus. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2014 Jan-Feb;22(1):23–33. doi: 10.1111/wrr.12135. [DOI] [PubMed] [Google Scholar]
- 51.Hao J, Shen W, Tian C, Liu Z, Ren J, Luo C, et al. Mitochondrial nutrients improve immune dysfunction in the type 2 diabetic Goto-Kakizaki rats. J Cell Mol Med. 2009 Apr;13(4):701–11. doi: 10.1111/j.1582-4934.2008.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.John CM, Ramasamy R, Al Naqeeb G, Dhiab Al-Nuaimi AH, Adam A. Enhanced CD4+CD25+ regulatory T cells with splenic proliferation and protection against oxidative stress by nicotinamide in gestational diabetes. Curr Med Chem. 2012 Aug 16; [PubMed] [Google Scholar]
- 53.Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14(9):3446–85. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol. 2011;52(4):1173–85. [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Z, Huang G, Wang B, Zhong Y. Inhibition of NF-kappaB activation by Pyrrolidine dithiocarbamate partially attenuates hippocampal MMP-9 activation and improves cognitive deficits in streptozotocin-induced diabetic rats. Behav Brain Res. 2013 Feb 1;238:44–7. doi: 10.1016/j.bbr.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 56.Sonnen JA, Larson EB, Brickell K, Crane PK, Woltjer R, Montine TJ, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009 Mar;66(3):315–22. doi: 10.1001/archneurol.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2014 Oct 23; doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maiese K, Chong ZZ, Hou J, Shang YC. New strategies for Alzheimer’s disease and cognitive impairment. Oxid Med Cell Longev. 2009 Nov-Dec;2(5):279–89. doi: 10.4161/oxim.2.5.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maiese K, Chong ZZ, Li F. Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr Neurovasc Res. 2005 Dec;2(5):425–46. doi: 10.2174/156720205774962692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aksu I, Ates M, Baykara B, Kiray M, Sisman AR, Buyuk E, et al. Anxiety correlates to decreased blood and prefrontal cortex IGF-1 levels in streptozotocin induced diabetes. Neurosci Lett. 2012 Dec 7;531(2):176–81. doi: 10.1016/j.neulet.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 61.Reagan LP. Diabetes as a chronic metabolic stressor: causes, consequences and clinical complications. Exp Neurol. 2012 Jan;233(1):68–78. doi: 10.1016/j.expneurol.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Busch S, Kannt A, Kolibabka M, Schlotterer A, Wang Q, Lin J, et al. Systemic treatment with erythropoietin protects the neurovascular unit in a rat model of retinal neurodegeneration. PLoS One. 2014;9(7):e102013. doi: 10.1371/journal.pone.0102013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu D, Wu M, Zhang J, Du M, Yang S, Hammad SM, et al. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Diabetologia. 2012 Nov;55(11):3128–40. doi: 10.1007/s00125-012-2692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang L, Zhang Y, Jiang Y, Willard L, Ortiz E, Wark L, et al. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood) 2011 Sep 1;236(9):1051–63. doi: 10.1258/ebm.2011.010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barthelmes D, Zhu L, Shen W, Gillies MC, Irhimeh MR. Differential gene expression in Lin−/VEGF-R2+ bone marrow-derived endothelial progenitor cells isolated from diabetic mice. Cardiovasc Diabetol. 2014;13(1):42. doi: 10.1186/1475-2840-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO Relies upon Novel Signaling of Wnt1 that Requires Akt1, FoxO3a, GSK-3beta, and beta-Catenin to Foster Vascular Integrity During Experimental Diabetes. Curr Neurovasc Res. 2011 May 1;8(2):103–20. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007 Aug;4(3):194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hou J, Chong ZZ, Shang YC, Maiese K. FoxO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010 Mar 4;321(2):194–206. doi: 10.1016/j.mce.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Q, Li J, Cheng R, Chen Y, Lee K, Hu Y, et al. Nitrosative stress plays an important role in wnt pathway activation in diabetic retinopathy. Antioxid Redox Signal. 2013 Apr 1;18(10):1141–53. doi: 10.1089/ars.2012.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L, Di L, Noguchi CT. Erythropoietin, a novel versatile player regulating energy metabolism beyond the erythroid system. Int J Biol Sci. 2014;10(8):921–39. doi: 10.7150/ijbs.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bagul PK, Banerjee SK. Insulin resistance, oxidative stress and cardiovascular complications: role of sirtuins. Curr Pharm Des. 2013;19(32):5663–77. doi: 10.2174/13816128113199990372. [DOI] [PubMed] [Google Scholar]
- 72.Liu J, Li J, Li WJ, Wang CM. The role of uncoupling proteins in diabetes mellitus. Journal of diabetes research. 2013;2013:585897. doi: 10.1155/2013/585897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peng Y, Huang S, Cheng B, Nie X, Enhe J, Feng C, et al. Mesenchymal stem cells: a revolution in therapeutic strategies of age-related diseases. Ageing research reviews. 2013 Jan;12(1):103–15. doi: 10.1016/j.arr.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 74.Weinberg E, Maymon T, Weinreb M. AGEs induce caspase-mediated apoptosis of rat BMSCs via TNFalpha production and oxidative stress. Journal of molecular endocrinology. 2014 Feb;52(1):67–76. doi: 10.1530/JME-13-0229. [DOI] [PubMed] [Google Scholar]
- 75.Xu YJ, Tappia PS, Neki NS, Dhalla NS. Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart failure reviews. 2014 Jan;19(1):113–21. doi: 10.1007/s10741-013-9379-6. [DOI] [PubMed] [Google Scholar]
- 76.Yang H, Jin X, Kei Lam CW, Yan SK. Oxidative stress and diabetes mellitus. Clin Chem Lab Med. 2011 Nov;49(11):1773–82. doi: 10.1515/CCLM.2011.250. [DOI] [PubMed] [Google Scholar]
- 77.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005 Feb;75(3):207–46. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010 Mar;45(3):217–34. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer’s disease. Brain Res Brain Res Rev. 2005 Jul;49(1):1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim S, Kang IH, Nam JB, Cho Y, Chung DY, Kim SH, et al. Ameliorating the Effect of Astragaloside IV on Learning and Memory Deficit after Chronic Cerebral Hypoperfusion in Rats. Molecules. 2015;20(2):1904–21. doi: 10.3390/molecules20021904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wright JW, Harding JW. The Brain Hepatocyte Growth Factor/c-Met Receptor System: A New Target for the Treatment of Alzheimer’s Disease. J Alzheimers Dis. 2015 Feb 3; doi: 10.3233/JAD-142814. [DOI] [PubMed] [Google Scholar]
- 82.Peng S, Zhao S, Yan F, Cheng J, Huang L, Chen H, et al. HDAC2 Selectively Regulates FOXO3a-Mediated Gene Transcription during Oxidative Stress-Induced Neuronal Cell Death. J Neurosci. 2015 Jan 21;35(3):1250–9. doi: 10.1523/JNEUROSCI.2444-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fraineau S, Pal CG, 2nd, Allan DS, Brand M. Epigenetic Regulation of Endothelial Cell-mediated Vascular Repair. The FEBS journal. 2014 Dec 24; doi: 10.1111/febs.13183. [DOI] [PubMed] [Google Scholar]
- 84.Jenwitheesuk A, Nopparat C, Mukda S, Wongchitrat P, Govitrapong P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. International journal of molecular sciences. 2014;15(9):16848–84. doi: 10.3390/ijms150916848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kilic U, Gok O, Bacaksiz A, Izmirli M, Elibol-Can B, Uysal O. SIRT1 Gene Polymorphisms Affect the Protein Expression in Cardiovascular Diseases. PLoS One. 2014;9(2):e90428. doi: 10.1371/journal.pone.0090428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maiese K. Epigenetics in the cerebrovascular system: changing the code without altering the sequence. Curr Neurovasc Res. 2014 Feb;11(1):1–3. doi: 10.2174/1567202610999131204122451. [DOI] [PubMed] [Google Scholar]
- 87.Xin YJ, Yuan B, Yu B, Wang YQ, Wu JJ, Zhou WH, et al. Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress. Scientific reports. 2015;5:7645. doi: 10.1038/srep07645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Asaithambi A, Ay M, Jin H, Gosh A, Anantharam V, Kanthasamy A, et al. Protein Kinase D1 (PKD1) Phosphorylation Promotes Dopaminergic Neuronal Survival during 6-OHDA-Induced Oxidative Stress. PLoS One. 2014;9(5):e96947. doi: 10.1371/journal.pone.0096947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen C, Xu Y, Song Y. IGF-1 gene-modified muscle-derived stem cells are resistant to oxidative stress via enhanced activation of IGF-1R/PI3K/AKT signaling and secretion of VEGF. Mol Cell Biochem. 2014 Jan;386(1–2):167–75. doi: 10.1007/s11010-013-1855-8. [DOI] [PubMed] [Google Scholar]
- 90.Palma HE, Wolkmer P, Gallio M, Correa MM, Schmatz R, Thome GR, et al. Oxidative stress parameters in blood, liver, and kidney of diabetic rats treated with curcumin and/or insulin. Mol Cell Biochem. 2014 Jan;386(1–2):199–210. doi: 10.1007/s11010-013-1858-5. [DOI] [PubMed] [Google Scholar]
- 91.Zeldich E, Chen CD, Colvin TA, Bove-Fenderson EA, Liang J, Tucker Zhou TB, et al. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J Biol Chem. 2014 Aug 29;289(35):24700–15. doi: 10.1074/jbc.M114.567321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akasaki Y, Alvarez-Garcia O, Saito M, Carames B, Iwamoto Y, Lotz MK. FOXO transcription factors support oxidative stress resistance in human chondrocytes. Arthritis & rheumatology (Hoboken, NJ) 2014 Sep 3;66(12):3349–58. doi: 10.1002/art.38868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aksu U, Yanar K, Terzioglu D, Erkol T, Ece E, Aydin S, et al. Effect of tempol on redox homeostasis and stress tolerance in mimetically aged drosophila. Archives of insect biochemistry and physiology. 2014 Jul 18; doi: 10.1002/arch.21176. [DOI] [PubMed] [Google Scholar]
- 94.Bowes Rickman C, Farsiu S, Toth CA, Klingeborn M. Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci. 2013 Dec;54(14):ORSF68–80. doi: 10.1167/iovs.13-12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng PW, Ho WY, Su YT, Lu PJ, Chen BZ, Cheng WH, et al. Resveratrol Decrease Fructose-Induced Oxidative Stress Mediated by NADPH Oxidase via an AMPK-Dependent Mechanism. Br J Pharmacol. 2014 Feb 18;171(11):2739–50. doi: 10.1111/bph.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo R, Li W, Liu B, Li S, Zhang B, Xu Y. Resveratrol protects vascular smooth muscle cells against high glucose-induced oxidative stress and cell proliferation in vitro. Medical science monitor basic research. 2014;20:82–92. doi: 10.12659/MSMBR.890858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maiese K, Chong ZZ, Shang YC, Hou J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009 May;29(3):395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moghaddasi M, Javanmard SH, Reisi P, Tajadini M, Taati M. The effect of regular exercise on antioxidant enzyme activities and lipid peroxidation levels in both hippocampi after occluding one carotid in rat. The journal of physiological sciences : JPS. 2014 Sep;64(5):325–32. doi: 10.1007/s12576-014-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patel SA, Velingkaar NS, Kondratov RV. Transcriptional Control of Antioxidant Defense by the Circadian Clock. Antioxid Redox Signal. 2014 Jan 3;20(18):2997–3006. doi: 10.1089/ars.2013.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turunc Bayrakdar E, Uyanikgil Y, Kanit L, Koylu E, Yalcin A. Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Abeta(1-42)-induced rat model of Alzheimer’s disease. Free Radic Res. 2014 Feb;48(2):146–58. doi: 10.3109/10715762.2013.857018. [DOI] [PubMed] [Google Scholar]
- 101.Guo R, Liu B, Wang K, Zhou S, Li W, Xu Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-kappaB pathway. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2014 Mar;11(2):92–102. doi: 10.1177/1479164113520332. [DOI] [PubMed] [Google Scholar]
- 102.Lee Y, Hong Y, Lee SR, Chang KT. Autophagy contributes to retardation of cardiac growth in diabetic rats. Lab Anim Res. 2012 Jun;28(2):99–107. doi: 10.5625/lar.2012.28.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Das F, Dey N, Venkatesan B, Kasinath BS, Ghosh-Choudhury N, Choudhury GG. High glucose upregulation of early-onset Parkinson’s disease protein DJ-1 integrates the PRAS40/TORC1 axis to mesangial cell hypertrophy. Cell Signal. 2011 Aug;23(8):1311–9. doi: 10.1016/j.cellsig.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mao XY, Cao DF, Li X, Yin JY, Wang ZB, Zhang Y, et al. Huperzine A ameliorates cognitive deficits in streptozotocin-induced diabetic rats. International journal of molecular sciences. 2014;15(5):7667–83. doi: 10.3390/ijms15057667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010 May;7(2):95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ceriello A, dello Russo P, Amstad P, Cerutti P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes. 1996 Apr;45(4):471–7. doi: 10.2337/diab.45.4.471. [DOI] [PubMed] [Google Scholar]
- 107.Kurban S, Mehmetoglu I, Yerlikaya HF, Gonen S, Erdem S. Effect of chronic regular exercise on serum ischemia-modified albumin levels and oxidative stress in type 2 diabetes mellitus. Endocr Res. 2011;36(3):116–23. doi: 10.3109/07435800.2011.566236. [DOI] [PubMed] [Google Scholar]
- 108.Yano M, Hasegawa G, Ishii M, Yamasaki M, Fukui M, Nakamura N, et al. Short-term exposure of high glucose concentration induces generation of reactive oxygen species in endothelial cells: implication for the oxidative stress associated with postprandial hyperglycemia. Redox Rep. 2004;9(2):111–6. doi: 10.1179/135100004225004779. [DOI] [PubMed] [Google Scholar]
- 109.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006 Apr 12;295(14):1681–7. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 110.Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007 Feb;4(1):63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Z, Stanojevic V, Brindamour LJ, Habener JF. GLP1-derived nonapeptide GLP1(28-36)amide protects pancreatic beta-cells from glucolipotoxicity. J Endocrinol. 2012 May;213(2):143–54. doi: 10.1530/JOE-11-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Noriega-Cisneros R, Cortes-Rojo C, Manzo-Avalos S, Clemente-Guerrero M, Calderon-Cortes E, Salgado-Garciglia R, et al. Mitochondrial response to oxidative and nitrosative stress in early stages of diabetes. Mitochondrion. 2013 Nov;13(6):835–40. doi: 10.1016/j.mito.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 113.Ribeiro MC, Barbosa NB, de Almeida TM, Parcianello LM, Perottoni J, de Avila DS, et al. High-fat diet and hydrochlorothiazide increase oxidative stress in brain of rats. Cell Biochem Funct. 2009 Oct;27(7):473–8. doi: 10.1002/cbf.1599. [DOI] [PubMed] [Google Scholar]
- 114.Rachek LI, Thornley NP, Grishko VI, LeDoux SP, Wilson GL. Protection of INS-1 cells from free fatty acid-induced apoptosis by targeting hOGG1 to mitochondria. Diabetes. 2006 Apr;55(4):1022–8. doi: 10.2337/diabetes.55.04.06.db05-0865. [DOI] [PubMed] [Google Scholar]
- 115.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002 Oct;51(10):2944–50. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 116.Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, et al. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006 Apr;49(4):784–91. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y, Meng N, Lv Z, Li H, Qu Y. The gene polymorphisms of UCP1 but not PPAR gamma and TCF7L2 are associated with diabetic retinopathy in Chinese type 2 diabetes mellitus cases. Acta ophthalmologica. 2014 Oct 1; doi: 10.1111/aos.12542. [DOI] [PubMed] [Google Scholar]
- 118.Criscuolo F, Mozo J, Hurtaud C, Nubel T, Bouillaud F. UCP2, UCP3, avUCP, what do they do when proton transport is not stimulated? Possible relevance to pyruvate and glutamine metabolism. Biochim Biophys Acta. 2006 Sep-Oct;1757(9–10):1284–91. doi: 10.1016/j.bbabio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 119.Li B, Nolte LA, Ju JS, Han DH, Coleman T, Holloszy JO, et al. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat Med. 2000 Oct;6(10):1115–20. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- 120.Bernal-Mizrachi C, Weng S, Li B, Nolte LA, Feng C, Coleman T, et al. Respiratory uncoupling lowers blood pressure through a leptin-dependent mechanism in genetically obese mice. Arterioscler Thromb Vasc Biol. 2002 Jun 1;22(6):961–8. doi: 10.1161/01.atv.0000019404.65403.71. [DOI] [PubMed] [Google Scholar]
- 121.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001 Jun 15;105(6):745–55. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 122.Huppertz C, Fischer BM, Kim YB, Kotani K, Vidal-Puig A, Slieker LJ, et al. Uncoupling protein 3 (UCP3) stimulates glucose uptake in muscle cells through a phosphoinositide 3-kinase-dependent mechanism. J Biol Chem. 2001 Apr 20;276(16):12520–9. doi: 10.1074/jbc.M011708200. [DOI] [PubMed] [Google Scholar]
- 123.MacLellan JD, Gerrits MF, Gowing A, Smith PJ, Wheeler MB, Harper ME. Physiological increases in uncoupling protein 3 augment fatty acid oxidation and decrease reactive oxygen species production without uncoupling respiration in muscle cells. Diabetes. 2005 Aug;54(8):2343–50. doi: 10.2337/diabetes.54.8.2343. [DOI] [PubMed] [Google Scholar]
- 124.Damasceno DC, Sinzato YK, Bueno A, Netto AO, Dallaqua B, Gallego FQ, et al. Mild diabetes models and their maternal-fetal repercussions. Journal of diabetes research. 2013;2013:473575. doi: 10.1155/2013/473575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert opinion on therapeutic targets. 2012 Dec;16(12):1203–14. doi: 10.1517/14728222.2012.719499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamada E, Singh R. Mapping autophagy on to your metabolic radar. Diabetes. 2012 Feb;61(2):272–80. doi: 10.2337/db11-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008 May;14(5):219–27. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maiese K. Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann Med. 2014 Aug 8;46(8):587–96. doi: 10.3109/07853890.2014.941921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao Y, Scott NA, Fynch S, Elkerbout L, Wong WW, Mason KD, et al. Autoreactive T cells induce necrosis and not BCL-2-regulated or death receptor-mediated apoptosis or RIPK3-dependent necroptosis of transplanted islets in a mouse model of type 1 diabetes. Diabetologia. 2014 Oct 10; doi: 10.1007/s00125-014-3407-5. [DOI] [PubMed] [Google Scholar]
- 130.Chong ZZ, Shang YC, Wang S, Maiese K. A Critical Kinase Cascade in Neurological Disorders: PI 3-K, Akt, and mTOR. Future Neurol. 2012;7(6):733–48. doi: 10.2217/fnl.12.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fong Y, Lin YC, Wu CY, Wang HM, Lin LL, Chou HL, et al. The antiproliferative and apoptotic effects of sirtinol, a sirtuin inhibitor on human lung cancer cells by modulating Akt/beta-catenin-Foxo3a axis. Scientific World Journal. 2014;2014:937051. doi: 10.1155/2014/937051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo X, Chen Y, Liu Q, Wu J, Wang L, Tang X, et al. Ac-cel, a novel antioxidant, protects against hydrogen peroxide-induced injury in PC12 cells via attenuation of mitochondrial dysfunction. J Mol Neurosci. 2013 Jul;50(3):453–61. doi: 10.1007/s12031-013-9955-1. [DOI] [PubMed] [Google Scholar]
- 133.Kimura R, Okouchi M, Kato T, Imaeda K, Okayama N, Asai K, et al. Epidermal growth factor receptor transactivation is necessary for glucagon-like peptide-1 to protect PC12 cells from apoptosis. Neuroendocrinology. 2013;97(4):300–8. doi: 10.1159/000345529. [DOI] [PubMed] [Google Scholar]
- 134.Choi D, Schroer SA, Lu SY, Wang L, Wu X, Liu Y, et al. Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J Exp Med. 2010 Dec 20;207(13):2831–42. doi: 10.1084/jem.20100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deng X, Cheng J, Zhang Y, Li N, Chen L. Effects of caloric restriction on SIRT1 expression and apoptosis of islet beta cells in type 2 diabetic rats. Acta Diabetol. 2009 Oct 30; doi: 10.1007/s00592-009-0159-7. [DOI] [PubMed] [Google Scholar]
- 136.Miao XY, Gu ZY, Liu P, Hu Y, Li L, Gong YP, et al. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides. 2013 Jan;39:71–9. doi: 10.1016/j.peptides.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 137.Hamed S, Ullmann Y, Egozi D, Daod E, Hellou E, Ashkar M, et al. Fibronectin potentiates topical erythropoietin-induced wound repair in diabetic mice. J Invest Dermatol. 2011 Jun;131(6):1365–74. doi: 10.1038/jid.2011.15. [DOI] [PubMed] [Google Scholar]
- 138.Dang J, Jia R, Tu Y, Xiao S, Ding G. Erythropoietin prevents reactive oxygen species generation and renal tubular cell apoptosis at high glucose level. Biomed Pharmacother. 2010 Dec;64(10):681–5. doi: 10.1016/j.biopha.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 139.Nakazawa J, Isshiki K, Sugimoto T, Araki S, Kume S, Yokomaku Y, et al. Renoprotective effects of asialoerythropoietin in diabetic mice against ischaemia-reperfusion-induced acute kidney injury. Nephrology (Carlton) 2010 Feb;15(1):93–101. doi: 10.1111/j.1440-1797.2009.01170.x. [DOI] [PubMed] [Google Scholar]
- 140.Toba H, Sawai N, Morishita M, Murata S, Yoshida M, Nakashima K, et al. Chronic treatment with recombinant human erythropoietin exerts renoprotective effects beyond hematopoiesis in streptozotocin-induced diabetic rat. Eur J Pharmacol. 2009 Jun 10;612(1–3):106–14. doi: 10.1016/j.ejphar.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 141.Chen W, Sun Y, Liu K, Sun X. Autophagy: a double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res. 2014 Jun 15;9(12):1210–6. doi: 10.4103/1673-5374.135329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maiese K. Therapeutic targets for cancer: current concepts with PI 3-K, Akt, & mTOR. The Indian journal of medical research. 2013 Feb;137(2):243–6. [PMC free article] [PubMed] [Google Scholar]
- 143.Lim YM, Lim H, Hur KY, Quan W, Lee HY, Cheon H, et al. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nature communications. 2014;5:4934. doi: 10.1038/ncomms5934. [DOI] [PubMed] [Google Scholar]
- 144.Liu Y, Shi S, Gu Z, Du Y, Liu M, Yan S, et al. Impaired autophagic function in rat islets with aging. Age (Dordr) 2013 Jul 28;35(5):1531–44. doi: 10.1007/s11357-012-9456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012 Jan 26;481(7382):511–5. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nakka VP, Prakash-Babu P, Vemuganti R. Crosstalk Between Endoplasmic Reticulum Stress, Oxidative Stress, and Autophagy: Potential Therapeutic Targets for Acute CNS Injuries. Mol Neurobiol. 2014 Dec 9; doi: 10.1007/s12035-014-9029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of beta-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr Neurovasc Res. 2012 Apr 4;9(2):89–99. doi: 10.2174/156720212800410858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martino L, Masini M, Novelli M, Beffy P, Bugliani M, Marselli L, et al. Palmitate activates autophagy in INS-1E beta-cells and in isolated rat and human pancreatic islets. PLoS ONE. 2012;7(5):e36188. doi: 10.1371/journal.pone.0036188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee JH, Lee JH, Jin M, Han SD, Chon GR, Kim IH, et al. Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp Mol Med. 2014;46:e111. doi: 10.1038/emm.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kibbe C, Chen J, Xu G, Jing G, Shalev A. FOXO1 competes with carbohydrate response element-binding protein (ChREBP) and inhibits thioredoxin-interacting protein (TXNIP) transcription in pancreatic beta cells. J Biol Chem. 2013 Aug 9;288(32):23194–202. doi: 10.1074/jbc.M113.473082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao Y, Yu Y, Tian X, Yang X, Li X, Jiang F, et al. Association Study to Evaluate FoxO1 and FoxO3 Gene in CHD in Han Chinese. PLoS One. 2014;9(1):e86252. doi: 10.1371/journal.pone.0086252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Geldman A, Pallen CJ. Protein tyrosine phosphatases in mast cell signaling. Methods in molecular biology (Clifton, NJ) 2015;1220:269–86. doi: 10.1007/978-1-4939-1568-2_17. [DOI] [PubMed] [Google Scholar]
- 153.Maiese K. WISP1: Clinical Insights for a Proliferative and Restorative Member of the CCN Family. Curr Neurovasc Res. 2014 Sep 12;11(4):378–89. doi: 10.2174/1567202611666140912115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Murahovschi V, Pivovarova O, Ilkavets I, Dmitrieva RM, Docke S, Keyhani-Nejad F, et al. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes. 2014 Oct 3; doi: 10.2337/db14-0444. [DOI] [PubMed] [Google Scholar]
- 155.Berschneider B, Konigshoff M. WNT1 inducible signaling pathway protein 1 (WISP1): a novel mediator linking development and disease. Int J Biochem Cell Biol. 2010 Mar;43(3):306–9. doi: 10.1016/j.biocel.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 156.Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, et al. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci U S A. 1998 Dec 8;95(25):14717–22. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Davies SR, Davies ML, Sanders A, Parr C, Torkington J, Jiang WG. Differential expression of the CCN family member WISP-1, WISP-2 and WISP-3 in human colorectal cancer and the prognostic implications. Int J Oncol. 2010 May;36(5):1129–36. doi: 10.3892/ijo_00000595. [DOI] [PubMed] [Google Scholar]
- 158.Berwick DC, Harvey K. The regulation and deregulation of Wnt signaling by PARK genes in health and disease. J Mol Cell Biol. 2014 Feb;6(1):3–12. doi: 10.1093/jmcb/mjt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007 Jun;19(6):1150–62. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chong ZZ, Shang YC, Hou J, Maiese K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010 Mar-Apr;3(2):153–65. doi: 10.4161/oxim.3.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jian YC, Wang JJ, Dong S, Hu JW, Hu LJ, Yang GM, et al. Wnt-induced secreted protein 1/CCN4 in liver fibrosis both in vitro and in vivo. Clinical laboratory. 2014;60(1):29–35. doi: 10.7754/clin.lab.2013.121035. [DOI] [PubMed] [Google Scholar]
- 162.Klinke DJ., 2nd Induction of Wnt-inducible signaling protein-1 correlates with invasive breast cancer oncogenesis and reduced type 1 cell-mediated cytotoxic immunity: a retrospective study. PLoS computational biology. 2014 Jan;10(1):e1003409. doi: 10.1371/journal.pcbi.1003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006 Jan;21(1):103–24. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Loilome W, Bungkanjana P, Techasen A, Namwat N, Yongvanit P, Puapairoj A, et al. Activated macrophages promote Wnt/beta-catenin signaling in cholangiocarcinoma cells. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014 Feb 19; doi: 10.1007/s13277-014-1698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008 Apr;118(1):58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ortiz-Masia D, Cosin-Roger J, Calatayud S, Hernandez C, Alos R, Hinojosa J, et al. Hypoxic macrophages impair autophagy in epithelial cells through Wnt1: relevance in IBD. Mucosal immunology. 2014 Jul;7(4):929–38. doi: 10.1038/mi.2013.108. [DOI] [PubMed] [Google Scholar]
- 167.Park HY, Toume K, Arai MA, Sadhu SK, Ahmed F, Ishibashi M. Calotropin: a cardenolide from calotropis gigantea that inhibits Wnt signaling by increasing casein kinase 1alpha in colon cancer cells. Chembiochem. 2014 Apr 14;15(6):872–8. doi: 10.1002/cbic.201300786. [DOI] [PubMed] [Google Scholar]
- 168.Saidak Z, Le Henaff C, Azzi S, Marty C, Marie PJ. Low dose PTH increases osteoblast activity via decreased Mef2c/Sost in senescent osteopenic mice. J Endocrinol. 2014 Jul 23; doi: 10.1530/JOE-14-0249. [DOI] [PubMed] [Google Scholar]
- 169.Thorfve A, Lindahl C, Xia W, Igawa K, Lindahl A, Thomsen P, et al. Hydroxyapatite coating affects the Wnt signaling pathway during peri-implant healing in vivo. Acta biomaterialia. 2014 Mar;10(3):1451–62. doi: 10.1016/j.actbio.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 170.van den Bosch MH, Blom AB, van Lent PL, van Beuningen HM, Blaney Davidson EN, van der Kraan PM, et al. Canonical Wnt signaling skews TGF-beta signaling in chondrocytes towards signaling via ALK1 and Smad 1/5/8. Cell Signal. 2014 May;26(5):951–8. doi: 10.1016/j.cellsig.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 171.Yeger H, Perbal B. The CCN family of genes: a perspective on CCN biology and therapeutic potential. J Cell Commun Signal. 2007 Dec;1(3–4):159–64. doi: 10.1007/s12079-008-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002 Jan 1;16(1):46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Venkatesan B, Prabhu SD, Venkatachalam K, Mummidi S, Valente AJ, Clark RA, et al. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010 May;22(5):809–20. doi: 10.1016/j.cellsig.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 neuroprotection requires FoxO3a post-translational modulation with autoregulatory control of SIRT1. Curr Neurovasc Res. 2013 Nov 12;10(1):54–60. doi: 10.2174/156720213804805945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002 Apr 29;157(3):429–40. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang S, Chong ZZ, Shang YC, Maiese K. Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr Neurovasc Res. 2012 Feb;9(1):20–31. doi: 10.2174/156720212799297137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chong ZZ, Li F, Maiese K. Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005 Jan;20(1):299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang M, Zhao X, Liu Y, Tian Y, Ran X, Jiang Y. A role for WNT1-inducible signaling protein-1 in airway remodeling in a rat asthma model. International immunopharmacology. 2013 Oct;17(2):350–7. doi: 10.1016/j.intimp.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 179.Reddy VS, Valente AJ, Delafontaine P, Chandrasekar B. Interleukin-18/WNT1-inducible signaling pathway protein-1 signaling mediates human saphenous vein smooth muscle cell proliferation. J Cell Physiol. 2011 Dec;226(12):3303–15. doi: 10.1002/jcp.22676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shang YC, Chong ZZ, Wang S, Maiese K. Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr Neurovasc Res. 2013 Feb;10(1):29–38. doi: 10.2174/156720213804806007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bayod S, Menella I, Sanchez-Roige S, Lalanza JF, Escorihuela RM, Camins A, et al. Wnt pathway regulation by long-term moderate exercise in rat hippocampus. Brain Res. 2014 Jan 16;1543:38–48. doi: 10.1016/j.brainres.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 182.Borrell-Pages M, Romero JC, Badimon L. LRP5 negatively regulates differentiation of monocytes through abrogation of Wnt signalling. J Cell Mol Med. 2014 Feb;18(2):314–25. doi: 10.1111/jcmm.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chong ZZ, Li F, Maiese K. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim, and beta-Catenin During Oxidative Stress. Curr Neurovasc Res. 2006 May;3(2):107–17. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Maiese K. The Many Facets of Cell Injury: Angiogenesis to Autophagy. Curr Neurovasc Res. 2012 Apr 19;9(2):1–2. doi: 10.2174/156720212800410911. [DOI] [PubMed] [Google Scholar]
- 185.Wei L, Sun C, Lei M, Li G, Yi L, Luo F, et al. Activation of Wnt/beta-catenin Pathway by Exogenous Wnt1 Protects SH-SY5Y Cells Against 6-Hydroxydopamine Toxicity. J Mol Neurosci. 2013 Jan;49(1):105–15. doi: 10.1007/s12031-012-9900-8. [DOI] [PubMed] [Google Scholar]
- 186.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert opinion on therapeutic targets. 2012 Feb;16(2):167–78. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chun P. Role of sirtuins in chronic obstructive pulmonary disease. Archives of pharmacal research. 2014 Oct 11; doi: 10.1007/s12272-014-0494-2. [DOI] [PubMed] [Google Scholar]
- 188.De Bonis ML, Ortega S, Blasco MA. SIRT1 Is Necessary for Proficient Telomere Elongation and Genomic Stability of Induced Pluripotent Stem Cells. Stem cell reports. 2014 May 6;2(5):690–706. doi: 10.1016/j.stemcr.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Oblong JE. The evolving role of the NAD+/nicotinamide metabolome in skin homeostasis, cellular bioenergetics, and aging. DNA Repair (Amst) 2014 Nov;23:59–63. doi: 10.1016/j.dnarep.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 190.Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014 May;32(5):1183–94. doi: 10.1002/stem.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Albiero M, Poncina N, Tjwa M, Ciciliot S, Menegazzo L, Ceolotto G, et al. Diabetes causes bone marrow autonomic neuropathy and impairs stem cell mobilization via dysregulated p66Shc and Sirt1. Diabetes. 2014 Apr;63(4):1353–65. doi: 10.2337/db13-0894. [DOI] [PubMed] [Google Scholar]
- 192.Wang L, Teng R, Di L, Rogers H, Wu H, Kopp JB, et al. PPARalpha and Sirt1 mediate erythropoietin action in increasing metabolic activity and browning of white adipocytes to protect against obesity and metabolic disorders. Diabetes. 2013 Dec;62(12):4122–31. doi: 10.2337/db13-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin Employs Cell Longevity Pathways of SIRT1 to Foster Endothelial Vascular Integrity During Oxidant Stress. Curr Neurovasc Res. 2011 Aug 1;8(3):220–35. doi: 10.2174/156720211796558069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010 Mar 12;285(11):8375–82. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chong ZZ, Shang YC, Wang S, Maiese K. PRAS40 Is an Integral Regulatory Component of Erythropoietin mTOR Signaling and Cytoprotection. PLoS ONE. 2012;7(9):e45456. doi: 10.1371/journal.pone.0045456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shang YC, Chong ZZ, Wang S, Maiese K. WNT1 Inducible Signaling Pathway Protein 1 (WISP1) Targets PRAS40 to Govern beta-Amyloid Apoptotic Injury of Microglia. Curr Neurovasc Res. 2012 Aug 6;9(4):239–49. doi: 10.2174/156720212803530618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull’s-eye for neurological disorders. Oxid Med Cell Longev. 2010 Nov-Dec;3(6):374–91. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kopp C, Hosseini A, Singh SP, Regenhard P, Khalilvandi-Behroozyar H, Sauerwein H, et al. Nicotinic Acid Increases Adiponectin Secretion from Differentiated Bovine Preadipocytes through G-Protein Coupled Receptor Signaling. International journal of molecular sciences. 2014;15(11):21401–18. doi: 10.3390/ijms151121401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013 Jan;19(1):51–60. doi: 10.1016/j.molmed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Martinez de Morentin PB, Martinez-Sanchez N, Roa J, Ferno J, Nogueiras R, Tena-Sempere M, et al. Hypothalamic mTOR: the rookie energy sensor. Curr Mol Med. 2014 Jan;14(1):3–21. doi: 10.2174/1566524013666131118103706. [DOI] [PubMed] [Google Scholar]
- 201.Maiese K. Driving neural regeneration through the mammalian target of rapamycin. Neural Regen Res. 2014 Aug 1;9(15):1413–7. doi: 10.4103/1673-5374.139453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008 Apr 25;30(2):214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Paiva MA, Rutter-Locher Z, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, et al. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011 Jun;300(6):H2123–34. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jessen N, Koh HJ, Folmes CD, Wagg C, Fujii N, Lofgren B, et al. Ablation of LKB1 in the heart leads to energy deprivation and impaired cardiac function. Biochim Biophys Acta. 2010 Jul-Aug;1802(7–8):593–600. doi: 10.1016/j.bbadis.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lai CS, Tsai ML, Badmaev V, Jimenez M, Ho CT, Pan MH. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARgamma and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. Journal of agricultural and food chemistry. 2012 Feb 1;60(4):1094–101. doi: 10.1021/jf204862d. [DOI] [PubMed] [Google Scholar]
- 206.Guan FY, Gu J, Li W, Zhang M, Ji Y, Li J, et al. Compound K protects pancreatic islet cells against apoptosis through inhibition of the AMPK/JNK pathway in type 2 diabetic mice and in MIN6 beta-cells. Life Sci. 2014 Jun 27;107(1–2):42–9. doi: 10.1016/j.lfs.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 207.Lough D, Dai H, Yang M, Reichensperger J, Cox L, Harrison C, et al. Stimulation of the follicular bulge LGR5+ and LGR6+ stem cells with the gut-derived human alpha defensin 5 results in decreased bacterial presence, enhanced wound healing, and hair growth from tissues devoid of adnexal structures. Plastic and reconstructive surgery. 2013 Nov;132(5):1159–71. doi: 10.1097/PRS.0b013e3182a48af6. [DOI] [PubMed] [Google Scholar]
- 208.Jung DW, Kim WH, Williams DR. Reprogram or reboot: small molecule approaches for the production of induced pluripotent stem cells and direct cell reprogramming. ACS Chem Biol. 2014 Jan 17;9(1):80–95. doi: 10.1021/cb400754f. [DOI] [PubMed] [Google Scholar]
- 209.Yang CS, Lopez CG, Rana TM. Discovery of nonsteroidal anti-inflammatory drug and anticancer drug enhancing reprogramming and induced pluripotent stem cell generation. Stem Cells. 2011 Oct;29(10):1528–36. doi: 10.1002/stem.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Heo J, Ahn EK, Jeong HG, Kim YH, Leem SH, Lee SJ, et al. Transcriptional characterization of Wnt pathway during sequential hepatic differentiation of human embryonic stem cells and adipose tissue-derived stem cells. Biochem Biophys Res Commun. 2013 May 3;434(2):235–40. doi: 10.1016/j.bbrc.2013.02.109. [DOI] [PubMed] [Google Scholar]
- 211.Lim HW, Lee JE, Shin SJ, Lee YE, Oh SH, Park JY, et al. Identification of differentially expressed mRNA during pancreas regeneration of rat by mRNA differential display. Biochem Biophys Res Commun. 2002 Dec 20;299(5):806–12. doi: 10.1016/s0006-291x(02)02741-9. [DOI] [PubMed] [Google Scholar]
- 212.Price RM, Tulsyan N, Dermody JJ, Schwalb M, Soteropoulos P, Castronuovo JJ., Jr Gene expression after crush injury of human saphenous vein: using microarrays to define the transcriptional profile. J Am Coll Surg. 2004 Sep;199(3):411–8. doi: 10.1016/j.jamcollsurg.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 213.Liu H, Dong W, Lin Z, Lu J, Wan H, Zhou Z, et al. CCN4 regulates vascular smooth muscle cell migration and proliferation. Mol Cells. 2013 Aug;36(2):112–8. doi: 10.1007/s10059-013-0012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Du J, Klein JD, Hassounah F, Zhang J, Zhang C, Wang XH. Aging increases CCN1 expression leading to muscle senescence. Am J Physiol Cell Physiol. 2014 Jan 1;306(1):C28–36. doi: 10.1152/ajpcell.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Marchand A, Atassi F, Gaaya A, Leprince P, Le Feuvre C, Soubrier F, et al. The Wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell. 2011 Apr;10(2):220–32. doi: 10.1111/j.1474-9726.2010.00661.x. [DOI] [PubMed] [Google Scholar]
- 216.Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS. Modulating mTOR in aging and health. Interdisciplinary topics in gerontology. 2015;40:107–27. doi: 10.1159/000364974. [DOI] [PubMed] [Google Scholar]
- 217.Maiese K. Cutting through the Complexities of mTOR for the Treatment of Stroke. Curr Neurovasc Res. 2014 Apr 7;11(2):177–86. doi: 10.2174/1567202611666140408104831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Neasta J, Barak S, Hamida SB, Ron D. mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. J Neurochem. 2014 Jul;130(2):172–84. doi: 10.1111/jnc.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tang Z, Baykal AT, Gao H, Quezada HC, Zhang H, Bereczki E, et al. mTor Is a Signaling Hub in Cell Survival: A Mass-Spectrometry-Based Proteomics Investigation. Journal of proteome research. 2014 Apr 14;13(5):2433–44. doi: 10.1021/pr500192g. [DOI] [PubMed] [Google Scholar]
- 220.Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012 Aug 15;99(2):128–48. doi: 10.1016/j.pneurobio.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chong ZZ, Shang YC, Maiese K. Cardiovascular Disease and mTOR Signaling. Trends Cardiovasc Med. 2011 Jul;21(5):151–5. doi: 10.1016/j.tcm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhou J, Wu J, Zheng F, Jin M, Li H. Glucagon-like peptide-1 analog-mediated protection against cholesterol-induced apoptosis via mammalian target of rapamycin activation in pancreatic betaTC-6 cells. Journal of diabetes. 2014 Jun, Sep; doi: 10.1111/1753-0407.12177. [DOI] [PubMed] [Google Scholar]
- 223.Pasini E, Flati V, Paiardi S, Rizzoni D, Porteri E, Aquilani R, et al. Intracellular molecular effects of insulin resistance in patients with metabolic syndrome. Cardiovasc Diabetol. 2010;9:46. doi: 10.1186/1475-2840-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Peng N, Meng N, Wang S, Zhao F, Zhao J, Su L, et al. An activator of mTOR inhibits oxLDL-induced autophagy and apoptosis in vascular endothelial cells and restricts atherosclerosis in apolipoprotein E(−/−) mice. Scientific reports. 2014;4:5519. doi: 10.1038/srep05519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002 Dec 3;106(23):2973–9. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 226.Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008 Jun;85(2):194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Maiese K, Hou J, Chong ZZ, Shang YC. Erythropoietin, forkhead proteins, and oxidative injury: biomarkers and biology. Scientific World Journal. 2009;9:1072–104. doi: 10.1100/tsw.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang H, Zhang Q, Wen Q, Zheng Y, Philip L, Jiang H, et al. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012 Jan;24(1):17–24. doi: 10.1016/j.cellsig.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 229.Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 Govern Pathways of mTOR, Apaf-1, and XIAP in Inflammatory Microglia. Curr Neurovasc Res. 2011 Oct 19;8(4):270–85. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012 Mar 3;4(3):187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kim J, Jung Y, Sun H, Joseph J, Mishra A, Shiozawa Y, et al. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012 Jan;113(1):220–8. doi: 10.1002/jcb.23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang GB, Ni YL, Zhou XP, Zhang WF. The AKT/mTOR pathway mediates neuronal protective effects of erythropoietin in sepsis. Mol Cell Biochem. 2014 Jan;385(1–2):125–32. doi: 10.1007/s11010-013-1821-5. [DOI] [PubMed] [Google Scholar]
- 233.Sanghera KP, Mathalone N, Baigi R, Panov E, Wang D, Zhao X, et al. The PI3K/Akt/mTOR pathway mediates retinal progenitor cell survival under hypoxic and superoxide stress. Mol Cell Neurosci. 2011 Jun;47(2):145–53. doi: 10.1016/j.mcn.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 234.Marfia G, Madaschi L, Marra F, Menarini M, Bottai D, Formenti A, et al. Adult neural precursors isolated from post mortem brain yield mostly neurons: an erythropoietin-dependent process. Neurobiol Dis. 2011 Jul;43(1):86–98. doi: 10.1016/j.nbd.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 235.Yu T, Li L, Chen T, Liu Z, Liu H, Li Z. Erythropoietin Attenuates Advanced Glycation Endproducts-Induced Toxicity of Schwann Cells In Vitro. Neurochem Res. 2015 Jan 14; doi: 10.1007/s11064-015-1516-2. [DOI] [PubMed] [Google Scholar]
- 236.Malaguarnera R, Sacco A, Morcavallo A, Squatrito S, Migliaccio A, Morrione A, et al. Metformin inhibits androgen-induced IGF-IR upregulation in prostate cancer cells by disrupting membrane initiated androgren signaling. Endocrinology. 2014 Jan 17;155(4):1207–21. doi: 10.1210/en.2013-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ullah I, Ullah N, Naseer MI, Lee HY, Kim MO. Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci. 2012;13:11. doi: 10.1186/1471-2202-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sheng B, Liu J, Li GH. Metformin preconditioning protects Daphnia pulex from lethal hypoxic insult involving AMPK, HIF and mTOR signaling. Comp Biochem Physiol B Biochem Mol Biol. 2012 May 4;163(1):51–8. doi: 10.1016/j.cbpb.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 239.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011 Jun;60(6):1770–8. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014 Jul;171(13):3146–57. doi: 10.1111/bph.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhu Z, Yan J, Jiang W, Yao XG, Chen J, Chen L, et al. Arctigenin effectively ameliorates memory impairment in Alzheimer’s disease model mice targeting both beta-amyloid production and clearance. J Neurosci. 2013 Aug 7;33(32):13138–49. doi: 10.1523/JNEUROSCI.4790-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Russo E, Andreozzi F, Iuliano R, Dattilo V, Procopio T, Fiume G, et al. Early molecular and behavioral response to lipopolysaccharide in the WAG/Rij rat model of absence epilepsy and depressive-like behavior, involves interplay between AMPK, AKT/mTOR pathways and neuroinflammatory cytokine release. Brain Behav Immun. 2014 Jul 3;42:157–68. doi: 10.1016/j.bbi.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 243.Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M. AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem. 2011 Aug;118(4):460–74. doi: 10.1111/j.1471-4159.2011.07331.x. [DOI] [PubMed] [Google Scholar]
- 244.Kang S, Chemaly ER, Hajjar RJ, Lebeche D. Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J Biol Chem. 2011 May 27;286(21):18465–73. doi: 10.1074/jbc.M110.200022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gong H, Pang J, Han Y, Dai Y, Dai D, Cai J, et al. Age-dependent tissue expression patterns of Sirt1 in senescence-accelerated mice. Molecular medicine reports. 2014 Oct 15;10(6):3296–302. doi: 10.3892/mmr.2014.2648. [DOI] [PubMed] [Google Scholar]
- 246.Duan W. Sirtuins: from metabolic regulation to brain aging. Frontiers in aging neuroscience. 2013;5:36. doi: 10.3389/fnagi.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Srivastava S, Haigis MC. Role of sirtuins and calorie restriction in neuroprotection: implications in Alzheimer’s and Parkinson’s diseases. Curr Pharm Des. 2011;17(31):3418–33. doi: 10.2174/138161211798072526. [DOI] [PubMed] [Google Scholar]
- 248.Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006;13(8):883–95. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chen S, Xiao X, Feng X, Li W, Zhou N, Zheng L, et al. Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. The Journal of nutritional biochemistry. 2012 Sep;23(9):1100–12. doi: 10.1016/j.jnutbio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 250.Chong ZZ, Wang S, Shang YC, Maiese K. Targeting cardiovascular disease with novel SIRT1 pathways. Future Cardiol. 2012 Jan;8(1):89–100. doi: 10.2217/fca.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jin X, Chen M, Yi L, Chang H, Zhang T, Wang L, et al. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the AMPK/SIRT1 signaling pathway. Molecular nutrition & food research. 2014 Oct;58(10):1941–51. doi: 10.1002/mnfr.201400161. [DOI] [PubMed] [Google Scholar]
- 252.Xiong S, Salazar G, Patrushev N, Alexander RW. FoxO1 Mediates an Autofeedback Loop Regulating SIRT1 Expression. J Biol Chem. 2011 Feb 18;286(7):5289–99. doi: 10.1074/jbc.M110.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Canto C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009 Sep;20(7):325–31. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008 May;14(5):661–73. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Herranz D, Serrano M. SIRT1: recent lessons from mouse models. Nat Rev Cancer. 2010 Dec;10(12):819–23. doi: 10.1038/nrc2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Marampon F, Gravina GL, Scarsella L, Festuccia C, Lovat F, Ciccarelli C, et al. Angiotensin-converting-enzyme inhibition counteracts angiotensin II-mediated endothelial cell dysfunction by modulating the p38/SirT1 axis. Journal of hypertension. 2013 Oct;31(10):1972–83. doi: 10.1097/HJH.0b013e3283638b32. [DOI] [PubMed] [Google Scholar]
- 257.Kim DW, Kim YM, Kang SD, Han YM, Pae HO. Effects of Resveratrol and trans-3,5,4′-Trimethoxystilbene on Glutamate-Induced Cytotoxicity, Heme Oxygenase-1, and Sirtuin 1 in HT22 Neuronal Cells. Biomolecules & therapeutics. 2012 May;20(3):306–12. doi: 10.4062/biomolther.2012.20.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhang J, Feng X, Wu J, Xu H, Li G, Zhu D, et al. Neuroprotective effects of resveratrol on damages of mouse cortical neurons induced by beta-amyloid through activation of SIRT1/Akt1 pathway. BioFactors (Oxford, England) 2013 Oct 17;40(2):258–67. doi: 10.1002/biof.1149. [DOI] [PubMed] [Google Scholar]
- 259.Sansone L, Reali V, Pellegrini L, Villanova L, Aventaggiato M, Marfe G, et al. SIRT1 silencing confers neuroprotection through IGF-1 pathway activation. J Cell Physiol. 2013 Aug;228(8):1754–61. doi: 10.1002/jcp.24334. [DOI] [PubMed] [Google Scholar]
- 260.Wang T, Cui H, Ma N, Jiang Y. Nicotinamide-mediated inhibition of SIRT1 deacetylase is associated with the viability of cancer cells exposed to antitumor agents and apoptosis. Oncology letters. 2013 Aug;6(2):600–4. doi: 10.3892/ol.2013.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Du G, Song Y, Zhang T, Ma L, Bian N, Chen X, et al. Simvastatin attenuates TNFalpha induced apoptosis in endothelial progenitor cells via the upregulation of SIRT1. Int J Mol Med. 2014 Jul;34(1):177–82. doi: 10.3892/ijmm.2014.1740. [DOI] [PubMed] [Google Scholar]
- 262.Saini A, Al-Shanti N, Sharples AP, Stewart CE. Sirtuin 1 regulates skeletal myoblast survival and enhances differentiation in the presence of resveratrol. Experimental physiology. 2012 Mar;97(3):400–18. doi: 10.1113/expphysiol.2011.061028. [DOI] [PubMed] [Google Scholar]
- 263.Zhao Y, Luo P, Guo Q, Li S, Zhang L, Zhao M, et al. Interactions between SIRT1 and MAPK/ERK regulate neuronal apoptosis induced by traumatic brain injury in vitro and in vivo. Exp Neurol. 2012 Oct;237(2):489–98. doi: 10.1016/j.expneurol.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 264.Xiong H, Dai M, Ou Y, Pang J, Yang H, Huang Q, et al. SIRT1 expression in the cochlea and auditory cortex of a mouse model of age-related hearing loss. Exp Gerontol. 2014 Mar;51:8–14. doi: 10.1016/j.exger.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 265.Chiara B, Ilaria C, Antonietta C, Francesca C, Marco M, Lucia A, et al. SIRT1 Inhibition Affects Angiogenic Properties of Human MSCs. BioMed research international. 2014;2014:783459. doi: 10.1155/2014/783459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Paschalaki KE, Starke RD, Hu Y, Mercado N, Margariti A, Gorgoulis VG, et al. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells. 2013 Dec;31(12):2813–26. doi: 10.1002/stem.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hong EH, Lee SJ, Kim JS, Lee KH, Um HD, Kim JH, et al. Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase. J Biol Chem. 2010 Jan 8;285(2):1283–95. doi: 10.1074/jbc.M109.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kozako T, Aikawa A, Shoji T, Fujimoto T, Yoshimitsu M, Shirasawa S, et al. High expression of the longevity gene product SIRT1 and apoptosis induction by sirtinol in adult T-cell leukemia cells. Int J Cancer. 2012 Nov 1;131(9):2044–55. doi: 10.1002/ijc.27481. [DOI] [PubMed] [Google Scholar]
- 269.Balaiya S, Ferguson LR, Chalam KV. Evaluation of sirtuin role in neuroprotection of retinal ganglion cells in hypoxia. Invest Ophthalmol Vis Sci. 2012;53(7):4315–22. doi: 10.1167/iovs.11-9259. [DOI] [PubMed] [Google Scholar]
- 270.Dong L, Zhou S, Yang X, Chen Q, He Y, Huang W. Magnolol protects against oxidative stress-mediated neural cell damage by modulating mitochondrial dysfunction and PI3K/Akt signaling. J Mol Neurosci. 2013 Jul;50(3):469–81. doi: 10.1007/s12031-013-9964-0. [DOI] [PubMed] [Google Scholar]
- 271.Qi XF, Li YJ, Chen ZY, Kim SK, Lee KJ, Cai DQ. Involvement of the FoxO3a pathway in the ischemia/reperfusion injury of cardiac microvascular endothelial cells. Experimental and molecular pathology. 2013 Oct;95(2):242–7. doi: 10.1016/j.yexmp.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 272.Yang Y, Su Y, Wang D, Chen Y, Wu T, Li G, et al. Tanshinol attenuates the deleterious effects of oxidative stress on osteoblastic differentiation via Wnt/FoxO3a signaling. Oxid Med Cell Longev. 2013;2013:351895. doi: 10.1155/2013/351895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Min JJ, Huo XL, Xiang LY, Qin YQ, Chai KQ, Wu B, et al. Protective effect of Dl-3n-butylphthalide on learning and memory impairment induced by chronic intermittent hypoxia-hypercapnia exposure. Scientific reports. 2014;4:5555. doi: 10.1038/srep05555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Jang SY, Kang HT, Hwang ES. Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J Biol Chem. 2012 Jun 1;287(23):19304–14. doi: 10.1074/jbc.M112.363747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, et al. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell. 2014 May 8;157(4):882–96. doi: 10.1016/j.cell.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, et al. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res. 2011 Nov;89(11):1723–36. doi: 10.1002/jnr.22725. [DOI] [PubMed] [Google Scholar]
- 277.Zhang S, Cai G, Fu B, Feng Z, Ding R, Bai X, et al. SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence. Mech Ageing Dev. 2012 Jun;133(6):387–400. doi: 10.1016/j.mad.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 278.Shi J, Yin N, Xuan LL, Yao CS, Meng AM, Hou Q. Vam3, a derivative of resveratrol, attenuates cigarette smoke-induced autophagy. Acta Pharmacol Sin. 2012 Jul;33(7):888–96. doi: 10.1038/aps.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008 Oct 10;283(41):27810–9. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chong ZZ, Maiese K. Enhanced Tolerance against Early and Late Apoptotic Oxidative Stress in Mammalian Neurons through Nicotinamidase and Sirtuin Mediated Pathways. Curr Neurovasc Res. 2008 Aug;5(3):159–70. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007 Oct;6(4):307–19. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 282.Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011 Nov;121(11):4477–90. doi: 10.1172/JCI46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Frojdo S, Durand C, Molin L, Carey AL, El-Osta A, Kingwell BA, et al. Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol Cell Endocrinol. 2011 Mar 30;335(2):166–76. doi: 10.1016/j.mce.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 284.Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, et al. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Science signaling. 2011;4(182):ra46. doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- 285.Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006 Feb;4(2):e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Du LL, Chai DM, Zhao LN, Li XH, Zhang FC, Zhang HB, et al. AMPK Activation Ameliorates Alzheimer’s Disease-Like Pathology and Spatial Memory Impairment in a Streptozotocin-Induced Alzheimer’s Disease Model in Rats. J Alzheimers Dis. 2014 Aug 11; doi: 10.3233/JAD-140564. [DOI] [PubMed] [Google Scholar]
- 287.Czubak P, Bojarska-Junak A, Tabarkiewicz J, Putowski L. A modified method of insulin producing cells’ generation from bone marrow-derived mesenchymal stem cells. Journal of diabetes research. 2014;2014:628591. doi: 10.1155/2014/628591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Schulz TC, Young HY, Agulnick AD, Babin MJ, Baetge EE, Bang AG, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7(5):e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Mazzoccoli G, Tevy MF, Borghesan M, Delle Vergini MR, Vinciguerra M. Caloric restriction and aging stem cells: the stick and the carrot? Exp Gerontol. 2014 Feb;50:137–48. doi: 10.1016/j.exger.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 290.Tang AH, Rando TA. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. EMBO J. 2014 Oct 14;33(23):2782–97. doi: 10.15252/embj.201488278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Chen J, Xavier S, Moskowitz-Kassai E, Chen R, Lu CY, Sanduski K, et al. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence. Am J Pathol. 2012 Mar;180(3):973–83. doi: 10.1016/j.ajpath.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Passariello CL, Zini M, Nassi PA, Pignatti C, Stefanelli C. Upregulation of SIRT1 deacetylase in phenylephrine-treated cardiomyoblasts. Biochem Biophys Res Commun. 2011 Apr 15;407(3):512–6. doi: 10.1016/j.bbrc.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 293.Lemarie CA, Shbat L, Marchesi C, Angulo OJ, Deschenes ME, Blostein MD, et al. Mthfr deficiency induces endothelial progenitor cell senescence via uncoupling of eNOS and downregulation of SIRT1. Am J Physiol Heart Circ Physiol. 2011 Mar;300(3):H745–53. doi: 10.1152/ajpheart.00321.2010. [DOI] [PubMed] [Google Scholar]
- 294.Liu X, Chen H, Zhu W, Chen H, Hu X, Jiang Z, et al. Transplantation of SIRT1-engineered aged mesenchymal stem cells improves cardiac function in a rat myocardial infarction model. J Heart Lung Transplant. 2014 Oct;33(10):1083–92. doi: 10.1016/j.healun.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 295.Choudhery MS, Khan M, Mahmood R, Mohsin S, Akhtar S, Ali F, et al. Mesenchymal stem cells conditioned with glucose depletion augments their ability to repair-infarcted myocardium. J Cell Mol Med. 2012 Oct;16(10):2518–29. doi: 10.1111/j.1582-4934.2012.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Yuen DA, Zhang Y, Thai K, Spring C, Chan L, Guo X, et al. Angiogenic dysfunction in bone marrow-derived early outgrowth cells from diabetic animals is attenuated by SIRT1 activation. Stem cells translational medicine. 2012 Dec;1(12):921–6. doi: 10.5966/sctm.2012-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Balestrieri ML, Servillo L, Esposito A, D’Onofrio N, Giovane A, Casale R, et al. Poor glycaemic control in type 2 diabetes patients reduces endothelial progenitor cell number by influencing SIRT1 signalling via platelet-activating factor receptor activation. Diabetologia. 2013 Jan;56(1):162–72. doi: 10.1007/s00125-012-2749-0. [DOI] [PubMed] [Google Scholar]
- 298.Liu DJ, Hammer D, Komlos D, Chen KY, Firestein BL, Liu AY. SIRT1 knockdown promotes neural differentiation and attenuates the heat shock response. J Cell Physiol. 2014 Sep;229(9):1224–35. doi: 10.1002/jcp.24556. [DOI] [PubMed] [Google Scholar]
- 299.Zhang Y, Wang J, Chen G, Fan D, Deng M. Inhibition of Sirt1 promotes neural progenitors toward motoneuron differentiation from human embryonic stem cells. Biochem Biophys Res Commun. 2011 Jan 14;404(2):610–4. doi: 10.1016/j.bbrc.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 300.Saharan S, Jhaveri DJ, Bartlett PF. SIRT1 regulates the neurogenic potential of neural precursors in the adult subventricular zone and hippocampus. J Neurosci Res. 2013 May;91(5):642–59. doi: 10.1002/jnr.23199. [DOI] [PubMed] [Google Scholar]
- 301.Aranha MM, Santos DM, Sola S, Steer CJ, Rodrigues CM. miR-34a regulates mouse neural stem cell differentiation. PLoS One. 2011;6(8):e21396. doi: 10.1371/journal.pone.0021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lee JS, Park JR, Kwon OS, Lee TH, Nakano I, Miyoshi H, et al. SIRT1 is required for oncogenic transformation of neural stem cells and for the survival of “cancer cells with neural stemness” in a p53-dependent manner. Neuro-oncology. 2014 Aug 5;17(1):95–106. doi: 10.1093/neuonc/nou145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Maiese K, Chong ZZ, Shang YC. “Sly as a FOXO”: New paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007 Nov;4(4):295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Maiese K, Hou J, Chong ZZ, Shang YC. A fork in the path: Developing therapeutic inroads with FoxO proteins. Oxid Med Cell Longev. 2009 Jul;2(3):119–29. doi: 10.4161/oxim.2.3.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yang ZH, Zheng R, Gao Y, Zhang Q, Zhang H. Abnormal gene expression and gene fusion in lung adenocarcinoma with high-throughput RNA sequencing. Cancer Gene Ther. 2014 Feb;21(2):74–82. doi: 10.1038/cgt.2013.86. [DOI] [PubMed] [Google Scholar]



