Abstract
Context
Depression, fatigue, and sleep disturbances have been identified as a symptom cluster among breast cancer patients. However, few longitudinal studies have examined the temporal relations between these symptoms surrounding diagnosis and treatment.
Objectives
The current study investigated the co-occurrence of and interrelations between non-somatic depressive symptoms, fatigue, and sleep disturbances in breast cancer patients at three time points: prior to, after, and six to eight months following adjuvant chemotherapy treatment.
Methods
Separate samples of premenopausal (N = 67) and postmenopausal (N = 67) breast cancer patients completed self-report measures of depression, fatigue, and sleep disturbances at all three time points. Path analysis was used to explore within- and cross-symptom paths across time.
Results
Depression, fatigue, and sleep disturbances were correlated within each time point. Continuity paths, whereby prior levels of symptom severity tended to predict subsequent severity of the same symptom at the subsequent time point, were significant in both samples, except for depression in the premenopausal sample. Instead, significant cross-symptom paths emerged whereby baseline fatigue predicted post-chemotherapy depression, and post-chemotherapy fatigue predicted depression at follow-up in the premenopausal patients. No significant cross-symptom paths emerged for the postmenopausal sample.
Conclusion
Findings supported the notion that depression, fatigue, and sleep disturbances manifest as a symptom cluster. Fatigue may precede non-somatic symptoms of depression among premenopausal breast cancer patients and represents a potential intervention target.
Keywords: cancer, oncology, fatigue, depression, sleep disturbance, symptom cluster
Introduction
Receiving a breast cancer diagnosis and undergoing cancer treatment is commonly associated with depression, fatigue, and sleep disturbances (1–3). These distressing symptoms not only affect patients at diagnosis and during cancer treatment, but also persist years beyond the end of treatment (4, 5). Given the growing number of breast cancer survivors (6) and the impact of receiving a cancer diagnosis and undergoing treatment on mood and quality of life, it is important to understand cancer-related mental health symptoms in order to inform treatment and prevention efforts.
Considerable research has examined individual symptoms of depression, fatigue, and sleep disturbances in cancer populations (7–9); however, a growing body of literature indicates that certain symptoms tend to co-occur as symptom clusters in cancer patients. A symptom cluster consists of three or more concurrent symptoms that are correlated to each other (10). Extant literature supports the notion that depression, fatigue, and insomnia are a symptom cluster across cancer types, as indicated by moderate, positive correlations between all pairings of these symptoms when measured concurrently (11, 12).
Recent longitudinal studies have reported mixed findings in the investigation of temporal relationships between cancer-related symptoms. Brown and colleagues (13) failed to find a directional relationship between depression and fatigue in a heterogeneous sample of cancer patients. However, in a recent study that examined anxiety, depression, insomnia, fatigue, and pain over an 18-month period in mixed cancer patients, fatigue predicted depression, insomnia, and pain at subsequent time points, indicating that fatigue was the most contributive predictor over time (14). In another heterogeneous sample of cancer patients, fatigue at baseline prospectively predicted depressive mood after treatment, rather than the opposite relation (15). Current research implicates biological and behavioral mechanisms in the development of depression, fatigue, and sleep disturbances. Some evidence indicates that elevated inflammatory processes contribute to fatigue, but not sleep disturbances or depression (16). However, further research is needed to support the notion that fatigue may be a primary or “core” symptom in depression. Moreover, it remains to be demonstrated whether this pattern generalizes to a homogeneous breast cancer sample. Further investigation also is needed around the extent to which these symptoms occur alone versus in combination to better delineate directionality and to aid the development of effective interventions.
The current project makes a unique contribution to the literature as the first longitudinal study that utilizes path analysis to examine symptom interrelations in a purported symptom cluster in breast cancer patients. The current project had three major aims: 1) to test a purported symptom cluster in the cancer literature, 2) to explore the individual course of depression, fatigue, and sleep disturbances, and 3) to explore the predictive nature of each symptom on the other two symptoms. We examined the co-occurrence, course, and interrelations between depression, fatigue, and sleep disturbances at three important time points surrounding adjuvant chemotherapy (CT) treatment for early stage breast cancer (i.e., before, after, and six to eight months post-treatment). Because this was a secondary analysis of data from two separate studies that examined the effects of CT on cognitive functioning, we explored these symptom relations in two different samples of breast cancer patients: older, postmenopausal women (Study 1) and younger, premenopausal women (Study 2).
We proposed to test an exploratory model of all possible associations between depressive symptoms, fatigue, and sleep disturbances. In accordance with Aim 1, we expected to find correlations similar in value to those previously reported in support of the assertion that depression, fatigue, and sleep disturbances co-occur as a symptom cluster. In order to address Aim 2, we hypothesized that the severity of each symptom would predict severity of the same symptom at later time points. We anticipated that this pattern would be maintained even after adjusting for concurrent and previous levels of the other two symptoms. Last, in accordance with Aim 3, we hypothesized that baseline severity of each symptom would predict subsequent levels of the other two symptoms. Given that past studies have indicated that fatigue is an important predictor of other symptoms in cancer patients (14, 15), we expected that fatigue would be a stronger predictor of the other two symptoms at later time points, compared with depression and sleep disturbances.
Methods
Participants
Participants were recruited for one of two studies from the patient pool of collaborating breast surgeons and oncologists affiliated with Columbia University Medical Center in New York City. Study 1 was conducted between 2001 and 2007, whereas Study 2 was conducted between 2005 and 2011. Patients across both studies were females, newly diagnosed with Stage I-IIIa breast cancer. Possible adjuvant treatments included CT, radiation, and endocrine therapy. Exclusion criteria included prior breast cancer diagnosis; prior exposure to CT or radiation; neoadjuvant CT; and neurological, psychological, or medical comorbidities that might affect cognitive functioning.
Eligibility criteria across the two studies differed primarily in patient age, menopausal status, and use of CT treatment. Study 1 included postmenopausal women aged 45–70 with postmenopausal status defined as no menstrual cycles for at least 12 months or surgical menopause. From a total of 129 patients who were approached, 81 patients completed a screen for eligibility, of which 67 eligible patients were enrolled in Study 1. Approximately half of the patients in Study 1 received adjuvant CT (n = 36), whereas the others were treated with surgery but no CT (n = 31). Chemotherapy treatment was decided between the patients and their oncologists prior to study enrollment. Study 2 included younger, premenopausal women aged 21–50, who reported regular menstrual cycles in the past year. From a total of 272 patients who were approached for Study 2, 156 patients completed a screen for eligibility, of which 67 eligible patients participated in Study 2. All patients in Study 2 received CT treatment (N = 67).
Study Designs
Both studies included three time points when self-report psychological and quality of life questionnaires were administered: baseline or T1 (i.e., at least two weeks after breast surgery but prior to beginning CT), T2 (i.e., within one month after completing a three- to six-month CT regimen, or approximately six months after T1 for patients who did not receive CT in Study 1), and T3 (i.e., six to eight months after T2). Because CT treatment has been associated with more severe depressive and fatigue symptoms and circadian rhythm changes (17, 18), and cancer-related impairment tends to extend beyond the course of treatment (5, 8), these time points were especially relevant in assessing symptom changes.
Measures
Participants completed the following self-report measures at each of the three time points to assess depression, fatigue, and sleep disturbances. Study 1 and Study 2 used the same measures of fatigue and sleep disturbances, but different measures of depression.
Beck Depression Inventory®-Second Edition (BDI-II)
Postmenopausal women in Study 1 completed the BDI-II (19), a self-report measure that assesses current depressive symptom severity. The BDI-II has been used widely in medical populations and demonstrates good reliability and validity (19). Confirmatory factor analysis of the BDI-II supports a two-factor structure that maps onto underlying cognitive-affective and somatic depressive symptoms (20). In Study 1 analyses, we used the cognitive-affective subscale to capture non-somatic depressive symptoms, in an effort to exclude items that overlapped with the other two symptoms of interest. In this sample, the alpha coefficient of 0.90 indicated excellent internal consistency across all three time points.
Hospital Anxiety and Depression Scale (HADS)
Premenopausal women in Study 2 completed the HADS depression subscale (HADS-D) to assess symptoms of depression. The HADS (21) is a brief, self-administered rating scale that assesses for depression and anxiety in patients with physical illness. Somatic symptoms such as dizziness, headaches, insomnia, and fatigue were excluded from the HADS in order to prevent false positives resulting from underlying medical conditions. The HADS-D score was calculated by summing all seven items that comprise the depression subscale. The HADS has shown good validity and test-retest reliability in a variety of medical populations, including breast cancer patients (22). The alpha coefficient of 0.84 demonstrated good reliability across time in Study 2.
Multidimensional Fatigue Symptom Inventory- Short Form (MFSI-SF)
The MFSI-SF (23) contains 30 items that comprise five subscales to assess somatic, affective, cognitive, behavioral, and global fatigue. The total fatigue score is calculated by subtracting the vigor scale from the sum of the other four scales (general, physical, emotional, and mental fatigue). The MFSI-SF is a reliable and valid tool for assessing the full spectrum of symptoms that characterize cancer-related fatigue (CRF) (24). The alpha coefficient values across time, 0.85 and 0.88 in Study 1 and Study 2, respectively, indicated a high degree of internal consistency.
Pittsburgh Sleep Quality Index (PSQI)
The PSQI (25) is a 19-item questionnaire that was used to assess subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The total PSQI score was calculated by summing the domain scores. The PSQI has demonstrated internal consistency reliability and construct validity in breast cancer patients (26). In the current project, the reliability coefficients across time were 0.65 and 0.78 in Study 1 and Study 2, respectively.
Statistical Analysis
Preliminary Analyses
Study 1 and Study 2 were analyzed separately for the purposes of this project because of the use of different measures of depression and different study designs. Participant and outcome measure descriptive statistics were examined in SPSS v. 21 (SPSS, Inc./IBM, Chicago, IL). Bivariate associations between measures of depressive symptoms, fatigue, and sleep disturbances were examined at each time point across all groups.
Primary Analyses
Path analysis was employed to model associations from a saturated model (i.e., df = 0; Fig. 1). All models were tested with Mplus v. 6.11 (Muthén & Muthén, Los Angeles, CA) (27) and used maximum likelihood estimation with robust standard errors to account for skewed data. Standard fit statistics were examined to evaluate nested model fit: Chi-square (χ2: P > 0.05 excellent), Comparative Fit Index (CFI; > 0.90 acceptable, > 0.95 excellent), Root Mean Square Error of Approximation (RMSEA; < 0.08 acceptable, < 0.05 excellent) and the Standardized Root Mean Square Residual (SRMR; < 0.08 acceptable, < 0.05 excellent) (28, 29). Missing data were treated as missing at random, and full information maximum likelihood estimation techniques were used for inclusion of all available data at each time point. The Model Indirect command was utilized to calculate standardized indirect effect parameters and biased-corrected bootstrap confidence intervals.
Fig. 1.
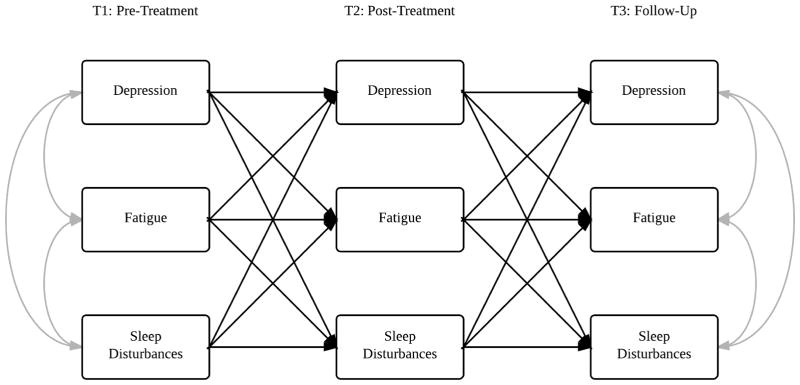
Saturated model of within- and cross-symptom temporal relations in breast cancer patients. Note: Between symptom correlations at T2, and T1–T3 within- and cross-symptom paths are not depicted in order to keep the figure legible.
Control Variables
The effects of covariates were explored with multiple-indicator/multiple-cause (MIMIC) models (30), in which all major constructs at each time point in the final model were regressed on the covariates. If paths in the model remained significant with the inclusion of a given variable, it was concluded that the covariate did not influence the relations among variables in the model. Covariates included sociodemographics (i.e., age, ethnicity, race, years of education), medically relevant variables (i.e., cancer stage, duration of CT, adjuvant radiation and endocrine therapy), and psychological history (i.e., past diagnosis of a depressive or anxiety disorder) variables.
Results
Study 1: Postmenopausal Sample
Participants
A total of 70 postmenopausal women with breast cancer were eligible and enrolled in Study 1. The study utilized data from 67 women who provided data for at least one time point. Missing data were consistent across measures of depression, fatigue, and sleep disturbances: 1.5% at T1, 10.4% at T2, and 23.9% at T3. Table 1 presents descriptive statistics for sociodemographic and medical variables. Additional descriptives are reported by Tager et al. (31). The CT and no CT treatment groups were generally well-balanced on sociodemographics, medical variables, and psychological history. However, they significantly differed in stage of cancer diagnosis (χ2(2) = 24.44, P < 0.001); treatment with CT was associated with higher cancer stage.
Table 1.
Sociodemographic and Medical Variables at Baseline (T1) in the Postmenopausal (Study 1) and Premenopausal (Study 2) Samples
| Study 1: Postmenopausal Sample | Study 2: Premenopausal Sample | |||
|---|---|---|---|---|
|
| ||||
| Total (N = 67) | CT Group (n = 36) | No CT Group (n = 31) | Total (N = 67) | |
| Age, yrs, M (SD) | 60.0 (5.9) | 59.4 (5.6) | 60.6 (6.3) | 41.4 (5.2) |
|
| ||||
| Highest Education in Years, M (SD) | 16.3 (3.2) | 16.6 (3.2) | 16.1 (3.2) | 15.6 (2.4) |
|
| ||||
| Ethnicity | ||||
| Asian or Pacific Islander | 6/67 = 9% | 4/36 = 11% | 2/31 = 7% | 3/67 = 5% |
| African American | 9/67 = 13% | 6/36 = 17% | 3/31 = 10% | 21/67 = 31% |
| Hispanic | 7/67 = 10% | 5/36 = 14% | 2/31 = 7% | 11/67 = 16% |
| White | 45/67 = 67% | 21/36 = 58% | 24/31 = 77% | 31/67 = 46% |
| Other | 0/67 = 0% | 0/36 = 0% | 0/31 = 0% | 1/67 = 2% |
|
| ||||
| Main Surgery Type | ||||
| Biopsy Only | 0/67 = 0% | 0/36 = 0% | 0/31 = 0% | 0/67 = 0% |
| Lumpectomy | 45/67 = 67% | 22/36 = 61% | 23/31 = 74% | 28/67 = 42% |
| Mastectomy | 22/67 = 33% | 14/36 = 39% | 8/31 = 26% | 39/67 = 58% |
|
| ||||
| Cancer Stage | ||||
| Invasive DCIS | 11/67 = 16% | 0/36 = 0% | 11/31 = 36% | 3/67 = 5% |
| Stage I | 29/67 = 43% | 13/36 = 36% | 16/31 = 52% | 23/67 = 34% |
| Stage II, IIIa | 27/67 = 40% | 23/36 = 64% | 4/31 = 13% | 40/67 = 60% |
|
| ||||
| CT Regimen | ||||
| AC | 9/67 = 13% | 9/36 = 25% | 10/67 = 14.9% | |
| T | 0/67 = 0% | 0/36 = 0% | 8/67 = 11.9% | |
| ACT | 16/67 = 24% | 16/36 = 44% | 46/67 = 68.7% | |
| CMF | 11/67 = 16% | 11/36 = 31% | 3/67 = 4.5% | |
|
| ||||
| Other Adjuvant Treatment | ||||
| Radiation | 41/67 = 61% | 22/36 = 61% | 19/31 = 61% | 29/67 = 43.3% |
| Endocrine therapy | 49/67 = 73% | 26/36 = 72% | 23/31 = 74% | 46/67 = 68.7% |
|
| ||||
| Psychological Historya | ||||
| Past Depressive Disorder | 17/67 = 25% | 8/36 = 22% | 9/31 = 29% | 10/67 = 15% |
| Past Anxiety Disorder | 12/67 = 18% | 4/36 = 11% | 8/31 = 26% | 4/67 = 6% |
Data are presented as Mean (SD) or proportions, as indicated.
CT = chemotherapy; No-CT = no chemotherapy; AC = doxorubicin, cyclophosphamide; ACT = doxorubicin, cyclophosphamide, docetaxel; CMF = cyclophosphamide, methotrexate, 5-flourouracil.
Psychological history was ascertained by diagnostic categories on the Structured Clinical Interview for DSM-IV Axis I Disorders.
Preliminary Analyses
Table 2 presents the depression, fatigue, and sleep disturbance statistics at each time point. Symptom correlations were examined across CT status and time. We found no significant differences between symptom correlations for patients treated with versus without CT. Across treatments, the overall correlations were 0.77 for depression and fatigue, 0.34 for fatigue and sleep disturbances, and 0.44 for depression and sleep disturbances (all Ps < 0.01). Depression and fatigue were more strongly correlated at all three time points compared with the correlations between other symptom pairs. In general, symptom correlations increased after CT treatment (i.e., at T2 and T3) compared with baseline.
Table 2.
Descriptive Statistics for Outcome Variables at Each Time Point in the Postmenopausal and Premenopausal Samples
| T1: Pre-Tx Mean (SD) |
T2: Post-Tx Mean (SD) |
T3: Follow-Up Mean (SD) |
|
|---|---|---|---|
|
Study 1: Postmenopausal Women
| |||
| BDI-II (Depression) | |||
| CT | 4.11 (4.07) | 4.07 (4.23) | 3.24 (3.82) |
| No-CT | 5.13 (6.26) | 4.45 (6.03) | 5.81 (7.14) |
| Total | 4.57 (5.34) | 4.27 (5.19) | 4.55 (5.85) |
| MFSI-SF (Fatigue) | |||
| CT | 8.50 (18.98) | 9.52 (16.94) | 4.04 (14.77) |
| No-CT | 7.10 (14.81) | 5.55 (17.59) | 7.50 (21.13) |
| Total | 7.86 (17.10) | 7.47 (17.25) | 5.80 (18.20) |
| PSQI (Sleep Quality) | |||
| CT | 6.94 (3.32) | 6.55 (3.00) | 6.36 (4.05) |
| No-CT | 8.00 (3.96) | 6.94 (3.91) | 8.08 (4.58) |
| Total | 7.42 (3.63) | 6.75 (3.47) | 7.24 (4.37) |
|
| |||
|
Study 2: Premenopausal Women
| |||
| HADS-D (Depression) | 3.25 (3.04) | 3.95 (3.13) | 3.55 (3.60) |
| MFSI-SF (Fatigue) | 9.11 (18.30) | 17.88 (20.33) | 11.82 (23.54) |
| PSQI (Sleep Quality) | 7.35 (4.19) | 8.94 (4.35) | 7.28 (4.10) |
CT = chemotherapy group; No-CT = no chemotherapy group; BDI-II = Beck Depression Inventory-Second Edition (Cognitive-Affective subscale); PSQI = Pittsburgh Sleep Quality Index; MFSI-SF = Multidimensional Fatigue Symptom Inventory-Short Form; HADS-D = Hospital Anxiety and Depression Scale-Depression subscale.
Primary Analyses
The saturated model (Fig. 1) was tested to explore associations between the three symptoms of interest across the three time points, using the entire sample (N = 67) of postmenopausal women. We failed to observe any significant within-or cross-symptom parameters between T1 and T3; therefore, these paths were dropped in order to create a more parsimonious model. The final model demonstrated excellent fit (χ2 (9, N = 67) = 7.69, P > 0.05, CFI = 1.00, RMSEA = 0.00, 90% CI 0.00, 0.12, SRMR = 0.02).
Table 3 presents the statistically significant standardized path coefficients in the final model (Fig. 2 represents the corresponding model). All within-time symptom correlations were significant, with the exception of fatigue and sleep disturbances at T3. As expected, continuity paths for all three symptoms emerged as significant. Contrary to hypotheses, we did not observe significant cross-symptom paths. The indirect effect from each major construct at T1 to the same construct at T3 through T2 was significant for depression (β = 0.39, P = 0.01), fatigue (β = 0.20, P = 0.01), and sleep disturbances (β = 0.50, P < 0.001). All pathways were unaffected by the inclusion of covariates in MIMIC models.
Table 3.
Statistically Significant Paths in Models 1 & 2
| Model 1: Postmenopausal Sample | Model 2: Premenopausal Sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Time | Estimated Path | β | S.E. | 95% CI | P | Time | Estimated Path | β | S.E. | 95% CI | P |
| Within-Time Point Correlations | Within-Time Point Correlations | ||||||||||
|
| |||||||||||
| T1 | Depression – Fatigue | .63 | .07 | .49 to .77 | < 0.001 | T1 | Depression – Fatigue | .69 | .06 | .56 to .81 | < 0.001 |
| Depression – Sleep | .26 | .10 | .07 to .46 | 0.008 | Depression – Sleep | .43 | .09 | .26 to .60 | < 0.001 | ||
| Fatigue – Sleep | .24 | .12 | .01 to .47 | 0.038 | Fatigue – Sleep | .66 | .08 | .51 to .81 | < 0.001 | ||
| T2 | Depression – Fatigue | .77 | .06 | .64 to .89 | < 0.001 | T2 | Depression – Fatigue | .75 | .05 | .65 to .85 | < 0.001 |
| Depression – Sleep | .33 | .14 | .29 to .65 | 0.019 | Depression – Sleep | .51 | .09 | .34 to .69 | < 0.001 | ||
| Fatigue – Sleep | .47 | .09 | .29 to .65 | < 0.001 | Fatigue – Sleep | .47 | .09 | .29 to .66 | < 0.001 | ||
| T3 | Depression – Fatigue | .55 | .07 | .42 to .68 | < 0.001 | T3 | Depression – Fatigue | .70 | .07 | .57 to .82 | < 0.001 |
| Depression – Sleep | .33 | .12 | .10 to .57 | 0.006 | Fatigue – Sleep | .35 | .14 | .07 to .62 | 0.013 | ||
|
| |||||||||||
| Within-Symptom Paths | Within-Symptom Paths | ||||||||||
|
| |||||||||||
| T1 → T2 | Depression | .60 | .16 | .28 to .93 | < 0.001 | T1 → T2 | Fatigue | .60 | .15 | .30 to .89 | < 0.001 |
| Fatigue | .38 | .12 | .13 to .62 | 0.002 | Sleep | .53 | .12 | .29 to .78 | < 0.001 | ||
| Sleep | .70 | .08 | .55 to .85 | < 0.001 | T2 → T3 | Fatigue | .66 | .16 | .35 to .98 | < 0.001 | |
| T2 → T3 | Depression | .64 | .15 | .34 to .94 | < 0.001 | Sleep | .62 | .13 | .37 to .87 | < 0.001 | |
|
|
|||||||||||
| Fatigue | .53 | .14 | .26 to .81 | < 0.001 | Cross-Symptom Paths | ||||||
|
|
|||||||||||
| Sleep | .72 | .09 | .55 to .89 | < 0.001 | T1 → T2 | Fatigue - Depression | .38 | .15 | .08 to .67 | 0.013 | |
| T2 → T3 | Fatigue - Depression | .47 | .16 | .15 to .79 | 0.004 | ||||||
S.E. = standard error; CI = confidence interval.
COMP: pls add placeholder zeros where appropriate.
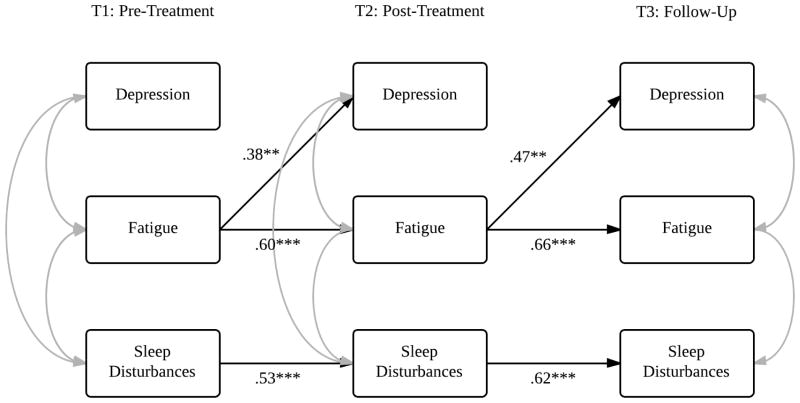
Fig. 2.
Model 1: Depression, fatigue, and sleep disturbances in the postmenopausal sample. Note: Only statistically significant pathways are displayed in the model. **P ≤ 0.01, ***P ≤ 0.001. Refer to Table 3 for model statistics.
Study 2: Premenopausal Sample
Participants
Table 1 presents descriptive information for sociodemographic and medical variables in Study 2. A total of 67 patients completed at least one assessment. Missing data were consistent across measures of depression, fatigue, and sleep disturbances: 3.0% at T1, 4.5% at T2, and 10.4% at T3.
Preliminary Analyses
Table 2 presents outcome measure descriptive statistics. Overall correlations were 0.79 for depression and fatigue, 0.59 for fatigue and sleep disturbances, and 0.45 for depression and sleep disturbances (all Ps < 0.01). Depression and fatigue emerged as the most strongly correlated pair of symptoms at all three time points and became more strongly correlated over time. Contrastingly, the strength of association between fatigue and sleep disturbances decreased over time.
Primary Analyses
The saturated model (Fig. 1) was tested in the premenopausal cancer sample (N = 67). We did not observe significant within- or cross-symptom parameters between T1 and T3 as proposed in the conceptual model; therefore, these paths were dropped. The final model demonstrated excellent fit of the data (χ2 (9, N = 67) = 11.32, P > 0.05, CFI = 0.99, RMSEA = 0.06, 90% CI 0.00, 0.16, SRMR = 0.04).
Table 3 presents all statistically significant standardized path coefficients in the final model (Fig. 3). Symptom correlations at each time point were significant, with the exception of depression and sleep disturbances at T3. As expected, continuity paths for fatigue and sleep disturbances were highly significant. Indirect effects from fatigue (β = 0.40, P < 0.01) and sleep disturbances (β = 0.33, P < 0.001) at T1 to the same construct at T3 through T2 were significant. The continuity path between depressive symptoms at T1 and T2 approached significance, whereas depressive symptoms at T2 did not predict T3. However, two significant cross-symptom paths emerged: fatigue at T1 → depression at T2, and fatigue at T2 → depression at T3. According to MIMIC models, the path from depressive symptoms at T2 → depressive symptoms at T3 decreased in significance (Ps ranged from 0.08 to 0.13) after the inclusion of education level and past depression or anxiety; however, the standardized estimate remained close to original value. All other pathways were unaffected by the inclusion of covariates.
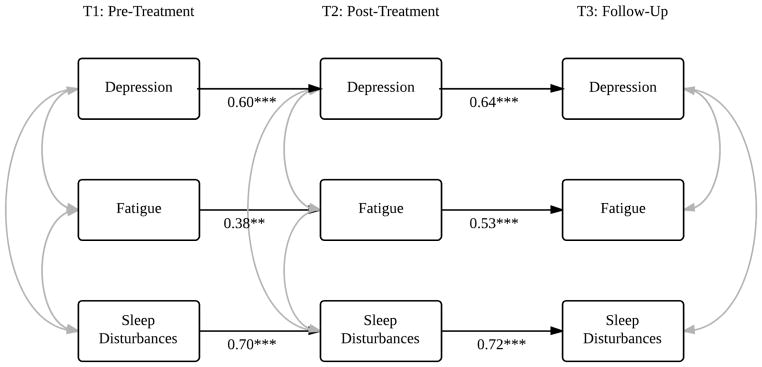
Fig. 3.
Model 2: Depression, fatigue, and sleep disturbances in the premenopausal sample. Note: Only statistically significant pathways are displayed in the model. **P ≤ 0.01, ***P ≤ 0.001. Refer to Table 3 for model statistics.
Discussion
The primary aim of this study was to examine the predictive utility of three symptoms belonging to a putative symptom cluster over a one-year period in postmenopausal and premenopausal breast cancer patients. We specifically aimed to demonstrate that depression, fatigue, and sleep disturbances co-occur as a symptom cluster over the course of breast cancer treatment, and explored the extent to which the severity of each symptom predicted severity of the same symptom and the other two symptoms at subsequent time points.
Consistent with the literature (32, 33) and stated hypotheses, findings revealed moderate to large, positive bivariate associations between the symptoms of interest across both samples. These findings support the notion that depression, fatigue, and sleep disturbances co-occur as a symptom cluster, prior to and following adjuvant chemotherapy treatment in postmenopausal and premenopausal women with breast cancer. Depression and fatigue emerged as the most strongly correlated symptom pair in both samples and were more strongly correlated across time.
Next, we tested a model of all temporal associations between depression, fatigue, and sleep disturbances. Results generally supported within-symptom continuity paths such that symptom severity at T1 and T2 predicted severity of the same symptom at the subsequent time point. Interestingly, the final model in the premenopausal sample pointed to fatigue being a predictor of depressive symptoms at subsequent time points, above and beyond prior levels of depressive symptoms and sleep disturbances. These findings are consistent with fatigue being a significant predictor of subsequent fatigue and depression in heterogeneous cancer patients (14, 15). We demonstrated that this pattern might generalize uniquely to premenopausal women with breast cancer. It is worth considering why fatigue did not significantly predict subsequent depressive symptoms in the postmenopausal sample. This may be explained by younger age being a strong risk factor for psychological distress and a better predictor of depressive symptoms in breast cancer patients (34). Depression epidemiology research has found that women between ages 40 to 59 may be at higher risk of depression compared to women older than 60 (35), thus suggesting different pathways to depression across age groups. Fatigue may represent one potential pathway that gives rise to depression in younger women with breast cancer. Further research with larger samples and uniform measures of depression is needed to replicate this finding. Other variables associated with age also may account for differing pathways. Hormonal changes may be particularly important, in light of the effect of CT on the acceleration of menopause (36) and the effect of endocrine therapies on hormone levels.
There are several plausible reasons why fatigue may lead to subsequent mood problems in cancer patients. Decreased energy may lead to reduced activity levels, which may result in less positive reinforcement derived from pleasurable activities and the development of depression (14). Alternatively, the development of fatigue and depression in cancer patients may result from shared biological or pathophysiological factors (37, 38). Additional research is needed to elucidate the interrelations between fatigue, depression, and sleep disturbances surrounding diagnosis and treatment of breast cancer.
Understanding mechanisms for symptom relations over time could inform the development of effective psychological interventions for breast cancer patients. A variety of non-pharmacological interventions have been recommended for treating CRF, including exercise, psychosocial, nutritional, and sleep interventions (38–40). Further research should examine whether multimodal treatment reduces CRF and, in turn, reduces depressive symptoms.
There were a number of limitations to the current project. First, temporal relations explored were correlational and do not imply causality. Second, it is important to point out that sample sizes were small; however, several statistically significant paths emerged and four of six within-symptom paths were replicated across both study samples. Another drawback to the current project was the inability to directly compare results between samples because of the use of different measures of depression, although we used well-validated measures.
The current study also had a number of strengths. First, the longitudinal study design allowed us to examine temporal relations at three important time points in relation to adjuvant CT treatment. Second, the study aims and proposed model were explored in two separate samples. Study replication provided us with higher confidence in our findings that spanned both samples. Third, we utilized MIMIC models to examine covariates and observed that pathways were not significantly affected by demographic, medical, or psychological variables. Lastly, we used the cognitive-affective subscale of the BDI-II and the HADS-D to focus on non-somatic depressive symptoms. The use of these measures prevented inflation of cross-symptom parameters in the symptom cluster of interest.
The current study demonstrated that depression, fatigue, and sleep disturbances tend to co-occur during the first year after diagnosis, thus providing further evidence for this symptom cluster in two distinct samples of breast cancer patients. Path analyses indicated that prior severity predicted subsequent severity of the same symptom, whereas significant cross-symptom paths were limited to fatigue at T1 and T2 predicting depression at T2 and T3, respectively, in premenopausal breast cancer patients. The clinical implications of this study may be important for assessment, prevention, and intervention of psychological symptoms related to breast cancer diagnosis and treatment. In addition to reducing severity of a given symptom by treating the same symptom earlier in time, this work provides preliminary evidence that targeting fatigue may have a meaningful effect on other related symptoms over time and potentially offset the development of depressive symptoms in younger women with breast cancer.
Acknowledgments
Funding for this project was provided by the National Cancer Institute (R03-CA96422; Principal Investigator: Felice A. Tager, PhD) and American Cancer Society (RSGPB-05-010-01; Principal Investigator: Felice A. Tager, PhD).
Footnotes
Disclosures
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fann JR, Thomas-Rich AM, Katon W, et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiat. 2008;30:112–126. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12:4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 3.Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage. 2002;24:471–480. doi: 10.1016/s0885-3924(02)00500-6. [DOI] [PubMed] [Google Scholar]
- 4.Burgess C, Cornelius V, Love S, et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. Brit Med J. 2005;330:702–705. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. [Accessed July 16, 2013];American Cancer Society breast cancer facts & figures, 2011–2012. Available from http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-030975.pdf.
- 7.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 8.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 9.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 10.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28:465–470. [PubMed] [Google Scholar]
- 11.Brown LF, Kroenke K. Cancer-related fatigue and its associations with depression and anxiety: A systematic review. Psychosomatics. 2009;50:440–447. doi: 10.1176/appi.psy.50.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan KA, Jacobsen PB. Fatigue, depression, and insomnia: evidence for a symptom cluster in cancer. Semin Oncol Nurs. 2007;23:127–135. doi: 10.1016/j.soncn.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Brown LF, Rand KL, Bigatti SM, et al. Longitudinal relationships between fatigue and depression in cancer patients with depression and/or pain. Health Psychol. 2013;32:1199–1208. doi: 10.1037/a0029773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trudel-Fitzgerald C, Savard J, Ivers H. Which symptoms come first? Exploration of temporal relationships between cancer-related symptoms over an 18-month period. Ann Behav Med. 2013;45:329–337. doi: 10.1007/s12160-012-9459-1. [DOI] [PubMed] [Google Scholar]
- 15.Visser MR, Smets EM. Fatigue, depression and quality of life in cancer patients: how are they related? Support Care Cancer. 1998;6:101–108. doi: 10.1007/s005200050142. [DOI] [PubMed] [Google Scholar]
- 16.Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2010;18:105–114. doi: 10.1007/s00520-009-0636-0. [DOI] [PubMed] [Google Scholar]
- 18.Roscoe JA, Morrow GR, Hickok JT, et al. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer. 2002;10:329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Steer RA, Brown GK. Beck Depression Inventory--2nd edition manual. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 20.Whisman MA, Perez JE, Ramel W. Factor structure of the Beck Depression Inventory-Second Edition (BDI-II) in a student sample. J Clin Psychol. 2000;56:545–551. doi: 10.1002/(sici)1097-4679(200004)56:4<545::aid-jclp7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiat Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 23.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 24.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh sleep quality index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 27.Muthén LK, Muthén BO. Mplus. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- 28.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Thousand Oaks, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- 29.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 30.Muthén BO. Latent variable modeling in heterogeneous populations. Psychometrika. 1989;54:557–585. [Google Scholar]
- 31.Tager FA, McKinley PS, Schnabel FR, et al. The cognitive effects of chemotherapy in breast cancer patients: a controlled longitudinal study. Breast Cancer Res Treat. 2010;123:25–34. doi: 10.1007/s10549-009-0606-8. [DOI] [PubMed] [Google Scholar]
- 32.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byar KL, Berger AM, Bakken SL, Cetak MA. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 2006;33:18–26. doi: 10.1188/06.ONF.E18-E26. [DOI] [PubMed] [Google Scholar]
- 34.Wong-Kim EC, Bloom JR. Depression experienced by young women newly diagnosed with breast cancer. Psychooncology. 2005;14:564–573. doi: 10.1002/pon.873. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease and Control. [Accessed September 7, 2014];QuickStats: Prevalence of current depression among persons ≥ 12 years, by age group and sex – United States, National Health and Nutrition Examination Survey, 2007–2010. Available from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6051a7.htm.
- 36.Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2365. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 37.Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. Oncologist. 2007;12:22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 38.Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer-related fatigue: evolving concepts in evaluation and treatment. Cancer. 2003;98:1786–1801. doi: 10.1002/cncr.11742. [DOI] [PubMed] [Google Scholar]
- 39.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue. Cancer. 2012;118:2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 40.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: a systematic meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;134:700–741. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]