Abstract
During cholestatic liver disease, there is dysregulation in the balance between biliary growth and loss in bile duct–ligated (BDL) rats modulated by neuroendocrine peptides via autocrine/paracrine pathways. Gonadotropin-releasing hormone (GnRH) is a trophic peptide hormone that modulates reproductive function and proliferation in many cell types. We evaluated the autocrine role of GnRH in the regulation of cholangiocyte proliferation. The expression of GnRH receptors was assessed in a normal mouse cholangiocyte cell line (NMC), sham, and BDL rats. The effect of GnRH administration was evaluated in normal rats and in NMC. GnRH-induced biliary proliferation was evaluated by changes in intrahepatic bile duct mass and the expression of proliferation and function markers. The expression and secretion of GnRH in NMC and isolated cholangiocytes was assessed. GnRH receptor subtypes GnRHR1 and GnRHR2 were expressed in cholangiocytes. Treatment with GnRH increased intrahepatic bile duct mass as well as proliferation and function markers in cholangiocytes. Transient knockdown and pharmacologic inhibition of GnRHR1 in NMC decreased proliferation. BDL cholangiocytes had increased expression of GnRH compared with normal rats, accompanied by increased GnRH secretion. In vivo and in vitro knockdown of GnRH decreased intrahepatic bile duct mass/cholangiocyte proliferation and fibrosis. GnRH secreted by cholangiocytes promotes biliary proliferation via an autocrine pathway. Disruption of GnRH/GnRHR signaling may be important for the management of cholestatic liver diseases.
Cholangiocytes, which line the intrahepatic biliary epithelium, play key roles in the modification of canalicular bile and are the target of chronic cholestatic liver diseases (ie, cholangiopathies). Cholangiopathies are characterized by dysregulation of the balance between biliary proliferation and loss, leading to chronic liver injury, liver failure, and, ultimately, liver transplantation.1 Among several gastrointestinal hormones/peptides regulating biliary functions, secretin is a key stimulatory factor that promotes ductal secretion and cholangiocyte proliferation.2,3 Secretin stimulates bicarbonate secretion by interaction with its receptor (SR), which causes phosphorylation of the cAMP-dependent cystic fibrosis transmembrane conductance regulator (CFTR) with subsequent activation of the Cl−/HCO3- anion exchanger 2 (AE2).4–6 Secretin-stimulated ductal secretion increases in parallel to enhanced biliary hyperplasia but decreases during biliary damage, suggesting that the secretin–SR–CFTR–AE2 axis may be an important functional index/regulator of changes in biliary proliferation/loss.2,5,7,8
Several animal models including bile duct ligation (BDL) mimic human cholangiopathies.2,7,8 For example, in rodents with BDL, there is enhanced proliferation of large cAMP-dependent cholangiocytes that leads to increased intrahepatic bile duct mass (IBDM).3,9 The proliferative response of the biliary epithelium to liver injury is characterized by cholangiocytes acquiring neuroendocrine phenotypes, which allows these cells to secrete a number of peptides and hormones [such as follicle-stimulating hormone (FSH), secretin, melatonin, and vascular endothelial factor] that are key for maintaining the homeostasis of the biliary epithelium by both autocrine/paracrine mechanisms.3,8,10 In addition, these factors modulate the liver fibrosis that accompanies the onset of cholestatic liver diseases.11 The BDL model of cholestatic injury also is characterized by marked hepatic fibrosis, which starts primarily around the biliary epithelium.12 Activation of hepatic stellate cells and myofibroblasts contribute to the progression of fibrosis, although some studies have highlighted the contribution of cholangiocytes in this process.13
The peptide gonadotropin-releasing hormone (GnRH), which is synthesized and released from neurons within the hypothalamus,14 mediates the release of FSH and luteinizing hormone from the anterior pituitary gland. GnRH exerts its effects by interacting with two GnRH receptor (GnRHR) subtypes, GnRHR1 and GnRHR2, which are expressed in mammals.15 There is evidence of an extrahypothalamic origin for GnRH as well as an extrapituitary presence of GnRHR in numerous peripheral tissues including reproductive organs such as testis, ovary, oviduct, and mammary glands.16 The mRNA for GnRHRs has been shown to be expressed in various nonreproductive human tissues such as liver, pancreas, colon, kidney, heart, and pituitary.17,18 Several studies have shown that GnRH exerts its cellular functions both in vivo and in vitro by activation of cAMP levels.19,20 With regard to inositol triphosphate (IP3)-dependent signaling (an important pathway modulating biliary function),21,22 some studies have shown that GnRH exerts its effects by increasing IP3 levels,23,24 although other studies have shown that GnRH has no effects on IP3 levels.25 GnRH has been shown to promote or inhibit cell proliferation (depending on the cell type) in various carcinomas of the breast, ovary, endometrium, pancreas, and liver.26 A study has shown higher expression of both GnRH and GnRHRs in grade I and grade II human hepatocellular carcinoma, suggesting modulation of hepatocellular growth by this neuroendocrine hormone.27 However, limited information exists regarding the cellular localization of GnRH and its receptors in the liver and particularly in the biliary epithelium, and the effect of GnRH on liver pathophysiology.26,28 Thus, we performed studies to show the paracrine/autocrine role of GnRH in the regulation of biliary mass in normal and cholestatic BDL rats.
Materials and Methods
Materials
Reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO) unless otherwise indicated. The rabbit monoclonal antibody against GnRHR1 and the mouse monoclonal antibody against GnRHR2 were obtained from Sigma-Aldrich Co. The substrate for γ-glutamyltranspeptidase, N (γ-L-glutamyl)-4-methoxy-2-naphthylamide, was purchased from Polysciences, Inc. (Warrington, PA). The mouse monoclonal antibody against proliferating cell nuclear antigen (PCNA) and the rabbit polyclonal antibody against GnRH were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The mouse antibody for cytokeratin-19 was purchased from Leica Biosystems Newcastle Ltd. (Newcastle, UK). The RNeasy kit for the purification of total RNA, real-time PCR primers for mRNA analysis, and shRNA constructs were purchased from Qiagen (Valencia, CA). Primers for mRNA analysis of GnRHR2, α-smooth muscle actin (α-SMA), collagen type I α 1, and fibronectin-1, and reagents for real-time PCR were obtained from BioRad Life Sciences (Hercules, CA). GnRH Vivo-Morpholino as well as mismatched-morpholinos were obtained from Gene Tools, LLC (Philomath, OR). The Nova Ultra Sirius Red Stain kit to detect interstitial collagen deposition was purchased from IHC World (Woodstock, MD). The Enzyme Immunoassay kits to measure intracellular cAMP levels were purchased from Cayman Chemicals Company (Ann Arbor, MI). The kits for evaluating IP3 levels (2IP1PEA–IP-One Elisa Assay) were purchased from Cisbio Bioassays (Burlington, MA). The EIA kits to measure GnRH levels were purchased from Phoenix Pharmaceutical Inc. (Burlingame, CA). The GnRHR1 antagonist, cetrorelix acetate,29 was purchased from R&D Systems (Minneapolis, MN).
Animals
Male Fischer 344 rats were purchased from Charles River (Wilmington, MA) and maintained in a temperature-controlled environment (20°C to 22°C) with 12:12-hour light-dark cycles. Animals were fed standard rat chow and had access to drinking water ad libitum. We treated normal rats with saline or GnRH (1.0 μg/day)30 for 1 week by i.p. implanted Alzet (Cupertino, CA) osmotic minipumps. In separate experiments, BDL rats (immediately after surgery)2 were treated with Vivo-Morpholino sequences against GnRH (5′-GATCGTTTCCATTCTGTTTGGATGT-3′, 1.0 mg/kg body weight/day to reduce the hepatic GnRH expression) or mismatch-morpholino sequences (5′-GAACCTTTCGATTCTCTTTCGATGT-3′) administered by an implanted portal vein catheter for 1 week.31 In these two groups of animals, GnRH expression was evaluated by immunohistochemistry in liver sections and enzyme-linked immunosorbent assay kits in the media of short-term cultures of isolated cholangiocytes. Before collection of serum, tissue, or liver perfusion, animals were injected with Euthasol (Fort Worth, TX) following the regulations of the panel of euthanasia of American Veterinarian Medical Association and protocols approved by Baylor Scott & White Institutional Animal Care and Use Committee. Liver and body weight as well as liver to body weight ratio, an index of liver cell growth including cholangiocytes,2 was measured in all animals.
Cholangiocyte Purification
Pure cholangiocytes (by γ-glutamyltranspeptidase histochemistry)32 were isolated by immunoaffinity separation using a monoclonal antibody [IgM; which was a gift from Dr. Ronald A. Faris (Brown University, Providence, RI)] that recognizes an unidentified antigen expressed by all intrahepatic cholangiocytes.33 Cell number and viability were assessed by trypan blue exclusion. The in vitro experiments were performed in our immortalized NMC line.34,35
Evaluation of GnRH Receptor Expression
The expression of GnRHR1 and GnRHR2 was evaluated by both immunofluorescence and semiquantitative immunohistochemistry in liver sections (4- to 5-μm thick) and by immunofluorescence36 in NMC smears. Immunofluorescence in liver sections was performed by simultaneous incubation with antibodies against GnRHR1 or GnRHR2 together with an antibody against cytokeratin-19 (a cholangiocyte marker).8 Images were obtained by using Leica AF 6000 Modular Systems (Leica Biosystems Newcastle Ltd.).
Measurement of Biliary Proliferation, Liver Fibrosis, and Morphology and Serum Chemistry
Cholangiocyte proliferation was evaluated by measuring IBDMs in paraffin-embedded liver sections (4- to 5-μm thick) by semiquantitative immunohistochemistry for cytokeratin-19, a biliary-specific marker.8 IBDM was evaluated as the area occupied by CK-19 positive bile ducts/total area × 100. Sections were examined with an Image Analysis System (Delta Sistemi, Rome, Italy). Biliary proliferation also was evaluated in isolated cholangiocytes by measuring PCNA expression by real-time PCR.37 Fibrosis in liver sections was assessed by Sirius red staining to evaluate interstitial collagen deposition identified by red color in 4- to 5-μm–thick paraffin-embedded liver sections.38 Changes in the fibrotic reaction in response to knockdown of GnRH in the liver (by GnRH Vivo-Morpholino) were evaluated by measuring the mRNA expression of fibrotic markers (ie, fibronectin-1, α-SMA, and collagen type IαI) in RNA from cholangiocytes and total liver by real-time PCR.38 All reactions were compared with glyceraldehyde-3-phosphate dehydrogenase to ensure proper RNA loading. Data were expressed as relative mRNA levels ± SEM of PCNA to glyceraldehyde-3-phosphate dehydrogenase ratio. The morphology of other organs including the liver was determined by H&E staining in 4- to 5-μm–thick paraffin-embedded sections. The serum levels of transaminases (glutamate pyruvate transaminases and serum glutamic oxaloacetic transaminase), alkaline phosphatase, and total bilirubin were measured by a Dimension RxL Max Integrated Chemistry system (Dade Behring, Inc., Deerfield, IL) at Baylor Scott & White.
Effect of GnRH on cAMP and IP3 Levels, and the Expression of SR, CFTR, and AE2 in Cholangiocytes
In cholangiocytes from normal rats treated with saline or GnRH we evaluated intracellular cAMP and IP3 levels and the expression of SR, CFTR, and AE2 (functional indices of biliary growth)2,5–7,9 by real-time PCR. After purification, cholangiocytes were incubated for 1 hour at 37°C33 and subsequently incubated for 5 minutes (cAMP) or 10 minutes (IP3) at room temperature before evaluating the levels of these messengers.9,39
Biliary Expression and Secretion of GnRH
To validate our models, we measured GnRH mRNA expression in the hypothalamus and cholangiocytes by real-time PCR, and GnRH levels by EIA kits40 in the serum and the media of primary cultures (after incubation for 6 hours) of cholangiocytes from the selected groups of animals. GnRH expression was evaluated by immunohistochemistry in 4- to 5-μm–thick liver sections and by real-time PCR and immunofluorescence in NMC. The levels of GnRH secreted from basolateral and apical domains of NMC was measured by plating the cell lines for 72 hours on collagen-coated filters of tissue culture inserts to produce a confluent monolayer.3
In Vitro Effect of GnRH on NMC Proliferation
NMC were stimulated with 0.2% bovine serum albumin (basal) or 10 to 100 nmol/L GnRH41 for 48 to 72 hours before measuring cell growth by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] proliferation assays.21 In other experiments, NMC were treated with bovine serum albumin or 50 nmol/L GnRH41 for 48 hours before evaluating proliferation by real-time PCR for PCNA36 and for 5 or 10 minutes before evaluating basal cAMP or IP3 levels, respectively. In separate experiments, NMC were incubated with 50 nmol/L GnRH in the absence/presence of 10 nmol/L GnRHR1 antagonist cetrorelix acetate before evaluating cell proliferation by MTS assays (48 hours’ incubation). To determine which receptor subtype mediates GnRH effects, control, siRNA-GnRHR1, or siRNA-GnRHR2 NMC were treated with bovine serum albumin or GnRH (50 nmol/L for 48 hours) before measuring cell proliferation by MTS assays. The transient knockdown of GnRHR1 or GnRHR2 in NMC was evaluated by real-time PCR. In other experiments, NMC were incubated with cholangiocyte media from BDL rats (72 hours’ incubation in the absence/presence of 10 ng/mL GnRH antibody) before evaluating cell proliferation by MTS assays.
The autocrine role of GnRH in the regulation of NMC proliferation was evaluated in cells after targeted knockdown of GnRH. These cell lines were established following a protocol described previously37 by using SureSilencing shRNA plasmids (SABiosciences, Valencia, CA) for mouse GnRH, which also confers resistance to neomycin for the selection of stably transfected cells. Transfected cells were selected by the addition of 0.5 mg/mL neomycin into the media, and the selection process was allowed to continue for 3 to 4 weeks.37 Surviving cells (designated shGnRH) were assessed for the relative expression of GnRH compared with the mock-transfected control cell line (Neg-transfected) by real-time PCR, and the clone with the greatest degree of knockdown was selected for the subsequent experiments. In both shGnRH and Neg-transfected NMC, we measured cell proliferation by MTS after incubation for 48 hours. The effect of conditioned media from shGnRH and Neg-transfected NMC on the proliferation of NMC also was evaluated by MTS assays.21
Statistical Analysis
Data are expressed as means ± SEM. Differences between groups were analyzed by the Student's unpaired t-test when two groups were analyzed and by analysis of variance when more than two groups were analyzed, followed by an appropriate post hoc test.
Results
Cholangiocytes Express GnRH Receptors
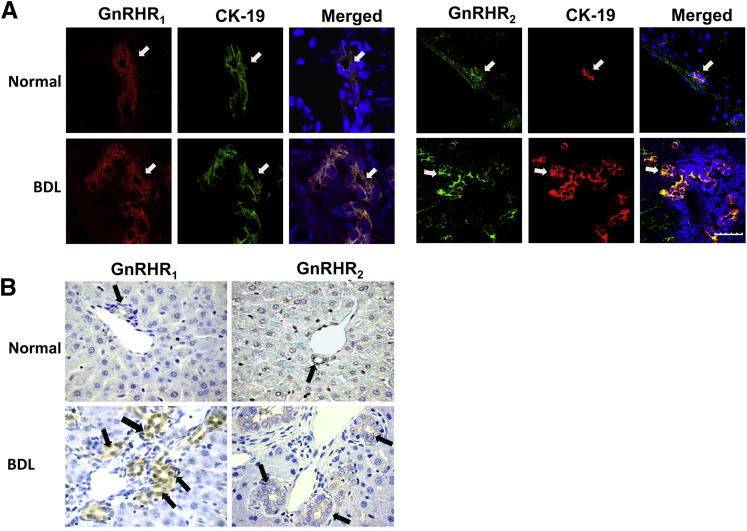
To begin to understand the mechanisms by which GnRH affects cholangiocyte function, it is important to show the localization of GnRH receptors in the biliary epithelium. By immunofluorescence in liver sections from normal and BDL rats, we showed the expression of GnRHR1 or GnRHR2, in bile ducts (Figure 1A). By immunohistochemistry, GnRHR1 and GnRHR2 were expressed by normal bile ducts, expression that was higher in normal rats treated with GnRH, and in BDL compared with normal rats (Figure 1B and Table 1); the biliary expression of GnRHR1 and GnRHR2 decreased in BDL rats treated with GnRH Vivo-Morpholino compared with mismatch controls (Table 1). BDL rats treated with mismatch-morpholino did not show any difference from BDL rats with respect to the parameters measured here. Therefore, these terms were used interchangeably.
Figure 1.
A: Representative immunofluorescence for GnRHR1 (red) and GnRHR2 (green) in liver sections co-stained with cytokeratin-19 (CK-19) show their expression on bile ducts. Receptor and CK-19 localization in bile ducts are indicated by white arrows. B: Immunohistochemistry showing the expression of GnRHR1 and GnRHR2 in bile ducts, the expression increases after bile duct ligation (BDL) (Table 1). Positive staining for GnRHR1 and GnRHR2 in bile ducts is shown by black arrows. Scale bar = 25 μm (A). Original magnification: ×40 (B).
Table 1.
Comparison of Parameters in Different Animal Groups
| Parameters | Normal rats + saline | Normal rats + GnRH | BDL rats + mismatch morpholino | BDL rats + GnRH Vivo-Morpholino |
|---|---|---|---|---|
| Body weight (g) | 217.7 ± 5.8 (n = 9) | 213.5 ± 2.3 (n = 8) | 165.3 ± 4.3∗ (n = 3) | 189.10 ± 2.7† (n = 3) |
| Liver weight (g) | 8.7 ± 0.7 (n = 9) | 8.5 ± 0.6 (n = 8) | 9 ± 0.1 (n = 3) | 8.7 ± 0.5 (n = 3) |
| Liver-to–body weight ratio (%) | 4.30 ± 0.2 (n = 9) | 3.9 ± 0.2 (n = 8) | 5.4 ± 0.1† (n = 3) | 4.6 ± 0.3‡ (n = 3) |
| GnRH (% cholangiocytes) | 16.2 ± 0.1 | 24.5 ± 1.0∗ | 80.2 ± 3.4† | 50.4 ± 0.3‡ |
| Serum GnRH levels (ng/mL) | 1.72 ± 0.4 (n = 24) | 2.63 ± 0.03∗ (n = 18) | 3.82 ± 1.18∗ (n = 12) | 1.89 ± 0.04‡ (n = 12) |
| IBDM (%) | 0.48 ± 0.1 | 0.71 ± 0.08∗ | 4.2 ± 0.4† | 2.4 ± 0.3‡ |
| GnRHR1 (% cholangiocytes) | 6.01 ± 0.3 | 14.51 ± 1.0∗ | 70.0 ± 3.8† | 51.0 ± 3.1‡ |
| GnRHR2 (% cholangiocytes) | 1.0 ± 0.1 | 3.02 ± 0.3∗ | 65.1 ± 1.1† | 61.4 ± 1.0‡ |
| SGPT (U/L) | 57.3 ± 2.4 (n = 12) | 52.5 ± 1.8 (n = 12) | 286.3 ± 2.5† (n = 9) | 235 ± 1.5‡ (n = 9) |
| SGOT (U/L) | 100.7 ± 3.5 (n = 12) | 111.8 ± 1.9 (n = 12) | 863 ± 2† (n = 9) | 571 ± 3.2‡ (n = 9) |
| Total bilirubin (mg/dL) | <0.1 (n = 12) | <0.1 (n = 12) | 11.5 ± 1.2† (n = 9) | 4.7 ± 1.3‡ (n = 9) |
| ALP (U/L) | 305.7 ± 1.7 (n = 12) | 263.7 ± 7 (n = 12) | 447.3 ± 3.8∗† (n = 9) | 360.3 ± 1.5‡ (n = 9) |
ALP, alkaline phosphatase; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamate pyruvate transaminase.
P < 0.05 versus normal rats.
P < 0.05 versus normal rats treated with GnRH.
P < 0.05 versus BDL rats treated with mismatch-Morpholino.
Measurement of Biliary Proliferation, Liver Fibrosis and Morphology, Serum Chemistry, cAMP and IP3 Levels, and Ductal Secretion
There was no significant difference in body weight and liver-to–body weight ratio (an index of liver growth)2 between normal rats treated with saline or GnRH (Table 1). Liver-to–body weight ratio increased in BDL compared with normal rats,2 but decreased in BDL rats treated with GnRH Vivo-Morpholino compared with BDL controls (Table 1), confirming the trophic effects of GnRH on the liver.
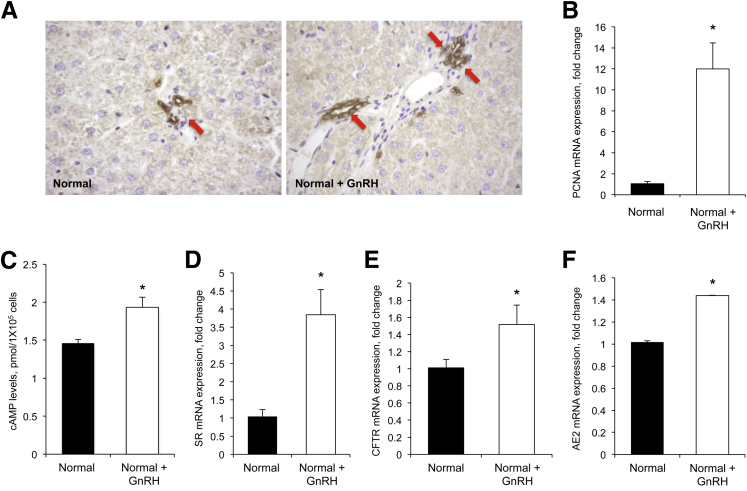
We next determined the in vivo effect of GnRH on biliary growth and the levels of cAMP, SR, CFTR, and AE2 (functional indices of biliary proliferation).2,5,7,42 Administration of GnRH to normal rats increased IBDM of cholangiocytes (Figure 2A and Table 1) and PCNA expression in cholangiocytes compared with saline-treated rats (Figure 2B). In cholangiocytes from normal rats treated with GnRH, there was an increase in basal cAMP (but not IP3, not shown) levels, and enhanced expression of SR, CFTR, and AE2 compared with cholangiocytes from controls (Figure 2, C–F) showing that GnRH mediates its proliferative effects by activation of cAMP-dependent signaling.
Figure 2.
A: Administration of gonadotropin-releasing hormone (GnRH) to normal rats enhances intrahepatic bile duct mass (IBDM) compared with saline-treated rats. Data are means ± SEM of 10 cumulative values obtained from two slides for each group. For semiquantitative analysis, see Table 1. Cytokeratin-19–positive bile ducts are indicated by red arrows. B: PCNA expression increases in cholangiocytes from rats treated with GnRH compared with controls. Data are means ± SEM of five replicates. C–F: cAMP levels and expression of SR, CFTR, and AE2 increases in cholangiocytes from GnRH-treated rats compared with control rats. Data are means ± SEM of three evaluations. ∗P < 0.05 versus cholangiocytes from control rats. Original magnification: ×40 (A).
To evaluate the effect of GnRH on liver morphology, H&E staining of liver sections was performed; we found no significant changes in the lobular structure and inflammation of normal rats treated with saline or GnRH (not shown). In BDL rats, we observed reactive mildly inflamed portal areas with biliary proliferation, with no significant parenchymal necrosis or inflammation compared with normal rats (not shown). The livers of BDL rats treated with GnRH Vivo-Morpholino showed mildly reactive portal tracts with minimal duct proliferation and minimal parenchymal necrosis or inflammation (not shown). No significant changes in the structure of other organs including kidney, stomach, intestine, and testicles between the latter two groups of animals were observed. Treatment of normal rats with GnRH did not alter the serum levels of alkaline phosphatase, transaminases, or bilirubin compared with control rats (Table 1). The serum levels of alkaline phosphatase, transaminases, and bilirubin increased in BDL rats (treated with mismatch-morpholino) compared with normal rats, but decreased in BDL rats treated with GnRH Vivo-Morpholino compared with matched controls (Table 1).
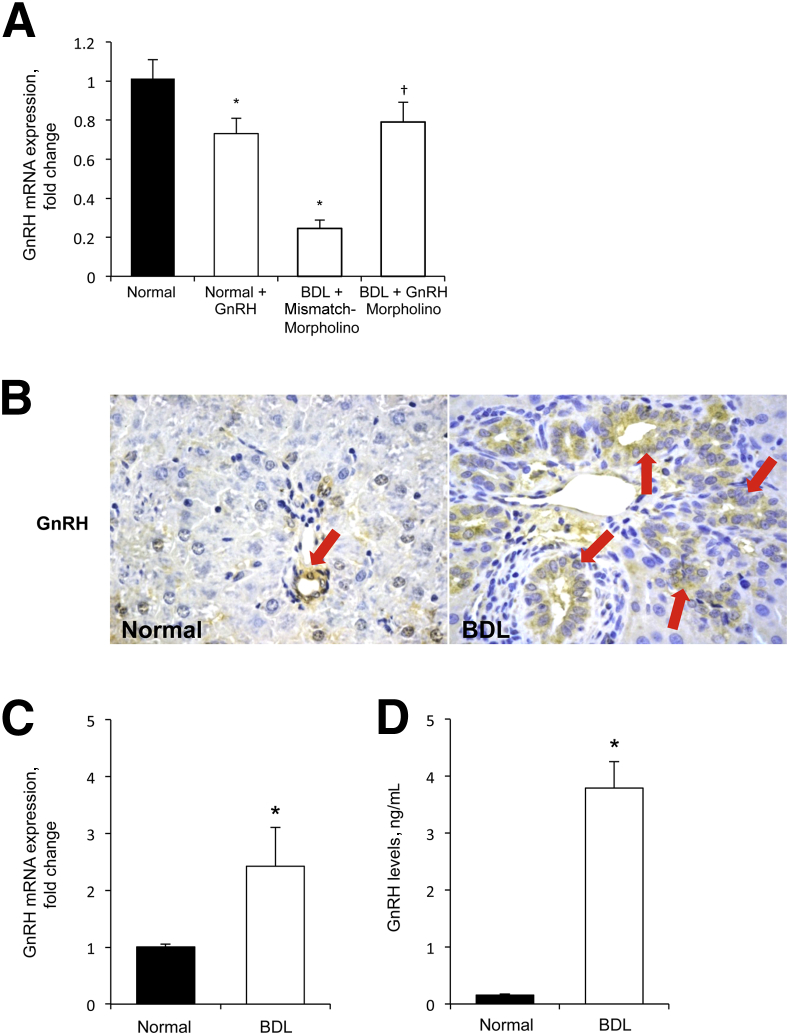
As validation of our in vivo models, there was reduced GnRH mRNA expression in the hypothalamus of normal rats treated with GnRH and BDL rats compared with normal rats, and increased GnRH mRNA expression in the hypothalamus of BDL rats treated with GnRH Vivo-Morpholino compared with matched controls (Figure 3A). In GnRH-treated normal rats, there was increased biliary immunoreactivity for GnRH in liver sections, and enhanced GnRH serum levels compared with control rats (Table 1). We observed an increase in GnRH serum levels in BDL compared with normal rats, and a reduced concentration of serum GnRH in BDL rats treated with GnRH Vivo-Morpholino compared with matched controls (Table 1).
Figure 3.
A: Gonadotropin-releasing hormone (GnRH) expression decreases in the hypothalamus of normal rats treated with GnRH and in bile duct-ligated (BDL) rats compared with normal rats, and increases in the hypothalamus of BDL rats treated with GnRH Vivo-Morpholino compared with BDL control rats. Data are means ± SEM of three experiments. B: Representative immunohistochemistry for GnRH in liver sections from normal and BDL rats. The immunoreactivity of GnRH increases in bile ducts from BDL compared with normal rat (Table 1). Red arrows indicate bile ducts showing positivity for GnRH. C and D: GnRH expression and levels increase in cholangiocytes (C) and cholangiocyte supernatant (D) from BDL compared with normal rats, respectively. Data are means ± SEM of six evaluations. ∗P < 0.05 versus normal rats; †P < 0.05 versus BDL rats. Original magnification: ×40 (B).
Role of GnRH in the Autocrine Regulation of Cholangiocyte Growth
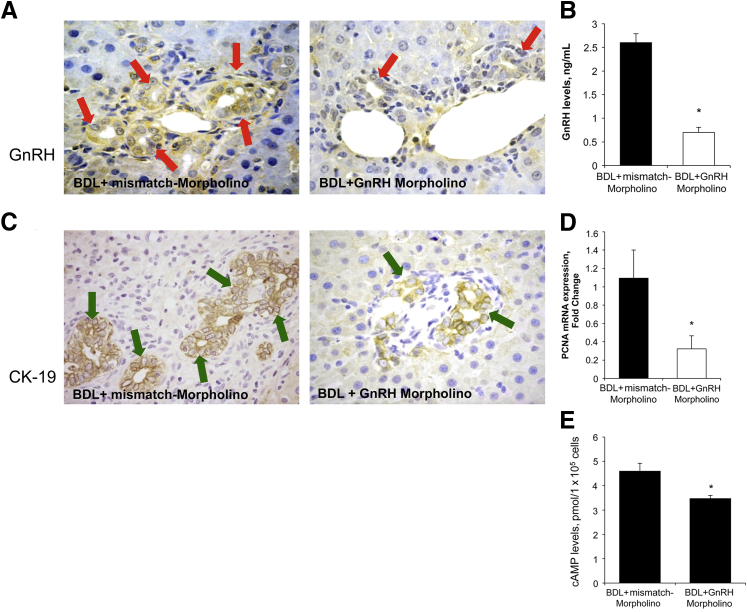
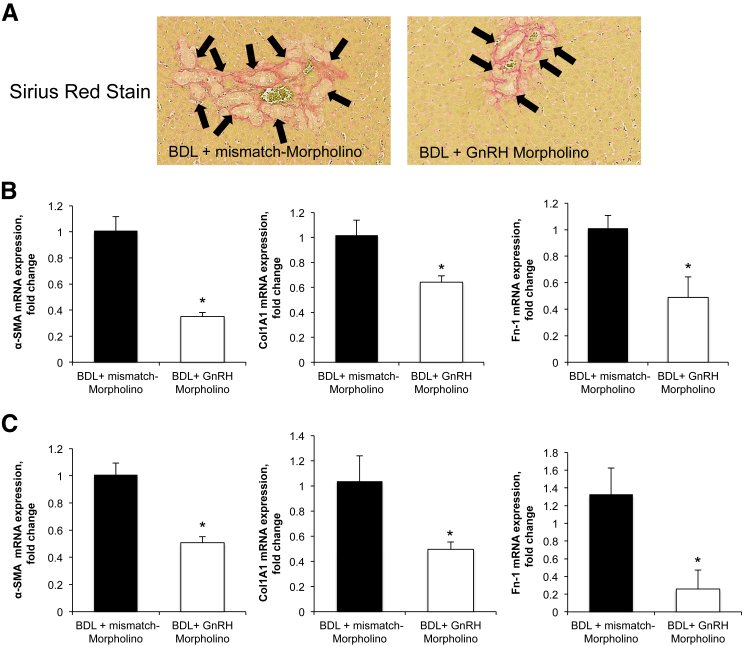
To establish the autocrine role of GnRH in biliary proliferation, we evaluated the expression and secretion of GnRH in the selected groups of animals. The immunoreactivity of GnRH increased in bile ducts from BDL compared with normal rats (Figure 3B and Table 1). In BDL rats, there was increased GnRH mRNA expression in cholangiocytes and GnRH levels in cholangiocyte supernatant compared with normal rats (Figure 3, C and D). Because the biliary expression/secretion of GnRH increases after BDL (Figure 3, B–D), we evaluated the effect of decreased expression of hepatic GnRH (by administration of GnRH Vivo-Morpholino) in the modulation of biliary hyperplasia during cholestasis. The decrease of GnRH in BDL rats treated with GnRH-specific Vivo-Morpholino was shown by immunohistochemistry in liver sections and enzyme-linked immunosorbent assay kits in cholangiocyte supernatant (Figure 4, A and B and Table 1). The administration of GnRH Vivo-Morpholino to BDL rats significantly decreased serum levels of transaminases, alkaline phosphatase, as well as total bilirubin (Table 1), IBDM (Figure 4C), PCNA expression (Figure 4D), and cAMP levels (Figure 4E) compared with matched control rats. Consistent with previous studies,38,43 we found enhanced fibrosis (evidenced by Sirius red staining) in liver sections obtained from BDL rats treated with mismatch-Morpholino compared with normal rats (data not shown). Inhibition of the GnRH/cAMP axis by targeted knockdown of GnRH (by administration of GnRH Vivo-Morpholino) in BDL rats resulted in a decrease in interstitial collagen deposition as well as reduced expression of α-SMA, collagen type I α, and fibronectin-1 (Figure 5) compared with mismatch control rats.
Figure 4.
A and B: The administration of gonadotropin-releasing hormone (GnRH) Vivo-Morpholino to bile duct-ligated (BDL) rats decreases the immunoreactivity of GnRH in liver sections (red arrows) (Table 1) and GnRH secretion in cholangiocyte supernatant. C–E: The administration of GnRH Vivo-Morpholino to BDL rats decreases intrahepatic bile duct mass (IBDM) in liver sections (green arrows; C). Proliferating cell nuclear antigen (PCNA) expression, and cAMP levels compared with BDL control rats. n = 3 (D); n = 6 (E). ∗P < 0.05 versus BDL rats treated with mismatch-Morpholino. Original magnification: ×20 (A and C). CK-19, cytokeratin-19.
Figure 5.
A: The administration of gonadotropin-releasing hormone (GnRH) Vivo-Morpholino to bile duct-ligated (BDL) rats decreases the immunoreactivity of interstitial collagen in liver sections evaluated by Sirius red staining compared with BDL rats treated with mismatch-Morpholino. Collagen deposition around bile ducts is indicated by black arrows. B and C: The administration of GnRH Vivo-Morpholino to BDL rats decreases the expression of α-SMA, collagen type I α(Col1A1), and fibronectin-1 (Fn-1) in purified cholangiocytes (B) and total liver tissue (C) compared with BDL control rats. Data are means ± SEM of 12 real-time PCR reactions. ∗P < 0.05 versus BDL rats treated with mismatch-Morpholino. Original magnification: ×20 (A).
Effect of GnRH on NMC Proliferation and Effect of Silencing of GnRHR1 and GnRHR2 on GnRH-Induced NMC Proliferation
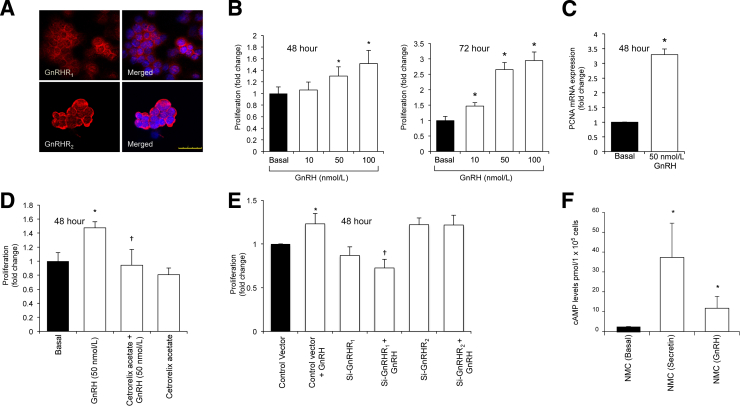
To validate the direct proliferative effect of GnRH on cholangiocytes, we performed in vitro studies to show that GnRH stimulates biliary proliferation by interaction with GnRH receptors. First, we showed by immunofluorescence that NMC express both GnRHR1 and GnRHR2 (Figure 6A). GnRH (10 to 100 nmol/L, 48 and 72 hours) increased the proliferation of NMC in a dose-dependent manner (Figure 6B). There also was increased PCNA expression in NMC treated with GnRH (50 nmol/L, 48 hours) compared with basal levels (Figure 6C). The GnRH-induced increase in NMC proliferation was blocked by cetrorelix acetate and by silencing of GnRHR1, but not GnRHR2 (Figure 6, D and E). The proliferative effects of GnRH on NMC were associated with enhanced cAMP levels; secretin (positive control)7,42 also increased cAMP levels of these cells (Figure 6F).
Figure 6.
A: By immunofluorescence, normal mouse cholangiocyte cell line (NMC) expresses both GnRHR1 and GnRHR2. Specific immunoreactivity of representative fields is shown in red and nuclei were stained with DAPI (blue). B: GnRH (10 to 100 nmol/L) induces the proliferation of NMC in a dose-dependent manner. C: Gonadotropin-releasing hormone (GnRH) increases PCNA expression in NMC compared with basal levels. Data are means ± SEM of five replicates. D: GnRH (50 nmol/L)-induced increase in NMC proliferation is blocked when NMC are treated with the specific GnRHR1 antagonist. E: GnRH-mediated increase in NMC proliferation is preferentially blocked when GnRHR1 is silenced. Basal proliferative activity decreases in si-GnRHR1 NMC compared with control NMC. Data are means ± SEM of five replicates. F: cAMP levels increase in NMC treated with GnRH. NMC treated with secretin was used as the positive control. Data are means ± SEM of six replicates. ∗P < 0.05 versus basal (C and E) or versus control NMC (F); †P < 0.05 versus NMC treated with GnRH alone (B and D) or versus cells treated with control vector and GnRH (E). Scale bar = 25 μm (A).
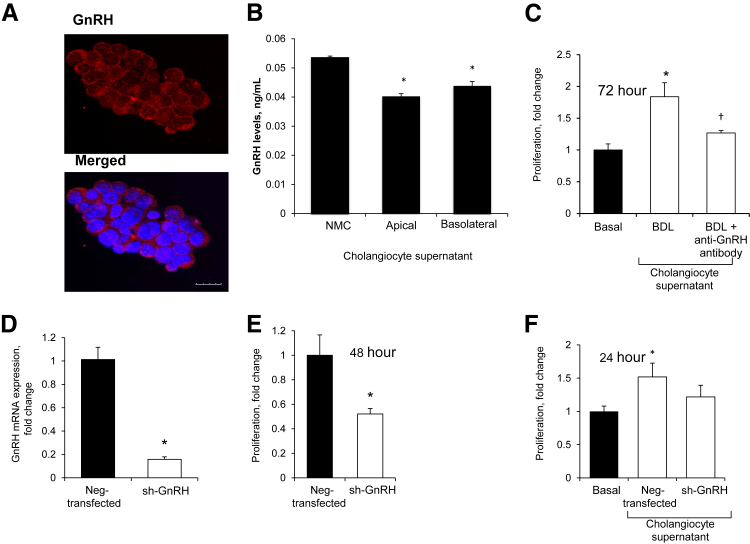
To provide direct evidence of the autocrine role of GnRH in biliary proliferation, we evaluated the effect of stable knockdown of GnRH on NMC proliferation. By immunofluorescence, NMC express GnRH (Figure 7A) and secrete GnRH in both the apical and basolateral domains (Figure 7B). Incubation of NMC with BDL cholangiocyte supernatant increased the proliferation of these cells that was blocked by preincubation with a GnRH-neutralizing antibody (Figure 7C). We have shown that shGnRH NMC (approximately 90% knockdown compared with Neg-transfected NMC) (Figure 7D) showed lower proliferative activity compared with Neg-transfected NMC (Figure 7E). The supernatant of shGnRH NMC (containing lower amounts of GnRH) increased NMC proliferation at a lower extent compared with the supernatant of Neg-transfected NMC, which secrete more GnRH (Figure 7F).
Figure 7.
A and B:In vitro, normal mouse cholangiocyte cell line (NMC) also express gonadotropin-releasing hormone (GnRH) and secrete GnRH at both the apical and basolateral domains. C: Incubation of NMC with the media of short-term cultures of bile duct-ligated (BDL) cholangiocytes increases the proliferation of these cells. GnRH-stimulation of NMC proliferation is blocked by preincubation with a GnRH-neutralizing antibody. Data are means ± SEM of four replicates. D and E: The shGnRH NMC (approximately 90% knockdown) shows lower proliferative activity compared with Neg-transfected NMC. Data are means ± SEM of five replicates. F: The supernatant of shGnRH NMC increases NMC proliferation at a lower extent compared with the supernatant of Neg-transfected NMC, which display a higher proliferative capacity because they secrete more GnRH. Data are means ± SEM of three experiments. ∗P < 0.05 versus basal; †P < 0.05 versus Neg-transfected NMC. Scale bar = 10 μm.
Discussion
Here, we showed the paracrine/autocrine stimulatory role of GnRH on biliary proliferation in normal and cholestatic rats. Specifically, we showed that bile ducts and isolated and cholangiocyte lines (NMC) express GnRHR1 and GnRHR2. Administration of GnRH to normal rats increased cholangiocyte proliferation and IBDM concomitant with increased cAMP levels and expression of SR, CFTR, and AE2. In addition to the hypothalamus, cholangiocytes also express GnRH and secrete GnRH at both the basolateral and apical domains. After BDL, there was an increase in GnRH serum levels that likely was owing to enhanced secretion of GnRH from cholangiocytes, because there was reduced expression of GnRH from the hypothalamus. Reduction of biliary GnRH expression/secretion (by administration of GnRH Vivo-Morpholino) in BDL rats was associated with reduced biliary proliferation and cAMP levels. We also showed the following: i) NMCs express GnRHR1 and GnRHR2; ii) GnRH increased cAMP-dependent proliferation of NMC, which was prevented by pharmacologic inhibition or silencing of GnRHR1; iii) NMCs express and secrete GnRH; iv) incubation of NMC with the conditioned media of cholangiocyte cultures increased the proliferation of these cells, and this increase was prevented by preincubation with a GnRH-neutralizing antibody; v) the supernatant of shGnRH NMC increased NMC proliferation at a lower extent compared with Neg-transfected NMC; and vi) shGnRH NMC displayed lower proliferative activity compared with Neg-transfected NMC.
Several studies have shown that during the progression of biliary damage (eg, after BDL) cholangiocytes secrete neuroendocrine factors, which regulate biliary homeostasis by both autocrine/paracrine mechanisms.10,31,44,45 For example, whereas secretin, vascular endothelial factor, and nerve growth factor stimulate biliary proliferation, other factors including melatonin and serotonin inhibit biliary hyperplasia.10,31,44 As validation of our model, the increase in GnRH serum levels (observed after prolonged GnRH administration or BDL) likely was owing to enhanced GnRH biliary expression because GnRH expression was decreased in the hypothalamus in these two models, supporting an autocrine role of GnRH in modulating biliary growth. The increase in GnRH expression observed in the hypothalamus in BDL rats treated with GnRH Vivo-Morpholino likely was owing to a compensatory mechanism because of the reduced expression/secretion of GnRH in cholangiocytes.
In support of our findings, GnRH and its receptors were shown to be present at higher levels in grades I to II hepatocellular carcinoma cells, suggesting a role for GnRH in the modulation of hepatocellular carcinoma growth.27 Furthermore, one study showed the presence of GnRHR in nonreproductive tissues such as human liver, heart, kidney, and pituitary.18 GnRH and its receptors also have been expressed in the rat gastrointestinal tract, pancreas, and submaxillary glands.46 A recent study also showed that cystic fibrosis epithelial cells express GnRHR, and GnRH improves the chloride transport defect in these cells.47 Supporting the finding that GnRH improves the chloride transport defect, we found that GnRH increased cAMP-dependent expression of SR, CFTR, and AE2. Our data provide the first evidence that GnRH stimulated biliary proliferation by interacting selectively with GnRHR1 (but not GnRHR2), supporting the concept that functional type II receptors are expressed by humans but not rodents.48 This view also is supported by the fact that administration of GnRH to normal rats induces an increase in the expression of GnRHR1 but not GnRHR2 (Table 1). Consistently, activation of GnRHR2 induces a decrease in proliferation of SK-OV-3 ovarian cancer cells that were negative for GnRHR1, but positive for GnRHR2.49
GnRHR subtypes function via activation of IP3 signaling as well as other pathways such as adenylyl cyclase/cAMP/protein kinase A.15,50,51 Parallel to the latter studies, our data showed that GnRH stimulates biliary proliferation by cAMP-dependent signaling because GnRH increases in vivo IBDM of large cholangiocytes that function by activation of cAMP-dependent activation of SR, CFTR, and AE2. Supporting our concept, GnRH did not increase IBDM of small cholangiocytes (that function by activation of IP3/Ca2+/CaMK I–dependent signaling)8,21 and IP3 levels of NMC. Consistent with the concept that cAMP is key in GnRH-modulation of biliary growth, we observed increased expression of SR/CFTR/AE2 that are functional proliferative indices of large, cAMP-dependent cholangiocytes.2,5,7,42
We next showed that cholangiocytes express/secrete GnRH, which plays a key trophic role in the maintenance of bile duct mass. In support of our data, several studies showed the expression of GnRH in other nonreproductive epithelia including the pancreas.52 Relevant to our findings, depletion of enteric GnRH is found in some patients suffering from severe gastrointestinal dysmotility.53 However, because GnRH regulates the synthesis and secretion of FSH and FSH induces biliary hyperplasia,8 we have to consider that GnRH effects on biliary proliferation also may be mediated partly by enhanced release of FSH. Future studies are warranted to elucidate this point.
Conclusion
In conclusion, our study provides novel insights into the role of the GnRH/GnRHR axis in the modulation of biliary growth/loss in normal and cholestatic diseased conditions. Thus, this study opens up a possible avenue for targeting this signaling pathway for treating liver diseases.
Acknowledgment
The monoclonal antibody for isolation of pure cholangiocytes was provided by Dr. Ronald A. Faris (Brown University, Providence, RI).
Footnotes
Supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White; a VA Research Career Scientist Award; VA Merit Awards (F.M., S.G., and S.D); NIH grants DK054811 and DK062975 (G.F., F.M., and S.G.) and R01DK082435 (S.D.); VA CDA-2 Award IK2 BX001760 (H.F.); and Research Project funds from the University of Rome La Sapienza and the Fondo investimenti in ricerca di base Accordi di Programma 2010 #RBAP10Z7FS (E.G.).
This material is the result of work supported by resources at the Central Texas Veterans Health Care System. The views expressed herein are those of the authors and do not necessarily reflect the views of the National Institutes of Health or the Department of Veterans Affairs.
Disclosures: None declared.
Contributor Information
Gianfranco Alpini, Email: galpini@tamu.edu.
Shannon S. Glaser, Email: sglaser@tamu.edu.
References
- 1.Lazaridis K.N., Strazzabosco M., LaRusso N.F. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Alpini G., Lenzi R., Sarkozi L., Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glaser S., Meng F., Han Y., Onori P., Chow B.K., Francis H., Venter J., McDaniel K., Marzioni M., Invernizzi P., Ueno Y., Lai J.M., Huang L., Standeford H., Alvaro D., Gaudio E., Franchitto A., Alpini G. Secretin stimulates biliary cell proliferation by regulating expression of MicroRNA 125b and MicroRNA let7a in mice. Gastroenterology. 2014;146:1795–1808. doi: 10.1053/j.gastro.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanno N., LeSage G., Glaser S., Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–G625. doi: 10.1152/ajpgi.2001.281.3.G612. [DOI] [PubMed] [Google Scholar]
- 5.Alpini G., Ulrich C.D., 2nd, Phillips J.O., Pham L.D., Miller L.J., LaRusso N.F. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1994;266:G922–G928. doi: 10.1152/ajpgi.1994.266.5.G922. [DOI] [PubMed] [Google Scholar]
- 6.Banales J.M., Saez E., Uriz M., Sarvide S., Urribarri A.D., Splinter P., Tietz Bogert P.S., Bujanda L., Prieto J., Medina J.F., LaRusso N.F. Up-regulation of microRNA 506 leads to decreased Cl-/HCO3- anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology. 2012;56:687–697. doi: 10.1002/hep.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alpini G., Ulrich C., Roberts S., Phillips J.O., Ueno Y., Podila P.V., Colegio O., LeSage G., Miller L.J., LaRusso N.F. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1997;272:G289–G297. doi: 10.1152/ajpgi.1997.272.2.G289. [DOI] [PubMed] [Google Scholar]
- 8.Mancinelli R., Onori P., Gaudio E., DeMorrow S., Franchitto A., Francis H., Glaser S., Carpino G., Venter J., Alvaro D., Kopriva S., White M., Kossie A., Savage J., Alpini G. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol. 2009;297:G11–G26. doi: 10.1152/ajpgi.00025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.LeSage G., Glaser S., Marucci L., Benedetti A., Phinizy J.L., Rodgers R., Caligiuri A., Papa E., Tretjak Z., Jezequel A.M., Holcomb L.A., Alpini G. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1289–G1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 10.Alvaro D., Mancino M.G., Glaser S., Gaudio E., Marzioni M., Francis H., Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Tuchweber B., Desmouliere A., Bochaton-Piallat M.L., Rubbia-Brandt L., Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest. 1996;74:265–278. [PubMed] [Google Scholar]
- 12.Schmitt J., Roderfeld M., Sabrane K., Zhang P., Tian Y., Mertens J.C., Frei P., Stieger B., Weber A., Mullhaupt B., Roeb E., Geier A. Complement factor C5 deficiency significantly delays the progression of biliary fibrosis in bile duct-ligated mice. Biochem Biophys Res Commun. 2012;418:445–450. doi: 10.1016/j.bbrc.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 13.Grappone C., Pinzani M., Parola M., Pellegrini G., Caligiuri A., DeFranco R., Marra F., Herbst H., Alpini G., Milani S. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol. 1999;31:100–109. doi: 10.1016/s0168-8278(99)80169-x. [DOI] [PubMed] [Google Scholar]
- 14.Wierman M.E., Bruder J.M., Kepa J.K. Regulation of gonadotropin-releasing hormone (GnRH) gene expression in hypothalamic neuronal cells. Cell Mol Neurobiol. 1995;15:79–88. doi: 10.1007/BF02069559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison G.S., Wierman M.E., Nett T.M., Glode L.M. Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocr Relat Cancer. 2004;11:725–748. doi: 10.1677/erc.1.00777. [DOI] [PubMed] [Google Scholar]
- 16.Ramakrishnappa N., Rajamahendran R., Lin Y.M., Leung P.C. GnRH in non-hypothalamic reproductive tissues. Anim Reprod Sci. 2005;88:95–113. doi: 10.1016/j.anireprosci.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Aguilar-Rojas A., Huerta-Reyes M. Human gonadotropin-releasing hormone receptor-activated cellular functions and signaling pathways in extra-pituitary tissues and cancer cells (review) Oncol Rep. 2009;22:981–990. doi: 10.3892/or_00000525. [DOI] [PubMed] [Google Scholar]
- 18.Kakar S.S., Jennes L. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non-reproductive human tissues. Cancer Lett. 1995;98:57–62. [PubMed] [Google Scholar]
- 19.Lariviere S., Garrel G., Robin M.T., Counis R., Cohen-Tannoudji J. Differential mechanisms for PACAP and GnRH cAMP induction contribute to cross-talk between both hormones in the gonadotrope LbetaT2 cell line. Ann N Y Acad Sci. 2006;1070:376–379. doi: 10.1196/annals.1317.048. [DOI] [PubMed] [Google Scholar]
- 20.Limonta P., Moretti R.M., Montagnani Marelli M., Motta M. The biology of gonadotropin hormone-releasing hormone: role in the control of tumor growth and progression in humans. Front Neuroendocrinol. 2003;24:279–295. doi: 10.1016/j.yfrne.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Francis H., Glaser S., DeMorrow S., Gaudio E., Ueno Y., Venter J., Dostal D., Onori P., Franchitto A., Marzioni M., Vaculin S., Vaculin B., Katki K., Stutes M., Savage J., Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–C513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alpini G., Franchitto A., DeMorrow S., Onori P., Gaudio E., Wise C., Francis H., Venter J., Kopriva S., Mancinelli R., Carpino G., Stagnitti F., Ueno Y., Han Y., Meng F., Glaser S. Activation of alpha(1)-adrenergic receptors stimulate the growth of small mouse cholangiocytes via calcium-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1. Hepatology. 2011;53:628–639. doi: 10.1002/hep.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobkin-Bekman M., Naidich M., Pawson A.J., Millar R.P., Seger R., Naor Z. Activation of mitogen-activated protein kinase (MAPK) by GnRH is cell-context dependent. Mol Cell Endocrinol. 2006;252:184–190. doi: 10.1016/j.mce.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 24.Azad N., LaPaglia N., Kirsteins L., Uddin S., Steiner J., Williams D.W., Lawrence A.M., Emanuele N.V. Jurkat cell proliferative activity is increased by luteinizing hormone-releasing hormone. J Endocrinol. 1997;153:241–249. doi: 10.1677/joe.0.1530241. [DOI] [PubMed] [Google Scholar]
- 25.Poulin B., Rich N., Mas J.L., Kordon C., Enjalbert A., Drouva S.V. GnRH signalling pathways and GnRH-induced homologous desensitization in a gonadotrope cell line (alphaT3-1) Mol Cell Endocrinol. 1998;142:99–117. doi: 10.1016/s0303-7207(98)00114-2. [DOI] [PubMed] [Google Scholar]
- 26.Park M.K., Kanaho Y., Enomoto M. Regulation of the cell proliferation and migration as extra-pituitary functions of GnRH. Gen Comp Endocrinol. 2013;181:259–264. doi: 10.1016/j.ygcen.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J., Huang G., Huang W. Gonadotropin releasing hormone and its receptor in the tissue of human hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi. 1998;78:343–346. [Chinese] [PubMed] [Google Scholar]
- 28.Pati D., Habibi H.R. Inhibition of human hepatocarcinoma cell proliferation by mammalian and fish gonadotropin-releasing hormones. Endocrinology. 1995;136:75–84. doi: 10.1210/endo.136.1.7828560. [DOI] [PubMed] [Google Scholar]
- 29.Britten J.L., Malik M., Levy G., Mendoza M., Catherino W.H. Gonadotropin-releasing hormone (GnRH) agonist leuprolide acetate and GnRH antagonist cetrorelix acetate directly inhibit leiomyoma extracellular matrix production. Fertil Steril. 2012;98:1299–1307. doi: 10.1016/j.fertnstert.2012.07.1123. [DOI] [PubMed] [Google Scholar]
- 30.Massol J., Martin P., Adessi G., Peuch A.J. Gonadotropin-releasing hormone (GnRH) as antidepressant: a psychopharmacological animal study. Eur J Pharmacol. 1989;160:395–399. doi: 10.1016/0014-2999(89)90095-2. [DOI] [PubMed] [Google Scholar]
- 31.Renzi A., DeMorrow S., Onori P., Carpino G., Mancinelli R., Meng F., Venter J., White M., Franchitto A., Francis H., Han Y., Ueno Y., Dusio G., Jensen K.J., Greene J.J., Jr., Glaser S., Gaudio E., Alpini G. Modulation of the biliary expression of arylalkylamine N-acetyltransferase alters the autocrine proliferative responses of cholangiocytes in rats. Hepatology. 2013;57:1130–1141. doi: 10.1002/hep.26105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutenburg A.M., Kim H., Fischbein J.W., Hanker J.S., Wasserkrug H.L., Seligman A.M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969;17:517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- 33.Ishii M., Vroman B., LaRusso N.F. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989;97:1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- 34.Renzi A., Glaser S., DeMorrow S., Mancinelli R., Meng F., Franchitto A., Venter J., White M., Francis H., Han Y., Alvaro D., Gaudio E., Carpino G., Ueno Y., Onori P., Alpini G. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol. 2011;301:G634–G643. doi: 10.1152/ajpgi.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueno Y., Alpini G., Yahagi K., Kanno N., Moritoki Y., Fukushima K., Glaser S., LeSage G., Shimosegawa T. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int. 2003;23:449–459. doi: 10.1111/j.1478-3231.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- 36.DeMorrow S., Glaser S., Francis H., Venter J., Vaculin B., Vaculin S., Alpini G. Opposing actions of endocannabinoids on cholangiocarcinoma growth: recruitment of Fas and Fas ligand to lipid rafts. J Biol Chem. 2007;282:13098–13113. doi: 10.1074/jbc.M608238200. [DOI] [PubMed] [Google Scholar]
- 37.DeMorrow S., Francis H., Gaudio E., Ueno Y., Venter J., Onori P., Franchitto A., Vaculin B., Vaculin S., Alpini G. Anandamide inhibits cholangiocyte hyperplastic proliferation via activation of thioredoxin 1/redox factor 1 and AP-1 activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G506–G519. doi: 10.1152/ajpgi.00304.2007. [DOI] [PubMed] [Google Scholar]
- 38.Han Y., Onori P., Meng F., DeMorrow S., Venter J., Francis H., Franchitto A., Ray D., Kennedy L., Greene J., Renzi A., Mancinelli R., Gaudio E., Glaser S., Alpini G. Prolonged exposure of cholestatic rats to complete dark inhibits biliary hyperplasia and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G894–G904. doi: 10.1152/ajpgi.00288.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato A., Gores G.J., LaRusso N.F. Secretin stimulates exocytosis in isolated bile duct epithelial cells by a cyclic AMP-mediated mechanism. J Biol Chem. 1992;267:15523–15529. [PubMed] [Google Scholar]
- 40.Shahabi S., Jorsaraei S.G., Moghadamnia A.A., Zabihi E., Aghajanpour S.M., Mousavi Kani S.N., Pourbagher R., Hosseini S.A., Esmaili M., Yoonesi A.A., Zarghami A., Alinezhad F. Central effects of camphor on GnRH and sexual hormones in male rat. Int J Mol Cell Med. 2012;1:191–196. [PMC free article] [PubMed] [Google Scholar]
- 41.Tanriverdi F., Gonzalez-Martinez D., Hu Y., Kelestimur F., Bouloux P.M. GnRH-I and GnRH-II have differential modulatory effects on human peripheral blood mononuclear cell proliferation and interleukin-2 receptor gamma-chain mRNA expression in healthy males. Clin Exp Immunol. 2005;142:103–110. doi: 10.1111/j.1365-2249.2005.02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francis H., Glaser S., Ueno Y., LeSage G., Marucci L., Benedetti A., Taffetani S., Marzioni M., Alvaro D., Venter J., Reichenbach R., Fava G., Phinizy J.L., Alpini G. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–537. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg P., Sjostrom M., Soderberg C., Kinnman N., Stal P., Hultcrantz R. Attenuated liver fibrosis after bile duct ligation and defective hepatic stellate cell activation in neural cell adhesion molecule knockout mice. Liver Int. 2011;31:630–641. doi: 10.1111/j.1478-3231.2011.02486.x. [DOI] [PubMed] [Google Scholar]
- 44.Marzioni M., Glaser S., Francis H., Marucci L., Benedetti A., Alvaro D., Taffetani S., Ueno Y., Roskams T., Phinizy J.L., Venter J., Fava G., LeSage G., Alpini G. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology. 2005;128:121–137. doi: 10.1053/j.gastro.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Gaudio E., Barbaro B., Alvaro D., Glaser S., Francis H., Ueno Y., Meininger C.J., Franchitto A., Onori P., Marzioni M., Taffetani S., Fava G., Stoica G., Venter J., Reichenbach R., De Morrow S., Summers R., Alpini G. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q., Xiao X., Li M., Li W., Yu M., Zhang H., Ping F., Wang Z., Zheng J. Berberine moderates glucose metabolism through the GnRH-GLP-1 and MAPK pathways in the intestine. BMC Complement Altern Med. 2014;14:188. doi: 10.1186/1472-6882-14-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benz N., Le Hir S., Norez C., Kerbiriou M., Calvez M.L., Becq F., Trouve P., Ferec C. Improvement of chloride transport defect by gonadotropin-releasing hormone (GnRH) in cystic fibrosis epithelial cells. PLoS One. 2014;9:e88964. doi: 10.1371/journal.pone.0088964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schang A.L., Querat B., Simon V., Garrel G., Bleux C., Counis R., Cohen-Tannoudji J., Laverriere J.N. Mechanisms underlying the tissue-specific and regulated activity of the Gnrhr promoter in mammals. Front Endocrinol. 2012;3:162. doi: 10.3389/fendo.2012.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grundker C., Gunthert A.R., Millar R.P., Emons G. Expression of gonadotropin-releasing hormone II (GnRH-II) receptor in human endometrial and ovarian cancer cells and effects of GnRH-II on tumor cell proliferation. J Clin Endocrinol Metab. 2002;87:1427–1430. doi: 10.1210/jcem.87.3.8437. [DOI] [PubMed] [Google Scholar]
- 50.Lariviere S., Garrel G., Simon V., Soh J.W., Laverriere J.N., Counis R., Cohen-Tannoudji J. Gonadotropin-releasing hormone couples to 3′,5′-cyclic adenosine-5′-monophosphate pathway through novel protein kinase Cdelta and -epsilon in LbetaT2 gonadotrope cells. Endocrinology. 2007;148:1099–1107. doi: 10.1210/en.2006-1473. [DOI] [PubMed] [Google Scholar]
- 51.Tipsmark C.K., Weber G.M., Strom C.N., Grau E.G., Hirano T., Borski R.J. Involvement of phospholipase C and intracellular calcium signaling in the gonadotropin-releasing hormone regulation of prolactin release from lactotrophs of tilapia (Oreochromis mossambicus) Gen Comp Endocrinol. 2005;142:227–233. doi: 10.1016/j.ygcen.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Wang L., Xie L.P., Huang W.Q., Yao B., Pu R.L., Zhang R.Q. Presence of gonadotropin-releasing hormone (GnRH) and its mRNA in rat pancreas. Mol Cell Endocrinol. 2001;172:185–191. doi: 10.1016/s0303-7207(00)00369-5. [DOI] [PubMed] [Google Scholar]
- 53.Hammar O., Ohlsson B., Veress B., Alm R., Fredrikson G.N., Montgomery A. Depletion of enteric gonadotropin-releasing hormone is found in a few patients suffering from severe gastrointestinal dysmotility. Scand J Gastroenterol. 2012;47:1165–1173. doi: 10.3109/00365521.2012.706826. [DOI] [PubMed] [Google Scholar]