Abstract
Although keratosis pilaris (KP) is common, its etiopathogenesis remains unknown. KP is associated clinically with ichthyosis vulgaris and atopic dermatitis and molecular genetically with filaggrin-null mutations. In 20 KP patients and 20 matched controls, we assessed the filaggrin and claudin 1 genotypes, the phenotypes by dermatoscopy, and the morphology by light and transmission electron microscopy. Thirty-five percent of KP patients displayed filaggrin mutations, demonstrating that filaggrin mutations only partially account for the KP phenotype. Major histologic and dermatoscopic findings of KP were hyperkeratosis, hypergranulosis, mild T helper cell type 1-dominant lymphocytic inflammation, plugging of follicular orifices, striking absence of sebaceous glands, and hair shaft abnormalities in KP lesions but not in unaffected skin sites. Changes in barrier function and abnormal paracellular permeability were found in both interfollicular and follicular stratum corneum of lesional KP, which correlated ultrastructurally with impaired extracellular lamellar bilayer maturation and organization. All these features were independent of filaggrin genotype. Moreover, ultrastructure of corneodesmosomes and tight junctions appeared normal, immunohistochemistry for claudin 1 showed no reduction in protein amounts, and molecular analysis of claudin 1 was unremarkable. Our findings suggest that absence of sebaceous glands is an early step in KP pathogenesis, resulting in downstream hair shaft and epithelial barrier abnormalities.
Keratosis pilaris (KP) is a disorder of keratinization, characterized by prominent keratinous plugging of follicular orifices, and various degrees of perifollicular erythema.1–3 Although the proximal extremities are a common area of involvement, other sites of predilection include the buttocks, trunk, and face.1,2 The onset of KP usually occurs during the first two decades of life with a peak at puberty,4 followed by either improvement with increasing age in 35%, persistence into adulthood in 43%, or worsening in 22% of the patients.5 Prevalence estimates for KP range between 4% and 34%,6,7 and earlier studies report a prevalence up to 80% in British girls,8 demonstrating that the KP phenotype is common.
Although family studies suggest an autosomal dominant pattern of inheritance,5 no single gene mutation has yet been linked to KP. KP is associated with atopic dermatitis in large population samples,1,4,5,7 ichthyosis vulgaris, and hence filaggrin (FLG) mutations.7,9–12
The clinical expression of KP is likely determined not only by genetic predisposition but also by environmental factors. Some persons with KP experience improvement during the summer, compared with winter months, suggesting a role for reduced environmental humidity in disease expression.4,5 A stronger association was found between FLG mutations and KP in populations residing in colder and drier temperate zones than in equatorial populations.13 An acquired increase in the incidence of KP-like lesions is observed in obesity, diabetes, pregnancy, menopause, and malnutrition, particularly in association with vitamin A deficiency.14–18 Treatment of KP has focused on preventing excessive skin dryness with moisturizers, softening and thinning the follicular plugs with keratolytic agents, peelings, or topical retinoids, and/or decreasing associated erythema with low-potency topical steroids.2,19
The pathogenesis of KP remains unknown, and the literature about this topic is limited. Because both ichthyosis vulgaris and atopic dermatitis are strongly associated with KP,9–11 it is possible that the follicular abnormalities observed in KP result from FLG deficiency. FLG, one major structural protein of the epidermis, not only aggregates keratin filaments into corneocytes but also is hydrolyzed into osmotically active amino acids, forming approximately 50% of the natural moisturizing factor of the stratum corneum (SC), and playing a key role in photoprotection and acidifying the skin surface.20,21 Null mutations in FLG result in reduced natural moisturizing factor in the SC, leading to xerosis cutis and epithelial barrier abnormality.20,22,23
In normal epidermis, the permeability barrier is formed by corneocytes surrounded by the hydrophobic extracellular lamellar bilayers of the SC, which derive from the secretion of lamellar body (LB) contents, with an additional potential role of tight junctions (TJs) of the subjacent stratum granulosum (SG).24 Corneodesmosomes regulate the integrity and cohesion of the SC.25 Together, these structures provide the epidermis with a formidable barrier against the outward loss of water and electrolytes, while also preventing transcutaneous entry of exogenous xenobiotics.26–28
We assessed here the FLG genotype of 20 KP patients and the morphology of KP skin by light and electron microscopy. Furthermore, we asked whether KP is associated with changes in epidermal structure and whether the putative barrier impairment is only observed in KP patients with FLG null mutations.
Materials and Methods
Human Subjects and Dermatoscopic Evaluation
The study was approved by the institutional review boards of the Innsbruck Medical University and the University of California, San Francisco, and complied with the Declaration of Helsinki Principles. Twenty adult Caucasians with KP, defined as rough folliculocentric keratotic papules on the proximal upper arms, thighs, and buttocks, and 20 matched healthy controls (all displayed Fitzpatrick skin pigment phenotype I-II or II) were included between June and July 2011 (Table 1). All subjects provided written informed consent before enrollment. Ten subjects lived in coastal California and 10 patients in the temperate region of Austria. Specifically excluded were persons with KP and ichthyosis vulgaris and/or atopic dermatitis, endocrinologic disorders, and patients receiving systemic immunosuppressives or retinoids. Skin examination and dermatoscopy, using PhotoMAX Plus digital image capture device (Equipmed, London, UK), were performed by experienced dermatologists.
Table 1.
Demographic Characteristics
| Characteristics | Keratosis pilaris patients | Controls |
|---|---|---|
| Age, years, median (range) | 31 (22–69) | 33 (23–76) |
| Sex, n = 20 | 12 females, 8 males | 11 females, 9 males |
| Frequency of filaggrin mutations, % | 35 | 5 |
| Subjects with specific mutation, n | ||
| 2282del4/2282del4 | 1 | |
| 2282del4/wt | 4 | |
| R501∗/wt | 2 | |
| R2447∗/wt | 1 |
Genotyping
Genomic DNA was extracted from EDTA-blood by using the GenoM48-automated-extractor (Qiagen, Vienna, Austria) or from saliva according to the manufacturer's instructions (Oragene DNA Self-Collection Kit; DNA Genotek Inc., Kanata, ON, Canada). European FLG variants 2282del4, R501∗, R2447∗, and S3247∗ were screened by allele-specific PCR; FLG variants 3702delG and 6867delAG were screened by sizing of fluorescently labeled PCR products.29,30 Claudin 1 gene (CLDN1) sequence analysis was done by amplifying each exon and exon/intron boundaries (http://www.ncbi.nlm.nih.gov/gene; Accession no. NM_021101.4) by PCR. Products were directly sequenced with the BigDye Terminator-v.1.1 Cycle-Sequencing Kit on a 3730 DNA Analyzer (Applied Biosystems, Foster City, CA). Primer sequences are as follows: exon 1 forward primer, 5′-CCCGACCCAGAGCTTCTC-3′, and reverse primer, 5′-GCTTTCCTCAAACCAGGATTC-3′; exon 2 forward primer, 5′-CCAGCGGAAACATCAGTATG-3′, and reverse primer, 5′-TTGCAGTTTGCCTTAGAGACTG-3′; exon 3 forward primer, 5′-GGACTTCTAATCTCCCTAATACC-3′, and reverse primer, 5′-CCCAGTTATACAGTTGAAAAGC-3′; exon 4 forward primer, 5′-GGGATATTCAGGGGTTATTTTC-3′, and reverse primer, 5′-TTAAGCCATGTTTAGCACTGAG-3′.
Functional Measurements
Measurements were performed on all 40 study subjects on the dorsal site of the upper arm (lesional KP) and the ventral site of the upper arm (nonlesional KP) (Supplemental Table S1). Subjects were not allowed to use topical formulations on the test sites at least 3 days before the experiments. Skin surface pH was measured with a Skin-pH-Meter, SC hydration with a Corneometer (Courage & Khazaka, Cologne, Germany), and is shown as a change in electrical capacitance (absolute units). Transepidermal water loss was performed with an Evaporimeter (Servomed, Stockholm, Sweden). Subjects underwent at least 20 seconds of preassessment rest period. Measurements were taken in accordance with published guidelines.31–33 Environment-related variables were ambient air temperature of 20°C to 24°C, skin surface temperature of 30.5°C to 32.5°C, ambient air humidity of 35% to 45%, and water vapor pressure of 4.7 to 10.9 mmHg.
Light Microscopy and Immunohistochemistry
Punch biopsies were taken from lesional and nonlesional KP sites of the upper arm in 13 study subjects and five controls (Supplemental Table S1), fixed in 4% formaldehyde, paraffin embedded, sectioned, and stained with hematoxylin and eosin. Semithin sections for electron microscopy were dyed with toluidin blue. For immunohistochemistry, paraffin-embedded sections were stained with monoclonal mouse/anti-human FLG antibody (dilution 1:50; Novocastra Laboratories Ltd., Newcastle on Tyne, UK), using the automated preparation system BenchMark XT (Ventana Medical Systems, Tucson, AZ) and the high-temperature antigen unmasking technique according to the manufacturer's instructions. For TJ protein CLDN1, sections were processed as described,34 except for a higher dilution of CLDN1 antibody (dilution 1:5000).
Images were generated with the laser optic system ProgRes-C10plus (Jenoptik, Jena, Germany) and PicEd-Cora software (Windows 7) or an Axiophot-II microscope (Carl Zeiss, Goettingen, Germany) and an Openlab software version 2.0.9 (Improvision, Coventry, UK).
Transmission Electron Microscopy
Punch biopsies were taken from the extensor site of the upper arm in 10 subjects and five controls (Supplemental Table S1). For evaluation of ultrastructure we used an algorithmic sequence similar to that previously applied to different ichthyoses.29,35–40 The combination of reduced osmium tetroxide and ruthenium tetroxide postfixation protocols with pyridine pretreatment and acid lipase visualization allowed assessment of the cornified envelope scaffold in relation to the extracellular lamellar bilayer system and LB secretory system.29,35,41–43 The permeability pathway was assessed by colloidal lanthanum nitrate.36,44,45 Ultrathin sections (600 Å) were mounted on Formvar-coated grids, counterstained with uranyl acetate and lead nitrate, and examined in a Jeol-JEM100CX electron microscope (80 kV; JEOL Ltd., Tokyo, Japan).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 5 software (GraphPad Inc., San Diego, CA). Numeric results were analyzed with the Mann-Whitney rank sum test to compare individual groups and the Kruskal-Wallis test for across-group significance. P < 0.05 was considered statistically significant. All graphs show means ± SEM. For ultrastructural analysis, two observers (R.G. and D.C.) were blinded to the case-control status of the study subjects.
Results
Phenotypical Variation and Low Prevalence of FLG Mutations in KP
In lesional KP sites, 13 patients (65%) exhibited mild and 7 patients (35%) exhibited moderate cutaneous xerosis. Three patients (15%) showed mild, 13 patients (65%) moderate, and 4 patients (20%) severe follicular papules, whereas 10 patients (50%) featured mild, 9 patients (45%) moderate, and 1 patient (5%) prominent erythema (Figure 1, A and B, and Supplemental Table S1). These findings were independent of FLG genotype. Seven of 20 KP patients (35%) displayed common FLG mutations (one homozygote for c.2282del4, four heterozygotes for c.2282del4, two heterozygotes for p.R501∗), 13 KP patients (65%), and 19 controls (95%) did not show a common FLG mutation, whereas one control (5%) was heterozygous for the FLG mutation p.R2447∗ (Table 1). In summary, common null mutations in FLG were present in approximately one-third of our KP cohort, which was a significantly higher frequency than in controls.
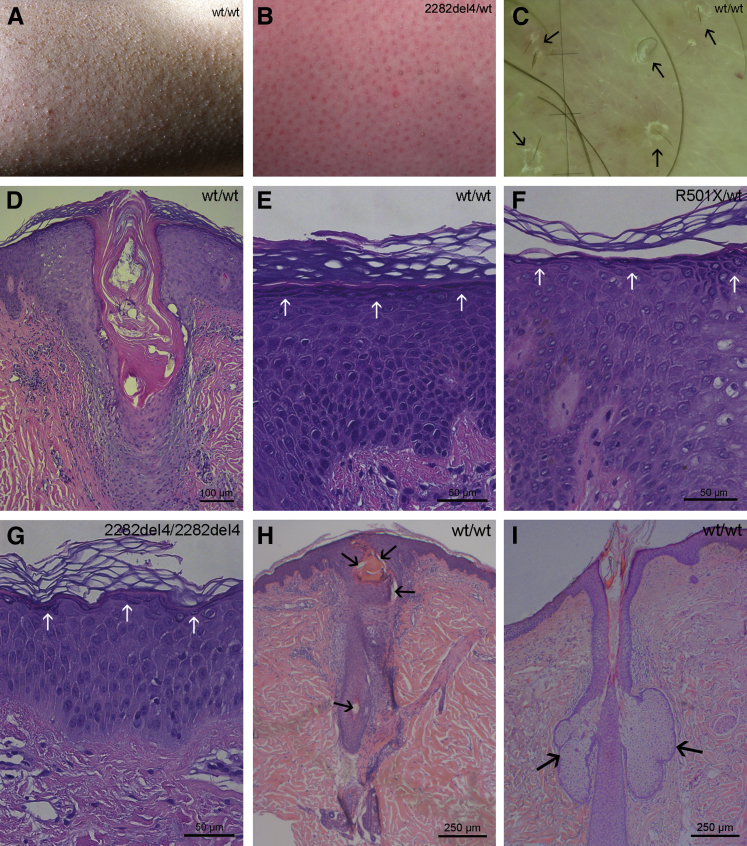
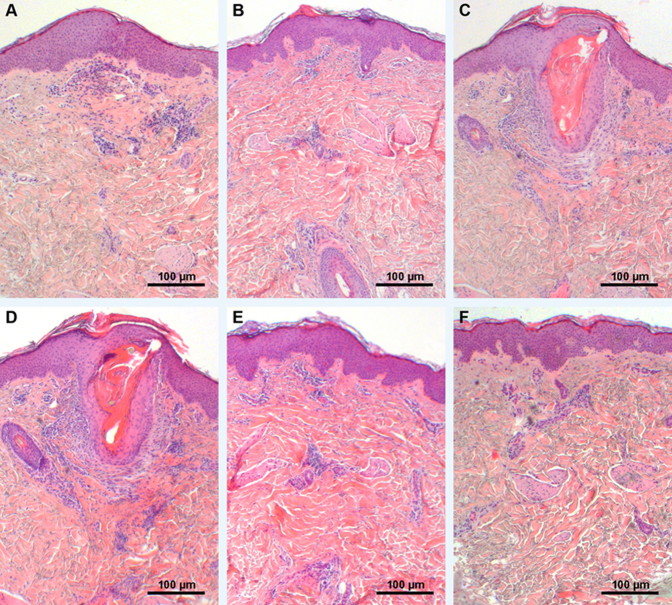
Figure 1.
Phenotypical, dermatoscopic, and histologic abnormalities in KP. A: Moderate xerosis and follicular papules and mild perifollicular rim of erythema. B: Mild xerosis and follicular papules but moderate erythema. C: Dermatoscopy of lesional KP with short, thin, and coiled hair shafts (black arrows). D–H: Hyperkeratosis, restrained acanthosis, marked plugging of follicular orifices, absence of sebaceous glands, and mild dermal lymphocytic infiltration in all KP specimens, regardless of FLG mutation status. Although KP mostly shows hypergranulosis (E, white arrows), two FLG single-allele mutant subjects presented a normal SG (F, white arrows) and one FLG double-allele mutant an attenuated SG (G, white arrows). H: Absence of sebaceous glands in lesional KP. Note the semicircular hair shaft embedded superficially within the SC (black arrows). I: Presence of sebaceous glands (black arrows) in nonlesional KP. Stained with hematoxylin and eosin (D–G) and toluidine blue (H and I). FLG, filaggrin; KP, keratosis pilaris; SG, stratum granulosum; WT, wild-type.
Histologic and Dermatoscopic Abnormalities in KP
Histology displayed hyperkeratosis, slight acanthosis, marked plugging of individual follicular orifices, and mild-to-moderate dermal lymphocytic infiltration, without eosinophils or mast cells in all KP specimens, regardless of FLG mutation status (Figure 1, D–I). Serial sections of KP lesions showed a striking absence of sebaceous glands (Figure 1, D and H, and Supplemental Figure S1), but normal-appearing sebaceous glands were present in uninvolved skin sites from the same subjects (Figure 1I). In addition, hematoxylin and eosin staining (Figure 1E) and immunohistochemistry for FLG (Supplemental Figure S2) showed hypergranulosis in most lesional KP, with the exception of one homozygote subject for the FLG mutation c.2282del4 and two of the heterozygote subjects (for mutation p.R501∗ and 2282del4), who demonstrated a clearly attenuated (Figure 1G) and a normal SG layer (Figure 1F), respectively.
Dermatoscopy of the papules in lesional KP sites revealed abnormalities of hair shaft structure in most terminal follicles in all patients. Hair shafts were thin and short, coiled or semicircular, and sometimes embedded superficially within the SC (Figure 1C and Supplemental Table S1). In follicles with only small keratinous plugs and no rim of erythema, which are occasionally found in larger fields of KP, normal hair shafts were present. Perifollicular scaling was seen in seven (35%) of the KP patients, and various degrees of erythema were identified in all patients. Thus, both characteristic histologic and dermatoscopic abnormalities were observed in KP lesions.
Changes in Epidermal Function in KP
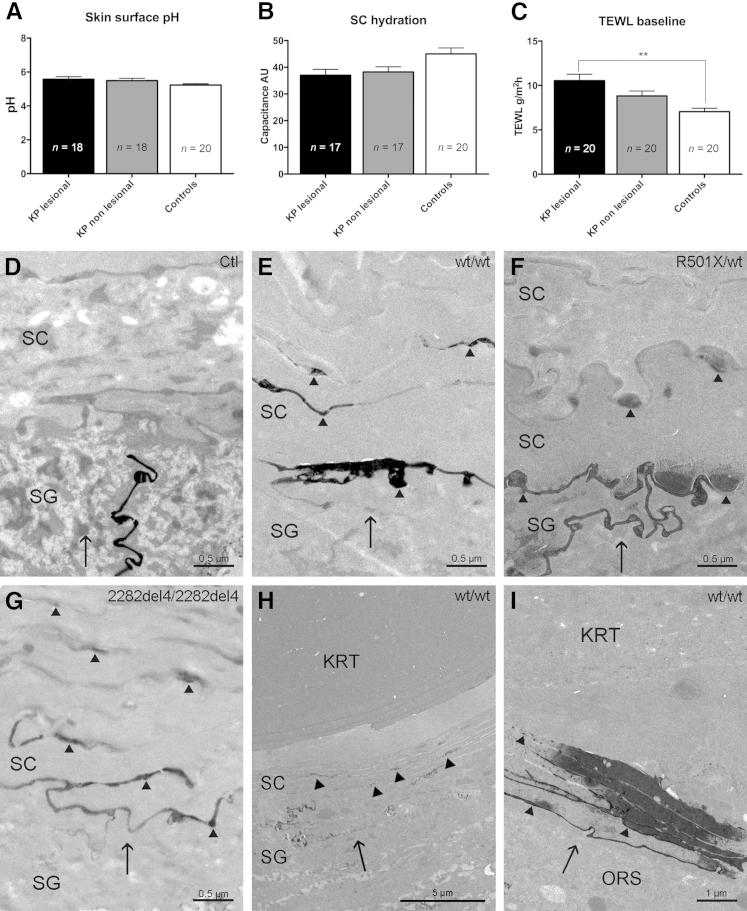
In our KP cohort, skin surface pH was statistically normal (Figure 2A), although patients with FLG-deficient epidermis showed a tendency toward higher pH values in both lesional and uninvolved skin. SC hydration seemed decreased in KP patients compared with controls, in particular in lesional skin, but this was not statistically significant (Figure 2B). Baseline transepidermal water loss rates were elevated in lesional skin compared with uninvolved skin in the same KP patients and significantly increased in comparison with controls (Figure 2C).
Figure 2.
Changes in epidermal function and abnormal paracellular barrier defect in KP. A: Normal skin surface pH in KP. B: Normal SC hydration in KP. C: Significantly increased TEWL in lesional KP versus controls. D–I: Lanthanum with osmium tetroxide postfixation. D: Exclusion of lanthanum tracer from both the interfollicular SC and the infundibular SC of the hair follicles in controls. E–G: Moderate tracer movement through and above the SG-SC interface by a paracellular route in WT and FLG-deficient KP. H: Lanthanum leakage in the infundibular SC in KP. I: Lanthanum leakage in the ORS of the follicular profound segments in KP. Black arrows indicate direction of tracer movement; arrowheads, lanthanum within intercellular spaces. Values are expressed as means ± SEM. ∗∗P < 0.01. AU, arbitrary unit; FLG, filaggrin; KP, keratosis pilaris; KRT, keratin; ORS, outer root sheath; SC, stratum corneum; SG, stratum granulosum; TEWL, transepidermal water loss; WT, wild-type.
The perfusion pathway of colloidal lanthanum nitrate was evaluated in KP skin. Although in controls lanthanum was excluded from the interfollicular SC and from the SC of hair follicle infundibula (Figure 2D), tracer movement was detectable throughout and above the SG-SC interface by a paracellular route in KP (Figure 2, E–H). The extent of lanthanum leakage did not correlate with the presence of a FLG genotype. Although one FLG wild-type patient and one FLG-deficient patient showed severe leakage, respectively, three FLG wild-type patients and three FLG-deficient patients showed moderate leakage, two FLG wild-type patients showed mild leakage, and no leakage was seen in four controls (Supplemental Table S2). Similar to these findings, lanthanum leakage was also obvious below the hair follicle infundibula in KP (Figure 2I and Supplemental Table S2). Together, these results indicate a paracellular permeability barrier abnormality of the interfollicular and follicular SC in lesional KP, independent of FLG genotype.
Basis for the Abnormal Permeability Barrier in KP
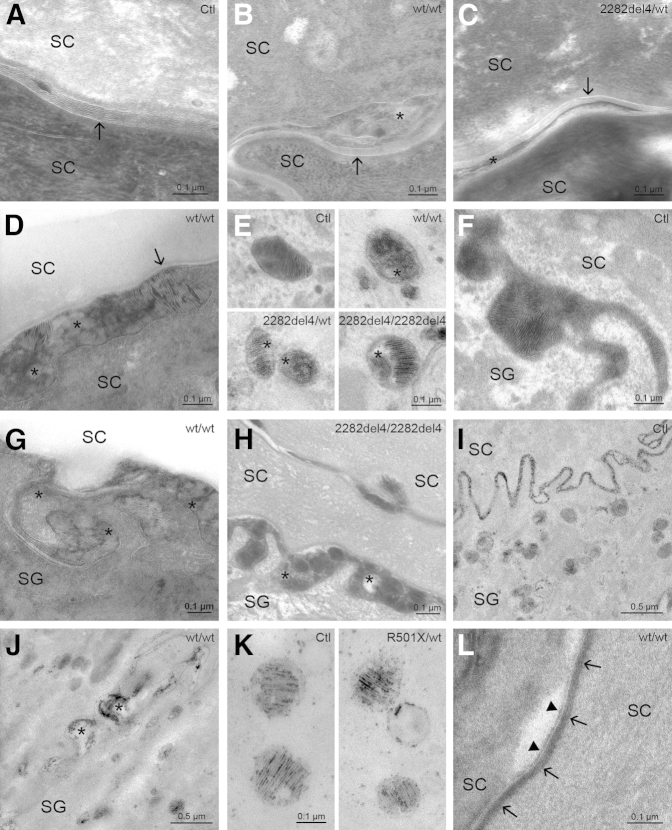
Paracellular permeability barrier impairment correlated with abnormalities in the maturation and organization of the extracellular lamellar bilayer system, assessed by electron microscopy (osmium tetroxide and ruthenium tetroxide postfixation). In all controls and in nonlesional skin sites of 17 KP patients (85%), LB-derived membrane structures were secreted at the SG-SC interface, and mature lamellar bilayers occurred one cell layer above the SG-SC interface in both the interfollicular epidermis and the infundibular epithelium (Figure 3A). In contrast, in the SC of lesional KP, and to a lesser extent in nonlesional skin of three KP patients with FLG mutations (15%), lamellar membrane arrays were disrupted by nonlamellar domains (Figure 3, B and C). In addition, evidence suggested delayed processing of secreted lipids in the interfollicular epidermis and between corneocytes in the upper parts of hair follicles (Figure 3D). Although LB density seemed normal in KP with and without FLG mutations, all KP patients displayed aberrant LB internal structures (Figure 3E), suggesting defective loading into the organelles. Secretion of LB contents appeared inhomogeneous in KP (Figure 3, G and H) compared to controls (Figure 3F). LB entombment, suggesting entrapment of nonsecreted LB contents within corneocytes, was seen in approximately 50% of patients with KP (Supplemental Table S2). In normal SC, the LB content marker, acid lipase, dispersed homogeneously within the SC interstices (Figure 3I). However, enzyme activity was nonuniform within the extracellular SC spaces in KP (Figure 3J). Concentration of acid lipase within LBs was normal in both KP and controls (Figure 3K). Assessment of cornified envelopes and corneocyte lipid envelopes, using the absolute pyridine technique,46 was normal in KP (Figure 3L). These findings show that abnormalities in permeability barrier function in KP likely reflect an impairment in lamellar bilayer architecture.
Figure 3.
Ultrastructural abnormalities are independent of FLG genotype in KP. A: Mature lamellar bilayers (arrow) one cell layer above the SG-SC interface in controls. B and C: In contrast, membrane arrays (arrows) were disrupted by nonlamellar domains (asterisks) in WT and FLG-deficient KP. D: Delayed postsecretory lipid processing, with both immature (asterisks) and mature (arrow) lamellar material above the SG-SC interface in KP. E: Partially empty or amorphous LB contents (asterisk) in KP. F: Normal LB secretion in controls. G and H: Inhomogeneous LB secretion, showing foci of nonlamellar, vesicular contents (asterisks) at the SG-SC interface in WT and FLG-deficient KP. I:In toto secretion of acid lipase and homogeneous dispersion within the SC interstices in controls. J: Nonuniform distribution of enzyme activity (asterisks) within the extracellular spaces in KP. K: Regular concentration of acid lipase within LBs in controls and KP. L: Normal cornified envelope (arrows) and corneocyte lipid envelope (arrowheads) in the mid SC of KP. Transmission electron microscopy: ruthenium tetroxide postfixation (A–D and G), pretreatment with pyridine (L) or acid lipase (I–K), and osmium tetroxide postfixation (E, F, and H). Clt, control; FLG, filaggrin; KP, keratosis pilaris; LB, lamellar body; SC, stratum corneum; SG, stratum granulosum; WT, wild-type.
Normal Corneodesmosomes and CLDN1 in KP
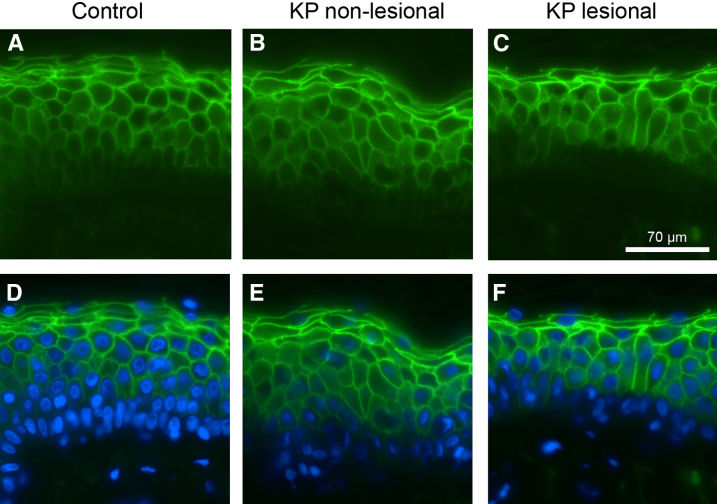
Both the density and the internal structure of corneodesmosomes were comparable in KP and control subjects (Supplemental Table S2). Although the lanthanum leakage reported in Changes in Epidermal Function in KP could result in part from a deficiency of TJ-like structures, these appeared ultrastructurally normal in KP. Immunohistochemical staining for a major TJ protein, CLDN1, which contributes to TJ resistance and reduced epidermal permeability,47,48 showed no reduction in protein amounts in both lesional and nonlesional KP (Figure 4). Finally, sequence analysis of CLDN1 in all KP patients did not reveal any disease-related mutations.
Figure 4.
Claudin 1 localization in KP. Epifluorescence images of claudin 1 (green) in control skin (A and D) and in nonlesional (B and E) and lesional skin (C and F) from patients with KP. D–F: Staining of cell nuclei with DAPI (blue). KP, keratosis pilaris.
Discussion
Although a widely recognized hypothesis of KP pathogenesis suggests that the papules are caused by excessive accumulation of keratin debris within the follicular orifices,1–5 it is uncertain if this is a primary or a secondary feature. Interestingly, we found a striking absence of sebaceous glands in all KP lesions. This absence of sebaceous glands was previously described by Giovannini49 in 1902 but fell into oblivion until our present study. Early KP lesions, characterized by small keratinous plugs and no hair shaft abnormalities, showed atrophic sebaceous glands. Yet, sebaceous glands appeared normal in nonlesional KP and controls. The resulting paucity of sebaceous gland-derived products may lead to defective corneocyte shedding from infundibula, hyperkeratinization of the acroinfundibula, and hair shaft abnormalities. Our findings confirm data that show coiled hair shafts within affected follicular infundibula in KP3 and studies in the asebia-2J mouse that highlight the importance of sebaceous glands in hair fiber sheath dissociation.50 In the asebia mutant mouse, characterized by a lack in stearoyl-CoA desaturase 1, which desaturates secreted fatty acids, rudimentary sebaceous glands and changes in skin surface lipids lead to chronic inflammation, followed by follicle loss and scarring.51 However, patients with KP do not develop scarring alopecia. Our study does not include the spectrum of KP atrophicans, such as KP atrophicans faciei, atrophoderma vermiculatum, keratosis follicularis spinulosa decalvans, or folliculitis spinulosa decalvans. All disorders of follicular keratinization may be part of a broad spectrum, in which the sebaceous gland plays the central role. Yet, it is not known why some disorders of follicular keratinization result in severe scarring alopecias, whereas KP shows a mild course. Further studies are needed to address this question.
The inflammation seen in KP could be caused by repeated mechanical irritation of the hyperkeratotic plugs, including scratching, but also by a decrease in antimicrobial peptides with accreted bacteria colonization, due to loss of sebaceous gland-derived antimicrobial peptides.52 In addition, an increase in skin surface pH due to reduced levels of natural moisturizing factor, as occurs in FLG-deficient epidermis, could facilitate bacterial colonization and inflammation. It is possible that multipotent hair bulge stem cells fail to regenerate sebaceous glands in KP, instead they could differentiate into interfollicular epidermal cells to compensate for a damaged barrier.53 Likely, the acanthosis and hypergranulosis that we observed in KP reflect hyperplasia due to barrier abnormalities. The abnormal barrier in KP could thus be a precondition for the development of atopic dermatitis, because it allows increased allergen ingress through the epidermis, followed by an initiation of immune responses mediated by type 2 T helper cells. The association of atopic dermatitis with KP could result from this pathogenic sequence. Histologic findings and transepidermal water loss measurements show an absence of sebaceous glands and an abnormal permeability barrier only in involved KP skin, but not in nonlesional skin sites of the same subjects, indicating that a lack of sebaceous glands is likely a secondary phenomenon and that it seems unlikely that a mutation in a single gene causes KP.
It was reported previously that in most patients with ichthyosis vulgaris sebaceous glands were absent or small in biopsies from the upper arms54,55; however, in another study the frequency of sebaceous glands in biopsies from the lateral surface of the upper arms from ichthyosis vulgaris was not considered to deviate from normal.56 This conflicting result could be explained by the strong association of ichthyosis vulgaris with KP, because these studies did not phenotypically distinguish between ichthyosis vulgaris with or without concomitant KP, and all biopsies were taken from specific KP sites.
Because of the observed lanthanum tracer leakage in lesional interfollicular and follicular epidermis in KP, we explored abnormalities in corneodesmosomes and TJs, which were previously described in ichthyoses and atopic dermatitis.26,29,57 Corneodesmosomes and TJs appeared normal under the electron microscope, and further immunohistochemical staining for CLDN1 and analysis of CLDN1 were unremarkable. However, because CLDN1 is not the only TJ protein, we cannot exclude that there might be a down-regulation in other TJ proteins.
In our study cohort, 35% of KP patients displayed common null mutations in FLG, which is much higher than the averaged carrier frequency of 4% to 9% in Europe.30,58,59 Even when taking into account the possibility that we could have missed uncommon FLG mutations, we still conclude that FLG mutations alone are unlikely to be solely responsible for the KP phenotype. However, FLG-deficient skin is at higher risk of developing KP, and this could be explained as follows. Epithelial barrier abnormalities caused by FLG mutations may lead to increased serum vitamin D3 concentrations,60 which may exert antiproliferative effects on sebocytes,61 potentially resulting in atrophy of sebaceous glands. Yet, FLG wild-type skin can also show KP in the presence of several environmental and hormonal factors. There is evidence that decreased sebocyte proliferation, with ensuing hypofunction of sebaceous glands, follows from reduced amounts of IGF-1, insulin, or decreased activation of peroxisome proliferator-activated receptor α and peroxisome proliferator-activated receptor γ1 in sebocytes, as reported for atopic dermatitis.53,62 In addition, low environmental humidities in cold and dry temperate zones, aging, and decreased serum androgens, estrogens, and cortisol can all cause xerotic skin.4,5,13,14,53
Why KP lesions mainly occur on the proximal dorsal extremities and on the buttocks, but infrequently on the trunk and face,1,2 was not clarified in this work. However, in disorders of cornification such as ichthyosis vulgaris or mild forms of autosomal recessive congenital ichthyoses, the predilection sites of scaling are often also on the extensor surfaces of the extremities and to a less degree on the face.63 This suggests that KP develops on body sites with higher levels of skin dryness and not on sites with a high sebum production such as the seborrheic area.
In summary, in KP atrophy/absence of sebaceous glands is an important early feature, resulting in follicular plugging, downstream hair shaft defects, and epithelial barrier abnormalities.
Acknowledgments
We thank the patients and control subjects for participating in this study and Ewa Wladykowski and Germar Schüring for skillful technical assistance.
Footnotes
Supported by MFF Tirol grant 194, EU-FP7 COST ActionBM0903, the Rene Touraine Foundation, NIH grants AR051930 and AG028492, and an unrestricted fellowship from the Lundbeck Foundation (J.P.T.).
R.G. and J.L.S. contributed equally to this work.
Disclosures: None declared.
Supplemental Data
Supplemental Figure S1.
A–F: Serial sections through a papule of lesional keratosis pilaris from the upper arm show a complete absence of sebaceous glands. Stained with hematoxylin and eosin.
Immunohistochemistry for filaggrin shows hypergranulosis (arrows) in WT keratosis pilaris. Paraffin-embedded sections were stained with monoclonal mouse/anti-human FLG antibody (dilution 1:50). FLG, filaggrin; WT, wild-type.
References
- 1.Hwang S., Schwartz R.A. Keratosis pilaris: a common follicular hyperkeratosis. Cutis. 2008;82:177–180. [PubMed] [Google Scholar]
- 2.Lateef A., Schwartz R.A. Keratosis pilaris. Cutis. 1999;63:205–207. [PubMed] [Google Scholar]
- 3.Thomas M., Khopkar U.S. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255–258. doi: 10.4103/0974-7753.111215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman L. Keratosis pilaris. Br J Dermatol. 1954;66:279–282. doi: 10.1111/j.1365-2133.1954.tb12634.x. [DOI] [PubMed] [Google Scholar]
- 5.Poskitt L., Wilkinson J.D. Natural history of keratosis pilaris. Br J Dermatol. 1994;130:711–713. doi: 10.1111/j.1365-2133.1994.tb03406.x. [DOI] [PubMed] [Google Scholar]
- 6.Popescu R., Popescu C.M., Williams H.C., Forsea D. The prevalence of skin conditions in Romanian school children. Br J Dermatol. 1999;140:891–896. doi: 10.1046/j.1365-2133.1999.02821.x. [DOI] [PubMed] [Google Scholar]
- 7.Brown S.J., Relton C.L., Liao H., Zhao Y., Sandilands A., McLean W.H., Cordell H.J., Reynolds N.J. Filaggrin haploinsufficiency is highly penetrant and is associated with increased severity of eczema: further delineation of the skin phenotype in a prospective epidemiological study of 792 school children. Br J Dermatol. 2009;161:884–889. doi: 10.1111/j.1365-2133.2009.09339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stannus H.S. Vitamin A and the skin. Proc R Soc Med. 1945;38:337–342. [PMC free article] [PubMed] [Google Scholar]
- 9.Mevorah B., Marazzi A., Frenk E. The prevalence of accentuated palmoplantar markings and keratosis pilaris in atopic dermatitis, autosomal dominant ichthyosis and control dermatological patients. Br J Dermatol. 1985;112:679–685. doi: 10.1111/j.1365-2133.1985.tb02336.x. [DOI] [PubMed] [Google Scholar]
- 10.Sergeant A., Campbell L.E., Hull P.R., Porter M., Palmer C.N., Smith F.J., McLean W.H., Munro C.S. Heterozygous null alleles in filaggrin contribute to clinical dry skin in young adults and the elderly. J Invest Dermatol. 2009;129:1042–1045. doi: 10.1038/jid.2008.324. [DOI] [PubMed] [Google Scholar]
- 11.Winge M.C., Bilcha K.D., Lieden A., Shibeshi D., Sandilands A., Wahlgren C.F., McLean W.H., Nordenskjold M., Bradley M. Novel filaggrin mutation but no other loss-of-function variants found in Ethiopian patients with atopic dermatitis. Br J Dermatol. 2011;165:1074–1080. doi: 10.1111/j.1365-2133.2011.10475.x. [DOI] [PubMed] [Google Scholar]
- 12.Thyssen J.P., Godoy-Gijon E., Elias P.M. Ichthyosis vulgaris: the filaggrin mutation disease. Br J Dermatol. 2013;168:1155–1166. doi: 10.1111/bjd.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai S.C., Chen H., Koh W.P., Common J.E., van Bever H.P., McLean W.H., Lane E.B., Giam Y.C., Tang M.B. Filaggrin mutations are associated with recurrent skin infection in Singaporean Chinese patients with atopic dermatitis. Br J Dermatol. 2012;166:200–203. doi: 10.1111/j.1365-2133.2011.10541.x. [DOI] [PubMed] [Google Scholar]
- 14.Barth J.H., Wojnarowska F., Dawber R.P. Is keratosis pilaris another androgen-dependent dermatosis? Clin Exp Dermatol. 1988;13:240–241. doi: 10.1111/j.1365-2230.1988.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 15.Jackson J.B., Touma S.C., Norton A.B. Keratosis pilaris in pregnancy: an unrecognized dematosis of pregnancy? W V Med J. 2004;100:26–28. [PubMed] [Google Scholar]
- 16.Yosipovitch G., DeVore A., Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007;56:901–916. doi: 10.1016/j.jaad.2006.12.004. quiz 917–920. [DOI] [PubMed] [Google Scholar]
- 17.Plascencia Gomez A., Vega Memije M.E., Torres Tamayo M., Rodriguez Carreon A.A. Skin disorders in overweight and obese patients and their relationship with insulin. Actas Dermosifiliogr. 2014;105:178–185. doi: 10.1016/j.ad.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Yosipovitch G., Hodak E., Vardi P., Shraga I., Karp M., Sprecher E., David M. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care. 1998;21:506–509. doi: 10.2337/diacare.21.4.506. [DOI] [PubMed] [Google Scholar]
- 19.Kaune K.M., Haas E., Emmert S., Schon M.P., Zutt M. Successful treatment of severe keratosis pilaris rubra with a 595-nm pulsed dye laser. Dermatol Surg. 2009;35:1592–1595. doi: 10.1111/j.1524-4725.2009.01282.x. [DOI] [PubMed] [Google Scholar]
- 20.Kezic S., O'Regan G.M., Yau N., Sandilands A., Chen H., Campbell L.E., Kroboth K., Watson R., Rowland M., McLean W.H., Irvine A.D. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy. 2011;66:934–940. doi: 10.1111/j.1398-9995.2010.02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barresi C., Stremnitzer C., Mlitz V., Kezic S., Kammeyer A., Ghannadan M., Posa-Markaryan K., Selden C., Tschachler E., Eckhart L. Increased sensitivity of histidinemic mice to UVB radiation suggests a crucial role of endogenous urocanic acid in photoprotection. J Invest Dermatol. 2011;131:188–194. doi: 10.1038/jid.2010.231. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa N., Sakai S., Matsumoto M., Yamada K., Nagano M., Yuki T., Sumida Y., Uchiwa H. Relationship between NMF (lactate and potassium) content and the physical properties of the stratum corneum in healthy subjects. J Invest Dermatol. 2004;122:755–763. doi: 10.1111/j.0022-202X.2004.22317.x. [DOI] [PubMed] [Google Scholar]
- 23.Rawlings A.V., Harding C.R. Moisturization and skin barrier function. Dermatol Ther. 2004;17(Suppl 1):43–48. doi: 10.1111/j.1396-0296.2004.04s1005.x. [DOI] [PubMed] [Google Scholar]
- 24.Kirschner N., Houdek P., Fromm M., Moll I., Brandner J.M. Tight junctions form a barrier in human epidermis. Eur J Cell Biol. 2010;89:839–842. doi: 10.1016/j.ejcb.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Haftek M., Simon M., Kanitakis J., Marechal S., Claudy A., Serre G., Schmitt D. Expression of corneodesmosin in the granular layer and stratum corneum of normal and diseased epidermis. Br J Dermatol. 1997;137:864–873. [PubMed] [Google Scholar]
- 26.Proksch E., Brandner J.M., Jensen J.M. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 27.Feingold K.R., Schmuth M., Elias P.M. The regulation of permeability barrier homeostasis. J Invest Dermatol. 2007;127:1574–1576. doi: 10.1038/sj.jid.5700774. [DOI] [PubMed] [Google Scholar]
- 28.Elias P.M. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 29.Gruber R., Elias P.M., Crumrine D., Lin T.K., Brandner J.M., Hachem J.P., Presland R.B., Fleckman P., Janecke A.R., Sandilands A., McLean W.H., Fritsch P.O., Mildner M., Tschachler E., Schmuth M. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol. 2011;178:2252–2263. doi: 10.1016/j.ajpath.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandilands A., Terron-Kwiatkowski A., Hull P.R., O'Regan G.M., Clayton T.H., Watson R.M., Carrick T., Evans A.T., Liao H., Zhao Y., Campbell L.E., Schmuth M., Gruber R., Janecke A.R., Elias P.M., van Steensel M.A., Nagtzaam I., van Geel M., Steijlen P.M., Munro C.S., Bradley D.G., Palmer C.N., Smith F.J., McLean W.H., Irvine A.D. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007;39:650–654. doi: 10.1038/ng2020. [DOI] [PubMed] [Google Scholar]
- 31.Pinnagoda J., Tupker R.A., Agner T., Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164–178. doi: 10.1111/j.1600-0536.1990.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 32.Nyren M., Hagstromer L., Emtestam L. On assessment of skin reactivity using electrical impedance. Ann N Y Acad Sci. 1999;873:214–220. doi: 10.1111/j.1749-6632.1999.tb09469.x. [DOI] [PubMed] [Google Scholar]
- 33.Parra J.L., Paye M., EEMCO Group EEMCO guidance for the in vivo assessment of skin surface pH. Skin Pharmacol Appl Skin Physiol. 2003;16:188–202. doi: 10.1159/000069756. [DOI] [PubMed] [Google Scholar]
- 34.Kirschner N., Poetzl C., von den Driesch P., Wladykowski E., Moll I., Behne M.J., Brandner J.M. Alteration of tight junction proteins is an early event in psoriasis: putative involvement of proinflammatory cytokines. Am J Pathol. 2009;175:1095–1106. doi: 10.2353/ajpath.2009.080973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elias P.M., Fartasch M., Crumrine D., Behne M., Uchida Y., Holleran W.M. Origin of the corneocyte lipid envelope (CLE): observations in harlequin ichthyosis and cultured human keratinocytes. J Invest Dermatol. 2000;115:765–769. doi: 10.1046/j.1523-1747.2000.00124-5.x. [DOI] [PubMed] [Google Scholar]
- 36.Schmuth M., Yosipovitch G., Williams M.L., Weber F., Hintner H., Ortiz-Urda S., Rappersberger K., Crumrine D., Feingold K.R., Elias P.M. Pathogenesis of the permeability barrier abnormality in epidermolytic hyperkeratosis. J Invest Dermatol. 2001;117:837–847. doi: 10.1046/j.0022-202x.2001.01471.x. [DOI] [PubMed] [Google Scholar]
- 37.Schmuth M., Fluhr J.W., Crumrine D.C., Uchida Y., Hachem J.P., Behne M., Moskowitz D.G., Christiano A.M., Feingold K.R., Elias P.M. Structural and functional consequences of loricrin mutations in human loricrin keratoderma (Vohwinkel syndrome with ichthyosis) J Invest Dermatol. 2004;122:909–922. doi: 10.1111/j.0022-202X.2004.22431.x. [DOI] [PubMed] [Google Scholar]
- 38.Elias P.M., Schmuth M., Uchida Y., Rice R.H., Behne M., Crumrine D., Feingold K.R., Holleran W.M. Basis for the permeability barrier abnormality in lamellar ichthyosis. Exp Dermatol. 2002;11:248–256. doi: 10.1034/j.1600-0625.2001.110308.x. [DOI] [PubMed] [Google Scholar]
- 39.Hachem J.P., Wagberg F., Schmuth M., Crumrine D., Lissens W., Jayakumar A., Houben E., Mauro T.M., Leonardsson G., Brattsand M., Egelrud T., Roseeuw D., Clayman G.L., Feingold K.R., Williams M.L., Elias P.M. Serine protease activity and residual LEKTI expression determine phenotype in Netherton syndrome. J Invest Dermatol. 2006;126:1609–1621. doi: 10.1038/sj.jid.5700288. [DOI] [PubMed] [Google Scholar]
- 40.Demerjian M., Crumrine D.A., Milstone L.M., Williams M.L., Elias P.M. Barrier dysfunction and pathogenesis of neutral lipid storage disease with ichthyosis (Chanarin-Dorfman syndrome) J Invest Dermatol. 2006;126:2032–2038. doi: 10.1038/sj.jid.5700332. [DOI] [PubMed] [Google Scholar]
- 41.Elias P.M. Stratum corneum architecture, metabolic activity and interactivity with subjacent cell layers. Exp Dermatol. 1996;5:191–201. doi: 10.1111/j.1600-0625.1996.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 42.Menon G.K., Elias P.M., Lee S.H., Feingold K.R. Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res. 1992;270:503–512. doi: 10.1007/BF00645052. [DOI] [PubMed] [Google Scholar]
- 43.Menon G.K., Grayson S., Elias P.M. Cytochemical and biochemical localization of lipase and sphingomyelinase activity in mammalian epidermis. J Invest Dermatol. 1986;86:591–597. doi: 10.1111/1523-1747.ep12355263. [DOI] [PubMed] [Google Scholar]
- 44.Elias P.M., Fritsch P.O., Lampe M., Williams M.L., Brown B.E., Nemanic M., Grayson S. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981;44:531–540. [PubMed] [Google Scholar]
- 45.Elias P.M., Ahn S., Brown B., Crumrine D., Feingold K.R. Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol. 2002;119:1269–1274. doi: 10.1046/j.1523-1747.2002.19622.x. [DOI] [PubMed] [Google Scholar]
- 46.Elias P.M., Gruber R., Crumrine D., Menon G., Williams M.L., Wakefield J.S., Holleran W.M., Uchida Y. Formation and functions of the corneocyte lipid envelope (CLE) Biochim Biophys Acta. 2014;184:314–318. doi: 10.1016/j.bbalip.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirschner N., Rosenthal R., Furuse M., Moll I., Fromm M., Brandner J.M. Contribution of tight junction proteins to ion, macromolecule, and water barrier in keratinocytes. J Invest Dermatol. 2013;133:1161–1169. doi: 10.1038/jid.2012.507. [DOI] [PubMed] [Google Scholar]
- 48.Sugawara T., Iwamoto N., Akashi M., Kojima T., Hisatsune J., Sugai M., Furuse M. Tight junction dysfunction in the stratum granulosum leads to aberrant stratum corneum barrier function in claudin-1-deficient mice. J Dermatol Sci. 2013;70:12–18. doi: 10.1016/j.jdermsci.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Giovannini S. Zur histologie der keratosis pilaris. Archiv Dermatol Syphilis. 1902;63:163–212. [Google Scholar]
- 50.Sundberg J.P., Boggess D., Sundberg B.A., Eilertsen K., Parimoo S., Filippi M., Stenn K. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067–2075. doi: 10.1016/S0002-9440(10)65078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Y., Eilertsen K.J., Ge L., Zhang L., Sundberg J.P., Prouty S.M., Stenn K.S., Parimoo S. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–270. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
- 52.Lee D.Y., Yamasaki K., Rudsil J., Zouboulis C.C., Park G.T., Yang J.M., Gallo R.L. Sebocytes express functional cathelicidin antimicrobial peptides and can act to kill propionibacterium acnes. J Invest Dermatol. 2008;128:1863–1866. doi: 10.1038/sj.jid.5701235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zouboulis C.C., Schagen S., Alestas T. The sebocyte culture: a model to study the pathophysiology of the sebaceous gland in sebostasis, seborrhoea and acne. Arch Dermatol Res. 2008;300:397–413. doi: 10.1007/s00403-008-0879-5. [DOI] [PubMed] [Google Scholar]
- 54.Feinstein A., Ackerman A.B., Ziprkowski L. Histology of autosomal dominant ichthyosis vulgaris and X-linked ichthyosis. Arch Dermatol. 1970;101:524–527. [PubMed] [Google Scholar]
- 55.Wells R.S. Ichthyosis. Br Med J. 1966;2:1504–1506. doi: 10.1136/bmj.2.5528.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fraki J.E., Kuokkanen K., Hosu-Havu V.K. Morphometric analysis of the dominant and sex-linked forms of ichthyosis vulgaris. Acta Derm Venereol. 1973;53:299–305. [PubMed] [Google Scholar]
- 57.De Benedetto A., Rafaels N.M., McGirt L.Y., Ivanov A.I., Georas S.N., Cheadle C., Berger A.E., Zhang K., Vidyasagar S., Yoshida T., Boguniewicz M., Hata T., Schneider L.C., Hanifin J.M., Gallo R.L., Novak N., Weidinger S., Beaty T.H., Leung D.Y., Barnes K.C., Beck L.A. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127:773–786.e1-7. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gruber R., Janecke A.R., Fauth C., Utermann G., Fritsch P.O., Schmuth M. Filaggrin mutations p.R501X and c.2282del4 in ichthyosis vulgaris. Eur J Hum Genet. 2007;15:179–184. doi: 10.1038/sj.ejhg.5201742. [DOI] [PubMed] [Google Scholar]
- 59.Palmer C.N., Irvine A.D., Terron-Kwiatkowski A., Zhao Y., Liao H., Lee S.P., Goudie D.R., Sandilands A., Campbell L.E., Smith F.J., O'Regan G.M., Watson R.M., Cecil J.E., Bale S.J., Compton J.G., DiGiovanna J.J., Fleckman P., Lewis-Jones S., Arseculeratne G., Sergeant A., Munro C.S., El Houate B., McElreavey K., Halkjaer L.B., Bisgaard H., Mukhopadhyay S., McLean W.H. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 60.Thyssen J.P., Thuesen B., Huth C., Standl M., Carson C.G., Heinrich J., Kramer U., Kratzsch J., Berg N.D., Menne T., Johansen J.D., Carlsen B.C., Schwab S., Thorand B., Munk M., Wallaschofski H., Heickendorff L., Meldgaard M., Szecsi P.B., Stender S., Bonnelykke K., Weidinger S., Bisgaard H., Linneberg A. Skin barrier abnormality caused by filaggrin (FLG) mutations is associated with increased serum 25-hydroxyvitamin D concentrations. J Allergy Clin Immunol. 2012;130:1204–1207.e2. doi: 10.1016/j.jaci.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 61.Sato T., Imai N., Akimoto N., Sakiguchi T., Kitamura K., Ito A. Epidermal growth factor and 1alpha,25-dihydroxyvitamin D3 suppress lipogenesis in hamster sebaceous gland cells in vitro. J Invest Dermatol. 2001;117:965–970. doi: 10.1046/j.0022-202x.2001.01516.x. [DOI] [PubMed] [Google Scholar]
- 62.Alestas T., Ganceviciene R., Fimmel S., Muller-Decker K., Zouboulis C.C. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med (Berl) 2006;84:75–87. doi: 10.1007/s00109-005-0715-8. [DOI] [PubMed] [Google Scholar]
- 63.Schmuth M., Martinz V., Janecke A.R., Fauth C., Schossig A., Zschocke J., Gruber R. Inherited ichthyoses/generalized Mendelian disorders of cornification. Eur J Hum Genet. 2013;21:123–133. doi: 10.1038/ejhg.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunohistochemistry for filaggrin shows hypergranulosis (arrows) in WT keratosis pilaris. Paraffin-embedded sections were stained with monoclonal mouse/anti-human FLG antibody (dilution 1:50). FLG, filaggrin; WT, wild-type.