Abstract
Objective
To describe the recruitment methods, study participation rate, and baseline characteristics of a representative sample of outpatients with COPD eligible for pulmonary rehabilitation participating in a trial of a lifestyle behavioral intervention to increase physical activity.
Setting and Design
A patient registry was developed for recruitment using an administrative database from primary care and specialty clinics of an academic medical center in northeast Texas for a parallel group randomized trial.
Results
The registry was comprised of 5,582 patients and over the course of the 30 month recruitment period 325 patients were enrolled for an overall study participation rate of 35.1%. After a 6-week COPD self-management education period provided to all enrolled patients, 305 patients were randomized into either Usual Care (UC; n=156) or the Physical Activity Self-Management intervention (PASM; n=149). There were no clinically significant differences in demographics, clinical characteristics, or health status indicators between the randomized groups.
Conclusion
The results of this recruitment process demonstrate the successful use of a patient registry for enrolling a representative sample of outpatients eligible for pulmonary rehabilitation with COPD from primary and specialty care. Moreover, this approach to patient recruitment provides a model for future studies utilizing administrative databases and electronic health records.
Keywords: randomized clinical trial, recruitment, chronic obstructive pulmonary disease, physical activity, self-management
Introduction
COPD is a leading cause of disability and mortality worldwide, largely as a result of cigarette smoking and aging population [1,2]. While prevention through tobacco control has the greatest potential to decrease the burden of COPD, there remains an ongoing need for effective treatments. The goals of treatment are to manage symptoms and exacerbations, improve functional performance, improve quality of life, and decrease emergency care, hospitalizations, and mortality [3].
Over the past two decades an increasing number of efficacious options for achieving these management goals have become available including pharmacological and nonpharmacological interventions [3]. However, effectiveness of these interventions in “real-world” clinical settings is often sub-optimal partly because of the limited external validity associated with clinical trials [4–6]. A major limitation to effectiveness is non-adherence to treatment that may result from factors at multiple levels including policy, community, delivery system, health care team, and patient [7]. To optimize adherence, multi-pronged approaches of patient support are needed in the clinical setting to assist patients in mastering a complex set of self-management behaviors [8,9].
Patient self-management includes adherence to medications, action plans for exacerbations, and lifestyle changes such as smoking cessation and increased physical activity [9]. While all of these behaviors affect patient outcomes, health behavior change is complex, which makes simultaneous change attempts for multiple behaviors difficult [10]. Moreover, sustained behavior change even for single behaviors often takes months and years of intermittent relapse and may never be permanent [11]. Due to these challenges, studies of self-management support interventions often focus on single behaviors such as exacerbation action plans and smoking cessation. Despite compelling evidence for the adverse effects of physical inactivity [12,13] and for the benefits of exercise rehabilitation programs [14,15] there have only been a few small-scale investigations of behavioral interventions to increase lifestyle physical activity among patients with COPD independent of pulmonary rehabilitation [16–20].
To address the limited evidence on interventions to increase physical activity among patients with COPD we designed and implemented the COPD Self-management Activation Research Trial (SMART) [21]. The goals of this paper are to: 1) describe the methods of patient recruitment, which were designed to optimize generalizability as recommended by the CONSORT (Consolidated Standards of Reporting Trials) statement [22] 2) estimate study participation rate [23], and 3) report baseline patient demographic and clinical characteristics after randomization.
Methods
Details of the rationale, design, intervention, measures, and statistical methods have been previously described [21]. In brief, this is a pragmatic, single-site, parallel group randomized trial. Patients with physician-diagnosed and spirometry confirmed COPD were actively recruited from primary and specialty care clinics of the University of Texas Health Science Center-Tyler (UTHSCT), an academic medical center with training programs limited to primary care in a large rural region of northeast Texas. Care for these patients was provided by a total of 49 health-care providers, which included family medicine faculty (n=5), family medicine trainees (n=28), internal medicine faculty (n=5), physician assistants (n=2), and pulmonary disease specialists (n=9). The institution does not provide pulmonary rehabilitation services. The intervention was comprised of two components: 1) structured COPD self-management education, and 2) lifestyle physical activity behavioral intervention [21]. COPD self-management education was provided to all patients over 6 weeks using a workbook and was supported by weekly telephone calls from a trained health coach. After 6 weeks, patients were randomized to usual care (UC) or the physical activity self-management intervention (PASM). The PASM component lasted 20 weeks with outcomes evaluated at 6, 12, 18 months.
Recruitment
The target population was all outpatients with COPD who were eligible for pulmonary rehabilitation. To optimize external validity of the relationship between the study sample to the target population, recruitment and enrollment was conducted using criteria applied in the clinical setting for selection and referral of patients with COPD for pulmonary rehabilitation [21]. Specifically, criteria for exclusion of patients were largely limited to safety concerns or inability to participate in minimal physical activity rather than exclusion due motivation, recent exacerbations, or common co-morbid conditions. To meet enrollment goals two recruitment methods were used; a patient registry and provider referral.
Registry
The primary method of recruitment was a patient registry. An initial registry was developed from clinical administrative data for the time period 1/1/2004 – 11/3/2009. Patients ≥ 45 years of age with COPD were identified using ICD-9 diagnosis codes 491, 492, 493.2, and 496. This list of patients was randomly ordered by the data coordinating center and patients subsequently screened for eligibility as described below. A permuted block design was used for randomization in order to ensure that an equal number of subjects were randomized to each study arm within each block [21]. The patient registry was expanded in 2011 for the period 8/1/2009 - 11/17/2011.
A number of steps were taken by the principal investigator (DBC) and research coordinator (RR) before the registry was used for recruitment to ensure enrollment was efficient, met goals for representativeness of the sample, and adhered to Health Insurance Portability and Accountability Act (HIPAA) regulations. These steps included elimination of duplicate records, deceased patients, disqualifying spirometry, and records lacking an identifiable primary care or pulmonary physician. The protocol for development and use of the registry for recruitment of patients was approved by the UTHSCT institutional review board (IRB). All physicians provided written permission for the study team to contact their patients to determine interest in participating.
Clinical data from medical records were reviewed to determine potential eligibility. Potentially eligible patients were mailed informational materials about the study in groups of 30 to 500. The materials included a letter of introduction signed by their physician and principal investigator (DC) and other informational materials. Informational materials included a brochure explaining the study in more detail and how to begin the registration process using an automated computer assisted telephone (CAT) (TeleMinder™ Los Altos, CA) answering system. Research staff conducted follow-up telephone calls to all patients to determine further interest, screen for eligibility, and schedule an enrollment visit for final determination of eligibility.
The CAT system was used for several purposes including: 1) the enrollment process, 2) delivery of the intervention, and 3) collection of health care utilization data [21]. As part of the enrollment process, the automated CAT system was accessible to patients 24 hours per day and used to further screen patients for interest in, and eligibility for participation in the study (Supplemental Table 1). The mailed introductory materials provided patients with the options of starting the enrollment process using the CAT system by answering four screening questions or to opt out of participation.
Direct referral
While the registry was the primary source for recruitment and enrollment of patients, direct referrals were also obtained from UTHSCT primary care and pulmonary physicians. These physicians were made aware of the study through small group meetings and written materials. Direct referrals were either self-initiated by the physician or prompted by research staff. Potential candidates for the study were identified from lists of scheduled appointments obtained from the clinic registration system. Physicians were notified by staff about potential candidates and referrals were sought the day prior to, or the day of the patient's appointment.
Patient Enrollment
Patient enrollment was conducted over a 30 month period (April 2010 through September 2012) with two major objectives: 1) to obtain written informed consent and final determination of eligibility, and 2) to obtain baseline data and introduce patients to the COPD self-management intervention [21]. Final determination of eligibility was made by the principal investigator or project coordinator based on clinical characteristics used by physicians to refer patients for pulmonary rehabilitation [21]. Participants were required to have a documented physician diagnosis of COPD; self-reported dyspnea on exertion that causes “trouble when hurrying on the level or walking up a slight hill;” a post-bronchodilator FEV1/FVC <0.7, FEV1 < 0.7; and be able to walk at least 110 meters during a standardized six minute walk test (6MWT) [24]. Patients were allowed to use assistive devices (e.g., cane, rollator) during the 6MWT if they routinely used them in their daily activities. Spirometry was performed for stable patients who did not have results available within 12 months. To ensure standardization and patient safety all testing was performed by trained technicians with oversight by the project coordinator. After final determination of eligibility, baseline data collection was completed, and patients were introduced to the health coach and COPD self-management intervention [21].
Variables of interest
Details of the study measures have been described previously and include patient demographics, lifestyle behaviors, and clinical characteristics along with primary, secondary, and process outcomes [21]. The primary outcome measures were the Chronic Respiratory Questionnaire dyspnea domain (CRQD) [25] and the 6MWT [24]. Secondary outcomes included the CRQ domains of fatigue, emotion, and mastery; Medical Outcomes Study 12-item Short Form (SF-12) health survey [26], and self-reported health care utilization. Self-reported health care utilization included: (1) Visiting a medical office to see a physician/nurse/nurse practitioner/physician's assistant for lung or non-lung disease related issues; (2) Been to urgent care or emergency room and was not hospitalized for lung or non-lung disease related issues; (3) Has been hospitalized for lung or non-lung disease related issues, and (4) Having used any home health services. In addition, process measures included the Rapid Assessment of Physical Activity (RAPA) [27], Charlson co-morbidity index (CCI) [28], and Geriatric Depression Scale (GDS) [29]. The RAPA measures a patient's readiness to meet physical activity goals using nine self-reported questions about intensity and duration of physical activity. Patient scores are calculated based on their highest degree of physical activity and the scores are further classified into three mutually exclusive categories of activity: 1) sedentary-low, 2) underactiveintermediate, and 3) active [27]. A patient categorized as active is consistent with participation in at least 30 minutes of moderate level physical activity daily. The CCI is a self-reported assessment of 22 conditions. Each condition is assigned a weight and the weights are totaled to calculate the index [28]. The GDS is a 15-item survey based on self-reports that are suggestive of depressive symptoms. Items that suggest depressive symptoms are assigned a value of one and all items are totaled to obtain the score; scores greater than or equal to 6 suggests clinically significant depression [29].
Statistical Methods
The data are presented as means and standard deviations (SD) or medians and interquartile ranges (IQR) for continuous variables, and frequency and proportions for categorical variables. We estimated study participation rate in two ways. The first referred to as actual study participation rate was calculated as the number of patients enrolled divided by the number of potentially eligible patients regardless of their willingness to participate in the study [23]. The second referred to as estimated participation rate adjusts for the proportion of patients who refuse and based on actual exclusions (20.3%) are likely to be found ineligible at an enrollment visit (Supplemental Figure 1 and Table 2). Effectiveness of the randomization process was assessed by comparisons of the baseline characteristics of the two randomized groups. No formal hypothesis testing to examine between group differences at baseline was performed. Any baseline differences observed would be by definition due to chance since we utilized a permuted block design. For baseline results, the reporting of p-values is not appropriate and not reported.[30–32]
Results
Recruitment, Enrollment, and Participant Flow
A total of 325 patients were enrolled from the registry during the 30 month enrollment period and 305 completed the 6 week run-in period and were randomized to UC (n=156) and PASM intervention (n=149) (Figure 1). Of the total registry (n=5,582), 98.3% of medical records were reviewed and of these, 3,485 were determined to be ineligible from record review and an additional 431 individuals were found to be ineligible after contact for a total of 3,916 ineligible patients with 1,251 unable to contact, refused, or failed to attend scheduled appointment. Supplement Table 2 provides specific reasons for ineligibility at all stages of the contact and enrollment process including non-qualifying spirometry or no physician diagnosis of COPD (n=1,871 [47.8%]), did not meet other inclusion/exclusion criteria (n=994 [25.4%]), were unable to participate in physical activity (n=282 [7.2%]), or were deceased (n=769 [19.6%]).
Figure 1. Patient flow from registry to randomization.
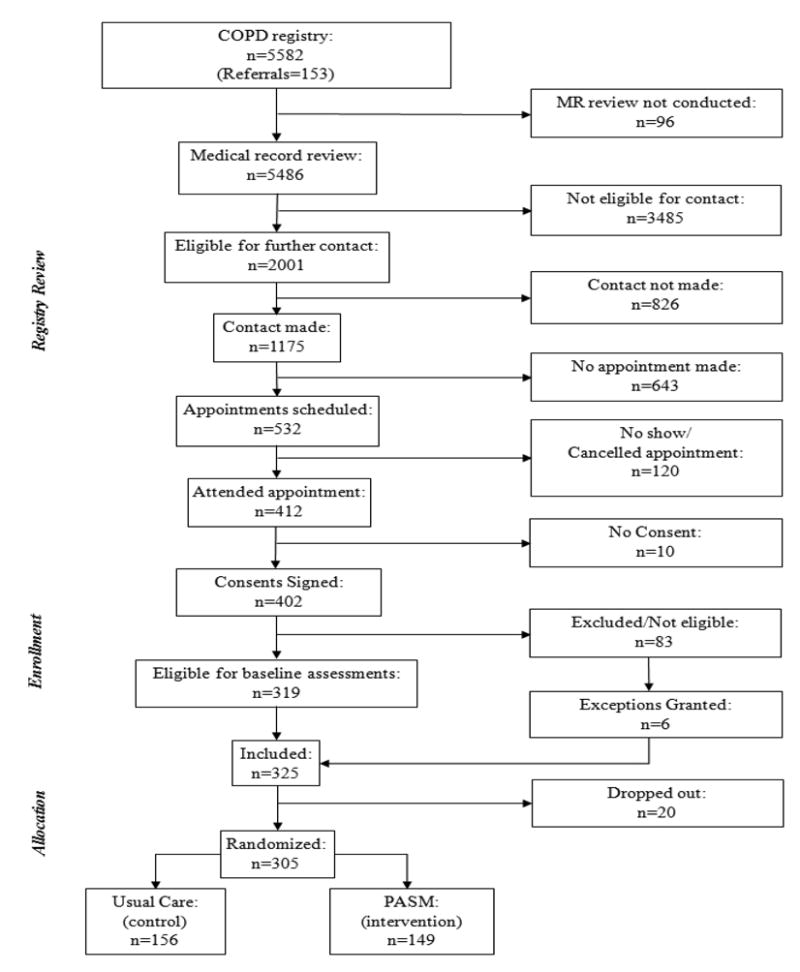
Details for exclusion of patients at all phases of recruitment through enrollment are summarized in Supplemental Table 2. Medical record (MR) review was discontinued (n=96) when the pre-specified enrollment goal was achieved.
After final evaluation for eligibility by medical record review, completion of eligibility screening questions, or at an enrollment visit a total of 924 patients were determined to be potentially eligible from 2,001 who were initially considered eligible from review of medical records alone. Appointments for an enrollment visit were scheduled for 532 and 412 attended. At the enrollment visit, 402 (97.6%) consented to participate and 325 (80.8%) were enrolled. The reasons patients were no longer potentially eligible included inability to make an initial contact (n=826), inability to re-contact (n=32), failure to meet inclusion criteria (n=213), or completion of enrollment (n=6). With the assumption that all 924 potentially eligible patients would be eligible and enrollment of 325, the actual study enrollment rate was 35.2%.
Baseline Group Characteristics
Of the 325 patients enrolled from the registry, 20 patients dropped-out during the 6-week run-in period and were not randomized (data not shown). Compared to the randomized patients (n=305), those who dropped out had worse SF-12 general health status (31.6 vs. 37.6) and CRQemotional function (4.1 vs. 4.6). The mean ages of patients who dropped out were 4 years younger (66.3 vs. 70.3 years), and had a clinically significant lower CRQ Fatigue score (3.2 vs. 3.7), and shorter 6MWD (309.5m vs. 340.1m).
Among the 305 patients randomized there were no clinically meaningful differences at baseline in demographics, lifestyle characteristics, co-morbid conditions, severity of COPD, or measures of health status between the UC and PASM intervention groups (Tables 1-4). Overall, the mean age (SD) was 68.6 years (9.54), 50.5% female, and 92.5% non-Hispanic white. The majority were married (57.7%), and 49.3% lived in a rural area. About half of the patients had a high school education or less and the vast majority (84.9%) reported being either retired or disabled. A slight majority (57.2%) had a total annual household income <$25,000 (Table 1).
Table 1. Baseline Characteristics of Randomized Patients (N=305).
| Variables | TOTAL | UC | PASM | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| N=305 | % | N=156 | % | N=149 | % | |
| Age (years), mean (SD) | 68.61 | 9.54 | 68.15 | 9.51 | 69.09 | 9.57 |
|
| ||||||
| Female, n(%) | 154 | 50.49 | 79 | 50.64 | 75 | 50.34 |
|
| ||||||
| Race/Ethnicity, n(%) | ||||||
| Hispanic | 4 | 1.31 | 2 | 1.28 | 2 | 1.34 |
| NH White | 282 | 92.46 | 140 | 89.74 | 142 | 95.30 |
| NH Black | 18 | 5.9 | 13 | 8.33 | 5 | 3.36 |
| NH Other | 1 | 0.33 | 1 | 0.64 | 0 | 0.00 |
|
| ||||||
| Marital Status, n(%) | ||||||
| Single | 26 | 8.52 | 13 | 8.33 | 13 | 8.72 |
| Married | 176 | 57.7 | 87 | 55.77 | 89 | 59.73 |
| Widowed | 58 | 19.02 | 30 | 19.23 | 28 | 18.79 |
| Separated | 45 | 14.75 | 26 | 16.67 | 19 | 12.75 |
|
| ||||||
| Work Status, n(%) | ||||||
| Fulltime | 23 | 7.57 | 10 | 6.41 | 13 | 8.78 |
| Part time | 12 | 3.95 | 5 | 3.21 | 7 | 4.73 |
| Unemployed | 11 | 3.62 | 4 | 2.56 | 7 | 4.73 |
| Disabled | 71 | 23.36 | 41 | 26.28 | 30 | 20.27 |
| Retired | 187 | 61.51 | 96 | 61.54 | 91 | 61.49 |
|
| ||||||
| Education, n(%) | ||||||
| No HS | 60 | 19.67 | 28 | 17.95 | 32 | 21.48 |
| HS Graduate | 91 | 29.84 | 49 | 31.41 | 42 | 28.19 |
| Some College | 102 | 33.44 | 53 | 33.97 | 49 | 32.89 |
| College Graduate | 40 | 13.11 | 22 | 14.10 | 18 | 12.08 |
| Post Graduate | 12 | 3.93 | 4 | 2.56 | 8 | 5.37 |
|
| ||||||
| Income Level, n(%) | ||||||
| ≤15K | 98 | 33 | 49 | 32.45 | 49 | 33.56 |
| 15-25K | 72 | 24.24 | 35 | 23.18 | 37 | 25.34 |
| 25-35K | 46 | 15.49 | 24 | 15.89 | 22 | 15.07 |
| 35-50K | 34 | 11.45 | 20 | 13.25 | 14 | 9.59 |
| ≥50K | 47 | 15.82 | 23 | 15.23 | 24 | 16.44 |
|
| ||||||
| Rural* | 150 | 49.34 | 81 | 51.92 | 69 | 46.62 |
|
| ||||||
| Insurance | ||||||
| Medicare | 242 | 79.34 | 125 | 80.13 | 117 | 78.52 |
| Medicaid | 13 | 4.26 | 10 | 6.41 | 3 | 2.01 |
| Veterans Affair | 1 | 0.33 | 1 | 0.64 | 0 | 0.00 |
| Commercial | 30 | 9.84 | 11 | 7.05 | 19 | 12.75 |
| Other | 3 | 0.98 | 2 | 1.28 | 1 | 0.67 |
| No Insurance | 16 | 5.25 | 7 | 4.49 | 9 | 6.04 |
UC-Usual Care; PASM-Physical Activity Self-Management; SD-Standard Deviation; NH-Non Hispanic; HS-High School; IQR-Interquartile Range;
-Rural Residence based off of Urban Influence Codes.
Table 4. COPD-related Clinical Characteristics of Randomized Patients (N=305).
| Variables | Total | UC | PASM | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| N=305 | % | N=156 | % | N=149 | % | ||
| BODE Index | BODE Index | 4.37 | 1.98 | 4.38 | 2.03 | 4.36 | 1.93 |
| BMI, mean(SD) | 28.93 | 7.06 | 28.52 | 6.26 | 29.35 | 7.81 | |
| FEV1 (%), mean(SD) | 46.45 | 13.09 | 47.33 | 45.20 | 45.53 | 43.49 | |
| MMRC Dyspnea, mean(SD) | 1.83 | 0.95 | 1.85 | 0.94 | 1.81 | 0.96 | |
| 6MW Greater than 350m, n(%) | 151 | 49.51 | 75 | 48.08 | 76 | 51.01 | |
|
| |||||||
| 6 Minute Walk | Total distance walked in 6 minutes(m), mean (SD) | 340.05 | 93.68 | 337.50 | 96.37 | 342.80 | 91.03 |
|
| |||||||
| GOLD Stage | GOLD Stage, n(%) | ||||||
| II | 134 | 43.93 | 75 | 48.08 | 59 | 39.60 | |
| III | 130 | 42.62 | 59 | 37.82 | 71 | 47.65 | |
| IV | 41 | 13.44 | 22 | 14.10 | 19 | 12.75 | |
|
| |||||||
| Oxygen Use | Currently using oxygen, n(%) | ||||||
| Yes | 122 | 40.00 | 67 | 42.95 | 55 | 36.91 | |
| No | 183 | 60.00 | 89 | 57.05 | 94 | 63.09 | |
UC-Usual Care; PASM-Physical Activity Self-Management; SD-Standard deviation; BODE-Body Mass Index, Degree of Airflow Obstruction and Dyspnea, and Exercise Capacity; FEV1-Forced Expiratory Volume in 1 second; MMRC-modified medical research council; 6MW-6 Minute Walk; GOLD-Global Initiative for chronic Obstructive Lung Disease
A number of indicators were used to characterize the status of patients’ health-related conditions (Tables 2-4). Overall, 92.5% were ever-smokers with a mean (SD) of 58.2 (36.8) pack-years. The distribution of self-reported physical activity included 56.6% active, 28.0% intermediate, and 15.5% low. Patients reported a mean of three co-morbid conditions with the most common associated conditions being cardiovascular disorders, diabetes, and depression (Table 2). Moderate to severe depressive symptoms as measured by the GDS were reported by over a quarter of the patients (26.6%).
Table 2. Health-related Behaviors and Comorbid Conditions of Randomized Patients (N=305).
| Health-related Behaviors Variables | TOTAL | UC | PASM | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| N=305 | % | N=156 | % | N=149 | % | |
| Smoking Status, n(%) | ||||||
| Current Smoker | 76 | 24.92 | 46 | 29.49 | 30 | 20.13 |
| Ex-Smoker | 206 | 67.54 | 99 | 63.46 | 107 | 71.81 |
| Never smoker | 23 | 7.54 | 11 | 7.05 | 12 | 8.05 |
| Pack Years, Mean(SD) | 58.22 | 36.76 | 57.05 | 34.32 | 59.47 | 39.27 |
| Drink Alcoholic Beverages | 107 | 35.2 | 54 | 34.84 | 53 | 35.57 |
| Rapid Assessment of Physical Activity | ||||||
| Sedentary | 47 | 15.46 | 23 | 14.74 | 24 | 16.22 |
| Underactive | 85 | 27.96 | 45 | 28.85 | 40 | 27.03 |
| Active | 172 | 56.58 | 88 | 56.41 | 84 | 56.76 |
| Co-morbid Conditions Variables | ||||||
|
| ||||||
| Geriatric Depression Scale, n(%) | ||||||
| No Depression | 224 | 73.44 | 111 | 71.15 | 113 | 75.84 |
| Moderate Depression | 71 | 23.28 | 40 | 25.64 | 31 | 20.81 |
| Severe Depression | 10 | 3.28 | 5 | 3.21 | 5 | 3.36 |
|
| ||||||
| CCI, median(IQR) | 5 | (4,6) | 5 | (4,6) | 5 | (4,6) |
| Congestive heart failure | 45 | 15.52 | 21 | 14.00 | 24 | 17.14 |
| Myocardial infarction | 39 | 13.78 | 20 | 13.70 | 19 | 13.87 |
| Peripheral vascular disease | 20 | 7.35 | 6 | 4.35 | 14 | 10.45 |
| Cerebrovascular disease | 3 | 1.08 | 1 | 0.70 | 2 | 1.48 |
| Dementia | 1 | 0.34 | 1 | 0.68 | 0 | 0.00 |
| Chronic pulmonary disease | 277 | 93.58 | 140 | 92.72 | 137 | 94.48 |
| Connective tissue disease | 16 | 5.82 | 12 | 8.33 | 4 | 3.05 |
| Ulcer disease | 24 | 8.28 | 14 | 9.52 | 10 | 6.99 |
| Mild liver disease | 14 | 4.75 | 8 | 5.30 | 6 | 4.17 |
| Diabetes | 62 | 20.74 | 25 | 16.45 | 37 | 25.17 |
| Depression | 105 | 35.23 | 50 | 32.68 | 55 | 37.93 |
| Use of warfarin | 32 | 10.92 | 13 | 8.55 | 19 | 13.48 |
| Hypertension | 174 | 58.19 | 92 | 60.53 | 82 | 55.78 |
| Hemiplagia | 1 | 0.34 | 0 | 0.00 | 1 | 0.69 |
| Moderate or severe renal disease | 6 | 2.05 | 3 | 2.00 | 3 | 2.10 |
| Diabetes with end organ damage | 1 | 0.34 | 0 | 0.00 | 1 | 0.69 |
| Lymphoma | 3 | 1.00 | 2 | 1.31 | 1 | 0.68 |
| Skin ulcers/cellulitis | 11 | 3.70 | 4 | 2.63 | 7 | 4.83 |
| Moderate or severe liver disease | 11 | 3.68 | 7 | 4.58 | 4 | 2.74 |
| Metastatic Cancer | 4 | 1.36 | 1 | 0.67 | 3 | 2.08 |
SD-Standard deviation; IQR-Interquartile range;
Overall, patients randomized reported taking a mean (SD) of 11.5 (5.5) medications (Table 3). Medication types reported by patients were consistent with treatment for a diagnosis of COPD and the most common self-reported co-morbid conditions (Table 2). Of the medications reported the 10 most frequent drug classes included antihypertensives/cardiac (85.3%), electrolytes/ minerals/vitamins (62.0%), inhaled anti-cholinergics (58.4%), inhaled beta-agonists (51.8%), analgesics (51.8%), long-acting beta-agonist and corticosteroid combinations (46.6%), gastrointestinal (43.6%), psychiatric (40.3%), nebulized bronchodilators (38.4%), diuretics (37.1%), and endocrine (33.4%).
Table 3. Medication Types used by Randomized Patients (N=305).
| Variable | TOTAL | UC | PASM | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| N=305 | % | N=156 | % | N=149 | % | |
| Mean(SD) Number of Medications | 11.47 | 5.51 | 11.42 | 5.42 | 11.51 | 5.63 |
| Beta-agonist | 158 | 51.80 | 76 | 48.72 | 82 | 55.03 |
| Anti-cholinergic | 178 | 58.36 | 91 | 48.33 | 87 | 58.39 |
| Inhaled Corticosteroid | 44 | 14.43 | 21 | 13.46 | 23 | 15.44 |
| Long Acting Beta Agonist/Corticosteroid combination | 142 | 46.56 | 78 | 50.00 | 64 | 42.95 |
| Nebulizer | 117 | 38.36 | 57 | 36.54 | 60 | 40.27 |
| Systemic bronchodilator | 18 | 5.90 | 9 | 5.77 | 9 | 6.04 |
| Oral corticosteroid | 49 | 16.07 | 28 | 17.95 | 21 | 14.09 |
| Antibiotic | 46 | 15.08 | 24 | 15.38 | 22 | 14.77 |
| Anti-tussive/mucolytic | 35 | 11.48 | 20 | 12.82 | 15 | 10.07 |
| Cardiac/Antihypertensive | 260 | 85.25 | 134 | 85.90 | 126 | 84.56 |
| Diuretic | 113 | 37.05 | 57 | 36.54 | 56 | 37.58 |
| Endocrinologic | 102 | 33.44 | 48 | 30.77 | 54 | 36.24 |
| Allergy | 142 | 46.56 | 75 | 48.08 | 67 | 44.97 |
| Gastrointestinal | 133 | 43.61 | 64 | 41.03 | 69 | 46.31 |
| Electrolytes/vitamins/minerals | 189 | 61.97 | 100 | 64.10 | 89 | 59.73 |
| Gout | 7 | 2.30 | 6 | 3.85 | 1 | 0.67 |
| Opthamologic | 20 | 6.56 | 9 | 5.77 | 11 | 7.38 |
| Neurologic | 55 | 18.03 | 24 | 15.38 | 31 | 20.81 |
| Psychiatric | 123 | 40.33 | 66 | 42.31 | 57 | 38.26 |
| Urologic | 45 | 14.75 | 25 | 16.03 | 20 | 13.42 |
| Analgesic | 158 | 51.80 | 86 | 55.13 | 72 | 48.32 |
| Antiviral | 3 | 0.98 | 1 | 0.64 | 2 | 1.34 |
| Complementary/Alternative | 21 | 6.89 | 10 | 6.41 | 11 | 7.38 |
| Oxygen | 122 | 40.00 | 67 | 42.95 | 55 | 36.91 |
| Osteoporosis | 25 | 8.20 | 14 | 8.97 | 11 | 7.38 |
| Antienetic | 4 | 1.31 | 2 | 1.28 | 2 | 1.34 |
| Oncology/Immunosuppressive/anti-inflammatory | 11 | 3.61 | 5 | 3.21 | 6 | 4.03 |
| Smoking cessation | 5 | 1.64 | 2 | 1.28 | 3 | 2.01 |
| Topical Steroid/Dermatologic | 14 | 4.59 | 11 | 7.05 | 3 | 2.01 |
| Alpha 1 Antitrypsin | 2 | 0.66 | 1 | 0.64 | 1 | 0.67 |
UC-Usual Care; PASM-Physical Activity Self-Management; SD- Standard deviation
Objective measures of health status included BMI, spirometry, and 6MWD (Table 4). Patients were overweight or obese with a BMI mean (SD) of 28.9 (7.1). The PASM intervention group had a slightly higher prevalence of severe and very severe FEV1 impairment compared to the UC group, 60.4% vs. 51.9%, respectively. Overall, the 6MWD mean (SD) was 340.1 m (93.7 m) with no difference between the two groups. The BODE index mean (SD) for both groups, which combines BMI, FEV1, 6MWD, along with self-reported level of dyspnea, was 4.4 (2.0), with no difference between the groups.
Patient reported indicators of health status included CRQ, SF-12, and health care utilization (Table 5). The mean CRQ domain scores (SD) were in the mid-range of the scale (1=worst to 7=least) with the lowest score for fatigue (3.7 [1.2]) and a high score for emotional functioning (4.6 [0.9]). Of the two SF-12 component scores the mean physical component score (SD) was lowest (32.1 [8.7]) compared to the mental component score (50.5 [11.1]). Self-reported health care utilization during the previous six months was highest for office visits and similar for lung-related (74.4%) and non-lung-related (72.0%) conditions. The prevalence of other types of health care utilization was low with urgent and emergent care visits being slightly higher for non-lung-related (13.4%) compared to lung-related (7.5%) conditions. However, the frequency of utilization was reversed for lung-related hospitalizations (13.8%) compared to non- lung-related (8.9%) conditions. Finally, the overall utilization of home health services was low (12.1%).
Table 5. Health Status Measures of Randomized Patients (N=305).
| Variables | TOTAL | UC | PASM | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| N=305 | % | N=156 | % | N=149 | % | ||
| CRQ | Chronic Respiratory Questionnaire, mean(SD) | ||||||
| Dyspnea | 4.40 | 1.33 | 4.61 | 1.31 | 4.77 | 1.27 | |
| Fatigue | 3.67 | 1.22 | 3.69 | 1.23 | 3.65 | 1.23 | |
| Emotional functioning | 4.57 | 0.88 | 4.59 | 0.89 | 4.55 | 0.87 | |
| Mastery | 4.28 | 0.76 | 4.31 | 0.76 | 4.26 | 0.76 | |
|
| |||||||
| SF-12 | Physical Component Score, mean(SD) | 32.1 | 8.72 | 32.01 | 8.79 | 32.19 | 8.68 |
| Mental Component Score, mean(SD) | 50.5 | 11.13 | 49.91 | 11.44 | 51.12 | 10.81 | |
|
| |||||||
| Healthcare Utilization | Visited a medical office to see a physician, nurse, nurse practitioner, or physician's assistant for lung disease, n(%) | 227 | 74.43 | 115 | 73.72 | 112 | 75.17 |
| Visited a medical office to see a physician, nurse, nurse practitioner, or physician's assistant for non-lung disease, n(%) | 219 | 72.04 | 112 | 72.26 | 107 | 71.81 | |
| Has been to urgent care or emergency room for lung disease and was not hospitalized, n(%) | 23 | 7.54 | 11 | 7.05 | 12 | 8.05 | |
| Has been to urgent care or emergency room for non-lung disease and was not hospitalized, n(%) | 41 | 13.44 | 17 | 10.90 | 24 | 16.11 | |
| Has been hospitalized for lung disease, n(%) | 42 | 13.77 | 22 | 14.10 | 20 | 13.42 | |
| Has been hospitalized for non-lung disease, n(%) | 27 | 8.85 | 10 | 6.41 | 17 | 11.41 | |
| Has used any home health services, n(%) | 37 | 12.13 | 17 | 10.90 | 20 | 13.42 | |
UC-Usual Care; PASM-Physical Activity Self-Management; SD-Standard deviation; CRQ-Chronic Respiratory Questionnaire; SF-Medical Outcomes Study 12-item Short Form Health Survey
Discussion
The objectives of this manuscript were to describe the COPD SMART recruitment methods, determine study participation rate, and to examine the effectiveness of randomization. Results of the recruitment, enrollment, and randomization processes for this pragmatic trial demonstrate the successful use of an administrative database to develop a patient registry for recruitment of a real world sample of patients with COPD. From this registry primary and specialty care patients were proactively recruited to participate in the study. The inability to enroll more than about one-third of potentially eligible patients was largely the result of inability to contact, refusal, or failure to attend a scheduled visit (see Supplemental Figure 1). Once enrolled, the majority of patients (93.9%) completed six weeks of COPD self-management education and was randomized. There were no significant differences in demographics, clinical characteristics, or health status between the randomized groups.
Randomized clinical trials are considered the “gold standard” for determining the efficacy of interventions. However, a number of factors in the recruitment of patients for trials may result in biased study populations including the study setting, methods of recruitment, selection criteria, study participation rate, and sample size. The potential consequences to these variations in recruitment processes are a sample population not representative of the target population, results that are inconsistent between different clinical trials, and ultimately lack of effectiveness of the intervention when applied in the “real world” setting.
While the majority of clinical trials among patients with COPD are focused on efficacy of pharmacological treatments, there is a growing recognition of the need for investigations to examine non-pharmacological interventions [3,9]. These interventions have included patient education, exacerbation action plans, self-management, pulmonary rehabilitation, and integrated care [9]. There is strong evidence for the efficacy of pulmonary rehabilitation [14] and growing evidence for self-management interventions [33]. However, results for some nonpharmacological interventions have been inconsistent [34,35], which may be partly attributed to limited external validity and emphasize the need for further research.
The reach, efficacy, adoption, implementation, and maintenance (RE-AIM) framework posits that evaluation of the effectiveness of an intervention is a complex process that includes systematic consideration of a chronic illness intervention's strengths and weaknesses [36]. Reach is the proportion of the target population who participates in the intervention. Efficacy refers to the success rate of the intervention protocol implemented under ideal conditions. At the organizational level adoption is the proportion of settings that adopt the intervention. Similarly, implementation is the extent to which the intervention is implemented by an organization as intended. Finally, maintenance is the extent to which the program is sustained over time by individuals and organizations.
Study populations for most pharmacological and non-pharmacological trials of patients with COPD are often not representative of patients with COPD [4,5,37,38]. In pharmacological trials patients are often excluded because of co-morbid conditions or current medication use [34]. Patients for non-pharmacological trials have been recruited from pulmonary specialty clinics [39,40] or after exacerbations or hospitalizations [34,35,41]. For most clinical interventions, available evidence to assess effectiveness is usually limited to efficacy with limited evidence on reach, and little or no evidence on adoption, implementation, or maintenance.
To optimize the reach in recruitment of patients with COPD we used a patient registry and proactive patient contact in primary care and specialty clinics. With these approaches we are able to provide a clear description of the study population, proportion who participated, and factors that affected participation. Moreover, the proactive recruitment process of calling patients helped minimize volunteer bias associated with passive recruitment such as through media advertising [42] or mailed invitations [20].
While the study participation rate is an important component for assessment of external validity of clinical trials it is infrequently reported and there is often uncertainty about what constitutes the eligible population [23]. Moreover, few trials of patients with COPD use recruitment methods that provide a representative sample of patients and are unable to determine the actual number of potentially eligible patients. In contrast, with our recruitment method we were able to determine this number and calculated an overall actual study enrollment rate of 35.2%. However, this rate is an underestimate because it assumes that all potentially eligible patients will meet criteria after further evaluation (i.e., spirometry, dyspnea, ability to participate). In our study 20.3% of subjects who attended an enrollment visit were determined to be ineligible or refused, which results in an estimated study participation rate of 40.5% (see further details in supplementary materials). This suggests a range of study participation of 35.2% - 40.5%, which is similar to reports of uptake of pulmonary rehabilitation among patients with COPD from the outpatient setting [43,44]. Moreover, our study participation rate is higher than reported in other clinical trials. In a behavioral intervention of patients with congestive heart failure and COPD, Culley and colleagues reported an enrollment rate of 6.5% [45]. Taken together these observations provide further support for the external validity of our study results.
The results of our findings are limited by our single site setting, which may have had a potential effect on the generalizability of the findings. As a result of institutional and regional sociocultural characteristics selected subgroups of the target population may only be represented at a single site. However, because the institution is not a tertiary referral center and training is limited to primary care the patients enrolled are typical of outpatients cared for by primary care providers and pulmonary specialists. Moreover, because the institution is located in a large rural region of Northeast Texas, approximately half of the patients live in rural areas, which we have found associated with more severe impairment and higher health care utilization compared to urban populations [46,47].
Conclusions
A patient registry with proactive contact is an effective method for recruitment of a representative sample of patients with COPD for a clinical trial that attempts to replicate the real world clinical environment. Moreover, this approach to patient recruitment provides a model for future studies utilizing administrative databases and electronic health records. Because patients have substantial differences in motivation and other factors that may affect behavior change (e.g., knowledge, social support, environment), a sample representative of the target population is critical to determine the effectiveness of behavioral interventions. Moreover, results from this recruitment process and study participation rate provide evidence of the large gap between the need for patients to increase physical activity and their ability or interest in participating in an intervention that was designed to minimize their burden of participation. Additional approaches are needed to broaden the reach of programs to engage patients in greater physical activity.
Supplementary Material
Supplement Figure 1.) Enrollment Flowchart for Calculation of Participation Rates
Supplement Table 2.) Inclusion and Exclusion Criteria for Patients in the COPD Registry
Acknowledgments
This study was funded by the National Institutes of Health-National Heart, Lung, and Blood Institute R18 HL092955. Clinicaltrials.gov identifier NCT1108991. Special thanks to the study participants and research staff Toyua Akers, Ginny Harleston, and staff of Cardiopulmonary Services.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- SMART
self-management activation research trial
- UC
usual care
- PASM
physical activity self-management
- CRQ
Chronic Respiratory Questionnaire
- 6MWT
six-minute walk test
- SD
standard deviation
- ICD-9
International Classification of Diseases 9th revision
- RAPA
Rapid Assessment of Physical Activity
- GDS
Geriatric Depression Scale
- CCI
Charlson Co-morbidity Index
Footnotes
Disclaimer: The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–73. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJL, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–57. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 3.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. 2014;176 doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 4.Herland K, Akselsen JP, Skjønsberg OH, Bjermer L. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir Med. 2005;99:11–9. doi: 10.1016/j.rmed.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Bjoernshave B, Korsgaard J, Nielsen CV. Does pulmonary rehabilitation work in clinical practice? A review on selection and dropout in randomized controlled trials on pulmonary rehabilitation. Clin Epidemiol. 2010;2:73–83. doi: 10.2147/clep.s9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson SS, Goss CH, Patel SR, Anzueto A, Au DH, Elborn S, et al. An official American Thoracic Society research statement: comparative effectiveness research in pulmonary, critical care, and sleep medicine. Am J Respir Crit Care Med. 2013;188:1253–61. doi: 10.1164/rccm.201310-1790ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viswanathan M, Golin C, Jones C, Ashok M, Blalock S, Wines R, et al. Medication Adherence Interventions: Comparative Effectiveness Closing the Quality Gap: Revisiting the State of the Science. Rockville, MD: 2012. AHRQ Publication 12 -E010-1. [PMC free article] [PubMed] [Google Scholar]
- 8.Adams SG, Smith PK, Allan PF, Anzueto A, Pugh JA, Cornell JE. Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Arch Intern Med. 2007;167:551–61. doi: 10.1001/archinte.167.6.551. [DOI] [PubMed] [Google Scholar]
- 9.Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 10.Prochaska JJ, Prochaska JO. A Review of Multiple Health Behavior Change Interventions for Primary Prevention. Am J Lifestyle Med. 2011;5 doi: 10.1177/1559827610391883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray J, Fenton G, Honey S, Bara AC, Hill KM, House A. A qualitative synthesis of factors influencing maintenance of lifestyle behaviour change in individuals with high cardiovascular risk. BMC Cardiovasc Disord. 2013;13:48. doi: 10.1186/1471-2261-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SK, Richardson CR, Holleman RG, Larson JL. Physical activity in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003-2006) Heart Lung. 2013;42:235–40. doi: 10.1016/j.hrtlng.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimeno-Santos E, Frei A, Steurer-Stey C, de Batlle J, Rabinovich RA, Raste Y, et al. Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax. 2014 doi: 10.1136/thoraxjnl-2013-204763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006:CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011:CD005305. doi: 10.1002/14651858.CD005305.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Puente-Maestu L, Sánz ML, Sánz P, Cubillo JM, Mayol J, Casaburi R. Comparison of effects of supervised versus self-monitored training programmes in patients with chronic obstructive pulmonary disease. Eur Respir J. 2000;15:517–25. doi: 10.1034/j.1399-3003.2000.15.15.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu WT, Wang CH, Lin HC, Lin SM, Lee KY, Lo YL, et al. Efficacy of a cell phone-based exercise programme for COPD. Eur Respir J. 2008;32:651–9. doi: 10.1183/09031936.00104407. [DOI] [PubMed] [Google Scholar]
- 18.Hospes G, Bossenbroek L, Ten Hacken NHT, van Hengel P, de Greef MHG. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns. 2009;75:274–8. doi: 10.1016/j.pec.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Donesky D, Janson SL, Nguyen HQ, Neuhaus J, Neilands TB, Carrieri-Kohlman V. Determinants of frequency, duration, and continuity of home walking in patients with COPD. Geriatr Nurs. 2011;32:178–87. doi: 10.1016/j.gerinurse.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Martinez CH, Moy ML, Nguyen HQ, Cohen M, Kadri R, Roman P, et al. Taking Healthy Steps: rationale, design and baseline characteristics of a randomized trial of a pedometer-based internet-mediated walking program in veterans with chronic obstructive pulmonary disease. BMC Pulm Med. 2014;14:12. doi: 10.1186/1471-2466-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashmore J, Russo R, Peoples J, Sloan J, Jackson BE, Bae S, et al. Chronic obstructive pulmonary disease self-management activation research trial (COPD-SMART): design and methods. Contemp Clin Trials. 2013;35:77–86. doi: 10.1016/j.cct.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Michael BH. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. 2008;2390:1–10. doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohanpal R, Hooper R, Hames R, Priebe S, Taylor S. Reporting participation rates in studies of non-pharmacological interventions for patients with chronic obstructive pulmonary disease: a systematic review. Syst Rev. 2012;1:66. doi: 10.1186/2046-4053-1-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 25.Schünemann HJ, Griffith L, Jaeschke R, Goldstein R, Stubbing D, Austin P, et al. A comparison of the original chronic respiratory questionnaire with a standardized version. Chest. 2003;124:1421–9. doi: 10.1378/chest.124.4.1421. [DOI] [PubMed] [Google Scholar]
- 26.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Friedman B, Heisel MJ, Delavan RL. Psychometric properties of the 15-item geriatric depression scale in functionally impaired, cognitively intact, community-dwelling elderly primary care patients. J Am Geriatr Soc. 2005;53:1570–6. doi: 10.1111/j.1532-5415.2005.53461.x. [DOI] [PubMed] [Google Scholar]
- 30.Knol M, Groenwold R, Grobbee D. P-values in baseline tables of randomised controlled trials are inappropriate but still common in high impact journals. Eur J Prev Cardiol. 2012;19:231–2. doi: 10.1177/1741826711421688. [DOI] [PubMed] [Google Scholar]
- 31.Roberts C, Torgerson DJ. Understanding controlled trials: Baseline imbalance in randomised controlled trials. BMJ. 1999;319:185–185. doi: 10.1136/bmj.319.7203.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senn S. Testing for baseline balance in clinical trials. Stat Med. 1994;13:1715–26. doi: 10.1002/sim.4780131703. [DOI] [PubMed] [Google Scholar]
- 33.Zwerink M, Brusse-Keizer M, van der Valk PDLPM, Zielhuis GA, Monninkhof EM, van der Palen J, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3:CD002990. doi: 10.1002/14651858.CD002990.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2010;182:890–6. doi: 10.1164/rccm.200910-1579OC. [DOI] [PubMed] [Google Scholar]
- 35.Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156:673–83. doi: 10.7326/0003-4819-156-10-201205150-00003. [DOI] [PubMed] [Google Scholar]
- 36.Glasgow RE, McKay HG, Piette JD, Reynolds KD. The RE-AIM framework for evaluating interventions: what can it tell us about approaches to chronic illness management? Patient Educ Couns. 2001;44:119–27. doi: 10.1016/s0738-3991(00)00186-5. [DOI] [PubMed] [Google Scholar]
- 37.Travers J, Marsh S, Caldwell B, Williams M, Aldington S, Weatherall M, et al. External validity of randomized controlled trials in COPD. Respir Med. 2007;101:1313–20. doi: 10.1016/j.rmed.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Halpin DMG. Lessons from the major studies in COPD: problems and pitfalls in translating research evidence into practice. Prim Care Respir J. 2010;19:170–9. doi: 10.4104/pcrj.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maltais F, Bourbeau J, Shapiro S, Lacasse Y, Perrault H, Baltzan M, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2008;149:869–78. doi: 10.7326/0003-4819-149-12-200812160-00006. [DOI] [PubMed] [Google Scholar]
- 40.Holland AE, Mahal A, Hill CJ, Lee AL, Burge AT, Moore R, et al. Benefits and costs of home-based pulmonary rehabilitation in chronic obstructive pulmonary disease - a multi-centre randomised controlled equivalence trial. BMC Pulm Med. 2013;13:57. doi: 10.1186/1471-2466-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupré A, Raymond B, et al. Reduction of Hospital Utilization in Patients With Chronic Obstructive Pulmonary Disease<subtitle>A Disease-Specific Self-management Intervention</subtitle>. Arch Intern Med. 2003;163:585. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 42.Berry MJ, Rejeski WJ, Miller ME, Adair NE, Lang W, Foy CG, et al. A lifestyle activity intervention in patients with chronic obstructive pulmonary disease. Respir Med. 2010;104:829–39. doi: 10.1016/j.rmed.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris D, Hayter M, Allender S. Improving the uptake of pulmonary rehabilitation in patients with COPD: qualitative study of experiences and attitudes. Br J Gen Pract. 2008;58:703–10. doi: 10.3399/bjgp08X342363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spruit MA, Franssen FME. What to do before pulmonary rehabilitation to improve adherence? Chron Respir Dis. 2010;7:131–3. doi: 10.1177/1479972310377226. [DOI] [PubMed] [Google Scholar]
- 45.Cully JA, Stanley MA, Deswal A, Hanania NA, Phillips LL, Kunik ME. Cognitive-behavioral therapy for chronic cardiopulmonary conditions: preliminary outcomes from an open trial. Prim Care Companion J Clin Psychiatry. 2010;12:1–10. doi: 10.4088/PCC.09m00896blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson BE, Suzuki S, Coultas D, Su F, Lingineni R, Singh KP, et al. Safety-net facilities and hospitalization rates of chronic obstructive pulmonary disease: a cross-sectional analysis of the 2007 Texas Health Care Information Council inpatient data. Int J Chron Obstruct Pulmon Dis. 2011;6:563–71. doi: 10.2147/COPD.S26072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson BE, Coultas DB, Suzuki S, Singh KP, Bae S. Rural-urban disparities in quality of life among patients with COPD. J Rural Health. 2013;29(Suppl 1):s62–9. doi: 10.1111/jrh.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1.) Enrollment Flowchart for Calculation of Participation Rates
Supplement Table 2.) Inclusion and Exclusion Criteria for Patients in the COPD Registry


