Abstract
Atrial fibrillation (AF) is common in patients with life threatening cancer and those undergoing active cancer treatment. However, data from persons with a history of non-life threatening cancer and those who do not require active cancer treatment are lacking. A total of 15,428 (mean age: 66 ± 8.9 years; 47% women; 45% blacks) participants from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study with baseline data on prior cancer diagnosis and AF were included. Participants with life threatening cancer and active cancer treatment within 2 years of study enrollment were excluded. History of cancer was identified using computer-assisted telephone interviews. AF cases were identified from baseline electrocardiogram data and by a self-reported history of a previous diagnosis. Logistic regression was used to examine the cross-sectional association between cancer diagnosis and AF. A total of 2,248 (15%) participants had a diagnosis of cancer and 1,295 (8.4%) had AF. In a multivariable logistic regression model adjusted for socio-demographics (age, sex, race, education, income, and region of residence) and cardiovascular risk factors (systolic blood pressure, high-density lipoprotein cholesterol, total cholesterol, C-reactive protein, body mass index, smoking, diabetes, antihypertensive and lipid-lowering agents, left ventricular hypertrophy, and cardiovascular disease), those with cancer were more likely to have prevalent AF than those without cancer (OR=1.19, 95%CI=1.02, 1.38). Subgroup analyses by age, sex, race, cardiovascular disease, and C-reactive protein yielded similar results. In conclusion, AF was more prevalent in participants with a history of non-life threatening cancer and those who did not require active cancer treatment in REGARDS.
Keywords: atrial fibrillation, cancer, epidemiology
Introduction
The development of atrial fibrillation (AF) after cancer surgery is well-known.1-3 Several studies have suggested that the association between cancer and AF is not limited to the postoperative period.4-8 Case-control studies have reported associations of colorectal and breast cancers with AF,4-6 and registry data from Denmark have reported similar associations with cancers of the colon, lung, kidney, and ovary.7,8 Notably, these reports from non-surgical populations focused on persons with recent cancer diagnoses or those who were admitted to the hospital for cancer treatment.4-8 However, data from persons with a history of non-life threatening cancer and those who do not require active cancer treatment are lacking. An association between AF and cancer from this population would support that cancer represents a comorbid state predisposing to AF. Therefore, the purpose of this study was to examine the association between cancer (non-life threatening or requiring active treatment) and AF using data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study.
Methods
Details of REGARDS have been published previously.9 Briefly, this prospective cohort study was designed to identify causes of regional and racial disparities in stroke mortality. The study population over sampled blacks and residents of the stroke belt (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana). Between January 2003 and October 2007, a total of 30,239 participants were recruited from a commercially available list of residents using postal mailings and telephone data. Demographic information and medical histories were obtained using a computer-assisted telephone interview (CATI) system that was conducted by trained interviewers. Additionally, a brief in-home physical examination was performed approximately 3 to 4 weeks after the telephone interview. During the in-home visit, trained staff collected information regarding medications, blood and urine samples, and a resting electrocardiogram. This analysis examined the cross-sectional association between cancer and AF. Participants were excluded if they were missing the following at baseline: cancer data, AF data, or baseline covariate data.
Cancer diagnosis was determined by a positive response to the following question during the CATI: “Have you ever been diagnosed with cancer?” Persons with life-threatening cancers or those who were receiving or received active cancer treatment within 2 years of study enrollment were excluded from participation in REGARDS. We assumed that participants with an affirmative response to the above question had survived cancer, no longer required treatment, or had cancers with indolent courses (non-life threatening).
AF was identified at baseline by the scheduled electrocardiogram and also from a self-reported history of a physician diagnosis during the CATI surveys. The electrocardiograms were read and coded at a central reading center (EPICARE, Wake Forest School of Medicine, Winston-Salem, NC) by analysts who were blind to other REGARDS data. Self-reported AF was defined as an affirmative response to the following question: “Has a physician or a health professional ever told you that you had atrial fibrillation?”10
Participant characteristics collected during the initial REGARDS in-home visit were used in this analysis. Age, sex, race, income, education, and smoking status were self-reported. Annual household income was dichotomized at <$20,000 or ≥$20,000. Similarly, education was categorized into “high school or less,” or “some college or more.” Smoking was defined as ever (e.g., current and former) or never smoker. Fasting blood samples were obtained and assayed for total cholesterol, high-density lipoprotein (HDL) cholesterol, glucose, creatinine, C-reactive protein (CRP), and urine albumin-to-creatinine ratio (ACR). Diabetes was defined as a fasting glucose level ≥126 mg/dL (or a non-fasting glucose, ≥200 mg/dL among those failing to fast) or self-reported diabetes medication use. Regular aspirin use and antihypertensive and lipid-lowering medication use were defined by the self-reported current use of these medications during the CATI surveys. Body mass index was computed as the weight in kilograms divided by the square of the height in meters. After the participant rested for 5 minutes in a seated position, blood pressure was measured using a sphygmomanometer. Two values were obtained following a standardized protocol and averaged. Using baseline electrocardiogram data, left ventricular hypertrophy was defined by the Sokolow-Lyon Criteria.11 Cardiovascular disease was defined as a history of coronary heart disease or stroke. Coronary heart disease was confirmed by self-reported history of myocardial infarction, coronary artery bypass grafting, coronary angioplasty or stenting, or if evidence of prior myocardial infarction was present on the baseline electrocardiogram. Prior stroke was ascertained by participant self-reported history.
Categorical variables were reported as frequency and percentage while continuous variables were reported as mean ± standard deviation. Statistical significance of differences for categorical variables was tested using the chi-square method and the Wilcoxon rank-sum procedure for continuous variables. Logistic regression was used to compute odds ratios (OR) and 95% confidence intervals (CI) for the association between cancer diagnosis and AF. Multivariable models were adjusted as follows: Model 1 adjusted for age, sex, race, education, income, and geographic region; Model 2 included covariates in Model 1 with the addition of systolic blood pressure, HDL-cholesterol, total cholesterol, log(CRP), body mass index, smoking, diabetes, aspirin, antihypertensive medications, lipid-lowering therapies, left ventricular hypertrophy, and cardiovascular disease; Model 3 included Model 2 covariates plus serum creatinine and log(ACR). Additionally, subgroup analyses were performed to evaluate effect modification by age (dichotomized at 65 years), sex, race, cardiovascular disease, and CRP (dichotomized at the median value) by including interaction terms in the model. Statistical significance for all comparisons including interactions was defined as p <0.05. SAS Version 9.3 (Cary, NC) was used for all analyses.
Results
Of the 30,239 participants from the original REGARDS cohort, 56 were excluded for data anomalies and 12,105 participants had missing data regarding prior cancer. Of those that remained, 362 participants with missing AF data and 2,288 participants with either missing baseline characteristics or missing medication data also were excluded. A total of 15,428 study participants (mean age: 66 ± 8.9 years; 47% women; 45% blacks) were included in the final analysis.
A total of 2,248 (15%) participants reported a cancer diagnosis and 1,295 (8.4%) had evidence of AF. Those with a history of cancer (n=249, 11%) were more likely to have AF than those without (n=1,046, 7.9%) (p<0.0001). The prevalence of AF in those with and without cancer by age, sex, race, cardiovascular disease, and CRP is shown in Figure 1.
Figure 1. Prevalence of Atrial Fibrillation in Participants with and without Cancer.
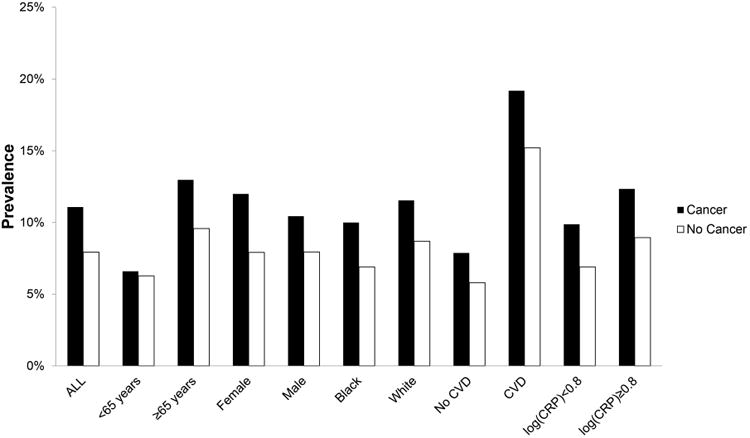
CVD=cardiovascular disease; CRP=C-reactive protein.
Baseline characteristics by cancer diagnosis are shown in Table 1. Persons with a cancer diagnosis were more likely to be older, male, white, and to report a history of lower educational attainment, smoking, aspirin use, and cardiovascular disease than those without a cancer diagnosis. Those with cancer also were more likely to have lower values for body mass index, total cholesterol, and HDL-cholesterol, and to have higher values for serum creatinine and log(ACR) compared with persons without a cancer diagnosis.
Table 1. Baseline Characteristics (N=15,428).
| Characteristic | Prior Cancer (n=2,248) | No Prior Cancer (n=13,180) | P-value* |
|---|---|---|---|
| Age, mean ± SD (years) | 70 ± 8.6 | 65 ± 8.7 | <0.0001 |
| Male | 1,322 (59%) | 6,856 (52%) | <0.0001 |
| Black | 680 (30%) | 5,601 (43%) | <0.0001 |
| Region | |||
| Stroke buckle | 331 (15%) | 2,047 (16%) | |
| Stroke belt | 806 (36%) | 4,628 (35%) | |
| Non-belt | 1,111 (49%) | 6,505 (49%) | 0.57 |
| Education, high school or less | 815 (36%) | 5,280 (40%) | 0.0006 |
| Annual income, <$20,000 | 397 (18%) | 2,508 (19%) | 0.13 |
| Body mass index, mean ± SD (kg/m2) | 28 ± 5.5 | 29 ± 6.0 | <0.0001 |
| Ever smoker | 1,354 (60%) | 7,549 (57%) | 0.0088 |
| Diabetes mellitus | 460 (20%) | 2,912 (22%) | 0.084 |
| Systolic blood pressure, mean ± SD (mm Hg) | 129 ± 16 | 129 ± 17 | 0.72 |
| Total cholesterol, mean ± SD (mg/dL) | 187 ± 39 | 192 ± 40 | <0.0001 |
| HDL-cholesterol, mean ± SD (mg/dL) | 50 ± 16 | 52 ± 16 | 0.0006 |
| Aspirin use | 1,097 (49%) | 5,976 (45%) | 0.0024 |
| Antihypertensive medication use | 1,233 (55%) | 7,014 (53%) | 0.15 |
| Lipid-lowering medication use | 768 (34%) | 4,311 (33%) | 0.17 |
| Log(CRP), mean ± SD (mg/L) | 0.79 ± 1.2 | 0.81 ± 1.2 | 0.17 |
| Left ventricular hypertrophy | 242 (11%) | 1,428 (11%) | 0.92 |
| Cardiovascular disease | 636 (28%) | 2,993 (23%) | <0.0001 |
| Serum creatinine, mean ± SD (mg/dL) | 0.99 ± 0.59 | 0.93 ± 0.48 | <0.0001 |
| Log(ACR), mean ± SD (mg/g) | 2.5 ± 1.3 | 2.4 ± 1.3 | <0.0001 |
Statistical significance for categorical variables tested using the chi-square method and for continuous variables the Wilcoxon-rank sum was used.
ACR=albumin-to-creatinine ratio; AF=atrial fibrillation, CRP=C-reactive protein; HDL=high-density lipoprotein; SD=standard deviation.
Those with a history of cancer were more likely to have AF at baseline than those without a diagnosis (unadjusted OR=1.45, 95%CI=1.25, 1.67). After adjustment for demographics, cardiovascular risk factors, and potential confounders, cancer was significantly associated with AF (Table 2). Similar results were observed when the analysis was stratified by age, sex, race, cardiovascular disease, and CRP (Table 3).
Table 2. Association of Cancer with Atrial Fibrillation (N=15,428).
| AF cases | Model 1* OR (95%CI) | P-value | Model 2† OR (95%CI) | P-value | Model 3‡ OR (95%CI) | P-value | |
|---|---|---|---|---|---|---|---|
| No Cancer | 1,046/13,180 | 1.0 | - | 1.0 | - | 1.0 | - |
| Cancer | 249/2,248 | 1.23 (1.06, 1.43) | 0.0072 | 1.19 (1.02, 1.38) | 0.028 | 1.18 (1.01, 1.38) | 0.036 |
Adjusted for age, sex, race, education, income, and geographic region
Adjusted for Model 1 covariates plus systolic blood pressure, HDL-cholesterol, total cholesterol, log(CRP), body mass index, smoking, diabetes, antihypertensive medications, lipid-lowering therapies, left ventricular hypertrophy, and prior history of cardiovascular disease.
Adjusted for Model 2 covariates plus serum creatinine and log(ACR).
ACR=albumin-to-creatinine ratio; AF=atrial fibrillation; CI=confidence interval; CRP=C-reactive protein; HDL=high-density lipoprotein; OR=odds ratio.
Table 3. Subgroup Analyses (N=15,428).
| Variable | Model 1* OR (95%CI) | P-value | Model 2† OR (95%CI) | P-value | Model 3‡ OR (95%CI) | P-value | Interactionδ P-value |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| <65 | 1.10 (0.80, 1.52) | 0.57 | 1.05 (0.75, 1.45) | 0.79 | 1.04 (0.75, 1.45) | 0.80 | 0.11 |
| ≥65 | 1.34 (1.13, 1.59) | 0.0007 | 1.27 (1.07, 1.51) | 0.0066 | 1.26 (1.06, 1.50) | 0.011 | |
| Sex | |||||||
| Female | 1.44 (1.15, 1.80) | 0.0015 | 1.34 (1.07, 1.69) | 0.011 | 1.37 (1.08, 1.72) | 0.0084 | 0.37 |
| Male | 1.08 (0.88, 1.32) | 0.46 | 1.06 (0.87, 1.31) | 0.56 | 1.04 (0.84, 1.28) | 0.72 | |
| Race | |||||||
| Black | 1.49 (1.13, 1.97) | 0.0045 | 1.41 (1.06, 1.86) | 0.018 | 1.34 (1.003, 1.80) | 0.048 | 0.59 |
| White | 1.12 (0.94, 1.34) | 0.21 | 1.09 (0.91, 1.31) | 0.36 | 1.10 (0.92, 1.32) | 0.31 | |
| Cardiovascular disease | |||||||
| No | 1.22 (0.99, 1.50) | 0.055 | 1.19 (0.97, 1.47) | 0.092 | 1.19 (0.97, 1.47) | 0.10 | 0.79 |
| Yes | 1.21 (0.96, 1.51) | 0.11 | 1.17 (0.93, 1.46) | 0.19 | 1.15 (0.91, 1.45) | 0.24 | |
| Log(CRP) (mg/L) | |||||||
| <0.80 | 1.26 (1.01, 1.57) | 0.042 | 1.23 (0.98, 1.53) | 0.075 | 1.21 (0.96, 1.51) | 0.11 | 0.69 |
| ≥0.80 | 1.19 (0.97, 1.46) | 0.097 | 1.15 (0.94, 1.42) | 0.18 | 1.15 (0.93, 1.42) | 0.19 |
Adjusted for age, sex, race, education, income, and geographic region
Adjusted for Model 1 covariates plus systolic blood pressure, HDL-cholesterol, total cholesterol, log(CRP), body mass index, smoking, diabetes, antihypertensive medications, lipid-lowering therapies, left ventricular hypertrophy, and prior history of cardiovascular disease.
Adjusted for Model 2 covariates plus serum creatinine and log(ACR).
Interactions tested using Model 2.
ACR=albumin-to-creatinine ratio; CI=confidence interval; CRP=C-reactive protein; HDL=high-density lipoprotein; OR=odds ratio.
Discussion
In this analysis from REGARDS, AF was more prevalent among those with a history of cancer (non-life threatening or requiring active treatment). Our results were consistent across several subgroups of REGARDS participants.
Prior studies have reported an association between cancer and AF in non-surgical populations, suggesting that cancer itself is a comorbid condition that predisposes to AF instead of a postoperative complication.4-8 A case-control study consisting of 12,304 veterans in the United States showed that AF preceded a diagnosis of colon cancer.4 Similar results were observed in a small case-control study of 1,463 patients admitted for surgical treatment of colon cancer in Italy, with colon cancer patients having twice the prevalence of AF compared with controls.5 Additionally, a population-based case-control study from northern Denmark consisting of 28,833 AF cases and 283,260 sex-, age-, and county-matched population controls showed that persons with AF were more likely to be diagnosed with colorectal cancer within 90 days before their AF diagnosis (OR=11.8, 95%CI=9.3, 14.9).7 AF also has been significantly associated with breast cancer patients in a case-control study of 2,339 patients admitted to a surgical service in Italy.6 Furthermore, a cohort study using Danish registry data showed that persons who were diagnosed with AF had an increased risk (standardized incidence ratios) of a cancer diagnosis (e.g., lung, kidney, colon) within 3 months of their AF diagnosis.8
The aforementioned studies support the claim that cancer predisposes to AF development. However, these reports were limited to persons who were recently diagnosed with malignancy or to those who were undergoing active treatment (e.g., admission to the hospital for cancer treatment).4-8 To our knowledge, we are the first to describe an association between cancer and AF among persons who survived their cancer or no longer required active treatment, suggesting that the risk of AF extends beyond the diagnosis and treatment periods. Participation in REGARDS required no evidence of life threatening cancer or active treatment at enrollment. Therefore, our results suggest that the observed association between cancer and AF exists among milder forms of malignancy (e.g., skin cancer). Additionally, our findings are generalizable to the black and white population aged 45 years and older in the United States and are not limited to specific populations or patients who are admitted to the hospital for cancer treatment. Although our results suggest that cancer is associated with a higher prevalence of AF, further studies are needed to confirm our findings and also to examine if AF is more prevalent with certain cancer types and if the association differs by cancer treatment (e.g., chemotherapy, radiation therapy).
The underlying mechanisms that explain the increased prevalence of AF among those with cancer remain unclear. Shared risk factors (e.g., advanced age, obesity, diabetes, smoking) for both cancer and AF possibly explain this association.12-15 Additionally, alterations in inflammation and/or hemostasis are common in both malignancy and AF.16-18 Potentially, dysfunctional regulation of these processes in cancer increases AF risk through remodeling of the left atria and pulmonary veins, structures that have been implicated in the ectopy of AF.19 Also, the development of AF possibly represents a complication of medical cancer therapy as numerous chemotherapeutic agents are associated with AF arrhythmiogenesis.20 However, our study was unable to determine the underlying mechanisms for this association and further research is needed to explore these hypotheses.
Our results should be interpreted in the context of certain limitations. We had to exclude many participants with missing cancer data. Although these data were collected, the response to the cancer question was not updated until 2005 which resulted in missing values. However, these data were missing randomly based on the date participants were seen and were unlikely to affect our estimates. Also, the prevalence of AF in those with missing cancer data was similar to that in those with available data (participants with cancer data=8.4%; participants without cancer data=8.6%; p=0.55). Similar to other cross-sectional analyses, we were unable to establish the temporal relationship between cancer and AF. While we offer several pathways through which cancer could lead to AF, it is difficult to speculate on a mechanism through which AF would lead to cancer. Therefore, our results suggest that the direction of causation is cancer increasing the risk of AF, rather than the opposite. Cancer type was not recorded in REGARDS and the association between cancer and AF may differ by type of cancer. The date of cancer diagnoses including last treatment and whether active cancer was present at baseline was not recorded. Also, the development of AF potentially varies by cancer stage and previous treatment type (e.g., surgery, radiation, and chemotherapy) and these data were not recorded. Finally, although we adjusted for several potential confounders, residual confounding remains a possibility.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Funding: This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Onaitis M, D'Amico T, Zhao Y, O'Brien S, Harpole D. Risk factors for atrial fibrillation after lung cancer surgery: analysis of the Society of Thoracic Surgeons general thoracic surgery database. Ann Thorac Surg. 2010;90:368–374. doi: 10.1016/j.athoracsur.2010.03.100. [DOI] [PubMed] [Google Scholar]
- 2.Imperatori A, Mariscalco G, Riganti G, Rotolo N, Conti V, Dominioni L. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg. 2012;7:4. doi: 10.1186/1749-8090-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardinale D, Martinoni A, Cipolla CM, Civelli M, Lamantia G, Fiorentini C, Mezzetti M. Atrial fibrillation after operation for lung cancer: clinical and prognostic significance. Ann Thorac Surg. 1999;68:1827–1831. doi: 10.1016/s0003-4975(99)00712-2. [DOI] [PubMed] [Google Scholar]
- 4.Muller AD, Sonnenberg A, Wasserman IH. Diseases preceding colon cancer. A case-control study among veterans. Dig Dis Sci. 1994;39:2480–2484. doi: 10.1007/BF02087670. [DOI] [PubMed] [Google Scholar]
- 5.Guzzetti S, Costantino G, Sada S, Fundaro C. Colorectal cancer and atrial fibrillation: a case-control study. Am J Med. 2002;112:587–588. doi: 10.1016/s0002-9343(02)01029-x. [DOI] [PubMed] [Google Scholar]
- 6.Guzzetti S, Costantino G, Vernocchi A, Sada S, Fundaro C. First diagnosis of colorectal or breast cancer and prevalence of atrial fibrillation. Intern Emerg Med. 2008;3:227–231. doi: 10.1007/s11739-008-0124-4. [DOI] [PubMed] [Google Scholar]
- 7.Erichsen R, Christiansen CF, Mehnert F, Weiss NS, Baron JA, Sorensen HT. Colorectal cancer and risk of atrial fibrillation and flutter: a population-based case-control study. Intern Emerg Med. 2012;7:431–438. doi: 10.1007/s11739-011-0701-9. [DOI] [PubMed] [Google Scholar]
- 8.Ostenfeld EB, Erichsen R, Pedersen L, Farkas DK, Weiss NS, Sorensen HT. Atrial fibrillation as a marker of occult cancer. PLoS One. 2014;9:e102861. doi: 10.1371/journal.pone.0102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 10.Soliman EZ, Howard G, Meschia JF, Cushman M, Muntner P, Pullicino PM, McClure LA, Judd S, Howard VJ. Self-reported atrial fibrillation and risk of stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2011;42:2950–2953. doi: 10.1161/STROKEAHA.111.621367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 13.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Kearney J, Willett WC. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. J Natl Cancer Inst. 1994;86:183–191. doi: 10.1093/jnci/86.3.183. [DOI] [PubMed] [Google Scholar]
- 15.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 16.Sorensen HT, Svaerke C, Farkas DK, Christiansen CF, Pedersen L, Lash TL, Prandoni P, Baron JA. Superficial and deep venous thrombosis, pulmonary embolism and subsequent risk of cancer. Eur J Cancer. 2012;48:586–593. doi: 10.1016/j.ejca.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 18.Siemes C, Visser LE, Coebergh JW, Splinter TA, Witteman JC, Uitterlinden AG, Hofman A, Pols HA, Stricker BH. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24:5216–5222. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, Takeishi Y, Hirono O, Itoh M, Matsui M, Nakamura K, Tamada Y, Kubota I. C-reactive protein elevation predicts the occurrence of atrial structural remodeling in patients with paroxysmal atrial fibrillation. Heart Vessels. 2005;20:45–49. doi: 10.1007/s00380-004-0800-x. [DOI] [PubMed] [Google Scholar]
- 20.Kaakeh Y, Overholser BR, Lopshire JC, Tisdale JE. Drug-induced atrial fibrillation. Drugs. 2012;72:1617–1630. doi: 10.2165/11633140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]


