Abstract
Remembering the sequence of events is critical for deriving meaning from our experiences and guiding behavior. Prior investigations into the function of the human hippocampus have focused on its more general role in associative binding, but recent work has focused on understanding its specific role in encoding and preserving the temporal order of experiences. In this review, we summarize recent work in humans examining hippocampal contributions to sequence learning. We distinguish the learning of sequential relationships through repetition from the rapid, episodic acquisition of sequential associations. Together, this research begins to clarify the link between hippocampal representations and the preservation of the order of events.
Keywords: hippocampus, sequence memory, episodic memory, context, prediction
Sequences in memory
Much of our experience is perceived and understood through the sequences of events that occur. Episodic memory, which allows us to relive events from our past, is defined by the recovery of the unique context in which the event occurred [1]. The context can, but need not always, include spatial details and various forms of temporal details including how the event unfolded in time. Furthermore, many of our everyday experiences are repeated sequences of highly similar events, such as one’s morning commute to work. Thus, learning the sequential order of events that are commonly encountered allows us to form predictions about the impending future and plan upcoming actions accordingly. Since sequential representations play such a defining role in learning and memory, understanding how sequences of events are encoded in a way that preserves their temporal order is fundamental to understanding memory.
The importance of the human hippocampus in associative encoding more broadly is well established (for reviews, see [2–5]). However, whether and how the human hippocampus encodes sequential representations is a strong focus of current investigations. Initial evidence that the hippocampus plays an important role in representing sequential representations was revealed by the groundbreaking result from rodent electrophysiology that hippocampal place cells replay (see Glossary) in the same sequential order as during a prior learning experience [6]. More recently, new evidence has emerged that hippocampal cells, referred to as ‘time cells’ (see Glossary), may code for specific moments in time, or temporal positions [7,8]. While studies on rodents and nonhuman primates are beyond the scope of this review (but see Box 1), these findings highlight potential hippocampal mechanisms for encoding and preserving the sequence of encountered events. However, the vast majority of the studies identifying sequential neural firing during an experience and its post-experience replay are of rodents who are navigating through space over hundreds of trials. Thus, many questions remain regarding how a sequence of events is encoded after only a single experience and in the absence of spatial navigation. Furthermore, which aspects of the temporal coding of experience are related to the successful recovery of temporal information in memory is still not well understood. Thus, the current review will highlight recent investigations of the role of the human hippocampus in the encoding and representation of temporally extended sequences. We organize our discussion by offering a potential distinction between the representation of sequences acquired over multiple learning repetitions and the episodic encoding of novel sequences.
Box 1. Contributions of research from nonhuman animals.
Although the focus of this review is the human hippocampus, much of the existing literature on sequence learning comes from work in non-human animals. These studies offer the unique ability to directly record neuronal activity from healthy tissue, as well as create focal lesions to assess the necessity of a region for a behavioral task. Thus, we provide some discussion of this here but refer readers to other recent reviews for a more in depth discussion [8,65–68].
Lesion work in rodents clearly demonstrates the necessity of the hippocampus for sequence memory [69,70]. Complementary electrophysiological data have allowed researchers to characterize changes in the hippocampal neural signature with sequence repetition. For example, place cells (see Glossary) that initially fire late in a theta cycle (see Glossary) have been found to fire at earlier phases of theta as the rodent repeatedly traverses a track or maze. This process, dubbed ‘theta phase precession’, is interpreted as evidence for a prospective code in the hippocampus that may be used to predict upcoming locations [71]. Furthermore, representations of recent and upcoming locations in place cell assemblies are coded within the theta cycle as compressed, ordered sequences [66,67]. Importantly, the content of these theta sequences depends on environmental context and distance from surrounding landmarks [72,73], supporting the notion that these sequences provide a memory-based prediction of possible upcoming spatial locations. This sequential representation of items in theta may provide a cellular mechanism for sequence learning through repetition described in the main text
In addition to hippocampal representations of place cell sequences, recent work provides evidence for hippocampal neurons that respond at particular moments in time, or temporal positions, during a delay period (dubbed ‘time cells’, see [8] for review). Coding of temporal position has also been reported in the monkey hippocampus during performance of an order memory task [74]. Beyond these relatively short-scale temporal representations, a recent study found that place cells in hippocampal subregion CA1 show a gradually changing firing pattern across multiple days compared to a more stable pattern in subregion CA3 [12]. Together these data suggest that hippocampal neural activity may provide a substrate for representing temporal information across multiple time scales. The more stable signals may provide a cellular mechanism that could potentially support context-mediated episodic sequence encoding discussed in the main text.
Laying the groundwork
Theoretical models have proposed various potential mechanisms by which hippocampal processes could bridge temporally disparate events into coherent, bound associative memories. One proposal is that context-sensitive cells may develop from background neural firing in the hippocampal subregion CA3 due to its recurrent excitatory connections [9]. Associations formed between cells coding for items in a sequence and these background context cells could result in indirect associations between items that span a temporal delay. Similarly, other models have proposed that integrator or time cells in the medial temporal lobe (MTL), which change their firing rates slowly, may provide a background context representation that can serve as a substrate for linking items across time [10,11]. Interestingly, recent evidence has shown that population activity in hippocampal subregion CA1 changes gradually over time [12,13], consistent with this notion of a slowly changing background context representation. Another proposed theory highlights the potential role of slow hippocampal oscillations in linking sequential items through their subsequent maintenance [14]. Specifically, the theory posits that recently active items can be maintained in a temporally compressed buffer within the hippocampal theta oscillation such that cells representing each item can fire sequentially within the short time range of long-term potentiation (also see Box 1).
In experimental work, recent neuroimaging data have linked the magnitude of the hippocampal response with successful mnemonic binding of representations across time. For example, the fMRI signal in the hippocampus, as well as in the MTL cortex, is significantly greater during the successful versus unsuccessful encoding of associations presented across temporal delays [15–17]. Furthermore, the magnitude of this hippocampal subsequent memory effect has been shown to increase with the degree of spatiotemporal discontinuity between the studied representations [18].
Interestingly, the role of the hippocampus in bridging representations across time does not appear to be limited to episodic memories. It has been shown that patients with hippocampal damage are intact on delay conditioning (see Glossary) but impaired on the acquisition of trace conditioning (see Glossary) when the conditioned and unconditioned stimuli do not overlap in time [19] and likewise show impairments in probabilistic learning when a short delay intervenes before feedback [20]. Thus, it is clear that the demand on hippocampal processing increases with temporal gaps and that this is related to the successful binding of the presented representations. However, these studies do not directly address to what extent hippocampal function is related to maintaining the fidelity of sequential associations such that their temporal order can later be retrieved. Thus, the remainder of the review focuses on recent empirical work aimed at addressing how the human hippocampus might support the encoding and recovery of sequential relationships.
Two routes to sequence learning?
It is important to consider that there may be multiple cognitive and neural mechanisms that support hippocampal sequence learning. In particular, we suggest that there may be a distinction between single-trial or episodic sequence encoding and the representation of a well-learned, repeated, predictable sequence, because each re-exposure to a sequence may modify the learned representation. Thus, recent work examining changes in hippocampal activation as a function of many sequence repetitions will be summarized separately from work examining episodic encoding of novel sequences.
Sequence learning through repetition refers to the learning of sequential relationships over multiple repeated trials that can, but does not have to, occur without explicit awareness. The notion is that repeated exposures to temporal regularities might drive the development and strengthening of a predictive code in the hippocampus [21] that contains information about the order in which the sequence of items typically occurs (see Fig 1A). Through repetition, it may be more adaptive for sequential associations to be supported by features that are invariant across repetitions (in this case, the relationships between items). By contrast, episodic sequence encoding describes the encoding of a novel sequence of events. In this case, by definition, there is no repetition of the same item pairings, and, thus, sequential encoding may be biased to rely more on the unique contextual features of the event. Similarity in contextual features across items in a sequence may promote binding of those representations in a manner that preserves the temporal structure of the event. The contextual features that may be shared could include a slowly drifting temporal context representation (e.g., [22]; see Glossary), but may also include other stable internal or external features, such as a spatial context (e.g., [23]), a schema (e.g., [24]), or an event model (e.g., [25]; see Fig 2A).
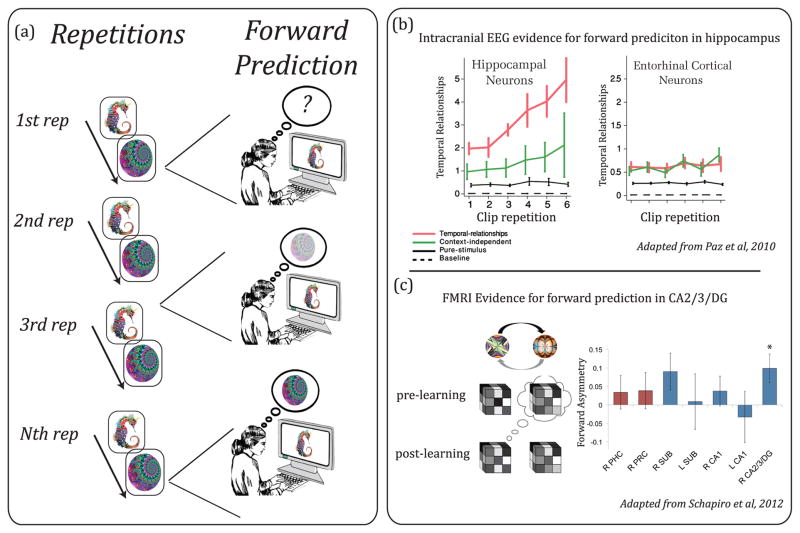
Figure 1. Sequence learning through repetition.
(A) Schematic of learning the temporal relationship between sequential items with repetition. Across repeated exposures to of the first item (N) followed by the second item (N+1), a forward prediction gradually emerges such that exposure to item N triggers the recovery of item N+1. (B) Neural evidence for forward prediction in the hippocampus. With repeated exposure to the same movie clips, firing rates of neurons at time N became more similar to firing rates at time N+1 in single-units of the hippocampus but not the entorhinal cortex. Red line: temporal relationships during clip presentation; Green line: temporal relationships during blank screen preceding clip; Black line: relationship between timepoints shuffled across repetitions (i.e, stimulus-driven); Dashed line: relationship between timepoints shuffled within clip (i.e., baseline). Reproduced, with permission, from [39]. (C) Functional MRI evidence for forward prediction in the hippocampus. After repeated exposure to two stimuli (fractals) N and N+1, fMRI patterns in right CA2/3/dentate gyrus elicited by item N became more similar to the prelearning pattern of N+1 compared to the extent to which the pattern elicited by N+1 becomes similar to the prelearning pattern of N. This measure is referred to as forward asymmetry. Reproduced, with permission, from [42].
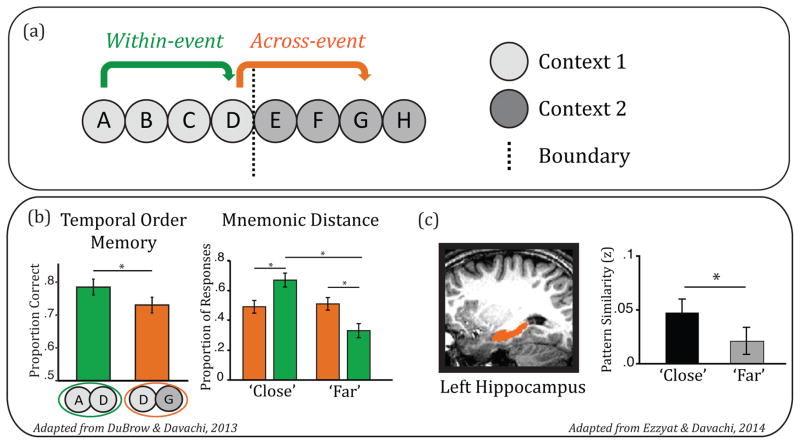
Figure 2. Episodic sequence encoding.
(A) Schematic of a sequence of items in which the first four items share a context whereas the second four items share a different context. Sequence memory for items within a shared context may be enhanced compared to items in different contexts that span a boundary. (B) Behavioral data showing that, compared to across boundaries, order memory is relatively greater within events (left, [60]) and items within events are rated as having occurred more closely together (right, reproduced, with permission, from [61]). (C) Functional MRI evidence that the hippocampus contributes to temporal memory. Patterns of activation in the left hippocampus during encoding of items in a sequence show greater similarity when those items are later rated as having occurred more closely together [61].
Sequence learning through repetition
There is now growing evidence that the human hippocampus is sensitive to repeating sequences. Prior fMRI work across a variety of sequence learning paradigms has shown that hippocampal activation is generally enhanced during sequence learning [26–28], and a recent case study showed that a patient with complete loss of bilateral hippocampus and some surrounding cortex failed to show learning of even simple sequential associations despite showing intact item memory [29].
Recent fMRI work has begun to test specific mechanistic hypotheses by examining hippocampal activation on individual trials embedded in repeating sequences. For example, it has been shown that activation in the hippocampus is enhanced both when encountered items predict the next item in a sequence [30] and at the branch point of two overlapping sequences where there may be ambiguity over the content of the next item in the sequence [27,28,31,32]. In these cases where there are multiple possible transitions, the increased hippocampal activation may reflect hippocampal pattern completion. That is, when presented with a partial input of the first one or more items in a previously experienced sequence, hippocampal subregion CA3 may serve the role of completing the prior pattern, or remaining elements of the sequence, thereby generating a prediction about what will be seen next [33–35]. Thus, this pattern completion may underlie the increase in the hippocampal response at the time in which the prediction is triggered, perhaps especially at branch points where uncertainty about the next item in the sequence could result in the generation of multiple competing predictions in the hippocampal network that need to be resolved. Furthermore, ambiguity might recruit additional top-down retrieval mechanisms that have also previously been shown to enhance the hippocampal response [36].
In cases where sequence acquisition involves the generation of predictions about the content of the next possible item, subsequent presentation of that item may result in an attenuated response in the same way that an attenuated response is observed on the second presentation of perceptually repeating items [37]. In this way, predicting and then seeing an item may look very similar to seeing an item twice and, hence, result in repetition attenuation. Indeed, an enhanced repetition attenuation effect has been observed in the parahippocampal gyrus for repeated scenes predicted by the preceding sequence [38].
Another approach to identifying whether sequence acquisition is associated with forward prediction has been to look for evidence of representational change across repetitions such that the pattern of neural firing and fMRI activation during earlier parts of a sequence become more similar to those present in later parts of a sequence, suggestive of a process of forward reinstatement. Consistent with this notion, recent data have shown that the firing rate of hippocampal neurons becomes increasingly similar with repetition. Researchers [39] recorded from the hippocampi of epileptic patients while the patients viewed repeating movie clips. They found that the firing rates of hippocampal neurons between successive timepoints gradually became more similar to each other as the movie clips were repeated (see Fig 1B). Specifically, the population firing at time N began to resemble that seen at N+1, and this effect was observed only in the hippocampus and not in surrounding entorhinal cortex. Furthermore, the extent to which an individual showed an increase in hippocampal temporal similarity with repetition was related to his or her later memory for the content of the movie clips.
Similarly, using fMRI, two recent experiments provide evidence that hippocampal patterns of activation also increase in similarity with sequence learning. In one study, [40] participants were scanned while encoding repeating sequences of letters for immediate serial recall. Across repetitions of identical sequences, the pattern of hippocampal activation gradually increased in similarity. Interestingly, of the few brain regions that showed this increase, only the hippocampus additionally showed that the similarity between distinct sequences decreased with repetition. Importantly, the repeating and distinct sequences all contained the same items, thus the enhanced and decreased hippocampal pattern similarity with learning highlights that the hippocampal representation is sensitive to the order of items in the sequences.
Another study examined fMRI responses to individual objects in learned sequences [41], allowing examination of position effects. They observed that pattern similarity in the hippocampus was greater for objects in their learned sequential positions than for the same objects in random positions and different objects in identical positions. This pattern was observed only in the hippocampus, whereas perirhinal cortex showed enhanced similarity for identical objects regardless of position, and parahippocampal cortex showed enhanced similarity to the same positions within random sequences. Interestingly, they also observed greater dissimilarity to identical items shared across different sequences, suggesting that the hippocampal response to the overlapping item was related to the other distinct items that made the sequences unique. Thus, using different methodologies, these studies converge in showing that hippocampal patterns elicited by sequences differentiate and stabilize with repetition.
While the prior data are consistent with the idea that individual items learned in a sequence come to predict the upcoming items, items in learned sequences were never tested out of sequence. Thus, it is unclear whether intact sequential context is necessary to show sequence prediction or whether sequence learning alters the representation of individual items. Furthermore, the prior data could result from symmetric sequential representations (e.g., in an ABC sequence, B is equally predictive of A and C) rather than forward-biased pattern completion (e.g., a tendency for B to predict C over A). A recent high-resolution fMRI study directly tested the notion that sequence acquisition is associated with representational change of individual items in a forward manner [42]. After multiple presentations of pairs of items whose temporal relationships were kept constant and, hence, presentation of N was always followed by N+1, they found an increase in the similarity of hippocampal and MTL activation patterns elicited by the N and N+1 stimuli. Furthermore, critical to the interpretation of a forward prediction, the authors were able to measure directionality in the change in similarity with experience by including sessions before and after learning where the individual stimuli were presented in random order. They found that the change in pattern similarity before and after learning showed asymmetry in the CA2/3/dentate gyrus subregions of the hippocampus such that the pattern elicited by the first item of the pair (N) became more similar to the pre-learning pattern of the second item (N+1) than the pattern of the second item (N+1) became similar to the first item (N) (see Fig 1C). This asymmetry suggests that after repeated exposures to a reliable sequential relationship, exposure to the first fractal leads to a forward prediction, but not vice versa.
Taken together, these data show that exposure to repeating sequences is associated with changes in the hippocampus that yield important insights into the potential underlying mechanisms supporting sequence memory. In particular, there is preliminary evidence that after repeated exposures, the onset of a sequence is associated with the forward reinstatement of a hippocampal representation of the remainder of the sequence. Thus, forward prediction via hippocampal pattern completion may be an important feature of sequence learning.
Episodic sequence encoding
One particular challenge for the development of theoretical approaches to understanding the mechanisms of sequence memory formation is that, outside of the laboratory setting, sequence memory is often formed even after a single exposure and, hence, repetition as a necessary component of learning is not viable. In paradigms testing the free recall of once-presented sequences, one of the most ubiquitous findings is the tendency for recall to exhibit temporal clustering [43], meaning that recall transitions tend to be to neighboring items from encoding. This contiguity effect has been found to be asymmetric such that recall transitions also tend to be to items that appeared after the recalled item in the sequence. Thus, despite there being no constraint on the order of recall, memory behavior shows that recall is structured by the sequence of presentation as if this is rapidly and automatically encoded.
The temporal context model [22] was developed, in part, to account for this asymmetry in memory recall. In the model, during encoding, items are bound by the hippocampus to a slowly drifting temporal context representation, which in turn drives the context state forward [44]. During recall, it is hypothesized that when item N is recalled, this reinstates item N’s corresponding temporal context and this then facilitates the recall of neighboring items (N−1 and N+1) that shared a similar context state. Critically, due to the asymmetrical nature of the temporal context representation, namely that the representation of N is encoded in N+1 but not vice versa, this theory can account for the asymmetry in the contiguity effect. Thus, it is possible that contextual asymmetry provides sequence information that enables the reconstruction of sequential order from memory.
Recent behavioral evidence suggests that even after a single experience, context repetition can cue sequence reactivation. One such study demonstrated that after exposure to a triplet sequence (ABC), simply repeating the first two items (AB) of the sequence led to better memory for the third (C) item [45]. Using a similar paradigm, another group recently reported that upon repeating the first two items (AB) in the sequence and then presenting an unpredicted novel item (D), the extent to which there was neural evidence for the prediction of the third item (C) was related to forgetting of that initial third item [46]. Despite demonstrating different behavioral outcomes, both results support the idea that items from a sequence can be reinstated after a single exposure when cued with their prior context.
The role of the hippocampus in episodic sequence encoding and retrieval has been explored in recent neuroimaging studies. Hippocampal and MTL cortical activation has been found to be enhanced during the successful versus unsuccessful encoding of temporal order [47,48], as well as during successful order retrieval [49–52]. Furthermore, the hippocampus has been shown to exhibit a sequential mismatch response where, after a single exposure, there is enhanced hippocampal activation when either the latter elements of the sequence are reordered (i.e., violation of a prediction; [53]) or the entire sequence is reordered [54].
One approach to examining the role of shared context in the acquisition of sequential information is to directly manipulate or effect abrupt changes in context during the encoding of items while holding constant the actual time passed. These changes have been referred to as event boundaries [25], and have been shown to have a lasting effect on associative memory. Specifically, long-term explicit and implicit measures of sequential memory are enhanced for items encountered within events compared to across event boundaries [55,56]. This event segmentation process may be analogous to hierarchical grouping in short-term serial recall (e.g., [57–59]). Recent work has shown that temporal order memory is more accurate for items presented within the same context as compared to across event boundaries ([60], see Fig 2B, left), and that that items encountered within the same event context are rated as having appeared closer together ([61], see Fig 2B, right). Together, these behavioral data provide evidence that encoding context influences multiple forms of temporal memory and raise the possibility that shared context provides mnemonic support for the encoding of sequences after a single experience.
In a study designed to investigate the neural mechanisms influencing the modulation of long-term memory by event structure, participants were scanned while reading narratives that contained temporally-defined event boundaries [55]. Fluctuations in the fMRI response in MTL cortex (as well as in the caudate and medial prefrontal cortex; see Box 2) were sensitive to events in a manner that predicted the structuring of those same representations in memory. Specifically, these regions exhibited increasing activation across sentences within the same event that dropped off at event boundaries. The extent to which these regions showed event-level modulation of fMRI activity predicted aspects of later temporal memory. These results suggest that an increase in the neural response across sequential items within an event context may support their sequential binding. One possibility is that this univariate activity may reflect increased working memory maintenance or integration across the event such that the items within the current event remain accessible and become associated in memory (e.g., [62]).
Box 2. Examining sequence memory outside of the MTL.
While this review is focused on the hippocampus, it is important to note that regions outside of the MTL also contribute to temporal memory. In particular the prefrontal cortex (PFC) and striatum have both been implicated in the acquisition of sequential relationships and temporal memory. Thus, characterizing the unique contributions of these regions is an important step in building a systems-based view of sequence learning.
Research has shown that frontal lobe damage is associated with disproportionate impairments in temporal order memory and sequence learning even when compared to patients with MTL damage [75–83]. Furthermore, the associative encoding of items that span a temporal gap evokes enhanced fMRI activation in PFC [15,62,84,85] and is associated with enhanced frontal theta power [86]. Furthermore, both PFC and MTL regions exhibit successful encoding effects. For example, one study [47] reported a region in left ventrolateral PFC whose activation during encoding predicted subsequent order memory success and another [55] found that activation in ventromedial PFC was related to the sequential binding of items within events. Furthermore, another group found that the degree of fMRI pattern change in rostrolateral PFC was related to subsequent coarse temporal estimates [48]. Together these results suggest that PFC plays an important role in remembering when items were encountered, and this is consistent with its hypothesized role in maintaining a representation of temporal context [44]. However, the fMRI work also highlights a great deal of heterogeneity in the precise localization of regions in the PFC that contribute to temporal memory, and whether and how these regions contribute to general executive functions, such as strategic processing, need to be addressed [87].
The striatum is another region often implicated in neuroimaging studies of temporal memory. As with the PFC, striatal activation has been associated with successful temporal memory encoding [48], memory for the relative order of multiple items [47], and within-event forward recall [55]. While this review is focused on sequences of stimulus-stimulus associations, the striatum has been consistently associated with encoding sequences of stimulus-response associations [26,78]. Thus, its role in sequence acquisition may be related to the learning of rigid stimulus-response associations, which may complement more flexible sequence learning by the hippocampus [88]. The striatum has also been implicated in representing time on the order of seconds to minutes [89], whereas the hippocampus has been shown to be detrimental at such short timescales [90]. Finally, the striatum and hippocampus may interact to calculate the reward value of a spatial sequence and support value-based action selection [91].
Finally, more recent work has used multivariate approaches to examine the role of across-trial stability in preserving the sequence of presented items. Two recent reports showed that pattern similarity in hippocampal activation patterns across extended sequences is related to subsequent judgments of temporal distance ([61]; Fig 2C) and temporal order [63]. This suggests that hippocampal stability across a presented sequence promotes the associative binding across items in a sequence. Furthermore, temporal order judgments after a single encoding experience were associated with behavioral and neural evidence for the reactivation of intervening items in the sequence [60,63]. Specifically, when presented with items A and E from the studied sequence (ABCDE) and asked to recover the temporal order (i.e., “which was more recent?”), there was neural evidence of category-level reactivation of the intervening items as well as greater behavioral priming to those items. Thus, taken together, these data suggest that the hippocampus plays a role in acquiring and expressing episodic sequence memory through encoding stability and reactivation during retrieval.
Concluding Remarks
Here we have reviewed emerging evidence characterizing the human hippocampal involvement in sequence learning. We draw a possible distinction between sequence learning that may involve extracting temporal regularities through multiple repetitions and rapid, novel episodic sequence encoding. Further, we propose that the underlying processes and representations by which sequence information is acquired in these two cases may be biased by their learning context. On the one hand, episodic sequence encoding may be more likely to be supported by the binding of novel sequential items to their shared context that helps to delineate their temporal order. Binding according to shared context is theoretically able to operate on longer time-scales and thus is well suited to mediate encoding of sequences that occur over large temporal gaps. On the other hand, sequence representations acquired through multiple repetitions may be additionally supported by a growing tendency for individual items to trigger forward pattern completion of the sequence, a process that may be mediated by the hippocampus. Importantly, this type of learning may operate on relatively shorter time-scales (i.e., short delays between items in a sequence).
While we find it useful to characterize the mechanisms that support episodic versus repeated sequence learning as distinct, it is important to highlight that these processes are by no means mutually exclusive. Robust single-trial sequence encoding may result in subsequent forward prediction (e.g., [64]), and shared context may benefit sequence acquisition through repetition to the extent that encoded contextual features are also repeated. Instead, we suggest that the episodic or repeated nature of an experienced sequence may bias the source of temporal information for that sequence. Temporal information based on a single experience may be biased towards relying on contextual features (e.g., [60,61]), whereas repetition may bias the source of such information away from a specific spatiotemporal context and towards cued prediction of subsequent items in a sequence. Importantly, the current experiments have not been designed to test these two possibilities simultaneously and, thus, future work will be needed to address this possible distinction. While many open questions remain (see Box 3), converging evidence across both human and animal studies (see Box 1) suggests that the hippocampus learns and represents the sequential structure of events both by encoding information about items in their contexts as well as by exhibiting a predictive code of upcoming events.
Box 3. Open questions.
If different ways of acquiring sequence information lead to distinct memory representations, do they offer unique contributions to adaptive behavior? For example, does sequence acquisition dependent on shared context provide a more flexible representation whereby indirect item-item relationships can be accessed?
It has been shown that stable hippocampal patterns across time promote binding [60,61] but also that coarse temporal memory is better for items showing greater dissimilarity with neighboring items [48]. Thus, one possibility is that similarity and dissimilarity promote associative encoding and item encoding, respectively.
Are there human homologues of the hippocampal time cells and theta sequences observed in nonhuman animals? If discovered, this may help us understand the unique contributions of each to memory behavior as well as the relationship between them.
What are the unique contributions of MTL cortical regions to sequence encoding and retrieval? MTL sequence learning effects have mirrored those observed in the hippocampus in some cases [42,47] but not in others [41,61,74].
Highlights.
The hippocampus shows a forward prediction signal with sequence repetition.
Encoding novel sequences is facilitated by shared context.
Stability in hippocampal activation patterns across items relates to the successful encoding of temporal relationships between those items.
Acknowledgments
Supported by the National Institute of Mental Health (R01-MH074692) and the National Science Foundation (DGE – 0813964).
Glossary
- Delay conditioning
conditioning in which the conditioned and unconditioned stimulus overlap in time
- Place cells
hippocampal neurons that fire when an animal is at a particular location in space
- Replay
sequential pattern of hippocampal place cell responses during offline periods that corresponds to the response pattern during a prior experience
- Temporal context
defined by the temporal context model ([22]) as a slowly drifting representation that binds to and updates with each newly encountered item. Retrieval of temporal context has been hypothesized to support associative recall
- Theta rhythm
oscillatory pattern in the 4–12 Hz range observed most strongly in the rodent hippocampus during action and REM sleep
- Time cells
hippocampal neurons that fire at specific moments in time, or serial positions, within a temporally structured event while controlling for an animal’s location and movement (see [8] for review)
- Trace conditioning
conditioning in which the conditioned stimulus ends before the unconditioned stimulus begins and therefore requires bridging a temporal gap to learn the association
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lila Davachi, Email: lila.davachi@nyu.edu, Department of Psychology, New York University, 6 Washington Place, Rm 871A, New York NY 10003, www.davachilab.org.
Sarah DuBrow, Email: sdubrow@nyu.edu, Department of Psychology, New York University, 6 Washington Place, Rm 871, New York NY 10003.
References
- 1.Tulving E. Episodic Memory: From Mind to Brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- 2.Eichenbaum H, et al. The medial temporal lobe and recognition memory. Annu Neview Neurosci. 2007;30:123–52. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Squire LR, et al. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 4.Mayes AR, et al. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Skaggs WE, et al. Theta Phase Precession in Hippocampal Neuronal Populations and the Compression of Temporal Sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald CJ, et al. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichenbaum H. Time cells in the hippocampus: a new dimension for mapping memories. Nat Rev Neurosci. 2014;15(11):732–44. doi: 10.1038/nrn3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallenstein GV, et al. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;21(8):317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 10.Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 2005;18:1172–1190. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard MW, Eichenbaum H. The hippocampus, time, and memory across scales. J Exp Psychol Gen. 2013;142:1211–1230. doi: 10.1037/a0033621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mankin Ea, et al. Neuronal code for extended time in the hippocampus. Proc Natl Acad Sci U S A. 2012;109:19462–7. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manns JR, et al. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Hales JB, Brewer JB. The timing of associative memory formation: frontal lobe and anterior medial temporal lobe activity at associative binding predicts memory. J Neurophysiol. 2011;105:1454–63. doi: 10.1152/jn.00902.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hales JB, Brewer JB. Activity in the hippocampus and neocortical working memory regions predicts successful associative memory for temporally discontiguous events. Neuropsychologia. 2010;48:3351–3359. doi: 10.1016/j.neuropsychologia.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin S, et al. Probing the transformation of discontinuous associations into episodic memory: An event-related fMRI study. NeuroImage. 2007;38:212–222. doi: 10.1016/j.neuroimage.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Staresina B, Davachi L. Mind the Gap: Binding Experiences across Space and Time in the Human Hippocampus. Neuron. 2009;63:267–276. doi: 10.1016/j.neuron.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- 20.Foerde K, et al. A role for the medial temporal lobe in feedback-driven learning: evidence from amnesia. J Neurosci. 2013;33:5698–704. doi: 10.1523/JNEUROSCI.5217-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bar M. The proactive brain: memory for predictions. Philos Trans R Soc Lond B Biol Sci. 2009;364:1235–1243. doi: 10.1098/rstb.2008.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard MW, Kahana MJ. A distributed representation of temporal context. J Math Psychol. 2002;46:269–299. [Google Scholar]
- 23.Burgess N, et al. Memory for events and their spatial context: models and experiments. Philos Trans R Soc Lond B Biol Sci. 2001;356:1493–1503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Kesteren MT, et al. How schema and novelty augment memory formation. Trends Neurosci. 2012;35:211–219. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Zacks JM, et al. Event Perception: A Mind – Brain Perspective. Psych Bull. 2007;133(2):273–293. doi: 10.1037/0033-2909.133.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schendan HE, et al. An fMRI Study of the Role of the Medial Temporal Lobe in Implicit and Explicit Sequence Learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 27.Kumaran D, Maguire Ea. The dynamics of hippocampal activation during encoding of overlapping sequences. Neuron. 2006;49:617–629. doi: 10.1016/j.neuron.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 28.Ross RS, et al. The Retrieval of Learned Sequences Engages the Hippocampus: Evidence From fMRI. Hippocampus. 2009;799:790–799. doi: 10.1002/hipo.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schapiro AC, et al. The necessity of the medial temporal lobe for statistical learning. J Cogn Neurosci. 2014;26:1736–1747. doi: 10.1162/jocn_a_00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turk-browne NB, et al. Implicit Perceptual Anticipation Triggered by Statistical Learning. 2010;30:11177–11187. doi: 10.1523/JNEUROSCI.0858-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown TI, et al. Which way was I going? Contextual retrieval supports the disambiguation of well learned overlapping navigational routes. J Neurosci. 2010;30:7414–7422. doi: 10.1523/JNEUROSCI.6021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bornstein AM, Daw ND. Dissociating hippocampal and striatal contributions to sequential prediction learning. Eur J Neurosci. 2012;35:1011–23. doi: 10.1111/j.1460-9568.2011.07920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norman Ka, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 2003;110:611–46. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 34.Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 2007;14:745–57. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- 35.Rolls ET. The mechanisms for pattern completion and pattern separation in the hippocampus. Front Syst Neurosci. 2013;7:74. doi: 10.3389/fnsys.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duncan K, et al. Distinct memory signatures in the hippocampus: intentional States distinguish match and mismatch enhancement signals. J Neurosci. 2009;29:131–139. doi: 10.1523/JNEUROSCI.2998-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henson RNA, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioural priming. 2003;41:263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- 38.Turk-Browne N, et al. Scene representations in parahippocampal cortex depend on temporal context. J Neurosci. 2012;32:7202–7207. doi: 10.1523/JNEUROSCI.0942-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paz R, Gelbard-Sagiv H. A neural substrate in the human hippocampus for linking successive events. PNAS. 2010;107(13):6046–6051. doi: 10.1073/pnas.0910834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalm K, et al. Individual sequence representations in the medial temporal lobe. J Cogn Neurosci. 2013;25:1111–1121. doi: 10.1162/jocn_a_00378. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh L-T, et al. Hippocampal activity patterns carry information about objects in temporal context. Neuron. 81:1165–1178. doi: 10.1016/j.neuron.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schapiro A, et al. Shaping of Object Representations in the Human Medial Temporal Lobe Based on Temporal Regularities. Curr Biol. 2012;22:1622–1627. doi: 10.1016/j.cub.2012.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahana MJ. Associative retrieval processes in free recall. Mem Cognit. 1996;24:103–109. doi: 10.3758/bf03197276. [DOI] [PubMed] [Google Scholar]
- 44.Polyn SM, Kahana MJ. Memory search and the neural representation of context. Trends Cogn Sci. 2008;12:24–30. doi: 10.1016/j.tics.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith TA, et al. The Context Repetition Effect: Predicted Events Are Remembered Better, Even When They Don’t Happen. J Exp Psychol Gen. 2013;142:1298–1308. doi: 10.1037/a0034067. [DOI] [PubMed] [Google Scholar]
- 46.Kim G, et al. Pruning of memories by context-based prediction error. PNAS. 2014;111:8997–9002. doi: 10.1073/pnas.1319438111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tubridy S, Davachi L. Medial temporal lobe contributions to episodic sequence encoding. Cereb cortex. 2011;21(2):272–280. doi: 10.1093/cercor/bhq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jenkins LJ, Ranganath C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J Neurosci. 2010;30:15558–15565. doi: 10.1523/JNEUROSCI.1337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehn H, et al. A specific role of the human hippocampus in recall of temporal sequences. J Neurosci. 2009;29:3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacques PS, et al. The Short and Long of It: Neural Correlates of Temporal-order Memory for Autobiographical Events. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dudukovic NM, Wagner AD. Goal-dependent modulation of declarative memory: neural correlates of temporal recency decisions and novelty detection. Neuropsychologia. 2007;45:2608–2620. doi: 10.1016/j.neuropsychologia.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 52.Konishi S, et al. Activation shift from medial to lateral temporal cortex associated with recency judgements following impoverished encoding. Cereb Cortex. 2006;16:469–474. doi: 10.1093/cercor/bhi126. [DOI] [PubMed] [Google Scholar]
- 53.Kumaran D, Maguire Ea. An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol. 2006;4(2):e424. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barnett AJ, et al. The human hippocampus is sensitive to the durations of events and intervals within a sequence. Neuropsychologia. 2014;64:1–12. doi: 10.1016/j.neuropsychologia.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 55.Ezzyat Y, Davachi L. What constitutes an episode in episodic memory? Psychol Sci. 2011;22:243–252. doi: 10.1177/0956797610393742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zwaan RA. Processing narrative time shifts. J Exp Psychol Learn Mem Cogn. 1996;22(5):1196–1207. [Google Scholar]
- 57.Wickelgren WA. Rehearsal grouping and hierarchical organization of serial position cues in short-term memory. Q J Exp Psychol. 1967;19:97–102. doi: 10.1080/14640746708400077. [DOI] [PubMed] [Google Scholar]
- 58.Ryan J. Grouping and short-term memory: different means and patterns of grouping. Q J Exp Psychol. 1969;21:137–47. doi: 10.1080/14640746908400206. [DOI] [PubMed] [Google Scholar]
- 59.Farrell S. Temporal clustering and sequencing in short-term memory and episodic memory. Psychological Review. 2012;119(2):223–271. doi: 10.1037/a0027371. [DOI] [PubMed] [Google Scholar]
- 60.DuBrow S, Davachi L. The influence of context boundaries on memory for the sequential order of events. J Exp Psychol Gen. 2013;142:1277–1286. doi: 10.1037/a0034024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ezzyat Y, Davachi L. Similarity Breeds Proximity: Pattern Similarity within and across Contexts Is Related to Later Mnemonic Judgments of Temporal Proximity. Neuron. 2014;81:1179–89. doi: 10.1016/j.neuron.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26:916–25. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DuBrow S, Davachi L. Temporal memory is shaped by encoding stability and intervening item reactivation. J Neurosci. 2014;34:13998–14005. doi: 10.1523/JNEUROSCI.2535-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith Ta, et al. The context repetition effect: predicted events are remembered better, even when they don’t happen. J Exp Psychol Gen. 2013;142:1298–308. doi: 10.1037/a0034067. [DOI] [PubMed] [Google Scholar]
- 65.Eichenbaum H. Memory on time. Trends Cogn Sci. 2013;17:81–8. doi: 10.1016/j.tics.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dragoi G. Internal operations in the hippocampus: single cell and ensemble temporal coding. Front Syst Neurosci. 2013;7:46. doi: 10.3389/fnsys.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colgin LL. Mechanisms and functions of theta rhythms. Annu Rev Neurosci. 2013;36:295–312. doi: 10.1146/annurev-neuro-062012-170330. [DOI] [PubMed] [Google Scholar]
- 68.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–8. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fortin NJ, et al. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–62. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kesner RP, et al. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci. 2002;116:286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- 71.Lisman J, Redish AD. Prediction, sequences and the hippocampus. Philos Trans R Soc London Ser B, Biol Sci. 2009;364:1193–1201. doi: 10.1098/rstb.2008.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta AS, et al. Segmentation of spatial experience by hippocampal θ sequences. Nat Neurosci. 2012;15:1032–1039. doi: 10.1038/nn.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jezek K, et al. Theta-paced flickering between place-cell maps in the hippocampus. Nature. 2011;478:246–9. doi: 10.1038/nature10439. [DOI] [PubMed] [Google Scholar]
- 74.Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Sci (New York, NY) 2011;333:773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- 75.Shimamura AP, et al. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- 76.McAndrews MP, Milner B. The frontal cortex and memory for temporal order. Neuropsychologia. 1991;29:849–859. doi: 10.1016/0028-3932(91)90051-9. [DOI] [PubMed] [Google Scholar]
- 77.Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- 78.Meier B, et al. Implicit task sequence learning in patients with Parkinson’s disease, frontal lesions and amnesia: The critical role of fronto–striatal loops. Neuropsychologia. 2013;51:3014–3024. [PubMed] [Google Scholar]
- 79.Marshuetz C. Order information in working memory: An integrative review of evidence from brain and behavior. Psychol Bull. 2005;131:323–339. doi: 10.1037/0033-2909.131.3.323. [DOI] [PubMed] [Google Scholar]
- 80.Butters MA, et al. Recency Discrimination Deficits in Frontal Lobe Patients. 1994;8:343–353. [Google Scholar]
- 81.Milner B, et al. Frontal-lobe contribution to recency judgements. Neuropsychologia. 1991;29:601–18. doi: 10.1016/0028-3932(91)90013-x. [DOI] [PubMed] [Google Scholar]
- 82.Milner B, et al. Frontal lobes and the temporal organization of memory. Hum Neurobiol. 1985;4:137–42. [PubMed] [Google Scholar]
- 83.Kesner RP, et al. Item and order dissociation in humans with prefrontal cortex damage. Neuropsychologia. 1994;32:881–91. doi: 10.1016/0028-3932(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 84.Murray LJ, Ranganath C. The Dorsolateral Prefrontal Cortex Contributes to Successful Relational Memory Encoding. 2007;27:5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hales JB, et al. Dissociation of frontal and medial temporal lobe activity in maintenance and binding of sequentially presented paired associates. J Cogn Neurosci. 2009;21:1244–1254. doi: 10.1162/jocn.2009.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hsieh LT, et al. Neural oscillations associated with item and temporal order maintenance in working memory. J Neurosci. 2011;31:10803–10810. doi: 10.1523/JNEUROSCI.0828-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mangels Ja. Strategic processing and memory for temporal order in patients with frontal lobe lesions. Neuropsychology. 1997;11:207–221. doi: 10.1037//0894-4105.11.2.207. [DOI] [PubMed] [Google Scholar]
- 88.Albouy G, et al. Hippocampus and striatum: Dynamics and interaction during acquisition and sleep-related motor sequence memory consolidation. Hippocampus. 2013;23:985–1004. doi: 10.1002/hipo.22183. [DOI] [PubMed] [Google Scholar]
- 89.Meck WH, et al. Cortico-striatal representation of time in animals and humans. 2008;18:145–152. doi: 10.1016/j.conb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 90.Jacobs NS, et al. Critical role of the hippocampus in memory for elapsed time. J Neurosci. 2013;33:13888–93. doi: 10.1523/JNEUROSCI.1733-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pezzulo G, et al. Internally generated sequences in learning and executing goal-directed behavior. Trends Cogn Sci. 2014;18(12):647–57. doi: 10.1016/j.tics.2014.06.011. [DOI] [PubMed] [Google Scholar]