Abstract
Cells reside in a complex and dynamic extracellular matrix where they interact with a myriad of biophysical and biochemical cues that direct their function and regulate tissue homeostasis, wound repair, and even pathophysiological events. There is a desire in the biomaterials community to develop synthetic hydrogels to recapitulate facets of the ECM for in vitro culture platforms and tissue engineering applications. Advances in synthetic hydrogel design and chemistries, including user-tunable platforms, have broadened the field’s understanding of the role of matrix cues in directing cellular processes and enabled the design of improved tissue engineering scaffolds. This review focuses on recent advances in the development and fabrication of hydrogels and discusses what aspects of ECM signals can be incorporated to direct cell function in different contexts.
Keywords: Hydrogels, Extracellular matrix, Three-dimensional culture, Peptides
SETTING THE CONTEXT
In our tissues and organs, the extracellular matrix (ECM) is a complex and dynamic structure where cells reside, remodel and interact over a range of length scales to maintain tissue homeostasis, growth and repair. 37,56 The ECM is bioactive, locally sequestering biomacromolecules that can promote cell adhesion, spreading, survival, proliferation, migration and even, differentiation. The synergistic and antagonistic interplay of these cues, in coordination with the biophysical properties of the surrounding matrix, ultimately directs cell fate. However, how cells receive, process, and exchange information with the ECM is often difficult to elucidate, and it typically involves coordinated presentation of multiple factors that can be presented over multiple time scales.
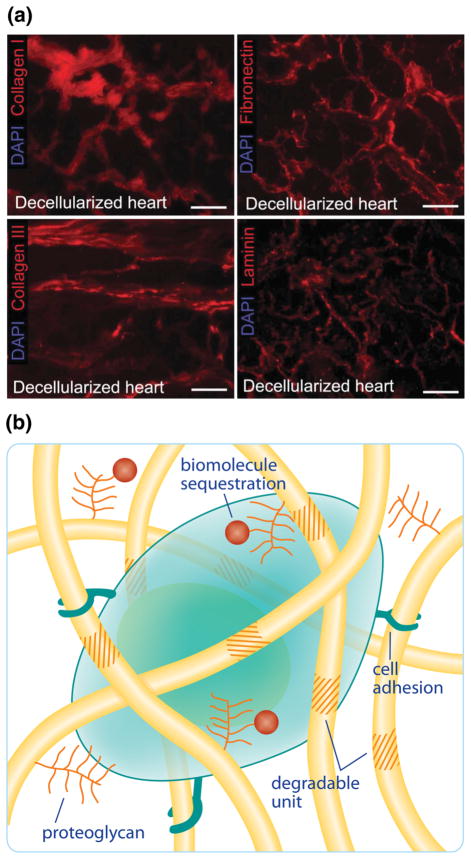
In the field of regenerative medicine, decellularized ECM matrices have played a long-standing role as scaffolds for tissue engineering.43 Decellularized ECM scaffolds are typically prepared by treating tissues with detergents to remove cells and antigens, and subsequently repopulated with host cells, allowing them to be transplanted in vivo while minimizing the immune response. 15 As an example, Ott et al. used decellularlized whole hearts as a structural architecture that was repopulated with cardiac or endothelial cells and the cell-laden matrix led to nascent pumping function.45 Collagen I and III, laminin and fibronectin were all preserved within the decellularized heart as shown in the immuofluoresence micrographs in Fig. 1a. While matrices derived from native ECM have the benefit of capturing the complex structure and composition found in tissues (e.g., a pre-formed vascular network); the ability to recolonize this dense matrix with multiple cell types and to control their functional properties on relevant length and time scales is difficult to achieve and predict.
FIGURE 1.
(a) The extracellular matrix is composed of many different proteins, including collagen, fibonectin, and laminin, which were carefully preserved in decellularized heart tissue in a study by Ott et al.45 (b) The cellular microenvironment is a complex biophysical and biochemical environment where cells reside. This microenvironment includes degradable structural fibers, adhesive binding domains, and proteoglycans for biomolecule sequestration.
As a result, there are numerous efforts to engineer materials that recapitulate important facets of the ECM, and synthetic hydrogels represent one such class of materials that have received widespread interest as tissue engineering scaffolds. Hydrogels are crosslinked biomacromolecules that absorb large amounts of water, without dissolving, and this high water content imparts physiologically relevant soft tissue mechanics, and allows facile transport and diffusion of cell secreted molecules. While the structure of synthetic hydrogels can be tuned to allow a range of material properties, the chemistry is devoid of functionalities that are recognized by cells. So when designing suitable hydrogels for culturing cells and regenerating tissues, the question becomes “How simple is complex enough?”. What minimal biological signals are necessary to guide desired cellular functions, and how might temporal addition and/or removal of these matrix cues play an important role in directing bioscaffold design, especially for the regeneration of functional tissues of future.
THE ECM HAS A COMPLEX COMPOSITION
The ECM provides a myriad of cues to resident cells that in combination with cell–cell and cell–matrix signaling regulate functional outputs (Fig. 1b). The composition of the ECM is complex and consists of high molecular weight proteins (e.g., collagen, fibronectin, and laminin) and branched glycosaminoglycan structures (e.g., heparin sulfate and chondroitin sulfate).27 These macromolecules are typically organized into a fibrillar network that provides a unique biophysical and bioactive environment for cells to reside. The most abundant protein in the ECM is collagen, which forms trimeric protein rods that provide tensile strength to the network. Proteoglycans represent another major component of the ECM; these highly branched biomacromolecules arise from covalent attachment of glycosaminoglycans (GAGs) to high molecular weight proteins. Negatively charged GAGs indirectly sequester water molecules through a cationic intermediary, resulting in a water-rich network and unique biophysical properties (e.g., high compressive strength, viscoelastic effects, and streaming potentials).
The multiple ECM components also contain adhesive binding sites for cell binding, such as those found in fibronectin and laminin, which facilitate attachment, spreading, migration, and transduction of mechanical signals from the local microenvironment.23,25 GAGs are also known to non-specifically sequester and bind growth factors and signaling proteins that cells interact with through surface receptors, imparting local bioactivity. Finally, the ECM should be considered as a dynamic material, where the local milieu can be remodeled through cell-mediated secretion and deposition of molecules or degraded through cell secreted enzymes called matrix metalloproteinases (MMPs).
SYNTHETIC HYDROGELS AS ECM MIMICS
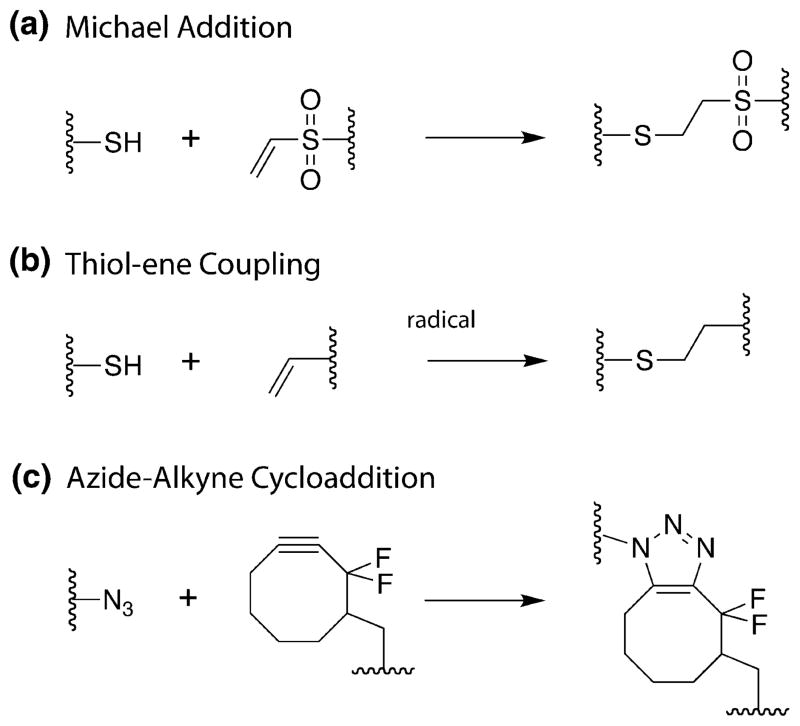
Synthetic hydrogels can serve as simple platforms to culture primary cells in three-dimensions and test the role of ECM interactions on functional outputs. For example, water soluble polymers, such as poly(ethylene glycol) (PEG), poly(vinyl alcohol), poly(2-hydroxyethyl methacrylate), can be crosslinked to form elastic materials that recreate basic aspects of ECM mechanics of soft tissues.1 Complementary bioconjugation methods have also been employed to impart biological functionality to these synthetic materials.28 The relatively bioinert nature and hydrophilicity of PEG, in particular, enables the design of water-rich “blank” cellular microenvironments. Typically, PEG macromolecules are crosslinked or functionalized by modifying the hydroxyl end groups with reactive groups, such as alkenes, alkynes, azides, maleimides, thiols, NHS esters, vinyl sulfones, and norbornenes, which can then be polymerized under cytocompatible conditions via numerous reactions: chain polymerization, Michael addition (Fig. 2a), thiol-ene (Fig. 2b), and strain-promoted azide alkyne cycloaddition (Fig. 2c).2,5,22,24 The initial macromolecular molecular weight and the functionality of the monomer can be used along with processing conditions to control the final network structure and properties.
FIGURE 2.
Examples of prominent reactions used for bioconjugation and/or hydrogel crosslinking: (a) base catalyzed thiol-vinyl sulfone Michael addition, (b) radical mediated thiolene, (c) strain-promoted azide-alkyne cycloaddition (SPAAC).
In this review, focus is first placed on hydrogels as simplified structural mimics of the ECM, capturing basic mechanical aspects and allowing three-dimensional cell culture that direct basic cellular outputs, such as adhesion and morphology (Fig. 1b). Then, the topic transitions to more complex material environments aimed towards controlling and manipulating cellular processes, such as directing differentiation or in vivo engraftment. Clearly, the ECM is a reservoir filled with a rich biochemical context that coordinates to regulate cell function by sequestering growth factors and providing adhesive cellular binding sites. How a cell interprets these bioactive signals can depend on the biophysical inputs a cell is receiving concurrently, so numerous contemporary topics in bioscaffold design focus on understanding and recapitulating the dynamic interplay between cells and their local ECM. In this regard, synthetic hydrogels have been engineered with properties that change with time, such as degrading through specific mechanisms (e.g., hydrolytic, enzymatic) and on specific time scales (Fig. 1c). These processes can better capture aspects of cells degrading and/or remodeling their local microenvironment in vivo, while simultaneously allowing a researcher to investigate how cells exchange and interpret information received from their niche. However, in designing synthetic hydrogels as ECM mimics, the complex biophysical and biochemical cues present should be motivated by the desired clinical application or hypothesis being tested. The following sections look to explore how these passive and active matrix cues might be incorporated into synthetic hydrogels to better understand how simplified and defined signals affect cell-ECM interactions and then place this in the broader context for the future design of hydrogels as bioscaffolds for tissue engineering applications.
Towards the goal of synthesizing a simplified ECM mimic, initial efforts demonstrated how one could introduce peptide sequences into the network structure to provide intregrin-binding sites for adhesion and enzymatically degradable linkers to allow for cell-mediated degradation. Hern et al. copolymerized PEG-diacrylate macromolecules with monoacrylated PEGs modified with a pendant RGD sequence to promote adhesion and survival of human foreskin fibroblasts.20 Specifically, the RGD sequence was coupled to an assymetric, linear PEG with an acrylate functional group on one end, and an N-hydroxy succinimide ester on the other end. Since this early demonstration, RGD and other adhesive epitopes have been widely used in bioscaffold design to direct cell migration7,11,58 or even spatially control cell spreading in three-dimensions.9,14
Beyond controlling cell–matrix adhesion, these synthetic methods have also been used to mimic aspects of ECM degradation and remodeling. For example, West and Hubbell incorporated a collagenase-sensitive peptide sequence into a PEG diacrylate crosslinker, rendering it degradable by cell-secreted MMPs.57 To further control the hydrogel connectivity and combine both adhesive and degradable functionalities, Lutolf and Hubbell used a base catalyzed Michael addition to react multi-arm PEG with vinyl-sulfone end groups with cysteine containing peptides (e.g., a bis-cysteine peptide that was MMP-cleavable and a mono-cysteine RGD sequence).38 This reaction scheme allowed encapsulation of fibroblasts and the study of their invasion into the matrix, as a function of its adhesivity and susceptibility to degradation.
Towards building in complexity and allowing spatial control of matrix functionality others exploited thiol-ene photochemistry to show the versatility of the reaction in forming PEG-peptide ECM mimics14 with gradient functionalities,10 conjugation of multiple epitopes in spatially distinct scaffold regions,9 and two-photon patterning of complex features.8 More recent work has expanded the library of “bio-click” chemistries, such as strain-promoted azide-alkyne cycloaddition, that allow sequential and orthogonal reactions for bioconjugation.9 This reductionist view of the ECM and the expansion of cytocompatible chemistries afford new opportunities to design innovative experiments that should improve the field’s understanding of cell–matrix signaling and the design of the next generation of tissue engineering scaffolds.
THE ACTIVE ROLE OF BIOPHYSICAL CUES
The ECM in its simplest interpretation is a 3D structure where cells physically reside. However, cells interact and receive important regulatory cues from the physical properties (e.g., stiff and soft matrices) and structure of the ECM, and these cues in turn direct cell outputs such as spreading, migration, and proliferation (Fig. 3a). Many of these physical attributes can be engineered and recapitulated within synthetic hydrogels. For example, moderately crosslinked PEG-based hydrogels imbibe large amounts of water (>95%), and this renders them with properties that can be tuned over a large range of elastic moduli, diffusivity, and structural information. However, this tunability of properties is highly coupled, as all of these properties depend directly on the network crosslinking density.
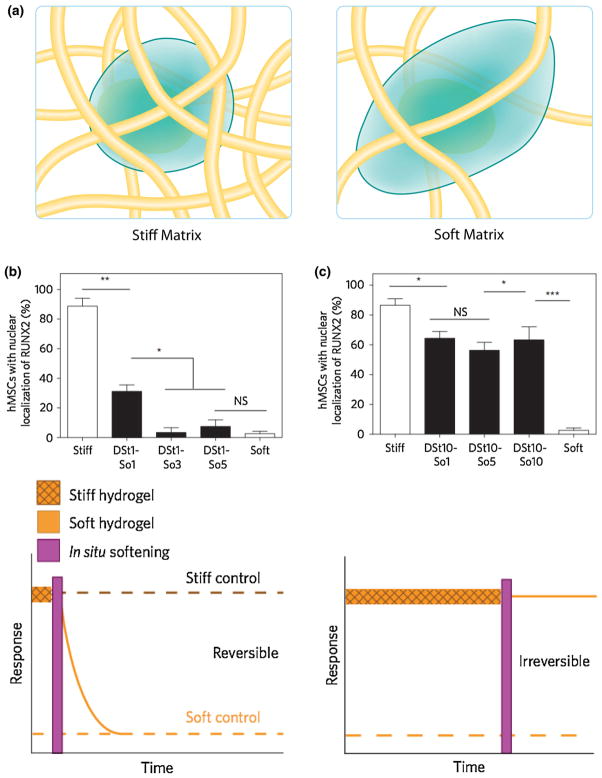
FIGURE 3.
(a) The ECM provides important biophysical cues, such as matrix elasticity, illustrated here with a stiff, dense matrix (Left) and a soft, loose matrix (Right). Synthetic hydrogels can recapitulate these properties by tuning their mechanical properties. (b) Hydrogels with phototunable elasticity provide a versatile platform to study the role of biophysical cues in directing cell function. Yang et al.59 cultured MSC on stiff substrates for 1 or 10 days and then softened the hydrogels in situ using a pre-determined dose of light. RUNX2 and YAP nuclear localization was then observed for up to 10 days, and they both followed similar trends. Shown here, the percent of hMSC with nuclear RUNX2 localization returned to basal levels after being cultured for only 1 day on stiff substrates (DSt1) and different durations on soft substrates. The stiff and soft controls correspond to the average RUNX2 localization for cells only cultured on stiff substrates (i.e., not softened with light) or only on soft substrates (i.e., softened with light) and represent full and basal levels of activation, respectively. (c) When hMSC were cultured on stiff substrates for 10 days (DSt10), RUNX2 localization persisted at active levels even after culture on soft substrates, indicating that 10 days on stiff substrates induced irreversible activation of RUNX2. The data are plotted as the mean ± SEM. NS, not significant; *p <0.05; **p<0.01; ***p <0.001.
One of the most widely studied biophysical properties is the matrix elasticity. Although still not fully understood, correlative studies suggest cells interpret elasticity through mechanotransduction and integrin binding to the matrix triggers outside-in signaling cascades. For example, in the seminal work from Engler et al., polyacrylamide hydrogels were used to create substrates with a range of elastic moduli, and this simple biophysical variation was found to manipulate the differentiation of mesenchymal stem cell (hMSC).13 Building on this pioneering work, hydrogels with phototunable elasticities were then synthesized to allow users the ability to study the effects of mechanical ‘dosing’ (e.g., culturing cells on TCPS for extended periods of time) on hMSC fate. Work by Yang et al. synthesized PEG substrates with elasticities that could be tuned on demand by exposure to light (Figs. 3b and 3c).59 Briefly, hMSC were cultured on stiff (10 kPa) gels for 1 (Fig. 3b), 7 (data not shown) or 10 days (Fig. 3c) and then the hydrogels were softened to 2 kPa in situ. The nuclear localization of two transcriptional factors, YAP and RUNX2, was studied, and found to depend upon the time of culture in stiff environments (Figs. 3b and 3c). These findings taught that cells may have a ‘memory’ related to their previous culture treatment and highlight the importance of carefully considering the context of cells during their ex vivo culture and expansion as this may influence their long-term function after transplantation.
While these studies point to the importance of mechanotransduction, a major unanswered question for the field is how does this signaling occur. Using the same photodegradable hydrogels described above,59 Wang et al. identified the PI3K/AKT pathway as one of the key signaling pathways that regulate mechanosensing and activation of primary valvular interstitial cells (VICs).55 This study showed that through a reduction in the material stiffness the myofibroblast to fibroblast activation of VICs could be reversed. As another example, Gilbert et al. also illustrated the efficacy of recapitulating appropriate ECM substrate stiffness on muscle stem cell (MuSC) function.17 Specifically, PEG-based hydrogel culture platforms that mimicked the elastic modulus of muscle (~12 kPa) were used as a culture platform for MuSC. The MuSC cultured on the muscle-like hydrogel substrates had heightened self-renewal and potency, as well as increased engraftment rates during muscle regeneration compared to MuSC cultured on TCPS.
THE ECM AS A RESERVOIR OF BIOCHEMICAL SIGNALS
Beyond serving as a structural framework, the ECM presents a myriad of biochemical cues that regulate cell function and direct macroscopic tissue development and regeneration. These active cues consist of adhesive domains present on ECM proteins (e.g., fibronectin, laminin), soluble molecules that diffuse through the ECM, and others that remain sequestered or substrate-bound. Here, the focus is to review some of the primary methods to present these biochemical signals in hydrogels by functionalizing them with short mimetic peptides of larger proteins or with functionalities that can physically sequester native biomacromolecules through affinity interactions (Fig. 4a). Finally, the contextual presentation in which cells receive these cues affects the biomolecules efficacy and the cellular output that results; therefore, it is important to understand the interplay between biochemical and biophysical cues.
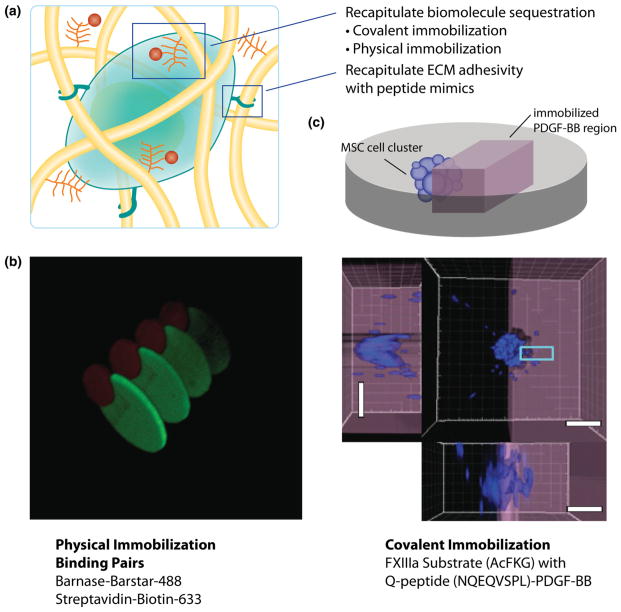
FIGURE 4.
(a) Techniques based on covalent and physical immobilization are being developed for spatial biomolecule patterning to recapitulate aspects of biomolecule sequestration. Further, peptide mimics have been shown to be effective for recapitulating cell adhesion in otherwise non-adhesive hydrogels. (b) Using barstar-barnase and biotin-streptavidin binding pairs, Wylie et al.58 spatially tethered fluorescently labeled sonic hedgehog and ciliary neurotrophic factor into a hydrogel through physical immobilization. (c) Moseweicz et al.44 spatially patterned PDGF-BB to direct the invasion of encapsulated MSC. A confocal micrograph shows that MSC, labeled with DAPI (blue), more efficiently invaded the patterned region of a hydrogel with covalently immobilized PDGF-BB (purple).
Within the in vivo milieu the ECM is known to sequester and bind many different molecules that then interact with cellular receptors. Recent advances in synthetic hydrogels have provided accessible techniques to recapitulate aspects of this biomolecule sequestration using versatile and bio-orthogonal chemistries for both specific and non-specific binding in a spatially defined manner. Some of these bioconjugation methods include thiol-ene, azide-alkyne, maleimide-thiol, diels–alder, oxime, hydrazine and hydroxysuccinimide-amine reactions, which enable facile chemical modification of the target molecule and subsequent conjugation to hydrogel scaffolds at physiologically relevant concentrations while retaining the bioactivity of the target molecule.2,26 More recent developments in bio-orthogonal “click” chemistries have further enabled highly specific patterning of biomolecules in the presence of cells and biological moieties. For example, 3T3 fibroblast cells were encapsulated in PEG gels using a copper-free azide-alkyne reaction, and the cellular environments were subsequently modified in a spatially defined manner to direct cell migration using a thiol-ene photoconjugation reaction combined with photolithography.7
For certain applications, peptides provide distinct advantages, compared to utilizing full proteins. Solid phase peptide synthesis allows bioactive cues to be readily synthesized and introduced into amatrix without some of the difficulties in maintaining the bioactivity of the target protein. For example, the adhesive ligand RGDS is routinely incorporated in hydrogels to promote cell attachment and increase survival.48 Along with RGDS, many other common adhesive binding sites found in the ECM have been incorporated in hydrogels and an excellent review of this topic can be found in Brennan et al.4 Towards studying the role of matrix binding epitopes on cell secretory properties, Gould et al. used a combination of RGDS, VGVAPG, and P15 peptides and showed marked differences in the activation of valvular interstitial cells to myofibroblasts, depending on the ratio of these adhesive ligands.18 Peptides have also been used as mimics of cell–cell interactions where Bian et al. presented covalently-bound N-cadherin mimetic peptides to MSC encapsulated in HA-based gels and observed accelerated chondrogenesis resulting in increased cartilage formation in vitro and in vivo.3 Beyond peptide mimics of adhesive ligands, there is also significant effort to identify sequences that mimic active sites of growth factors, such as stromal derived factor 1 (preserved C terminus and N terminus), a known chemoattractant, and bone morphogenetic proteins (e.g., DWIVA, KIPKASSVPTELSAISTLYL), a potent regulator of MSC differentiation.21,39,40 However, peptides do not possess the activity of full proteins and in many instances, designing synthetic ECMs for local protein delivery is critical.
In vivo, many growth factors are physically bound and stored in the ECM through affinity interactions with local macromolecules, such as glycosaminoglycans. This results in localized concentration profiles of the target protein, reduced enzymatic cleavage, and effective presentation of bioactive ligands for cell receptor binding. Building from this notion, synthetic hydrogels have been designed to recapitulate aspects of biomolecule sequestration, where early work in the field demonstrated the use of heparin. Heparin is known to bind growth factors, proteases, and chemokines, and its presentation in hydrogels can be exploited to locally sequester cell secreted molecules to control local signaling.36,50 For example, a recent work by Purcell et al. used dextran-sulfate, as a heparin mimetic, within an MMP-degradable HA hydrogel to bind to TIMP-3, an MMP inhibitor, through electrostatic interactions.49 This stimuli-responsive scaffold allowed for cell secreted MMPs to degrade the network and dictate spatially relevant release of TIMP-3. The scaffold was injected into myocardial infarcted (MI) pig hearts and pathophysiological MMP expression was reduced by 14 days post-MI.
Correspondingly, there is a push to develop more highly defined synthetic scaffolds utilizing specific binding pairs for sequestration of target molecules in a spatially defined method. Wylie et al. took advantage of the specific interactions of barnase-barstar and streptavidin–biotin by spatially patterning barnase and streptavidin into an agarose hydrogel using photolithography (Fig. 4b).58 Next, proteins functionalized with either barstar or biotin, were swollen into the hydrogel and immobilized only in locations where the binding partner was located to direct 3D adult neural precursor cell invasion. In a complementary approach, Mosiewicz et al. used enzymatically degradable hydrogels combined with photopatterning of growth factors to direct MSC migration (Fig. 4c).44 First, MSC clusters were encapsulated in an MMP-degradable PEG hydrogel, and platelet derived growth factor B (PDGF-BB), a known MSC chemoattractant, was conjugated in precise regions near the cell cluster. MSC were shown to migrate in a biased direction towards the conjugated PDGF-BB as quantified through the cell densities within the patterned and unpatterned regions (Fig. 3c). An interesting facet of this work focused on binding Fc-chimeric proteins to spatially tethered protein A molecules within the hydrogel, which has broad appeal for immobilizing biomolecules as many Fc-chimeric proteins are commercially available.
The context in which these biochemical cues are presented to cells can play a critical role in the efficacy and cellular output. Using highly defined synthetic scaffolds, compelling work is beginning to elucidate the synergistic and antagonistic relationships between biophysical and biochemical cues. For example, work from Chen and coworkers found that varying the rigidity of polyacrylamide gels modified the functional response of TGF-β1 for mammary gland cells and kidney epithelial cells in inducing higher levels of apoptosis (less rigid substrate) or epithelial-mesenchymal transition (more rigid substrates).35 This knowledge is also advancing translational studies for clinical applications. Cosgrove et al. showed that inhibition of p38α and p38β on soft hydrogel culture platforms promoted higher yields of functional stem cell populations relative to inhibition on TCPS for muscle regeneration application.6 In this example, the biochemical inhibition and the biophysical hydrogel culture platform worked synergistically, resulting in a desired cellular output for a clinical application. Complementary, there is a focus within the induced pluripotent stem cell community to use hydrogels to retain pluripotency and increase proliferation through the synergistic presentation of biochemical and biophysical cues which is discussed in depth by Dingal et al.12 Synthetic hydrogels provide a simple, versatile and highly defined culture platform to elucidate the interplay between biochemical and biophysical cues on cell function and further to engineer more effective tissue engineering scaffolds for regenerative medicine applications.
CELL-DIRECTED AND USER-DIRECTED MICROENVIRONMENTAL ECM REMODELING
The ECM is a highly dynamic environment that is locally degraded and remodeled during development, homeostasis, wound repair, and even pathophysiological events. Degradation of the ECM primarily occurs through enzymatic cleavage by cell-secreted proteases, allowing cells to remodel their local microenvironment or move through this dense matrix (Fig. 5a). To capture the dynamic nature of ECM remodeling, synthetic hydrogels are often engineered to degrade through hydrolytic mechanisms, user-tunable mechanisms (e.g., light cleavable, exogenous delivery of chemicals), or enzymatic mechanisms (e.g., protease secretion).28 This section will focus on cell-mediated and user-dictated degradation mechanisms, the former allowing the synthesis of hydrogels to observe cells in a native-like environment and the latter allowing user manipulation of the hydrogel to better understand matrix signaling.
FIGURE 5.
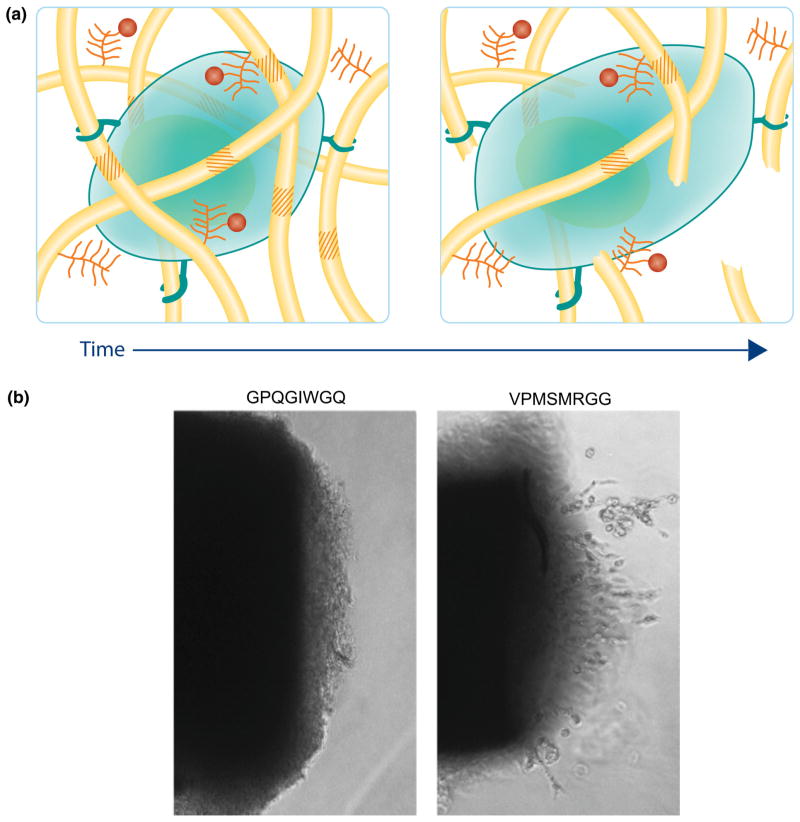
(a) Cellular remodeling and degradation play an important role in regulating the dynamic nature of the ECM. The introduction of hydrogels that degrade in response to cell-secreted enzymes begins to recapitulate this natural process. (b) Patterson et al.47 synthesized PEG hydrogels that were crosslinked with two different peptide sequences that vary in degradation rates. Cells from the aortic chick ring invaded the MMP-degradable network at different rates based on the cleavage rate of the peptide sequence, GPQGIWG (slower) and VPMSMRGG (faster).
To examine how stiffness and structure affect cell function, photodegradable hydrogels were developed that allow for user-tunable material properties in space and time. Photodegradable gels have been used in a number of studies that investigate how temporal manipulation of the synthetic hydrogel regulates cell function. As discussed previously, these user-tunable hydrogels have advanced our understanding of cellular mechanotransduction and mechanical ‘memory’.55,59 Photodegradable hydrogels have also been used to study how cells receive mechanical information from the environment by spatially controlling cell adhesion on a sub-cellular length scale. Tibbitt et al. used photodegradable PEG hydrogels to study cytoskeletal pretension through sub-cellular focal adhesion detachment of encapsulated and spread cells within 3D hydrogels.54 Further, photodegradable hydrogels in combination with two-photon lithography allow for structural changes such as developing 3D channels and shapes to be formed. Kloxin et al. used this technique to develop a culture platform that recapitulated the alveolar structure for epithelial lung cell alignment.30 Using similar techniques, cyst-like and vascular-like structures can be created within a 3D scaffold for tissue regeneration applications.
Synthetic hydrogels are routinely engineered to degrade in response to cell secreted enzymes, serving as robust in vitro models of tissue matrices or useful cell delivery vehicles. Seminal work from Lutolf et al. showed that by crosslinking a PEG-based hydrogel with an MMP-cleavable peptide sequence (Ac-GCRD-GPQGIWGQ-DRCG) cells locally degraded the material through a physiological mechanism allowing for cellular invasion into the hydrogel.38 This ushered in a major advance towards synthetic scaffolds that more closely mimic the ECM. Similar materials and approaches are now being pursued to design biomaterials that can actively promote infiltration by endogenous cells or dispense delivered cells at sites of injury.31
From a fundamental perspective, these materials are useful as in vitro culture platforms where cell-mediated matrix degradation has been implicated in determining cell differentiation fate. Khetan et al. encapsulated mesenchymal stem cells into hyaluronic acid (HA) based hydrogels and concluded that differentiation pathways were determined through degradation-mediated cellular traction and not a function of cell shape or elastic modulus of the material.29 Finally, cell-mediated degradation can be tuned through the introduction of a myriad of enzymatically-degradable peptide sequences with varying cleavage kinetics enabling the design of materials that are application and hypothesis specific through the simple functionalization of effective peptide sequences.47 For example, Patterson et al. studied the degradation rates of a large subset of enzymatically degradable peptides in the presence of MMP-1 or MMP-2 to better engineer hydrogels for 3D cellular invasion from aortic chick rings into PEG based hydrogels. Shown in Fig. 5b, is cell invasion into a hydrogel with a slower cleaving peptide (GPQGIWGQ) or a faster cleaving peptide (VPMSMRGG) for the MMPs secreted by the migrating cells.
While advances in real time tracking have fueled our understanding of cell matrix interactions in 3D, less is understood about dynamic changes in material properties that occur as a result of degradation of the hydrogel scaffold itself. To this end, fluorescently quenched peptides have been developed as sensors to directly detect cleavage of sequences (un-quenching the fluorescence), as a measure of MMP within a hydrogel matrix.32,34,46 These peptides are conjugated as ligands isotropically within the network, and when cleaved become fluorescent and can be visualized using fluorescent microscopy or quantified using a plate reader. There is also a focus on understanding how the material structure in the pericellular region is being degraded and remodeled, and multiple particle tracking is one technique that has emerged to characterize the gel-sol transition surrounding a cell.51,52 Cells also attach to the network through focal adhesions and pull on the network to spread and migrate. Here, traction force microscopy has proven to be a useful tool to measure this force in both 2D and 3D hydrogel cultures. 29,33,53 Together these microrheological methods and spectroscopic tools for characterizing degradation will provide the field with a much deeper understanding of the complex changes that occur during cell-mediated matrix remodeling.
FUTURE OUTLOOK AND PERSPECTIVE
Synthetic hydrogels provide a versatile and reductionist platform to effectively recapitulate aspects of the ECM through the introduction of biophysical and biochemical facets to direct cell function. Future questions in the field will continue to focus on improving the understanding as to how important inputs interact synergistically or antagonistically to elicit responses in cells. Large data sets that evolve as biological measurements advance will provide a wealth of information, but sifting through this data to determine major signaling pathways, to define concentration space, and to discover synergistic cell-material interactions will be critical to guide bioscaffold design and translational application.
An ever-growing library of techniques to create functional scaffolds and conjugate various bioactive signals are being developed, and high-throughput techniques for processing and screening will play a critical role in parsing out synergistic and antagonistic effects. Synthetic hydrogels allow for creation of 3D environments where cellular functions are readily imaged. However, 3D culture and analysis is more complicated than 2D assays, and advanced methods for real time tracking of cell signaling and functions while embedded in hydrogels will be very insightful when applied to systematically engineered cellular microenvironments. For example, Gupat et al. utilized thiol-ene chemistry to develop versatile and tunable high-throughput culture platforms to interrogate spatially defined cell attachment. 19 Similar techniques will provide probing methods for informed hypothesis generation followed by detailed mechanistic understanding of these results. For example, high-throughput techniques would allow for the study of the contextual presentation of soluble or substrate-bound biochemical cues in different biophysical contexts to better understand their relational effects on cell function. Further, investigations over broad concentration ranges of biochemical cues that are spatially defined will facilitate the identification of effective and physiologically relevant presentation techniques, especially when multiple factors are being delivered. Collectively, these types of experiments will facilitate an improved understand of the relevant time and length scales upon which one needs to direct cell-material interactions to promote tissue regeneration.
Beyond biological analysis, new chemistries and materials that allow dynamic probing of cell matrix interactions are needed, and some innovations of note include materials with reversibly tunable mechanics and biochemical functionalities. Here, covalently adaptable networks that recapitulate the stress relaxation nature of certain tissues (e.g., muscle) may fill a niche in material design and allow deeper insight as to how cells interact within environments with physiologically relevant viscoelastic properties.41,42 Others have shown the use of additional fragmentation chain transfer reactions, which have significantly impacted the synthesis of polymers with controlled molecule weight and composition, and applied them to hydrogel cell culture systems for the reversible introduction of matrix signaling ligands.16
Increased investigation into how cells engage with ECM cues will provide a better working model to develop in situ and in vitro platforms for cell culture, as well as guide the engineering of substrates for regenerative medicine applications. Using synthetic hydrogels, the opportunity exists to simplify the design strategy and take advantage of local signals present at an injury site, such as sequestration resulting in a more sustained and amplified signal. Additionally, scaffolds might be designed with critical signals to slightly perturb a non-healing wound into one that results in healing. Future design of bioactive and bioresponsive hydrogels will take advantage of many of the synthetic and engineering techniques described above to direct degradability, mechanotransduction, and local biochemical molecule delivery.
In summary, synthetic hydrogels afford great opportunity to recapitulate important aspects of the ECM using advances in polymer chemistry and engineering material properties. Techniques are being developed to test large experimental spaces of these different facets to better understand their interplay in directing cell output that should ultimately translate into more effective in vivo applications. More detailed analysis of individual and collective cellular interactions within scaffolds on various length and time scales will help elucidate the engineering parameters towards the rational design of materials for regenerative medicine. Collaboration with biologists and biochemists will result in well-defined hypotheses to test and experimental setups that will focus on important cellular outputs. These well-defined hypotheses and translational applications will be increasingly important to guide future tissue engineering research focused on cells derived from multiple sources (e.g., primary cells, embryonic cells, induced pluripotent stem cells), delivery of newly discovered signals (e.g., miRNAs, siRNAs, peptides, small molecules), and advances in the chemistry of hydrogels used to deliver these signals.
Acknowledgments
The authors would like to especially thank Emily Kyburz for her figure design and illustrations and Sharon Wang for valuable insight and discussion. Funding for this work was provided in part by the Howard Hughes Medical Institute and Grants from the National Institutes of Health (RO1DE016523) and National Science Foundation (CBET 1236662).
References
- 1.Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, et al. 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. Adv Mater. 2014;26:85–124. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azagarsamy MA, Anseth KS. Bioorthogonal click chemistry: an indispensable tool to create multifaceted cell culture scaffolds. ACS Macro Lett. 2013;2:5–9. doi: 10.1021/mz300585q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bian L, Guvendiren M, Mauck RL, Burdick JA. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci USA. 2013;110:10117–10122. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan AB, Kirschner CM Society for Biomaterials. Bio-Inspired Materials for Biomedical Engineering. New York: Wiley; 2014. [Google Scholar]
- 5.Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. Second-generation difluorinated cyclooctynes for copper-free click chemistry. J Am Chem Soc. 2008;130:11486–11493. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255–264. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeForest CA, Anseth KS. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat Chem. 2011;3:925–931. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeForest CA, Anseth KS. Photoreversible patterning of biomolecules within click-based hydrogels. Angew Chem Int Edit. 2012;51:1816–1819. doi: 10.1002/anie.201106463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 2009;8:659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeForest CA, Sims EA, Anseth KS. Peptide-functionalized click hydrogels with independently tunable mechanics and chemical functionality for 3D cell culture. Chem Mater. 2010;22:4783–4790. doi: 10.1021/cm101391y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26:3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Dingal PC, Discher DE. Combining insoluble and soluble factors to steer stem cell fate. Nat Mater. 2014;13:532–537. doi: 10.1038/nmat3997. [DOI] [PubMed] [Google Scholar]
- 13.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 14.Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv Mater. 2009;21:5005. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faulk DM, Johnson SA, Zhang L, Badylak SF. Role of the extracellular matrix in whole organ engineering. J Cell Physiol. 2014;229:984–989. doi: 10.1002/jcp.24532. [DOI] [PubMed] [Google Scholar]
- 16.Gandavarapu NR, Azagarsamy MA, Anseth KS. Photo-click living strategy for controlled, reversible exchange of biochemical ligands. Adv Mater. 2014;26:2521–2526. doi: 10.1002/adma.201304847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould ST, Darling NJ, Anseth KS. Small peptide functionalized thiol-ene hydrogels as culture substrates for understanding valvular interstitial cell activation and de novo tissue deposition. Acta Biomater. 2012;8:3201–3209. doi: 10.1016/j.actbio.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta N, Lin BF, Campos LM, Dimitriou MD, Hikita ST, Treat ND, et al. A versatile approach to high-throughput microarrays using thiol-ene chemistry. Nat Chem. 2010;2:138–145. doi: 10.1038/nchem.478. [DOI] [PubMed] [Google Scholar]
- 20.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Hiesinger W, Frederick JR, Atluri P, McCormick RC, Marotta N, Muenzer JR, et al. Spliced stromal cell-derived factor-1 alpha analog stimulates endothelial progenitor cell migration and improves cardiac function in a dose-dependent manner after myocardial infarction. J Thorac Cardiov Sur. 2010;140:1174–1180. doi: 10.1016/j.jtcvs.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyle CE, Bowman CN. Thiol-ene click chemistry. Angew Chem. 2010;49:1540–1573. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 23.Hubbell JA. Biomaterials in tissue engineering. Bio-Technology. 1995;13:565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 24.Hudalla GA, Eng TS, Murphy WL. An approach to modulate degradation and mesenchymal stem cell behavior in poly(ethylene glycol) networks. Biomacromolecules. 2008;9:842–849. doi: 10.1021/bm701179s. [DOI] [PubMed] [Google Scholar]
- 25.Humphries MJ. The molecular-basis and specificity of integrin ligand interactions. J Cell Sci. 1990;97:585–592. doi: 10.1242/jcs.97.4.585. [DOI] [PubMed] [Google Scholar]
- 26.Jabbari E. Bioconjugation of hydrogels for tissue engineering. Curr Opin Biotechnol. 2011;22:655–660. doi: 10.1016/j.copbio.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karp G. Cell and Molecular Biology: Concepts and Experiments. 3. New York: Wiley; 2002. [Google Scholar]
- 28.Kharkar PM, Kiick KL, Kloxin AM. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem Soc Rev. 2013;42:7335–7372. doi: 10.1039/c3cs60040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12:458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kloxin AM, Lewis KJ, DeForest CA, Seedorf G, Tibbitt MW, Balasubramaniam V, et al. Responsive culture platform to examine the influence of microenvironmental geometry on cell function in 3D. Integr Biol. 2012;4:1540–1549. doi: 10.1039/c2ib20212c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyburz KA, Anseth KS. Three-dimensional hMSC motility within peptide-functionalized PEG-based hydrogels of varying adhesivity and crosslinking density. Acta Biomater. 2013;9:6381–6392. doi: 10.1016/j.actbio.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SH, Moon JJ, Miller JS, West JL. Poly(ethylene glycol) hydrogels conjugated with a collagenase-sensitive fluorogenic substrate to visualize collagenase activity during three-dimensional cell migration. Biomaterials. 2007;28:3163–3170. doi: 10.1016/j.biomaterials.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Legant WR, Miller JS, Blakely BL, Cohen DM, Genin GM, Chen CS. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat Methods. 2010;7:969–971. doi: 10.1038/nmeth.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leight JL, Alge DL, Maier AJ, Anseth KS. Direct measurement of matrix metalloproteinase activity in 3D cellular microenvironments using a fluorogenic peptide substrate. Biomaterials. 2013;34:7344–7352. doi: 10.1016/j.biomaterials.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-beta1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell. 2012;23:781–791. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang YK, Kiick KL. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014;10:1588–1600. doi: 10.1016/j.actbio.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu PF, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci USA. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacArthur JW, Jr, Purcell BP, Shudo Y, Cohen JE, Fairman A, Trubelja A, et al. Sustained release of engineered stromal cell-derived factor 1-alpha from injectable hydrogels effectively recruits endothelial progenitor cells and preserves ventricular function after myocardial infarction. Circulation. 2013;128:S79–S86. doi: 10.1161/CIRCULATIONAHA.112.000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madl CM, Mehta M, Duda GN, Heilshorn SC, Mooney DJ. Presentation of BMP-2 mimicking peptides in 3D hydrogels directs cell fate commitment in osteoblasts and mesenchymal stem cells. Biomacromolecules. 2014;15:445–455. doi: 10.1021/bm401726u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKinnon DD, Domaille DW, Cha JN, Anseth KS. Bis-aliphatic hydrazone-linked hydrogels form most rapidly at physiological ph: identifying the origin of hydrogel properties with small molecule kinetic studies. Chem Mater. 2014;26:2382–2387. [Google Scholar]
- 42.McKinnon DD, Domaille DW, Cha JN, Anseth KS. Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Adv Mater. 2014;26:865–872. doi: 10.1002/adma.201303680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moroni F, Mirabella T. Decellularized matrices for cardiovascular tissue engineering. Am J Stem Cells. 2014;3:1–20. [PMC free article] [PubMed] [Google Scholar]
- 44.Mosiewicz KA, Kolb L, van der Vlies AJ, Martino MM, Lienemann PS, Hubbell JA, et al. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat Mater. 2013;12:1072–1078. doi: 10.1038/nmat3766. [DOI] [PubMed] [Google Scholar]
- 45.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 46.Packard BZ, Artym VV, Komoriya A, Yamada KM. Direct visualization of protease activity on cells migrating in three-dimensions. Matrix Biol. 2009;28:3–10. doi: 10.1016/j.matbio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 48.Perlin L, MacNeil S, Rimmer S. Production and performance of biomaterials containing RGD peptides. Soft Matter. 2008;4:2331–2349. [Google Scholar]
- 49.Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellars KN, et al. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat Mater. 2014;13:653–661. doi: 10.1038/nmat3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabenstein DL. Heparin and heparan sulfate: structure and function. Nat Prod Rep. 2002;19:312–331. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]
- 51.Schultz KM, Anseth KS. Monitoring degradation of matrix metalloproteinases-cleavable PEG hydrogels via multiple particle tracking microrheology. Soft Matter. 2013;9:1570–1579. [Google Scholar]
- 52.Schultz KM, Furst EM. Microrheology of biomaterial hydrogelators. Soft matter. 2012;8:6198–6205. [Google Scholar]
- 53.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc Natl Acad Sci USA. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tibbitt MW, Kloxin AM, Dyamenahalli KU, Anseth KS. Controlled two-photon photodegradation of PEG hydrogels to study and manipulate subcellular interactions on soft materials. Soft Matter. 2010;6:5100–5108. doi: 10.1039/C0SM00174K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Tibbitt MW, Langer SJ, Leinwand LA, Anseth KS. Hydrogels preserve native phenotypes of valvular fibroblasts through an elasticity-regulated PI3K/ AKT pathway. Proc Natl Acad Sci USA. 2013;110:19336–19341. doi: 10.1073/pnas.1306369110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watt FM, Huck WTS. Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Bio. 2013;14:467–473. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- 57.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32(1):241–244. [Google Scholar]
- 58.Wylie RG, Ahsan S, Aizawa Y, Maxwell KL, Morshead CM, Shoichet MS. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat Mater. 2011;10:799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- 59.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]