Abstract
Objective
To investigate whether elevated IFN-α early in pregnancy is associated with poor pregnancy outcomes and examine its relationship to angiogenic imbalance.
Methods
Women were enrolled in a case-control longitudinal study of lupus pregnancies. Serum samples obtained monthly through pregnancy were assayed for IFN-α and for antiangiogenic factor, sFlt1, and proangiogenic factor, (PlGF). Each of 28 SLE patients with poor pregnancy outcome was matched to an SLE patient with an uncomplicated pregnancy and to a pregnant healthy control. The effects of IFN-α and/or sFlt1 on-human endothelial cells and endothelial-trophoblast interactions was assessed.
Results
Compared to SLE patients with uncomplicated pregnancies, patients with preeclampsia had increased IFN-α before clinical symptoms. Non-autoimmune patients destined for preeclampsia did not have increased IFN-α. In SLE patients with low IFN-α, marked angiogenic imbalance (higher sFlt1, lower PlGF and higher sFlt1/PlGF ratios) precedes maternal manifestations of preeclampsia, whereas in SLE with high IFN-α, preeclampsia occurs without evidence of systemic angiogenic imbalance. Treatment of human endothelial cells with sFlt1 induced expression of sFlt1 mRNA, and IFN-α dramatically amplified responses to sFlt1. In a model of spiral artery transformation, only IFN-α and sFlt1 together disrupted the ability of trophoblast cells to remodel endothelial tube structures.
Conclusions
Our studies identify a new mechanism by which IFN-α induces an antiangiogenic milieu, increases the sensitivity of endothelial cells to sFlt1, and suggest that elevated IFN-α may contribute to pathogenesis of preeclampsia in some SLE pregnancies.
Systemic lupus erythematosus (SLE), the prototypic systemic autoimmune disease, predominantly afflicts women and presents during reproductive years. Pregnancy in patients with SLE is associated with increased risk for maternal and fetal morbidity and mortality, including premature birth, miscarriage, fetal growth restriction, preeclampsia and neonatal death (1-3). Placenta dysfunction plays a major role in these complications.
Normal placenta development requires coordinated expression of angiogenic growth factors, vascular endothelial growth factor (VEGF) and placenta growth factor (PlGF), as well as expression of their receptors on invasive trophoblasts, VEGF receptor-1 (VEGFR-1; also known as fms-like tyrosine kinase-1, Flt1) and VEGFR-2 (4, 5). Placental trophoblasts release a splice variant of VEGFR-1, soluble VEGFR-1 (sVEGFR-1, also known as sFlt1) that sequesters circulating VEGF and PlGF, prevents their binding to trophoblast and endothelial cell receptors, and thus acts as a potent antiangiogenic growth factor (6). Adequate placental perfusion requires remodeling of uterine spiral arteries into dilated, flaccid vessels, a process dependent upon trophoblast invasion and replacement of endothelium (7). Imbalance of angiogenic factors is associated with abnormal placental invasion and subsequent hypoperfusion and fetal growth restriction (8-10).
Circulating sFlt1 levels normally increase slowly throughout pregnancy, but in women destined for preeclampsia, sFlt1 levels are markedly increased in blood and placenta and sFlt1/PlGF ratios are increased, leading to the clinical manifestations of preeclampsia – widespread endothelial dysfunction, hypertension and proteinuria (4, 11, 12). Inflammatory mediators, specifically TNF-α and oxidants, have also been associated with placental insufficiency, fetal growth restriction and renal structural alterations characteristic of preeclampsia (13). It is not known whether immune dysregulation associated with SLE contributes to risk for poor pregnancy outcomes.
Type I interferons (IFN), particularly IFN-α, are thought to play a central role in the pathogenesis of SLE (14). Extensive data from patients with SLE demonstrate an association of IFN pathway activation, identified as an IFN signature in peripheral blood mononuclear cells, kidney and skin tissue, with more severe disease and greater disease activity (15). Notably, IFN-α is a potent antiangiogenic factor, contributing to down-regulation of pro-angiogenic molecules such as VEGF, decrease in hematopoietic progenitor cells involved in vascular remodeling and impairment of vasculogenesis (16-20). Recent studies have linked type I IFNs to vascular damage and dysfunction in SLE, in part related to transcriptional repression of angiogenic factors (16, 17). Given the requirement for VEGF by certain vascular beds, such as those in glomeruli (21), we considered the possibility that the endothelium of SLE patients exposed to elevated IFN-α levels may be more vulnerable to the angiogenic imbalance induced by a dysfunctional placenta. We hypothesized that IFN-α contributes to increased risk of preeclampsia and other complications in patients with SLE. Accordingly, we investigated whether pregnant SLE patients destined for poor outcomes had increased IFN-α activity early in pregnancy and examined the relationship between IFN-α and angiogenic imbalance in the pathogenesis of pregnancy complications.
PATIENTS AND METHODS
Subjects
We performed a case-control study of SLE patients in the PROMISSE Study (Predictors of Pregnancy Outcome: Biomarkers In Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus). Patients had the following characteristics: age 18-45 years, live single intrauterine pregnancy confirmed by ultrasound, pregnancy less than 12 weeks’ gestation, Hct >26% and capacity to give informed consent. Detailed medical and obstetrical information, and serial blood samples were obtained monthly beginning at 6 weeks of pregnancy.
Pregnant SLE patients met ACR criteria (22). Exclusions for enrollment into PROMISSE at screening were: prednisone >20mg, active renal disease (proteinuria >1gm/24 hr or protein/creatinine ratio >1000 mg, protein/gram creatinine on spot urine, or RBC casts or and serum creatinine >1.2 mg/dl), diabetes mellitus (type I and Type II) antedating pregnancy, hypertension (BP ≥140/90 mmHg) and multiple fetal gestations. Disease activity was measured with SLEPDAI, an instrument designed for pregnant SLE patients (23). Cases were SLE patients who had a poor pregnancy outcome, defined as one or more of the following: fetal death >12 weeks (n=7); pre-term delivery at <36 weeks’ gestation due to placental insufficiency, gestational hypertension or preeclampsia (n=16), severe growth restriction (birth weight <5th percentile) (24-26) (n=8); these events occurred most often by 28-31 weeks’ gestation. Preeclampsia was defined as new onset of elevated systolic blood pressure (≥140 mm Hg) and/or elevated diastolic blood pressure (≥90 mm Hg) after 20 weeks’ gestation on two occasions at least four hours apart and proteinuria of 300 mg or greater in a 24h urine specimen or ≥1+ on dipstick on two occasions at least 4 hours apart in the absence of pyelonephritis or hematuria (25, 27).
Each of 28 cases (SLE/outcome) was matched by age and ethnicity/race to an SLE patient with an uncomplicated pregnancy (SLE/no outcome) and to a pregnant healthy control. Pregnant healthy control subjects had to have had ≥1 normal pregnancy and could not have a history of fetal death at >10 weeks’ gestation, or >1 miscarriage at <10 weeks’ gestation. The racial composition of each group was 50% White, 25% Black, 14% Asian and 11% mixed. Ethnicity was 20% Hispanic and 80% non-Hispanic. This study was approved by Hospital for Special Surgery Institutional Review Board. We analyzed a second group of non-autoimmune patients followed through pregnancy at Beth Israel Deaconess Medical Center, Boston MA, including 9 patients who developed preeclampsia and 6 patients from that cohort with uncomplicated pregnancies. Stored frozen samples from both PROMISSE and Beth Israel Deaconess cohorts were used in IFN and angiogenic factor assays.
IFN-α reporter: WISH cell assay
Serum samples from the 3 matched pregnancies were assayed simultaneously for IFN-α activity using a reporter cell assay (28). A total of 400 samples prospectively collected during pregnancy were tested. All analyses for Relative Expression (RE) of MX1 mRNA were done on samples collected at 6-27 weeks’ gestation, a time before poor outcomes were clinically apparent. Results for each culture condition are reported as relative expression (RE) compared with WISH cells cultured with medium alone. Details of the real-time quantitative PCR method and data analysis have been described previously (28). In preliminary studies, we confirmed that neutralizing antibodies to IFN-α decreased elevated IFN score in SLE patients to levels of healthy donors. MX1 was the gene transcript that drove the IFN score in this group of patients and was chosen as a single transcript to quantify serum IFNα activity.
Maternal angiogenic factor assays
sFlt1 and PlGF were measured by ELISA in duplicate with commercial kits (R&D Systems) as described previously (29). The minimal detectable doses in the assays for sFlt1 and PlGF were 5 pg/ml for both assays, and inter-assay coefficients of variation were 7% and 11% respectively. sFlt1 and PlGF levels are reported as pg/ml and as sFlt1/PlGF ratio.
Endothelial cell culture and reagents
Human umbilical vein endothelial cells (HUVECs) were cultured as previously described (30) and used between passages 1 to 8. For gene expression analysis, HUVECs (50,000 cells/well) were cultured for 3 days, then incubated for 6 h with or without recombinant IFN-α (100 U/ml, PBL Interferon Source), recombinant sFlt1 (5 ng/ml, R&D Systems) or both, then RNA was purified as below.
The human endometrial endothelial cell line (HEEC) (31) was maintained as previously described (32). For analysis of sFlt1 secretion, HEECs were plated into 60mm tissue culture dishes overnight, and then treated in serum-free OptiMEM (Invitrogen) with or without recombinant IFN-α (1000 U/ml). After 24 h, sFlt1 in supernatants was measured by ELISA.
Endothelial cell gene expression analysis by q-RT PCR
RNA was isolated using RNeasy kit (QIAGEN) and 0.5 μg of RNA per condition was used for the cDNA synthesis using iScript Reverse Transcription Supermix (BIO-RAD), and an ABI Veriti Thermocycler (Life Technologies). SsoAdvance Universal SYBR Green Supermix and CFX96 Real Time System from BIO-RAD were used following manufacturer’s instructions for volumes and reaction settings. Primer sequences for sFlt1, MX-1 and GAPDH genes were previously described (28, 33, 34), and purchased from IDT Integrated DNA Technologies.
Endothelial-trophoblast co-culture assay
Trophoblast-endothelial interactions were evaluated using an established 3-D culture system (35). Briefly, HEECs (31) stained with red PKH26 (Sigma) were seeded onto 24-well tissue culture plates coated with Matrigel. After 24hrs, tube-like structures were formed (35). Media was removed and the human first trimester extravillous trophoblast cell line, HTR8 (36) stained with green PKH67, was added in serum-free OptiMEM in the presence or absence of either IFNα (100U/ml), sFlt1 (5ng/ml), or both for 24hrs. 6 fields per well were recorded by fluorescent microscopy and the number of tubes per field counted.
Statistical analysis
RE MX1 levels were obtained at multiple time points between 6–27 weeks’ gestation from each patient. Because levels did not vary systematically between visits, the median value of each subject’s available measurements over the time period of interest was computed and used as the unit of analysis in subsequent statistical tests. All samples for each patient were included. The repeated measures data were also analyzed using linear mixed effects models based on the log transformed data but results are not shown because they were nearly identical to those of the first approach. RE MX1 and other continuous variables were compared between disease groups with the Wilcoxon Rank Sum test. Spearman rank correlation coefficients were estimated to assess relationships between RE MX1 with SLEPDAI, anti-Ro, and antibody titers. Unadjusted and adjusted odds ratios for preeclampsia were estimated by fitting logistic regression models. A two-sided p-value <0.05 was considered statistically significant. Endothelial gene expression data were analyzed using the ΔΔCT methodology and the GraphPad Prism version 6.0b software.
RESULTS
In early pregnancy, SLE patients destined for preeclampsia have higher functional IFN-α activity
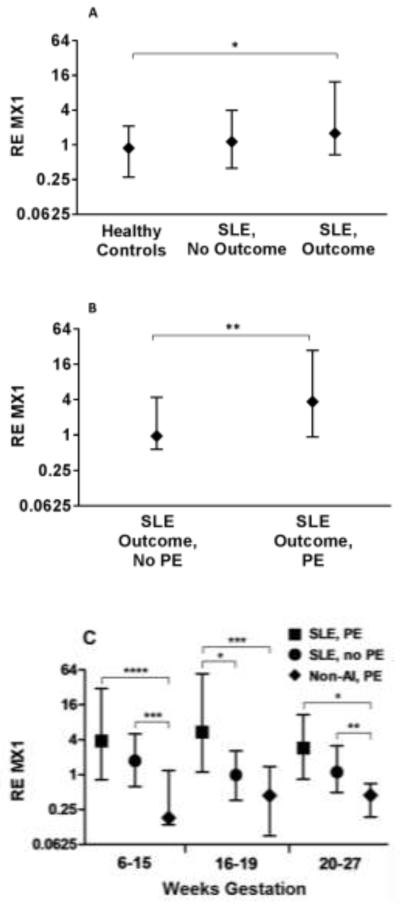
Clinical features of SLE patients at enrollment in the PROMISSE study were similar for patients with poor pregnancy outcomes and uncomplicated pregnancies (Table I). Patients with poor outcome were more likely to have anti-DNA antibodies at screening. All patients had low disease activity assessed by SLEPDAI scores at screening. Serum samples were obtained monthly, and we assayed samples from 6-27 weeks’ gestation (average of 3.6 samples/patient), a period that generally antedated clinically-apparent pregnancy complications in our patients. IFN-α activity was assessed using the WISH reporter cell assay, and the serial measures for each subject were summarized using the median of RE of MX1 (an IFN-α-responsive gene). As expected, levels of IFN-α activity, defined as RE of MX1 expression, were higher in serum from the SLE group than that of controls (all SLE patients vs. healthy controls medians: 1.50 vs. 0.71, p<0.007). IFN-α activity was greater in SLE patients with poor outcome compared to the healthy controls (p<0.004), but there was no statistically significant difference compared to the SLE without poor outcome group, (Figure 1A). When patients with poor outcome were further stratified according to whether or not their pregnancy was complicated by preeclampsia (Figure 1B), patients with preeclampsia showed higher median levels of IFN-α activity than SLE patients with poor outcome other than preeclampsia (4.33 vs 1.25; p<0.006) and compared to SLE pregnancies without poor outcome (4.33 vs 1.33; p=0.04).
Table I.
Clinical and Laboratory Features of SLE Patients at Screening.
| Poor Pregnancy Outcome N (%) |
Uncomplicated Pregnancy N (%) |
|
|---|---|---|
|
| ||
| Clinical * | ||
| Cutaneous | 18/28 (64%) | 15/27 (59%) |
| Arthritis | 20/28 (71%) | 19/27 (70%) |
| Serositis | 5/28 (18%) | 7/27 (26%) |
| Hematologic | 14/28 (50%) | 14/27 (52%) |
| Renal | 15/28 (54%) | 10/27 (37%) |
| Laboratory | ||
| Anti-DNA | 16/28 (57%) | 9/26 (35%) |
| Anti-Ro | 9/25 (36%) | 16/25 (64%) † |
| Anti-La | 2/25 (8%) | 6/25 (24%) |
| Low C3 | 16/28 (57%) | 7/27 (26%) § |
| Medications | ||
| Hydroxychloroquine use | 17/28 (61%) | 16/27 (59%) |
| Steroids use | 16/28 (57%) | 11/27 (41%) |
|
| ||
| Age (Mean ± SD) | 31.6 ± 3.9 | 31.4 ± 4.5 |
| SLEPDAI (Mean ± SD) | 4.6 ± 3.5 | 2.2 ± 2.7 ‡ |
defined according to ACR criteria for SLE
p = 0.05
p = 0.02
p < 0.01
Figure 1. IFN-α Activity in PROMISSE Study Patients.
Samples from 6 - 27 weeks’ gestation (average 3.6/patient), a period that antedated clinically-apparent pregnancy complications, were assayed for IFN-α activity using the WISH reporter cell assay. Data are reported as median values of RE of MX1 (an IFN-α-responsive gene) from all samples collected for each patient. Lines represent 25-75th percentile. (A) Median values of RE MX1 are shown for healthy controls (n=28 patients), SLE patients without poor outcomes (No Outcome) (n=27) and SLE patients with poor outcomes (Outcome) (n=28). *Healthy controls vs. SLE/Outcome: p<0.004; Healthy controls vs. SLE/No Outcome: p=0.07; SLE/Outcome vs. SLE/No Outcome: p=0.23. (B) Median values of RE MX1 are shown for SLE patients with poor outcomes other than PE (n=14) and SLE patients with PE (n=14). (**p=0.006). (C) Samples from healthy, non-autoimmune patients who developed PE (Non-AI PE, n=9), SLE patients without PE (no outcomes and outcomes but not PE) (SLE, no PE, n=41) and SLE patients with PE (SLE, PE, n=14) were assayed and data presented group by weeks of gestation. *p=0.02; **p=0.04; ***p=0.008; ****p<0.005. Non-AI, non-autoimmune; PE, preeclampsia
Because RE MX1 levels were similar in SLE patients without poor outcome and those with outcomes other than preeclampsia (p=0.83), we combined these groups and compared them to SLE with preeclampsia to increase sample size to further evaluate the association between RE MX1 and preeclampsia. RE MX1 levels were higher in the SLE with preeclampsia group compared to the combined SLE without preeclampsia group (p=0.006). For every 10 unit increase in RE MX1, the odds ratio for preeclampsia from a logistic regression analysis was 1.37 (95% CI: 1.09-1.72). RE MX1 levels were consistently higher in the preeclampsia group throughout pregnancy, with the largest difference occurring between 16 – 19 weeks (p=0.02; Figure 1C). We performed similar analyses using an IFN score calculated by measuring RE of 3 IFN-α-responsive genes (IFT1, IFI44 and MX1) as previously described (28). Results showed trends similar to those of MX1, but were less robust (data not shown).
The difference in IFN-α activity in patients with who developed preeclampsia is not attributable to disease activity or autoantibody profile. In non-pregnant SLE patients, higher disease activity and specific autoantibody profiles are associated with IFN signature (15). At baseline, SLEPDAI values were low and not different for SLE patients who developed preeclampsia compared to those who had other poor outcomes (4.6 ± 3.8 vs. 4.6 ± 3.5, p = 0.98); though SLEPDAI scores for SLE patients with any complication were higher than those without (4.6 ± 3.5 vs. 2.2 ± 2.7, p = 0.007). We found no correlation between SLEPDAI and RE MX1. Similarly, the association between RE MX1 and anti-Ro antibodies at 6-11 weeks’ gestation was not significant. Paradoxically, anti-Ro antibodies were less frequent in patients who had poor pregnancy outcomes (Table I).
Antimalarials, potent inhibitors of endosomal Toll-like receptors, have been associated with relatively low IFN-α levels in SLE (15). The use of hydroxychloroquine among patient groups did not differ (Table I). In addition, the differences in RE MX1 in patients taking hydroxychloroquine compared to those not taking the medication at screening was not significant (median 1.1 vs. 2.4; p=0.94). Taken together, these results argue that there is increased IFN-α activity before clinical symptoms in SLE patients destined to develop preeclampsia, and this increase in IFN-α is not related to levels of disease activity, autoantibody profiles, or exposure to hydroxychloroquine. After adjusting for these factors in a multivariable logistic regression model, the odds ratio for preeclampsia associated with a 10 unit increase in RE MX1 is 1.85 (95% CI: 1.27-2.68; p=0.001).
Non-autoimmune patients destined for preeclampsia do not have increased functional IFN-α
To determine whether the increase in functional IFN-α present before the onset of preeclampsia in patients with SLE is also a harbinger of preeclampsia in non-autoimmune patients, we assessed RE MX1 induced in WISH cells by serum obtained at 12-28 weeks’ gestation in 9 healthy patients (average of 2.7 samples/patient) who subsequently developed preeclampsia and compared them to healthy controls who did not develop preeclampsia (6 patients from the same cohort and 28 patients from PROMISSE). IFN-α activity was not increased by serum from these non-autoimmune patients. SLE patients destined for preeclampsia showed higher RE MX1 than the non-autoimmune patients as early as 6-15 weeks’ gestation (3.31 vs. 0.18, p=0.003), and this difference persisted through 20-27 weeks’ gestation (Figure 1C). These data indicate that preeclampsia, in and of itself, is not associated with elevated IFN-α activity.
Minimal angiogenic factor dysregulation is associated with preeclampsia in patients with elevated IFN-α, in contrast to those with low IFN-α
The major clinical phenotypes of preeclampsia, hypertension and proteinuria, are due, at least in part, to excess antiangiogenic factor, sFlt1, and reduced placenta growth factor (PIGF) in the maternal circulation (12). IFN-α represses transcription of angiogenic factors in endothelial cells and consequently could increase the vulnerability of vascular beds that require VEGF, such as those in glomeruli (21). If this were true, pregnant SLE patients with high IFN-α could manifest endothelial dysfunction in the absence of marked alterations in sFlt1/PlGF, whereas those with preeclampsia without marked elevations in IFN-α activity should demonstrate angiogenic imbalance. To test this possibility, we divided SLE patients with preeclampsia into 2 groups, high IFN-α and low IFN-α defined as above or below the median RE MX1 in SLE patients with uncomplicated pregnancies (1.33) and compared sFlt1 and PlGF levels at 18-21 weeks’ gestation, a time before clinical manifestations of preeclampsia were present.
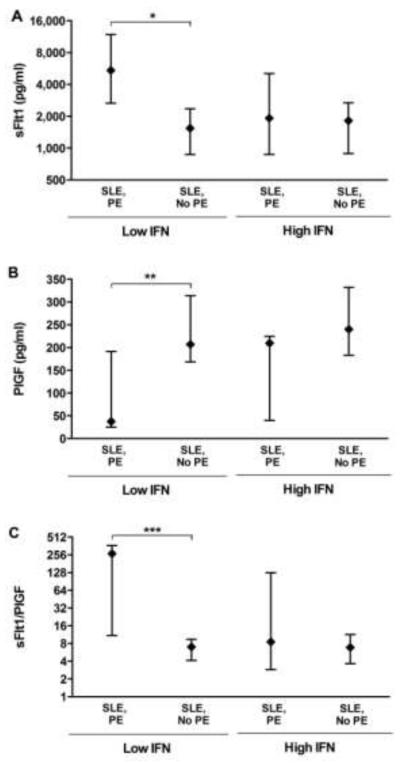
As expected, SLE patients with lower IFN-α who developed preeclampsia had higher sFlt1 levels than those who did not (medians: 5425 ng/ml vs. 1793, respectively; p<0.08) (Figure 2A). In contrast, in SLE patients with higher IFN-α, there was no difference in sFlt1 levels between those who did and those who did not develop preeclampsia (1916 ng/ml vs. 1873, respectively, p=0.83), and these patients showed minimal elevations in sFlt1 compared to healthy pregnant patients. For reference, in healthy controls enrolled in the PROMISSE study, the median level of sFlt1 at 18-21 weeks was 1026 pg/ml, and for non-pregnant SLE patients median level of sFlt1 was 81 pg/ml, over 10-fold higher.
Figure 2. Angiogenic Factor Levels in SLE Patients Who Developed Preeclampsia.
SLE patients were grouped as low IFN-α (n=22) or high IFN-α (n=17). Plasma from 18-21 weeks was assayed for sFlt1 and PlGF by ELISA. Lines represent 25-75th percentile. (A) Median levels of sFlt1 are shown in low IFN-α group with PE (n=5) compared to those without PE (no outcome and non PE outcomes) (n=17) (*p=0.08, Wilcoxon rank sum) and in high IFN-α patients with PE (n=7) compared to those without PE (n=10). (B) Median levels of PlGF are similarly compared in these groups (**p=0.03). (C) Ratios of sFlt1/PlGF are shown (***p=0.07). For reference, in healthy controls enrolled in the PROMISSE study the median levels of angiogenic factors at 18-21 weeks were: sFlt1 1026 pg/ml, PlGF 249 pg/ml, and sFlt1/PlGF ratio 6.21.
Likewise, PlGF levels were significantly decreased (medians: 37 pg/ml vs. 240) and sFlt1/PlGF ratios (268 vs. 8.0) were increased in low IFN-α patients destined for preeclampsia compared to those who did not develop preeclampsia (p<0.03 and p<0.07, respectively). Again, in SLE patients with high IFN-α, levels of angiogenic factors were indistinguishable between those who did and did not develop preeclampsia (240 pg/ml vs. 210 and 10.1 vs. 8.5, respectively) (Figures 2B and 2C). The median level of PlGF was 249 pg/ml and the sFlt1/PlGF ratio was 6.21 in pregnant healthy controls. Taken together, these data indicate that marked angiogenic imbalance precedes maternal manifestations of preeclampsia in SLE patients with low IFN-α, whereas in patients with high IFN-α preeclampsia occurs without evidence of systemic levels of antiangiogenic factors in the range associated with preeclampsia.
Exposure to IFN-α increases sensitivity of HUVEC to sFlt1
There is a slow and steady increase of sFlt1 during normal pregnancy, but the rate of change and levels are markedly greater in those destined for preeclampsia (12). Given that maternal endothelial dysfunction, manifested as preeclampsia, occurs in the absence of elevated antiangiogenic factors in SLE patients with high IFN-α, we considered the possibility that IFN-α increases the vulnerability of endothelial cells to the relative VEGF deficiency induced by increasing levels of sFlt1. We discovered that incubation of HUVEC with sFlt1 (5 ng/ml, levels comparable to those in the second trimester of pregnancy) induced the expression of sFlt1 mRNA. The effects of sFlt1 on MX-1 (an IFN-α responsive gene) (Figure 3) and VEGF mRNA expression were minimal (data not shown). When modest doses of IFN-α (100 IU/ml) were present (16), a greater than 10-fold increase in the capacity of sFlt1 to amplify its own production was seen (Figure 3B). Our results identify a novel mechanism by which IFN-α can potentiate the antiangiogenic effects of sFlt1. IFN-α can lower the threshold for endothelial dysfunction by antagonizing the autocrine function of VEGF and amplify the feed-forward loop of sFlt1 production, and as shown by others, decreasing the production of VEGF.
Figure 3. Regulation of gene expression in HUVECs by IFN-α and sFLT1.
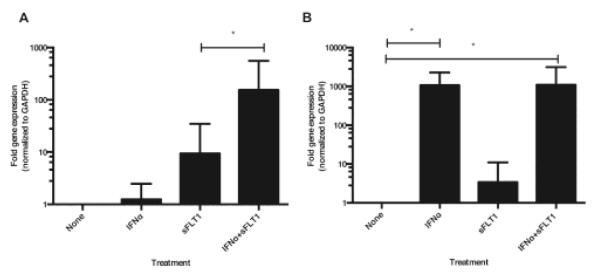
The transcriptional regulation of sFLT1 (A) and MX-1 (B) in HUVECs following treatment with recombinant IFN-α and sFlt1 was analyzed by q-RT PCR. HUVECs were incubated for 6 h with no treatment (None), IFN-α (100 U/ml), sFlt1 (5 ng/ml) or both proteins. The fold of gene expression was calculated using ΔΔCt normalized to the housekeeping gene GAPDH and to the baseline condition without treatment. Results represent the mean ± standard error of fold-gene expression of at least 5 different experiments; *:p<0.05 by two-tailed Wilcoxon matched-pairs signed rank test.
IFN-α and sFlt1 in combination disrupt endothelial-trophoblast interactions
During normal placentation, the trophoblast invades the maternal uterine vasculature and remodels it by replacing endothelial cells (37, 38). Insufficient spiral artery transformation is a hallmark of preeclampsia (39). Using an in vitro model of spiral artery transformation (35, 40), we explored the implications of the antiangiogenic effects of IFN-α and sFlt1 on HEEC interactions with extravillous trophoblast cells (HTR8). In this in vitro system, HTR8 cells stabilize HEEC tube structures over time (Figure 4). As shown in Figure 5A, compared to untreated control (NT), IFN-α or sFlt1 alone had no effect on the ability of HTR8 cells to invade the tube-like structures, replacing the HEECs. However, the combination of IFN-α and sFlt1 dramatically reduced the ability of the HTR8 cells to remodel HEEC tubes, resulting in their destabilization (Figure 5A), reflected in a significant reduction in the number of tubes (Figure 5B). Moreover, fewer HTR8 (green) cells were observed within the HEEC (red) tube structures under these conditions. These changes were not due to altered viability of HEEC or HTR8 cells in the presence of IFN-α, sFlt1, or both. Ater 24 hrs, cell viability measured by colormetric CellTiter assay (Promega) was > 95%.
Figure 4. HTR8 cells stabilize HEEC tubes over time.
HEEC cells were seeded in matrigel overnight to allow tube formation. Media was then removed from the wells and replaced with or without the first trimester extravillous trophoblast cell line, HTR8. After 24hrs, in absence of HTR8 cells, HEEC tubes are established. However, by 48hrs, the HEEC tubes begin to destabilize and by 72hrs they are mostly disintegrated. In contrast, in the presence of HTR8 cells, the tube structures are maintained over 72hrs, and increase in size as the trophoblast cells remodel the endothelium.
Figure 5. Effect of IFN-α and sFlt1 on endothelial-trophoblast interactions.
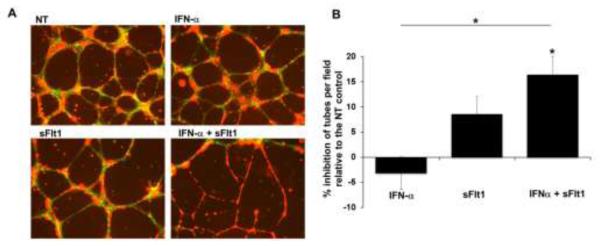
HTR8 cells were added to HEEC tube cultures in the presence of no treatment (NT), IFN-α (100U/ml), sFlt1 (5ng/ml) or both (IFN-α + sFtl-1) and cultured for 24hrs. (A) HEEC (red)-HRT8 (green) interactions were visualized by fluorescent microscopy. Images are from one representative experiment. Co-localization of the cells is seen as yellow. (B) Bar chart shows the % inhibition of the number of tubes per field expressed as the mean ± SEM relative to the NT control from 4 independent experiments. For NT controls, the number of tubes was 18.1 ± 1.0. *p<0.05 by ANOVA with Bonferroni correction.
To expand upon our observations showing that IFN-α amplifies sFlt1 mRNA in HUVECs, we exposed HEECs to recombinant IFN-α and found IFN-α increased sFlt protein secretion by 27.3± 8.9% (p=0.03). These data show that in two different endothelial cells lines, IFN--α modulates sFlt1 expression.
DISCUSSION
Preeclampsia complicates 13-35% of SLE pregnancies, compared to 4-6% of all first pregnancies and less than 1% of subsequent pregnancies in otherwise healthy women (41). We have shown, in a case-control study, that SLE patients destined to develop preeclampsia are more likely to have increased circulating IFN-α activity before the onset of clinical symptoms compared to patients who do not develop preeclampsia, that this increase cannot be explained by higher levels of disease activity, autoantibodies, or exposure to hydroxychloroquine, and, in a group of non-autoimmune patients, that preeclampsia, in and of itself, is not associated with elevated IFN-α.
In non-autoimmune patients, preeclampsia has been associated with excessive placenta-derived circulating antiangiogenic proteins, such as sFlt1, which causes a shift toward a more “antiangiogenic” state in the mother (29). We found that in SLE patients with lower levels of IFN-α, there was also a marked increase in sFlt1 and sFtl-1/PlGF ratios before clinical signs of preeclampsia. In contrast, SLE patients with higher IFN-α and destined for preeclampsia showed no evidence, in serum, for angiogenic factor dysregulation. Unexpectedly, their sFlt1 levels were similar to levels in patients who would go on to uncomplicated pregnancies or complications other than preeclampsia. These findings suggested that elevated IFN-α may contribute to the pathogenesis of preeclampsia in some women with lupus by sensitizing the maternal vascular endothelium to the antiangiogenic effects of even normal levels of sFlt1, as well as by inhibiting transcription of proangiogenic VEGF. Indeed, our in vitro studies showed that incubation with physiological doses of sFlt1 induces expression of endogenous sFlt1 mRNA in endothelial cells and that IFN-α dramatically increases this effect, thereby potentiating the blockade of autocrine VEGF signaling.
There is precedent for the concept that endothelial health and angiogenesis, which is promoted by VEGF, is antagonized by type I IFNs, and multiple mechanisms have been described. IFN-α has been shown to induce an antiangiogenic signature in endothelial progenitor cells from lupus patients, as well those from healthy subjects; addition of VEGF restores their capacity to survive, differentiate and proliferate (17). IFN-α inhibits VEGF activation of FAK, ERK, Akt, and eNOS, and induces transcription of hypoxia-inducible factor-α in human endothelial cells which limits their proliferative capacity (19, 42). Recent studies have shown that inhibition of type I IFN receptor signaling, as a consequence of VEGF-induced degradation of receptors for type I IFNs, is required for efficient VEGF-stimulated angiogenesis (43). That IFN-α induces an antiangiogenic state in vivo in non-pregnant SLE patients is suggested by findings of vascular rarefaction in renal blood vessels and repression of VEGF in kidney and serum (16, 17).
Our in vitro studies identify a new mechanism by which IFN-α induces an antiangiogenic milieu locally in the vasculature. We show that treatment with exogenous sFlt1, which creates a VEGF-deficient state, induces expression of endogenous sFlt1 mRNA in HUVEC, leading to a local positive feedback loop, and that IFN-α dramatically amplifies the response to sFlt1. Enhanced production of sFlt1 promoted by IFN-α could blunt uterine spiral artery remodeling early in pregnancy and alter placental perfusion. Indeed, using an in vitro model of the uterine vasculature, our studies also show that IFN-α, in combination with sFlt1, has a dramatic effect on the ability of trophoblast cells to interact with and transform the endothelium. Similarly, later in pregnancy, IFN-α could sensitize the maternal vasculature to the effects of even modest elevations of sFlt1 (median post-partum level: 81 pg/ml) and intensify local VEGF insufficiency. This is particularly important in glomerular endothelial beds which require podocyte production of VEGF for health; loss of free VEGF in experimental models promotes renal microvascular injury and thrombotic microangiopathy, clinically manifested as proteinuria and hypertension, characteristics of preeclampsia (6, 21). Our findings support the possibility that glomerular endothelial cells primed by IFN-α in SLE have exaggerated responses to decreased VEGF availability. Recent studies have shown that sFlt1 also sensitizes endothelial cells to the proinflammatory and proadhesive effects of TNF-α (44).
We propose that in a subset of SLE patients, the gradual increase in sFlt1 that occurs in all pregnancies, superimposed upon the increase in sFlt1 and previously described decrease in VEGF produced by endothelial cells in response to IFN-α, is sufficient to disrupt podocyte-derived VEGF function. Indeed, the increased frequency of preeclampsia in patients who have active SLE early in pregnancy, particularly active nephritis (45-48), may be related, in part, to vasculopathic effects of elevated IFN-α present in many such patients. Although the major source of sFlt1 in pregnant patients is placenta (4, 5), that circulating levels of sFlt1 are not correlated with IFN-α suggests that modest local increases in sFlt1 production by endothelial cells have adverse clinical consequences in vulnerable vascular beds.
The limitation of our study is the small number of patients. We matched cases and controls according demographics and excluded those with other risk factors for preeclampsia. Nonetheless, SLE patients are highly heterogeneous. We focused on sFlt1 and PlGF, but there are multiple other factors that may contribute to risk for preeclampsia, including soluble endoglin, microparticles, and agonistic antiangiotensin receptor antibodies (29, 49, 50). Our small pilot study could not address more analytes. In addition, it is possible that concentrations of cytokines and angiogenic factors at the maternal fetal interface or within glomeruli, which may not be reflected in the circulation, alter trophoblast function and trigger vasculopathic effects. Despite these limitations, our study provides a new model to explain the increased vulnerability of some SLE patients to antiangiogenic factors.
Our findings extend the role of type I IFNs to risk for maternal vascular disease characteristic of preeclampsia. They support the concept that in the presence of elevated IFN-α, endothelial cell-trophoblast interactions are impaired, a potential basis for inadequate spiral artery remodeling, and the resultant placental dysfunction and angiogenic dysregulation, even if modest, may lead to maternal endothelial responses characteristic of preeclampsia. In addition, they raise the possibility that elevated IFN-α may be used to risk stratify SLE pregnancies.
ACKNOWLEDGMENTS
We are grateful to the PROMISSE investigators for recruiting and collecting data and samples on these informative patients and to Marta Guerra for help preparing the manuscript.
SOURCES OF FUNDING Supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), (grants R01-AR-49772 and R01-AR-49772-07S2) (JES, MK, DA, PBR), the National Council of Technological and Scientific Development of Brazil (CNPq) (grant 200591/2008 to DA) and the March of Dimes (to VMA). AMA is a fellow of Colciencias and was supported by Colciencias Grant #1115-49326157. LB and MJK were supported by the Intramural Research Program, NIAMS. SAK is an investigator of the Howard Hughes Medical Institute.
Footnotes
CONFLICT(S) OF INTEREST/DISCLOSURE(S) SAK is a co-inventor on patents related to angiogenic biomarkers for use in preeclampsia. SAK is a consultant to Siemens Diagnostics and has financial interest in Aggamin Therapeutics that is developing therapies for vascular disorders.
REFERENCES
- 1.Petri M, Allbritton J. Fetal outcome of lupus pregnancy: a retrospective case-control study of the Hopkins Lupus Cohort. The Journal of rheumatology. 1993;20(4):650–6. [PubMed] [Google Scholar]
- 2.Georgiou PE, Politi EN, Katsimbri P, Sakka V, Drosos AA. Outcome of lupus pregnancy: a controlled study. Rheumatology (Oxford) 2000;39(9):1014–9. doi: 10.1093/rheumatology/39.9.1014. [DOI] [PubMed] [Google Scholar]
- 3.Clowse ME, Jamison M, Myers E, James AH. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol. 2008;199(2):127, e1–6. doi: 10.1016/j.ajog.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46(5):1077–85. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 6.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27(9-10):939–58. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–92. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 9.McMahon K, Karumanchi SA, Stillman IE, Cummings P, Patton D, Easterling T. Does soluble fms-like tyrosine kinase-1 regulate placental invasion? Insight from the invasive placenta. Am J Obstet Gynecol. 2014;210(1):68, e1–4. doi: 10.1016/j.ajog.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99(9):2152–64. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160(4):1405–23. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 13.Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, Graham CH. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med. 2014;211(1):165–79. doi: 10.1084/jem.20130295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crow MK. Interferon-alpha: a therapeutic target in systemic lupus erythematosus. Rheum Dis Clin North Am. 2010;36(1):173–86. doi: 10.1016/j.rdc.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 16.Denny MF, Thacker S, Mehta H, Somers EC, Dodick T, Barrat FJ, et al. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007;110(8):2907–15. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thacker SG, Berthier CC, Mattinzoli D, Rastaldi MP, Kretzler M, Kaplan MJ. The detrimental effects of IFN-alpha on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. J Immunol. 2010;185(7):4457–69. doi: 10.4049/jimmunol.1001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee PY, Li Y, Richards HB, Chan FS, Zhuang H, Narain S, et al. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2007;56(11):3759–69. doi: 10.1002/art.23035. [DOI] [PubMed] [Google Scholar]
- 19.Gerber SA, Pober JS. IFN-alpha induces transcription of hypoxia-inducible factor-1alpha to inhibit proliferation of human endothelial cells. J Immunol. 2008;181(2):1052–62. doi: 10.4049/jimmunol.181.2.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albini A, Marchisone C, Del Grosso F, Benelli R, Masiello L, Tacchetti C, et al. Inhibition of angiogenesis and vascular tumor growth by interferon-producing cells: A gene therapy approach. Am J Pathol. 2000;156(4):1381–93. doi: 10.1016/S0002-9440(10)65007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358(11):1129–36. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 23.Buyon JP, Kalunian KC, Ramsey-Goldman R, Petri MA, Lockshin MD, Ruiz-Irastorza G, et al. Assessing disease activity in SLE patients during pregnancy. Lupus. 1999;8(8):677–84. doi: 10.1191/096120399680411272. [DOI] [PubMed] [Google Scholar]
- 24.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 25.ACOG practice bulletin Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002 Jan;99(1):159–67. doi: 10.1016/s0029-7844(01)01747-1. Number 33. 2002. [DOI] [PubMed] [Google Scholar]
- 26.American College of, Obstetricians and Gynecologists ACOG Practice bulletin no. 134: fetal growth restriction. Obstet Gynecol. 2013;121(5):1122–33. doi: 10.1097/01.AOG.0000429658.85846.f9. [DOI] [PubMed] [Google Scholar]
- 27.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 28.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54(6):1906–16. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 29.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 30.Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184(6):3284–97. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krikun G, Mor G, Huang J, Schatz F, Lockwood CJ. Metalloproteinase expression by control and telomerase immortalized human endometrial endothelial cells. Histology and histopathology. 2005;20(3):719–24. doi: 10.14670/HH-20.719. [DOI] [PubMed] [Google Scholar]
- 32.Krikun G, Potter JA, Abrahams VM. Human endometrial endothelial cells generate distinct inflammatory and antiviral responses to the TLR3 agonist, Poly(I:C) and the TLR8 agonist, viral ssRNA. American journal of reproductive immunology. 2013;70(3):190–8. doi: 10.1111/aji.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung JJ, Tiwari A, Inamdar SM, Thomas CP, Goel A, Choudhury A. Secretion of soluble vascular endothelial growth factor receptor 1 (sVEGFR1/sFlt1) requires Arf1, Arf6, and Rab11 GTPases. PLoS One. 2012;7(9):e44572. doi: 10.1371/journal.pone.0044572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myoung H, Hong SD, Kim YY, Hong SP, Kim MJ. Evaluation of the anti-tumor and anti-angiogenic effect of paclitaxel and thalidomide on the xenotransplanted oral squamous cell carcinoma. Cancer Lett. 2001;163(2):191–200. doi: 10.1016/s0304-3835(00)00701-1. [DOI] [PubMed] [Google Scholar]
- 35.Aldo PB, Krikun G, Visintin I, Lockwood C, Romero R, Mor G. A novel three-dimensional in vitro system to study trophoblast-endothelium cell interactions. American journal of reproductive immunology. 2007;58(2):98–110. doi: 10.1111/j.1600-0897.2007.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Experimental cell research. 1993;206(2):204–11. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 37.Ashton SV, Whitley GS, Dash PR, Wareing M, Crocker IP, Baker PN, et al. Uterine spiral artery remodeling involves endothelial apoptosis induced by extravillous trophoblasts through Fas/FasL interactions. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(1):102–8. doi: 10.1161/01.ATV.0000148547.70187.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyall F, Bulmer JN, Duffie E, Cousins F, Theriault A, Robson SC. Human trophoblast invasion and spiral artery transformation: the role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol. 2001;158(5):1713–021. doi: 10.1016/S0002-9440(10)64127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341(8858):1447–51. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 40.Kalkunte S, Lai Z, Tewari N, Chichester C, Romero R, Padbury J, et al. In vitro and in vivo evidence for lack of endovascular remodeling by third trimester trophoblasts. Placenta. 2008;29(10):871–8. doi: 10.1016/j.placenta.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakravarty EF, Nelson L, Krishnan E. Obstetric hospitalizations in the United States for women with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54(3):899–907. doi: 10.1002/art.21663. [DOI] [PubMed] [Google Scholar]
- 42.Ma B, Dela Cruz CS, Hartl D, Kang MJ, Takyar S, Homer RJ, et al. RIG-like helicase innate immunity inhibits vascular endothelial growth factor tissue responses via a type I IFN-dependent mechanism. Am J Respir Crit Care Med. 2011;183(10):1322–35. doi: 10.1164/rccm.201008-1276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng H, Qian J, Carbone CJ, Leu NA, Baker DP, Fuchs SY. Vascular endothelial growth factor-induced elimination of the type 1 interferon receptor is required for efficient angiogenesis. Blood. 2011;118(14):4003–6. doi: 10.1182/blood-2011-06-359745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cindrova-Davies T, Sanders DA, Burton GJ, Charnock-Jones DS. Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovasc Res. 2011;89(3):671–9. doi: 10.1093/cvr/cvq346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lockshin MD, Qamar T, Druzin ML. Hazards of lupus pregnancy. J Rheumatol Suppl. 1987;14(Suppl 13):214–7. [PubMed] [Google Scholar]
- 46.Nicklin JL. Systemic lupus erythematosus and pregnancy at the Royal Women’s Hospital, Brisbane 1979-1989. Aust N Z J Obstet Gynaecol. 1991;31(2):128–33. doi: 10.1111/j.1479-828x.1991.tb01800.x. [DOI] [PubMed] [Google Scholar]
- 47.Julkunen H, Jouhikainen T, Kaaja R, Leirisalo-Repo M, Stephansson E, Palosuo T, et al. Fetal outcome in lupus pregnancy: a retrospective case-control study of 242 pregnancies in 112 patients. Lupus. 1993;2(2):125–31. doi: 10.1177/096120339300200211. [DOI] [PubMed] [Google Scholar]
- 48.Kleinman D, Katz VL, Kuller JA. Perinatal outcomes in women with systemic lupus erythematosus. J Perinatol. 1998;18(3):178–82. [PubMed] [Google Scholar]
- 49.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 50.Xia Y, Zhou CC, Ramin SM, Kellems RE. Angiotensin receptors, autoimmunity, and preeclampsia. J Immunol. 2007;179(6):3391–5. doi: 10.4049/jimmunol.179.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]