Abstract
Purpose
To compare the in-vitro fertilization (IVF) outcomes of cancer patients who underwent oocyte retrieval and embryo/oocyte cryopreservation prior to gonadotoxic therapy to those of age and time-matched controls with tubal factor infertility.
Methods
All cancer patients who underwent embryo/oocyte cryopreservation at our institution from 1997 to 2014 were reviewed. Primary outcomes were total dose of gonadotropins used, number of oocytes retrieved, and number of 2pn embryos obtained. Outcomes were compared to age-matched controls with tubal-factor infertility who underwent a fresh embryo transfer within the same relative time period as the IVF cycle of the cancer patient.
Results
Sixty-three cancer patients underwent 65 IVF cycles, and 21 returned for frozen embryo transfer. One hundred twenty-two age-matched controls underwent IVF cycles with fresh transfer, and 23 returned for frozen embryo transfer. No difference was seen between cancer patients and controls with respect to total ampules of gonadotropin used (38.0 vs. 35.6 respectively; p = 0.28), number of oocytes retrieved (12.4 vs. 10.9 respectively; p = 0.36) and number of 2pn embryos obtained (6.6 vs. 7.1 respectively; p = 0.11). Cumulative pregnancy rate per transfer for cancer patients compared to controls was 37 vs. 43 % respectively (p = 0.49) and cumulative live birth rate per transfer was 30 vs. 32 % respectively (p = 0.85). Cancer patients had a higher likelihood of live birth resulting in twins (44 vs. 14 %; p = 0.035).
Conclusions
Most IVF outcomes appear comparable for cancer patients and age-matched controls. Higher twin pregnancy rates in cancer patients may reflect lack of underlying infertility or need for cancer-specific transfer guidelines.
Keywords: Cancer, In-vitro fertilization, Fertility preservation
Introduction
The Centers for Disease Control and Prevention estimate that there are over 275,000 women of reproductive age in the US who are cancer survivors [1]. Approximately 125,000 women age less than 50 are diagnosed with cancer each year in the United States, according to the 2011 Surveillance, Epidemiology and End Results (SEER) statistics [2]. Cancer treatments continue to improve but are also often gonadotoxic, resulting in an ever-increasing number of young cancer survivors seeking individualized fertility preservation strategies [3, 4]. Loss of reproductive ability negatively impacts quality of life (QOL) for young cancer survivors [5, 6], and women diagnosed with cancer indicate that the ability to have a biological child in the future is of high importance [7]. In fact, in young women diagnosed with cancer, the potential loss of the ability to have a child can sometimes be more stressful than the cancer diagnosis itself [8]. The American Society of Clinical Oncology recommends that as part of education and informed consent before cancer therapy, health care providers address the risk of infertility with patients treated during their reproductive years and be prepared to discuss fertility preservation options and/or refer all patients to reproductive specialists [9]. These referrals are essential, as studies indicate that receiving pre-cancer treatment counseling regarding fertility preservation significantly increases QOL scores after cancer treatment in reproductive-age women [10]. Furthermore, receiving this counseling from a fertility specialist and subsequently attempting fertility preservation are both associated with increased QOL as compared to women who have been counseled solely by an oncologist [10].
It is well established that cryopreservation of embryos and, more recently oocytes [11], are feasible options for young women facing potentially gonadotoxic cancer treatment who wish to have genetically related children in the future, but a paucity of data exists to facilitate patient counseling in terms of outcomes [3, 4, 9, 12–14]. The aim of this study is to compare the ovarian stimulation and IVF outcomes, most notably total dose of gonadotropins used, number of oocytes retrieved, and number of 2pn embryos obtained, for cancer patients who underwent embryo cryopreservation prior to gonadotoxic treatment to those of an age-matched comparison group with tubal infertility who underwent a fresh IVF cycle within the same three-year period as the cancer patient.
Methods
This study was approved by the Partners Healthcare Human Research Committee.
Patient selection
All patients with a cancer diagnosis seeking fertility preservation prior to chemotherapy/radiation therapy from January 1, 1997 to March 31, 2014 at the Massachusetts General Hospital Fertility Center were reviewed (“cancer group”). All patients with a cancer diagnosis who had undergone potentially gonadotoxic treatment prior to oocyte retrieval were excluded. These subjects were retrospectively compared to an age-matched cohort with tubal factor infertility from 2002 to 2014 (“comparison group”). The comparison group was unable to be queried prior to 2002 due to inability to access the appropriate paper charts. Each cancer patient was matched to controls of the same age (cancer patient date of birth was within 1 year of control patient’s date of birth) who underwent an initial fresh IVF cycle within 3 years of the cancer patient IVF cycle. Those cancer patients who cycled prior to 2002 were matched with a control from 2002. Only the first fresh IVF cycle of the comparison group was considered for analysis, however for both the cancer and comparison groups, all cryothaw cycles resulting from the embryos preserved from the initial stimulation were included in the analysis. Variables observed were age, cancer diagnosis, gravidity, parity, body mass index (BMI), prior infertility, evidence of subfertility, stimulation protocol used, insemination method (conventional in vitro or intracytoplasmic sperm injection (ICSI)), day three estradiol (E2) level, day three follicle stimulating hormone (FSH) level, peak E2 level, day of human chorionic gonadotropin (HCG) injection, ampules of gonadotropin medication used, number of oocytes retrieved, fertilization rate, number of pronuclear (2pn) embryos, use of a gestational carrier, day of embryo transfer, presence of a yolk sac on ultrasound indicating a pregnancy, live births, number of twin deliveries, number of embryos remaining in storage, number of embryos discarded, and patient deaths.
Stimulation, cryopreservation, thaw and embryo transfer protocols
Three controlled ovarian hyperstimulation protocols were used as previously described by Styer et al. [15]. Briefly, the luteal phase GnRh agonist protocol (“Low Dose Luteal Lupron” or LDLL), used for anticipated normal responders, consists of pituitary desensitization and down-regulation with the gonadotropin releasing hormone agonist (GnRH-a) Lupron (leuprolide acetate, 0.5 mg/day SC; TAP Pharmaceuticals Inc., Lake Forest, IL) overlapping with the final 5 days of oral contraceptive pill (OCP) treatment and continuing for a minimum of 10 days, followed by reduction of the leuprolide acetate dosage on day three after menses. Gonadotropin stimulation is initiated on cycle day three once suppression has been documented. The follicular phase GnRH agonist (Flare) protocol, used for anticipated low responders, utilizes OCP pre-treatment, then Lupron 1 mg is started 5 days after stopping OCPs. Gonadotropins are initiated on cycle day three, and on cycle day five Lupron dosage is decreased to 0.25 mg. The antagonist protocol, used at our institution for both anticipated normal and low responders, consists of OCP pre-treatment, then gonadotropins are started 5 days after stopping OCPs. When the lead follicle is 14 mm or greater, or estradiol level reaches 1000, the antagonist is initiated. Antagonists used include ganirelix acetate or cetrorelix acetate, 250 μg SC per d (Organon Inc., West Orange, NJ; Merck, Inc, Whitehouse Station, NJ). Both the GnRH-a and GnRH antagonist are continued through the day of trigger with human chorionic gonadotropin (hCG) (Ferring pharmaceuticals, Parsippany, NJ). Gonadotropins included Gonal-F (follitropin-alpha, Serono, Rockland, MA), Follistim (follitropin-beta; Organon Inc.), Menopur and Repronex (hMG, Ferring Pharmaceuticals, Tarrytown, NY). Per institutional protocol, hCG trigger was initiated with three or more follicles reached a size of 16 mm or greater and serum estradiol reached at least 600 pg/mL.
Embryo cryopreservation for cancer patients is performed at the two pronuclear (2pn) stage for all embryos. Up until November 2004, 2pn embryos were cryopreserved using a traditional controlled-rate slow-freeze protocol as described by Testart et al. [16] using a three step loading of the cryoprotectants propanediol and sucrose, and thawed using a three step cryoprotectant removal. Since November 2004, 2pn embryos are cryopreserved using a controlled-rate slow freeze protocol utilizing the Quinn’s Advantage® Embryo Freeze Kit (Cooper Surgical Inc, Trumbull, CT). For the comparison group, women undergoing IVF for tubal factor infertility, supernumerary embryos not transferred during a fresh cycle are observed in culture until the blastocyst stage. Embryos that reach an appropriate stage of blastocyst development by day 5 or 6 and are of good quality are cryopreserved using a slow-freeze cryopreservation technique described by Berin et al. [17].
Patients returning for transfer of cryopreserved embryos undergo daily Lupron for pituitary down-regulation starting the last 5 days of OCP use. Hormone replacement with transdermal estradiol (Vivelle-Dot; Novartis Pharmaceuticals) and Aspirin 81 mg [18–20] are initiated on day one of cycle. On day eighteen of the cycle, Lupron is stopped and the following day progesterone is initiated. Day five blastocyst transfer occurs on day 24 of the recipient cryothaw preparation cycle. Luteal support is maintained with intramuscular (50 mg daily) or vaginal (100 mg twice daily) micronized progesterone in addition to transdermal estradiol (0.2 mg every other day; Vivelle-Dot; Novartis Pharmaceuticals) until 13 weeks gestational age [21].
At the time of this study, no IRB approved protocol for ovarian tissue cryopreservation, an experimental technique for fertility preservation [22], was available at our institution. Patients were counseled that this option was available elsewhere, however to our knowledge none chose to pursue it.
Statistical analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at Massachusetts General Hospital. Normally distributed data is represented as a mean ± standard deviation. Nonparametric data is represented as median/range. Pearson chi-square test was used for dichotomous variables, and Mann–Whitney nonparametric tests were used for continuous variables. A p-value of < 0.05 was used to determine significance. Statistical analysis was carried out using Stata Software Version 10.
Results
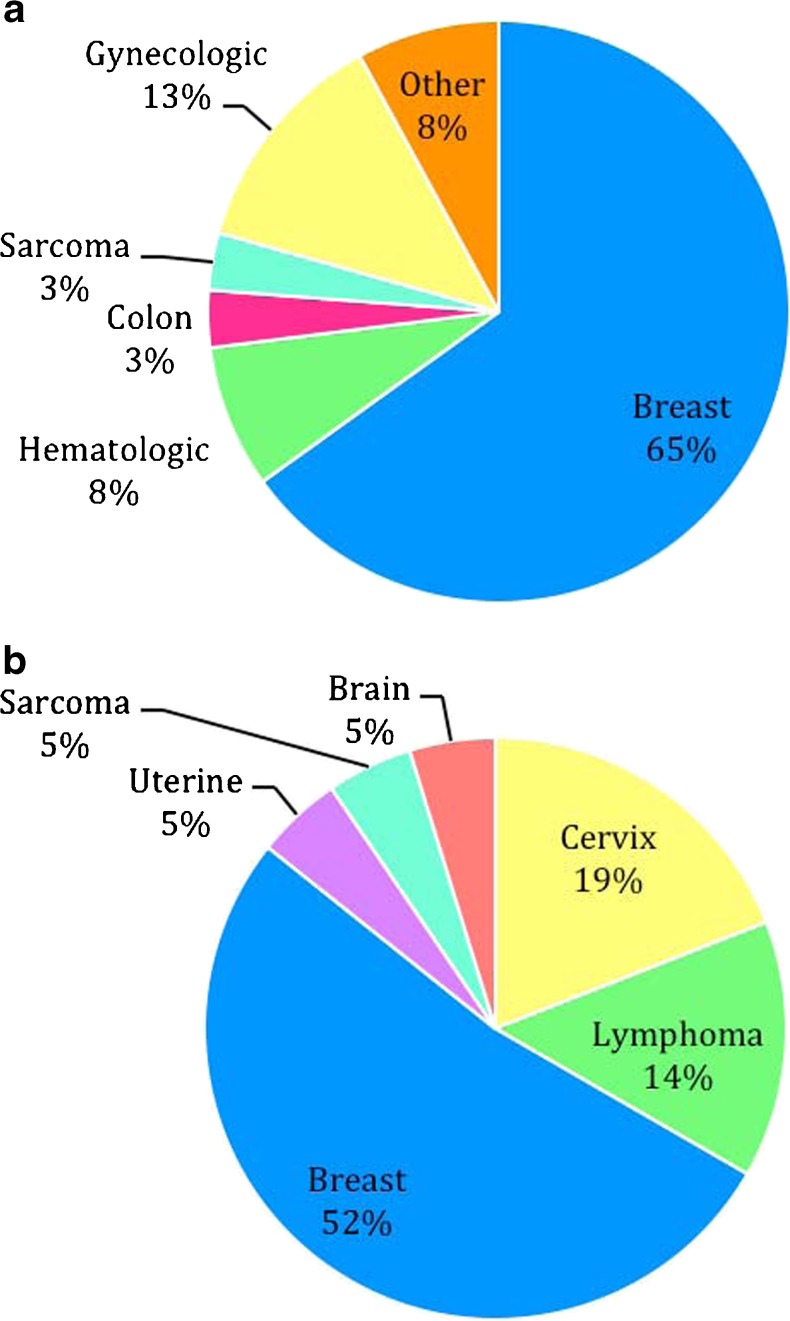
Sixty-three cancer patients who underwent 65 IVF cycles with oocyte retrieval and cryopreservation of either embryos or oocytes were identified and compared to 122 age-matched controls who underwent 122 IVF cycles within the same 3-year time period as the cancer patient’s IVF cycle. Additionally, the control patients underwent 23 subsequent cryothaw cycles resulting from their original IVF cycle. Cancer diagnoses of the 63 patients who presented for fertility preservation are detailed in Fig. 1a, with breast cancer representing the majority. Baseline data for cancer and control patients is shown in Table 1. There was no difference seen between age, BMI and day 3 E2 levels. A significantly lower day 3 FSH level was seen in the cancer group vs. comparison group (6.4 vs 7.3, p = .01).
Fig. 1.
Distribution of Cancer Diagnoses. a: Distribution of cancer diagnoses among all cancer patients who presented for fertility preservation (n = 63): Breast (41), Hematologic (5), Gynecologic (8: Cervix [4], Uterine [2], ovarian [1], fallopian tube [1]), Colon (2), Sarcoma (2), Other (5: Lung [1], Brain [1], Adrenal [1], Esthesioneuroblastoma [1], Thyroid [1]). b: Distribution of cancer diagnoses among cancer patients who returned for thawed embryo transfer (n = 21): Breast (11), Gynecologic (5: Cervix [4], Uterine [1]), Hematologic (3: Lymphoma [3]), Other (2: Sarcoma [1], Brain [1])
Table 1.
Demographics
| Baseline characteristics | Cancer patients (63) | Controls (122) | p-value |
|---|---|---|---|
| Age | 33.7 ± 4.1 | 34.5 ± 3.5 | 0.08 |
| BMI | 24.0 ± 4.8 | 24.8 ± 4.4 | 0.12 |
| Day 3 FSH | 6.4 ± 2.2 | 7.3 ± 2.2 | 0.01 |
| Day 3 Estradiol | 49.8 ± 25.7 | 45.1 ± 16.3 | 0.91 |
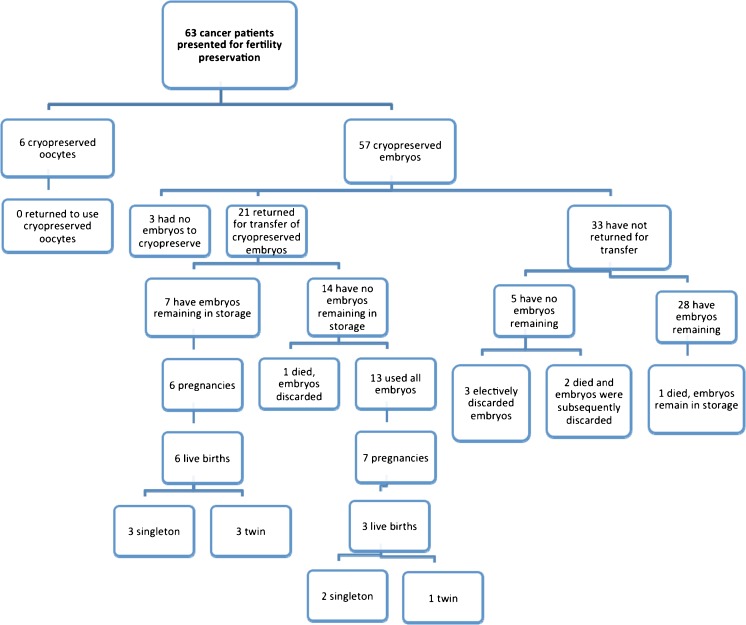
The majority of cancer patients chose to freeze embryos vs. oocytes (90.5 vs. 9.5 %, Table 2). Six out of 63 cancer patients (9.5 %) carried a prior infertility diagnosis, while 11 (17.5 %) had evidence of subfertility during the IVF process (Table 2). None of the cancer patients had undergone gonadotoxic treatment prior to their stimulation. Of these cancer patients, three (4.8 %) had cycles that were either cancelled or had no embryos obtained. No cases of moderate to severe ovarian hyperstimulation syndrome (OHSS) that resulted in hospitalization or delay of chemotherapy occurred in either group. Outcomes of the 63 cancer patients who cryopreserved oocytes and embryos are described in Fig. 2. None of the patients who cryopreserved oocytes have returned for transfer. Twenty-one (36.8 %) of the 57 cancer patients who cryopreserved embryos returned for transfer of the embryos. Cancer diagnoses of the 21 cancer patients who returned for transfer of their cryopreserved embryos are detailed in Fig. 1b. Of the 21 cancer patients who returned for transfer, thirteen (61.9 %) had one transfer, eight (38.1 %) had two or more transfers. Thirty-three of the cancer patients who cryopreserved embryos (57.9 %) have not yet returned for transfer. Of the 21 cancer patients who underwent transfers, seven (33.3 %) currently have embryos remaining in storage. Of the 33 patients who did not return for transfer, 28 (84.8 %) have embryos remaining. Ten cancer patients (three with cervical cancer, six with breast cancer and one with uterine cancer) who returned for transfer used gestational carriers. Four deaths occurred, three prior to transfer and one subsequent to transfer. Of the patients who did not survive to undergo embryo transfer, one died of lung cancer 5 years after her IVF cycle, one died of invasive breast cancer 1 year after her IVF cycle and one died of metastatic breast cancer 3 years after her IVF cycle. The single death that occurred after transfer was due to recurrent AML 3 years after transfer.
Table 2.
Characteristics of cancer patients (n = 63)
| Cancer patient IVF data | n (%) |
|---|---|
| Number of IVF cycles | 65 (N/A) |
| Evidence of subfertility | 11 (17.5) |
| Prior infertility diagnosis | 6 (9.5) |
| Number electing to freeze embryos with partner | 56 (89.9) |
| Number electing to freeze embryos with sperm donor | 1 (1.5) |
| Number electing to freeze oocytes | 6 (9.5) |
Fig. 2.
Flow chart of current outcomes for all cancer patients who underwent IVF
IVF stimulation and outcomes are outlined in Table 3. A significantly smaller proportion of cancer patients were placed on the Low Dose Luteal Lupron (LDLL) protocol vs. comparison group (60.0 vs. 83.6 %, p = 0.0005). Conversely, significantly more cancer patients were placed on the antagonist protocol (27.7 vs. 7.4 %, p = 0.0004). A random-start protocol [23, 24] was not used for any of the cases reported in this study.
Table 3.
IVF stimulation cycle outcomes in cancer vs. controls
| IVF parameters | Cancer n (%) ± sd 65 cycles | Control n (%) ± sd 122 cycles | p-value | |
|---|---|---|---|---|
| Protocol type | LDL | 39 (60.0) | 102 (83.6) | 0.0005 |
| Flare | 8 (12.3) | 11 (9.0) | 0.48 | |
| Antagonist | 18 (27.7) | 9 (7.4) | 0.0004 | |
| Ampules of gonadotropin | 38.0 ± 2.0 | 35.6 ± 1.5 | 0.28 | |
| Day of hCG administration | 11.7 ± 0.2 | 11.9 ± 0.1 | 0.15 | |
| Peak E2 | 2049.9 ± 133.5 | 2162.2 ± 71.7 | 0.21 | |
| Number of oocytes | 12.4 ± 7.8 | 10.9 ± 5.2 | 0.36 | |
| Insemination typea | Regular | 34 (57.6) | 111 (91.0) | <0.0001 |
| ICSI | 25 (42.4) | 11 (9.0) | <0.0001 | |
| Fertilization rate | Overall | 0.68 ± 0.3 | 0.74 ± 0.2 | 0.34 |
| ICSI | 0.77 ± 0.18 | 0.87 ± 0.18 | 0.052 | |
| Conventional | 0.71 ± 0.25 | 0.73 ± 0.18 | 0.33 | |
| Number of 2pn embryos | 6.6 ± 0.7 | 7.1 ± 0.4 | 0.11 | |
| Day of transferǂ | Cancer (n) | Control (fresh) (n) | 0.013 | |
| 2 | 2 | 8 | ||
| 3 | 15 | 72 | ||
| 5 | 0 | 42 | ||
aNot applicable for six patients who underwent oocyte cryopreservation
ǂIncomplete data for cancer patients
Primary study outcomes, including total dose of gonadotropins received, number of oocytes retrieved and number of 2pn embryos obtained, are detailed in Table 3. No difference was seen between cancer patients and controls with respect to total ampules of gonadotropin used (38.0 vs. 35.6 respectively; p = 0.28), number of oocytes retrieved (12.4 vs. 10.9 respectively; p = 0.36) and number of 2pn embryos obtained (6.6 vs. 7.1 respectively; p = 0.11). In addition, no significant differences were seen between cancer patients and controls with respect to day of hCG administration (11.7 vs. 11.9 respectively; p = 0.15), peak E2 level (2049.9 vs. 2162.2 respectively; p = 0.21), and fertilization rate (0.68 vs. 0.74 respectively; p = 0.34). A greater proportion of cancer patients than controls used ICSI for insemination (42.4 vs. 9.0 %, p < 0.0001). No statistically significant difference in fertilization rate was seen between groups overall, or when stratified by insemination type (ICSI vs. conventional insemination). A difference was noted when comparing day of transfer for cancer patient cryothaw cycles versus control patient fresh stimulation cycles (p = 0.013).
Primary outcomes were then stratified by protocol type (Table 4). A statistically significant difference was noted between cancer and control patients who underwent a Flare protocol with respect to peak estradiol level (1091.8 vs. 1731.4 respectively, p = 0.015), total number of oocytes retrieved (3.3 vs. 7.2 respectively, p = 0.006), and number of 2pn embryos obtained (1.9 vs. 4.7 respectively p = 0.001), as well as between cancer and control patients who underwent an Antagonist protocol with respect to total dose of gonadotropins received (38.8 vs. 48.8 respectively, p = 0.022). Among cancer and control patients on an Antagonist protocol, a difference in peak estradiol level and total number of 2pn embryos was observed but did not reach significance.
Table 4.
Primary outcomes stratified by protocol type
| Variable | Protocol | Cancer ± sd | Control ± sd | p-value |
|---|---|---|---|---|
| Total ampules of gonadotropin | LDLL | 33.2 ± 15.1 | 33.2 ± 16.5 | 0.511 |
| Flare | 56.3 ± 16.3 | 45.6 ± 8.3 | 0.960 | |
| Antagonist | 38.8 ± 12.3 | 48.8 ± 9.8 | 0.022 | |
| Peak estradiol level | LDLL | 2411.2 ± 175.1 | 2207.1 ± 79.5 | 0.886 |
| Flare | 1091.8 ± 160.1 | 1731.4 ± 196.6 | 0.015 | |
| Antagonist | 1635.3 ± 218.8 | 2180.4 ± 244.7 | 0.069 | |
| Total oocytes retrieved | LDLL | 14.8 ± 1.2 | 11.2 ± 0.5 | 0.999 |
| Flare | 3.3 ± 0.8 | 7.2 ± 1.0 | 0.006 | |
| Antagonist | 10.7 ± 1.9 | 11.9 ± 2.1 | 0.353 | |
| Number of 2pn embryos | LDLL | 8.0 ± 0.9 | 7.2 ± 0.4 | 0.820 |
| Flare | 1.9 ± 0.4 | 4.7 ± 0.6 | 0.001 | |
| Antagonist | 4.8 ± 1.4 | 8.6 ± 1.6 | 0.054 |
Secondary study outcomes, cumulative pregnancy and live birth rates, are detailed in Table 5. Table 5 reflects the outcomes of cancer patients’ cumulative IVF cycle (includes cryothaw only) compared to the control patients’ cumulative cycle (includes fresh plus cryothaw). The two groups had a comparable average number of embryos transferred with similar implantation rates observed. Pregnancy rates per transfer (36.7 vs. 43.4 %, p = 0.49) and cumulative pregnancy rate for IVF cycle (52.4 vs. 51.6 %, p = 0.95) were no different between cancer and comparison groups, respectively. Similarly, live birth rate per transfer (30.0 vs. 31.7 %, p = 0.85) and per IVF cycle (46.9 vs. 37.7 %, p = 0.65) were not significantly different between cancer and comparison groups. A significant increase in the percentage of live births resulting in twins was seen in the cancer group (44.0 vs. 14.0 %, p = 0.035). No higher order multiples were seen in either group.
Table 5.
Cumulative pregnancy and live birth outcomes (175 total transfers)
| IVF outcomes | Cancer | Controls | p-value | OR |
|---|---|---|---|---|
| No. of embryos transferred | 1.97 | 1.97 | 0.79 | – |
| Implantation rate | 27.8 % | 29.9 % | 0.79 | – |
| Pregnancy rate per transfer | 36.7 % (11/30) | 43.4 % (63/145) | 0.49 | 0.75 |
| Pregnancy rate per IVF cycle | 52.4 % (11/21) | 51.6 % (63/122) | 0.95 | 1.03 |
| Live birth rate per transfer | 30 % (9/30) | 31.7 % (46/145) | 0.85 | 0.92 |
| Live birth rate per IVF cycle | 46.9 % (9/21) | 37.7 % (46/122) | 0.65 | 1.24 |
| Live births with twins | 44 % (4/9) | 14.0 % (6/46) | 0.035 | 4.93 |
OR odds ratio
LBR and PR per transfer resulting exclusively from cryothaw cycles were compared between cancer patients and controls (Table 6), as no cancer patients underwent fresh transfer. Again, no differences were observed (LBR 30.0 vs. 34.8 % respectively, p = 0.71; PR 36.7 vs. 56.5 %, respectively, p = 0.15).
Table 6.
Cryothaw cycle specific IVF outcomes
| Outcome | Cancer | Controls | p-value | Odds ratio |
|---|---|---|---|---|
| Live births per transfer | 30 % (9/30) | 34.8 % (8/23) | 0.71 | 0.80 |
| Pregnancy rate per transfer | 36.7 % (11/30) | 56.5 % (13/23) | 0.15 | 0.45 |
Discussion
The goal of this study was to evaluate outcomes for cancer patients who cryopreserved oocytes or embryos for fertility preservation in anticipation of gametotoxic treatments including chemotherapy and/or radiotherapy. We compared stimulation parameters and cycle outcomes including total dose of gonadotropins administered, number of oocytes retrieved and number of 2pn embryos obtained, in addition to pregnancy and live birth rates, with the goal of providing cancer patients with better information about IVF outcomes relative to a similarly aged population undergoing IVF. Currently, limited long-term outcome data is available in the cancer population who underwent embryo cryopreservation prior to gonadotoxic therapy, making it difficult to counsel patients with respect to overall probability of success. The present study offers one of the largest cohorts of cancer patients (63) and the longest follow-up period (17 years) of currently available publications.
We selected age-matched controls with tubal factor infertility undergoing their first IVF cycle, consistent with previously published literature [25–27], since in this circumstance, infertility is thought to be primarily mechanical and presumably not due to ovarian dysfunction or other factors that may confound results. Controls were age matched to within 1 year of the cancer patient, as female age is the greatest prognostic indicator of IVF success [28]. Patients were included over 17 years, during which time technology changes and protocols evolve. For this reason, controls were chosen who had cycled within the same 3-year time period as the cancer patient, to minimize the likelihood that results are confounded by changes in laboratory protocol. In that context, it is also important to emphasize that the comparison group for this study is provided primarily to give the background for the center’s general practice and IVF outcomes.
This study found no significant differences between cancer patients and controls in terms of baseline characteristics (with the exception of day 3 FSH level). The lower day 3 FSH among cancer patients may reflect a lack of underlying infertility in this population. Among all patients, no differences were seen between cancer patients and controls with respect to stimulation parameters and IVF outcomes including total ampules of gonadotropins used, peak estradiol level, number of oocytes retrieved, fertilization rate, and number of 2pn embryos obtained. The similarity between cancer patients and controls with respect to mean number of oocytes retrieved [26, 29–33] as well as number of 2pn embryos obtained [26, 34] is consistent with the existing literature. However, contrary to our results, the literature to date suggests that cancer patients typically receive a lower mean-dose of gonadotropins [34, 35], achieve a lower peak estradiol level [27, 34–36], and according to some studies, have a lower mean number of oocytes retrieved [27, 34, 36] compared to controls. These differences could be accounted for by the fact that aromatase inhibitors [37] were not used as a part of the stimulation protocol in this study, though were commonly used in the stimulation protocol for cancer patients in comparison studies [29, 34, 35]. These differences also may be a reflection of the fact that in our study, cancer patients were more likely to undergo a Flare or Antagonist protocol compared to controls, which are typically reserved for limited responders, despite the mean day 3 FSH being lower in the cancer group than the control group. This discrepancy in protocol type may be a reflection of a desire to maximize the number of oocytes retrieved from a cancer patient prior to gonadotoxic treatment, as it likely represents her only opportunity for ovarian stimulation and fertility preservation. Alternatively, the Antagonist protocol has a shorter time to start of stimulation, and therefore may be chosen more frequently for cancer patients in order to minimize the time to initiation of cancer treatments. Finally, this discrepancy could be a reflection of the fact that the majority of the patients in this study had a diagnosis of breast cancer, and a very low percentage had a hematologic cancer, as evidence suggests that breast cancer patients respond more similarly to age matched controls than those with other cancer diagnoses such as hematologic [27].
When outcomes were stratified by protocol type, no differences were seen among normal responder cancer patients and normal responder controls (who underwent a LDLL protocol) with respect to primary outcomes of total dose of gonadotropin administered, number of oocytes retrieved, and number of 2pn embryos obtained, as well as peak estradiol level. However, a difference in primary outcomes was noted between cancer and control patients who were on an Antagonist or Flare protocol, typically reserved for poorer responders. One possible explanation for this is that our program may have a different threshold for providing treatment to a patient with limited time and options, such as cancer patients, as opposed to a control patient with similarly diminished ovarian reserve. It is also possible that the cancer patients requiring an Antagonist or Flare protocol have underlying physiology related to their cancer that makes them poorer responders. Some theories that support this include cancer patients being in a catabolic state with high stress hormone levels resulting in lower gonadotropin levels [38], as well as some evidence that women carrying the BRCA mutation may have decreased ovarian reserve and a poorer response to ovarian stimulation [39, 40]. It is of note, however, that only 8 cancer patients and 11 control patients underwent a Flare protocol and only 18 cancer patients and 9 control patients underwent an Antagonist protocol, therefore it is difficult to interpret these results. More research is needed with larger numbers to understand if IVF outcomes are different between cancer and control patients who are poor responders.
There was a significantly higher use of ICSI among the cancer population. Elective ICSI is often employed for cancer patients to avoid a situation of unexpected failure of fertilization. While the risk is low when semen parameters are normal, many cancer patients elect for ICSI given that there will only be one opportunity to obtain embryos. No difference was seen in fertilization rate between cancer patients and controls when stratified by insemination type, therefore downstream outcomes of this study such as number of 2pn embryos obtained were unlikely to be impacted by the difference in insemination types.
There were no cases of moderate to severe ovarian hyperstimulation syndrome (OHSS) that resulted in hospitalization or delay of chemotherapy that occurred in either the cancer or control groups of this study. Generally, our protocols as described above are very conservative so as to minimize risk of OHSS. This is relevant, as great caution must be taken to prevent OHSS in the cancer population as it could pose significant health risks and result in a delay in the start of chemotherapy.
Cancer patients were more likely to undergo a day 2 or day 3 embryo transfer compared to control patients who were more likely to undergo a day 5 blastocyst transfer. At our institution, contrary to a fresh IVF cycle for non-cancer patients where the entire cohort of embryos is often observed until the blastocyst stage (day 5) in order to select the “best” embryo, as few cancer patients’ frozen embryos as possible are thawed at a single time in the interest of preserving as many embryos as possible. Thus, for cancer patients returning for cryopreserved embryos, the “best” embryos are chosen from a smaller cohort. Once this can be achieved, there is rarely a benefit to extended culture for embryo selection, therefore in cancer patients embryos are frequently transferred at the cleavage stage (day 3) of development to avoid cohort dropout.
Our secondary outcomes, pregnancy rate and live birth rate, show no difference between cancer patients and controls. This is in contrast to prior studies, which have shown that live birth and pregnancy rates for cancer patients who undergo cryopreservation of embryos are lower than that of the general population seeking infertility treatment, however these studies are limited by their inclusion of only a small number of cancer patients [41, 42]. Michaan et al. reported similar clinical pregnancy rates between cancer patients and controls with tubal factor infertility, however their study is limited by small numbers (21 cancer patients) and by the fact that only 4 patients returned for transfer resulting two live births [26]. It should be noted, however, that in the comparison group only high quality blastocysts are cryopreserved, while in cancer patients all 2pn embryos are cryopreserved which may not all be of high quality. In control patients only the highest quality embryos are chosen for fresh transfer, while no cancer patients underwent a fresh transfer. In both cases, the best available embryos at the time are selected for frozen transfer. However, in the control patients, the best embryos were chosen for transfer at the time of fresh cycle, and the remaining high quality blastocysts are available for frozen embryo transfer. Alternatively, in the cancer patients, efforts are made to thaw a minimum number of 2pn embryos, though enough are thawed so that the cohort can be observed until the highest quality embryos can be chosen for transfer. This comparison between groups is therefore not identical and thus may represent a limitation of the study.
While pregnancy and live birth rates are similar between groups, it is interesting to note that there are a significantly higher number of twin births in the cancer group as compared to the tubal factor infertility group, despite no significant difference in the number of embryos transferred or implantation rate. This may be a reflection of cancer patients generally not having an underlying diagnosis of infertility and thus producing higher quality embryos, however the similar implantation rates we report between groups makes this explanation less likely. Given similar implantation rates but higher rates of twin gestations, it is possible that cancer patients are less likely to have a spontaneous (or elective) reduction to a singleton pregnancy, perhaps due to their lack of underlying infertility, or in the latter case, reluctance given inability to make more embryos in the future. However data was not available to explain this outcome or support this theory. It is important to note that while multiple gestations pose maternal and fetal risks to all patients, the associated morbidity may be even higher in cancer patients, who may have sequelae from their chemotherapy and/or radiation putting them at even higher risk for complications. Further research is needed understand the cause of this high twin rate, and efforts must be taken to avoid multifetal gestation in this population while maximizing singleton pregnancy rates. It is possible that cancer patients who underwent embryo cryopreservation prior to gonadotoxic treatment should not be subject to the same transfer guidelines as patients with a diagnosis of infertility, however more data is needed to make this determination.
While there was no difference in the number of 2pn embryos per cycle between cancer patients and controls, the mean number of embryos cryopreserved for cancer patients was 6.6 embryos. This by no means provides a guarantee of a live birth, as studies of donor oocyte cycles have indicated that 15 embryos available for transfer are needed to achieve a 92.4 % likelihood of achieving a live birth [43]. This is further highlighted by the fact that 14 of the 21 cancer patients who returned for transfer with the hope of a live birth have no embryos remaining, and only 3 of those 14 patients in fact had a live birth. Four patients have passed from their cancer after preserving embryos, one of which still has embryos remaining in storage. This is a somber reminder of the unique issues surrounding fertility preservation for a cancer diagnosis, including the need to appropriately counsel patients regarding prognosis and plans for disposition of unused embryos. Cancer patients undergoing fertility preservation are therefore counseled to the best of our ability regarding their likelihood of success in this process, risks, benefits and alternatives. Patients engage in discussion with both physicians and social workers regarding alternatives if cryopreserved oocytes or gametes do not result in a live birth, including assessment of ovarian reserve for natural conception or a potential fresh cycle, oocyte donation and adoption.
Limitations of this study include the small sample size of 63 cancer patients, and particularly the small sample size once results were stratified by stimulation protocol, although this still represents a relatively large cohort when compared to previously published literature [26, 29–33]. Additionally, although the number (21) of cancer patients who underwent transfer is one of the largest reported in the literature, the numbers are still quite small, making it difficult to generalize results obtained in this study to a broader population. No patients who banked oocytes have returned thus far, and as a result, this study is unable to comment on pregnancy and live birth rates for patients utilizing this method for fertility preservation. Furthermore, due to the limited representation of specific cancer diagnoses in our study, one must be cautious in overgeneralizing this study’s findings, as different types of cancers and different therapies may result in different long-term outcomes.
In summary, controlled ovarian hyperstimulation and pronuclear embryo freezing with delayed embryo transfer for normal responder cancer patients may lead to successful outcomes, similar to those of age matched controls with tubal factor infertility, including total dose of gonadotropins administered, number of oocytes retrieved, number of 2pn embryos obtained, as well as similar pregnancy and live birth rates. More research is needed to understand if and why limited responder patients with a diagnosis of cancer do not achieve similar outcomes to age matched controls. Patients should be carefully counseled to consider their reproductive goals, likelihood of success, and the potential effects of delaying cancer therapy.
Footnotes
Eden R. Cardozo and Alexcis P. Thomson contributed equally to this work.
Capsule We report outcomes of 63 women who underwent IVF for fertility preservation prior to chemotherapy. In general they can expect similar outcomes to age matched controls with tubal factor infertility in terms of IVF cycle parameters and outcomes.
References
- 1.Cancer Survivors — United States, 2007 [Internet]. [cited 2014 Jun 3]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6009a1.htm.
- 2.National Cancer Institute. Surveillance Epidemiology and End Results. Fast Stats. Statistics stratified by age. Available at: http://seer.cancer.gov/faststats/selections.php. Accessed on 12 Oct 2014.
- 3.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360(9):902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9(12):735–749. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 5.Loprinzi CL, Wolf SL, Barton DL, Laack NN. Symptom management in premenopausal patients with breast cancer. Lancet Oncol. 2008;9(10):993–1001. doi: 10.1016/S1470-2045(08)70256-0. [DOI] [PubMed] [Google Scholar]
- 6.Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Hum Reprod Update. 2009;15(5):587–597. doi: 10.1093/humupd/dmp015. [DOI] [PubMed] [Google Scholar]
- 7.Reh AE, Lu L, Weinerman R, Grifo J, Krey L, Noyes N. Treatment outcomes and quality-of-life assessment in a university-based fertility preservation program: results of a registry of female cancer patients at 2 years. J Assist Reprod Genet. 2011;28(7):635–641. doi: 10.1007/s10815-011-9559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schover LR. Patient attitudes toward fertility preservation. Pediatr Blood Cancer. 2009;53(2):281–284. doi: 10.1002/pbc.22001. [DOI] [PubMed] [Google Scholar]
- 9.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol: Off J Am Soc Clin Oncol. 2013;31(19):2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118(6):1710–1717. doi: 10.1002/cncr.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rienzi L, Cobo A, Paffoni A, Scarduelli C, Capalbo A, Vajta G, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27(6):1606–1612. doi: 10.1093/humrep/des088. [DOI] [PubMed] [Google Scholar]
- 12.Ethics Committee of American Society for Reproductive M Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2013;100(5):1224–1231. doi: 10.1016/j.fertnstert.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Deal AM, Balthazar U, Kondapalli LA, Gracia C, Mersereau JE. Fertility preservation consultation for women with cancer: are we helping patients make high-quality decisions? Reprod Biomed Online. 2013;27(1):96–103. doi: 10.1016/j.rbmo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Practice Committees of American Society for Reproductive M, Society for Assisted Reproductive T Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99(1):37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Styer AK, Wright DL, Wolkovich AM, Veiga C, Toth TL. Single-blastocyst transfer decreases twin gestation without affecting pregnancy outcome. Fertil Steril. 2008;89(6):1702–1708. doi: 10.1016/j.fertnstert.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 16.Testart J, Lassalle B, Belaisch-Allart J, Hazout A, Forman R, Rainhorn JD, et al. High pregnancy rate after early human embryo freezing. Fertil Steril. 1986;46(2):268–272. doi: 10.1016/s0015-0282(16)49524-5. [DOI] [PubMed] [Google Scholar]
- 17.Berin I, McLellan ST, Macklin EA, Toth TL, Wright DL. Frozen-thawed embryo transfer cycles: clinical outcomes of single and double blastocyst transfers. J Assist Reprod Genet. 2011;28(7):575–581. doi: 10.1007/s10815-011-9551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frattarelli JL, Miller BT, Scott RT., Jr Adjuvant therapy enhances endometrial receptivity in patients undergoing assisted reproduction. Reprod Biomed Online. 2006;12(6):722–729. doi: 10.1016/S1472-6483(10)61084-X. [DOI] [PubMed] [Google Scholar]
- 19.Waldenstrom U, Hellberg D, Nilsson S. Low-dose aspirin in a short regimen as standard treatment in in vitro fertilization: a randomized, prospective study. Fertil Steril. 2004;81(6):1560–1564. doi: 10.1016/j.fertnstert.2004.02.082. [DOI] [PubMed] [Google Scholar]
- 20.Rubinstein M, Marazzi A, Polak de Fried E. Low-dose aspirin treatment improves ovarian responsiveness, uterine and ovarian blood flow velocity, implantation, and pregnancy rates in patients undergoing in vitro fertilization: a prospective, randomized, double-blind placebo-controlled assay. Fertil Steril. 1999;71(5):825–829. doi: 10.1016/S0015-0282(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 21.Veeck LL, Bodine R, Clarke RN, Berrios R, Libraro J, Moschini RM, et al. High pregnancy rates can be achieved after freezing and thawing human blastocysts. Fertil Steril. 2004;82(5):1418–1427. doi: 10.1016/j.fertnstert.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 22.Practice Committee of American Society for Reproductive M Ovarian tissue cryopreservation: a committee opinion. Fertil Steril. 2014;101(5):1237–1243. doi: 10.1016/j.fertnstert.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 23.Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013;100(6):1673–1680. doi: 10.1016/j.fertnstert.2013.07.1992. [DOI] [PubMed] [Google Scholar]
- 24.Keskin U, Ercan CM, Yilmaz A, Babacan A, Korkmaz C, Duru NK, et al. Random-start controlled ovarian hyperstimulation with letrozole for fertility preservation in cancer patients: case series and review of literature. JPMA J Pak Med Assoc. 2014;64(7):830–832. [PubMed] [Google Scholar]
- 25.Klock SC, Zhang JX, Kazer RR. Fertility preservation for female cancer patients: early clinical experience. Fertil Steril. 2010;94(1):149–155. doi: 10.1016/j.fertnstert.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Michaan N, Ben-David G, Ben-Yosef D, Almog B, Many A, Pauzner D, et al. Ovarian stimulation and emergency in vitro fertilization for fertility preservation in cancer patients. Eur J Obstet Gynecol Reprod Biol. 2010;149(2):175–177. doi: 10.1016/j.ejogrb.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Pavone ME, Hirshfeld-Cytron J, Lawson AK, Smith K, Kazer R, Klock S. Fertility preservation outcomes may differ by cancer diagnosis. J Human Reprod Sci. 2014;7(2):111–118. doi: 10.4103/0974-1208.138869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Practice Committee of the American Society for Reproductive M Aging and infertility in women. Fertil Steril. 2006;86(5 Suppl 1):S248–S252. doi: 10.1016/j.fertnstert.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Devesa M, Martinez F, Coroleu B, Rodriguez I, Gonzalez C, Barri PN. Ovarian response to controlled ovarian hyperstimulation in women with cancer is as expected according to an age-specific nomogram. J Assist Reprod Genet. 2014;31(5):583–588. doi: 10.1007/s10815-014-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson LN, Dillon KE, Sammel MD, Efymow BL, Mainigi MA, Dokras A, et al. Response to ovarian stimulation in patients facing gonadotoxic therapy. Reprod Biomed Online. 2013;26(4):337–344. doi: 10.1016/j.rbmo.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nurudeen SK, Douglas NC, Mahany EL, Sauer MV, Choi JM. Fertility preservation decisions among newly diagnosed oncology patients: a single-center experience. Am J Clin Oncol. 2014 doi: 10.1097/COC.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 32.Knopman JM, Noyes N, Talebian S, Krey LC, Grifo JA, Licciardi F. Women with cancer undergoing ART for fertility preservation: a cohort study of their response to exogenous gonadotropins. Fertil Steril. 2009;91(4 Suppl):1476–1478. doi: 10.1016/j.fertnstert.2008.07.1727. [DOI] [PubMed] [Google Scholar]
- 33.Quintero RB, Helmer A, Huang JQ, Westphal LM. Ovarian stimulation for fertility preservation in patients with cancer. Fertil Steril. 2010;93(3):865–868. doi: 10.1016/j.fertnstert.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Friedler S, Koc O, Gidoni Y, Raziel A, Ron-El R. Ovarian response to stimulation for fertility preservation in women with malignant disease: a systematic review and meta-analysis. Fertil Steril. 2012;97(1):125–133. doi: 10.1016/j.fertnstert.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Velasco JA, Domingo J, Cobo A, Martinez M, Carmona L, Pellicer A. Five years’ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil Steril. 2013;99(7):1994–1999. doi: 10.1016/j.fertnstert.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Domingo J, Guillen V, Ayllon Y, Martinez M, Munoz E, Pellicer A, et al. Ovarian response to controlled ovarian hyperstimulation in cancer patients is diminished even before oncological treatment. Fertil Steril. 2012;97(4):930–934. doi: 10.1016/j.fertnstert.2012.01.093. [DOI] [PubMed] [Google Scholar]
- 37.Reddy J, Oktay K. Ovarian stimulation and fertility preservation with the use of aromatase inhibitors in women with breast cancer. Fertil Steril. 2012;98(6):1363–1369. doi: 10.1016/j.fertnstert.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal A, Said TM. Implications of systemic malignancies on human fertility. Reprod Biomed Online. 2004;9(6):673–679. doi: 10.1016/S1472-6483(10)61779-8. [DOI] [PubMed] [Google Scholar]
- 39.Oktay K, Moy F, Titus S, Stobezki R, Turan V, Dickler M, et al. Age-related decline in DNA repair function explains diminished ovarian reserve, earlier menopause, and possible oocyte vulnerability to chemotherapy in women with BRCA mutations. J Clin Oncol: Off J Am Soc Clin Oncol. 2014;32(10):1093–1094. doi: 10.1200/JCO.2013.53.5369. [DOI] [PubMed] [Google Scholar]
- 40.Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28(2):240–244. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson AD, Missmer SA, Ginsburg ES. Embryo yield after in vitro fertilization in women undergoing embryo banking for fertility preservation before chemotherapy. Fertil Steril. 2011;95(2):588–591. doi: 10.1016/j.fertnstert.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 42.Sabatini ME, Wolkovich AM, Macklin EA, Wright DL, Souter I, Toth TL. Pronuclear embryo cryopreservation experience: outcomes for reducing the risk of ovarian hyperstimulation syndrome and for fertility preservation in cancer patients. J Assist Reprod Genet. 2011;28(3):279–284. doi: 10.1007/s10815-010-9515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrido N, Bellver J, Remohi J, Alama P, Pellicer A. Cumulative newborn rates increase with the total number of transferred embryos according to an analysis of 15,792 ovum donation cycles. Fertil Steril. 2012;98(2):341–346 e1-2. doi: 10.1016/j.fertnstert.2012.04.039. [DOI] [PubMed] [Google Scholar]