Abstract
Purpose
To investigate the impact of elevated serum estradiol (E2) levels on the day of hCG trigger on the birth weight of term singletons after fresh In Vitro Fertilization (IVF)-Embryo Transfer (ET) cycles.
Methods
Retrospective cohort study of all patients initiating fresh IVF-ET cycles resulting in live births between January 2004 and February 2013. The incidence of low birthweight (LBW) term singletons in patients with E2 levels on day of hCG trigger above or below the 95 % cutoff for E2 values in our clinic (3,069.2 pg/mL) was estimated. Multiple gestations and vanishing twin pregnancies were excluded.
Results
Two thousand nine hundred thirty-nine singleton live births were identified for inclusion. One hundred forty seven (5 %) and 2792 (95 %) live singleton births occurred in patients with peak E2 levels above and below 3,069.2 pg/mL, respectively. The overall incidence of term LBW was 5.4 % in the >3,069.2 pg/mL group compared to 2.4 % in the ≤3,069.2 pg/mL group (P = .038). An E2 level >3,069.2 pg/mL on the day of hCG administration was associated with increased odds of LBW term singletons (OR = 2.29; 95 % CI = 1.03–5.11). The increased odds remained unchanged when adjusting for maternal age (aOR = 2.29; 95 % CI = 1.02–5.14; P = .037), gestational age at delivery (aOR = 2.04; 95 % CI = 1.22–3.98; P = .025), and day 3 versus blastocyst transfer (aOR = 2.5; 95 % CI = 1.11–5.64; P = .023).
Conclusions
Peak E2 level >3,069.2 pg/mL is associated with increased odds of LBW term singletons after fresh IVF-ET cycles. Conservative stimulation protocols aiming not to exceed an E2 level of 3,000 pg/mL may be advantageous for placentation and fetal growth if a fresh transfer is planned.
Keywords: Estradiol level, Birth weight, Small for gestational age, IVF, Controlled ovarian hyperstimulation
Introduction
The use of Assisted Reproductive Technology (ART) to overcome the problem of infertility continues to increase steadily [1]. Overall, ART contributed to 1.5 % of all live U.S. births in 2012 [2]. In recent years, there has been concern about the safety of ART, particularly IVF [3–5]. The majority of singleton births after IVF are uncomplicated; however, studies have suggested that IVF pregnancies may be independently associated with increased risks for low birthweight (LBW) [6–8], preterm birth [9], and perinatal mortality [7, 8] compared with spontaneous singleton conceptions. Although the pathogenesis of LBW in IVF singletons still remains unknown, recent data have suggested that the supraphysiologic hormonal milieu during ovarian hyperstimulation (COH) may be a possible mediator of LBW in IVF singletons [10, 11]. To address this hypothesis, we set out to investigate the impact of elevated E2 levels on the day of hCG administration on the birth weight of term singletons after fresh IVF-ET cycles at our center.
Materials and methods
Cycle inclusion criteria
The institutional review board at Weill Cornell Medical College approved our study protocol. All patients initiating fresh IVF-ET cycles at the Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine resulting in live births between January 2004 and February 2013 were analyzed for potential inclusion. Cycles that resulted in multiple births, selective reduction, vanishing twins, or had incomplete records were excluded. Preterm birth was defined as any live birth ≤37 weeks of gestational age. Preterm birth <34 weeks of gestation was defined as early preterm birth, while preterm between >34 and ≤37 weeks of gestation was defined as late preterm birth [12]. LBW was defined as birth weight <2,500 g irrespective of gestational age [13]. Very low birth weight (VLBW) was defined as birth weight <1,500 g irrespective of gestational age [9, 13]. E2 levels of cycles resulting in singleton live births were analyzed for E2 level, and the 95th percentile was defined as elevated E2.
Clinical and laboratory protocols
COH, hCG trigger, oocyte retrieval, embryo culture, and ET were carried out per our standard protocols [14–16]. Some patients were down regulated using a GnRH agonist (Lupron; Abbott Pharmaceuticals) followed by stimulation with gonadotropins (Follistim [Merck]; Gonal-F [EMD-Serono]; and/or Menopur [Ferring]). Flare protocols were used as clinically indicated. Alternatively, patients were stimulated with gonadotropins followed by pituitary suppression with a GnRH antagonist (Ganirelix Acetate, 0.25 mg [Organon]; or Cetrotide, 0.25 mg [EMD-Serono]). Patients who required pretreatment for follicular synchronization were started on oral contraceptive (OC) pills (Ortho-Novum, Janssen Pharmaceuticals), or 0.1 mg E2 patches (Climara, Bayer Healthcare Pharmaceuticals) with or without Ganirelix. For GnRH antagonist cycles, patients were started on 0.25 mg of Ganirelix or Cetrotide based on a flexible protocol as previously described [15]. Ovarian stimulation was carried out to maximize follicular response while minimizing risk of ovarian hyperstimulation syndrome (OHSS). Selection of the initial gonadotropin dose was based on multiple factors such as patient age, weight, antral follicle count, day 3 FSH/E2, antimüllerian hormone (subsequent to 2010), and previous response to stimulation.
Oocyte maturation was triggered via hCG (Profasi [EMDSerono]; Novarel [Ferring Pharmaceuticals]; or Pregnyl [Schering-Plough]), according to a dosing scheme ranging from 10,000 to 3,300 IU based on serum E2 levels [14–16]. In general, the hCG trigger was administered when the two lead follicles attained a mean diameter ≥17 mm. For GnRH antagonist cycles considered to be at high risk for OHSS, an ovulatory trigger of 2 mg leuprolide acetate in conjunction with 1,500 IU hCG was administered, with appropriate E2 and progesterone (P) luteal support [17]. In all other cases, luteal support was begun the day after retrieval with 50 mg of intramuscular P. Oocyte retrieval was performed with transvaginal ultrasound guidance under conscious sedation 35–37 h after hCG administration. Fertilization was performed using conventional insemination or intracytoplasmic sperm injection according to the couple’s history and male partner’s semen analysis. Embryos were incubated in sequential in-house culture media. Most patients underwent ET on day 3; however, patients with several good-quality embryos on day 3 were eligible for and underwent blastocyst transfer on day 5. All embryo transfers were performed with Wallace catheters (Marlow/Cooper Surgical).
Study variables
Demographic characteristics extracted from patient charts included age, parity, body mass index (kg/m2), infertility diagnosis, number of previous IVF attempts, basal follicle stimulating hormone (FSH) level (mIU/mL), COH protocol during current IVF cycle, endometrial stripe thickness (mm) on day of hCG administration, total days of stimulation, total dosage of gonadotropins administered (IU), number of embryos transferred, and day of ET. Birth outcomes analyzed included birth weight, mode of delivery, incidence of preterm birth, and incidence of term LBW.
Statistical analysis
All statistical analyses were performed using STATA version 13 (College Station, TX: StataCorp LP). Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as number of cases (n) and percentage of occurrence (%). Wilcoxon rank sum test and student’s t-test were utilized for continuous variables. Chi-square (χ2) with Mantzel-Hansel correction and Fisher’s exact test were used for categorical variables. Odds ratios (OR) with 95 % confidence intervals (CI) for the incidence of term LBW were calculated. Adjusted odds ratios (aOR) were estimated using logistic regression controlling for maternal age, gestational age at delivery, hCG trigger dose, and day of ET. Statistical significance was set at P < .05.
Results
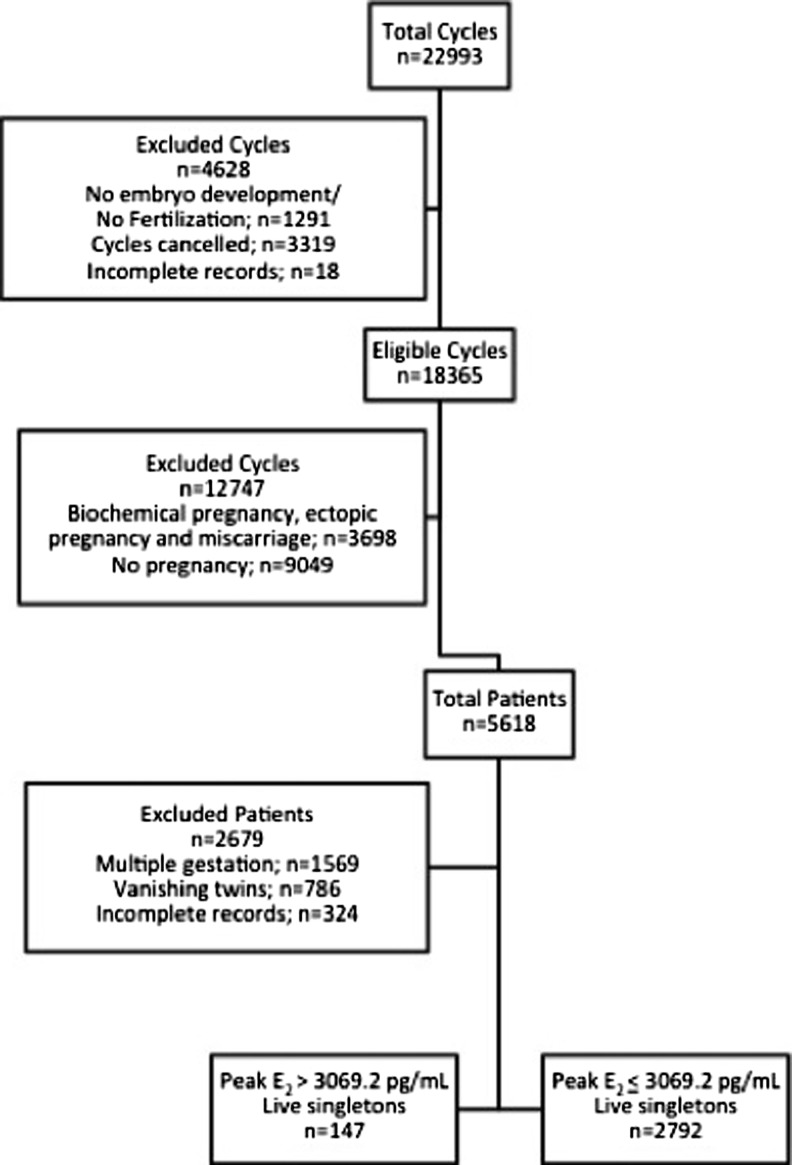
Figure 1 summarizes the selection of the study cohort. A total of 5618 patients underwent fresh IVF-ET cycles resulting in live birth during the study period. Of these, 1569 (27.9 %) patients were excluded due to multiple gestation, 786 (14 %) patients were excluded due to vanishing twins and 324 (5.8 %) patients were excluded for incomplete records. The remaining (52.3 %) patients accounted for 2939 live singleton births. The mean E2 level ± SD and the 95th percentile ± SD on the day of hCG administration for the study cohort was 1675.6 ± 748.1 pg/mL and 3069.2 ± 748.1 pg/mL, respectively. There were 147 live singleton births in the E2 > 3069.2 pg/mL group and 2792 live singleton births in the E2 ≤ 3069.2 pg/mL group.
Fig. 1.
Study cohort selection
Table 1 compares the demographic characteristics of patients with peak E2 levels above and below 3,069.2 pg/mL. Overall, there were no differences in mean age, parity, body mass index, number of previous IVF attempts, basal FSH levels, peak endometrial stripe, total days of stimulation, or mean embryos transferred between the two groups. There was also no difference in distribution of infertility diagnoses and COH protocols within each group. However, the total dosage of gonadotropins administered in the >3069.2 pg/mL group was significantly lower than the ≤3069.2 pg/mL group (P < .0001). The mean number of oocytes ± SD retrieved in the E2 > 3069.2 pg/mL group (17.3 ± 6.9) was higher compared to the E2 ≤ 3069.2 pg/mL group (11.4 ± 5.7). A significantly higher number of blastocyst transfers occurred in the E2 > 3069.2 pg/mL group (P = .003). Patients in the E2 > 3069.2 pg/mL group exhibited a robust response to gonadotropins, as evident from the number of oocytes retrieved in this group compared to the E2 ≤ 3069.2 pg/mL group. Due to this response, patients required comparatively lower doses of gonadotropins. In order to prevent OHSS in this group of patients, transfer was more often deferred until day 5, so as to observe patients for signs of early OHSS; thus, a higher number of blastocyst transfers occurred in the E2 > 3069.2 pg/mL group.
Table 1.
Baseline characteristics of study cohort (n = 2939)
| Parameter | Peak E2 > 3069.2 pg/mL (n = 147) |
Peak E2 ≤ 3069.2 pg/mL (n = 2792) |
P |
|---|---|---|---|
| Age (years) | 34.8 (±4.6) | 35.2 (±4.5) | .29 |
| Parity | .27 (±.58) | .33 (±.59) | .22 |
| BMI (kg/m2) | 22.5 (±4.5) | 22.9 (±6.4) | .45 |
| Infertility diagnoses | .99 | ||
| Ovulatory | 30 (20.4) | 579 (20.7) | |
| Tubal | 10 (6.8) | 169 (6.1) | |
| Endometriosis | 5 (3.4) | 119 (4.3) | |
| Male factor | 36 (24.5) | 628 (22.5) | |
| Idiopathic | 11 (7.5) | 227 (8.1) | |
| Other | 55 (37.4) | 1070 (38.3) | |
| Previous IVF attempts | 1.58 (±1.60) | 1.63 (±1.93) | .77 |
| Basal FSH (mIU/mL) | 4.23 (±2.03) | 4.46 (±2.74) | .19 |
| COH Protocol | .65 | ||
| Follicular phase GnRH-ant | 46 (31.3) | 947 (33.9) | |
| Follicular phase GnRH-a | 44 (30) | 929 (33.3) | |
| Luteal phase GnRH-a | 29 (19.7) | 453 (16.2) | |
| Other | 28 (19) | 463 (16.6) | |
| Peak endometrial stripe (mm) | 11.5 (±2.6) | 11.4 (±6.8) | .86 |
| Total stimulation days | 9.6 (±1.7) | 9.6 (±1.9) | 1 |
| Total gonadotropins administered (IU) | 1923.1 (±1007.1) | 2977.2 (±1772.1) | <.0001 |
| Total number of oocytes | 17.3 (±6.9) | 11.4 (±5.7) | <.0001 |
| Embryos transferred | 2.6 (±1.3) | 2.7 (±1.1) | .29 |
| Blastocyst transfer | 46 (31.3) | 391 (14) | .003 |
Data are presented as mean ± standard deviation and n (%)
BMI Body Mass Index
IVF In Vitro Fertilization
FSH Follicle Stimulating Hormone
COH Controlled Ovarian Hyperstimulation
GnRH-ant Gonadotropin Releasing Hormone-Antagonist
GnRH-a Gonadotropin Releasing Hormone-Agonist
Bolded numbers indicate significance
Birth outcomes were available for all 2939 births. Table 2 compares the birth outcomes between the two E2 groups; rates of vaginal and cesarean deliveries were comparable. Furthermore, no differences in the rates of term births (88.4 % vs. 90 %), late preterm births (9.5 % vs. 7.7 %), or early preterm birth (2.1 % vs. 2.3 %) were found between the two groups. There was also no difference in the mean overall birth weight (range 3261.6–3268.0 g) and mean term birth weight (range 3335.8–3354.0 g) when comparing the two groups. A significant difference was found in the incidence of term LBW singletons (5.4 % in the >3069.2 pg/mL group versus 2.4 % in the ≤3069.2 pg/mL group (P = .038). Patients with E2 levels >3,069.2 pg/mL on the day of hCG administration had more than twice the risk of giving birth to a LBW term infant as compared to patients with lower E2 levels (OR = 2.29; 95 % CI = 1.03–5.11). The increased odds remained unchanged when adjusting for maternal age (aOR = 2.29; 95 % CI = 1.02–5.14; P = .037), gestational age at delivery (aOR = 2.04; 95 % CI = 1.22–3.98; P = .025), hCG trigger dose (aOR = 1.99; 95 % CI = 1.08–3.99; P = .036), as well as adjusting for the day of embryo transfer (aOR = 2.5; 95 % CI = 1.11–5.64; P = .023). There were no term VLBW births in either group.
Table 2.
Birth outcomes of study cohort (n = 2939)
| Parameter | Peak E2 > 3069.2 pg/mL (n = 147) |
Peak E2 ≤ 3069.2 pg/mL (n = 2792) |
P |
|---|---|---|---|
| Mode of Delivery | .20 | ||
| Vaginal | 68 (46.3) | 1122 (40.2) | |
| Cesarean | 76 (51.7) | 1558 (43.8) | |
| Unknown | 3 (2.0) | 112 (4.0) | |
| Term Birth | 130 (88.4) | 2514 (90) | .26 |
| Preterm Birth | .30 | ||
| Late preterm | 14 (9.5) | 214 (7.7) | |
| Early preterm | 3 (2.1) | 64 (2.3) | |
| Overall Birth Weight (g) | 3261.6 (±547.0) | 3268.0 (±555.2) | .99 |
| Term LBW | 7 (5.4) | 61 (2.4) | .038 |
| Term VLBW | 0 | 0 | – |
Data are presented as mean ± standard deviation and n (%)
Term: >37 weeks gestational age
Preterm: ≤37 weeks gestational age
Late preterm: >34 and ≤37 weeks gestational age
Early preterm: ≤34 weeks gestational age
LBW Low Birth Weight i.e., birth weight <2500 g irrespective of gestational age
VLBW Very Low Birth Weight i.e., birth weight <1500 g irrespective of gestational age
Bolded numbers indicate significance
Discussion
Our study adds to the growing body of literature suggesting an association between supraphysiologic E2 levels during COH and altered placental dynamics in IVF singletons. Our data showed increased odds of term LBW at a lower E2 threshold than previously reported i.e., 3,069.2 pg/mL versus 3,450 pg/mL [11].
In 2002, Schieve et al. highlighted the potential association between IVF and LBW [6]. In that study, the authors found that the overall odds of LBW in IVF singletons were 1.8 times higher than spontaneous singletons. Since then, several studies have corroborated these findings [7, 8]. Latest data indicate that infants conceived with ART comprise 5.6 % of all LBW infants in the United States, though much of this morbidity is due to multiple gestations [1]. Putative mechanisms ranging from the intrinsic characteristics of the infertile couple to the hormonal milieu during COH for IVF have been suggested to explain the association between LBW in singletons and IVF, however the exact mechanisms still remain unknown [4, 5, 10, 11].
Studies reporting the association between supraphysiologic E2 levels during COH and LBW have begun to emerge [10, 11]. Kalra et al. [10] highlighted this potential association in the their analysis of birth weights in 56,792 singletons. The authors reported a 1.73 times higher odds of LBW at term in singletons from fresh autologous IVF as compared to frozen-thawed cycles. In another retrospective study of 292 IVF singletons, Imudia et al. [11] reported 9.4 times higher odds of delivering LBW singletons in patients with E2 levels >3,450 pg/mL on the day of hCG administration. Consistent with these findings, our data show twice the odds of term LBW singletons in patients with E2 levels >3,069.2 pg/mL on the day of hCG administration compared to patients with E2 levels below this cutoff.
The supraphysiologic hyperestrogenic milieu during COH may contribute to the pathogenesis of LBW, at least in animal models, by creating a poorer environment for implantation [4, 18]. For example, supraphysiologic E2 levels have been shown to have a toxic effect on the developing embryo in mice, leading to impairment in embryonic adhesion and implantation potential [19–21]. Elevated estrogen levels also impair the expression of implantation-associated genes in mice, leading to aberrant placentation [22]. Altered implantation physiology has been demonstrated in a baboon model, where elevated E2 levels are associated with attenuation of spiral artery invasion [23]. Consistent with these animal models, supraphysiologic E2 levels can modulate endometrial gene expression profiles in humans, thereby potentially affecting implantation and placentation [24–28]. Resulting modifications to trophoblastic invasion may lead to placental dysfunction and contribute to the pathogenesis of LBW and preeclampsia in IVF singletons [22].
The odds of delivering LBW singletons (2 times higher in our study cohort), was lower than the 9 times higher odds in the Imudia et al. study possibly because of the much larger size of our cohort. Although the overall rate of preterm birth (10 %) in our study cohort was higher than that reported by Imudia et al. (6.85 %), we found no difference in rates of preterm birth in patients with peak E2 levels above and below 3,069.2 pg/mL; moreover, this rate of preterm birth is consistent with overall rates of preterm birth (12–13 %) in spontaneously conceived pregnancies in the U.S. [29]. Similarly, Kalra et al. found no difference in the rate of preterm birth in their cohort. Although our findings remain unchanged even when adjusting for age and day of embryo transfer, we recognize that the confidence intervals remain relatively wide. Therefore, larger prospective studies to confirm these findings are encouraged. Of course, while several studies have implicated an endometrial effect underlying the observations delineated above, a primary adverse effect on gametes from vigorous stimulation remains to be further interrogated. It is also possible an elevated E2 level may merely be a surrogate for some other uncharacterized molecular marker [30]. Even if E2 levels are implicated in the pathogenesis of LBW in IVF singletons, the supraphysiologic threshold for such an effect still remains unknown [30].
The impact of the hyperestrogenic milieu during COH on implantation and placentation is an area of active investigation. Although maximizing follicular response during COH is important, it is more important to minimize the risks of OHSS and LBW associated with supraphysiologic E2 levels, especially given the correlation between LBW and adult cardiovascular disease, diabetes, and dyslipidemia [31–33]. While there has been a recent shift towards frozen embryo transfer cycles in the name of improved endometrial physiology, it is plausible that conservative stimulation protocols with autologous, fresh transfer offer the same benefits, provided serum E2 levels are carefully monitored.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule Peak estradiol levels and birth weight
Nigel Pereira and David E. Reichman contributed equally to this work.
References
- 1.Sunderam S, Kissin DM, Crawford S, Anderson JE, Folger SG, Jamieson DJ, et al. Assisted reproductive technology surveillance – United States, 2010. MMWR Surveill Summ. 2013;62(9):1–24. [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. Assisted Reproductive Technology (ART). Available at: http://www.cdc.gov/art. Accessed on 10/18/2014.
- 3.Kalra SK, Barnhart KT. In vitro fertilization and adverse childhood outcomes: what we know, where we are going, and how we will get there. A glimpse into what lies behind and beckons ahead. Fertil Steril. 2011;95(6):1887–9. doi: 10.1016/j.fertnstert.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondapalli LA, Perales-Puchalt A. Low birth weight: is it related to assisted reproductive technology or underlying infertility? Fertil Steril. 2013;99(2):303–10. doi: 10.1016/j.fertnstert.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnhart KT. Assisted reproductive technologies and perinatal morbidity: interrogating the association. Fertil Steril. 2013;99(2):299–302. doi: 10.1016/j.fertnstert.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with the use of assisted reproductive technology. N Engl J Med. 2002;346:731–7. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 7.Helmerhorst FM, Perquin DA, Donker D, Keirse MJ. Perinatal outcome of singletons and twins after assisted conception; a systematic review of controlled studies. BMJ. 2004;328:261–5. doi: 10.1136/bmj.37957.560278.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson R, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;130:551–63. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 9.McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146:138–48. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 10.Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol. 2011;118(4):863–71. doi: 10.1097/AOG.0b013e31822be65f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97(6):1374–9. doi: 10.1016/j.fertnstert.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Spong CY, Mercer BM, D’alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118(2 Pt 1):323–33. doi: 10.1097/AOG.0b013e3182255999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 14.Reichman DE, Greenwood E, Meyer L, Kligman I, Rosenwaks Z. Can in vitro fertilization cycles be salvaged by repeat administration of intramuscular human chorionic gonadotropin the day after failed injection? Fertil Steril. 2012;98:671–4. doi: 10.1016/j.fertnstert.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 15.Reichman DE, Chung P, Meyer L, Greenwood E, Davis O, Rosenwaks Z. Consecutive gonadotropin-releasing hormone-antagonist in vitro fertilization cycles: does the elapsed time interval between successive treatments affect outcomes? Fertil Steril. 2013;99:1277–82. doi: 10.1016/j.fertnstert.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 16.Reichman DE, Goldschlag D, Rosenwaks Z. Value of antimüllerian hormone as a prognostic indicator of in vitro fertilization outcome. Fertil Steril. 2014;98(5):1225–8. doi: 10.1016/j.fertnstert.2012.07.1056. [DOI] [PubMed] [Google Scholar]
- 17.Engmann L, Benadiva C. Agonist trigger: what is the best approach? Agonist trigger with aggressive luteal support. Fertil Steril. 2012;97:531–3. doi: 10.1016/j.fertnstert.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100:2963–8. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valbuena D, Martin J, de Pablo JL, Remohi J, Pellicer A, Simon C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76:962–8. doi: 10.1016/S0015-0282(01)02018-0. [DOI] [PubMed] [Google Scholar]
- 20.Bittner AK, Horsthemke B, Winterhager E, Grummer R. Hormone-induced delayed ovulation affects early embryonic development. Fertil Steril. 2011;95:2390–4. doi: 10.1016/j.fertnstert.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Ertzeid G, Storeng R. The impact of ovarian stimulation on implantation and fetal development in mice. Hum Reprod. 2001;16:221–5. doi: 10.1093/humrep/16.2.221. [DOI] [PubMed] [Google Scholar]
- 22.Kalra SK. Adverse perinatal outcome and in vitro fertilization singleton pregnancies: what lies beneath? Further evidence to support an underlying role of the modifiable hormonal milieu in in vitro fertilization stimulation. Fertil Steril. 2012;97(6):1295–6. doi: 10.1016/j.fertnstert.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 23.Albrecht ED, Bonagura TW, Burleigh DW, Enders AC, Aberdeen GW, Pepe GJ. Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during early baboon pregnancy. Placenta. 2006;27:483–90. doi: 10.1016/j.placenta.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Simon C, Cano F, Valbuena D, Remohi J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10:2432–7. doi: 10.1093/oxfordjournals.humrep.a136313. [DOI] [PubMed] [Google Scholar]
- 25.Valbuena D, Jasper M, Remohi J, Pellicer A, Simon C. Ovarian stimulation and endometrial receptivity. Hum Reprod. 1999;14(Suppl 2):107–11. doi: 10.1093/humrep/14.suppl_2.107. [DOI] [PubMed] [Google Scholar]
- 26.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–8. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 27.Dominguez F, Remohi J, Pellicer A, Simon C. Human endometrial receptivity: a genomic approach. Reprod Biomed Online. 2003;6:332–8. doi: 10.1016/S1472-6483(10)61853-6. [DOI] [PubMed] [Google Scholar]
- 28.Horcajadas JA, Riesewijk A, Polman J, van Os R, Pellicer A, Mosselman S, et al. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. Mol Hum Reprod. 2005;11:195–205. doi: 10.1093/molehr/gah150. [DOI] [PubMed] [Google Scholar]
- 29.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnhart KT. Introduction: are we ready to eliminate the transfer of fresh embryos in in vitro fertilization? Fertil Steril. 2014;102(1):1–2. doi: 10.1016/j.fertnstert.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grella PV. Low birth weight and early life origins of adult disease: insulin resistance and type 2 diabetes. Clin Exp Obstet Gynecol. 2007;34(1):9–13. [PubMed] [Google Scholar]
- 32.Nesterenko TH, Aly H. Fetal and neonatal programming: evidence and clinical implications. Am J Perinatol. 2009;26(3):191–8. doi: 10.1055/s-0028-1103027. [DOI] [PubMed] [Google Scholar]
- 33.Negrato C, Gomes M. Low birth weight: causes and consequences. Diabetol Metab Syndr. 2013;5(1):49. doi: 10.1186/1758-5996-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]