Abstract
Reactive oxygen species (ROS) play an important role in male fertility. Overproduction of reactive oxygen species (ROS) has been associated with a variety of male fertility complications, including leukocytospermia, varicocele and idiopathic infertility. The subsequent oxidative insult to spermatozoa can manifest as insufficient energy metabolism, lipid peroxidation and DNA damage, leading to loss of motility and viability. However, various studies have demonstrated that physiological amounts of ROS play important roles in the processes of spermatozoa maturation, capacitation, hyperactivation and acrosome reaction. It is therefore crucial to define and understand the delicate oxidative balance in male reproductive cells and tissues for a better understanding of both positive as well as negative impact of ROS production on the fertilizing ability. This review will discuss the specific physiological roles, mechanisms of action and effects that ROS have on the acquisition of structural integrity and physiological activity of spermatozoa.
Keywords: Male fertility, Reactive oxygen species, Spermatozoa, Capacitation, Hyperactivation, Acrosome reaction
Introduction
Male infertility is a major problem in human reproduction, affecting as many as 15 % of couples of reproductive age, with half of these cases stemming from male abnormalities [1]. The increasing importance of this problem has pushed scientists over the last decade to dwell on the study of the pathological role of ROS; a causative agent in male infertility [2]. On the other hand, ROS play an important physiological role in spermatozoa function following fertilization of the oocyte. Reactive oxygen species (ROS) have been extensively studied, and their detrimental effects on male fertility are well accepted. It has already been reported that pathological ROS levels have a deleterious effect on semen quality, including spermatozoa motility [3–5], viability, morphology and concentration [6, 7].
Accumulating evidence suggests that low levels of ROS are of paramount importance in the activation of intracellular pathways responsible for spermatozoa maturation, capacitation, hyperactivation, acrosomal reaction, chemotactic processes as well as fusion with the female gamete [8–11].
This review aims to highlight the importance of the physiological roles of ROS ensuring morphological and functional alterations of spermatozoa at different stages of development, thereby warranting the acquisition of fertilization ability. At the same time, it gives detailed information on the relationship between spermatozoa and ROS, which is crucial for an accurate understanding of the latest findings involving ROS and male reproduction.
Reactive oxygen species
An atom with two complementary electrons in its outermost shell spinning in opposite directions is defined to be in a ground state. This phenomenon stabilizes the atom and prevents it from reacting with its surroundings, thereby making it inert. Conversely, the reduction of diatomic oxygen (O2), which is essential for cellular respiration and survival, results in the creation of a highly reactive oxygen metabolite—the superoxide anion (O2−), which is capable of interfering with cellular functions. If this reduction is followed by the gain of another electron, peroxide (O22−) is formed. Interestingly, O22− is not considered a free radical [12]. The endogenous hydrogen peroxide (H2O2), a weak though abundant free radical, is subsequently generated by numerous metabolic reactions in the human body including the peroxisomal pathway via beta glycolate and monoamine oxidases. It is also produced by O2− dismutation [13].
Both O2− and H2O2 can undergo a series of cellular transformations to form the extremely reactive hydroxyl radical (OH•) through the Fenton and Haber Weiss reaction, which involves two steps. The first step consists of a reduction of ferric (Fe3+) to ferrous ion (Fe2+) in the presence of O2−. The second step consists of H2O2 conversion to OH•. Ferrous ions act as catalysts in this slow reaction [14]. Superoxide anion interacts with nitric oxide (NO) to form peroxynitrite (ONOO−). Nitric oxide is a reactive radical with an odd number of electrons catalyzed by the family of nitric oxide synthase (NOS) enzymes [15]. Other ROS species including ozone, organic peroxyl and alkoxy radicals may be present too, but are not biologically important [16].
ROS include all molecules containing the oxygen atom. Free radicals “steal” electrons from surrounding structures to achieve a ground state, thereby causing other molecules to become subsequent free radicals. This positive feedback amplifies the degree of disruption in the neighboring structures [17].
Endogenous sources of ROS in semen
Seminal fluid is an essential medium that nurtures spermatozoa during their migration from the epididymis to the female genital tract. Secretions from the prostate, seminal vesicles, Cowper’s glands and testicular secretions make up the ejaculate. The seminal fluid of healthy fertile men contains a variety of cells including leukocytes, macrophages, immature germ cells and Sertoli cells [18, 19].
Leukocytes
Activated leukocytes are a significant ROS producer in semen [20, 21]. Myeloperoxidase staining (Endz test) is an easy and inexpensive way to quantify leukocytes in unprocessed semen as a part of the semen profile [22]. According to the WHO, if the leukocyte concentration in the ejaculate exceeds a level of 1 × 106 per milliliter, leukocytospermia is present [23].
Numerous studies have examined the relationship between seminal leukocytes and male infertility, resulting in two different views. On the one hand, some studies failed to find a correlation between the leukocyte concentration and sperm damage [24], while on the other hand, certain studies reported a strong link between leukocytospermia and abnormal sperm parameters and function [25]. Another study concluded that ROS generated by abnormally high levels of leukocytes were associated with an increase in sperm DNA fragmentation [26]. However, Sharma et al. [27] observed that small numbers of white blood cells, even those below the WHO cut-off value for leukocytospermia, are responsible for oxidative stress and therefore, sub-threshold levels of leukocytes, as seen in normal ejaculates, may not be considered safe as previously thought. Furthermore, activated forms of white blood cells are responsible for a 100-fold increase in ROS production compared to non-activated leukocytes [28].
Leukocytes are activated by multiple factors, especially inflammation and infection [29]. This causes the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase to catalyze the formation of free radicals through the hexose monophosphate shunt in order to fight infections. The hexose monophosphate shunt consists of a number of cytosolic enzymes that bypass the glycolytic pathway through alternative reactions leading to the formation of reduced NADP+ (NADPH). NADPH oxidase is a membrane bound enzyme that transfers electrons from NADPH to oxygen to produce O2− [30].
Leukocytospermia has also been implicated in the increased ROS production by spermatozoa. This event is triggered by a direct cell-to-cell contact of the leukocyte with spermatozoa or by the release of soluble products acting on the sperm cell [21].
Subpopulations of leukocytes usually present in semen, mainly consist of polymorphonuclear (PMN) leukocytes (50–60 %) and macrophages (20–30 %) [31]. PMN leukocytes represent an important source of ROS due to their abundant presence in semen. At the same time, external stimuli induce the activation of macrophages, thereby causing an oxidative burst and overproduction of ROS. These differentiated monocytes are of paramount importance in defending the male reproductive tract from foreign invaders [32].
Immature spermatozoa
Immature spermatozoa with abnormal head morphology and cytoplasmic retention are another important source of ROS in semen [7]. Failure to extrude excess cytoplasm during sperm differentiation and maturation traps a surplus of enzymes, including glucose-6-phosphate dehydrogenase (G6PD) and NADPH oxidase, which lead to the generation of free radicals through the formation of the intermediate NADPH [33–35]. G6PD is an enzyme of the hexose monophosphate shunt. In the presence of NADP+ and glucose-6-phosphate, the reaction results in reduced NADPH. This hypothesis is supported by findings of a study showing that after density gradient separation of the human ejaculate, the layer of immature spermatozoa produces the highest levels of ROS [36].
Normal spermatozoa have the ability to produce ROS, which can be attributed to two different sources: NADPH oxidase at the level of the plasma membrane and NADH dependent oxidoreductase (diaphorase) in the mitochondria [37]. NADH diaphorase is a member of the Krebs cycle—a high energy producing biochemical cycle consisting of a series of chemical reactions that eventually lead to ATP formation. This pathway is utilized by all aerobic organisms and leads to acetate oxidation. After one cycle, three NADH molecules are created from NAD+. Electrons transported by NADH can later be used for ATP production in the electron transport chain. The mitochondrial contribution of ROS is generally considered to be very low [38]. According to de Lamirande et al. [39], any claim alluding to the generation of ROS by NADPH should be rejected for the reason that neither polymerase chain reaction (PCR) nor immunoblotting analysis has found oxidase components in the cell membrane.
Sertoli cells
Sertoli cells have also been shown to possess the ability to produce ROS—an effect inhibited by the addition of scavestrogens (J811 and J861). Derived in structure from 17alpha-estradiol, scavestrogens serve as potent radical scavengers that also inhibit iron-induced cell damage in vitro. Hence, it can be hypothesized that Sertoli cells assist in spermatogenesis mediated by ROS. Based on this evidence, there is a need to further investigate and clarify the function of Sertoli cells in the process of ROS formation [40].
Unfortunately, an exact and unanimously agreed upon ROS concentration, defining the physiological and pathological roles, has not been established as of yet.
Sperm transformational stages
The spermatozoon nucleus carries the haploid paternal DNA to complement its counterpart present in the female oocyte. The nuclear fusion of male and female gametes gives rise to a diploid fetus. Development of the spermatid into a normal mature spermatozoon is a key process to achieve fertilization. The immature germ cell is produced in the seminiferous tubules of the testis. Once formed, spermatozoa are delivered to the epididymis to be stored and acted upon by the epithelial cells. Non-motile spermatozoa undergo maturation at this stage and acquire the ability to swim. Furthermore, the nucleus condenses and the cytoplasm is extruded from the head of the sperm. During ejaculation, spermatozoa are mixed with secretions from different glands to form semen.
Semenogelin, which is released from the seminal gland, prostate and epididymis, is the most abundant protein in semen and is responsible for the coagulation of the ejaculate upon its release [41, 42]. The coagulum is later degraded by protein-specific antigens originating from the prostate. The coagulation and liquefaction process may be regulated by redox reactions. Hamada et al. reported that patients with high levels of ROS had higher levels of semenogelin in their spermatozoa [43]. On the other hand, Chatterjee et al. [44] showed that the liquefaction process is amplified by the presence of O2−. This discrepancy between the studies may be due to the fact that ROS activate different pathways or that Chatterjee et al. used a higher concentration of O2− in vitro compared to in vivo levels.
A mature spermatozoon is greatly compartmentalized. It consists of a head carrying the condensed chromatin in the nucleus in the almost absence of cytoplasm. The anterior half of the head is characterized by the presence of the acrosome, which is a membrane-enclosed structure filled with inactive enzymes. The midpiece is the “power house” of the spermatozoon, which generates energy through the mitochondria (only present in midpiece) in order to propel the flagellum [45]. The tail (flagellum) consists of microtubules and provides motility to the male gametes through a whip-like motion [46].
Mature spermatozoa enter the female reproductive system through ejaculation. At this point, a series of physiological modifications take place to prepare the spermatozoa to recognize and bind to the zona pellucida, a process called capacitation. This is a reversible process that lasts several hours. Various cellular modifications take place during this indispensable process including an increase in plasma membrane fluidity, cholesterol efflux, an upsurge in intracellular calcium (mediating the increase in adenylyl cyclase and cAMP), a rise in pH, protein tyrosine phosphorylation (P-Tyr), membrane hyper-polarization, and the induction of hyperactivated motility of the spermatozoon [47–49].
The acrosome reaction (AR) can start only if the capacitation process is completed and the spermatozoon has reached the oocyte [46]. The reaction involves exocytosis of enzymes following the fusion of the spermatozoon with the zona pellucida, at which point the acrosome is shedded and its contents released. These modifications permit the spermatozoon to penetrate the tough outer layers of the female oocyte [50]. The process is irreversible and is triggered by the zona pellucida glycoprotein 3 (ZP3) or progesterone [36]. Some investigators do not consider the AR and capacitation two independent processes. This might be due to the similarity in the biochemical modifications typical for both processes, including changes in pH as well as intracellular calcium and P-Tyr [36, 51, 52].
However, these two processes are distinctly separate and occur at a specific time during the fertilization process. Moreover, the triggering events, kinetics, amplitude of the modifications, enzyme targets and cellular compartmentalization are significantly different [53].
Starting with their production in the testes and finishing with oocyte fusion, spermatozoa undergo several maturational steps throughout their life, allowing them to perform various functions culminating in one sole purpose, i.e. achieving fertilization. The structural integrity and functional activity of the spermatozoon is facilitated by extracellular stimuli stemming from its surrounding environment, which varies as the cell migrates from the male to the female reproductive tract. ROS are such a stimulus needed by spermatozoa to gain their functional competence [54].
Physiological role of ROS during sperm maturation
Capacitation
Extensive research has identified the role physiological ROS levels play in potentiating capacitation. There is an overall agreement that the presence of ROS is essential for the amplification in P-Tyr of proteins with a molecular weight ~100 kDa [55, 56].
Aitken et al. inhibited ROS production in vitro by introducing 2-deoxyglucose—a modified glucose molecule that interacts with and inhibits the glycolysis pathway, which is an important intracellular source of ROS. This modification resulted in a decrease of tyrosine-phosphorylated proteins, justifying the hypotheses of ROS-induced phosphorylation [57]. Among the phosphorylated proteins, bands with a molecular weight of 80 and 105 kDa were identified as the A-kinase anchoring proteins (AKAP) that bind protein kinase A (PKA) to the fibrous sheath of the spermatozoon [58]. Their location suggests that they play a role in hyperactivated motility of spermatozoa.
PKA is an essential enzyme and acts by adding a phosphate group to a target protein, modifying its structure and regulating its activity. A cascade of events follows with the activation of adenylyl cyclase (AC) leading to the production of cyclic adenosine monophosphate (cAMP) from ATP. cAMP subsequently triggers PKA to function during the second messenger cascade known as the AC/cAMP/PKA pathway [59] (see Fig. 1).
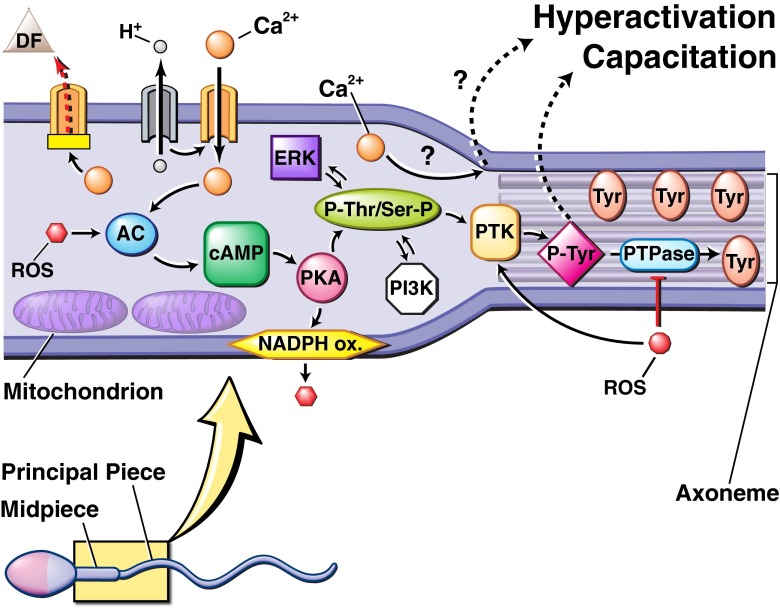
Fig. 1.
Biochemical pathway proposed to regulate sperm capacitation and hyperactivation. The process is initiated by an influx of Ca2+ and HCO3 −, possibly caused by the inactivation of an ATP-dependent Ca2+ regulatory channel (PMCA) and alkalization of the cytosol. Both Ca2+ and reactive oxygen species (ROS), specifically O2 −, activate adenylate cyclase (AC), which produces cyclic adenosine monophosphate (cAMP). cAMP activates downstream protein kinase A (PKA). PKA triggers a membrane bound NADPH oxidase to stimulate greater ROS production. In addition, PKA triggers phosphorylation of serine (Ser) and tyrosine (Tyr) residues that, in addition to other inter-connected pathways, lead to the activation of protein tyrosine kinase (PTK). PTK phosphorylates Tyr residues of the fibrous sheath surrounding the axoneme, the cytoskeletal component of the flagellum. ROS, specifically hydrogen peroxide (H2O2), increases the amount of Tyr phosphorylation by promoting PTK activity and inhibiting phosphotyrosine phosphatase (PTPase) activity, which normally de-phosphorylates Tyr residues. The enhanced Tyr phosphorylation observed in capacitation is the last known step in the process, but intermediate steps or other (in)direct methods may be involved
Correspondingly, an increase in cAMP nucleotides leads to the amplification of P-Tyr [60]. The common end product resulting from ROS and cAMP activity suggests that they work along the same pathway in order to produce P-Tyr within the cell. It is hypothesized that free radicals stimulate the AC synthesis through either a direct or indirect pathway involving secondary messengers such as calcium [61].
Leclerc et al. [62] argued that an increase in cellular calcium levels is required for cAMP and O2− generation, which eventually act on PKA through the previously mentioned cascade. They reached this conclusion after observing the effects caused by the addition of certain capacitation inducers including fetal cord serum ultrafiltrate (FCSu) and A23187. This ultrafiltrate triggered capacitation and amplification of P-Tyr when added to a medium containing spermatozoa. When BAPTA (a calcium chelating agent) was added, it prevented FCSu-induced capacitation and decreased O2− by 60 %. Similarly, A23187—a divalent calcium ionophore—mimicked the role of Ca2+ and activated capacitation, leading to increased phosphorylation.
Phosphatases are another family of enzymes in the AC/cAMP/ PKA pathway that catalyze the breakdown of cAMP into inactive metabolites, thereby terminating its function. In order for the enzyme to act, its cysteine residue in the catalytic domain must be reduced [63]. Because ROS are oxidizing agents, they inhibit the action of phosphatases, causing an increase in the intracellular levels of cAMP and subsequently, enhancing the protein phosphorylation.
Continuous production of ROS during sperm maturation is necessary for the intracellular pathways of phosphorylation to remain functional. In the absence of ROS, P-Tyr, intermediate signals and the capacity of NADH to produce more free radicals are blunted [57].
Similarly, Donà et al. [56] studied the correlation between endogenous ROS formation (measured by a luminometer with luminol as the source of fluorescence), P-Tyr and capacitation. Cells were collected from 12 healthy fertile individuals with normal sperm parameters according to WHO standards. In this experiment, the capacitation process showed 3 patterns of ROS production. An early phase characterized by a peak after 45 min of incubation was associated with an amplification of P-Tyr proteins with a molecular weight of 105 and 81 kDa. The following phase was characterized by a decrease in both ROS and P-Tyr due to high levels of ATP generated by NADH in the first phase, causing a negative feedback on ROS production and protein dephosphorylation. The last phase involved a gradual and continuous increase in ROS formation paralleling an increase in P-Tyr; levels peaked after 180 min. This might have been caused by the presence of NADH in the mitochondria, which maintains sperm hyperactivation.
Furthermore, de Lamirande et al. [64] confirmed the need for O2− to trigger a cascade of events early on through two key observations. Firstly, spermatozoa production of O2− started as soon as capacitation was induced and slowly declined over the next couple of hours. Secondly, the addition of an O2− scavenger, superoxide dismutase (SOD), did not stop capacitation. However, the same study suggested that a higher concentration of O2− is needed to trigger hyperactivation of spermatozoa and that a subsequent basal level of this free radical is required to ensure that the cell remains in this state. In fact, adding SOD to hyperactivated spermatozoa can reverse this process [62, 64].
In a later study, Donà et al. [65] determined that the threshold level for endogenous ROS ranged between 0.05 and 0.1 RLU (relative light units). If the amount of ROS generation in the sperm falls outside this range, metabolic pathways and subsequently cell function are disrupted. This idea was suggested when studying the effect of apocynin (APO), a NADPH oxidase inhibitor, on capacitation. Sperm suspensions treated with APO prevented free radical generation and caused cell death. On the other hand, addition of ascorbic acid (antioxidant) decreased ROS concentrations and did not induce apoptosis. The cells were maintained in a resting phase—an uncapacitated state ready to be triggered to undergo capacitation.
De Lamirande et al. showed that NO, O2−, and H2O2 play a significant role in the process of capacitation [39, 66]. Minor differences exist in the mode of action between O2− and NO; the latter is generated during the whole process of capacitation and it can activate P-Tyr through cyclic guanosine monophosphate (cGMP) and cAMP simultaneously [67]. Cyclic guanosine monophosphate is a secondary messenger resembling cAMP in its intracellular function. Furthermore, Revelli et al. [68] suggested that NOS are present in human spermatozoa for the production of NO, which is necessary for fertilization.
Nitric oxide and O2− can interact in regulating capacitation. Indeed de Lamirande et al. [39] introduced a new hypothesis of ROS-induced ROS generation implying that NO stimulates O2− formation and vice versa in sperm development. Furthermore, they suggested that these free radicals can react together to form ONOO−, an effector in capacitation. Peroxynitrite does not have any stimulating impact on NO and O2− production [39].
As previously stated, capacitation is associated with cellular changes that include basification of the environment. Indeed, ROS activity can only be achieved under similar conditions. If bicarbonate ions are removed from the medium, ROS production stops [57]. This cannot be reversed by the addition of cAMP or NADPH to bypass the inhibited ROS production. Therefore, the entire AC/cAMP/PKA cascade is sensitive to high pH [69].
Sodium hydrogen antiporter pumps play an important role in maintaining a basic intracellular medium. The free radical O2− was reported to enhance intracellular pH by acting on the Na+/H+ exchanger and stimulating H+ conducting pathways [70].
The extracellular signal regulated pathway (ERK) is also implicated in regulating the capacitation process. Its presence in spermatozoa is widely accepted [71, 72]. The mitogen activated protein kinase (MAPK)/ERK pathway involves an intracellular cascade of proteins activated by the binding of an extracellular mitogen to a membrane receptor. The successive stimulation of secondary messengers drives a signal from the cell surface towards the nucleus where it activates transcription factors for protein expression. The MAPK intracellular cascade causes the sequential stimulation of three protein kinases. The three enzymes of the ERK pathway are Raf (for Serine; Ser/Threonine; Thr phosphorylation), MEK (Dual specific Ser/ Thr and Tyrosine; Tyr) as well as ERK1, ERK2 (for Ser/Thr phosphorylation) [73, 74]. Once a ligand binds to the membrane receptor, Ras (a GTPase) exchanges GDP for a GTP and activates Raf, which phosphorylates the serine residue of MEK, thereby stimulating it. Similarly, MEK phosphorylates Thr and Tyr amino acids within the Thr-Glu-Tyr motif, which is present at the active site of ERK1 and ERK2 [75].
O’Flaherty et al. [76] identified the existence of a dual specificity kinase resembling MEK in the ERK pathway. To prove this theory, they subjected human spermatozoa to an anti-phospho-MEK antibody, which binds to a phosphorylated protein, allowing it to be visualized. They obtained protein bands with 55, 94 and 115 kDa, which clearly differed from MEK1 and MEK2 (45 kDa). Similar results were achieved in separate experiments. The phosphorylation levels of the discovered proteins, named MEK-like proteins, were exaggerated during capacitation. Additionally, inhibitors of MEK and MEK-like proteins (PD98059 and U126) prevented the phosphorylation of Thr-Glu-Tyr proteins in the spermatozoa and impeded capacitation, suggesting that these kinases play a role in regulating sperm maturation [77–79].
Human sperm capacitation induced by FCSu caused phosphorylation of ERK1/2 early on in the experiment (5 min), detected by immunoblotting analysis of the double phosphorylated Thr and Tyr residues in the Thr-Glu-Tyr motif. This trend declined during the next couple of hours [78]. Using the same techniques, Thundathil et al. [77] subjected a sperm preparation to aThr-Glu-Tyr motif specific antibody. Once the added complex interacted with the “antigen,” the initial antibody was exposed to a secondary antibody resulting in an immunoreactive band detected using chemiluminescence. This procedure demonstrated that phosphorylated proteins with the aforementioned motif have a molecular mass of 80 and 105 kDa and are present in the sperm principal piece containing the main portion of the tail. Furthermore, this study confirmed the presence of a higher amount of phosphorylated (P)-Thr-Glu-Tyr-P 60 min after incubation. Various agents mediate this phosphorylation including MEK, MEK-like proteins, receptor type PTK, and the ERK pathway, but not PKA and PKC.
Unlike stimulation of the cAMP pathway, ROS have been shown to possess diverse roles in the regulation of the ERK pathway. According to Thundathil et al. [79], O2− is not involved in phosphorylation of the Thr-Glu-Tyr motif in proteins with a molecular weight of 81 and 105 kDa. In fact, when SOD was introduced to the tested medium, it did not have any impact on the phosphorylation levels of the involved proteins. Similar conclusions were reached focusing on H2O2. Additionally, evidence showed a positive correlation between NO and P-Thr-Glu-Tyr-P. Nitric oxide synthase inhibitors decreased phosphorylation of the motif whereas NONOate—a compound that releases NO upon contact with water—has an opposing effect [79].
Phosphotyrosine kinases (PTKs) are a subgroup of protein kinases, involved in the process of capacitation. In fact, inhibitors of non-receptor type PKC and PKA prevented the phosphorylation of PKA substrates, which is not the case with the addition of inhibitors of receptor type PTK, MEK or PKC [71, 80]. Therefore, PTKs have been implicated in the late phosphorylation of tyrosine residues within the fibrous sheath of spermatozoa.
In a human somatic cell, the Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Protein Kinase B (PKB)/mechanistic target of rapamycin (MTOR) intracellular pathway is important in muscle growth and apoptosis. However, it is a complex and far from completely understood signaling process with a variety of effectors, inhibitors and secondary messengers. For the purpose of this review, only the most important cascades will be presented in a simplified manner.
When an extracellular ligand binds to the membrane receptor tyrosine kinase (RTK), two receptor monomers undergo dimerization followed by autophosphorylation. This structural modification serves to activate PI3K, which may undergo a direct stimulation from the membrane bound Ras-GTP. The kinase acts to phosphorylate phosphatidylinositol 4,5-bisphosphate (PIP2) present within the inner layer of the cell and converts it to phosphatidulinositol (3,4,5)-triphosphate (PIP3). PIP3 can act as a source of phosphate to PKB triggering P-Ser-Thr. Finally, MTOR is stimulated indirectly by PKB.
More recently, PI3K and PKB were identified in spermatozoa [81]. O’Flaherty et al. evaluated the importance of the PI3K/PKB axis in capacitation by studying the effect of Wortmannin (a PI3K inhibitor) and PKB inhibitors in the presence and absence of the capacitation-inducers FCSu and bovine serum albumin (BSA). Capacitation was evaluated by the induction of the AR with lysophosphatidylcholine. The concentration of spermatozoa undergoing the AR declined, which means that capacitation did not occur. The authors concluded that PI3K and PKB are necessary for sperm maturation. To investigate the role of this axis in P-Tyr, the same inhibitors were added to spermatozoa incubated with the similar inducers and two other inducing molecules: NO and H2O2. The experiment detected a decrease in P-Tyr in the spermatozoa that were incubated with BSA and FCSu, but not with NO and H2O2. Similar results were observed regarding P-Thr-Glu-Tyr-P (H2O2 acted like BSA in this case), but PI3K and PKB inhibitors did not affect the MEK-like protein phosphorylation. Consequently, the PI3K/PKB pathway is a mediator of P-Tyr and P-Thr-Glu-Tyr-P, but not P-MEK-like protein modifications. The authors hypothesized that PI3K and PKB act on PKA and ERK secondary messengers involved in capacitation through the phosphoinositide dependent kinase 1 (PDPK1) [72]. The reason behind the conflicting results obtained in the presence of NO can be a consequence of the PKB activation of NOS in order to produce NO [82]. Furthermore, NO and H2O2 were not affected by inhibitors when phosphorylating Tyr/Thr-Glu-Tyr and Tyr, respectively, due to their role downstream of PKB [72].
Acrosome reaction
The AR constitutes the last maturational stage of the spermatozoon in its journey to acquire fertilizing ability. It involves modifications of the anterior part of the spermatozoa head and the release of acrosomal enzymes following contact with the zona pellucida or extracellular layer of the oocyte. Acrosin is among the enzymes secreted in the vicinity of the oocyte during the AR. It is a protease that facilitates spermatozoa penetration of the zona pellucida by digesting this layer.
Following the discovery of the involvement of ROS in regulating capacitation, further studies investigated the prospective role free radicals play in potentiating the AR. Ichikawa et al. [83] compared the effect of small amounts of free radicals in stimulating the exocytosis and activation of acrosin. Samples were collected from normozoospermic and oligozoospermic patients. A cut-off value of 17.46 counts/viable spermatozoa was employed to classify the samples as containing low or high levels of ROS. The authors found a strong correlation between the presence of a small quantity of radical species in the sample and an increase in the number of fully mature spermatozoa. A high level of ROS, irrespective of the sample of origin, produced the opposite effect. However, no relationship was found between the acrosin activity and the level of free radicals. The AR was induced by low temperature, and the spermatozoa that underwent this process were identified with a triple stain technique [84].
Currently, there is no agreement as to which free radical is fully implicated in regulating the AR. On one hand, Aitken et al. [85] demonstrated the essential role H2O2 plays in sperm penetration of zona-free hamster eggs in the presence of Biggers, Whittem, Whittingham (BWW) medium containing A23187 ionophore and BSA. The aforementioned process stopped after catalase, an antioxidant enzyme directed against H2O2, was added. However, the process could be resumed by adding H2O2. At the same time, SOD (an O2− scavenger) did not have any effect on the ongoing experiment. Although H2O2, but not O2−, is required for the AR, no clear evidence was found regarding the source of H2O2 during this process [85]. On the other hand, Griveau et al. [86] recorded a 4- to 5-fold increase in the level of O2− in capacitated spermatozoa cultured in a B2 Menezo medium supplemented with the A23197 ionophore. Furthermore, their results showed a positive association between the O2− amplification and induction of the AR. In the presence of SOD, no change in the free radical level or AR was observed, further strengthening their claim that O2− is the free radical involved in the ionophore-induced AR.
Ultimately, de Lamirande and her team intended to clarify this controversy. In order to detect any variation in the results arising from the utilization of different inducers within the medium, the researchers tested various inducers of the AR including FCSu, follicular fluid ultrafiltrate (FFu), lipid disturbing agent (LDA) and A23187 ionophore. They stimulated the AR only in capacitated spermatozoa, and despite variability in the mechanism of action within the cell, their function was inhibited with the presence of both catalase and O2−. This observation indicates that both O2− and H2O2 play a role in the AR, which contradicts the results of the two previous studies [87].
Capacitation and AR occur in quick succession during sperm maturation. ROS regulatory pathways involved in capacitation, although not completely elucidated, are better understood than the intracellular cascades stimulated by free radicals in triggering the AR. ROS may be involved in the P-Tyr of the sperm proteins. In one of the earliest studies by Aitken et al. [85], a number of proteins were phosphorylated at their tyrosine residue after incubation for 3 h in BWW medium supplemented with human serum albumin and A23187. Antiphosphate antibody and western blotting showed different bands of proteins corresponding to different molecular weights (222, 220, 159, 133, 116, 82 kDa). One important limitation encountered in this study was the lack of a clear differentiation between capacitation and the AR as a cause for this phosphorylation [85]. A similar study was performed using the same medium and supplements. The results showed phosphorylation of two proteins (116 and 100 kDa) in capacitated spermatozoa [69]. Furthermore, capacitated spermatozoa stimulated by the presence of platelet-activated factor or progesterone exhibited? tyrosine amplification in proteins with a molecular weight of 75 and 97 kDa [88]. de Lamirande et al. determined that the Tyr-P proteins associated with induction of the AR in the presence of LDA and A23187 involved bands with a molecular weight of 70, 76, 81 and 105 kDa. The authors suggested the presence of a common pathway through which ROS regulate capacitation and the AR leading to P-Tyr of similar proteins. This hypothesis was based on the fact that both maturation processes were shown to be regulated by free radicals and result in phosphorylation of the same proteins [87]. Tyrosine-P of A-kinase anchor proteins (AKAPs) of 81 and 105 kDa raises questions about the role of fibrous sheath proteins in regulating the AR. During capacitation, these proteins were involved in hyperactivation as mentioned earlier. Inhibition of AKAP, but not PKA, resulted in decreased spermatozoa motility. Therefore, AKAP function in the cell is not limited to binding PKA to the fibrous sheath, but it is also involved in the regulation of intracellular pathways [89].
Reactive oxygen species may stimulate a variety of targets, including AC, PKC, and phospholipase A2 (PLA2), which have been shown to play a role in the AR [90–92].
Phospholipase A2 is an enzyme that catalyzes the release of fatty acid by cleaving the second carbon of the triglycerol backbone and is activated by phosphorylation. It was shown that both O2− and H2O2 may activate PLA2 [93]. The role played by ROS in inhibiting phosphatases has been described previously. When phosphatases are downregulated, PLA2 remains activated due to the presence of an attached phosphate group not removed by the weak enzyme [94]. Furthermore, phosphorylation and a subsequent activation of PLA2 may be achieved by a cascade of events involving ROS stimulating PTK, which will act upon PKC, leading to P-PLA2 and sperm membrane degradation [50, 95, 96] (see Fig. 2).
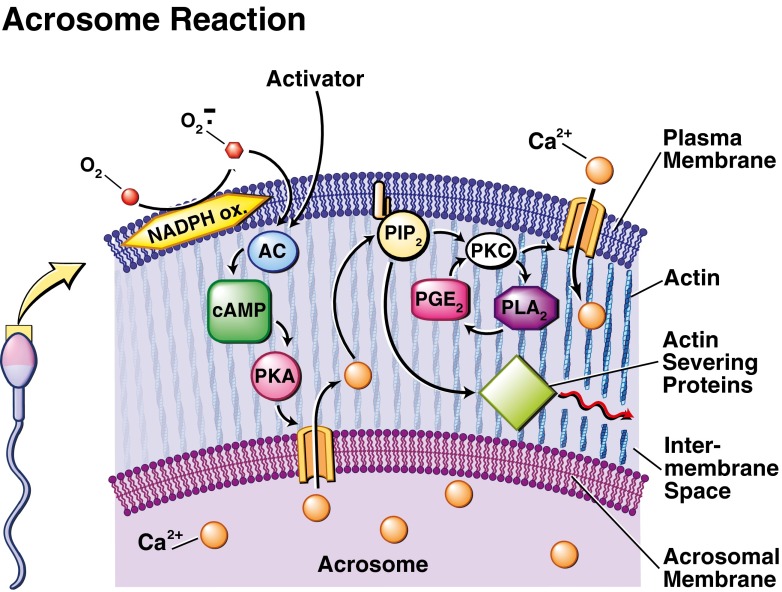
Fig. 2.
Biochemical pathway proposed to regulate the acrosome reaction (AR). Induction of the AR can occur by physiological and non-physiological activators, including the zona pellucida (ZP), progesterone, or reactive oxygen species (ROS). Subsequent release of Ca2+ from the acrosomal calcium store generated during capacitation causes the cleavage of phosphatidylinosital-4,5-biphosphate (PIP2), which forms diacylglycerol (DAG) and inosital triphosphate (IP3). The latter activates actin-severing proteins, which leads to the fusion of the acrosomal and plasma membranes, and eventual acrosomal exocytosis. DAG later activates protein kinase C (PKC), causing a second, greater influx of Ca2+ and activation of phospholipase A2 (PLA2). The release of large amounts of membrane fatty acids increases the fluidity of the plasma membrane necessary for later fusion with the oocyte
Griveau et al. [86] indicated that treatment of capacitated spermatozoa with potassium superoxide resulted in an oxidative insult to the plasma membrane by O2− and a subsequent de-esterification of the membrane phospholipids [86]. Activation of PLA2 leads to increased fluidity of the sperm plasma membrane, which means it plays an important function in the regulation of AR and contributes to successful sperm-oocyte fusion [86, 94].
Other stages of spermatozoa maturation requiring physiological levels of ROS
Following capacitation, spermatozoa migrate through the female reproductive tract against a concentration gradient of progesterone released by the cumulus cells surrounding the oocyte, taking advantage of chemotaxis. Sanchez et al. suggested that this phenomenon could be regulated by redox reactions. A short co-culture of spermatozoa with a ROS-inducer phorbolmyristateacetate (PMA) or PMA in combination with catalase did not result in a significant change in the process of chemotaxis at time zero. At the same time, spermatozoa culture supplemented with catalase only revealed a lower percentage of chemotactic cells.
On the other hand, a longer in vitro incubation revealed that PMA supplementation resulted in a lower number of chemotactic spermatozoa compared to the control or PMA with catalase. At the same time, the presence of catalase prevented this decrease. The results obtained suggest that a threshold of ROS must exist for normal chemotaxis to occur. High levels of ROS caused a state of oxidative stress leading to a lower percentage of chemotactic spermatozoa [11].
Measurement of the ROS threshold
Various research groups focused on establishing a reference value for seminal ROS in order to help predict fertility outcomes. However, these cut-off values did not correspond when the results from different studies were compared. This is probably due to the small sample size of patients included in a specific study, the involvement of patients with different pathogenesis, or the use of non-standardized assays to determine ROS [97]. Das et al. [98] determined a cut-off value of 0.075 × 106 counts per minute (cpm) per million spermatozoa, above which ROS cause a significant drop in fertilization and pregnancy outcomes [98]. However Desai et al. [99] concluded that a value of 0.0185 × 106 cpm/million spermatozoa was needed to distinguish between fertile and infertile men. Subsequently, the same group devised a new method of reporting ROS levels in semen. The reference value was initially set at <20 RLU/s/106 spermatozoa [100] and was recently revised to 93 RLU/s/106 spermatoza (unpublished data).
Conclusion
Maintaining a physiological level of ROS in spermatozoa and their surrounding environment is of paramount importance. Indeed, a proper physiological amount of ROS is necessary for the successful lifecycle journey of spermatozoa i.e. from production to fertilization of the oocyte. Along the way, redox reactions act as essential cofactors in the development of fully mature spermatozoa. Free radicals ensure the morphological reshaping of spermatozoa by activating intracellular pathways leading to activating processes such as chromatin condensation, motility, capacitation, acrosome reaction, and chemotaxis. Conversely, excessive amounts of ROS have pathological effects on spermatozoa ranging from diminished sperm concentration, decreased motility and reduced fertilization to apoptosis. ROS therefore act as a double edge sword. Finally we can say that the acquisition of male fertilization capacity is achieved by well balanced processes of formation and degradation of ROS acting at specific moments during the “life” of spermatozoa.
Despite the extensive research performed in this field, more studies are needed to identify the proteins that are expressed and phosphorylated in spermatozoa. Furthermore, scientists need to understand how ROS activate the intracellular machinery. A better understanding of the delicate oxidative balance affecting sperm function and regulation could be of paramount importance in the diagnosis, prevention and treatment of male infertility.
Acknowledgments
This study was supported by the financial support from the Center for Reproductive Medicine, Cleveland Clinic Foundation.
Disclosure
The authors declare that they have no relevant financial or competing interests.
Footnotes
Capsule This review discusses specific roles, mechanisms of action, and effects of reactive oxygen species (ROS) on the acquisition of structural and physiological properties of mammalian spermatozoa.
Contributor Information
Stefan S. Du Plessis, Email: ssdp@sun.ac.za
Ashok Agarwal, Phone: (216) 444-9485, Email: agarwaa@ccf.org.
Jacques Halabi, Email: jacqueshalbi@gmail.com.
Eva Tvrda, Email: evina.tvrda@gmail.com.
References
- 1.Jarow JP, Sharlip ID, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. J Urol. 2002;167(5):2138–44. [PubMed] [Google Scholar]
- 2.Agarwal A, Sharma RK, Sharma R, Assidi M, Abuzenadah AM, Alshahrani S, et al. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod Biol Endocrinol. 2014;12:33. doi: 10.1186/1477-7827-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khosrowbeygi A, Zarghami N. Levels of oxidative stress biomarkers in seminal plasma and their relationship with seminal parameters. BMC Clin Pathol. 2007;7:6. doi: 10.1186/1472-6890-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLeod J. The role of oxygen in the metabolism and motility of human spermatozoa. Am J Physiol. 1943;138:512–518. [Google Scholar]
- 5.Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of ROS by human spermatozoa. J Reprod Fertil. 1987;81:459–469. doi: 10.1530/jrf.0.0810459. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Mulgund A, Sharma R, Sabanegh E. Mechanisms of oligozoospermia: an oxidative stress perspective. Syst Biol Reprod Med. 2014;60(4):206–216. doi: 10.3109/19396368.2014.918675. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal A, Tvrda E, Sharma R. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol. 2014;12:45. doi: 10.1186/1477-7827-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod. 1997;2(1):48–54. doi: 10.1530/ror.0.0020048. [DOI] [PubMed] [Google Scholar]
- 9.de Lamirande E, Gagnon C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic Biol Med. 1993;14(2):157–66. doi: 10.1016/0891-5849(93)90006-g. [DOI] [PubMed] [Google Scholar]
- 10.Aitken RJ, Irvine DS, Wu FC. Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am J Obstet Gynecol. 1991;164(2):542–51. doi: 10.1016/s0002-9378(11)80017-7. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez R, Sepulveda C, Risopatron J, Villegas J, Giojalas LC. Human sperm chemotaxis depends on critical levels of reactive oxygen species. Fertil Steril. 2010;93(1):150–3. doi: 10.1016/j.fertnstert.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 12.Ford WC. Regulation of sperm function by reactive oxygen species. Hum Reprod Update. 2004;10(5):387–99. doi: 10.1093/humupd/dmh034. [DOI] [PubMed] [Google Scholar]
- 13.Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Lett. 2000;486(1):10–3. doi: 10.1016/s0014-5793(00)02197-9. [DOI] [PubMed] [Google Scholar]
- 14.Koppenol WH. The Haber-Weiss cycle—70 years later. Redox Rep. 2001;6(4):229–34. doi: 10.1179/135100001101536373. [DOI] [PubMed] [Google Scholar]
- 15.Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta. 1999;1411(2–3):273–89. doi: 10.1016/s0005-2728(99)00020-1. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B, Gutteridge JM. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986;246(2):501–14. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- 17.Goldfarb AH. Nutritional antioxidants as therapeutic and preventive modalities in exercise-induced muscle damage. Can J Appl Physiol. 1999;24(3):249–66. doi: 10.1139/h99-021. [DOI] [PubMed] [Google Scholar]
- 18.Smith DC, Barratt CL, Williams MA. The characterisation of non-sperm cells in the ejaculates of fertile men using transmission electron microscopy. Andrologia. 1989;21(4):319–33. [PubMed] [Google Scholar]
- 19.Fisher HM, Aitken RJ. Comparative analysis of the ability of precursor germ cells and epididymal spermatozoa to generate reactive oxygen metabolites. J Exp Zool. 1997;277(5):390–400. doi: 10.1002/(sici)1097-010x(19970401)277:5<390::aid-jez5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 20.Whittington K, Ford WC. Relative contribution of leukocytes and of spermatozoa to reactive oxygen species production in human sperm suspensions. Int J Androl. 1999;22(4):229–35. doi: 10.1046/j.1365-2605.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- 21.Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Nada EA, et al. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil Steril. 2002;78(6):1215–24. doi: 10.1016/s0015-0282(02)04237-1. [DOI] [PubMed] [Google Scholar]
- 22.Shekarriz M, Sharma RK, Thomas AJ, Jr, Agarwal A. Positive myeloperoxidase staining (Endtz test) as an indicator of excessive reactive oxygen species formation in semen. J Assist Reprod Genet. 1995;12(2):70–4. doi: 10.1007/BF02211372. [DOI] [PubMed] [Google Scholar]
- 23.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 24.Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg HR, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83(3):635–42. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Wolff H, Politch JA, Martinez A, Haimovici F, Hill JA, Anderson DJ. Leukocytospermia is associated with poor semen quality. Fertil Steril. 1990;53(3):528–36. [PubMed] [Google Scholar]
- 26.Mahfouz R, Sharma R, Thiyagarajan A, Kale V, Gupta S, Sabanegh E, et al. Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertil Steril. 2010;94(6):2141–6. doi: 10.1016/j.fertnstert.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Sharma RK, Pasqualotto AE, Nelson DR, Thomas AJ, Jr, Agarwal A. Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. J Androl. 2001;22(4):575–83. [PubMed] [Google Scholar]
- 28.Plante M, de Lamirande E, Gagnon C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil Steril. 1994;62(2):387–93. doi: 10.1016/s0015-0282(16)56895-2. [DOI] [PubMed] [Google Scholar]
- 29.Potts JM, Pasqualotto FF. Seminal oxidative stress in patients with chronic prostatitis. Andrologia. 2003;35(5):304–8. [PubMed] [Google Scholar]
- 30.Babior BM. NADPH oxidase: an update. Blood. 1999;93(5):1464–76. [PubMed] [Google Scholar]
- 31.Wolff H. The biologic significance of white blood cells in semen. Fertil Steril. 1995;63(6):1143–57. doi: 10.1016/s0015-0282(16)57588-8. [DOI] [PubMed] [Google Scholar]
- 32.Ochsendorf FR. Infections in the male genital tract and reactive oxygen species. Hum Reprod Update. 1999;5(5):399–420. doi: 10.1093/humupd/5.5.399. [DOI] [PubMed] [Google Scholar]
- 33.Aitken RJ, Fisher HM, Fulton N, Gomez E, Knox W, Lewis B, et al. Reactive oxygen species generation by human spermatozoa is induced by exogenous NADPH and inhibited by the flavoprotein inhibitors diphenylene iodonium and quinacrine. Mol Reprod Dev. 1997;47(4):468–82. doi: 10.1002/(SICI)1098-2795(199708)47:4<468::AID-MRD14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 34.Aitken J, Krausz C, Buckingham D. Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions. Mol Reprod Dev. 1994;39(3):268–79. doi: 10.1002/mrd.1080390304. [DOI] [PubMed] [Google Scholar]
- 35.Storey BT, Alvarez JG, Thompson KA. Human sperm glutathione reductase activity in situ reveals limitation in the glutathione antioxidant defense system due to supply of NADPH. Mol Reprod Dev. 1998;49(4):400–7. doi: 10.1002/(SICI)1098-2795(199804)49:4<400::AID-MRD7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 36.Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, Thomas AJ, Jr, et al. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod. 2001;16(9):1922–30. doi: 10.1093/humrep/16.9.1922. [DOI] [PubMed] [Google Scholar]
- 37.Gavella M, Lipovac V. NADH-dependent oxidoreductase (diaphorase) activity and isozyme pattern of sperm in infertile men. Arch Androl. 1992;28(2):135–41. doi: 10.3109/01485019208987691. [DOI] [PubMed] [Google Scholar]
- 38.Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab. 2008;93(8):3199–207. doi: 10.1210/jc.2007-2616. [DOI] [PubMed] [Google Scholar]
- 39.de Lamirande E, Lamothe G. Reactive oxygen-induced reactive oxygen formation during human sperm capacitation. Free Radic Biol Med. 2009;46(4):502–10. doi: 10.1016/j.freeradbiomed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Hipler UC, Görnig M, Hipler B, Römer W, Schreiber G. Stimulation and scavestrogen-induced inhibition of reactive oxygen species generated by rat sertoli cells. Arch Androl. 2000;44(2):147–54. doi: 10.1080/014850100262326. [DOI] [PubMed] [Google Scholar]
- 41.Lilja H, Lundwall A. Molecular cloning of epididymal and seminal vesicular transcripts encoding a semenogelin-related protein. Proc Natl Acad Sci U S A. 1992;89(10):4559–63. doi: 10.1073/pnas.89.10.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lundwall A, Bjartell A, Olsson AY, Malm J. Semenogelin I and II, the predominant human seminal plasma proteins, are also expressed in non-genital tissues. Mol Hum Reprod. 2002;8(9):805–10. doi: 10.1093/molehr/8.9.805. [DOI] [PubMed] [Google Scholar]
- 43.Hamada A, Sharma R, du Plessis SS, Willard B, Yadav SP, Sabanegh E, et al. Two-dimensional differential in-gel electrophoresis-based proteomics of male gametes in relation to oxidative stress. Fertil Steril. 2013;99(5):1216–26. doi: 10.1016/j.fertnstert.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 44.Chatterjee S, Laloraya M, Kumar PG. Free radical-induced liquefaction of ejaculated human semen: a new dimension in semen biochemistry. Arch Androl. 1997;38(2):107–11. doi: 10.3109/01485019708987887. [DOI] [PubMed] [Google Scholar]
- 45.Du Plessis SS, Agarwal A, Mohanty G, Van der Linde M. Oxidative phosphorylation versus glycolysis: what fuel do spermatozoa use? Asian J Androl. 2015;17:1–6. doi: 10.4103/1008-682X.135123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod. 1997;3(3):175–94. doi: 10.1093/molehr/3.3.175. [DOI] [PubMed] [Google Scholar]
- 47.Baldi E, Casano R, Falsetti C, Krausz C, Maggi M, Forti G. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J Androl. 1991;12(5):323–30. [PubMed] [Google Scholar]
- 48.Guraya SS. Cellular and molecular biology of capacitation and acrosome reaction in spermatozoa. Int Rev Cytol. 2000;199:1–64. doi: 10.1016/s0074-7696(00)99001-6. [DOI] [PubMed] [Google Scholar]
- 49.López-González I, Torres-Rodríguez P, Sánchez-Carranza O, Solís-López A, Santi CM, Darszon A, et al. Membrane hyperpolarization during human sperm capacitation. Mol Hum Reprod. 2014;20(7):619–29. doi: 10.1093/molehr/gau029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breitbart H. Signaling pathways in sperm capacitation and acrosome reaction. Cell Mol Biol. 2003;49(3):321–7. [PubMed] [Google Scholar]
- 51.Liguori L, de Lamirande E, Minelli A, Gagnon C. Various protein kinases regulate human sperm acrosome reaction and the associated phosphorylation of Tyr residues and of the Thr-Glu-Tyr motif. Mol Hum Reprod. 2005;11(3):211–21. doi: 10.1093/molehr/gah154. [DOI] [PubMed] [Google Scholar]
- 52.Olds-Clarke P. Unresolved issues in mammalian fertilization. Int Rev Cytol. 2003;232:129–84. doi: 10.1016/s0074-7696(03)32004-2. [DOI] [PubMed] [Google Scholar]
- 53.de Lamirande E, O’Flaherty C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta. 2008;1784(1):106–15. doi: 10.1016/j.bbapap.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 54.Kothari S, Thompson A, Agarwal A, du Plessis SS. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol. 2010;48(5):425–35. [PubMed] [Google Scholar]
- 55.Leclerc P, de Lamirande E, Gagnon C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med. 1997;22(4):643–56. doi: 10.1016/s0891-5849(96)00379-6. [DOI] [PubMed] [Google Scholar]
- 56.Donà G, Fiore C, Tibaldi E, Frezzato F, Andrisani A, Ambrosini G, et al. Endogenous reactive oxygen species content and modulation of tyrosine phosphorylation during sperm capacitation. Int J Androl. 2011;34(5 Pt 1):411–9. doi: 10.1111/j.1365-2605.2010.01097.x. [DOI] [PubMed] [Google Scholar]
- 57.Aitken RJ, Harkiss D, Knox W, Paterson M, Irvine DS. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci. 1998;111(Pt 5):645–56. doi: 10.1242/jcs.111.5.645. [DOI] [PubMed] [Google Scholar]
- 58.Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3′,5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod. 1996;55(3):684–92. doi: 10.1095/biolreprod55.3.684. [DOI] [PubMed] [Google Scholar]
- 59.Dimitriadis F, Giannakis D, Pardalidis N, Zikopoulos K, Paraskevaidis E, Giotitsas N, et al. Effects of phosphodiesterase-5 inhibitors on sperm parameters and fertilizing capacity. Asian J Androl. 2008;10(1):115–33. doi: 10.1111/j.1745-7262.2008.00373.x. [DOI] [PubMed] [Google Scholar]
- 60.Lefièvre L, Jha KN, de Lamirande E, Visconti PE, Gagnon C. Activation of protein kinase A during human sperm capacitation and acrosome reaction. J Androl. 2002;23(5):709–16. [PubMed] [Google Scholar]
- 61.Breitbart H. Intracellular calcium regulation in sperm capacitation and acrosomal reaction. Mol Cell Endocrinol. 2002;187(1–2):139–44. doi: 10.1016/s0303-7207(01)00704-3. [DOI] [PubMed] [Google Scholar]
- 62.Leclerc P, de Lamirande E, Gagnon C. Interaction between Ca2+, cyclic 3′,5′ adenosine monophosphate, the superoxide anion, and tyrosine phosphorylation pathways in the regulation of human sperm capacitation. J Androl. 1998;19(4):434–43. [PubMed] [Google Scholar]
- 63.Hecht D, Zick Y. Selective inhibition of protein tyrosine phosphatase activities by H2O2 and vanadate in vitro. Biochem Biophys Res Commun. 1992;188(2):773–9. doi: 10.1016/0006-291x(92)91123-8. [DOI] [PubMed] [Google Scholar]
- 64.de Lamirande E, Harakat A, Gagnon C. Human sperm capacitation induced by biological fluids and progesterone, but not by NADH or NADPH, is associated with the production of superoxide anion. J Androl. 1998;19(2):215–25. [PubMed] [Google Scholar]
- 65.Donà G, Fiore C, Andrisani A, Ambrosini G, Brunati A, Ragazzi E, et al. Evaluation of correct endogenous reactive oxygen species content for human sperm capacitation and involvement of the NADPH oxidase system. Hum Reprod. 2011;26(12):3264–73. doi: 10.1093/humrep/der321. [DOI] [PubMed] [Google Scholar]
- 66.de Lamirande E, Lamothe G, Villemure M. Control of superoxide and nitric oxide formation during human sperm capacitation. Free Radic Biol Med. 2009;46(10):1420–7. doi: 10.1016/j.freeradbiomed.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 67.Herrero MB, de Lamirande E, Gagnon C. Nitric oxide regulates human sperm capacitation and protein-tyrosine phosphorylation in vitro. Biol Reprod. 1999;61(3):575–81. doi: 10.1095/biolreprod61.3.575. [DOI] [PubMed] [Google Scholar]
- 68.Revelli A, Soldati G, Costamagna C, Pellerey O, Aldieri E, Massobrio M, et al. Follicular fluid proteins stimulate nitric oxide (NO) synthesis in human sperm: a possible role for NO in acrosomal reaction. J Cell Physiol. 1999;178(1):85–92. doi: 10.1002/(SICI)1097-4652(199901)178:1<85::AID-JCP11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 69.Aitken RJ, Harkiss D, Knox W, Paterson M, Irvine S. On the cellular mechanisms by which the bicarbonate ion mediates the extragenomic action of progesterone on human spermatozoa. Biol Reprod. 1998;58(1):186–96. doi: 10.1095/biolreprod58.1.186. [DOI] [PubMed] [Google Scholar]
- 70.Demaurex N, Downey GP, Waddell TK, Grinstein S. Intracellular pH regulation during spreading of human neutrophils. J Cell Biol. 1996;133(6):1391–402. doi: 10.1083/jcb.133.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic Biol Med. 2006;41(4):528–40. doi: 10.1016/j.freeradbiomed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 72.O’Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic Biol Med. 2006;40(6):1045–55. doi: 10.1016/j.freeradbiomed.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 73.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- 74.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79(1):143–80. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 75.Zhou G, Bao ZQ, Dixon JE. Components of a new human protein kinase signal transduction pathway. J Biol Chem. 1995;270(21):12665–9. doi: 10.1074/jbc.270.21.12665. [DOI] [PubMed] [Google Scholar]
- 76.O’Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species and protein kinases modulate the level of phospho-MEK-like proteins during human sperm capacitation. Biol Reprod. 2005;73(1):94–105. doi: 10.1095/biolreprod.104.038794. [DOI] [PubMed] [Google Scholar]
- 77.Thundathil J, de Lamirande E, Gagnon C. Different signal transduction pathways are involved during human sperm capacitation induced by biological and pharmacological agents. Mol Hum Reprod. 2002;8(9):811–6. doi: 10.1093/molehr/8.9.811. [DOI] [PubMed] [Google Scholar]
- 78.de Lamirande E, Gagnon C. The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol Hum Reprod. 2002;8(2):124–35. doi: 10.1093/molehr/8.2.124. [DOI] [PubMed] [Google Scholar]
- 79.Thundathil J, de Lamirande E, Gagnon C. Nitric oxide regulates the phosphorylation of the threonine-glutamine-tyrosine motif in proteins of human spermatozoa during capacitation. Biol Reprod. 2003;68(4):1291–8. doi: 10.1095/biolreprod.102.008276. [DOI] [PubMed] [Google Scholar]
- 80.O’Flaherty C, de Lamirande E, Gagnon C. Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: modulation and protein kinase A dependency. Mol Hum Reprod. 2004;10(5):355–63. doi: 10.1093/molehr/gah046. [DOI] [PubMed] [Google Scholar]
- 81.Nauc V, De Lamirande E, Leclerc P, Gagnon C. Inhibitors of phosphoinositide 3-kinase, LY294002 and wortmannin, affect sperm capacitation and associated phosphorylation of proteins differently: Ca2+-dependent divergences. J Androl. 2004;25(4):573–85. doi: 10.1002/j.1939-4640.2004.tb02828.x. [DOI] [PubMed] [Google Scholar]
- 82.Wymann MP, Marone R. Phosphoinositide 3-kinase in disease: timing, location, and scaffolding. Curr Opin Cell Biol. 2005;17(2):141–9. doi: 10.1016/j.ceb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 83.Ichikawa T, Oeda T, Ohmori H, Schill WB. Reactive oxygen species influence the acrosome reaction but not acrosin activity in human spermatozoa. Int J Androl. 1999;22(1):37–42. doi: 10.1046/j.1365-2605.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- 84.Talbot P, Chacon RS. A triple-stain technique for evaluating normal acrosome reactions of human sperm. J Exp Zool. 1981;215(2):201–8. doi: 10.1002/jez.1402150210. [DOI] [PubMed] [Google Scholar]
- 85.Aitken RJ, Paterson M, Fisher H, Buckingham DW, van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci. 1995;108(Pt 5):2017–25. doi: 10.1242/jcs.108.5.2017. [DOI] [PubMed] [Google Scholar]
- 86.Griveau JF, Renard P, Le Lannou D. Superoxide anion production by human spermatozoa as a part of the ionophore-induced acrosome reaction process. Int J Androl. 1995;18(2):67–74. doi: 10.1111/j.1365-2605.1995.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 87.de Lamirande E, Tsai C, Harakat A, Gagnon C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J Androl. 1998;19(5):585–94. [PubMed] [Google Scholar]
- 88.Luconi M, Bonaccorsi L, Krausz C, Gervasi G, Forti G, Baldi E. Stimulation of protein tyrosine phosphorylation by platelet-activating factor and progesterone in human spermatozoa. Mol Cell Endocrinol. 1995;108(1–2):35–42. doi: 10.1016/0303-7207(95)92576-a. [DOI] [PubMed] [Google Scholar]
- 89.Vijayaraghavan S, Goueli SA, Davey MP, Carr DW. Protein kinase A-anchoring inhibitor peptides arrest mammalian sperm motility. J Biol Chem. 1997;272(8):4747–52. doi: 10.1074/jbc.272.8.4747. [DOI] [PubMed] [Google Scholar]
- 90.Tan CM, Xenoyannis S, Feldman RD. Oxidant stress enhances adenylyl cyclase activation. Circ Res. 1995;77(4):710–7. doi: 10.1161/01.res.77.4.710. [DOI] [PubMed] [Google Scholar]
- 91.Gopalakrishna R, McNeill TH, Elhiani AA, Gundimeda U. Methods for studying oxidative regulation of protein kinase C. Methods Enzymol. 2013;528:79–98. doi: 10.1016/B978-0-12-405881-1.00005-7. [DOI] [PubMed] [Google Scholar]
- 92.Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J Physiol Pharmacol. 2013;64(4):409–21. [PubMed] [Google Scholar]
- 93.Sawada M, Carlson JC. Rapid plasma membrane changes in superoxide radical formation, fluidity, and phospholipase A2 activity in the corpus luteum of the rat during induction of luteolysis. Endocrinology. 1991;128(6):2992–8. doi: 10.1210/endo-128-6-2992. [DOI] [PubMed] [Google Scholar]
- 94.Goldman R, Ferber E, Zort U. Reactive oxygen species are involved in the activation of cellular phospholipase A2. FEBS Lett. 1992;309(2):190–2. doi: 10.1016/0014-5793(92)81092-z. [DOI] [PubMed] [Google Scholar]
- 95.Zor U, Ferber E, Gergely P, Szücs K, Dombrádi V, Goldman R. Reactive oxygen species mediate phorbol ester-regulated tyrosine phosphorylation and phospholipase A2 activation: potentiation by vanadate. Biochem J. 1993;295(Pt 3):879–88. doi: 10.1042/bj2950879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu J, Yu S, Sun AY, Sun GY. Oxidant-mediated AA release from astrocytes involves cPLA(2) and iPLA(2) Free Radic Biol Med. 2003;34(12):1531–43. doi: 10.1016/s0891-5849(03)00152-7. [DOI] [PubMed] [Google Scholar]
- 97.Chen SJ, Allam JP, Duan YG, Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet. 2013;288(1):191–9. doi: 10.1007/s00404-013-2801-4. [DOI] [PubMed] [Google Scholar]
- 98.Das S, Chattopadhyay R, Jana SK, Narendra BK, Chakraborty C, Chakravarty B, et al. Cut-off value of reactive oxygen species for predicting semen quality and fertilization outcome. Syst Biol Reprod Med. 2008;54(1):47–54. doi: 10.1080/19396360701883274. [DOI] [PubMed] [Google Scholar]
- 99.Desai N, Sharma R, Makker K, Sabanegh E, Agarwal A. Physiologic and pathologic levels of reactive oxygen species in neat semen of infertile men. Fertil Steril. 2009;92(5):1626–31. doi: 10.1016/j.fertnstert.2008.08.109. [DOI] [PubMed] [Google Scholar]
- 100.Kashou AH, Sharma R, Agarwal A. Assessment of oxidative stress in sperm and semen. Methods Mol Biol. 2013;927:351–61. doi: 10.1007/978-1-62703-038-0_30. [DOI] [PubMed] [Google Scholar]