Abstract
Purpose
Obesity is a growing public health concern now reaching epidemic status worldwide for children and adults due to multiple problems impacting on energy intake and expenditure with influences on human reproduction and infertility. A positive family history and genetic factors are known to play a role in obesity by influencing eating behavior, weight and level of physical activity and also contributing to human reproduction and infertility. Recent advances in genetic technology have led to discoveries of new susceptibility genes for obesity and causation of infertility. The goal of our study was to provide an update of clinically relevant candidate and known genes for obesity and infertility using high resolution chromosome ideograms with gene symbols and tabular form.
Methods
We used computer-based internet websites including PubMed to search for combinations of key words such as obesity, body mass index, infertility, reproduction, azoospermia, endometriosis, diminished ovarian reserve, estrogen along with genetics, gene mutations or variants to identify evidence for development of a master list of recognized obesity genes in humans and those involved with infertility and reproduction. Gene symbols for known and candidate genes for obesity were plotted on high resolution chromosome ideograms at the 850 band level. Both infertility and obesity genes were listed separately in alphabetical order in tabular form and those highlighted when involved with both conditions.
Results
By searching the medical literature and computer generated websites for key words, we found documented evidence for 370 genes playing a role in obesity and 153 genes for human reproduction or infertility. The obesity genes primarily affected common pathways in lipid metabolism, deposition or transport, eating behavior and food selection, physical activity or energy expenditure. Twenty-one of the obesity genes were also associated with human infertility and reproduction. Gene symbols were plotted on high resolution ideograms and their name, precise chromosome band location and description were summarized in tabular form.
Conclusions
Meaningful correlations in the obesity phenotype and associated human infertility and reproduction are represented with the location of genes on chromosome ideograms along with description of the gene and position in tabular form. These high resolution chromosome ideograms and tables will be useful in genetic awareness and counseling, diagnosis and treatment to improve clinical outcomes.
Keywords: Obesity, Obesity susceptibility genes, Gene symbols, Human infertility and reproduction, High resolution chromosome ideogram
Introduction
Obesity is a major public health concern and reaching epidemic status worldwide for both children and adults. Without intervention, estimates of 2 billion overweight and 1 billion obese individuals will be present by the year 2030 [1]. The worldwide prevalence of childhood overweight and obesity from 1990 to 2010 had increased from 4.2 to 6.7 % and is expected to reach over 9 % by 2020 [2]. Obesity now affects 35.7 % of the US population (aged 20 years and over [reproductive years]) and 61 % are overweight [3–5]. Correspondingly, 10–15 % of the US population experiences infertility with women in their mid-thirties having greater than 25 % chance of being infertile [6]. Both obesity and infertility are relatively prevalent conditions and clearly influenced by genetic and environmental factors. Increased weight gain during infancy does predispose to obesity later in life with an increased rate of developing type 2 diabetes, nonalcoholic fatty liver disease, cancer, sleep apnea, hypertension and infertility. The consequences of increased weight and obesity can shorten life expectancy as well as affecting reproduction with dysfunction in ovulation, spontaneous abortions, and overall infertility [3–7]. Adverse pregnancy outcomes including preeclampsia, fetal growth failure with premature delivery and gestational diabetes [8]. The prevalence of maternal obesity in the US population is increased with more women having obesity-related reproductive problems [3]. Gradual and sustained maternal weight loss is needed to improve menstrual cycles and ovulation and thus reproductive rates and outcomes [9]. Weight loss is considered the first line of treatment in those women with reproductive failure and obesity-related infertility [3, 9]. Bariatric surgical procedures and drug therapy options may also be considered in obese females to improve the likelihood of conception and delivery [10]. One of the most common causes of subfertility in women with obesity is polycystic ovarian syndrome which is associated with the androgen receptor (AR) gene [11]. Hence, obesity and infertility may have genes in common and their identification may provide insight into treatment options requiring further studies. One major goal of our study was to identify such genes and their relationship in both conditions.
Unhealthy calorically-dense diets, decreased physical activity and other common environmental causes of childhood obesity in western society illustrate an interaction with a complex obesogenic environment. A positive family history for obesity is a well-established risk predictor. For example, a child has a 2.5 to 4-fold higher risk of developing obesity if one parent is obese and a 10-fold risk if both parents are obese compared to both parents having a normal weight [12]. Strong heritability estimates for obesity and body mass index (BMI) indicate the role of genetics supported by twin and family studies. In twin studies, heritability estimates for BMI are between 20 and 86 % with an average of about 50 % in children and adults [13–16]. The definition of heritability is a measure of the fraction of the phenotypic variability that can be attributed to genetic variation or the relative contributions of genetic and non-genetic differences to the total phenotypic variation in a population. The highest heritability estimates are found in childhood obesity indicating the importance of genetic factors early in life. Important genetic influences have been found for body fat percentage, waist circumference, eating behavior, level of physical activity or energy expenditure [17–19].
Genetic forms of obesity can be grouped into Mendelian or single gene forms of obesity including recessive forms, partial gene deficiencies, genomic structural variations or copy number variants and polygenic forms [20–22]. Monogenic forms or single gene conditions causing obesity have been reported for at least eight genes including leptin (LEP), leptin receptor (LEPR), proopiomelanocortin (POMC), prohormone convertase 1 (PCSK1), melanocortin 4 receptor (MC4R), single-minded homolog 1 (SIM1), brain-derived neurotrophic factor (BDNF) and the neurotrophic tyrosine kinase receptor type 2 gene (NTRK2) [20, 21]. The hypothalamic leptin-melanocortin system is critical for regulating energy balance with disturbances leading to severe obesity disorders [20–25]. The latest update of the 2005 Human Obesity Gene Map reported in 2006 summarized 127 candidate genes for obesity and obesity-related traits [24].
Several obesity-related syndromic genetic disorders are identified in humans, both common and rare, but monogenic causes of morbid obesity are uncommon in the general population. Obesity and eating behavior (hyperphagia) are key features of several rare genetic syndromes including Prader-Willi, Alström, Bardet-Biedl, Albright hereditary osteodystrophy, Cohen and fragile X syndromes with recognized genes playing a role (e.g., SNRPN for Prader-Willi syndrome, GNAS1 for Albright hereditary osteodystrophy, FMR1 for fragile X syndrome) [25, 26]. Understanding the regulatory and molecular basis of these disorders should provide a better picture of mechanisms controlling food intake and energy balance in humans in the general population including epigenetics affecting gene expression without altering the DNA sequence. Environmental exposure or nutrition during critical periods of development can affect the epigenetic marks at the genome level with failures in imprinting or regulation of gene activity if they are genetic predispositions leading to extreme forms of obesity [26, 27]. In addition, coding and non-coding RNA expression patterns, specifically microRNAs and snoRNAs that play important regulatory roles in a variety of biological processes may impact appetite regulation, gene-environment interaction, adipocyte differentiation and biochemical pathways [20, 21, 25].
The advent of advanced genetic technology and genome-scanning technologies has led to the discovery that genetic differences among people can derive from copy number variants (CNVs) or structural rearrangements [20, 21, 28]. Rare deletions are also reported in the chromosome 16p11.2 region in about 0.5 % of individuals with severe obesity [29]. The SH2B adapter protein 1 (SH2B1) gene is localized in this chromosome region and linked to obesity [29, 30]. Common CNVs can also be found in linkage disequilibrium with single nucleotide polymorphisms (SNPs) and obesity including a 45-kb deletion near the NEGR1 gene and a 21-kb deletion upstream of the GPRC5B gene, both known to contribute to obesity. Genetic variants close to the MC4R and FTO genes will increase the body weight of an individual carrying these variants by approximately one pound, while mutations of the MC4R gene are present in about 2 % of all obese individuals with male and female heterozygous carriers weighing 15 to 30 kg more, respectively, than their relatives without the mutations [31]. More research is needed to examine for rare CNVs and novel insights into the genetic causation and architecture of obesity and infertility.
A summary of obesity genes recorded on the 2005 Human Obesity Gene Map was reported in 2006 and included 176 single-gene mutations in 11 different genes, 50 loci were related to known Mendelian syndromes, 244 murine adiposity related-genes, 408 animal model based quantitative trait loci (QTLs) and 253 QTLs from 61 genome-wide scans [24]. Current updated lists of clinically relevant known and candidate genes for obesity and infertility in humans are needed for genetic diagnosis and counseling purposes for patients with non-syndromic and syndromic obesity and those presenting with infertility for medical services.
Materials and methods
We used PubMed and other computer-based internet websites to search for combined key words such as obesity, body mass index, genetics, gene mutations or variants to identify documented evidence (clinical, functional or experimental) of the genes causing obesity in humans. Often the research publication would contain the key word obesity and gene in the title of the article. We focused our attention to an updated study on the 2005 Human Obesity Gene Map, the 2005 update which was published in 2006 [24], a primary source of clinically relevant known and candidate genes for obesity with description and cited evidence of support for causation and, in addition, more recent whole-genome wide association and DNA sequencing studies of families with obesity and functional gene expression profiles analyses. Other informative websites (e.g., Online Mendelian Inheritance in Man-www.OMIM.org) and Gene Cards (www.genecards.org) were then used to compile an updated list of genes from these major sources for a total of 370 genes. The genes recognized, to date, play a role in obesity susceptibility affecting common pathways in lipid metabolism, deposition or transport, eating behavior and food selection, physical activity and energy expenditure. We then included gene symbols, their expanded name and chromosome band location for the separate obesity genes and highlighted those genes associated with human infertility or reproduction. The position for each known or candidate gene for obesity susceptibility is then plotted on high resolution chromosome ideograms (850 band level). Similarly, the genes for human infertility and reproduction were found by searching the medical literature via PubMed with associated key words such as genetics, genes, infertility, reproduction, azoospermia, endometriosis, diminished ovarian reserve and estrogen and documented evidence of involvement in the causation of infertility in humans. The resulting gene list was cross-referenced with the identified obesity genes and the overlapped genes with infertility were highlighted.
Results
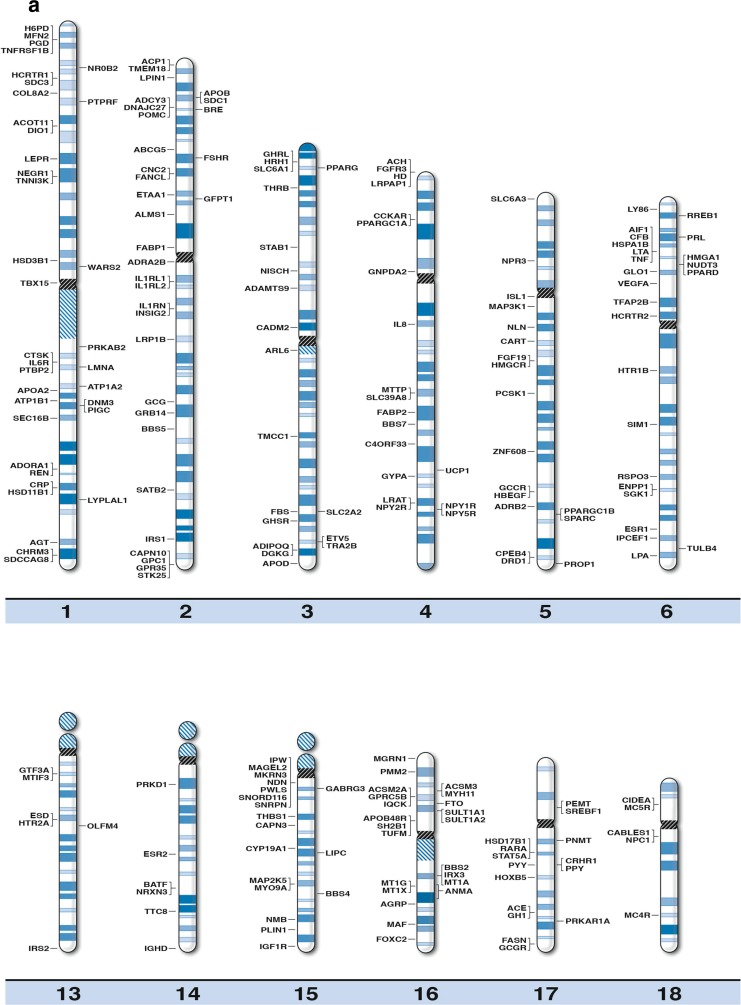
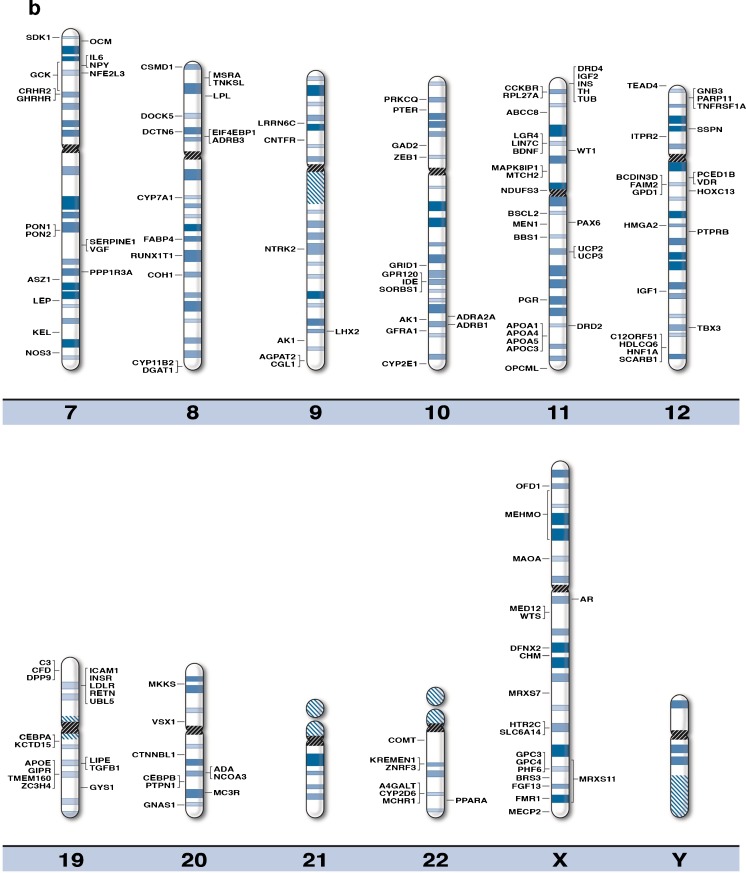
We generated high resolution chromosome ideograms (850 band level) and included gene symbols plotted on the ideogram at the precise chromosome region/band for each of the 370 genes found to play a role in obesity after searching the medical literature and internet websites (Fig. 1). Gene symbols, expanded name and chromosome location are listed in Table 1 in alphabetical order for each of the 370 obesity genes. Similarly, 153 genes were found to be associated with human infertility and reproduction (Table 2) when searching the medical literature and based on genome-wide association, linkage and gene mutation or variant studies and 21 of these genes had a recognized role in obesity [32–44].
Fig. 1.
High-resolution human chromosome ideograms (850 band level) with the obesity gene symbol positioned at the chromosome band or sub-band location. The centromere area, highlighted in black, separates the upper ‘p’ and lower ‘q’ arms for each chromosome. The gene symbol in alphabetical order, expanded name and precise chromosome band location are listed in Table 1




Table 2.
Known and candidate genes for human infertility and reproduction
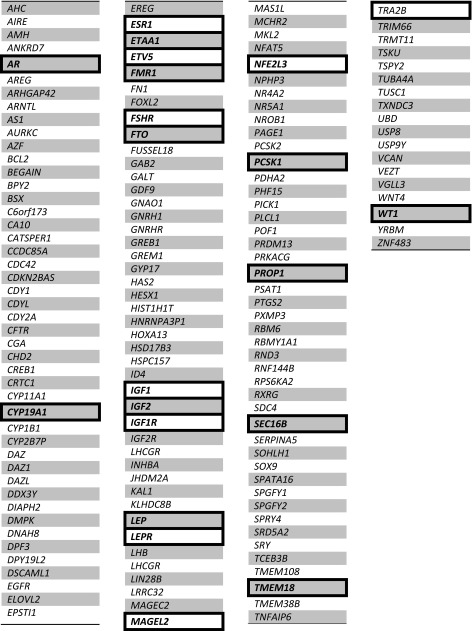
Bracketed genes represent those found to be involved with obesity. See Table 1 for list of obesity genes
Discussion
In this review, we summarize the evidence that obesity is a heritable disorder with an increasing number of involved genes, and briefly provide an update on the molecular basis and list 370 clinically relevant known or candidate genes for obesity in humans and their location on chromosome ideograms and 153 genes implicated in human infertility and reproduction. Some genes cause Mendelian forms of obesity while other loci are known to contribute to polygenic obesity and/or infertility. Rare and common structural variants are also associated with obesity or infertility, but most remain to be discovered.
The study of recessive single gene causes of extreme obesity have been useful in identifying and delineating the role of causative genes involving the leptin / melanocortin pathways but explain only a small percentage of the obesity seen in the general population. For example, only 14 individuals with complete leptin deficiency have been identified. Moreover, MC4R protein deficiency is the most common cause of monogenic obesity. Loss of function mutation in the MC4R gene occurs in 0.07 % of the general population with a prevalence of 0.5 to 1 % among obese adults and 1 to 6 % among children with obesity [23]. Haplo-insufficiency for BDNF, TRKB (NTRK2) and SIM1 genes have also been associated with severe obesity related to hyperphagia but often accompanied by syndromic features [20, 21].
Dasouki et al. [30] summarized structural chromosome abnormalities in the literature and reported new cases associated with onset of obesity in childhood. Some reports were classical such as the 15q11-q13 deletion seen in Prader-Willi syndrome while other reports were more rare. Usually structural chromosome defects are associated with congenital anomalies, growth retardation and developmental delay but occasionally can be associated with hyperphagia and obesity. Every chromosome except chromosome number 13 was found to be reported with structural anomalies (i.e., deletions, duplications) and related to obesity. These include chromosome 1 with a recognized 1p36 deletion obesity syndrome [45]; chromosome 6 with a 6q16.2 deletion involving SIM1 gene [46]; chromosome 11 with 11p deletions involving the BDNF gene [47]; chromosome 15 with the 15q11-q13 deletion seen in Prader-Willi syndrome [25]; chromosome 16 with 16p11.2 deletions involving the SH2B1 gene [25, 26] and 16q11.2 duplications involving the FTO gene [48]; chromosome 18 with 18q deletions involving the MC4R gene [49]; chromosome 20 with 20q deletions seen in Albright hereditary osteodystrophy involving the complex GNAS1 gene locus [50]; chromosome 22 with the 22q11.2 deletion seen in DiGeorge syndrome [51]; and chromosome X with a Xq27 deletion involving the FMR1 gene seen in fragile X syndrome [52]. These multiple chromosome abnormalities support the polygenic nature of obesity useful for the identification of candidate genes and their location for more precise genetic characterization and testing. Large cohorts of obese adults have been studied using genome-wide linkage scans and genotyping results with highly polymorphic microsatellite markers regularly spaced across the whole genome. Many of these cohorts would also be at risk for infertility as a clear association does exist between the two conditions (obesity and infertility). More than 80 genetic linkage studies of over 31,000 individuals have been reported to examine obesity-related traits and significant evidence for linkage were found [20]. For example, significant linkage in childhood obesity was found for chromosome 6q22.31-q23.2 [53] and severe obesity for chromosome 4p15-p14 [54].
Genome-wide association studies (GWAS) involving large numbers of individuals with obesity have been reported using thousands of genetic variants such as SNPs. Candidate genes that play a role in body weight regulation and obesity have been reported. Specifically, four GWAS meta-analyses involving several thousand individuals of European descent confirmed a strong association for the FTO locus and BMI with identification of 35 new SNPs found in 33 additional loci but many new loci are yet to be discovered [55–57]. For example, of 19 loci identified, five were associated with BMI / obesity (FTO, MC4R, TFAP2B, NRXN3, MSRA) [20, 58, 59]. Additional likely causative genes for extreme obesity include TMEM18, NPC1, PTER, PRL and SDCCAG8 [20, 60, 61]. The biological role of several candidate genes for obesity (e.g., FTO, MC4R, POMC, SH2B1, BDNF, NPC1, NRX3 and NEGR1) [20, 21] involve adipose tissue development and function or are known to act at the brain level indicating a role in regulation of eating behavior, hyperphagia and food intake. Obesity impacts fertility as well as reproduction and share overlap in genetic liability. Several reports indicate that the FTO gene, one of the most important obesity-related genes, is also implicated in human infertility and reproduction. A recent review of GWAS data showed that the onset of menarche involves at least 35 genes including not only FTO but also TRA2B, ETV5, TMEM18 and SEC16B which are also known to play a role in obesity [62]. Obesity in women impacts fertility status with an increased time to conception and a relative risk for anovulatory infertility estimated at 2.7 [63–65]. Spontaneous conceptions decrease with subsequent increases in BMI for women.
There are several mechanisms whereby obesity can cause subfertility including hormonal and peptide based disturbances, e.g., an increase in leptin levels produced by the adipose tissue and a decrease in adiponectin levels [66, 67]. Leptin inhibits ovarian steroidogenesis while lower adiponectin levels are associated with increased insulin levels thereby causing hyperandrogenemia. This process influences sex hormone binding and relationship with the androgen receptor. Obesity also acts on insulin production and insulin-like growth factor 1 (IGF1) which enhances lutenizing hormone (LH) mediated steroidogenesis in the ovary and thus increase ovarian androgens [68–70]. Increased levels of androgen can result in apoptosis of granulosa cells and conversion of androgens peripherally to estrogens in fat cells inhibiting gonadotrophen secretion further impacting hormone imbalance and fertility status in obese women [66, 71–73].
Polycystic ovary syndrome is a classical obesity-related disorder characterized by menstrual irregularities, hyperandrogenism and subfertility [74, 75]. Obesity is seen in 30–75 % of women with this disorder implicating several genes involved in hormonal and metabolic function. These obesity-related genes impacting hormone and related peptide production include LEP, IGF1 and IGF2 and receptors (AR, ESR1, FSHR, LEPR, IGF1R). Other reported obesity and infertility-related genes are involved with metabolism (CYP19A1) or testes function with spermatogonial cell production (ETV5), premature ovarian failure (FMR1), obesity formation or susceptibly (FTO, PCSK1), transcriptional activator (NFE2L3) , transcription factor for development of somatotrophs and gonadotrophs (PROP1), organization of endoplasmic reticulum and protein export (SEC16B) and recycling (MAGEL2), neuronal influence on body weight regulation (TMEM18), testes-specific RNA splicing factors (TRA2B) and transcription factors involved with genito-urinary development (WT1) (genes reviewed in OMIM-www.ncbi.nlm.hih.gov/omim/). Clearly, obesity in women impacts reproduction and fertility status with genetics playing a role. An increased BMI reduces the conception rate leading to infertility by requiring higher doses of gonadotrophins that respond more poorly to ovarian stimulation. These women often have fewer oocytes to harvest but weight loss improves the likelihood of reproductive outcomes. Weight loss should be sustained and gradual in order to improve the hormone imbalance leading to a successful pregnancy.
Current methods in genetic technology with improved computer software and advanced bioinformatics have increased genetic testing options and outcomes in the clinical setting. Several obesity-related genetic syndromes and causative genes are now recognized as well as non-syndromic targets. Identification of genetic defects in the causation of obesity are now made possible with high resolution microarray technology and next generation sequencing. The addition of DNA probes recognizing SNPs combined with copy number probes in microarrays not only identify segmental deletions and duplications in the genome at a 100 fold greater level than standard routine chromosome studies but can also identify regions of homozygosity. These regions can be used to identify genomic areas harboring recessive genes for obesity but also can be used to calculate the inbreeding coefficients to determine the consanguinity status of an individual as well as uniparental disomy of individual chromosomes. This added information has the potential to also identify genetic factors contributing to obesity and infertility.
A goal for next generation exome and/or RNA sequencing is to allow for discoveries of disease-causing genes and regulatory sequences required for normal function. The investigation of non-coding RNA and their coding gene targets may be fruitful. Identification of commonly disturbed mechanisms in the development of hyperphagia, energy expenditure, obesity and/or infertility may lead to targeted treatments aimed at metabolic processes and allow for management and eventually prevention of obesity in a significant number of individuals. Characterizing molecular signatures and disease-specific gene profiles and patterns of expression and their overlap with related conditions should lead to recognition of interconnected disturbed gene pathways in many diseases including a growing body of genetic evidence for obesity or infertility. Genetic dissection of obesity and its interface with infertility will help to characterize disease mechanisms and processes and provide new targets for drug design and therapy. Characterization of these relationships should lead to earlier diagnosis, potential treatment strategies and prevention in individuals with obesity and/or infertility.
Our summary of validated human genes associated with obesity susceptibility found in the medical literature plotted on high resolution chromosome ideograms along with tabular information for both obesity and infertility genes can be used to inform diagnosis and genetic testing options required for genetic counseling purposes of family members presenting for genetic services. The cross-reference of obesity genes against known fertility genes should enhance clinical application and relevance. The authors encourage the use of this current collection of clinically relevant known and candidate genes for obesity susceptibility and human infertility in their evaluation of patients and families in the clinical setting.
Acknowledgments
We thank Carla Meister for expert preparation of the manuscript, Dr. Syed Rafi for assistance in literature review and Lorie Gavulic for excellent artistic design and preparation of chromosome ideograms.
Funding
Partial funding support was provided by the Prader-Willi Syndrome Association (USA), the Headley Family Scholarship, the National Institute of Child Health and Human Development (NICHD) HD02528 and from the Angelman, Rett and Prader-Willi Syndromes Consortium (U54 HD06122) which is part of the National Institute of Health (NIH) Rare Disease Clinical Research Network (RDCRN) supported through collaboration between the NIH Office of Rare Disease Research (ORDR) at the National Center of Advancing Translational Science (NCATS) and NICHD. The content is solely the responsibility of the authors and does not necessarily represent the office views of the National Institutes of Health.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Capsule Obesity is impacted by genetic factors that also affect human reproduction and infertility. We provide an update of clinically relevant candidate and known genes for obesity and infertility using high resolution chromosome ideograms with gene symbols and location.
References
- 1.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 2.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 3.Marsh CA, Hecker E. Maternal obesity and adverse reproductive outcomes: reducing the risk. Obstet Gynecol Surv. 2014;69:622–628. doi: 10.1097/OGX.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Pandey S, Pandey S, Maheshwari A, Bhattacharya S. The impact of female obesity on the outcome of fertility treatment. J Hum Reprod Sci. 2010;3:62–67. doi: 10.4103/0974-1208.69332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silber SJ, Barbey N. Scientific molecular basis for treatment of reproductive failure in the human: an insight into the future. Biochim Biophys Acta. 1822;2012:1981–1996. doi: 10.1016/j.bbadis.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 8.Jungheim ES, Travieso JL, Carson KR, Moley KH. Obesity and reproductive function. Obstet Gynecol Clin North Am. 2012;39:479–493. doi: 10.1016/j.ogc.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kort JD, Winget C, Kim SH, Lathi RB. A retrospective cohort study to evaluate the impact of meaningful weight loss on fertility outcomes in an overweight population with infertility. Fertil Steril. 2014;101:1400–1403. doi: 10.1016/j.fertnstert.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 10.Guelinckx I, Devlieger R, Vansant G. Reproductive outcome after bariatric surgery: a critical review. Hum Reprod Update. 2009;15:189–201. doi: 10.1093/humupd/dmn057. [DOI] [PubMed] [Google Scholar]
- 11.Peng CY, Xie HJ, Guo ZF, Nie YL, Chen J, Zhou JM, et al. The association between androgen receptor gene CAG polymorphism and polycystic ovary syndrome: a case–control study and meta-analysis. J Assist Reprod Genet. 2014;31:1211–1219. doi: 10.1007/s10815-014-0286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hjelmborg J, Fagnani C, Silventoinen K, McGue M, Korkeila M, Christensen K, et al. Genetic influences on growth traits of BMI: a longitudinal study of adult twins. Obesity (Silver Spring) 2008;16:847–852. doi: 10.1038/oby.2007.135. [DOI] [PubMed] [Google Scholar]
- 14.Carnell S, Haworth CM, Plomin R, Wardle J. Genetic influence on appetite in children. Int J Obes (Lond) 2008;32:1468–1473. doi: 10.1038/ijo.2008.127. [DOI] [PubMed] [Google Scholar]
- 15.Mustelin L, Latvala A, Pietilainen KH, Piirila P, Sovijarvi AR, Kujala UM, et al. Associations between sports participation, cardiorespiratory fitness, and adiposity in young adult twins. J Appl Physiol. 2011;110:681–686. doi: 10.1152/japplphysiol.00753.2010. [DOI] [PubMed] [Google Scholar]
- 16.Bouchard C, Tremblay A, Nadeau A, Despres JP, Theriault G, Boulay MR, et al. Genetic effect in resting and exercise metabolic rates. Metabolism. 1989;38:364–370. doi: 10.1016/0026-0495(89)90126-1. [DOI] [PubMed] [Google Scholar]
- 17.Cheung WW, Mao P. Recent advances in obesity: genetics and beyond. ISRN Endocrinol. 2012; 536905. [DOI] [PMC free article] [PubMed]
- 18.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 19.Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666–670. doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choquet H, Meyre D. Genetics of obesity: what have we learned? Curr Genomics. 2011;12:169–179. doi: 10.2174/138920211795677895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choquet H, Meyre D. Molecular basis of obesity: current status and future prospects. Curr Genomics. 2011;12:154–168. doi: 10.2174/138920211795677921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356:237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govaerts C, Srinivasan S, Shapiro A, Zhang S, Picard F, Clement K, et al. Obesity-associated mutations in the melanocortin 4 receptor provide novel insights into its function. Peptides. 2005;26:1909–1919. doi: 10.1016/j.peptides.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 24.Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, et al. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- 25.Butler MG. Prader-Willi syndrome: obesity due to genomic imprinting. Curr Genomics. 2011;12(3):204–215. doi: 10.2174/138920211795677877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrera BM, Keildson S, Lindgren CM. Genetics and epigenetics of obesity. Maturitas. 2011;69:41–49. doi: 10.1016/j.maturitas.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20:63–68. doi: 10.1016/j.nut.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Feuk L, Carson AR, Scherer SW. Structural variation in the human genome. Nat Rev Genet. 2006;7:85–97. doi: 10.1038/nrg1767. [DOI] [PubMed] [Google Scholar]
- 29.Bachmann-Gagescu R, Mefford HC, Cowan C, Glew GM, Hing AV, Wallace S, et al. Recurrent 200-kb deletions of 16p11.2 that include the SH2B1 gene are associated with developmental delay and obesity. Genet Med. 2010;12:641–647. doi: 10.1097/GIM.0b013e3181ef4286. [DOI] [PubMed] [Google Scholar]
- 30.Dasouki MJ, Youngs EL, Hovanes K. Structural chromosome abnormalities associated with obesity: report of four new subjects and review of literature. Curr Genomics. 2011;12:190–203. doi: 10.2174/138920211795677930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Layman LC. Human gene mutations causing infertility. J Med Genet. 2002;39:153–161. doi: 10.1136/jmg.39.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada Y, Tateishi K, Zhang Y. Histone demethylase JHDM2A is involved in male infertility and obesity. J Androl. 2010;31:75–78. doi: 10.2164/jandrol.109.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosova G, Scott NM, Niederberger C, Prins GS, Ober C. Genome-wide association study identifies candidate genes for male fertility traits in humans. Am J Hum Genet. 2012;90:950–961. doi: 10.1016/j.ajhg.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Inati E, Muller J, Viville S. Autosomal mutations and human spermatogenic failure. Biochim Biophys Acta. 1822;2012:1873–1879. doi: 10.1016/j.bbadis.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Qiu R, Chen C, Jiang H, Shen L, Wu M, Liu C. Large genomic region free of GWAS-based common variants contains fertility-related genes. PLoS One. 2013;8:e61917. doi: 10.1371/journal.pone.0061917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferfouri F, Boitrelle F, Ghout I, Albert M, Molina Gomes D, Wainer R, et al. A genome-wide DNA methylation study in azoospermia. Andrology. 2013;1:815–821. doi: 10.1111/j.2047-2927.2013.00117.x. [DOI] [PubMed] [Google Scholar]
- 38.Albertsen HM, Chettier R, Farrington P, Ward K. Genome-wide association study link novel loci to endometriosis. PLoS One. 2013;8:e58257. doi: 10.1371/journal.pone.0058257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fragouli E, Lalioti MD, Wells D. The transcriptome of follicular cells: biological insights and clinical implications for the treatment of infertility. Hum Reprod Update. 2014;20:1–11. doi: 10.1093/humupd/dmt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bulun SE. Aromatase and estrogen receptor alpha deficiency. Fertil Steril. 2014;101:323–329. doi: 10.1016/j.fertnstert.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greene AD, Patounakis G, Segars JH. Genetic associations with diminished ovarian reserve: a systematic review of the literature. J Assist Reprod Genet. 2014;31:935–946. doi: 10.1007/s10815-014-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin Y, Ji J, Du G, Wu W, Dai J, Hu Z, et al. Comprehensive pathway-based analysis identifies associations of BCL2, GNAO1 and CHD2 with non-obstructive azoospermia risk. Hum Reprod. 2014;29:860–866. doi: 10.1093/humrep/deu013. [DOI] [PubMed] [Google Scholar]
- 43.Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT. Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update. 2014;20:702–716. doi: 10.1093/humupd/dmu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu Z, Li Z, Yu J, Tong C, Lin Y, Guo X, et al. Association analysis identifies new risk loci for non-obstructive azoospermia in Chinese men. Nat Commun. 2014;5:3857. doi: 10.1038/ncomms4857. [DOI] [PubMed] [Google Scholar]
- 45.D’Angelo CS, Da Paz JA, Kim CA, Bertola DR, Castro CI, Varela MC, et al. Prader-Willi-like phenotype: investigation of 1p36 deletion in 41 patients with delayed psychomotor development, hypotonia, obesity and/or hyperphagia, learning disabilities and behavioral problems. Eur J Med Genet. 2006;49:451–460. doi: 10.1016/j.ejmg.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Faivre L, Cormier-Daire V, Lapierre JM, Colleaux L, Jacquemont S, Genevieve D, et al. Deletion of the SIM1 gene (6q16.2) in a patient with a Prader-Willi-like phenotype. J Med Genet. 2002;39:594–596. doi: 10.1136/jmg.39.8.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gul D, Ogur G, Tunca Y, Ozcan O. Third case of WAGR syndrome with severe obesity and constitutional deletion of chromosome (11)(p12p14) Am J Med Genet. 2002;107:70–71. doi: 10.1002/ajmg.10013. [DOI] [PubMed] [Google Scholar]
- 48.van den Berg L, de Waal HD, Han JC, Ylstra B, Eijk P, Nesterova M, et al. Investigation of a patient with a partial trisomy 16q including the fat mass and obesity associated gene (FTO): fine mapping and FTO gene expression study. Am J Med Genet A. 2010;152A:630–637. doi: 10.1002/ajmg.a.33229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cody JD, Reveles XT, Hale DE, Lehman D, Coon H, Leach RJ. Haplosufficiency of the melancortin-4 receptor gene in individuals with deletions of 18q. Hum Genet. 1999;105:424–427. doi: 10.1007/s004390051125. [DOI] [PubMed] [Google Scholar]
- 50.Aldred MA, Aftimos S, Hall C, Waters KS, Thakker RV, Trembath RC, et al. Constitutional deletion of chromosome 20q in two patients affected with albright hereditary osteodystrophy. Am J Med Genet. 2002;113:167–172. doi: 10.1002/ajmg.10751. [DOI] [PubMed] [Google Scholar]
- 51.D’Angelo CS, Jehee FS, Koiffmann CP. An inherited atypical 1 Mb 22q11.2 deletion within the DGS/VCFS 3 Mb region in a child with obesity and aggressive behavior. Am J Med Genet A. 2007;143A:1928–32. [DOI] [PubMed]
- 52.Hirst M, Grewal P, Flannery A, Slatter R, Maher E, Barton D, et al. Two new cases of FMR1 deletion associated with mental impairment. Am J Hum Genet. 1995;56:67–74. doi: 10.1002/ajmg.1320560115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyre D, Lecoeur C, Delplanque J, Francke S, Vatin V, Durand E, et al. A genome-wide scan for childhood obesity-associated traits in French families shows significant linkage on chromosome 6q22.31-q23.2. Diabetes. 2004;53:803–811. doi: 10.2337/diabetes.53.3.803. [DOI] [PubMed] [Google Scholar]
- 54.Stone S, Abkevich V, Hunt SC, Gutin A, Russell DL, Neff CD, et al. A major predisposition locus for severe obesity, at 4p15-p14. Am J Hum Genet. 2002;70:1459–1468. doi: 10.1086/340670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 57.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindgren CM, Heid IM, Randall JC, Lamina C, Steinthorsdottir V, Qi L, et al. Genome-wide association scan meta-analysis identifies three Loci influencing adiposity and fat distribution. PLoS Genet. 2009;5:e1000508. doi: 10.1371/journal.pgen.1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chambers JC, Elliott P, Zabaneh D, Zhang W, Li Y, Froguel P, et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet. 2008;40:716–718. doi: 10.1038/ng.156. [DOI] [PubMed] [Google Scholar]
- 60.Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009;41:157–159. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- 61.Scherag A, Dina C, Hinney A, et al. Two new loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and german study groups. PLoS Genet. 2010;6:e1000916. doi: 10.1371/journal.pgen.1000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montgomery GW, Zondervan KT, Nyholt DR. The future for genetic studies in reproduction. Mole Hum Reprod. 2014;20(1):1–14. doi: 10.1093/molehr/gat058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22:414–420. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25:253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171:171–177. doi: 10.1016/0002-9378(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 66.Metwally M, Li TC, Ledger WL. The impact of obesity on female reproductive function. Obes Rev. 2007;8:515–523. doi: 10.1111/j.1467-789X.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- 67.Gil-Campos M, Canete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr. 2004;23:963–974. doi: 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 68.Brannian JD, Hansen KA. Leptin and ovarian folliculogenesis: implications for ovulation induction and ART outcomes. Semin Reprod Med. 2002;20:103–112. doi: 10.1055/s-2002-32501. [DOI] [PubMed] [Google Scholar]
- 69.Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertil Steril. 2002;77:433–444. doi: 10.1016/S0015-0282(01)03010-2. [DOI] [PubMed] [Google Scholar]
- 70.Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjo T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril. 1993;59:323–331. doi: 10.1016/s0015-0282(16)55675-1. [DOI] [PubMed] [Google Scholar]
- 71.Balen AH, Platteau P, Andersen AN, Devroey P, Sorensen P, Helmgaard L, et al. The influence of body weight on response to ovulation induction with gonadotrophins in 335 women with World Health Organization group II anovulatory infertility. BJOG. 2006;113:1195–1202. doi: 10.1111/j.1471-0528.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- 72.Zaadstra BM, Seidell JC, Van Noord PA, te Velde ER, Habbema JD, Vrieswijk B, et al. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. BMJ. 1993;306:484–487. doi: 10.1136/bmj.306.6876.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology–a systematic review. Hum Reprod Update. 2007;13:433–444. doi: 10.1093/humupd/dmm017. [DOI] [PubMed] [Google Scholar]
- 74.Pasquali R, Gambineri A. Polycystic ovary syndrome: a multifaceted disease from adolescence to adult age. Ann N Y Acad Sci. 2006;1092:158–174. doi: 10.1196/annals.1365.014. [DOI] [PubMed] [Google Scholar]
- 75.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]