Abstract
Purpose
To compare the outcomes of embryos selected via time lapse monitoring (TLM) versus those selected with conventional methods of selection in subfertile women undergoing ICSI.
Methods
The study population (239 women) was classified into two groups, based on the monitoring method used: Group 1 (TLM) and Group 2 (conventional monitoring). Groups were compared according to the clinical and ICSI cycle characteristics and reproductive outcomes, while transfers were performed at day 2 or 3. Subgroup analyses were performed, in women of both groups according to age and clinical parameters, and in embryos of Group 1 based on their cellular events.
Results
There was a statistically significant difference between the two study groups with regard to the outcome parameters, favoring Group 1 and especially in women >40 years of age. No differences were found in subgroup analyses in participants of both groups, regarding the stimulation protocol used, number of the oocytes retrieved and type of subfertility, while in Group 1 the percentages of “in range” cellular events were higher in certain divisions in ages 35–40, non-smokers, and the GnRH-agonist group, and in embryos that resulted in pregnancy.
Conclusion
Morphokinetic parameters of early embryo development via TLM are related to the characteristics of subfertile patients and associated with ICSI outcomes.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-015-0436-z) contains supplementary material, which is available to authorized users.
Keywords: Assisted reproductive techniques, ICSI, IVF, Time-Lapse Monitoring, Pregnancy
Introduction
Embryo staging, evaluation and selection remain a challenge in order to increase the current success rates in Assisted Reproduction Techniques (ART), with pregnancy and live birth rates to be around 35 % and 25 %, respectively [1]. Until recently, and even today in most ART centers rely on the use of the inverted microscope, which offers information based on morphological and developmental characteristics from the early cleavage till the blastocyst stage. However, this method has several limitations [2, 3]. Notably, transfer of top grade morphologically embryos often fails to result in clinical pregnancy, while embryos with poor scores sometimes result in live births. Apart from morphological assessment, invasive and non-invasive methods of selection have been developed. Single cell biopsy at the cleavage stage has been shown to not affect embryo progression to blastocyst [4]. Therefore, preimplantation genetic screening (PGS) has been used for embryo selection and aneuploidy screening, however its effectiveness has recently been placed under scrutiny [5]. Non-invasive techniques such as embryo oxygen consumption, testing of soluble HLA-G, amino acid turnover, proteomics and metabolomics, cumulus cell gene expression analysis, and time-lapse microscopy (TLM) are consequently becoming more popular options for embryo selection [6].
The consensus behind the use of TLM is based on the improved assessment of the embryos and their early cellular divisions, enabling to determine the timing of specific morphological occurrences and permit comparison between them, while also identifying anomalies (such as fragmentations and multinucleations), which otherwise would not be detected. This information can lead to more objective selection of embryos for transfer and/or cryopreservation, the reduction of the number of embryos to be biopsied in PGS and the improvement of the success rates for ART patients, and for those diagnosed with repeated implantation failures [7, 8]. Moreover, the potential undesirable shock or stress due to sudden changes in environmental parameters, such as temperature, associated with the use of a microscope are avoided [9–11].
The technology is easily assimilated into the ART laboratory and is used with any culture medium and environment. Images of the embryos are recorded at regular intervals without their removal from the culture environment, and images can be viewed instantly or merged to form a video showing complete development from the oocyte to blastocyst stage: their review can assist in identifying and selecting embryos with normal developmental profiles, and in deselecting those with abnormal phenotypes [12–14].
Reports on the modality have been numerous during the last 10 years or more. The target was to explore the dynamics of an embryo to implant successfully along with its various cleavage patterns and characteristics, by observing it at various stages of development [12, 15–21]. Recent studies reported information on the association of the type of fertilization [22], the IVF protocol used [23], female obesity [24], and smoking [25] on embryo kinetics and development. Interestingly, the sensitivity and specificity of the modality in terms of predicting the progression to the blastocyst stage was >90 % by measuring parameters at day 2 after fertilization, before embryonic genome activation [26, 27], while the bad prognosis of good-performing but unviable embryos reaches a specificity of 100 % [28]; moreover, the area under the receiver operating characteristic curve has been reported to be 0.74 for live birth [29] with high intra- and inter-observer correlation [30].
Regarding the use of TLM with pregnancy and implantation rates compared to the conventional incubator, literature data are conflicting, reporting both positive – even after accounting for confounding factors- [31, 32] and negative associations [33–35]. To date, most reports conclude that further studies are warranted to elucidate the relationship, either in the form of a large, age-adjusted data set or in a randomized controlled trial [36, 37]. We performed a prospective cohort trial to compare the effects of the TLM versus the conventional methods of selection on embryo implantation potential and reproductive outcome in subfertile women undergoing ICSI.
Materials and methods
Patient population and study design
This is a prospective, cohort trial performed at the Assisted Reproductive Unit of the MITERA Private Hospital with the scientific support of the Assisted Reproductive Unit of 3rd Department of Obstetrics and Gynecology, “Attikon” Hospital, of the Athens University School of Medicine. The study was approved by the Scientific and Ethics Committee of MITERA Hospital. A written informed consent was obtained from all patients enrolled in the current study.
Subjects
Two hundred thirty-nine cycles of ICSI in 239 women with primary or secondary subfertility were analyzed. Subfertility factors were recorded through the typical processes of the Unit, categorized as follows: female (tubal subfertility and ovulatory dysfunction), male factor and unexplained infertility. Exclusion criteria for the participation in the study were: age > 42 years old, basal hormonal levels of FSH at day 3 of the menstrual cycle > 15 IU/L, other protocols towards oocyte retrieval (natural or mild IVF cycles) and signs of ovarian hyperstimulation syndrome, as well as cases where fresh embryo transfers were a priori excluded. In addition, women with known previous poor ovarian response to ovarian stimulation were excluded.
Patients were enrolled consecutively during a 4 months period and participated in the study only once. The study population was classified into two groups, according to the monitoring modality, Group 1 (TLM) and Group 2 (conventional monitoring). Classification was achieved immediately after oocyte retrieval (OR): if more than five oocytes were retrieved, TLM or conventional monitoring was offered according to the number of the file of each patient (0 to 2 vs. 3 to 9 as a last digit), respectively. A consensus between the attending physician, the embryologist and the couple/ patient was placed in either groups. Of note, two patients in Group 1 and three in Group 2 did not agree with the allocated method and therefore were not included in the study.
Stimulation protocol
For the GnRH-agonist protocol, Triptorelin [Gonapeptyl, 0.1 mg (Ferring Pharmaceutical Hellas Α.Ε.) or Arvekap, 0.1 mg (Ipsen, EPE)] was administered subcutaneously daily during the midluteal phase or the second day of the menstrual cycle. For the GnRH antagonist protocol, ovarian stimulation began on the second day of the cycle and the antagonist, either Cetrorelix (Merck Serono Europe Limited, UK) or Orgalutran (Merck Sharp & Dohme Limited, UK) was initiated as soon as the leading follicle reached a diameter of 14 mm. Once pituitary down-regulation was achieved, ovarian stimulation with exogenous gonadotropins was started while GnRH agonist administration was continued concomitantly until the day of human chronic gonadotrophin (hCG) administration. Recombinant FSH in the form of either follitropin alpha (Gonal-F; Merck Serono Europe Ltd) or follitropin beta (Puregon Merck Sharp & Dohme Ltd) was administered subcutaneously.
The patients were monitored with serial transvaginal ultrasound and E2 levels every 2 ~ 4 days to monitor follicular growth and endometrial thickness. Starting doses were adjusted individually according to the age, FSH, AMH levels and previous response to IVF/ICSI cycles of each participant, while further adjustments and monitoring frequency were dependent upon women’s response to stimulation. When ≥2 follicles reached a diameter of 18 mm, human chorionic gonadotrophin [(10.000 IU Pregnyl (N. V. Organon, Netherlands) or 250mcg Ovitrelle (Merck Serono Europe Ltd, Germany)] was administered prior to transvaginal ultrasound–guided oocyte retrieval (OR) 36 h later.
According to the embryo quality, embryo transfer (ET) was performed either 2 or 3 days after the OR. Luteal phase support was achieved by transvaginal administration of progesterone in the form of either Utrogestan vaginal suppositories (Angelini Pharma Hellas Α.Β.Ε.Ε.) or Vasclor gel (Verisfield; U.K. Ltd) or a combination of both.
Fertilisation and preparation and embryo culture methods
All oocytes were stripped of cumulus cells and all M2 oocytes were microinjected through ICSI, 40 h post-hCG injection. The inseminated oocytes were placed in standard culture dishes containing 1 mL Universal IVF Medium™ (Origio, Denmark), overlaid with oil and left overnight in a standard Thermo Forma™ incubator (Thermo Scientific, Thermo Fisher Scientific, USA) at 37 °C and 6%CO2. Fertilisation was assessed 18 h post-insemination based on the presence of two pronuclei.
All zygotes in Group 1 were then transferred to a PrimoVision Embryo Culture Dish™ (Vitrolife, Sweden) containing 60–80 μl ISM1™ culture medium (Origio, Denmark), overlaid with oil and placed under a PrimoVision Time-Lapse Embryo Monitoring System™ (Vitrolife, Sweden) in a standard Thermo Forma™ incubator at 37 °C and 6%CO2. The monitoring system automatically took photographs of the embryos using an inverted microscope every 10 min, for the whole duration of culture. Zygotes from the control group were cultured in Nunc™ 4-Well Dishes (Thermo Scientific, Thermo Fisher Scientific, USA) containing 500 μl ISM1™, placed in a Thermo Forma™ incubator. Embryos in both groups were cultured until day 2 or 3, and then transferred, depending on their quality, and consensus between the embryologist and the attending physician of the couple.
Embryo evaluation and selection for transfer
Embryos in Group 2 were evaluated at 44 and 68 h (depending on day of ET) based on classic morphological criteria (number of blastomeres, fragmentation and multi-nucleation) on a scale of 1–5, with 1 representing a perfect morphological embryo, as previously described [38, 39]. Embryos in Group 1 were assessed without removing them from the incubator, using the Primo Vision–Analyser program (Vitrolife, Sweden) through which the exact time-points of each embryological event were marked, by viewing the TLM images. Evaluation of embryos was made based on the position of their time-points within the “normal” range, as proposed previously [39]. The events employed as morphological markers for embryo assessment and selection were:: t2: time to 2 cells (24–28 h), ii) cc2a: time between division from 2 to 3 cells (8–12 h), iii) t3: time to 3 cells (30–38 h), iv) s2: time between division from 3 to 4 cells (<45 min), v) t4: time to 4 cells (35–41 h), vi) cc3a: time between division from 4 to 5 cells (13–16 h), vii) t5: time to 5 cells (48–57 h), viii) s3: time between division from 5 to 8 cells (<6 h), ix) t8: time to 8 cells (50–59 h), as described elsewhere [39, 40]. The embryos that showed the most optimal morphokinetic parameters were chosen for transfer. All embryos were evaluated by a single operator. Missing data, mainly coming from uncertainty regarding the exact timing of cellular events, were avoided by classifying the event at the nearest time point. Up to three embryos were selected and then transferred per patient. Surplus embryos were frozen.
Outcome assessment
Groups were compared regarding the reproductive outcomes: clinical pregnancy, ongoing pregnancy and live birth rates. Subgroup analyses were performed according to participants’ age, stimulation protocol used, number of oocytes retrieved and type of subfertility. Clinical pregnancy was confirmed by a transvaginal ultrasound scan at 7 weeks of gestation, ongoing pregnancy as a positive heart beat at 12 weeks, while live birth was defied a viable pregnancy after 20 completed gestational weeks.
A further subgroup analysis was performed in embryos of Group 1, based on the timing of cellular events, age of the woman, smoking habit, stimulation protocol used, number of oocytes retrieved and reproductive outcomes.
Statistical methods
The study yielded categorical variables which were presented as percentages and analysed using χ2 test. Student t-test was used to analyse continuous data such as cleavage timing. Finally, the effect of age and infertility type was analysed by ANOVA. All analysis was performed on SPSS v17.0 (SPSS, Inc, Chicago, IL) and p values < 0.05 were considered to be significant.
Results
Two hundred thirty-nine women undergoing 239 ICSI cycles were enrolled. Women’s age were 33.7 ± 4.1 (mean ± SD) and 35.3 ± 4.2 years for Groups 1 and 2, respectively. Baseline and cycle characteristics in the two study groups were similar (Table 1). In Group 1, half of them underwent the long GnRH-agonist and half the antagonist protocol.
Table 1.
Baseline and cycle characteristics in the two groups studied
| Group 1 | Group 2 | P-value | |
|---|---|---|---|
| Number of patients | 70 | 169 | |
| Number of cycles | 70 | 169 | |
| Maternal age (years) | 33.7 ± 4.1 | 35.3 ± 4.2 | NS |
| Maternal BMI (kg/m2) | 22.3 ± 4.2 | 21.3 ± 3.2 | NS |
| Cause of subfertility | |||
| Male factor | 44.2 % | 40.2 % | NS |
| Tubal factor | 18.5 % | 20.5 % | NS |
| Unexplained | 22.8 % | 26.8 % | NS |
| Number of previous cycles | 1 (0–3) | 1 (0–3) | NS |
| Oocytes retrieved/cycle | 12.6 ± 7.1 | 10.7 ± 6.9 | NS |
| Fertilisation rate | 65.4 % ± 17 % | 61.1 % ± 23.8 % | NS |
| Day of embryo transfer | 2.7 ± 0.5 | 2.6 ± 1.0 | NS |
| Number of embryos transferred | 2.8 ± 0.5 | 2.3 ± 1 | NS |
| Number of cycles with embryo transfer | 70 | 169 | |
| Number of embryos cryopreserved | 2 (0–7) | 2 (0–5) | NS |
Continuous data are presented as mean ± SD
There was a statistically significant difference between the two study groups with regard to the outcome parameters: Group 1 demonstrated higher clinical (65.7 % vs. 39 %, p < 0.001), ongoing (55.7 % vs. 31.3 %, p < 0.001) and live birth rates (45.7 % vs. 28.4 %, p = 0.01) as compared with Group 2 (Table 2). Subgroup analysis according to women’s age, showed that this difference was retained between Group 1 and 2 regarding clinical pregnancy rates at the age of < 35 years (67 % vs. 44.12 %, respectively, p = 0.025), in ongoing pregnancy rates at the age of 35–40 (56 % vs. 32.39 %, respectively, p = 0.037) and in clinical (67 % vs. 20 %, respectively, p < 0.039), ongoing (67 % vs. 13.33 %, respectively, p < 0.014) and live birth rates (67 % vs. 13.33 %, respectively, p < 0.014), in women older than 40 years (Table 2). No differences were found among participants of both groups after subgroup analyses according to the stimulation protocol used, number of the oocytes retrieved and type of subfertility (all p values > 0.05) (Suppl Figures 1,2,3).
Table 2.
Outcome parameters of the two study groups in total and subgroup analysis based on participants’ age (<35, 35–40 and > 40 years of age)
| Group 1 | Group 2 | p value | Group 1 < 35 years | Group <35 years | p value | Group 1 35–40 years | Group 2 35–40 years | p value | Group > 40 years | Group >40 years | p value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPR | 65.71 % | 39.05 % | <0.001 | 67 % | 44.12 % | 0.025 | 64 % | 42.25 % | 0.061 | 67 % | 20 % | 0.039 |
| OPR | 55.71 % | 31.36 % | <0.001 | 54 % | 38.24 % | 0.117 | 56 % | 32.39 % | 0.037 | 67 % | 13.33 % | 0.014 |
| LBR | 45.71 % | 28.40 % | 0.01 | 44 % | 35.29 % | 0.259 | 44 % | 28.17 % | 0.115 | 67 % | 13.33 % | 0.014 |
CPR clinical pregnancy rate, OPR ongoing pregnancy rate, LBR live birth rate
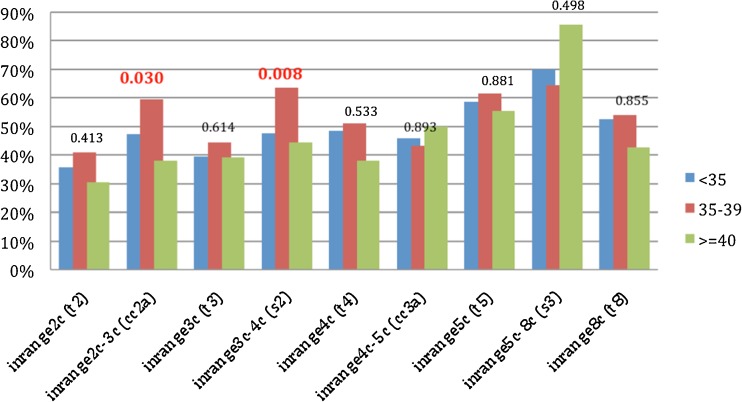
In Group 1, the percentages of “in range” cellular events in the embryos, expressed by the timings of the second and third cell cycles (2c–3c and 3c–4c), were found to be significantly higher in women aged 35–40 compared to those > 40 years of age (59.6 % vs. 38.1 %, p = 0.03 and 63.6 % vs. 44.4 %, p = 0.008, respectively) (Fig. 1). The percentage of “in range” cellular events was higher in non-smokers than in smokers, expressed by an increase in “in range” divisions in the fourth cell cycle (4c–5c) period (54.5 % vs. 37.9 %, p = 0.012) (Supp. Fig. 4). With regard to the protocol used, the cellular events were higher in the GnRH-agonist group, as compared to the GnRH-antagonist, in the timing to the eight cell cycle (65.8 % vs. 37.3 %, p = 0.001) (Supp. Fig. 5). Regarding the effect of the number of oocytes retrieved on the timing of cellular events, there was a higher length in the second cell cycle (2c-3c) in embryos derived from retrievals with > 10 oocytes, as compared to those with five to 10 (55 % vs. 43.6 %, p = 0.032); in contrast, a higher length was observed in the fifth cell cycle (5c–8c) in embryos derived from retrievals with > 10 oocytes, as compared to those with five to 10 (87.2 % vs. 62.3 %, p = 0.004) (data not shown).
Fig. 1.
Percentage in Group 1 (subgroup analysis) of “in range” cellular events of the embryos (from 2c up to 8c stage) according to participants’ age (groups <35, 35–39, and ≥40 years of age). P values are presented on the top of the columns
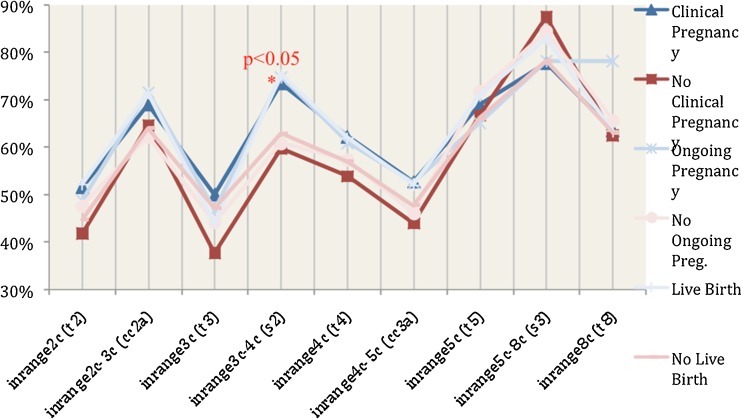
Also, the percentage of “in range” events in embryos that were transferred and resulted in successful outcomes was compared to that of embryos that did not. For the first cellular cycles (events 2c, 2c–3c, 3c, 3c–4c, 4c, 4c–5c) embryos that resulted in successful outcomes had 10–20 % higher probability of being “in range” than those that did not. However, this was significant only in the 3c–4c period (73.4 % vs. 59.7 %, p < 0.05). This trend was not observed in the more advanced cellular divisions (5c, 5c–8c, 8c) (Fig. 2).
Fig. 2.
Percentage of transferred embryos of Group 1 (TLM) that exhibited “in range” cellular events in patients with positive outcomes (Blue lines) and negative outcomes (Red lines)
Discussion
We performed a prospective cohort study of patients undergoing ICSI, comparing the reproductive outcomes between embryos whose evaluation was performed through TLM and those whose evaluation was performed with conventional methods. We found higher clinical, ongoing and live birth rates in participants whose embryos were monitored through TLM, as compared to those whose embryos were monitored by morphological assessment. This difference was maintained in women over 40 years. Early cellular events of the embryos monitored by TLM, were more “in range” in women aged 35–40 compared to those > 40 years (2c–3c, 3c–4c), in non-smokers compared to smokers (4c–5c), in the GnRH-agonist group, as compared to the GnRH-antagonist group (8c); also, these events were more “in range” in those embryos which resulted in pregnancy.
There was a statistically significant difference between the two study groups with regard to the outcome parameters, such as higher clinical, ongoing and live birth rates, favoring the use of TLM. These favourable results were maintained with regard to clinical pregnancy rates at the age of < 35 years, ongoing pregnancy rates at the age of 35–40 and, most interestingly, with regard to all outcomes at > 40 years of age. It should be noted, however, that in the case of the > 40 subgroup the sample size was very low (n = 6) causing the misleading impression of an extremely high LBR (Table 2). These results may be attributed to the detailed embryological assessment and improved embryo selection through the use of morphokinetic parameters for embryo selection under stable culture conditions that TLM offers. Similar reports have linked with high sensitivity and specificity the use of TLM with the ability of prediction of the cleavage stage embryos and their potential to reach the blastocyst stage, resulting in increased live births [12, 16, 19, 26, 31, 34, 39, 41]. On the other hand, other reports showed that TLM parameters are not able to predict live birth when compared with the conventional methods [33, 34, 42].
In fact, robust evidence in the literature on the effectiveness of TLM in improving success rates in ART has been scarce until recently. In a systematic review, after an initial yield of more than 1000 records, Polanski et al. [43], found only two randomized trials addressing this issue [44, 45]: authors concluded that there is no effect of TLM on live birth and congenital abnormality rates, while it was not correlated with a large change on the chance of achieving clinical and/or ongoing pregnancy when transferring blastocyst stage embryos. Although the present study is of lesser strength than these trials, it should be noted that cleavage stage transfers were performed and our positive results may be associated with significantly improved selection of high potential embryos which could have a similar effect on results as during blastocyst transfers. Following Polanski’s review, Rubio et al. [46] published the largest to date randomized control trial on TLM: authors reported that the use of the integrated EmbryoScope time-lapse monitoring system significantly increases the implantation and ongoing pregnancy rates while decreasing early pregnancy loss, when compared to a standard incubator embryo culture and selection based exclusively on morphology. Our study independently observed similar ongoing pregnancy rates in the TLM group and a similarly large increase, when compared to the control group. Of note, Rubio’s group performed embryo transers mainly at the cleavage stage, as we did in our study, as opposed to the previously mentioned trials. Nevertheless, regardless of its immediate effect on outcomes, all authors have acknowledged that apart from the reproductive outcomes, TLM is associated with advantages in the function of ART laboratories [47].
No significant effect was measured on the outcomes of TLM cycles by clinical/external factors, such as type of subfertility, type of down-regulation protocol used and number of oocytes retrieved (a measure of the effectiveness of stimulation). Smoking habit, BMI, certain types of subfertility (such as PCOS) and the type of ovarian stimulation used have been found to affect embryo development and morphokinetics, as well as reproductive outcomes following IVF/ICSI [25, 48, 49]. Our observations corroborate at least the morphokinetic conclusions of these studies since age, smoking and type of down-regulation were all found to affect at least one of the embryological events in the early cleavage stage of development. What is interesting is that the most affected events (and the only ones that achieved statistical significance) were the lengths of cell cycles (2c–3c, 3c–4c and 4c–5c) and not their exact timing. This is an encouraging result, as the length of each cell cycle is probably more indicative of embryo normality than the exact timing of events, which may vary from patient to patient without necessarily indicating some underlining defect of the embryo.
Moreover, the 3c–4c period was also significantly more likely to be “in range” in transferred embryos that resulted in successful outcomes than those linked with failed cycles. The 3c–4c period is very indicative of the synchronicity of the second cell division and therefore of the embryo’s competence. This result, in addition to the fact that younger women in our study exhibited shorter 3c–4c periods than older women seems to point to the importance of synchronicity in the early embryo development. The same time period (3c–4c) was also found to be an important predictor of implantation potential in the study of Meseguer et al. [39]: authors attempted to elucidate the “correct” timing of cellular events in the cleavage stage embryo by comparing the timings of embryos that implanted with those of embryos that failed to implant; by ranking the embryos according to their temporal “normality” they were able to identify embryos with the highest implantation potential. Synchronicity may also be an indicator of normal chromosomal profile, with long delays in divisions being related to aneuploidy [50]. Our study therefore suggests that synchronicity of early divisions can be used to select viable embryos with high sensitivity within the first 48 h post-insemination. In this context, TLM might be offered as an alternative to blastocyst culture in certain cases as a means of selection of viable embryos; the later carries the advantage of a high embryo selection potential, and has led to increased success rates coupled with reduced numbers of embryos transferred [51]. However, concerns have been raised on the effect of prolonged culture on embryo epigenetics and overall health of the resulting fetus [52].
The apparent limitations of our study are mainly attributed to its nature. The lack of power calculation and equality of the size of the groups studied, blinding, proper randomization and random allocation of the participants, is known to be linked with known and unknown confounders and selection and misclassification bias. Specifically, the allocation based on the number of patient’s file does not represent a formal random component in the sequence generation process, as stated in the Cochrane Handbook for Systematic Reviews of Interventions; the latter would necessitate referring to a random number table or generator [53]. Also, although the number of oocytes collected in each group was similar, the effect of the slightly higher number in the TLM group cannot be fully discounted and may be linked with the elevated success rate in this group, as compared to the control [54].
In addition, the present study is heavily influenced by that of Meseguer et al. [39] and, being comparable in terms of sample size, depends on the accuracy of the ranges of normal cleavage events. Comparing our study with Meseguer’s, we observed that synchronicity is possibly the most important parameter in early cleavage embryonic stage (specifically the synchronicity of the second cell cycle) and the most affected by factors such as age. Both studies, however, agree that early cellular events as opposed to later ones are more predictive of successful outcomes.
Conclusion(s)
The present prospective cohort trial on 239 subfertile women undergoing ICSI, found better reproductive outcomes in embryos whose evaluation was performed through TLM compared with those with conventional methods. The results were more evident in women aged more than 40 years. In contrast, there was no effect of TLM on outcomes, when clinical/external factors were taken under consideration. Also, we observed more “in range” cellular events in certain embryo cycles in women aged 35–40 compared to those > 40 years, in non-smokers than in smokers, in the GnRH-agonist group, as compared to the GnRH-antagonist, as well as in the embryos resulted in pregnancy compared to those that did not. In conjunction with similar studies, our results indicate the importance of the timing of cleavage events on embryo competence and the positive effect that embryo selection via TLM has on ICSI outcomes.
The evident selection and attrition bias of the current study make properly powered and conducted prospective studies a must, in order to support or reject these findings.
Electronic supplementary material
(DOC 316 kb)
Acknowledgments
The authors wish to thank the clinical, paramedical and laboratory team of Mitera Assisted Reproduction Unit. There was no finding for the current work. The study is a part of the Msc thesis of the second author.
Conflict of interest
All authors declare no conflict of interest.
Authors’ roles
CS: Study design, interpretation of findings and manuscript preparation.
MAK: Study design, data collection and data analysis.
AM: Data collection, data analysis and manuscript preparation.
AS, MB: Clinical embryology, data acquisition and interpretation of results.
GS, GM: data acquisition and interpretation of results.
GAP: Study concept and design, clinical embryology, data acquisition, data analysis and interpretation.
All authors critically reviewed and approved the final version of the manuscript.
Footnotes
Capsule
This prospective cohort study showed that the morphokinetic parameters of early embryo development observed via time lapse monitoring in subfertile women undergoing ICSI are related to the special characteristics of these women and are associated with ICSI outcomes.
References
- 1.Ferraretti AP, Goossens V, Kupka M, Bhattacharya S, de Mouzon J, Castilla JA, et al. Assisted reproductive technology in Europe, 2009: results generated from European registers by ESHRE. Hum Reprod. 2013;28:2318–31. doi: 10.1093/humrep/det278. [DOI] [PubMed] [Google Scholar]
- 2.Edwards RG, Purdy JM, Steptoe PC, Walters DE. The growth of human preimplantation embryos in vitro. Am J Obstet Gynecol. 1981;141:408–16. doi: 10.1016/0002-9378(81)90603-7. [DOI] [PubMed] [Google Scholar]
- 3.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 4.Palmer GA, Traeger-Synodinos J, Davies S, Tzetis M, Vrettou C, Mastrominas M, et al. Pregnancies following blastocyst stage transfer in PGD cycles at risk for beta-thalassaemic haemoglobinopathies. Hum Reprod. 2002;17:25–31. doi: 10.1093/humrep/17.1.25. [DOI] [PubMed] [Google Scholar]
- 5.Mastenbroek S, Twisk M, van der Veen F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update. 2011;17:454–66. doi: 10.1093/humupd/dmr003. [DOI] [PubMed] [Google Scholar]
- 6.Nel-Themaat L, Nagy ZP. A review of the promises and pitfalls of oocyte and embryo metabolomics. Placenta. 2011;32(Suppl 3):S257–63. doi: 10.1016/j.placenta.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Swain JE. Could time-lapse embryo imaging reduce the need for biopsy and PGS? J Assist Reprod Genet. 2013;30:1081–90. doi: 10.1007/s10815-013-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon A, Laufer N. Assessment and treatment of repeated implantation failure (RIF) J Assist Reprod Genet. 2012;29:1227–39. doi: 10.1007/s10815-012-9861-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anifandis G. Temperature variations inside commercial IVF incubators. J Assist Reprod Genet. 2013;30:1587–8. doi: 10.1007/s10815-013-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang JQ, Li XL, Peng Y, Guo X, Heng BC, Tong GQ. Reduction in exposure of human embryos outside the incubator enhances embryo quality and blastulation rate. Reprod Biomed Online. 2010;20:510–5. doi: 10.1016/j.rbmo.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Calzi F, Papaleo E, Rabellotti E, Ottolina J, Vailati S, Vigano P, et al. Exposure of embryos to oxygen at low concentration in a cleavage stage transfer program: reproductive outcomes in a time-series analysis. Clin Lab. 2012;58:997–1003. [PubMed] [Google Scholar]
- 12.Lemmen JG, Agerholm I, Ziebe S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod Biomed Online. 2008;17:385–91. doi: 10.1016/S1472-6483(10)60222-2. [DOI] [PubMed] [Google Scholar]
- 13.Conaghan J. Time-lapse imaging of preimplantation embryos. Semin Reprod Med. 2014;32:134–40. doi: 10.1055/s-0033-1363555. [DOI] [PubMed] [Google Scholar]
- 14.Chen AA, Tan L, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril. 2013;99:1035–43. doi: 10.1016/j.fertnstert.2013.01.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardarson T, Löfman C, Coull G, Sjögren A, Hamberger L, Edwards RG. Internalization of cellular fragments in a human embryo: time-lapse recordings. Reprod Biomed Online. 2002;5:36–8. doi: 10.1016/S1472-6483(10)61594-5. [DOI] [PubMed] [Google Scholar]
- 16.Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escriba MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98:1458–63. doi: 10.1016/j.fertnstert.2012.07.1135. [DOI] [PubMed] [Google Scholar]
- 17.Athayde Wirka K, Chen AA, Conaghan J, Ivani K, Gvakharia M, Behr B, et al. Atypical embryo phenotypes identified by time-lapse microscopy: high prevalence and association with embryo development. Fertil Steril. 2014;101:1637–48. doi: 10.1016/j.fertnstert.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 18.Dal Canto M, Coticchio G, Mignini Renzini M, De Ponti E, Novara PV, Brambillasca F, et al. Cleavage kinetics analysis of human embryos predicts development to blastocyst and implantation. Reprod Biomed Online. 2012;25:474–80. doi: 10.1016/j.rbmo.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto S, Kato N, Saeki K, Morimoto Y. Selection of high-potential embryos by culture in poly (dimethylsiloxane) microwells and time-lapse imaging. Fertil Steril. 2012;97:332–7. doi: 10.1016/j.fertnstert.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Stecher A, Vanderzwalmen P, Zintz M, Wirleitner B, Schuff M, Spitzer D, et al. Transfer of blastocysts with deviant morphological and morphokinetic parameters at early stages of in-vitro development: a case series. Reprod Biomed Online. 2014;28:424–35. doi: 10.1016/j.rbmo.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Zhang J, Salem SA, Liu X, Kuang Y, Salem RD, et al. Selection of competent blastocysts for transfer by combining time-lapse monitoring and array CGH testing for patients undergoing preimplantation genetic screening: a prospective study with sibling oocytes. BMC Med Genomics. 2014;7:38. doi: 10.1186/1755-8794-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joergensen MW, Agerholm I, Hindkjaer J, Bolund L, Sunde L, Ingerslev HJ, et al. Altered cleavage patterns in human tripronuclear embryos and their association to fertilization method: a time-lapse study. J Assist Reprod Genet. 2014;31:435–42. doi: 10.1007/s10815-014-0178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz M, Cruz M, Humaidan P, Garrido N, Perez-Cano I, Meseguer M. The type of GnRH analogue used during controlled ovarian stimulation influences early embryo developmental kinetics: a time-lapse study. Eur J Obstet Gynecol Reprod Biol. 2013;168:167–72. doi: 10.1016/j.ejogrb.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 24.Bellver J, Mifsud A, Grau N, Privitera L, Meseguer M. Similar morphokinetic patterns in embryos derived from obese and normoweight infertile women: a time-lapse study. Hum Reprod. 2013;28:794–800. doi: 10.1093/humrep/des438. [DOI] [PubMed] [Google Scholar]
- 25.Freour T, Dessolle L, Lammers J, Lattes S, Barriere P. Comparison of embryo morphokinetics after in vitro fertilization-intracytoplasmic sperm injection in smoking and nonsmoking women. Fertil Steril. 2013;99:1944–50. doi: 10.1016/j.fertnstert.2013.01.136. [DOI] [PubMed] [Google Scholar]
- 26.Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28:1115–21. doi: 10.1038/nbt.1686. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Moussavi F, Lorenzen P. Automated embryo stage classification in time-lapse microscopy video of early human embryo development. Med Image Comput Comput Assist Interv. 2013;16:460–7. doi: 10.1007/978-3-642-40763-5_57. [DOI] [PubMed] [Google Scholar]
- 28.Hlinka D, Kaľatová B, Uhrinová I, Dolinská S, Rutarová J, Rezáčová J, et al. Time-lapse cleavage rating predicts human embryo viability. Physiol Res. 2012;61:513–25. doi: 10.33549/physiolres.932287. [DOI] [PubMed] [Google Scholar]
- 29.Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Thornton S. Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time-lapse imaging without PGS. Reprod Biomed Online. 2013;27:140–6. doi: 10.1016/j.rbmo.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Sundvall L, Ingerslev HJ, Breth Knudsen U, Kirkegaard K. Inter- and intra-observer variability of time-lapse annotations. Hum Reprod. 2013;28:3215–21. doi: 10.1093/humrep/det366. [DOI] [PubMed] [Google Scholar]
- 31.Meseguer M, Rubio I, Cruz M, Basile N, Marcos J, Requena A. Embryo incubation and selection in a time-lapse monitoring system improves pregnancy outcome compared with a standard incubator: a retrospective cohort study. Fertil Steril. 2012;98:1481–9. doi: 10.1016/j.fertnstert.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Findikli N, Oral E. Time-lapse embryo imaging technology: does it improve the clinical results? Curr Opin Obstet Gynecol. 2014;26:138–44. doi: 10.1097/GCO.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 33.Cruz M, Gadea B, Garrido N, Pedersen KS, Martinez M, Perez-Cano I, et al. Embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients whose embryos were monitored by time-lapse imaging. J Assist Reprod Genet. 2011;28:569–73. doi: 10.1007/s10815-011-9549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkegaard K, Kesmodel US, Hindkjaer JJ, Ingerslev HJ. Time-lapse parameters as predictors of blastocyst development and pregnancy outcome in embryos from good prognosis patients: a prospective cohort study. Hum Reprod. 2013;28:2643–51. doi: 10.1093/humrep/det300. [DOI] [PubMed] [Google Scholar]
- 35.Kirkegaard K, Hindkjaer JJ, Grondahl ML, Kesmodel US, Ingerslev HJ. A randomized clinical trial comparing embryo culture in a conventional incubator with a time-lapse incubator. J Assist Reprod Genet. 2012;29:565–72. doi: 10.1007/s10815-012-9750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montag M. Morphokinetics and embryo aneuploidy: has time come or not yet? Reprod Biomed Online. 2013;26:528–30. doi: 10.1016/j.rbmo.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Ottolini C, Rienzi L, Capalbo A. A cautionary note against embryo aneuploidy risk assessment using time-lapse imaging. Reprod Biomed Online. 2014;28:273–5. doi: 10.1016/j.rbmo.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Veeck LL. Preembryo grading and degree of cytoplasmic fragmentation. New York: Parthenon Publishing; 1999. An Atlas of Human Gametes and Conceptuses: An Illustrated Reference for Assisted Reproductive Technology; pp. 46–51. [Google Scholar]
- 39.Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohí J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–71. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 40.Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M, et al. Time-lapse user group. Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod. 2014;29:2650–60. doi: 10.1093/humrep/deu278. [DOI] [PubMed] [Google Scholar]
- 41.Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, et al. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100:412–9. doi: 10.1016/j.fertnstert.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 42.Azzarello A, Hoest T, Mikkelsen AL. The impact of pronuclei morphology and dynamicity on live birth outcome after time-lapse culture. Hum Reprod. 2012;27:2649–57. doi: 10.1093/humrep/des210. [DOI] [PubMed] [Google Scholar]
- 43.Polanski LT, Coelho Neto MA, Nastri CO, Navarro PA, Ferriani RA, Raine-Fenning N, et al. Time-lapse embryo imaging for improving reproductive outcomes: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2014 doi: 10.1002/uog.13428. [DOI] [PubMed] [Google Scholar]
- 44.Kovacs P, Matyas S, Forgacs V, Sajgo A, Rarosi F, Pribenszky C. Time-lapse embryo selection for single blastocyst transfer-results of a multicenter, prospective, randomized clinical trial. Fertil Steril. 2013;100:S90. doi: 10.1016/j.fertnstert.2013.07.1736. [DOI] [Google Scholar]
- 45.Kahraman S, Cetinkaya M, Pirkevi C, Yelke H, Kumtepe Y. Comparison of blastocyst development and cycle outcome in patients with eSET using either conventional or time lapse incubators. A prospective study of good prognosis patients. J Reprod Stem Cell Biotechnol. 2013;3:55–61. [Google Scholar]
- 46.Rubio I, Galán A, Larreategui Z, Ayerdi F, Bellver J, Herrero J, et al. Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the EmbryoScope. Fertil Steril. 2014;102:1287–94. doi: 10.1016/j.fertnstert.2014.07.738. [DOI] [PubMed] [Google Scholar]
- 47.Aparicio B, Cruz M, Meseguer M. Is morphokinetic analysis the answer? Reprod BioMed Online. 2013;27:654–63. doi: 10.1016/j.rbmo.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 48.Muñoz M, Cruz M, Humaidan P, Garrido N, Pérez-Cano I, Meseguer M. Dose of recombinant FSH and oestradiol concentration on day of HCG affect embryo development kinetics. Reprod BioMed Online. 2012;25:382–9. doi: 10.1016/j.rbmo.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 49.Wissing ML, Hoest T, Mikkelsen AL. Slower early embryo development in women with polycystic ovary syndrome (PCOS) compared to regularly cycling women (controls) Fertil Steril. 2012;98(Suppl 3):S109. doi: 10.1016/j.fertnstert.2012.07.399. [DOI] [Google Scholar]
- 50.Davies S, Christopikou D, Tsorva E, Karagianni A, Handyside AH, Mastrominas M. Delayed cleavage divisions and a prolonged transition between 2-and 4-cell stages in embryos identified as aneuploid at the 8-cell stage by array CGH. Hum Reprod. 2012;27(Suppl 2):ii84. doi: 10.1093/humrep/27.s2.58. [DOI] [Google Scholar]
- 51.Mercader A, Garcia-Velasco JA, Escudero E, Remohi J, Pellicer A, Simon C. Clinical experience and perinatal outcome of blastocyst transfer after coculture of human embryos with human endometrial epithelial cells: a 5-year follow-up study. Fertil Steril. 2003;80:1162–8. doi: 10.1016/S0015-0282(03)01178-6. [DOI] [PubMed] [Google Scholar]
- 52.Kallen B, Finnstrom O, Lindam A, Nilsson E, Nygren KG, Olausson PO. Blastocyst versus cleavage stage transfer in in vitro fertilization: differences in neonatal outcome? Fertil Steril. 2010;94:1680–3. doi: 10.1016/j.fertnstert.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 53.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. <http://handbook.cochrane.org/> (2011). Accessed on 31 Dec 2014.
- 54.Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26:1768–74. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 316 kb)