Abstract
Purpose
The present study was designed to investigate the effect of L-carnitine treatment during IVM on nuclear and cytoplasmic maturation of immature oocytes selected by Brilliant Cresyle Blue (BCB) staining, and their subsequent developmental competence.
Materials & methods
Compact cumulus-oocyte complexes (COCs) were collected from NMRI mice ovaries and stained with BCB staining. BCB+ (colored cytoplasm) oocytes were then cultured in tissue culture medium (TCM) 199 with 0.0, 0.3 and 0.6 mg/ml L-carnitine.
Results
The both L-carnitine concentrations significantly increased the intracellular glutathione (P < 0.001), nuclear maturation (P < 0.01) and expression levels of cyclin-dependent kinase1 (CDK1) (P < 0.05). Moreover, treated oocytes with 0.6 mg/ml L-carnitine showed increased (P < 0.05) expression of mitogen-activated protein kinase1 (MAPK1) mRNA. Also, adding L-carnitine (0.6 mg/ml) to IVM medium significantly increased the cleavage rate (P < 0.05). The blastocyst development rate (BDR) in the both L-carnitine treated groups was significantly higher (P < 0.001) than the control group. L-carnitine had no significant effect on total blastocyst cell numbers.
Conclusions
These data indicated that L-carnitine supplementation during IVM of immature BCB+ oocytes improved preimplantation developmental competence of oocytes after IVF, probably by accelerating cytoplasmic and nuclear maturation of oocytes. It may provide a novel approach to improving ART outcomes in infertile couples.
Keywords: In vitro maturation, BCB staining, Glutathione, L-carnitine
Introduction
In vitro maturation (IVM) has emerged as an important issue in the field of assisted reproduction [1]. IVM can alleviate drawbacks of in vitro fertilization (IVF) such as high cost of gonadotropin administration, the risk of ovarian hyperstimulation syndrome (OHSS) [2], the possible association between repeated ovarian stimulation and hormone related cancer [3], and embryo aneuploidy [4]. Although the technique of IVM has been improved, its successful rate in human remains low compared with in vivo-derived oocytes [5], which means that IVM still needs to be optimized.
The efficiency of IVM and IVF is influenced by various factors including oocyte quality, and culture condition [6]. Oocytes are selected for IVM on the basis of morphological morphological assessment by observing the cumulus thickness, compactness granulation and homogeneity of the ooplasm. However, these criteria are often inaccurate, making it difficult to identify suitable oocytes [7]. The enzyme glucose-6-phosphate dehydrogenase (G6PDH) is active in growing oocytes, while its activity is reduced in fully grown oocytes. Brilliant Cresyle Blue (BCB) is a vital dye which stains the cytoplasm blue (BCB+) or colorless (BCB−) depending on G6PDH function and is a convenient test for selection for fully grown oocytes [8]. It has been reported that the competence of maturation and development increased in BCB+ oocytes in pig [9], sheep [10], and bovine [11].
Oxidative stress has recently appeared as one of the potential factors for low embryonic quality in IVF cycles [12]. Growth factors, cytokines such as leukemia inhibitory factor (LIF) [13] and antioxidants such as β-mercaptoethanol, glutathione (GSH), and melatonin [14, 15] have been shown to have positive effects on mammalian embryonic development during IVM and IVF [14]. L-carnitine (LC) is a small, water-soluble molecule, which can promote fatty acid and energy application by transporting long chain fatty acids across the inner mitochondria membrane for β-oxidation, thus enhance adenosine triphosphate (ATP) concentration [16]. Interestingly, mitochondrial organization and ATP levels of oocytes reflect their developmental capacity after IVF [17]. In addition, L-carnitine has antioxidant activity which can stabilize mitochondrial membrane and protect DNA against damage induced by ROS [18]. The anti-apoptotic activity of L-carnitine has also been reported in human lymphoma cells treated with GP7 (apoptosis-inducing agent) by inhibition of caspase-3 activity in this cells [19]. L-carnitine treatment was shown to down-regulate cytokines such as interleukin (IL)-1, and IL-6 in rats implanted subcutaneously with sarcoma tumor [20]. L-carnitine treatment of mouse embryos was able to neutralize anti-proliferative effect of tumor necrosis factor (TNF)-α compared with the group treated with TNF-α alone [21].
The antioxidant, anti-cytokines, and anti-apoptotic effects of L-carnitine have made it as a novel non-invasive agent for improving oocyte quality and subsequent embryonic development. Several studies examined the effect of L-carnitine supplementation on oocytes quality. However, Very limited data are available for gene expression of these oocytes and to our knowledge no study has reported the effect of L-carnitine on the oocytes maturation of immature oocytes selected by BCB test. Therefore, the present study aimed to investigate the effect of L-carnitine treatment during IVM of BCB+ mice oocytes on nuclear maturation of oocytes, intracellular glutathione concentration, and expression levels of several genes in metaphase II (MII) oocytes Such as growth differentiation factor-9: GDF-9, mitogen-activated protein kinase1: MAPK1, and cyclin-dependent kinase1: CDK1. In addition, we examined effect of L-carnitine supplementation during oocyte maturation process on developmental competence of IVF embryos.
Materials and methods
Materials
All chemicals used in this study were purchased from sigma-Aldrich Co. (St. Louis, MO, USA), unless otherwise indicated.
Animals
NMRI Mice were obtained from Pasteur Institute of Iran. Mice had ad libitum access to a standard diet and were maintained in a temperature- and light-controlled room (22 °C, 12L: 12D). All animal protocols were approved by the Shahid Beheshti University of medical science animal Ethics Committee and performed with accordance with the University guidelines.
Preparation of oocytes and BCB staining
Female NMRI mice (6–8 weeks old) were given an intraperitoneal injection of 10 IU pregnant Mare’s serum gonadotrophin (PMSG). Forty-eight hours after PMSG administration, mice were sacrified by cervical dislocation. Ovaries were dissected and situated in 1 ml warmed tissue cell culture medium (TCM) 199 Hepes supplemented with 5 mg/ml fetal bovine serum (FBS), immediately. Antral follicles were pierced with a pair of syringes with 26-gauge needles to release of cumulus oocyte complexes (COCs). At least 52 mice were used in the present study and approximately 25 COCs are obtained from ovaries of each mouse. The morphologically good quality compact COCs were collected and then were incubated in potassium simplex optimized medium (KSOM) supplemented with 4 mg/ml bovine serum albumin (BSA) containing 26 μM BCB for 90 min at 37 °C in humidified air atmosphere. After washing, the COCs were examined under a stereomicroscope and oocytes with any degree of blue coloration cytoplasm (BCB+) were selected.
In vitro maturation
The BCB+ COCs washed three times in maturation medium (TCM 199, with Earle’s salt and L-glutamine, Gibco, Life Tech. Co., UK) supplemented with 10 mg/ml FBS, 1 μg/ml 17-β estradiol, 24.2 mg/l sodium pyruvate, 10 μg/ml FSH, and 10 μg/ml LH. The BCB+ oocytes were randomly devoted to each treatment and cultured in IVM medium supplemented in 0.0, 0.3 and 0.6 mg/ml LC [21]. L-carnitine was dissolved in TCM-199 to give L-carnitine concentration and sterilized by filtration.
IVM of oocytes was performed at 37 °C with 5 % CO2 under maximum humidity for 24 h and were then utilized for further experiment as described below.
Measurement of intracellular GSH concentration in MII oocytes with MCB
Monochlorobimane (MCB) is a non-fluorescent molecule which reacts with GSH (or other sulfhydryl containing compounds) through a reaction catalysed by glutathione-S-transferase (GST) and produced a fluorescent product with an excitation peak at 395 nm [22]. The intracellular GSH levels in MII oocytes were measured by MCB. Briefly, oocytes from each treatment group were denuded from cumulus cells and washed 3 times in KSOM 4 mg/ml BSA. Then oocytes were incubated (in the dark) in 30 μl droplets of KSOM 4 mg/ml BSA supplemented with MCB for 45 min at 37 °C in a humidified 5 % CO2 atmosphere under mineral oil. Fluorescence was measured under inverted fluorescent microscope (with filter at 390 nm excitation). Image J software (National Institutes of Health, Bethesda, MD, USA) was used for analyzing intensity of fluorescence.
Measurement of ROS by chemiluminescence assay
In this assay, luminol (5-amino-2,3 dihydro-1,4 phthalazinedione) is added to the culture media that reacts with free radicals including O2−, OH and hydrogen peroxide. It does not differentiate between the types of free radicals and therefore measures global ROS in the medium. The byproduct of this reaction results in the production of photons of light [23, 24].
The culture media of MII oocytes was collected and immediately centrifuged for 7 min at 600×g. The supernatant was removed and then 1 μl of luminal (50 mM) prepared in dimethyl sulfoxide was added to 400 μl of the supernatant. The ROS levels were determined by measuring chemiluminescence with a luminometer (LKB 953, Wallac, Gaithersburg, MD) in the integrated mode for 15 min, and the results are expressed in relative light units (RLU)/s.
Assessment of nuclear status
IVM oocytes were denuded from cumulus cells after treatment with 1 mg/ml hyaloronidase for 1 min by gentle repeated pipetting. The nuclear status of oocytes was determined by chromatin dye (Hoechst 33342: 10 μg/ml) under an inverted fluorescent microscope (Olympus, Tokyo, Japan). Oocytes were classified as germinal vesicle stage (GV, containing an intact GV), germinal vesicle breakdown stage and metaphase I (GVBD and MI, containing a broken vesicle with the chromatin starting to condense and oocytes with a metaphase plate, but without a polar body, respectively), metaphase II stage (MII, containing a metaphasic plate and polar body), and degenerated oocyte (oocyte with membrane rupture or without visible chromosomes).
Simultaneous RNA extraction and cDNA synthesis
For extraction of total RNA from oocytes, three pools of biological replicates (each replicate containing ten denuded mature oocytes) were used. MII oocytes transferred to Eppendorf tube containing 1.5 μl lysis buffer [25]. Two microliters of poly N and 5 μl water were added to oocytes samples and placed in thermocycler for 5 min at 75 °C for the reaction to take place. The tubes were then placed on ice and 5 μl of 5× RT Buffer, 1 μl of RT Enzyme (200 u), 3 μl dNTP (10 mM), 0.25 μl RNase inhibitor (10 u) was added to the reaction. Reactions were performed on an applied Bio Rad thermocycler. The amplification program for the reverse transcription step was as follows: 25 °C (10 min), 37 °C (15 min), 42 °C (45 min), and 72 °C (10 min). After the reverse transcriptase reaction, the samples were kept at 4 °C overnight.
Gene expression analysis by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
Quantitative real-time PCR was performed to assess the expression of GDF-9, MAPK1 and CDK1 genes using Rotor Gene Q instrument (QIAGEN). Primer sequences and annealing temperature are showed in Table 1. Real time PCR reactions were carried out in a final volume of 13 μl according to the manuals for DNA Master SYBR Green I mix (Roche Applied Sciences). The primer concentrations were adjusted to 1 μl for each gene. The reaction conditions: 94 °C (30 min) for predenaturation; 40 cycles include 94 °C (30 s) for denaturation, 60 °C (45 s) for annealing, 72 °C (45 s), and final elongation step at 72 °C (7 min). PCR products were identified by generating a melting curve. Since, melting curve of a product is sequence specific, it can be used to distinguish between them. For the normalization, expression of HPRT1 (hypoxanthine phosphoribosyl transferase1) was examined as the internal reference gene. Three replications were performed and the mRNA level of each sample was normalized to that of HPRT1 mRNA level.
Table 1.
Details of primers for quantitative real-time PCR
| Genes name | Primer sequences (5′-3′) | Product size (bp) | Accession number |
|---|---|---|---|
| HPRT1a | Forward: TCCCAGCGTCGTGATTAG | 138 | NM_013556.2 |
| Reverse: CGAGCAAGTCTTTCAGTCC | |||
| GDF9 | Forward: CAACCAGGTGACAGGACC | 116 | NM_008110.2 |
| Reverse: AGGCAGAGTTGTTCAGAGTG | |||
| CDK1 | Forward: AGTTACTTACACCAAATCCTCC | 184 | NM_007659.3 |
| Reverse: ACAGCGTCACTACCTCGTG | |||
| MAPK1 | Forward: AAGTGATGAGCCCATTGC | 156 | NM_011949.3 |
| Reverse: CAGTCCTCTGAGCCCTTG |
aThe internal reference gene
The relative mRNA level was presented as 2−∆∆Ct; where Ct = threshold cycle for target amplification, ∆Ct = Ct target gene – Ct internal reference, and ∆∆Ct = ∆Ct sample – Ct calibrator [26].
Sperm preparation
Male mice (10–12 weeks of age) were sacrified by cervical dislocation. The cauda epididymis and vas deferens were cut and fragmented by using a pair of syringes with 26-gauge needles. Then, a dense mass of sperm was extruded into 1 ml Ham’s F10 medium supplemented with 5 mg/ml BSA. To allow the sperm to disperse into the medium, the dish was located in a 37 °C incubator for 15 min. The sperm medium without excess tissue centrifuged at 2000 rpm for 3 min. After discarding the supernatant, 0.5 ml Ham’s F10 supplemented 5 mg/ml BSA was carefully added to the sperm pellet. The sperm suspension was incubated for 45 min at 37 °C in a humidified 5 % CO2 atmosphere to allow the sperm to swim-up and capacitate.
In vitro fertilization and embryo culture
Groups of about five MII oocytes were placed to 50 μl droplets of fertilization medium (KSOM supplemented with 15 mg/ml BSA) and 15 μl of sperm suspension were added to each microdrop to give final spermatozoa concentration of about 3 × 106 sperm/ml in fertilization droplet. The dishes were incubated for 6 h at 37 °C in a humidified 5 % CO2 atmosphere. After co-incubation, presumptive zygotes were gently pipetted to remove the cumulus cells and adhered spermatozoa. Groups of 15 zygotes were transferred to 30 μl droplets of culture medium (KSOM supplemented with 4 mg/ml BSA) under mineral oil and placed in 5 % CO2 incubator at 37 °C. The day of insemination was considered as day 0. Cleavage rate was examined at 24 h after insemination. The BDR was recorded at the end of the 5th day. The total cell numbers of blastocyst were determined using Hoechst 33342 staining under an epifluorescent microscope.
Statistical analysis
Oocytes were randomly distributed in each experimental group and at least three replicates were conducted for each experiment. Data were analyzed using the IBM SPSS statistical program version 19. The relative expression of genes, the GSH levels, ROS levels, and total cell numbers of mouse blastocyst were analyzed using one-way ANOVA (post-hoc was Tukey test). Comparison of maturation rate, cleavage rate, and BDR between groups was performed by non-parametric analysis test (Chi-square). Values were considered statistically significant with P < 0.05 and presented as mean ± S.E.
Results
Effect of L-carnitine on intracellular GSH levels and ROS concentration
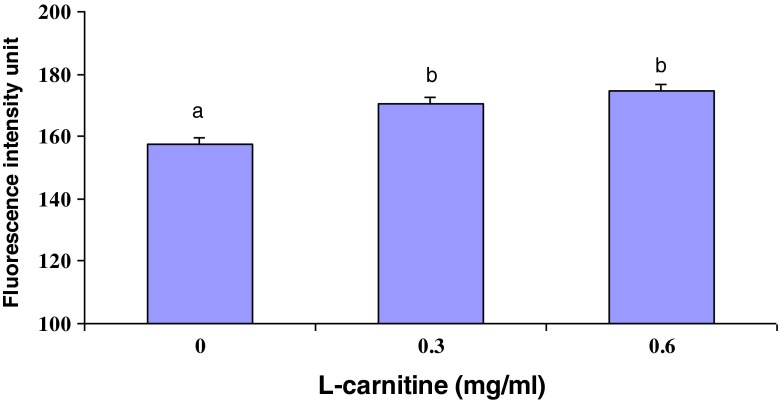
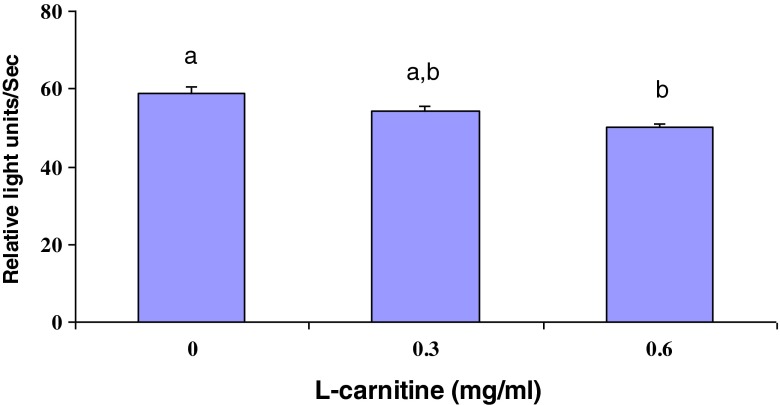
The intracellular levels of GSH were measured in L-carnitine treated oocytes after IVM. As shown in Fig. 1, L-carnitine treatment during IVM increased (P < 0.001) intracellular GSH levels (170.27 ± 2.09, and 174.60 ± 2.26 for 0.3 and 0.6 mg/ml L-carnitine, respectively) compared with control group (157.41 ± 2.01). Moreover, ROS concentration in maturation medium after IVM was significantly lower in oocytes treated with 0.6 mg/ml L-carnitine than in untreated group (44.99 ± 1.05 vs. 58.71 ± 1.86, respectively, p < 0.01) (Fig. 2).
Fig. 1.
Effect of L-carnitine treatment on level of GSH in matured oocytes selected by BCB test during IVM. GSH concentration is presented as the mean intensity of fluorescence emitted by MCB. Values are mean ± SEM. Different superscripts (a, b) indicate a significant difference (P < 0.001)
Fig. 2.
Effect of L-carnitine treatment on ROS concentration in maturation medium of mouse BCB+ oocytes during IVM. Values are mean ± SEM. Different superscripts (a, b) indicate a significant difference (P < 0.01)
Effect of L-carnitine on oocyte maturation
During IVM, the proportion of oocytes that reached the MII stage in both L-carnitine treated groups (73.42 % ± 2.63, and 73.44 % ± 1.93 for 0.3 and 0.6 mg/ml L-carnitine groups, respectively) was significantly (P < 0.01) higher than those of untreated group (59.56 % ± 3.46) (Table 2). There was also significant decrease (P < 0.01) in the percentage of degenerative oocytes between control group (11.27 % ± 2.47) and both L-carnitine treated groups (2.32 % ± 0.76, and 1.62 % ± 0.74 for 0.3 and 0.6 mg/ml L-carnitine groups, respectively) (Table 2).
Table 2.
Effect of L-carnitine (LC) treatment on nuclear status of mouse oocytes selected by BCB test during IVM
| Group | No. of COCs | Nuclear status | |||
|---|---|---|---|---|---|
| GV n (%) | GVBD and MI n (%) | MII n (%) | Deg. n (%) | ||
| Control | 333 | 19 (3.19 ± 1.28) | 74 (25.63 ± 2.94) | 205 (59.56 ± 3.46)a | 32 (11.27 ± 2.47)a |
| 0.3 mg/ml LC | 325 | 14 (3.60 ± 1.58) | 69 (20.66 ± 2.03) | 231 (73.42 ± 2.63)b | 11 (2.32 ± 0.76)b |
| 0.6 mg/ml LC | 358 | 10 (3.21 ± 1.17) | 71 (21.33 ± 1.84) | 268 (73.44 ± 1.93)b | 7 (1.62 ± 0.74)b |
Data are presented as mean ± SEM. Different superscripts (a, b) within the columns indicate a significant difference (P < 0.01)
COC cumulus oocyte complex, GV germinal vesicle oocyte, GVBD germinal vesicle breakdown, MI metaphase I oocyte, MII metaphase II oocyte, Deg. degenerative oocytes
Effect of L-carnitine on the gene expression of GDF-9, MAPK1, and CDK1
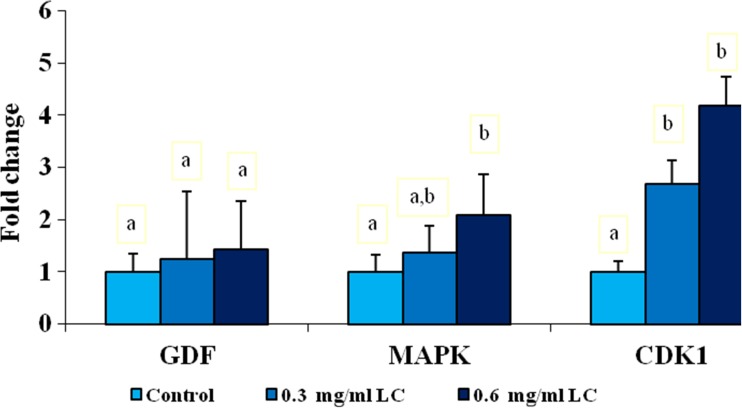
The relative expressions levels of the three maternal genes, including GDF-9, MAPK1, and CDK 1 were analyzed by quantitative real-time PCR (Fig. 3). The mRNA expression of GDF-9 in the oocytes was higher in both treated groups than in the control group, but this difference was not statistically significant. The relative transcript abundance of MAPK1 mRNA within oocytes was increased in both treated groups compared with untreated group. However, the enhancement of expression of MAPK1 mRNA was significant only in 0.6 mg/ml L-carnitine group (P < 0.05). In addition, the transcript abundance of CDK1 in MII oocytes treated with L-carnitine (0.3 and 0.6 mg/ml L-carnitine) during IVM increased compared with the control group (P < 0.05).
Fig. 3.
Relative mRNA expression of GDF-9, MAPK1, and CDK1 in MII oocytes treated with L-carnitine (LC) during IVM. Within the same mRNA, different superscripts (a, b) indicate a significant difference (P < 0.05). Data are presented as mean ± SEM
Effect of L-carnitine on embryonic development after IVF
Results of in vitro embryo development after IVF of BCB+ oocytes were shown in Table 3. Cleavage rate of embryos in 0.3 mg/ml L-carnitine treaeted group (69.65 % ± 3.9) was not significantly different with control group (61.04 % ± 3.45). However, 0.6 mg/ml of L-carnitine significantly increased (P < 0.05) the cleavage rate (73.94 % ± 2.97) compared with untreated group. L-carnitine treatment during IVM increased (P < 0.001) blastocyst development rate (BDR) (29.33 % ± 0.98, and 28.71 % ± 1.35 for 0.3 and 0.6 mg/ml L-carnitine groups, respectively) compared with control group (21.35 % ± 1.62). The total cell numbers of blastocyst was not significantly influenced by L-carnitine treatment.
Table 3.
Effect of L-carnitine (LC) treatment during IVM of BCB+ oocytes on embryonic development after IVF
| Groups | No. of oocytes cultured | Embryo development | Total cells in blastocyst (n) | |
|---|---|---|---|---|
| Cleavage rate (%) | BDR (%) | |||
| Control | 213 | 61.04 ± 3.45a | 21.35 ± 1.62c | 55.25 ± 2.17 |
| 0.3 mg/ml LC | 232 | 69.65 ± 3.91a,b | 29.33 ± 0.98d | 58.00 ± 1.94 |
| 0.6 mg/ml LC | 254 | 73.94 ± 2.97b | 28.71 ± 1.35d | 58.12 ± 1.69 |
Data are presented as mean ± SEM. Different superscripts (a, b) within the columns indicate a significant difference (P < 0.05). Different superscripts (c, d) within the columns indicate a significant difference (P < 0.001)
BDR blastocyst development rate
Discussion
In this study, we found that treatment of mouse BCB+ oocytes with L-carnitine during IVM effectively increased maturation rate of oocytes, the intracellular level of GSH, scavenged ROS in maturation medium, and expression levels of MAPK1 and CDK1 mRNA in MII oocytes. In addition, L-carnitine improved cleavage and blastocysts rates, but had no significant effect on total blastocyst cell numbers.
Developmental competence (quality) of oocytes is defined as their ability to resume meiosis, to be fertilized, to convert into a blastocyst, and finally to develop into viable offspring [27]. Oocytes selection using BCB test is a useful method to categorize competent oocytes. It has been reported that BCB+ oocytes have significantly higher mRNA level of genes involved in mitochondrial biogenesis, showing that this may be one of the factors for their high developmental competence [28].
In vitro environmental factors such as increased exposure to oxygen, light, and culture medium composition can induce metabolic alterations in oocytes and embryos, leading to an imbalance between the formation of ROS, and the antioxidant capacity [29]. This imbalance can lead to many pathological effects including lipid peroxidation, DNA fragmentation, and ultimately mitochondrial alterations, apoptosis, and fetal growth arrest [30]. ROS is involved in the pathophysiology of a number of female reproductive tract disorders such as endometriosis, polycystic ovary syndrome, preeclampsia maternal diabetes [31–33].
Oocytes are unavoidably exposed to oxidative stress during in vitro maturation [34]. Hence, during in vitro oocyte culture, oxidative stress must be regulated by addition of antioxidants to culture media. L-carnitine is an essential metabolite for energy production and glucose metabolism derived from both dietary sources and from endogenous biosynthesis [35]. L-carnitine was able to neutralize the embryotoxic effects of H2O2 (up to 500 μmol/l) in culture medium of mouse embryos [21]. Our result showed that adding 0.6 mg/ml L-carnitine into maturation medium of immature oocyte decreased ROS level in maturation medium. This result was in accordance with previous studies [36, 37]. The mechanism of the antioxidation of L-carnitine may be via an scavenging effect on 1,1-diphenyl-2-picryl-hydrazyl free radical (DPPH), superoxide anion radical, hydrogen peroxide [18].
GSH concentration in oocytes in the MII stage is relatively higher than that in the GV stage, while it is significantly reduced after fertilization. Thus, GSH content in oocytes reflects the maturity of the cytoplasm [38, 39]. Increased GSH levels in MII oocytes stimulate male pronuclear (MPN) formation and improve developmental competence of oocytes or embryos by protecting them against free radicals [39, 40]. Based on our results, L-carnitine improved GSH concentration in MII oocytes, which is in close accordance with a previous study which showed that supplementing of 0.5 mg/ml L-carnitine to IVM medium significantly increased intracellular GSH levels of porcine matured oocytes and improved development competence of parthenogenetic embryos; this was attributed to confront L-carnitine with ROS and thus preserve stores of GSH in porcine mature oocytes [41]. It appears that the positive effect of L-carnitine on oocyte cytoplasmic maturation occurs through increasing glutathione levels.
On the other hand, L-carnitine plays a key role in β-oxidation of long-chain fatty acids by providing a transmission system for free fatty acids and derivatives acyl-coenzyme A in mitochondria to generate cellular ATP [42]. The β-oxidation process is involved in the nuclear and cytoplasmic maturation of oocytes which finally results in oocyte developmental competence [43]. L-carnitine improved the nuclear maturation of porcine oocytes during in vitro culture by improving mitochondrial activity and preventing the apoptosis of cumulus cells [41]. In the present study, the treatment of immature oocytes with L-carnitine during IVM increased proportion of oocytes that reached the MII stage, and decreased degenerated oocytes rate. Thus, we concluded that L-carnitine treatment during IVM was effective on nuclear maturation through improving meiotic competence and cytoplasmic maturation through increasing GSH concentration. These results were similar with previous studies in porcine that adding L-carnitine to the IVM medium increased nuclear maturation and blastocyst development rate after parthenogenetic activation and IVF [36, 41]. The decreased degenerated oocyte rate in L-carnitine supplemented groups could also be attributed to its roles as an antioxidant and a free radical scavenger. The beneficial effects of L-carnitine are probably due to the reduction of intracellular levels of ROSs, which might lead to protection of micro-organelles such as mitochondria [37].
The mRNA stored in oocytes is a valuable molecular tool for determining oocyte quality. On the other hand, alternations in culture medium can influence the pattern of gene expression in oocytes and embryos [44–46]. Thus, we determined relative mRNA expression levels of three key maternal genes involved in cytoplasmic and nuclear maturation (GDF-9, MAPK1, and CDK1) in mice MII oocytes. GDF-9 (a member of the transforming growth factor-β family: TGF-β) is an oocyte-secreted growth factor that plays essential roles in oocyte maturation, cumulus expansion, and oogenesis [47]. It has been shown that the cytoplasmic maturation can be assayed through the expression of GDF-9 and GSH levels [38, 47, 48]. Exposure of COCs during oocyte maturation to oocyte-secreted factors (OSFs) such as GDF-9 significantly enhanced oocyte developmental competence [49]. Based on our results, the addition of L-carnitine to IVM medium of BCB+ immature oocytes had no significant effect on the expression of GDF-9.
In mammalian oocytes, the regulation of the meiotic cell cycle is controlled by a phosphorylation and/or dephosphorylation regulatory cascade of MAPK, and MPF (maturation promoting factor). MAPKs, also known as extracellular signal-regulated kinases (ERKs), have involved in many signal transduction pathways for meiotic competence acquisition [50]. Phosphorylation levels of MAPK are strongly associated with the MAPK activity which is in turn related to the expression of MAPK gene [8]. Our results showed that incubation of immature oocytes with L-Carnitine during IVM increased the expression of MAPK1 gene in oocytes. This indicates that BCB+ oocytes treated with L-carnitine have better quality than untreated BCB+ oocytes and L-carnitine treatment could create a beneficial microenvironment for stimulation of gene expression in mouse oocytes.
MPF activity is controlled by both subunits of MPF: CDK1, as a catalytic subunit and the cyclin B, as a regulatory subunit [50]. MPF activity depends upon the phosphorylation status of Thr161 in CDK1 [51]. The mRNAs, proteins, and regulatory molecules (function in the oocyte maturation, fertilization, and early embryogenesis) must be stored enough in competent oocytes to ensure that their progression would not be eliminated after fertilization [8]. MPF activity is required for sperm head decondensation and pronuclei formation [52]. MPF activity increased significantly at MII stage BCB+ oocytes [10]. Concurrently, we observed that L-carnitine caused a greater increase in the CDK1 transcript levels in mature oocytes. Although, it was not clear how L-carnitine supplementation during IVM of immature oocytes increased mRNA levels, it can be concluded that increased expression of cell cycle regulator genes have a positive effect on oocyte maturation.
Three stages of maturation processes of the oocyte (nuclear, cytoplasmic and molecular) have important roles in the oocytes developmental competence and the resulting embryo quality [27]. In the present study, L-carnitine improved cleavage and blastocysts rates when added to maturation medium of BCB+ oocytes, but had no significant effect on total blastocyst cell numbers. L-carnitine supplementation (1.5–3 mM) during embryo culture enhanced lipid metabolism, via β-oxidation generating ATP, in bovine embryos resulting in improved blastocyst development and blastocyst cell numbers [53]. The positive effects of L-carnitine treatment (10 mM) during IVM of pig oocytes on the developmental competence of somatic cell nuclear transfer (SCNT) embryos is probably due to increased intracellular GSH in recipient ooplasm, reduced ROS levels, and the stimulation of nuclear reprogramming [37]. More research is necessary to clarify the precise molecular and subcellular mechanisms underlying L-carnitine function in oocyte maturation and embryo development.
In conclusion, based on our results, improvement in the cytoplasmic and nuclear maturation of immature oocytes selected by BCB test and reduction in the level of ROS by supplementing the maturation medium with L-carnitine concentrations (0.3 and 0.6 mg/ml) is a new approach that have beneficial effects on early embryo development. The molecular mechanisms of L-carnitine that is causing these effects need to be investigated in detail in future study. This approach may have substantially clinical applications in the ART setting, and its use may improve fertility outcomes in infertile couples and prove to be affordable.
Acknowledgments
This project was financially supported by Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Footnotes
Capsule L-carnitine supplementation during IVM of immature BCB+ oocytes improved cytoplasmic and nuclear maturation of mouse oocytes and enhanced preimplantation developmental competence of embryos after IVF.
Zohreh Zare and Reza Masteri Farahani contributed equally to this work.
References
- 1.Albuz FK, Sasseville M, Lane M, Armstrong DT, Thompson JG, Gilchrist RB. Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod. 2010;25:2999–3011. doi: 10.1093/humrep/deq246. [DOI] [PubMed] [Google Scholar]
- 2.Whelan JG, 3rd, Vlahos NF. The ovarian hyperstimulation syndrome. Fertil Steril. 2000;73:883–896. doi: 10.1016/S0015-0282(00)00491-X. [DOI] [PubMed] [Google Scholar]
- 3.Wang N, Le F, Zhan QT, Li L, Dong MY, Ding GL, et al. Effects of in vitro maturation on histone acetylation in metaphase II oocytes and early cleavage embryos. Obstet Gynecol Int. 2010;2010:989278. doi: 10.1155/2010/989278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NG, Verhoeff A, et al. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980–988. doi: 10.1093/humrep/del484. [DOI] [PubMed] [Google Scholar]
- 5.Buckett WM, Chian RC, Holzer H, Dean N, Usher R, Tan SL. Obstetric outcomes and congenital abnormalities after in vitro maturation, in vitro fertilization, and intracytoplasmic sperm injection. Obstet Gynecol. 2007;110:885–891. doi: 10.1097/01.AOG.0000284627.38540.80. [DOI] [PubMed] [Google Scholar]
- 6.Nagai T. The improvement of in vitro maturation systems for bovine and porcine oocytes. Theriogenology. 2001;55:1291–1301. doi: 10.1016/S0093-691X(01)00483-6. [DOI] [PubMed] [Google Scholar]
- 7.Su J, Wang Y, Li R, Peng H, Hua S, Li Q, et al. Oocytes selected using BCB staining enhance nuclear reprogramming and the in vivo development of SCNT embryos in cattle. PLoS ONE. 2012;7:e36181. doi: 10.1371/journal.pone.0036181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torner H, Ghanem N, Ambros C, Holker M, Tomek W, Phatsara C, et al. Molecular and subcellular characterisation of oocytes screened for their developmental competence based on glucose-6-phosphate dehydrogenase activity. Reproduction. 2008;135:197–212. doi: 10.1530/REP-07-0348. [DOI] [PubMed] [Google Scholar]
- 9.Roca J, Martinez E, Vazquez JM, Lucas X. Selection of immature pig oocytes for homologous in vitro penetration assays with the brilliant cresyl blue test. Reprod Fertil Dev. 1998;10:479–485. doi: 10.1071/RD98060. [DOI] [PubMed] [Google Scholar]
- 10.Catala MG, Izquierdo D, Uzbekova S, Morato R, Roura M, Romaguera R, et al. Brilliant Cresyl Blue stain selects largest oocytes with highest mitochondrial activity, maturation-promoting factor activity and embryo developmental competence in prepubertal sheep. Reproduction. 2011;142:517–527. doi: 10.1530/REP-10-0528. [DOI] [PubMed] [Google Scholar]
- 11.Alm H, Torner H, Lohrke B, Viergutz T, Ghoneim IM, Kanitz W. Bovine blastocyst development rate in vitro is influenced by selection of oocytes by brillant cresyl blue staining before IVM as indicator for glucose-6-phosphate dehydrogenase activity. Theriogenology. 2005;63:2194–2205. doi: 10.1016/j.theriogenology.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 12.Karja NW, Wongsrikeao P, Murakami M, Agung B, Fahrudin M, Nagai T, et al. Effects of oxygen tension on the development and quality of porcine in vitro fertilized embryos. Theriogenology. 2004;62:1585–1595. doi: 10.1016/j.theriogenology.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Mo X, Wu G, Yuan D, Jia B, Liu C, Zhu S, et al. Leukemia inhibitory factor enhances bovine oocyte maturation and early embryo development. Mol Reprod Dev. 2014;81:608–618. doi: 10.1002/mrd.22327. [DOI] [PubMed] [Google Scholar]
- 14.Choe C, Shin YW, Kim EJ, Cho SR, Kim HJ, Choi SH, et al. Synergistic effects of glutathione and beta-mercaptoethanol treatment during in vitro maturation of porcine oocytes on early embryonic development in a culture system supplemented with L-cysteine. J Reprod Dev. 2010;56:575–582. doi: 10.1262/jrd.09-214H. [DOI] [PubMed] [Google Scholar]
- 15.Dehghani-Mohammadabadi M, Salehi M, Farifteh F, Nematollahi S, Arefian E, Hajjarizadeh A, et al. Melatonin modulates the expression of BCL-xl and improve the development of vitrified embryos obtained by IVF in mice. J Assist Reprod Genet. 2014;31:453–461. doi: 10.1007/s10815-014-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanella A, Russo A, Acquaviva R, Campisi A, Di Giacomo C, Sorrenti V, et al. L -propionyl-carnitine as superoxide scavenger, antioxidant, and DNA cleavage protector. Cell Biol Toxicol. 2000;16:99–104. doi: 10.1023/A:1007638025856. [DOI] [PubMed] [Google Scholar]
- 17.Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64:904–909. doi: 10.1095/biolreprod64.3.904. [DOI] [PubMed] [Google Scholar]
- 18.Gulcin I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006;78:803–811. doi: 10.1016/j.lfs.2005.05.103. [DOI] [PubMed] [Google Scholar]
- 19.Qi SN, Zhang ZF, Wang ZY, Yoshida A, Ueda T. L-carnitine inhibits apoptotic DNA fragmentation induced by a new spin-labeled derivative of podophyllotoxin via caspase-3 in Raji cells. Oncol Rep. 2006;15:119–122. [PubMed] [Google Scholar]
- 20.Winter BK, Fiskum G, Gallo LL. Effects of L-carnitine on serum triglyceride and cytokine levels in rat models of cachexia and septic shock. Br J Cancer. 1995;72:1173–1179. doi: 10.1038/bjc.1995.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdelrazik H, Sharma R, Mahfouz R, Agarwal A. L-carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertil Steril. 2009;91:589–596. doi: 10.1016/j.fertnstert.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 22.Cook JA, Pass HI, Iype SN, Friedman N, DeGraff W, Russo A, et al. Cellular glutathione and thiol measurements from surgically resected human lung tumor and normal lung tissue. Cancer Res. 1991;51:4287–4294. [PubMed] [Google Scholar]
- 23.Agarwal A, Allamaneni SS, Said TM. Chemiluminescence technique for measuring reactive oxygen species. Reprod BioMed Online. 2004;9:466–468. doi: 10.1016/S1472-6483(10)61284-9. [DOI] [PubMed] [Google Scholar]
- 24.Sharma RK, Agarwal A. Role of reactive oxygen species in gynecologic diseases. Reprod Med Biol. 2004;3:177–199. doi: 10.1111/j.1447-0578.2004.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuccotti M, Boiani M, Ponce R, Guizzardi S, Scandroglio R, Garagna S, et al. Mouse Xist expression begins at zygotic genome activation and is timed by a zygotic clock. Mol Reprod Dev. 2002;61:14–20. doi: 10.1002/mrd.1126. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Sirard MA, Richard F, Blondin P, Robert C. Contribution of the oocyte to embryo quality. Theriogenology. 2006;65:126–136. doi: 10.1016/j.theriogenology.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Opiela J, Lipinski D, Slomski R, Katska-Ksiazkiewicz L. Transcript expression of mitochondria related genes is correlated with bovine oocyte selection by BCB test. Anim Reprod Sci. 2010;118:188–193. doi: 10.1016/j.anireprosci.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Thiyagarajan B, Valivittan K. Ameliorating effect of vitamin E on in vitro development of preimplantation buffalo embryos. J Assist Reprod Genet. 2009;26:217–225. doi: 10.1007/s10815-009-9302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med. 1999;26:463–471. doi: 10.1016/S0891-5849(98)00216-0. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanti A, Santanam N, Morales AJ, Parthasarathy S, Murphy AA. Autoantibodies to markers of oxidative stress are elevated in women with endometriosis. Fertil Steril. 1999;71:1115–1118. doi: 10.1016/S0015-0282(99)00145-4. [DOI] [PubMed] [Google Scholar]
- 33.Karuputhula NB, Chattopadhyay R, Chakravarty B, Chaudhury K. Oxidative status in granulosa cells of infertile women undergoing IVF. Syst Biol Reprod Med. 2013;59:91–98. doi: 10.3109/19396368.2012.743197. [DOI] [PubMed] [Google Scholar]
- 34.Natarajan R, Shankar MB, Munuswamy D. Effect of alpha-tocopherol supplementation on in vitro maturation of sheep oocytes and in vitro development of preimplantation sheep embryos to the blastocyst stage. J Assist Reprod Genet. 2010;27:483–490. doi: 10.1007/s10815-010-9430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fenkci SM, Fenkci V, Oztekin O, Rota S, Karagenc N. Serum total L-carnitine levels in non-obese women with polycystic ovary syndrome. Hum Reprod. 2008;23:1602–1606. doi: 10.1093/humrep/den109. [DOI] [PubMed] [Google Scholar]
- 36.Wu GQ, Jia BY, Li JJ, Fu XW, Zhou GB, Hou YP, et al. L-carnitine enhances oocyte maturation and development of parthenogenetic embryos in pigs. Theriogenology. 2011;76:785–793. doi: 10.1016/j.theriogenology.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 37.You J, Lee J, Hyun SH, Lee E. L-carnitine treatment during oocyte maturation improves in vitro development of cloned pig embryos by influencing intracellular glutathione synthesis and embryonic gene expression. Theriogenology. 2012;78:235–243. doi: 10.1016/j.theriogenology.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 38.Rausell F, Pertusa JF, Gomez-Piquer V, Hermenegildo C, Garcia-Perez MA, Cano A, et al. Beneficial effects of dithiothreitol on relative levels of glutathione S-transferase activity and thiols in oocytes, and cell number, DNA fragmentation and allocation at the blastocyst stage in the mouse. Mol Reprod Dev. 2007;74:860–869. doi: 10.1002/mrd.20569. [DOI] [PubMed] [Google Scholar]
- 39.Luberda Z. The role of glutathione in mammalian gametes. Reprod Biol. 2005;5:5–17. [PubMed] [Google Scholar]
- 40.Yoshida M, Ishigaki K, Nagai T, Chikyu M, Pursel VG. Glutathione concentration during maturation and after fertilization in pig oocytes: relevance to the ability of oocytes to form male pronucleus. Biol Reprod. 1993;49:89–94. doi: 10.1095/biolreprod49.1.89. [DOI] [PubMed] [Google Scholar]
- 41.Somfai T, Kaneda M, Akagi S, Watanabe S, Haraguchi S, Mizutani E, et al. Enhancement of lipid metabolism with L-carnitine during in vitro maturation improves nuclear maturation and cleavage ability of follicular porcine oocytes. Reprod Fertil Dev. 2011;23:912–920. doi: 10.1071/RD10339. [DOI] [PubMed] [Google Scholar]
- 42.Jeulin C, Lewin LM. Role of free L-carnitine and acetyl-L-carnitine in post-gonadal maturation of mammalian spermatozoa. Hum Reprod Update. 1996;2:87–102. doi: 10.1093/humupd/2.2.87. [DOI] [PubMed] [Google Scholar]
- 43.Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod. 2010;83:909–918. doi: 10.1095/biolreprod.110.084145. [DOI] [PubMed] [Google Scholar]
- 44.Zhou W, Xiang T, Walker S, Farrar V, Hwang E, Findeisen B, et al. Global gene expression analysis of bovine blastocysts produced by multiple methods. Mol Reprod Dev. 2008;75:744–758. doi: 10.1002/mrd.20797. [DOI] [PubMed] [Google Scholar]
- 45.Racedo SE, Wrenzycki C, Lepikhov K, Salamone D, Walter J, Niemann H. Epigenetic modifications and related mRNA expression during bovine oocyte in vitro maturation. Reprod Fertil Dev. 2009;21:738–748. doi: 10.1071/RD09039. [DOI] [PubMed] [Google Scholar]
- 46.Salhab M, Dhorne-Pollet S, Auclair S, Guyader-Joly C, Brisard D, Dalbies-Tran R, et al. In vitro maturation of oocytes alters gene expression and signaling pathways in bovine cumulus cells. Mol Reprod Dev. 2013;80:166–182. doi: 10.1002/mrd.22148. [DOI] [PubMed] [Google Scholar]
- 47.Paradis F, Novak S, Murdoch GK, Dyck MK, Dixon WT, Foxcroft GR. Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Reproduction. 2009;138:115–129. doi: 10.1530/REP-08-0538. [DOI] [PubMed] [Google Scholar]
- 48.Lee SE, Kim EY, Choi HY, Moon JJ, Park MJ, Lee JB, et al. Rapamycin rescues the poor developmental capacity of aged porcine oocytes. Asian-Australas J Anim Sci. 2014;27:635–647. doi: 10.5713/ajas.2013.13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hussein TS, Thompson JG, Gilchrist RB. Oocyte-secreted factors enhance oocyte developmental competence. Dev Biol. 2006;296:514–521. doi: 10.1016/j.ydbio.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 50.Zhang DX, Park WJ, Sun SC, Xu YN, Li YH, Cui XS, et al. Regulation of maternal gene expression by MEK/MAPK and MPF signaling in porcine oocytes during in vitro meiotic maturation. J Reprod Dev. 2011;57:49–56. doi: 10.1262/jrd.10-087H. [DOI] [PubMed] [Google Scholar]
- 51.De Smedt V, Poulhe R, Cayla X, Dessauge F, Karaiskou A, Jessus C, et al. Thr-161 phosphorylation of monomeric Cdc2. Regulation by protein phosphatase 2C in Xenopus oocytes. J Biol Chem. 2002;277:28592–28600. doi: 10.1074/jbc.M202742200. [DOI] [PubMed] [Google Scholar]
- 52.Kim KH, Kim EY, Kim Y, Kim E, Lee HS, Yoon SY, et al. Gas6 downregulation impaired cytoplasmic maturation and pronuclear formation independent to the MPF activity. PLoS ONE. 2011;6:e23304. doi: 10.1371/journal.pone.0023304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi T, Inaba Y, Somfai T, Kaneda M, Geshi M, Nagai T, et al. Supplementation of culture medium with L-carnitine improves development and cryotolerance of bovine embryos produced in vitro. Reprod Fertil Dev. 2013;25:589–599. doi: 10.1071/RD11262. [DOI] [PubMed] [Google Scholar]