Abstract
CD44 is a cellular protein that has been intensively studied in relation to carcinogenesis over the last decade. It is altered during inflammatory responses and cellular malfunctioning during tumor progression. Tumors of epithelial origin express CD44 in multiple isoforms called variants; some isoforms are related to specific cancer cells. An increase of CD44 specific isoforms is detected in certain leukemic proliferations. Most published data indicates a partial involvement of CD44 in cancer cells, either in invasiveness or self-renewability. However, there is still uncertainty regarding the exact mechanism by which CD44 participates in growth of cancer or the inflammatory response. This review focuses on CD44 prevalence in cancer cell. It considers tumorigenic behavior of cells that highly express CD44 as an early marker for neoplastic stem cell proliferation. We will discuss multiple examples of tumor in this paper, with an emphasis of 2 solid tumors; namely, breast and colon cancer.
CD44 is a trans membrane glycoprotein that has various functions in cell division, migration, adhesion, and signaling. CD44 normally binds to its primary ligand hyaluronic acid (HA). This binding is thought to be responsible for cellular signaling, and regulating other biological process within cells. Cells within a tissue interact either through the intracellular matrix (ICM) or through cellular junctions. CD44 as an adhesion molecule is enables cell communication by cell-cell signal transduction.1 In addition, CD44 also mediates the signal transduction of human epidermal growth factor receptor (HER) and common cell signaling pathways regulates cell division (MET) receptor tyrosine kinases, and works as a platform for some growth factors as well as heparan sulphate proteoglycans.2 CD44 has a molecular weight of around 85-200 KDa. The studied trans-protein is widely expressed in almost all body cell types. These cell surface glycoproteins can be found in leukocytes, fibroblasts, epithelial, mesodermal, and neuroectodermal cells.1 This distribution helps the investigation, since it is also found in many cancer stem cells (CSCs). CD44 prevalence in cancer cell attracts our attention to focus on its relation to molecular onset of tumor progression. This study considers the tumorigenic behavior of cells that highly express CD44 as an early marker for neoplastic stem cell proliferation. We discuss multiple examples of tumors in this paper, with an emphasis on 2 solid tumors; namely, breast and colon cancer.
Genetic structure (Figure 1)
Figure 1.
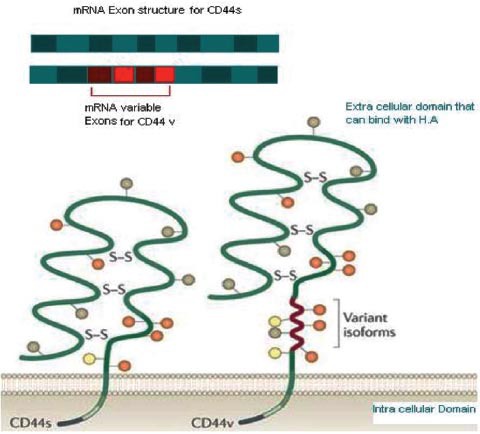
CD44 molecular structure, a comparison between standard (CD44s) and variant (CD44v) forms. Illustration of the different exons of CD44. The upper green section shows a constant region of messenger RNA included in every possible variant. The red section is a variant exon that might be selected via alternative splicing of RNA. The lower shows the different domains of a CD44 molecule. The trans-membrane domain, which contains 269-289 amino-acids (a.a); then, the extracellular domain where the variant isoform is located with 292-300 a.a. The extracellular domain forms and the binding site for hyaluronate acid is structurally maintained by a disulphide bond.28 Copyright permission from Nature Reviews Cancer. Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 2011; 11: 254-267.
Scientists studying CD44 genetic composition in human cells found that the CD44 gene is located on the short arm of chromosome 11. It contains 50 KB of human DNA. The CD44 chromosome consists of 20 exons, and 12 of them have played a role in splicing. Exons 1-5 encode the constant region of the extracellular domain, whereas exons 6-15 exons are encoded for variable sides of the extracellular domain. The proximal region of the extracellular domain is encoded by exon 16 and 17. Exon 18 is a constant exon that encodes the hydrophobic region and the first 3 amino acids of the cytoplasmic tail in CD44. Exon 19 is rich with adenine and thymine, it is an un-translated region believed to generate a 3 amino acid long but not short cytoplasmic tail. Exon 20 participates to produce the long cytoplasmic tail of CD44.
CD44 isoforms
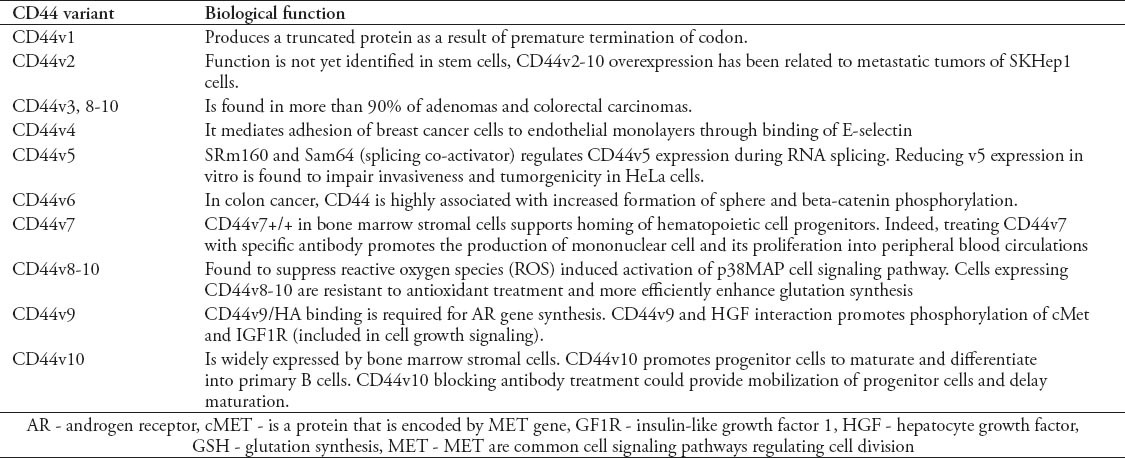
CD44 has more than 20 isoform generated out of RNA alternative splicing. These isoforms regulate and participate in cellular differentiation. The commonly known isoforms comprise 12 isoforms. These isoforms are known as variants, and are symbolized by “v”. The nomenclature for this is CD44v#. These variants can be isolated out of any normal functioning cells, and can also be observed in pathological cells. CD44H is a hematopoietic isoform, which is also known as the standard isoform because it is the simplest isoform. CD44H or CD44s is the most commonly distributed isoform. It is expressed in all cell types of blood, including white blood cells, and natural killers. Another good example of the wide diversity of CD44 isoforms is the CD44E, which is weakly expressed by epithelial cells. Mononuclear white blood cells also express the important isoforms, CD44 R1 and R2, which are isolated in granulocytes and some leukemia’s. However, specific CD44 variants have been found highly expressed in certain cancer metastatic cells stem cells.1 For example, CD44v6 is suggested to be involved in breast and colorectal cancers.3 The observation of over expression of certain variants with specific cancers, can lead to a correlated relation in incidence. The high frequency of variants CD44v6-v10 reported in metastatic growth, for instance, could indicate that exon v6 boost metastasis of cancer cells, especially in lymphoma;3 however, its function is not yet clear. Table 1 illustrates some biological functions of CD44 variants.
Table 1.
CD44 variants and related biological functions.7
Pathological role of CD44
CD44 is highly expressed in many diseases. It has been found in cancerous, inflammatory, and auto-immunological diseases. To find a precise relation of CD44 to specific cancers, scientists investigated the CD44 isoforms, to correlate specific isoform with certain types of CSCs and inflammatory immunological reactions. It was found that the CD44 levels were higher in many tumor malignancies, chronic inflammatory reactions, and autoimmune dysfunctions. For example, CD44v6 has been associated with the aggressiveness of human non-Hodgkin’s lymphomas.1 The immunological response is mediated through a variety of cellular and soluble elements. T-cell lymphocyte and dendritic cells are evolved with CD44 expression. CD44 is engaged in rearrangement of the cytoskeleton and adhesion of T-cell lymphocytes. The T cell receptor (TCR) signaling pathway is initiated by CD44 and associated tyrosine phospho-kinases p56lck/p59fyn.4 In dendritic cells (DC), cross-linking of CD44 with monoclonal antibody (mAb) promotes DC aggregation and maturation. It also increases secretion of cytokines such as interleukin (IL)-8, tumor necrosis factor (TNF)-alfa and IL-1beta. However, treating DC with anti-CD44 mAb inhibited T cell activity in vitro.5 The binding of the major histocompatibility (MHC) peptide with TCR is mediated through the adenomatous polyposis coli (APC) cell junction. These highly organized junctions are referred to as immunological synapses. The immunological synapse recruits CD44 during allogeneic binding between T cells and dendritic cells, which cause further activation in the T cells.4 Binding of CD44 to its ligand HA regulates the immuno-inflammatory response. For instance, IL-5 is the major interleukin responsible for eosinophilic inflammatory asthma. Interestingly, it has been seen to promote the expression of CD44 in human eosinophils and in murine B cells.1 CD44 promotes homing of CSCs in many types of solid tumors, such as in breast and prostate cancers. It mediates leukemia stem cells in homing to their niches. Interestingly, CD44 expression was down regulated when CSCs was introduced to cancer suppressor elements such as p53 and microRNA 34a.6
CD44 interactions in stem cells
The CD44 is a glycoprotein receptor that is activated by binding to its major ligand HA. This binding regulates stem cells homing. In hematopoietic stem cells, CD44 mediate these cells homing on bone marrow. Platelet derived growth factor stimulates mesenchymal stems cell (MSCs) to produce more CD44 that facilitates cell traveling through extracellular HA binding. This process is suggested to help in recruitment of MSCs during tissue development and healing.7 CD44/HA binding seems to interact with different intrinsic niche factors. One common pathway is the reaction with extracellular heparan sulphate. Heparan sulphate is known to bind with protein, and gives rise to heparan sulphate proteoglycan. The heparan sulphate moiety enables CD44v3 to bind with factors such fibroblast growth factor 2, and higher heparin-binding epidermal growth factor (HB-EGF).8 Growth factors mediate CD44 to link to TRK to work as cellular signal transducers. On the other hand, CD44 could be also interacting with integrin. Integrin is a cell adhesion molecule linking extracellular adhesion molecules to the intracellular actin cytoskeleton.9 Integrin generally is found to play a role in cell-to-cell signaling, cell differentiation, proliferation, and migration. So, CD44 and integrin association in these bimolecular interactions could lead to an assumption that CD44 mediates integrin functions in the cell.7
Cancer stem cells synthesizes HA (the primary ligand for CD44) to attract tumor-associated macrophages (TAM) in the CSC niches.10 The CSCs and TAMs produce oncogenic growth factor platelet derived growth factor (PDGF-BB) that maintains the tumor cell in a sustained proliferative phase. As a result, this interaction recruits stromal cells into the CSC niche. Stromal cells are known to produce multiple growth factors that regulate stem cells activity and reproductively.7
CD44s interaction with HA plays a crucial role in cell invasiveness. Flushing an in vitro culture of breast cancer cells with high molecular weight (HMW) motivate invasion of tumor cells by 45% cell invasion and metastisis can be inhibited by pre-incubating the Matrigel with anti-CD44s or HAOligo-6. This approach indicates that interaction between HA and CD44 is pivotal in this process. The mechanism of how HA affects invasion remains unclear. The HA is found more concentrated in premalignant and malignant cell than normal cells, supporting the theory that it plays a role in cancer invasiveness.11
CD 44 and breast cancer
Breasts cancer cells show a remarkable heterogeneity and diversity among breast cancers and within tumors. Examining breast cancer cells with a technique known as Gene Expression Profiling indicates that cancer cells show a diverse population of cell subtypes. CD44 and another surface protein, CD24, were both abnormally expressed in breast cancer cells.2 The CD44/CD24 ratio could be a guideline for early detection of neoplastic growth in human breast tissue. Over the last 10 years, clinical studies investigated the reason for elevated expression of CD44 found on cell surfaces isolated from breast cancer patients. Most studies claim no statistical significance between CD44 overexpression and growth/metastasis of breast tumor. However, Fillmore & Kuperwasser,12 reported a correlation between the irregularity of CD44+/CD24- ratio in breast cancer cells and the mesenchymal like phenotype (MDA. MB231, SUM159 and SUM1315). They also suggested that CD44 is a basal line cell that could be used as a marker for cases with a poor breast cancer prognosis, while CD24 expression was found in abundance in the luminal differentiated type of breast cancer.12
By reviewing recent and older studies on CD44 malfunctioning in breast cancer, we found discrepancies in the outcomes and justifications, including discrepancies in techniques used and/or sample storage/processing methods. In 2011, the study of Olsson et al2 used more advanced molecular techniques including real time polymerase chain reaction (RT-PCR) and Western blot to examine the associations between the mRNA expression of CD44 isoforms and the CSC phenotypes CD44 of 151 frozen breast tumor samples. Unlike antibody antigen dependent assays, this technique measures the level of various isoforms in breast CSCs. They found a heterogeneous expression of CD44 isoforms in different cell lines of breast cancer. Surprisingly, CD44s isoform were detected highly expressed in mesenchymal cell lines. CD44 plays an important role in different subtypes of breast cancer. ?It might acquire an oncogenic signaling pathway since it have been found interfering with the expression of well-known oncogenic markers such HER2, ER, and progesterone receptor (PgR).
Since the CD44+/CD24- ratio is highly expressed in cancerous cells in general, it is also important to investigate whether it has a role in chemotherapy resistance and relapsing of breast cancer. Li et al13 conducted an experiment on human cancer breast cells to identify the role of CD44 in breast cancer cells. Initially, breast cancer cells expressed high levels of CD44 and low CD24 levels. Conventional chemotherapy on in vitro isolated cancer cell showed an increased expression of CD44+/CD24- and formation of mammospheres, but there was a non-statistically significant reduction in expression when treated with lapitine. This indicates an intrinsic chemical resistance of cells has a high expression of CD44.13
Receptor tyrosine kinase and CD44
Receptor tyrosine kinase (RTKs) is a cellular surface receptor for many growth factors and cytokines that regulate cellular proliferation and functioning. The RTKs were identified as critical key factors in the initiation and development of many cancers.14 CD44 mediates and promotes the activity of RTKs in tumor cells. For example, in ovarian cancer, binding of HA to CD44/N-WASP is promoted by receptor tyrosine kinase 2 (ErBB2) kinase activity. As a result, beta-catenin phosphorylation increases, then phosphorylated beta-catenin diffuses into the nucleus and binds to T-cell factor/lymphoid enhancer factor (TCF/LEF), which promotes the transcription.15 Another example of growth factor/CD44 intervention is found with transforming growth factor-1ß (TGF-1ß) where the CD44/epidermal growth factor receptor interaction is promoted by binding of HA (the primary ligand for CD44). In turn, CD44/EGFR interaction activates the mitogen-activated protein kinases/extracellular signal-regulated kinases pathway leading to an increase in TGF-ß1 dependent fibroblast proliferation.16 In insulin like growth factor receptor-1 (GF1R), which is responsible for uncontrolled proliferation of cancer cell, CD44v6 along with IGF1R mediates homing of multiple myeloma cells to bone marrow.17
The CD44/RTKs signaling pathway regulates microRNA expression. Binding of CD44 to HER2 elevates the expression of metastasis-associated protein type 1 (MTA1) in gastric cancer cells. The MTA1 deactylates the H3 histone and silences the promoter region for miR139 leading to reduction in miR139 expression - unclear, please clarify. In turn, it inhibits the C-X-C receptor 4.18 An increase in HER2/CD44 expression along with a reduction of miRNA 139/CXC4 expression could be a comprehensive element for gastric tumor growth and metastasis. These examples indicate the influence of CD44 on RTKs physiocellular activity. Although, CD44 is not the primary factor in activating RTK’s mutations, it has been pointed out that CD44 were associated with RTK mutations.
CD44 in intestinal and colon cancers
In intestinal CSCs, CD44 variants 4-10 were found to be highly expressed in intestinal cancers of mice. It is questionable whether CD44 variants regulate or have any effect on the renewability of CSCs in intestinal human cancers. The CD44 standard isoform has never been isolated in CSC; however, other variants of CD44 were massively found in such tumors. Alternative splicing for CD44 mRNA produces many variants of CD44, which makes it difficult for researchers to identify the exact function for each isoform in CSCs. For example, CD44v4 that is known to mediate cell adhesion to endothelial monolayer in breast cancer,7 has also been found in colon cancer with an undetermined role. Indeed, multiple studies use CD44 antibodies, which are not precise enough to identify which exact variant initiates or regulates CSCs differentiation in the colon. Although researchers all agree on the tumorigenicity of CD44, the exact function of CD44 in the tumor cell is not clear.6
In 2011, Ishimoto et al19 conducted experiments to investigate oxidative stress resistance in CSCs in intestinal tumors. Normal tumor cells possess a defensive mechanism against oxidative free radicals that eradicates CSCs. Secretion of redox enzymes and other anti-oxidants is one strategy CSCs use to fight for survival. Reduced glutathione (GSH) is another factor to protect cancer cells from oxidative anticancer chemicals. Ishimoto et al19 assessed these metabolites in intestinal CD44v, and found them high. They concluded that the CD44v reactive oxygen species (ROS) resistance mechanism abrogates apoptosis and promotes longer life span by fighting against normal oxidative elements for such cells. The study suggested that by targeting CD44v ROS defense mechanisms, researchers could exterminate gastrointestinal cancers and force these cells to follow a normal apoptotic cellular cycle.19
CD44 and leukemia
Normal hematopoietic cell proliferation in adults occurs in bone marrow, with some further maturation in other organs. Evidence from recent studies20 suggests that CD44 regulates normal hematopoietic cell proliferation. In vitro treatment of long-term bone marrow culture (LTC) cells with anti-CD44 antibodies during differentiation stage shows a reduction in number of mature cell.20 The only feasible explanation for this is that CD44 plays a role in myeloid cell proliferation in bone marrow. Accordingly, we can assume that CD44 mediates some micro-environmental functions of bone marrow. Acute myeloid leukemia (AML) is characterized by extensive proliferation of blood cells progenitor producing blast cells that are functionally disabled to serve the body. The ligation of CD44 activated mAbs can cause reverse blockage in the differentiation AML blast in some myeloid leukemia subtypes such as M1, 2, and 6. CD44 ligation with mAbs can also inhibit proliferation and induce apoptosis.21 CD44 modulates the function of AML stem cells and facilitates the proper homing of stem cell to niches of the bone marrow microenvironment. Binding of CD44 to HA or mAbs regulates many functions of hematopoietic stem cells including differentiation of myeloid and lymphoid cells.21,22
CD44 as a therapeutic target
CD44 could be a useful tool to abrogate cancer cell proliferation and metastasis. Cancer hepatic cells, when treated with antisense oligonucleotide (ASO) showed a down regulation in CD44 expression combined with an increase in cellular apoptosis, which may reduce invasion and metastasis of the cancer.23 Down-regulated expression of CD44 increases the chemo-sensitivity of hepatic cancer cells to doxorubicin (anti-cancer chemotherapy). Therefore, making CD44 a potential target for cancer therapy. In patients with chronic cell leukemia (CLL), targeting CD44 expression in myeloid cells could avoid immature proliferation of white blood cells. A recent study24 proposed that treatment with a single dose of 1 mg/kg anti-CD44 mAb caused a complete eradication of engrafted chronic lymphoid leukemia cells. Antibody ligation to CD44 induces caspase dependent apoptosis in “ZAP-70Pos” myeloid cells. However, treatment with mAb requires close attention to avoid non-specific toxicity that might occur. Ligation of a specific antibody is a new way to enhance the success of chemotherapy in patients with AML. Cyclin dependent kinase inhibitor (p27) is a general tumor suppressor factor. Ligation of CD44 with anti-CD44 monoclonal antibodies up regulates the production of p27 in AML agent with CD44. Despite the mechanism by which AML blast is down regulated, increased levels of p27 have been combined with better outcomes in AML patient prognosis.25 An increase in the level of p27 seems to inhibit the proliferation of primary cells in AML. In addition to chemo radiotherapy, CD44 ligation could be a synergistic treatment administered to leukemia patients to improve the overall treatment plan. Leukemia stem cells (LSC) are suggested to be the primary cause for the relapse of leukemic cells after chemotherapy. Recently, many clinical trials26 reviewed the effect of an anti-CD44 agent in assisting in preventing remission of MAL. The LSC homing in bone marrow niches is assisted by CD44; namely, cell adhesion molecule down-expression of CD44 could impair LSC self-renewability and promote its sensitivity to chemotherapy. Early recognition of any disease will help in promoting the chances of treatment, and the prognosis of cancer specifically. Cancer markers help healthcare providers to assess the situation in early stages of tumor growth. CD44, and its isoform, seem to be good indicative markers for certain cancers. CD44 isoform expression changes during prostate epithelial differentiation.27 Therefore, variant v3-v10, for example, can be a marker of the early stages of cell differentiation in prostate cancer. By using the immunomagnetic cell sorting technique, CD44 could be employed as a tool for separating basal from luminal prostate cells.
In conclusion, over the last decade, CD44 molecular interactions have been intensively studied from different points of view. Researchers explored the pathological role of CD44 in patients with different types of tumor, investigating how it can nourish cancer growth. Some studies held in vitro revealed a significant increase of CD44 variants. However, when these same variants were studied in real patients, a normal expression of CD44 was found. Hence, we cannot be sure of the exact relation between cancer and overexpression of CD44 depending on a study of in vitro cell culture. In blood cancers, a number of scholars investigating human lymphoma in vivo reported overexpression of certain variants of CD44. In breast and colon cancers, CD44 could be used along with other parameters as a marker for early detection of a tumor. Studying CD44 overexpression in murine tumor is considered weak evidence to precisely determine the role of CD44 in humans.
Cancer biology indicates that not only one factor could lead to initiation of cancer; multiple reasons are required to begin an uncontrolled cell division. Antibody inactivation of CD44 in tumor cells could conclude the role of CD44 when reduction of tumor cell division is monitored. Most recent research claims that an abnormal increase of CD44 has been found in cancers cells without a clear explanation of the mechanism involved in this process. Therefore, considering CD44 as a viable reason for cancer progression requires further research to discover the role CD44 completely.
Upon our review of this topic, we can expect many applications for CD44 in medicine. CD44 could be a diagnostic marker for specific cancer cells. CD44 also can be employed as a therapeutic alternative for eradicating or sensitizing cancer cells for chemotherapy. We believe CD44 requires further investigation for use as a successful marker in the early detection of multiple cancer cell lines.
Footnotes
Related Articles.
Alsobhi EM, Hashim IA, Abdelaal MA, Aljifri AM, Alshamy AM. Elevated cerebrospinal fluid beta-2 microglobulin as a tumor marker in a patient with myeloma of the central nervous system. Saudi Med J 2007; 28: 128-130.
Vrdoljak DV, Knezevic F, Ramljak V. The relation between tumor marker Ca 15-3 and metastases in interpectoral lymph nodes in breast cancer patients. Saudi Med J 2006; 27: 460-462.
Yildirim A, Akkus M, Nergiz Y, Yuruker S. Immunohistochemical analysis of CD31, CD36, and CD44 antigens in human omentum. Saudi Med J 2004; 25: 308-312.
References
- 1.Gee K, Kryworuchko M, Kumar A. Recent advances in the regulation of CD44 expression and its role in inflammation and autoimmune diseases. Arch Immunol Ther Exp (Warsz) 2004;52:13–26. [PubMed] [Google Scholar]
- 2.Olsson E, Honeth G, Bendahl PO, Saal LH, Gruvberger-Saal S, Ringnér M, et al. CD44 isoforms are heterogeneously expressed in breast cancer and correlate with tumor subtypes and cancer stem cell markers. BMC Cancer. 2011;11:418. doi: 10.1186/1471-2407-11-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akisik E, Bavbek S, Dalay N. CD44 variant exons in leukemia and lymphoma. Pathol Oncol Res. 2002;8:36–40. doi: 10.1007/BF03033699. [DOI] [PubMed] [Google Scholar]
- 4.Hegde VL, Singh NP, Nagarkatti PS, Nagarkatti M. CD44 mobilization in allogeneic dendritic cell-T cell immunological synapse plays a key role in T cell activation. J Leukoc Biol. 2008;84:134–142. doi: 10.1189/jlb.1107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Termeer C, Averbeck M, Hara H, Eibel H, Herrlich P, Sleeman J, et al. Targeting dendritic cells with CD44 monoclonal antibodies selectively inhibits the proliferation of naive CD4+T-helper cells by induction of FAS-independent T-cell apoptosis. Immunology. 2003;109:32–40. doi: 10.1046/j.1365-2567.2003.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo W, Frenette PS. Alternative CD44 splicing in intestinal stem cells and tumorigenesis. Oncogene. 2014;33:537–538. doi: 10.1038/onc.2013.260. [DOI] [PubMed] [Google Scholar]
- 7.Williams K, Motiani K, Giridhar PV, Kasper S. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp Biol Med (Maywood) 2013;238:324–338. doi: 10.1177/1535370213480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett KL, Jackson DG, Simon JC, Tanczos E, Peach R, Modrell B, et al. CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol. 1995;128:687–698. doi: 10.1083/jcb.128.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delon I, Brown NH. Integrins and the actin cytoskeleton. Curr Opin Cell Biol. 2007;19:43–50. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Okuda H, Kobayashi A, Xia B, Watabe M, Pai SK, Hirota S, et al. Hyaluronan synthase HAS2 promotes tumor progression in bone by stimulating the interaction of breast cancer stem-like cells with macrophages and stromal cells. Cancer Res. 2012;72:537–547. doi: 10.1158/0008-5472.CAN-11-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afify A, Purnell P, Nguyen L. Role of CD44s and CD44v6 on human breast cancer cell adhesion, migration, and invasion. Exp Mol Pathol. 2009;86:95–100. doi: 10.1016/j.yexmp.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Fillmore C, Kuperwasser C. Human breast cancer stem cell markers CD44 and CD24: enriching for cells with functional properties in mice or in man? Breast Cancer Res. 2007;9:303. doi: 10.1186/bcr1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 14.Zwick E, Bange J, Ullrich A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr Relat Cancer. 2001;8:161–173. doi: 10.1677/erc.0.0080161. [DOI] [PubMed] [Google Scholar]
- 15.Bourguignon LY, Zhu H, Chu A, Iida N, Zhang L, Hung MC. Interaction between the adhesion receptor, CD44, and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J Biol Chem. 1997;272:27913–27918. doi: 10.1074/jbc.272.44.27913. [DOI] [PubMed] [Google Scholar]
- 16.Meran S, Luo DD, Simpson R, Martin J, Wells A, Steadman R, et al. Hyaluronan facilitates transforming growth factor-ß1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. J Biol Chem. 2011;286:17618–17630. doi: 10.1074/jbc.M111.226563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 18.Bao W, Fu HJ, Xie QS, Wang L, Zhang R, Guo ZY, et al. HER2 interacts with CD44 to up-regulate CXCR4 via epigenetic silencing of microRNA-139 in gastric cancer cells. Gastroenterology. 2011;141:2076–2087. doi: 10.1053/j.gastro.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 19.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Ghaffari S, Dougherty GJ, Lansdorp PM, Eaves AC, Eaves CJ. Differentiation-associated changes in CD44 isoform expression during normal hematopoiesis and their alteration in chronic myeloid leukemia. Blood. 1995;86:2976–2985. [PubMed] [Google Scholar]
- 21.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 22.Sconocchia G, Campagnano L, Adorno D, Iacona A, Cococcetta NY, Boffo V, et al. CD44 ligation on peripheral blood polymorphonuclear cells induces interleukin-6 production. Blood. 2001;97:3621–3627. doi: 10.1182/blood.v97.11.3621. [DOI] [PubMed] [Google Scholar]
- 23.Xie Z, Choong PF, Poon LF, Zhou J, Khng J, Jasinghe VJ, et al. Inhibition of CD44 expression in hepatocellular carcinoma cells enhances apoptosis, chemosensitivity, and reduces tumorigenesis and invasion. Cancer Chemother Pharmacol. 2008;62:949–957. doi: 10.1007/s00280-008-0684-z. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S, Wu CC, Fecteau JF, Cui B, Chen L, Zhang L, et al. Targeting chronic lymphocytic leukemia cells with a humanized monoclonal antibody specific for CD44. Proc Natl Acad Sci U S A. 2013;110:6127–6132. doi: 10.1073/pnas.1221841110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadhoum Z, Leibovitch MP, Qi J, Dumenil D, Durand L, Leibovitch S, et al. CD44: a new means to inhibit acute myeloid leukemia cell proliferation via p27Kip1. Blood. 2004;103:1059–1068. doi: 10.1182/blood-2003-04-1218. [DOI] [PubMed] [Google Scholar]
- 26.Van Etten RA. New insights into the normal and leukemic stem cell niche: a timely review. Cytometry B Clin Cytom. 2013;84:5–6. doi: 10.1002/cyto.b.21071. [DOI] [PubMed] [Google Scholar]
- 27.Alam TN, O’Hare MJ, Laczkó I, Freeman A, Al-Beidh F, Masters JR, et al. Differential expression of CD44 during human prostate epithelial cell differentiation. J Histochem Cytochem. 2004;52:1083–1090. doi: 10.1369/jhc.4A6256.2004. [DOI] [PubMed] [Google Scholar]
- 28.Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]


