Abstract
Objectives:
To describe characteristics of children developing Bacillus Calmette-Guérin (BCG) lymphadenitis, and to evaluate needle aspiration treatment.
Methods:
Children developing BCG lymphadenitis following BCG vaccination in Al-Rass General Hospital, Al-Rass, Saudi Arabia were prospectively studied from October 2008 to September 2013. Non-suppurative BCG lymphadenitis was conservatively managed, while suppurative lymphadenitis was treated by needle aspiration.
Results:
The mean (SD) age of children (n=23) was 4.1 (1.4) months, and symptoms duration was 2.08 (1.38) months. Fifteen (65.2%) children had suppurative, whereas 8 (34.8%) had non-suppurative lymphadenitis. Age, gender, birth weight, and duration of symptoms were not significantly different between children with suppurative and non-suppurative lymphadenitis (p>0.05). Children with suppurative adenitis had higher weight, and larger size of the involved lymph nodes compared with those with non-suppurative nodes (p=0.001). Most (91.3%) had axillary lesions; with a mean lymph node size of 3.2 cm. Abscesses were detected by ultrasound in 8 (80%). Non-suppurative lymphadenitis was conservatively managed, while suppurative was aspirated. The mean duration for resolution was 3.25 months. Lymph nodes aspirate were positive for acid and alcohol fast bacilli in 10 patients (66.6%), and 3 of them grew Staphylococcus aureus.
Conclusion:
Bacillus Calmette-Guérin adenitis occurs in young children, mainly as unilocular suppurative left axillary group with a mean size of 3.2 cm. Needle aspiration is safe in treatment of suppurative lymphadenitis. Mycobacteria stained positive in most of the suppurative lesions. Excision is not needed.
The Bacillus Calmette-Guérin (BCG) vaccine was derived by in vitro attenuation of Mycobacterium bovis strains in 1906, and the World Health Organization (WHO) incorporated the vaccine in the Expanded Program of Immunization (EPI) in 1974.1 It is estimated that 89% of the targeted children received BCG vaccination in the year 2012.2 The efficacy of the BCG vaccine for tuberculosis protection is controversial; however, it is known to be effective against disseminated tuberculosis (TB) and tuberculous meningitis.3 The most common complication of BCG vaccination is regional lymphadenitis. In Saudi children, the incidence was reported as 1.96 per 1000.4,5 Different methods of management are recommended; however, their beneficial effects remain controversial.6 Non-suppurated lymphadenitis resolves without treatment in 4-6 months.7 In a Saudi setting, the mean age of developing BCG-related complications in lymph nodes was 4.8 months. Suppurative adenitis was treated with total excision or incision and drainage.8 Needle aspiration is infrequently studied as a mode of treatment of suppurative BCG lymphadenitis. This study aimed to describe the clinical characteristics of children developing BCG lymphadenitis and to evaluate the value of needle aspiration in treatment of the suppurative type.
Methods
This descriptive hospital-based study was conducted at the pediatrics and surgical clinics at Al-Rass General Hospital (AGH), Qassim, Saudi Arabia from October 2008 to September 2013. Three trained nurses routinely injected the BCG vaccine (BCG SSI, Danish strain 1331) to all children either at birth, before discharge at the well baby unit, or within 7 days of birth at a vaccination clinic at AGH. A 0.05 ml of the vaccine was injected intradermally in the left arm at the deltoid insertion, using an auto-disabled syringe (BD Solo Shot), which is designed to give only 0.05 ml. All children with BCG-induced lymphadenitis defined as enlargement of the ipsilateral regional lymph node after BCG vaccination in the absence of local or systemic signs of inflammations were included.6 Children who were clinically suspected of having immunodeficiency were excluded on the basis of history. Those were children with a family history of immunodeficiency or had recurrent or chronic infections (cellulitis, abscesses, otitis media, pneumonia, lymphadenitis) within a year, serious infections at unusual sites and unusual pathogens and infections with common childhood pathogens, but of unusual severity.9 Full blood count (FBC), erythrocyte sedimentation rate (ESR), chest radiographs, and ultrasound (USS) of the lymph nodes were carried out for all children. Children were then followed up to document the course of the lymphadenitis in scheduled visits. At each visit, physical examination, especially of the lymph nodes were recorded. Patients with non-suppurative lymphadenitis, swollen firm non-fluctuant lymph nodes were managed conservatively. All patients with suppurative lymphadenitis, namely, swollen cystic fluctuant lesions were managed with needle aspiration. A general surgeon aspirated nodes as an outpatient procedure. After Xylocaine ointment was applied for one hour before the aspiration in all patients, aspiration in different directions was performed subcutaneously 2-3 cm from the periphery of the node to avoid sinus formation while manually compressing the node until there was no return in an 18-gauge needle 5 mL syringe. Recollection of pus within subsequent visits was aspirated in the same way for a maximum of 4 aspirations. The aspirates were sent to the laboratory of AGH for Acid-fast bacilli (AFB), gram staining and cultured in blood agar according to the local laboratory methods. The number of aspirations and the time required for complete resolution of the lymphadenitis were also documented during the follow up visits.
The children’s parents/caregivers provided their written consent after being informed of the study details. The Medical Research Ethics Committee of the College of Medicine, Qassim University approved this study.
Statistical analysis was made using IBM SPSS Statistics for Windows (IBM Corp., Armonk, NY, USA) Version 20.0. Continuous and categorized data between the suppurative and non-suppurative groups were compared using the 2-sample Student’s t-test and x2 tests (Fisher`s exact test). A p-value <0.05 was considered statistically significant.
Results
Twenty-three children were included during the study period. The mean (SD) age at presentation was 4.1 (1.4) months, with a male to female ratio of 12:11. The mean (SD) of birth weight was 3.3 (0.07) Kg. Symptoms had a mean (SD) duration of 2.08 (1.38) months, and the mean hemoglobin, white cell, and platelet count were within normal limits (Tables 1 & 2). Fifteen (65.2%) children had suppurative, whereas 8 (34.8%) had non-suppurative lymphadenitis. Age, gender, birth weight, weight at presentation, and duration of symptoms were not significantly different between children with suppurative and those with non-suppurative lymphadenitis. However, children with suppurative adenitis had significantly higher weight and larger size of the involved lymph nodes compared to those with non-suppurative nodes (Table 1).
Table 1.
Demographic and clinical variables comparison between patients with suppurative and those with non-suppurative Bacillus Calmette-Guérin lymphadenitis using Student’s t-test.
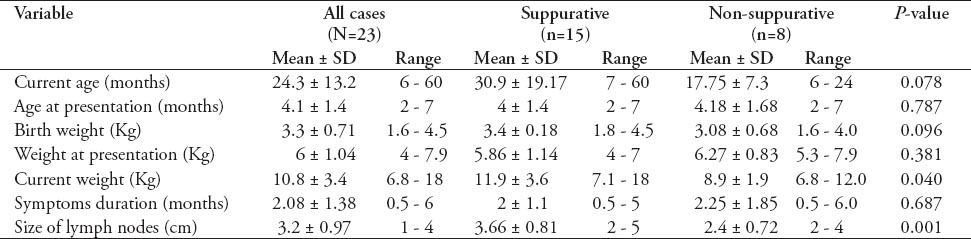
Table 2.
Comparison by Student’s t-test of the laboratory values and the time of resolution of the nodal lesion between patients with suppurative and those with non-suppurative Bacillus Calmette-Guérin lymphadenitis.
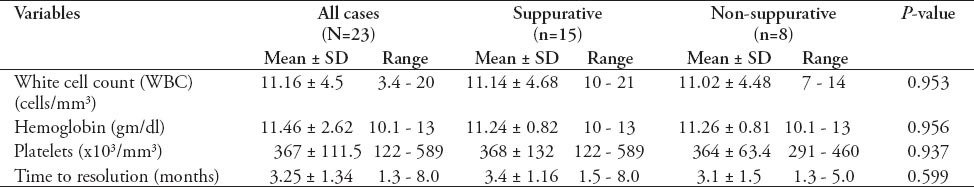
The left axillary lymph node was the site of lesion in 21 children (91.3%). One had the lesion in the left axillary, left supraclavicular, and left cervical nodes, and another had both left axillary and left chest wall abscess. The mean lymph node size was 3.2 cm. Except for 2 preterm born children, one had suppurative and the other had non-suppurative (gestational age 27 and 24 weeks). All patients were born at full term with a mean birth weight of 3.3 (range 1.6-4.7 kg). All children had normal chest radiographs. Ultrasonography, which was carried out for 10 (66.6%) of the 15 children with suppurative lymphadenitis, had demonstrated unilocular abscess in 8 (80%) and multilocular in 2 (20%). The ESR, which was carried out for 8 children, was found to be normal in 6 and was elevated in 2 (90 and 20 mm/hour). Children with non-suppurative lymphadenitis were managed conservatively. All suppurative lymphadenitis were aspirated, with a mean number of aspirations of 2.4 (range 1-4). However, 7 patients (48.6%) required more than 2 aspirations. The mean duration for complete resolution of the lymphadenitis was 3.25 months (range 1.5-8 months). Three patients required more than 4 months for healing, 2 of them had multilocular abscesses. Lymph nodes aspirate were positive for acid and alcohol fast bacilli in 10 patients (66.6%) and 3 of them grew Staphylococcus aureus (S. aureus) as well. Isoniazid (INH) and rifampicin were used to treat one patient whose ESR was 90 mm/hour and had disseminated lymphadenitis. However, this patient was lost in the subsequent follow-up. The antibiotics, amoxicillin, and erythromycin, were used to treat a coincidental respiratory infection in one patient, but not as part of the treatment protocol in this study, each with no effect on lymph nodes healing time. Only one patient developed sinus formation after aspiration and no patient needed excision. There was no difference in the mean white cell count, the hemoglobin concentration, and the platelet counts between patients with suppurative compared to those with non-suppurative lymph nodes. Moreover, there was no statistically significant difference in duration of the resolution of the lymph node lesion between the 2 groups (Table 2).
Discussion
The concentration of the live-attenuated BCG strains range from 50000 to 3 million particles per dosage. Tokyo172 and Glaxo are weak strains whereas Pasteur and Danish are strong; hence, complication rates differ according to the type.10-12 Introduction of an overdose or a new BCG vaccine may result in an increased rate of BCG-associated complications.13-15
In this study, children of either gender developed BCG adenitis at a relatively younger age. However, symptoms were chronic, and the left axillary nodes were the frequently involved groups. Most children had suppurative unilocular lymphadenitis following BCG vaccination and had normal routine laboratory values. There was no significant difference between suppurative and non-suppurative lymphadenitis concerning demographic characteristics such as age and gender. On average, more than twice aspirations were performed for the suppurative adenitis to resolve. Duration of the resolution was not different between children with suppurative and those with non-suppurative adenitis. Acid-fast bacilli and S. aureus were recovered from the aspirates.
In Saudi Arabia, an increased incidence from 0 to 1.96 per 1000 in BCG lymphadenitis was attributed to the change in the vaccine strain.5 The number of complications, during this study period (23 cases) might be attributed to the use of the same vaccine strain. However, the true incidence could not be determined and might well be higher, as not all post-BCG vaccination infants were routinely seen in this clinic. Improper vaccination skills are one of the main reasons for complications.16 In the present study; we had ascertained that trained staff vaccinated the infants, though we could not confirm the technique. Other factors influencing the incidence of BCG complications include dose, age, and immunogenicity of the vaccinated infants.17 Using an auto-disabled syringe rendered extra dose unlikely as a cause for the complications. Immunologically normal newborns are more likely to have a higher incidence of BCG lymphadenitis compared with older infants and children.18 Complications due to BCG vaccination can be mild as in regional lymphadenitis, or severe as in suppurative lymphadenitis, osteitis/osteomyelitis, and disseminated BCG infection.19 The finding of isolated enlarged axillary lymph nodes, ipsilateral to the site of BCG vaccination with no other identifiable cause for the adenitis is usually sufficient to make the diagnosis in most cases.6 This study confirmed that full blood count, ESR, and chest radiograph did not add to the diagnosis. Meanwhile, the ESR was significantly raised in one patient who had a more severe disseminated form of lymphadenitis. However, the ultrasonography may be helpful for the confirmation of the presence of pus in the suppurative lymphadenitis. This will influence the mode of treatment and assure the response to aspiration.
The cytopathology of the aspirate from BCG lymphadenitis is not different from that seen in tuberculous lymphadenitis.20 Although microbiological confirmation is generally not required, the finding of the acid-fast bacilli in the smear or recovery of the mycobacteria from the aspirated nodes material correlates with the diagnosis of BCG lymphadenitis. However, definitive identification of BCG by phage typing or gene analysis and immune workup are not necessary unless disseminated BCG infection is suspected.6 Isolation of pyogenic bacteria from the aspirate of suppurative lymphadenitis has been reported in immunocompromised children.21 In this study, AFB were positive in 66.6% of the aspirate, and 3 aspirates yielded S. aureus, and 2 of the patients received oral antibiotics with no effect on the healing of the lymphadenitis. However, those patients were not investigated for immunodeficiency because there were no warning signs of immunodeficiency.9
In the present study, we compared the current age and weight, age at presentation, birth weight, duration of symptoms, and size of lymph nodes in patients with suppurative with those with non-suppurative lymphadenitis. There were no significant differences except for the current weight and the lymph node size, which was higher in the suppurative group, suggesting that lymph node size is the only clinical characteristic, in this study that can differentiate between suppurative and non-suppurative lymphadenitis. Naturally, a suppurative node is expected to be larger than the non-suppurative one and the suppurative lymphadenitis patients were seen more frequently at the beginning of the study period.
Treatment of simple lymphadenitis with oral erythromycin or anti-tuberculous drugs did not hasten the regression or prevent progression into suppuration.6 In our study, 8 patients had non-suppurative lymphadenitis and they all healed on only conservative measures in a mean time of 3.1 months.
Because suppurative lymphadenitis is frequently complicated by spontaneous perforation with sinus formation, which may persist for months,6 we aspirated suppurative lesions, which proved to be effective in both prevention of rupture and shortening the time of healing. The mean time of complete resolution in this study was 3.4 months. This is comparable to Chan et al’s17 results, where most of the children’s lesions healed in an average of 4 months. Sataynarayana et al,20 have also obtained satisfactory results in non-drained suppurative adenitis after needle aspiration. Repeated aspirations may be needed for some patients.5,20 In this study, the mean number of aspirations was 2.4 with a range of 1-4, and approximately 49% of nodes required more than 2 aspirations. Some authors advocate intra-nodal injection of INH and others recommended local rifampicin after needle aspiration.21,22 However, our results suggested that this practice is even unnecessary. To avoid the only known complication of needle aspiration, iatrogenic sinus formation, it is recommended to aspirate subcutaneously 2-3 cm from the periphery of the node.20 In the present study, only one patient developed iatrogenic sinus formation, which healed in 8 months.
Surgical excision may be needed when needle aspiration has failed as in the case of matted and multiloculated lymph nodes,20 or when suppurative lymph nodes have already drained with sinus formation.22 However, surgical incision is not recommended even among immunocompromised patients.6 None of the affected children needed surgical incision in this cohort.
This study has its limitations. First the incidence of complications could not be determined because only part of the infant population received vaccination in this center. Second we could not control for the suppurative group for ethical considerations and due to the limited number of cases over a long period, which may constrain generalization of the results. Hence, inclusion of large number of facilities and time extension control for the cohort may, in the future, give better results.
Diagnosis of BCG lymphadenitis is clinical. Children develop BCG adenitis at an earlier age with no gender difference. They have average symptom duration of 2 months. The commonly affected group is the left axillary with a mean size of 3.2 cm. Most cases of adenitis are unilocular suppurative. There was no difference in duration of resolution of the lymph node lesion and in the mean white cell count, the hemoglobin concentration, and the platelet counts between patients with suppurative compared to those with non-suppurative lymphadenitis. Needle aspiration is a safe simple technique in the management of suppurative lymphadenitis. Tubercle bacilli are detected in most of suppurative lesions, but S. aureus was recovered from quite a few. Antibiotics have no effect on healing of BCG adenitis and excision of the affected lymph nodes is not needed.
Acknowledgment
The authors would like to thank Dr. Sami Abd Al Hakeem, Consultant Rheumatologist, Al-Rass General Hospital, for conducting the statistical analysis. We appreciate the valuable comments of Professor Ishag Adam, Epidemiologist and Consultant Obstetrics and Gynecology, College of Medicine, Qassim University, Qassim, Saudi Arabia.
Footnotes
Related Articles.
Al-Salem AH, Kothari MR, AlHani HM, Oquaish MM, Khogeer SS, Desouky MS. Safety of intradermal Bacillus Calmette-Guerin vaccine for neonates in Eastern Saudi Arabia. Saudi Med J 2012; 33: 172-176.
Al-Jassir FF, Aldeeri RA, Alsiddiky AM, Zamzam MM. Osteomyelitis following Bacille Calmette-Guerin vaccination. Saudi Med J 2012; 33: 87-90.
Al-Hassan AA, Ahsanullah AM. Bacillus Calmette-Guerins vaccination at birth causing tuberculous granulomatous lymphadenitis. Saudi Med J 2011; 32: 412-414.
References
- 1.WHO/UNICEF. Expanding immunization coverage. Immunization, vaccines and biological. [Updated 2014; Accessed 2014 September 4]. Available from URL: http://www.unicef.org/immunization/index_coverage.html.
- 2.Centers for Disease Control and Prevention. Morbidity and mortality weekly report. Global routine vaccine coverage. [Updated 2013 November 1, Accessed 2014 November 15]. Available from URL: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6243a4.htm.
- 3.Hawn TR, Day TA, Scriba TJ, Hatherill M, Hanekom WA, Evans TG, et al. Tuberculosis vaccines and prevention of infection. Microbil Mol Biol Rev. 2014;78:650–671. doi: 10.1128/MMBR.00021-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukhari E, Alzahrani M, Alsubaie S, Alrabiaah A, Alzamil F. Bacillus Calmette-Guerin lymphadenitis: A 6-year experience in two Saudi hospitals. Indian J Pathol Microbiol. 2012;55:202–205. doi: 10.4103/0377-4929.97869. [DOI] [PubMed] [Google Scholar]
- 5.Alrabiaah AA, Alsubaie SS, Bukhari EL, Gad A, Alzamel FA. Outbreak of Bacille Calmette-Guérin-related lymphadenitis in Saudi children at a university hospital after a change in the strain of vaccine. Ann Saudi Med. 2012;32:4–8. doi: 10.5144/0256-4947.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuttall JJ, Davies MA, Hussey GD, Eley BS. Bacillus Calmette-Guérin (BCG) vaccine-induced complications in children treated with highly active antiretroviral therapy. Int J Infect Dis. 2008;12:e99–e105. doi: 10.1016/j.ijid.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Duello-Garcia CA, Perez-Gaxiola G, Jemenez GC. Treating BCG-induced disease in children. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD008300.pub2. 10.1002/14651858.CD008300.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Salem AH, Kothari MR, Al Hani HM, Oquaish MM, Khogeer SS, Desouky MS. Safety of intradermal Bacillus Calmette-Guerin vaccine for neonates in Eastern Saudi Arabia. Saudi Med J. 2012;33:172–176. [PubMed] [Google Scholar]
- 9.Buckley RH. Evaluation of suspected immunodeficiency. In: Nelson KR, editor. Nelson textbook of pediatrics. 18th ed. Philadelphia (PA): Elsevier Saunders; 2008. pp. 715–721. [Google Scholar]
- 10.Dagg B, Hockley J, Rigsby P, Ho MM. The establishment of sub-strain specific WHO Reference Reagents for BCG vaccine. Vaccine. 2014;32:6390–6395. doi: 10.1016/j.vaccine.2014.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inamoto T, Ubai T, Nishida T, Fujisue Y, Katsuoka Y, Azuma H. Comparable effect with minimal morbidity of low-dose Tokyo 172 strain compared with regular dose Connaught strain as an intravesical bacillus Calmette-Guérin prophylaxis in nonmuscle invasive bladder cancer: Results of a randomized prospective comparison. Urol Ann. 2013;5:7–12. doi: 10.4103/0974-7796.106873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Tran V, Leung AS, Alexander DC, Zhu B. BCG vaccines: Their mechanisms of attenuation and impact on safety and protective efficacy. Hum Vaccine. 2009;5:70–78. doi: 10.4161/hv.5.2.7210. [DOI] [PubMed] [Google Scholar]
- 13.Ritz N, Tebruegge M, Streeton J, Curtis N. Too much of a good thing: Management of BCG vaccine overdose. Vaccine. 2009;27:5562–5564. doi: 10.1016/j.vaccine.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 14.Antas PR, Castello-Branco LR. New vaccines against tuberculosis: lessons learned from BCG immunisation in Brazil. Trans R Soc Trop Med Hyg. 2008;102:628–630. doi: 10.1016/j.trstmh.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Dommergues MA, de La Rocque F, Guy C, Lécuyer A, Jacquet A, Guérin N, Fagot JP, et al. Local and regional adverse reactions to BCG-SSI®vaccination: A 12-month cohort follow-up study. Vaccine. 2009;27:6967–6973. doi: 10.1016/j.vaccine.2009.09.073. [DOI] [PubMed] [Google Scholar]
- 16.Smith KC, Orme IM, Starke JR. Tuberculosis. Tuberculosis vaccines. In: Plotkin SA, Orenstein WA, Offit P, editors. Vaccines. 6th ed. Philadelphia (PA): WB Saunders; 2013. pp. 789–811. [Google Scholar]
- 17.Chan WM, Kwan YW, Leung CW. Management of Bacillus Calmette-Guérin Lymphadenitis. Hong Kong Journal of Paediatrics. 2011;16:85–94. [Google Scholar]
- 18.Hawkridge A, Hatherill M, Little F, Goetz MA, Barker L, Mahomed H, et al. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial. BMJ. 2008;337:a2052. doi: 10.1136/bmj.a2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadeghi-Shanbestari M, Ansarin K, Maljaei SH, Rafeey M, Pezeshki Z, Kousha A, et al. Immunologic aspects of patients with disseminated bacille Calmette-Guerin disease in north-west of Iran. Ital J Pediatr. 2009;35:42. doi: 10.1186/1824-7288-35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sataynarayana S, Mathur AD, Verma Y, Pradhan S, Bhandari MK. Needle aspiration as a diagnostic tool and therapeutic modality in suppurative lymphadenitis following Bacillus Calmette-Guerin vaccination. J Assoc Physicians India. 2002;50:788–791. [PubMed] [Google Scholar]
- 21.Kim MS, Jo DS, Kyung KM, Kim SJ, Kim JS. The effect of local rifampicin instillation on the treatment of suppurative BCG lymphadenitis. Korean J Pediatr. 2006;49:40–45. [Google Scholar]
- 22.Juzi JT, Sidler D, Moore SW. Surgical management of BCG vaccine-induced regional axillary lymphadenitis in HIV-infected children. S Afr J Surg. 2008;46:52–55. [PubMed] [Google Scholar]


