Abstract
Objectives:
To identify potential risk factors such as smoking, cardiovascular diseases (CVD), denture wearing, and consuming vitamin rich foods, and its relation to the development of sublingual varices (SLV).
Methods:
This cross-sectional observational study was conducted on patients who attended the Department of Dentistry at The University of Jordan Hospital, Amman, Jordan between February and May 2013. Clinical examinations and inspections of 391 patients (203 males and 188 females), 13-74 years of age were conducted to determine the presence of SLV. Sublingual varices were classified into 2 categories: grade 0 (few or none visible), and grade one (moderate or severe). Frequency distributions of both SLV and risk factors were obtained. Multiple logistic regression analysis and Chi-square test were used to analyze the influence of individual risk factors on the incidence of SLV.
Results:
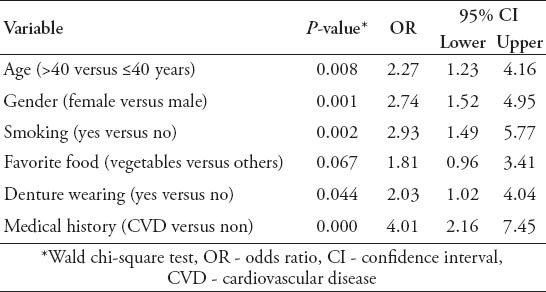
There were 88 subjects (22.5%) who had SLV. In the multivariate logistic regression model, SLV were significantly associated with age (odds ratio [OR]: 2.27, p=0.008) with highest occurrences in the eighth decade of life, gender (OR: 2.74, p=0.001), smoking (OR: 2.93, p=0.002), denture wearing (OR: 2.03, p=0.044), and CVD (OR: 4.01, p=0.00).
Conclusion:
The presence of SLV could be indicative of some potential risk factors including old age, female gender, and denture wearing, and may alert the dental clinician to recognize underlying systemic conditions, particularly CVD.
Sublingual varices (SLV) are dilated tortuous veins that may be seen along the ventral surface of the tongue or floor of mouth, and tend to become more prominent with age. However, in a young population, such vascular lesions could be part of Fabry, or Osler syndrome.1 Sublingual varices may be noticed by patients, or more commonly by dentists. They are often confused with the main veins running from the tip of the tongue backwards, and should be differentiated from primary malignant melanomas of the tongue base.1 Several explanations have been suggested concerning the pathogenesis of SLV; it is known that the ageing process, including changes in the connective tissues and venous walls is associated with an increase in the incidence of varices.2-4 Kaplan and Moskona5 reported that varicosities increased from 11.1-41.1% between ages 50-99 years. Few studies in the literature1,2-4,6-10 investigated the relation between SLV and some potential risk factors, such as cardiovascular diseases (CVD), smoking, denture wearing, and consuming foods rich in vitamins, such as vegetables. Furthermore, portal hypertension,6 and varicose veins of the leg2 have been claimed to have a possible connection and association with SLV. Differences in the incidence of SLV between males and females has been the subject of a recent survey.1 The relation between SLV and CVD remains controversial; whereas some studies found no relation,4,6 other older studies reported an association.2,3 A recent study1 of 281 consecutive adults aged 40-92 years demonstrated a strong association between CVD and SLV. Similarly, controversy regarding the relation between SLV and smoking still exists, and there is one study published in the English literature investigating the relation between SLV and smoking.1 Smoking was established as a predisposing factor for CVD, particularly hypertension.11 Hedström and Bergh1 found that SLV was significantly associated with smoking. However, Kroeger et al12 reported a preventive effect of smoking on the development of varicose veins of the leg. Ettinger and Manderson,2 in their study of SLV found a relation between sublingual veins and varicose veins of the leg. Sublingual varices were also ascribed to vitamin C deficiency in older age groups. A study of 22 elderly vegetarians aged 57-75 years found a much lower incidence of sublingual petechiae and varicosities than generally reported in an older population.7 However, the literature did not support a link between SLV and diabetes,13 or denture wear.8-10 In Jordan, a recent study showed that more than 48% of adult males were current smokers.14 In addition, more than 60% of patients who attended the United Nations Relief and Works Agency (UNRWA) primary health care clinics in Jordan were diagnosed with hypertension.15 Hence, it is expected that SLV could be a common finding among a Jordanian population. Therefore, the aim of this study was to assess the influence of potential risk factors including CVD, smoking, denture wearing, and consuming vitamin rich foods on the incidence of SLV among the young, middle aged, and elderly population.
Methods
This cross-sectional observational study was conducted on patients who attended the Department of Dentistry, The University of Jordan Hospital, Amman, Jordan between February and May 2013. This study was conducted according to the principles of the Helsinki Declaration. Ethical approval was not deemed necessary as the patients were not subjected to risk or discomfort. Patients were invited to participate in this study, consent was obtained, then they were medically interviewed, and clinically assessed for the presence, or absence of SLV. The examiner was blinded to the medical history of the patients and to the grading of SLV before examination. Sample size calculation was not deemed relevant by the authors. Patients under 13 years of the age, those unable to provide an accurate medical history, and those who refused participation in this study were all excluded. Patients completed a form that included personal details, relevant medical ailments including 4 risk factors; smoking, denture wearing, favorite food, and CVD. Smoking was defined according to the standard set by The Centers for Disease Control and Prevention (CDC) definition; smokers were identified as those who reported any type of smoking on a daily or occasional basis during the last 30 days (current smokers), or those who reported any type of smoking before, but reported cessation at the time of survey (ex-smokers). Non-smokers were defined as those who had never undertaken any type of smoking in their lifetime.16 In addition, type and duration of smoking were recorded. Types included: cigarette, water pipe (nargela), pipes, and cigar. As for medical status; 2 categories were identified: patients with CVD, and patient with no CVD. Cardiovascular diseases was defined according to the International Classification of Diseases version 10 (ICD-10),17 and included diseases such as hypertension, angina pectoris, myocardial infarction, stroke, atrial fibrillation, cardiac valve dysfunction, and others. These diseases could be present in isolation, or in association with each other, or with other systemic diseases. However, patients with no CVD comprised those who are healthy, and those having systemic disease(s) not involving the cardiovascular system. Clinical assessment of SLV was carried out by inspection and palpation with the help of the standard set of examinations. The examiner requested the patients to move the tongue upward and bilaterally, to examine the ventral surface and lateral borders of the tongue and floor of the mouth. Using a previously reported criteria,1 SLV were classified into 2 grades: grade 0 for those SLV, which were absent or only a few visible (Figure 1), and grade one for those SLV, which were present in a medium or severe form (Figure 2). The grading of questionable SLV was recorded after seeking the opinion of another oral medicine consultant at The University of Jordan Hospital. Finally, subjects who had SLV were asked whether they were aware of its presence.
Figure 1.
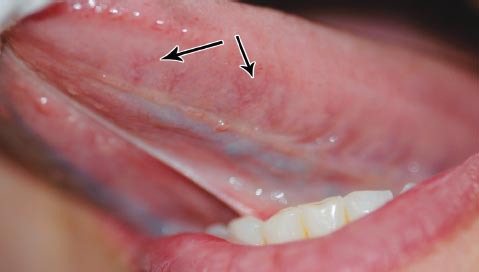
Grade 0 sublingual varices (SLV). There are few visible sublingual varices.
Figure 2.
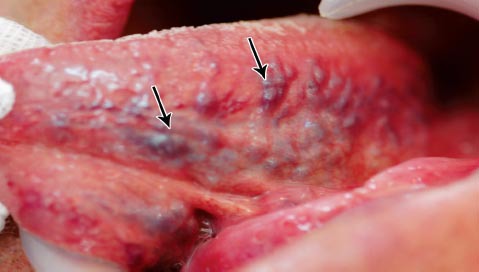
Grade 1 sublingual varices: present in a medium form.
The literature concerning SLV in relation to potential risk factors was searched by performing a comprehensive electronic search (PubMed-National Library of Medicine, NCBI). Key words employed in this search were sublingual varices, smoking, cardiovascular diseases, denture wearing, and vitamins. The search included only articles written in English, and published during the last 10 years from January 2004 to January 2014, to obtain recent and applied information concerning the subject. However, following thorough examination of the abstract, full text, and references of these articles, a lack of sufficient data and literature concerning the subject was found. Therefore, the abstract and full text of additional non-PubMed and older related research were used.
Statistical analysis was performed using the Statistical Package for Social Sciences for Windows version 17 (SPSS Inc., Chicago, IL, USA). Frequency distributions of SLV and risk factors were obtained. Multiple logistic regression analysis and Chi-Square test were used to analyze the influence of individual risk factors on the incidence of SLV, and to compare differences between groups. Statistical significance was set at p<0.05.
Results
A total of 391 patients were included in this study with 203 (51.9%) males, and 188 (48.1%) females. The patients were 13-74 years of age (mean 43.2 ± 14.6 years). There were 88 (22.5%) patients with SLV, only 30 patients (34.1%) were aware of its presence; 7 (23.3 %) by themselves, and 23 (76.7%) by their dentists. Table 1 shows the descriptive statistics of both SLV and potential risk factors against the age groups; the incidence of SLV, denture wearing, and CVD were more common with increasing age; however, the number of smokers and subjects not favoring vegetables decreased with increasing age. Univariate analysis was used (Table 2); and 4 potential risk factors were found to be significantly associated with SLV. The incidence of SLV increased with advancing age and smoking habit (p=0.0). Smokers were more likely to have SLV, and this difference was highly significant, and maintained its significance for those who had smoked for a longer duration. Other risk factors, such as denture wearing and pre-existing CVD also had a significant association (p=0.0) with SLV. However, although SLV was more common in females and in those favoring vegetables, the differences were not statistically significant. When multivariate regression analysis was used (Table 3), the above mentioned risk factors maintained their significant association with SLV, in addition, gender became significant. Sublingual varices were significantly associated with age, and denture wearing suggesting that older age (>40 years), and denture wearers have an approximately 2 times increase in risks of developing SLV. Additionally, female patients, and smokers were approximately 3 times more likely to have SLV. The presence of CVD was associated with an approximately 4 times greater chance of SLV.
Table 1.
Description of the study group (Jordanian dental patients) and the distribution of present smoking habits, cardiovascular diseases (CVD), denture wearing, favorite food, and sublingual varices (SLV) (N=391).
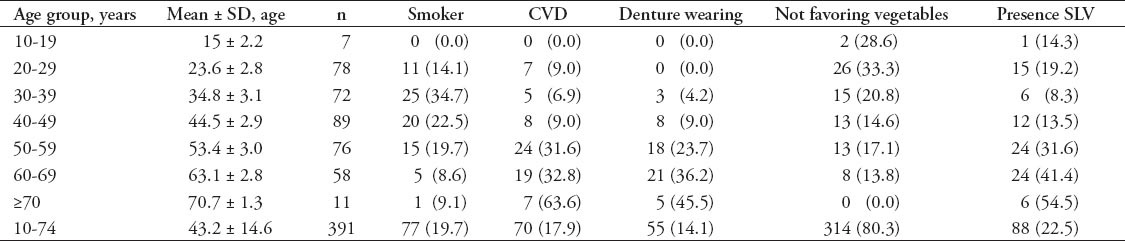
Table 2.
Incidence of sublingual varices (SLV) in relation to gender, age group, smoking, favorite food, denture wearing, and medical history (N=391) among Jordanian dental patients.
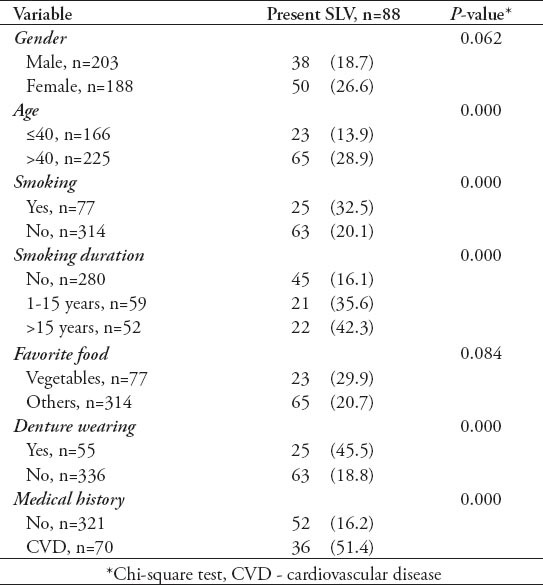
Table 3.
The influence of risk factors on sublingual varices by logistic regression analysis (N=391) among Jordanian dental patients.
Discussion
The incidence of SLV in this study was 22.5%, and is in agreement with previous studies (16-70%).1,2,4 This wide range of incidence reported could be explained by the variation in the included age groups. In this study, the presence of SLV increased with age, with highest occurrences in the eighth decade of life, which is in agreement with previous reports,1,2-4 and explained by the changes in the morphology of blood vessels and connective tissue, and in hemodynamic activity, such as increasing arterial pressure through arteriovenous shunts.18 Additionally, the action of smooth muscle fibers encircling the capillaries and terminal arterioles (metarterioles) at intermittent points causes the opening and closure of vessels leading to changes in the distribution of blood in the tissue, thus, pooling blood from arterioles to the venules without passing the capillary bed.1,19
This present study demonstrated that CVD was strongly associated with SLV, and this is in agreement with a recent study.1 Ettinger and Manderson2 demonstrated a relation between SLV and varicose veins of the legs, however, the authors did not provide an explanation for their findings. Similarly, and despite the fact that there is no recognized anastomoses between lingual venous drainage and portal circulation, it is suggested that SLV diagnosis should be considered in cases of portal hypertension.6 Sublingual varices were attributed to the fact that it is a frequent semiological finding in elderly patients, but, they may also be related to abnormalities in the circulatory system commonly occurring in specific diseases, such as CVD and diabetes.13 However, the association demonstrated in the current study between SLV and CVD disagrees with the double blind study by Kleinman3 who unsubstantiated the assumption of this association.
Smoking, despite its contradictory role is known to have a preventive effect on the development of varicose veins of the leg. This was attributed to the nicotine inducing constriction on venous vessels.12 However, the vasoconstrictive effect of smoking on the arterial side was considered as a predisposition for CVD, particularly hypertension.1,11 A recent study confirmed an association between smoking and SLV,1 and this is in agreement with our study. However, to the authors’ knowledge, duration and types of smoking were never considered in the literature. In this study, the duration of smoking had a significant effect on the incidence of varices; it could be possible that smoking could have dose-related vascular effects.
On gender, it is known that females are less susceptible to some of the SLV risk factors, such as CVD and smoking,20 and a recent study1 reported an insignificant relation between gender and SLV. This is in contrast to the relation found in the present study between gender and SLV; females were more likely to have SLV. This may indicate a hormonal influence on SLV. A previous study7 in the literature looked at consumed foods rich in vitamins, such as vegetables, and its role in SLV; SLV were found more common among non-vegetarians, therefore, a possible association was concluded particularly in the older population. These findings are consistent with previous studies on Jordanian populations reporting higher prevalence of risk factors for SLV, such as hypertension and obesity, among non-vegetarians compared to vegetarian subjects.21 In this study, there was no significant difference on the incidence of SLV between subjects who reported preferring vegetables, and those who reported not preferring vegetables. However, due to the subjective nature of the question it will be difficult to draw conclusions on this matter, particularly in light of the fact that a vegetarian dietary culture is not common in Jordan. Furthermore, reporting one’s favorite food does not necessarily reflect that one is actually consuming that food.
In the present study, we found a significant association between denture wearing and SLV; however, the duration of denture wearing was not considered. This association was not in agreement with the findings of previous reports,8-10 that looked at wearing dentures, without considering the duration, and its role in mucosal abnormalities such as SLV; they concluded no significant relation between mucosal alterations, such as SLV and removable-denture wearing. However, they emphasized the role of denture wearing, especially for a long duration in oral lesions, such as denture stomatitis and denture hyperplasia. These denture-induced lesions were believed to be related to poor oral hygiene and irritation caused by the use of a prosthesis.
This study is not without limitations; information concerning the stage and duration of CVD, accurate diagnosis of cardiovascular status for the possibility of undiagnosed CVD particularly in the elderly, the duration of denture wear, and the quantity of smoking were not considered. The difficulty in finding pure vegetarians led the authors to consider asking whether subjects favored eating vegetables or not, introducing bias in assessing foods rich in vitamins, and its role in SLV. Consequently, the above mentioned limitations have to be considered in future research to confirm the findings of this study.
In conclusion, further studies are needed to confirm the association of risk factors with SLV as reported risk factors may vary among reported studies. A better understanding of the potential risk factors associated with SLV may alert the dental clinician to recognize underlying systemic conditions, which require timely and appropriate management.
Acknowledgment
The authors would like to thank Prof. Yousef Khader for his help in statistical analysis of the data.
Footnotes
Clinical Practice Guidelines.
Clinical Practice Guidelines must include a short abstract. There should be an Introduction section addressing the objective in producing the guideline, what the guideline is about and who will benefit from the guideline. It should describe the population, conditions, health care setting and clinical management/diagnostic test. Authors should adequately describe the methods used to collect and analyze evidence, recommendations and validation. If it is adapted, authors should include the source, how, and why it is adapted? The guidelines should include not more than 50 references, 2-4 illustrations/tables, and an algorithm.
References
- 1.Hedström L, Bergh H. Sublingual varices in relation to smoking and cardiovascular diseases. Br J Oral Maxillofac Surg. 2010;48:136–138. doi: 10.1016/j.bjoms.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger RL, Manderson RD. A clinical study of sublingual varices. Oral Surg Oral Med Oral Pathol. 1974;38:540–545. doi: 10.1016/0030-4220(74)90084-x. [DOI] [PubMed] [Google Scholar]
- 3.Kleinman H. Lingual varicosities. Oral Surg Oral Med Oral Pathol. 1967;23:546–548. doi: 10.1016/0030-4220(67)90550-6. [DOI] [PubMed] [Google Scholar]
- 4.Bhaskar SN. Oral lesions in the aged population. A survey of 785 cases. Geriatrics. 1968;23:137–149. [PubMed] [Google Scholar]
- 5.Kaplan I, Moskona D. A clinical survey of oral soft tissue lesions in institutionalized geriatric patients in Israel. Gerodontology. 1990;9:59–62. doi: 10.1111/j.1741-2358.1990.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 6.Jassar P, Jaramillo M, Nunez DA. Base of tongue varices associated with portal hypertension. Postgrad Med J. 2000;76:576–577. doi: 10.1136/pmj.76.899.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eddy TP, Taylor GF. Sublingual varicosities and vitamin C in elderly vegetarians. Age Ageing. 1977;6:6–13. doi: 10.1093/ageing/6.1.6. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira RC, Magalhães CS, Moreira AN. Oral mucosal alterations among the institutionalized elderly in Brazil. Braz Oral Res. 2010;24:296–302. doi: 10.1590/s1806-83242010000300007. [DOI] [PubMed] [Google Scholar]
- 9.Freitas JB, Gomez RS, De Abreu MH, Ferreira E, Ferreira E. Relationship between the use of full dentures and mucosal alterations among elderly Brazilians. J Oral Rehabil. 2008;35:370–374. doi: 10.1111/j.1365-2842.2007.01782.x. [DOI] [PubMed] [Google Scholar]
- 10.Corbet EF, Holmgren CJ, Philipsen HP. Oral mucosal lesions in 65-74-year-old Hong Kong Chinese. Community Dent Oral Epidemiol. 1994;22:392–395. doi: 10.1111/j.1600-0528.1994.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 11.Whelan EM, editor. Smoking and peripheral vascular disease. In: American Council on Science and Health. Cigarettes: what the warning label doesn’t tell you. New York (NY): Prometheus Books; 1997. pp. 35–39. [Google Scholar]
- 12.Kroeger K, Ose C, Rudofsky G, Roesener J, Hirche H. Risk factors for varicose veins. Int Angiol. 2004;23:29–34. [PubMed] [Google Scholar]
- 13.Vasconcelos BC, Novaes M, Sandrini FA, Maranhão Filho AW, Coimbra LS. Prevalence of oral mucosa lesions in diabetic patients: a preliminary study. Braz J Otorhinolaryngol. 2008;74:423–428. doi: 10.1016/S1808-8694(15)30578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belbeisi A, Al Nsour M, Batieha A, Brown DW, Walke HT. A surveillance summary of smoking and review of tobacco control in Jordan. Global Health. 2009;5:18. doi: 10.1186/1744-8603-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khader A, Farajallah L, Shahin Y, Hababeh M, Abu-Zayed I, Zachariah R, et al. Hypertension and treatment outcomes in Palestine refugees in United Nations Relief and Works Agency primary health care clinics in Jordan. Trop Med Int Health. 2014;19:1276–1283. doi: 10.1111/tmi.12356. [DOI] [PubMed] [Google Scholar]
- 16.Sychareun V, Hansana V, Choummanivong M, Nathavong S, Chaleunvong K, Durham J. Cross-sectional survey: smoking among medical, pharmacy, dental and nursing students, University of Health Sciences, Lao PDR. BMJ Open. 2013;3:3042. doi: 10.1136/bmjopen-2013-003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Statistical Classification of Diseases and Related Health Problems, version 3. Diseases of the circulatory system (100-199) Geneva (CH): World Health Organization; 1998. [Google Scholar]
- 18.Southam JC, Ettinger RL. A histologic study of sublingual varices. Oral Surg. 1974;38:879–886. doi: 10.1016/0030-4220(74)90340-5. [DOI] [PubMed] [Google Scholar]
- 19.The microcirculation and the lymphatic system: capillary fluid exchange, interstitial fluid, and lymph flow. Local and humoral control of blood flow by the tissues. Guyton AC, Hall JE, editors. Textbook of medical physiology. 11th ed. New York (NY): WB Saunders; 2003. pp. 181–203.
- 20.Weidner G, Cain VS. The gender gap in heart disease: lessons from Eastern Europe. Am J Public Health. 2003;93:768–770. doi: 10.2105/ajph.93.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alrabadi NI. The effect of lifestyle food on chronic diseases: a comparison between vegetarians and non-vegetarians in Jordan. Glob J Health Sci. 2012;5:65–69. doi: 10.5539/gjhs.v5n1p65. [DOI] [PMC free article] [PubMed] [Google Scholar]


