Abstract
Preterm birth remains the leading cause of neonatal mortality and morbidity worldwide. There are currently few effective therapies and therefore an urgent need for novel treatments. Although there is much focus on trying to alter gestation of delivery, the primary aim of preterm birth prevention therapies should be to reduce prematurity related mortality and morbidity. Given the link between intrauterine infection and inflammation and preterm labour (PTL), we hypothesized that administration of lipoxins, key anti-inflammatory and pro-resolution mediators, could be a useful novel treatment for PTL. Using a mouse model of infection-induced PTL, we investigated whether 15-epi-lipoxin A4 could delay lipopolysaccharide (LPS)-induced PTL and reduce pup mortality. On D17 of gestation mice (n = 9–12) were pretreated with vehicle or 15-epi-lipoxin A4 prior to intrauterine administration of LPS or PBS. Although pretreatment with 15-epi-lipoxin A4 did not delay LPS-induced PTL, there was a significant reduction in the mortality amongst prematurely delivered pups (defined as delivery within 36 h of surgery) in mice treated with 15-epi-lipoxin A4 prior to LPS treatment, compared with those receiving LPS alone (P < 0.05). Quantitative real-time (QRT)-PCR analysis of utero-placental tissues harvested 6 h post-treatment demonstrated that 15-epi-lipoxin A4 treatment increased Ptgs2 expression in the uterus, placenta and fetal membranes (P < 0.05) and decreased 15-Hpgd expression (P < 0.05) in the placenta and uterus, suggesting that 15-epi-lipoxin A4 may regulate the local production and activity of prostaglandins. These data suggest that augmenting lipoxin levels could be a useful novel therapeutic option in the treatment of PTL, protecting the fetus from the adverse effects of infection-induced preterm birth.
Keywords: anti-inflammatory, lipoxin, parturition, preterm birth, resolution
Introduction
Preterm labour (PTL), defined as labour before 37 weeks gestation, remains a major obstetric problem estimated to affect between 5 and 18% of pregnancies worldwide, with ∼15 million babies born prematurely each year (March of Dimes, 2012). Despite advances in the medical care of preterm infants, there are currently few effective treatment options and premature birth remains the leading cause of neonatal mortality. Indeed, preterm birth is estimated to account for up to 75% of neonatal deaths (Goldenberg et al., 2008). Additionally, preterm birth is associated with an increased risk of a range of short-term morbidities and long-term disabilities, including cerebral palsy, bronchopulmonary dysplasia (BPD), retinopathy of prematurity and learning difficulties (Saigal and Doyle, 2008).
Spontaneous labour at term is now considered to be an inflammatory event that is associated with an immune cell infiltration into the cervix, myometrium and fetal membranes and increased production of pro-inflammatory mediators in the utero-placental tissues (Denison et al., 1998; Thomson et al., 1999; Sennstrom et al., 2000; Young et al., 2002; Osman et al., 2003). Although the causes of PTL are often unclear, many cases are associated with the presence of occult or overt intrauterine infection (Goldenberg et al., 2000) and the premature activation of these inflammatory pathways is likely responsible for PTL in this scenario. Animal models have confirmed a causal link between intrauterine infection and inflammation and PTL, given that injection of bacterial components, such as LPS or pro-inflammatory cytokines, such as tumour necrosis factor-α (TNF-α) or interleukin (IL)-1β reliably induce PTL (Romero et al., 1991; Elovitz et al., 2003; Sadowsky et al., 2006). Our own in vitro studies have shown that LPS directly induces contractions of isolated human myometrial cells (Hutchinson et al., 2013). Influx of immune cells likely also contributes to the process, although further work is required to define their precise roles (Timmons and Mahendroo, 2006; Murphy et al., 2009; Thaxton et al., 2009; Gonzalez et al., 2011; Rinaldi et al., 2014).
Given the link between inflammation and spontaneous labour onset, and the association between intrauterine infection and PTL, there has been a growing interest in examining whether anti-inflammatory agents could be effective novel therapeutic options for PTL (Rinaldi et al., 2011). Animal studies have been invaluable in demonstrating the potential of a number of anti-inflammatory agents to delay preterm delivery and improve pup survival, including IL-10 (Terrone et al., 2001; Rodts-Palenik et al., 2004; Robertson et al., 2006), TLR-4 signalling blockade (Adams Waldorf et al., 2008; Li et al., 2010), NFκB inhibitors (Nath et al., 2010; Chang et al., 2011) and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) (Pirianov et al., 2009).
The understanding that the resolution of inflammation is an active process involving the production of mediators with specific anti-inflammatory and pro-resolution actions has provided new pathways to target in the search for novel treatments for inflammation-associated pathologies (Gilroy et al., 2004; Serhan et al., 2008). The arachidonic acid-derived lipid mediators, lipoxins, were the first family of mediators recognized to have dual-acting anti-inflammatory and pro-resolution actions (Serhan et al., 1984; Ryan and Godson, 2010). During the resolution phase of an inflammatory response, in addition to the native lipoxins (lipoxin A4 and lipoxin B4), arachidonic acid can, in the presence of aspirin, also be converted to aspirin-triggered or 15-epi-lipoxins (15-epi-lipoxin A4 and 15-epi-lipoxin B4) (Chiang et al., 2005). The anti-inflammatory and pro-resolution actions of lipoxins and 15-epi-lipoxins include: inhibiting neutrophil activation, adhesion and chemotaxis (Papayianni et al., 1996; Filep et al., 2005; Maderna and Godson, 2009); counteracting neutrophil anti-apoptotic signals (El Kebir et al., 2007, 2009); triggering non-phlogistic phagocytosis of apoptotic neutrophils by macrophages (Godson et al., 2000); stimulating monocyte adhesion and migration (Maddox and Serhan, 1996); and down-regulating pro-inflammatory cytokine production (Wu et al., 2008; Kure et al., 2009). The therapeutic potential of lipoxins has been widely demonstrated in animal models of a range of inflammation-associated pathologies, including asthma (Levy et al., 2002) and lung injury (El Kebir et al., 2009), arthritis (Zhang et al., 2008; Conte et al., 2010) and inflammatory bowel diseases (Gewirtz et al., 2002). Within the reproductive tract, studies have identified a role for lipoxin signalling in endometriosis (Chen et al., 2010; Xu et al., 2012), embryo implantation (Xiong et al., 2013) and spontaneous miscarriage (Xu et al., 2013). To date, the role of lipoxins in regulating inflammation in parturition has been less well explored (Hutchinson et al., 2011). However, using an in vitro model, we previously showed that expression of the lipoxin receptor, FPR2/ALX, is increased in myometrial tissue obtained from women during term labour (compared with tissue obtained from non-labouring women); and that lipoxin treatment down-regulated LPS-induced inflammatory gene expression in myometrial explant culture (Maldonado-Perez et al., 2010).
Given evidence that lipoxins could be involved in regulating the inflammation associated with labour, and the therapeutic potential of lipoxin administration demonstrated in animal models of other inflammation-associated pathologies, we hypothesized that lipoxins could be effective therapeutic agents for the treatment of infection-induced PTL. In the study described here, using a mouse model of LPS-induced PTL, we evaluated the effect of pretreatment with 15-epi-lipoxin A4 on LPS-induced preterm delivery, pup mortality and the LPS-induced inflammatory response of the utero-placental tissues.
Materials and Methods
Mouse model of infection-induced PTL
All animal studies were conducted under a UK Home Office licence to JEN (60/4241) and were approved by the University's ethical board and the UK Home Office. Timed-pregnant CD-1 mice were obtained from Charles River Laboratories (Margate, UK) on D9-11 of gestation (the day vaginal plug was found was designated D1 of gestation). Mice were acclimatized for a minimum of 6 days prior to surgery. On D17 of gestation, a mini-laparotomy procedure was performed to expose the uterine horns, as previously described (Rinaldi et al., 2014). The number of viable pups in each horn was recorded prior to injection. In LPS dose–response experiments, the horn with the greater number of fetuses was injected with either LPS (5–20 µg; from Escherichia coli 0111:B4; Sigma-Aldrich, Poole, UK) or sterile PBS (Gibco, Life Technologies Ltd, Paisley, UK) each in a 25 μl volume using a 33-gauge Hamilton syringe. Injections were performed directly into the uterine cavity between the first and second anterior fetuses. Care was taken not to enter any amniotic sacs. The wound was then closed and mice received a subcutaneous injection of Vetergesic analgesia (Alstoe Ltd, York, UK) at a dose of 0.03 mg/ml in 60 μl.
Mice were kept at 30°C while they recovered from surgery, before being transferred to individual cages for continuous monitoring using individual CCTV cameras and a digital video recorder. The time to delivery was recorded and defined as the number of hours from the time of intrauterine injection, to delivery of the first pup. Preterm delivery was defined as delivery of the first pup within 36 h of intrauterine injection. Term delivery in CD1 mice occurs on D19-21 of gestation, and we previously reported that mean (±SEM) time to delivery in a ‘no surgery’ control group of CD1 mice was 51.34 ± 1.13 h (n = 8), with all these mice delivering on D19 of gestation (Rinaldi et al., 2014). Based on these data, delivery within 36 h of injection was chosen as preterm in our model. Within 12–24 h of delivery, the number of live/dead pups was recorded and the mortality rate per dam was calculated by dividing the number of dead pups by the number of viable pups counted in utero at the time of intrauterine injection.
In experiments to determine whether lipoxin administration could modulate LPS-induced preterm delivery and pup mortality, mice were pretreated with 15-epi-lipoxin A4 prior to intrauterine PBS or LPS administration. The 15-epi-lipoxin A4 analogue was chosen as several studies have reported that it is more stable, has a longer half-life in vivo and has more potent anti-inflammatory and pro-resolution effects, compared with lipoxin A4 (Serhan et al., 1995; Serhan, 1997; Gewirtz et al., 1998). Mice received an intra-peritoneal (i.p.) injection of vehicle (PBS + 1% ethanol) or 15-epi-lipoxin A4 (doses of 12.5 or 125 ng in a volume of 100 µl; Cayman Chemical, Ann Arbor, MI, USA), 1–2 h prior to intrauterine administration of PBS or 20 µg LPS. Therefore, there were five treatment groups: vehicle (i.p. injection of vehicle, followed by intrauterine PBS); 125 ng 15-epi-lipoxin A4 (i.p. injection of 125 ng 15-epi-lipoxin A4 followed by intrauterine PBS); LPS (i.p. injection of vehicle followed by intrauterine LPS); 12.5 ng 15-epi-lipoxin A4 + LPS (i.p. injection of 12.5 ng 15-epi-lipoxin A4 followed by intrauterine LPS) and 125 ng 15-epi-lipoxin A4 + LPS (i.p. injection of 125 ng 15-epi-lipoxin A4 followed by intrauterine LPS). The time to delivery and pup mortality rate was then recorded in each treatment group, as described earlier.
Tissue collection
In a separate cohort of mice, to examine the effect of pretreatment with 15-epi-lipoxin A4 on the LPS-induced inflammatory response of the utero-placental tissues, tissues were collected 6 h post-surgery from mice treated with either vehicle or 15-epi-lipoxin A4 (0.25 and 2.5 µg) 1–2 h prior to intrauterine administration of PBS or 20 µg LPS. Higher doses of 15-epi-lipoxin A4 were used in these 6 h experiments to maximize the potential anti-inflammatory actions of 15-epi-lipoxin A4. All doses of 15-epi-lipoxin A4 used in this study were chosen based on published literature, which shows that lipoxins can be tolerated and have strong anti-inflammatory and pro-resolution effects over a wide range of doses in vivo (Levy et al., 2002; El Kebir et al., 2009; Kure et al., 2009; Conte et al., 2010; Borgeson et al., 2011; Zhou et al., 2011). Mice were culled by lethal exposure to CO2 and all pups were removed from the uterine horns and decapitated. Uterine tissue was sampled from three fixed sites within the uterus; fetal membranes were dissected free from the placenta, and these tissues were collected from three separate gestational sacs. Tissues were stored in RNAlater® (Sigma-Aldrich) at −80°C until processing.
Quantitative real-time PCR
Total RNA was extracted from uterus, fetal membranes and placental tissue collected 6 h post-surgery using the RNeasy mini kit (Qiagen, Crawley, UK) as per the manufacturer's guidelines. Total RNA (300 ng) was reverse transcribed using the High Capacity cDNA Reverse Transcription kit (Applied biosystems, Life Technologies Ltd, Paisely, UK). Quantitative real-time PCR (qRT-PCR) was carried out to quantify the mRNA expression of specific genes of interest. Predesigned gene expression assays from Applied Biosystems were used to examine the expression of 15-hydroxy prostaglandin dehydrogenase (15-Hpgd) (Mm00515121_m1), Il-10 (Mm00439614_m1), Il-1β (Mm00434228_m1), Tnf-α (Mm99999068_m1), Cxcl1 (Mm04207460_m1), Cxcl2 (Mm00436450_m1) and Cxcl5 (Mm00436451_g1). Primer and probe sequences for β-actin, Ptgs2 and Il-6 were designed using Primer Express Software (version 3.0). Details of designed β-actin, Ptgs2 and Il-6 primer and probe sequences are given in Table I. Target gene expression was normalized for RNA loading using β-actin and the expression in each sample was calculated relative to a calibrator sample (untreated D18 uterus), which was included in all reactions, using the 2−ΔΔCt method of analysis. All qRT-PCR analysis was performed on an Applied Biosystems 7900HT instrument.
Table I.
Primer and probe sequences designed using Primer Express software.
| Gene | Primer/Probe | Sequence |
|---|---|---|
| β-actin | Forward | 5′-GCTTCTTTGCAGCTCCTTCGT-3′ |
| Reverse | 5′-GCGCAGCGATATCGTCATC-3′ | |
| Probe | 5′-CACCCGCCACCAGTTCGCCAT-3′ | |
| Ptgs2 | Forward | 5′-GCTTCGGGAGCACAACAG-3′ |
| Reverse | 5′-TGGTTTGGAATAGTTGCTC-3′ | |
| Probe | 5′-TGTGCGACATACTCAAGCA-3′ | |
| Il-6 | Forward | 5′-CCACGGCCTTCCCTACTTC-3′ |
| Reverse | 5′-TGCACAACTCTTTTCTCATTCCA-3′ | |
| Probe | 5′-TCACAGAGGATACCACTCCCAACAGACCTG-3′ |
Statistical analysis
Data are presented as mean ± SEM. Where data were not normally distributed they were transformed prior to analysis to achieve normal distribution. Time to delivery data was log-transformed before analysis; and the proportion of dead pups was arc-sin transformed prior to analysis. Data were analysed by one-way analysis of variance to compare treatment groups, followed by either Dunnett's or Newman–Keuls multiple comparison tests between treatment groups to identify significant differences. All statistical analyses were performed using GraphPad Prism 5.0 software (Graph Pad, San Diego, CA, USA). P < 0.05 was considered to indicate statistical significance.
Results
Intrauterine LPS administration dose-dependently increases pup mortality
As we have previously reported, intrauterine LPS administration dose-dependently induces PTL in a mouse model (Rinaldi et al., 2014). To assess the effects of intrauterine LPS treatment on pup mortality mice were treated with increasing doses of LPS and the pup mortality rate was calculated following delivery. Pup mortality was increased in response to administration of increasing doses of intrauterine LPS, with a significantly higher proportion of dead pups born to mice treated with 20 µg LPS, compared with the PBS control group (mean ± SEM proportion of dead pups 0.75 ± 0.05 versus 0.40 ± 0.06, respectively, P < 0.001; Fig. 1A). To further investigate whether this observed increase in pup mortality in the 20 µg LPS group was simply due to a higher proportion of preterm deliveries in this group, rather than a direct effect of the LPS treatment, pup mortality was also assessed only in mice delivering preterm (defined as delivery within 36 h of surgery). Even amongst mice delivering preterm, fetal mortality was still significantly greater in mice treated with 20 µg LPS group compared with PBS (mean ± SEM proportion of dead pups 0.85 ± 0.04 versus 0.49 ± 0.11, respectively, P < 0.01; Fig. 1B). Subsequent experiments were performed with 20 µg LPS as this dose has been shown to induce preterm delivery reliably in our model with the least variation (Rinaldi et al., 2014).
Figure 1.
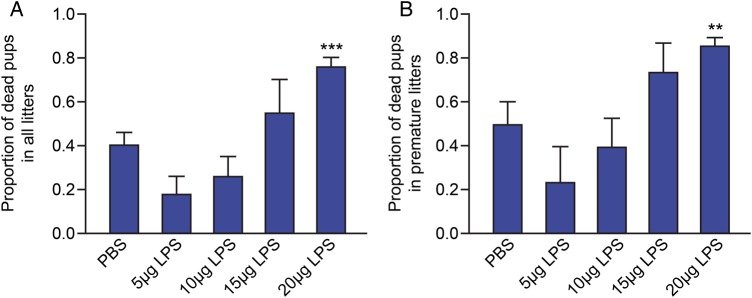
Effect of intrauterine LPS administration on pup mortality. The proportion of dead pups were determined in mice receiving intrauterine injection of either phosphate-buffered saline (PBS; n = 35), 5 μg LPS (n = 6), 10 μg LPS (n = 11), 15 μg LPS (n = 8) and 20 μg LPS (n = 42). (A) Proportion of dead pups in all litters. (B) Proportion of dead pups in premature litters (delivered within 36 h of surgery); [PBS (n = 14), 5 μg LPS (n = 3), 10 μg LPS (n = 6), 15 μg LPS (n = 6) and 20 μg LPS (n = 35)]. Data presented as mean ± SEM (error bars); **P < 0.01, ***P < 0.001, compared with PBS.
Pretreatment with 15-epi-lipoxin A4 reduces pup mortality without delaying LPS-induced preterm delivery
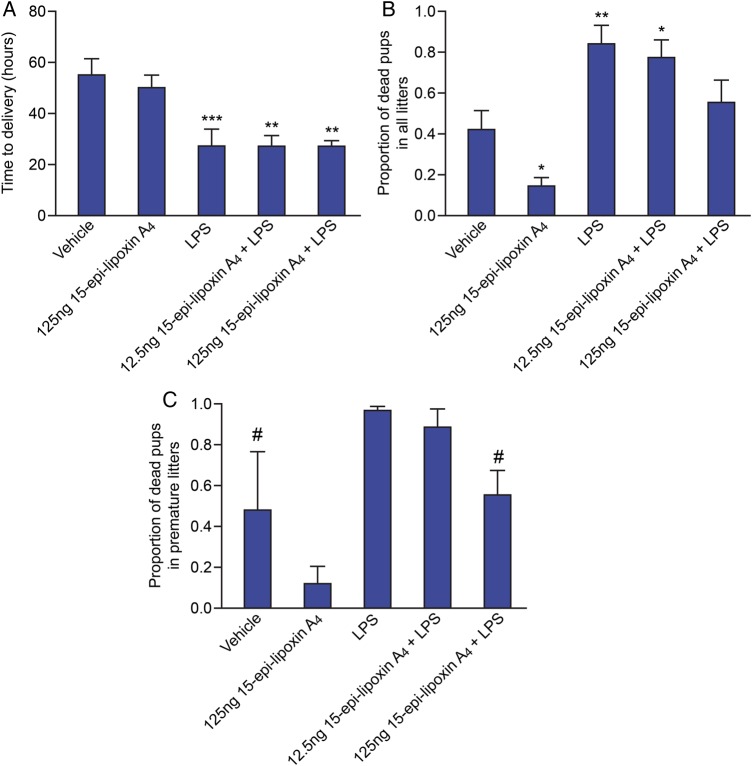
To investigate the therapeutic potential of lipoxin to delay preterm delivery and reduce prematurity induced fetal mortality, mice were pretreated with 15-epi-lipoxin A4 1–2 h prior to intrauterine LPS (20 µg) or PBS administration. Control mice were pretreated with vehicle prior to intrauterine LPS or PBS administration. Pretreatment with 125 ng 15-epi-lipoxin A4 prior to intrauterine PBS had no effect on time to delivery compared with the vehicle control group (Fig. 2A). As expected mice receiving intrauterine LPS delivered significantly earlier than the vehicle control group (LPS mean time to delivery: 27.54 h ± SEM 6.33; versus vehicle mean time to delivery: 55.40 h ± SEM 6.40; P < 0.001; Fig. 2A). Pretreatment with either 12.5 or 125 ng 15-epi-lipoxin A4 prior to intrauterine LPS administration did not delay LPS-induced PTL. Mice in these groups still delivered significantly earlier than the vehicle control group (mean ± SEM time to delivery 12.5 ng 15-epi-lipoxin A4 + LPS: 27.02 ± 4.57 h; mean time delivery in 125 ng 15-epi-lipoxin A4 + LPS: 26.82 ± 2.61; P < 0.01 versus vehicle).
Figure 2.
Effect of pretreatment with 15-epi-lipoxin A4 on time to delivery and pup mortality. Time to delivery and the proportion of dead pups was determined in mice pretreated with vehicle (n = 12) or 125 ng 15-epi-lipoxin A4 (n = 9), prior to intrauterine PBS; and in mice pretreated with vehicle (n = 12), 12.5 ng 15-epi-lipoxin A4 (n = 11) or 125 ng 15-epi-lipoxin A4 (n = 11), prior to intrauterine LPS (20 µg) administration. (A) Time to delivery. (B) Proportion of dead pups in all litters. (C) Proportion of dead pups in premature litters (delivered within 36 h of surgery); [Vehicle (n = 3), 125 ng 15-epi-lipoxin A4 (n = 2), LPS (n = 10), 12.5 ng 15-epi-lipoxin A4 (n = 7) or 125 ng 15-epi-lipoxin A4 (n = 10)]. The 15-epi-lipoxin A4 group was excluded from statistical analysis of the proportion of prematurely delivered dead pups due to n < 3. Data presented as mean ± SEM (error bars); *P < 0.05, **P < 0.01, ***P < 0.001, compared with vehicle; #P < 0.05 compared with LPS.
Again as expected, mice treated with LPS alone had significantly increased pup mortality compared with the vehicle group (mean ± SEM proportion of dead pups: 0.84 ± 0.09; P < 0.01; Fig. 2B). Interestingly, pretreatment with 125 ng 15-epi-lipoxin A4 prior to intrauterine PBS significantly reduced pup mortality, compared with the vehicle control group (mean ± SEM proportion of dead pups 0.13 ± 0.05 versus 0.42 ± 0.1, respectively, P < 0.05; Fig. 2B). Within the subgroup of mice delivering preterm (within 36 h of surgery), pretreatment with 125 ng 15-epi-lipoxin A4 prior to intrauterine LPS significantly reduced pup mortality, compared with mice receiving LPS alone (mean proportion ± SEM of dead pups 0.55 ± 0.12 versus 0.97 ± 0.02, respectively; P < 0.05; Fig. 2C).
Pretreatment with 15-epi-lipoxin A4 alters the expression of Ptgs2 and 15-Hpgd in the utero-placental tissues, but does not attenuate LPS-induced expression of classical pro-inflammatory markers
To examine whether pretreatment with 15-epi-lipoxin A4 affected the LPS-induced inflammatory response of the utero-placental tissues, qRT-PCR analysis was performed on uterus, placenta and fetal membranes collected 6 h post-surgery. The mRNA expression of several key inflammatory genes associated with parturition was quantified. The genes measured were: two of the key enzymes responsible for regulating prostaglandin synthesis and breakdown, respectively, Ptgs2 and 15-Hpgd; the pro-inflammatory cytokines Il-1β, Tnf-α and Il-6; and the chemokines Cxcl1, Cxcl2 and Cxcl5. To investigate the anti-inflammatory actions of 15-epi-lipoxin A4, we administered higher doses (0.25 and 2.5 µg) of 15-epi-lipoxin A4 1–2 h prior to LPS or vehicle to try to maximize the anti-inflammatory effects in these 6 h experiments. As stated earlier, all doses of 15-epi-lipoxin A4 used were within the range of effective doses used in vivo in previously published studies.
In the uterus, Ptgs2 expression was significantly elevated in response to 2.5 µg 15-epi-lipoxin A4 alone (P < 0.01), LPS alone (P < 0.01), and 0.25 µg and 2.5 µg 15-epi-lipoxin A4 + LPS (P < 0.001; Fig. 3A), compared with the vehicle control group. Co-treatment with 2.5 µg 15-epi-lipoxin A4 and LPS also significantly increased uterine Ptgs2 expression compared with treatment with LPS alone (P < 0.05; Fig. 3A). Conversely, uterine 15-Hpgd expression was significantly reduced in mice treated with 2.5 µg 15-epi-lipoxin A4 prior to intrauterine PBS, compared with vehicle (P < 0.01) and LPS alone (P < 0.05). LPS alone did not significantly alter 15-Hpgd expression; however, mice treated with 0.25 µg 15-epi-lipoxin A4 + LPS and 2.5 µg 15-epi-lipoxin A4 + LPS had significantly reduced uterine 15-Hpgd expression, compared with the vehicle group (P < 0.001). Additionally pretreatment with 0.25 µg 15-epi-lipoxin A4 and 2.5 µg 15-epi-lipoxin A4 prior to intrauterine LPS, significantly reduced uterine expression of 15-Hpgd, compared with LPS alone (P < 0.001; Fig. 3A).
Figure 3.
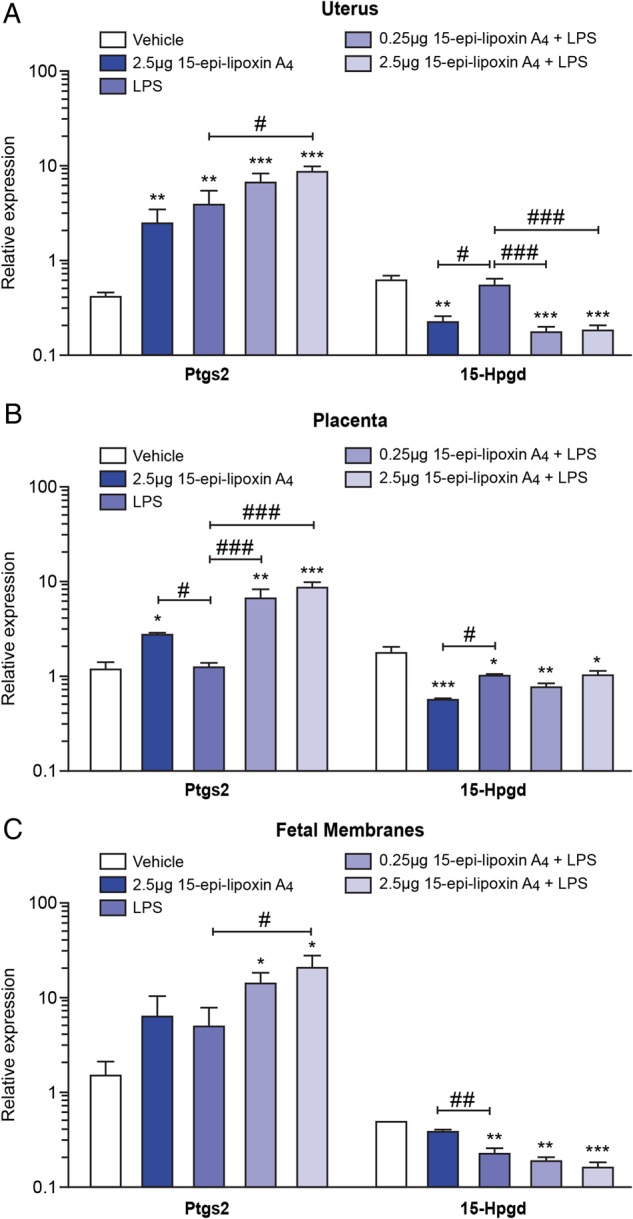
Effect of pretreatment with 15-epi-lipoxin A4 on mRNA expression of Ptgs2 and 15-Hpgd in the utero-placental tissues. Uterus, placenta and fetal membranes were collected 6 h post-surgery from mice pretreated with vehicle (n = 3) or 2.5 μg 15-epi-lipoxin A4 (n = 4), prior to intrauterine PBS; and in mice pretreated with vehicle (n = 5), 0.25 μg 15-epi-lipoxin A4 (n = 5) or 2.5 μg 15-epi-lipoxin A4 (n = 5), prior to intrauterine LPS administration. The mRNA expression of Ptgs2 and 15-Hpgd was quantified by quantitative real-time PCR. (A) Uterine expression. (B) Placental expression. (C) Expression in the fetal membranes. Data presented as mean fold change ± SEM (error bars); *P < 0.05, **P < 0.01, ***P < 0.001, compared with vehicle; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with LPS.
Placental Ptgs2 expression was significantly elevated in mice treated with 2.5 µg 15-epi-lipoxin A4 prior to intrauterine PBS, compared with vehicle (P < 0.05; Fig. 3B) and LPS alone (P < 0.05). Ptgs2 expression in the placenta was unaffected by LPS alone, but pretreatment with 15-epi-lipoxin A4 at both 0.25 and 2.5 µg prior to intrauterine LPS administration significantly increased Ptgs2 expression compared with both the vehicle control group (P < 0.01 and P < 0.001, respectively; Fig. 3B) and compared with LPS treatment alone (P < 0.001; Fig. 3B). Placental 15-Hpgd expression was significantly down-regulated in response to 2.5 µg 15-epi-lipoxin A4 alone (P < 0.001), LPS alone (P < 0.05), 0.25 µg 15-epi-lipoxin A4 + LPS (P < 0.01) and 2.5 µg 15-epi-lipoxin A4 + LPS (P < 0.05; Fig. 3B), compared with the vehicle control group.
In the fetal membranes, intrauterine LPS treatment alone did not significantly alter Ptgs2 expression; however, mice treated with 0.25 µg 15-epi-lipoxin A4 + LPS had significantly elevated Ptgs2 expression compared with the vehicle control group (P < 0.05; Fig. 3C); and mice treated with 2.5 µg 15-epi-lipoxin A4 + LPS had significantly elevated Cox-2 expression, compared with both the vehicle control group and LPS alone (P < 0.05; Fig. 3C). Expression of 15-Hpgd in the fetal membranes was significantly reduced in response to LPS treatment alone (P < 0.01), 0.25 µg 15-epi-lipoxin A4 + LPS (P < 0.01) and 2.5 µg 15-epi-lipoxin A4 + LPS (P < 0.001; Fig. 3B).
In contrast to the effects on Ptgs2 and 15-Hpgd, pretreatment with 15-epi-lipoxin A4 at either 0.25 or 2.5 µg prior to intrauterine LPS did not attenuate or amplify the LPS-induced expression of the classical inflammatory markers Tnf-α and Il-1β in the uterus (Fig. 4A), placenta (Fig. 4B) and fetal membranes (Fig. 4C). Similarly, pretreatment with 15-epi-lipoxin A4 did not alter the LPS-induced expression of the other inflammatory mediators examined, Il-6, Cxcl1, Cxcl2 and Cxcl5 (data not shown).
Figure 4.
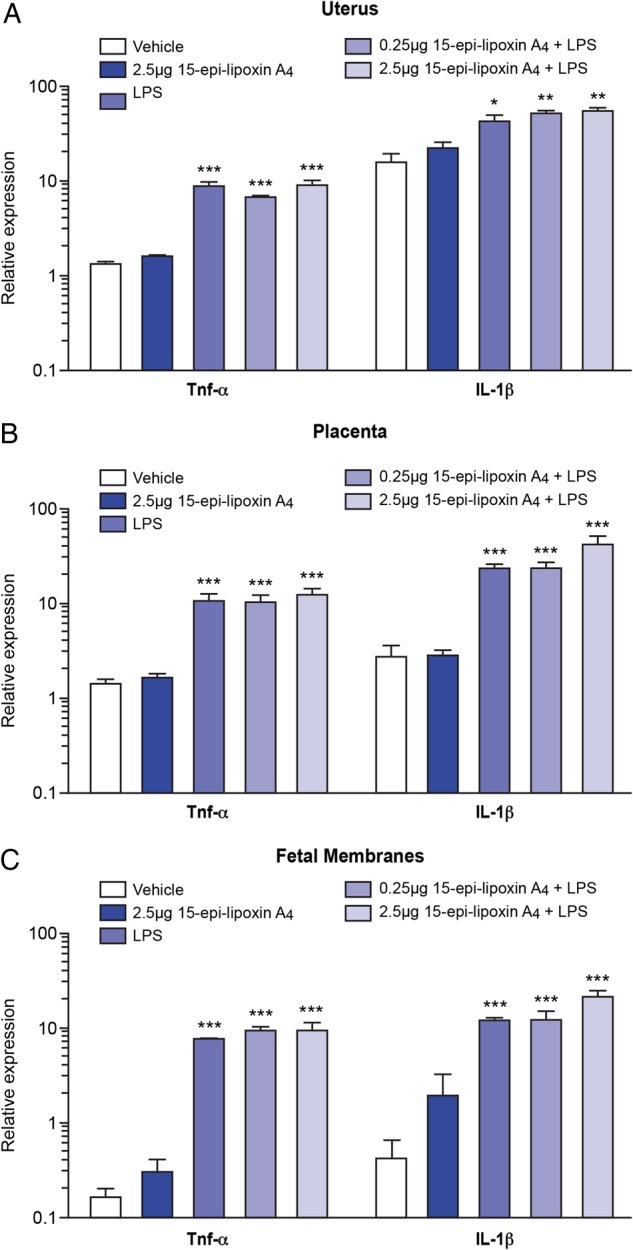
Effect of pretreatment with 15-epi-lipoxin A4 on mRNA expression of Tnf-α and Il-1β in the utero-placental tissues. Uterus, placenta and fetal membranes were collected 6 h post-surgery from mice pretreated with vehicle (n = 3) or 2.5 μg 15-epi-lipoxin A4 (n = 4), prior to intrauterine PBS; and in mice pretreated with vehicle (n = 5), 0.25 μg 15-epi-lipoxin A4 (n = 5) or 2.5 μg 15-epi-lipoxin A4 (n = 5), prior to intrauterine LPS administration. The mRNA expression of Tnf-α and Il-1β was quantified by quantitative real-time PCR. (A) Uterine expression. (B) Placental expression. (C) Expression in the fetal membranes. Data presented as mean fold change ± SEM (error bars); *P < 0.05, **P < 0.01, ***P < 0.001, compared with vehicle.
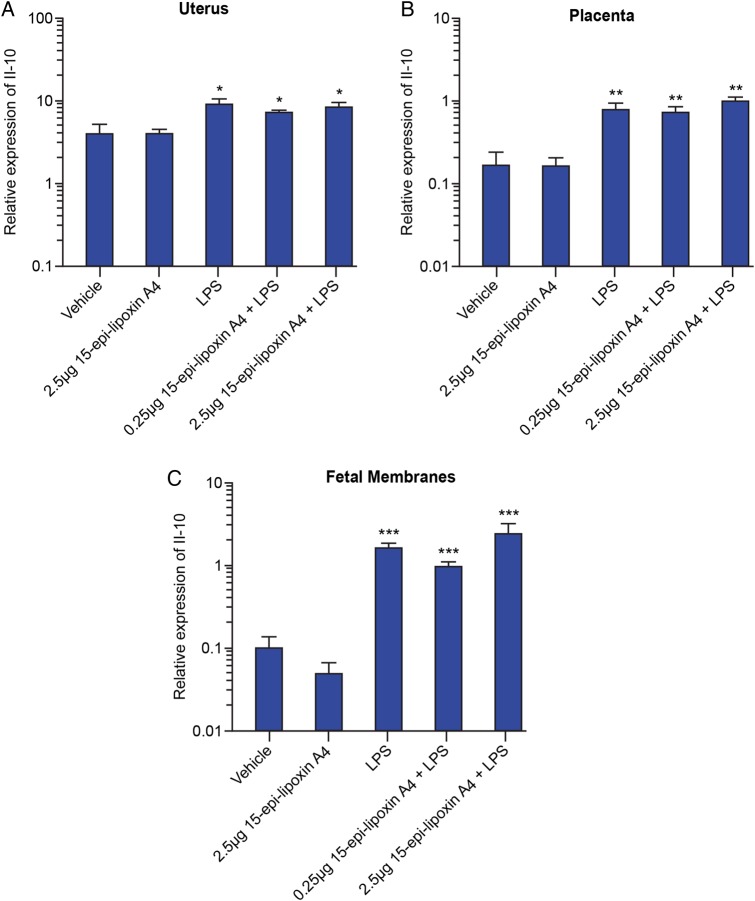
Pretreatment with 15-epi-lipoxin A4 does not further up-regulate the LPS-induced expression of Il-10 in the utero-placental tissues
Previous studies have reported that one mechanism by which lipoxins exert anti-inflammatory actions is by up-regulating the expression of the anti-inflammatory cytokine, IL-10 (Baker et al., 2009; Borgeson et al., 2011). Therefore, we investigated the mRNA expression of Il-10 in the utero-placental tissues 6 h post-surgery using qRT-PCR. Treatment with LPS alone resulted in significantly elevated expression of Il-10 in the uterus (P < 0.05; Fig. 5A), placenta (P < 0.01; Fig. 5B) and fetal membranes (P < 0.001; Fig. 5C). However, pretreatment with 15-epi-lipoxin A4, at either 0.25 or 2.5 µg, prior to intrauterine LPS treatment did not result in a further increase in Il-10 expression, compared with LPS alone.
Figure 5.
Effect of pretreatment with 15-epi-lipoxin A4 on mRNA expression of Il-10 in the utero-placental tissues. Uterus, placenta and fetal membranes were collected 6 h post-surgery from mice pretreated with vehicle (n = 3) or 2.5 μg 15-epi-lipoxin A4 (n = 4), prior to intrauterine PBS; and in mice pretreated with vehicle (n = 5), 0.25 μg 15-epi-lipoxin A4 (n = 5) or 2.5 μg 15-epi-lipoxin A4 (n = 5), prior to intrauterine LPS administration. The mRNA expression of Il-10 was quantified by quantitative real-time PCR. (A) Uterine expression. (B) Placental expression. (C) Expression in the fetal membranes. Data presented as mean fold change ± SEM (error bars); *P < 0.05, **P < 0.01, ***P < 0.001, compared with vehicle.
Discussion
We have previously shown the anti-inflammatory effects of the dual-acting anti-inflammatory and pro-resolution lipid mediators, lipoxins, in human gestational tissues in vitro (Maldonado-Perez et al., 2010). Here, we tested the efficacy of 15-epi-lipoxin A4 as a novel therapeutic agent in an in vivo mouse model of infection-induced PTL. Contrary to our original hypothesis, we did not observe a reduction in preterm delivery or reduced pro-inflammatory signalling in mice treated with 15-epi-lipoxin A4. We did, however, show that 15-epi-lipoxin A4 treatment reduced the mortality of prematurely delivered pups and altered basal and LPS-induced Ptgs2 and 15-Hpgd expression in the utero-placental tissues.
We believe that the finding that 15-epi-lipoxin A4 treatment resulted in a greater proportion of prematurely delivered pups being born alive is a novel and important discovery. Current treatment options for preterm birth are largely limited to tocolytic therapies that aim to block myometrial contractions and prolong gestation. However, there is little convincing evidence that these treatments actually result in improved neonatal outcome in the long-term. Given that preterm birth remains the single biggest cause of neonatal mortality and morbidity worldwide, there is an urgent requirement for novel therapeutic options which are capable of achieving the ultimate goal of preterm prevention therapies—reduced perinatal mortality and morbidity. Interestingly a recent paper has highlighted the potential of lipoxin treatment to treat the preterm-related lung disease, BPD (Martin et al., 2014). Using a mouse model of hyperoxia-induced lung injury Martin et al. (2014) reported that lipoxin A4 treatment given (post-natally) to neonatal pups reduced the morphologic and cellular characteristics of lung injury and improved pup growth; therefore, supporting the hypothesis that pre- and post-natal lipoxins could be useful novel therapeutic agents to improve neonatal outcome.
The pup mortality observed in our model is likely to be a result of the immaturity of the prematurely delivered pups, which if delivered on Day 17 or 18 of gestation are unlikely to be developmentally competent to survive, and also the LPS treatment given to the mice. Owing to the invasive nature of the model, which we have previously shown results in a local inflammatory response, even in mice treated with PBS (Rinaldi et al., 2014), some of the control mice do deliver prematurely, and therefore do experience some pup mortality. We are currently exploring other, less invasive methods, to reduce this preterm delivery in our control group. Importantly, however, we did observe a significant reduction in pup mortality in mice treated with intrauterine PBS if they were pretreated with 15-epi-lipoxin A4, suggesting that treatment with 15-epi-lipoxin A4 may be able to protect the fetus from the negative effects of the local inflammatory response induced by the surgery.
The mechanism by which 15-epi-lipoxin A4 reduces perinatal mortality in our model is currently unclear, although our data implicate prostanoid regulation via increased Ptgs2 and decreased 15-Hpgd expression in the uterus and placenta. This increased expression of Ptgs2 could result in increased production of prostaglandins with anti-inflammatory effects, such as PGE2, PGD2 and 15d-PGJ2, as has been described in other studies (Gilroy et al., 1999; Hodges et al., 2004; Fukunaga et al., 2005; Bonnans et al., 2006; Zheng et al., 2011; Font-Nieves et al., 2012). These prostaglandins may act to resolve the inflammatory environment surrounding the fetus, thus leading to the reduced pup mortality rate observed in mice treated with 15-epi-lipoxin A4. Support for this hypothesis comes from a study that reported that administration of 15d-PGJ2 increased pup survival in a mouse model of LPS-induced PTL (Pirianov et al., 2009).
Another potential mechanism by which 15-epi-lipoxin A4 could be acting to reduce pup mortality may be by promoting fetal lung maturation. PGE2 has been implicated in regulating fetal pulmonary surfactant production both in vitro (Acarregui et al., 1990) and in vivo in a sheep model of intra-amniotic infection (Westover et al., 2012); suggesting that the 15-epi-lipoxin A4-induced increase in utero-placental Ptgs2 expression may promote fetal lung maturation via increased local PGE2 production. Additionally, a recent study reported that administration of a synthetic analogue of 15-epi-lipoxin A4 restored expression of surfactant protein C in lung tissue in a model of bleomycin-induced pulmonary fibrosis (Guilherme et al., 2013); supporting the hypothesis that lipoxin administration can regulate lung surfactant production. Further work examining the inflammatory response at several time points is required to elucidate the relationship between Ptgs2 and 15-epi-lipoxin A4 in our model, and to identify whether alterations in prostanoid production are involved in the reduced pup mortality observed in this study.
Interestingly, the administration of low-dose aspirin to women during pregnancy has been associated with reduced perinatal death and other adverse perinatal outcomes (Bujold et al., 2010; Roberge et al., 2013). As 15-epi-lipoxins are produced in the presence of aspirin, it is possible that 15-epi-lipoxin A4 is involved in mediating any beneficial effects of aspirin treatment. Other studies have shown that low-dose aspirin administration to healthy volunteers leads to significantly elevated plasma levels of 15-epi-lipoxin A4 (Chiang et al., 2004), therefore, it would be interesting to assess whether similar mechanisms are acting during pregnancy.
Another important observation from our work which is worthy of further investigation is the finding that elevated levels of Ptgs2 were also observed in uterus and placental tissue obtained from mice treated with 15-epi-lipoxin A4 alone, even though mice in this treatment group did not go into PTL. Previous studies have demonstrated a central role for elevated Ptgs2 expression, and subsequent production of prostaglandins such as PGF2α and PGE2 in the onset of parturition in mice (Sugimoto et al., 1997; Gross et al., 1998, 2000; Tsuboi et al., 2003). However, mice in the 15-epi-lipoxin A4 group delivered at term, despite having elevated Ptgs2 expression, again suggesting that treatment with 15-epi-lipoxin A4 may be triggering an alternative prostanoid pathway, as has been reported in other systems (Zheng et al., 2011).
Interestingly, 15-epi-lipoxin A4 was unable to attenuate LPS-induced pro-inflammatory signalling in our model, which is in contrast to our previous work showing that lipoxin treatment in vitro attenuated IL-6 and IL-8 expression in human myometrial explant culture (Maldonado-Perez et al., 2010). The reasons for these differences are unclear, but may be a result of differences in the type and dose of lipoxin used in the two studies, and also the time-point at which tissues were collected from our in vivo model. Perhaps if tissues had been collected at a different time-point, we may have observed alterations in inflammatory signalling. Whilst it is often difficult to extrapolate between animal models and the clinical scenario in humans, importantly, our in vitro data suggests that lipoxin treatment may have a more profound impact on inflammatory signalling in human tissues.
This study demonstrates for the first time that 15-epi-lipoxin A4 reduces pup mortality in a mouse model of LPS-induced PTL. Although the mechanisms by which 15-epi-lipoxin A4 may be acting to protect the prematurely delivered pups from mortality are not currently clear, we propose that 15-epi-lipoxin A4 may be stimulating the resolution of the LPS-induced inflammatory and/or promoting fetal maturation via increased Ptgs2 expression and decreased 15-Hpgd expression in the utero-placental tissues. Collectively, these data suggest that lipoxins warrant further investigation as potential novel therapeutic options in the treatment of PTL, which may be useful in protecting the fetus from the adverse effects of infection-induced preterm birth.
Authors' roles
S.F.R., R.D.C. and J.W. performed the experiments. S.F.R. wrote the manuscript. S.F.R., R.D.C., J.W., A.G.R. and J.E.N. contributed to the design of the study, analysis and interpretation of the data, drafting of the article and final approval of the version to be published.
Funding
This work was supported by grants from Tommy's the baby charity and PiggyBank Kids. S.F.R. is supported by Medical Research Council (grant number MR/L002657/1). Funding to pay the Open Access publication charges for this article was provided by the Medical Research Council.
Conflict of interest
No authors declare any financial or other relationships that might lead to a conflict of interest.
Acknowledgements
The authors thank Ronnie Grant for graphic design and Prof Catherine Godson for advice and critical reading of this manuscript.
References
- Acarregui MJ, Snyder JM, Mitchell MD, Mendelson CR. Prostaglandins regulate surfactant protein A (SP-A) gene expression in human fetal lung in vitro. Endocrinology 1990;127:1105–1113. [DOI] [PubMed] [Google Scholar]
- Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci 2008;15:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N, O'Meara SJ, Scannell M, Maderna P, Godson C. Lipoxin A4: anti-inflammatory and anti-angiogenic impact on endothelial cells. J Immunol 2009;182:3819–3826. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol 2006;168:1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeson E, Docherty NG, Murphy M, Rodgers K, Ryan A, O'Sullivan TP, Guiry PJ, Goldschmeding R, Higgins DF, Godson C. Lipoxin A(4) and benzo-lipoxin A(4) attenuate experimental renal fibrosis. FASEB J 2011;25:2967–2979. [DOI] [PubMed] [Google Scholar]
- Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, Forest JC, Giguere Y. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol 2010;116:402–414. [DOI] [PubMed] [Google Scholar]
- Chang EY, Zhang J, Sullivan S, Newman R, Singh I. N-acetylcysteine attenuates the maternal and fetal proinflammatory response to intrauterine LPS injection in an animal model for preterm birth and brain injury. J Matern Fetal Neonatal Med 2011;24:732–740. [DOI] [PubMed] [Google Scholar]
- Chen QH, Zhou WD, Pu DM, Huang QS, Li T, Chen QX. 15-Epi-lipoxin A(4) inhibits the progression of endometriosis in a murine model. Fertil Steril 2010;93:1440–1447. [DOI] [PubMed] [Google Scholar]
- Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers anti-inflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA 2004;101:15178–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids 2005;73:163–177. [DOI] [PubMed] [Google Scholar]
- Conte FP, Menezes-de-Lima O, Jr, Verri WA, Jr, Cunha FQ, Penido C, Henriques MG. Lipoxin A(4) attenuates zymosan-induced arthritis by modulating endothelin-1 and its effects. Br J Pharmacol 2010;161:911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison FC, Kelly RW, Calder AA, Riley SC. Cytokine secretion by human fetal membranes, decidua and placenta at term. Hum Reprod 1998;13:3560–3565. [DOI] [PubMed] [Google Scholar]
- El Kebir D, Jozsef L, Khreiss T, Pan W, Petasis NA, Serhan CN, Filep JG. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J Immunol 2007;179:616–622. [DOI] [PubMed] [Google Scholar]
- El Kebir D, Jozsef L, Pan W, Wang L, Petasis NA, Serhan CN, Filep JG. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med 2009;180:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol 2003;163:2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filep JG, Khreiss T, Jozsef L. Lipoxins and aspirin-triggered lipoxins in neutrophil adhesion and signal transduction. Prostaglandins Leukot Essent Fatty Acids 2005;73:257–262. [DOI] [PubMed] [Google Scholar]
- Font-Nieves M, Sans-Fons MG, Gorina R, Bonfill-Teixidor E, Salas-Perdomo A, Marquez-Kisinousky L, Santalucia T, Planas AM. Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes. J Biol Chem 2012;287:6454–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol 2005;174:5033–5039. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, McCormick B, Neish AS, Petasis NA, Gronert K, Serhan CN, Madara JL. Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest 1998;101:1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, Williams IR, Neish AS, Madara JL. Lipoxin a4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol 2002;168:5260–5267. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med 1999;5:698–701. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov 2004;3:401–416. [DOI] [PubMed] [Google Scholar]
- Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol 2000;164:1663–1667. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342:1500–1507. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol 2011;179:838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GA, Imamura T, Luedke C, Vogt SK, Olson LM, Nelson DM, Sadovsky Y, Muglia LJ. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci USA 1998;95:11875–11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G, Imamura T, Vogt SK, Wozniak DF, Nelson DM, Sadovsky Y, Muglia LJ. Inhibition of cyclooxygenase-2 prevents inflammation-mediated preterm labor in the mouse. Am J Physiol Regul Integr Comp Physiol 2000;278:R1415–R1423. [DOI] [PubMed] [Google Scholar]
- Guilherme RF, Xisto DG, Kunkel SL, Freire-de-Lima CG, Rocco PR, Neves JS, Fierro IM, Canetti C, Benjamim CF. Pulmonary antifibrotic mechanisms aspirin-triggered lipoxin A(4) synthetic analog. Am J Respir Cell Mol Biol 2013;49:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges RJ, Jenkins RG, Wheeler-Jones CP, Copeman DM, Bottoms SE, Bellingan GJ, Nanthakumar CB, Laurent GJ, Hart SL, Foster ML, et al. Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin E(2) production. Am J Pathol 2004;165:1663–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JL, Rajagopal SP, Sales KJ, Jabbour HN. Molecular regulators of resolution of inflammation: potential therapeutic targets in the reproductive system. Reproduction 2011;142:15–28. [DOI] [PubMed] [Google Scholar]
- Hutchinson JL, Rajagopal SP, Yuan M, Norman JE. Lipopolysaccharide promotes contraction of uterine myocytes via activation of Rho/ROCK signaling pathways. FASEB J. 2013;28:94–105. [DOI] [PubMed] [Google Scholar]
- Kure I, Nishiumi S, Nishitani Y, Tanoue T, Ishida T, Mizuno M, Fujita T, Kutsumi H, Arita M, Azuma T, et al. Lipoxin A(4) reduces lipopolysaccharide-induced inflammation in macrophages and intestinal epithelial cells through inhibition of nuclear factor-kappaB activation. J Pharmacol Exp Ther 2009;332:541–548. [DOI] [PubMed] [Google Scholar]
- Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4). Nat Med 2002;8:1018–1023. [DOI] [PubMed] [Google Scholar]
- Li L, Kang J, Lei W. Role of Toll-like receptor 4 in inflammation-induced preterm delivery. Mol Hum Reprod 2010;16:267–272. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med 1996;183:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderna P, Godson C. Lipoxins: resolutionary road. Br J Pharmacol 2009;158:947–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Perez D, Golightly E, Denison FC, Jabbour HN, Norman JE. A role for lipoxin A4 as anti-inflammatory and proresolution mediator in human parturition. FASEB J 2010;25:569–575. [DOI] [PubMed] [Google Scholar]
- March of Dimes P, Save the Children, WHO. Born Too Soon: The Global Action Report on Preterm Birth. Geneva, World Health Organisation, 2012. [Google Scholar]
- Martin CR, Zaman MM, Gilkey C, Salguero MV, Hasturk H, Kantarci A, Van Dyke TE, Freedman SD. Resolvin D1 and lipoxin A4 improve alveolarization and normalize septal wall thickness in a neonatal murine model of hyperoxia-induced lung injury. PLoS One 2014;9:e98773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SP, Hanna NN, Fast LD, Shaw SK, Berg G, Padbury JF, Romero R, Sharma S. Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am J Obstet Gynecol 2009;200:308e301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath CA, Ananth CV, Smulian JC, Peltier MR. Can sulfasalazine prevent infection-mediated pre-term birth in a murine model? Am J Reprod Immunol 2010;63:144–149. [DOI] [PubMed] [Google Scholar]
- Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 2003;9:41–45. [DOI] [PubMed] [Google Scholar]
- Papayianni A, Serhan CN, Brady HR. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J Immunol 1996;156:2264–2272. [PubMed] [Google Scholar]
- Pirianov G, Waddington SN, Lindstrom TM, Terzidou V, Mehmet H, Bennett PR. The cyclopentenone 15-deoxy-delta 12,14-prostaglandin J(2) delays lipopolysaccharide-induced preterm delivery and reduces mortality in the newborn mouse. Endocrinology 2009;150:699–706. [DOI] [PubMed] [Google Scholar]
- Rinaldi SF, Hutchinson JL, Rossi AG, Norman JE. Anti-inflammatory mediators as physiological and pharmacological regulators of parturition. Expert Rev Clin Immunol 2011;7:675–696. [DOI] [PubMed] [Google Scholar]
- Rinaldi SF, Catalano RD, Wade J, Rossi AG, Norman JE. Decidual neutrophil infiltration is not required for preterm birth in a mouse model of infection-induced preterm labor. J Immunol 2014;192:2315–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol 2013;41:491–499. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol 2006;177:4888–4896. [DOI] [PubMed] [Google Scholar]
- Rodts-Palenik S, Wyatt-Ashmead J, Pang Y, Thigpen B, Cai Z, Rhodes P, Martin JN, Granger J, Bennett WA. Maternal infection-induced white matter injury is reduced by treatment with interleukin-10. Am J Obstet Gynecol 2004;191:1387–1392. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 1991;165:969–971. [DOI] [PubMed] [Google Scholar]
- Ryan A, Godson C. Lipoxins: regulators of resolution. Curr Opin Pharmacol 2010;10:166–172. [DOI] [PubMed] [Google Scholar]
- Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol 2006;195:1578–1589. [DOI] [PubMed] [Google Scholar]
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–269. [DOI] [PubMed] [Google Scholar]
- Sennstrom MB, Ekman G, Westergren-Thorsson G, Malmstrom A, Bystrom B, Endresen U, Mlambo N, Norman M, Stabi B, Brauner A. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod 2000;6:375–381. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, Samuelsson B. Trihydroxytetraenes: a novel series of compounds formed from arachidonic acid in human leukocytes. Biochem Biophys Res Commun 1984;118:943–949. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, Colgan SP, Madara JL. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry 1995;34:14609–14615. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): a jungle of cell–cell interactions or a therapeutic opportunity? Prostaglandins 1997;53:107–137. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008;8:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science 1997;277:681–683. [DOI] [PubMed] [Google Scholar]
- Terrone DA, Rinehart BK, Granger JP, Barrilleaux PS, Martin JN, Jr, Bennett WA. Interleukin-10 administration and bacterial endotoxin-induced preterm birth in a rat model. Obstet Gynecol 2001;98:476–480. [DOI] [PubMed] [Google Scholar]
- Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol 2009;183:1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod 1999;14:229–236. [PubMed] [Google Scholar]
- Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod 2006;74:236–245. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Iwane A, Nakazawa S, Sugimoto Y, Ichikawa A. Role of prostaglandin H2 synthase 2 in murine parturition: study on ovariectomy-induced parturition in prostaglandin F receptor-deficient mice. Biol Reprod 2003;69:195–201. [DOI] [PubMed] [Google Scholar]
- Westover AJ, Hooper SB, Wallace MJ, Moss TJ. Prostaglandins mediate the fetal pulmonary response to intrauterine inflammation. Am J Physiol Lung Cell Mol Physiol 2012;302:L664–L678. [DOI] [PubMed] [Google Scholar]
- Wu SH, Liao PY, Dong L, Chen ZQ. Signal pathway involved in inhibition by lipoxin A(4) of production of interleukins induced in endothelial cells by lipopolysaccharide. Inflamm Res 2008;57:430–437. [DOI] [PubMed] [Google Scholar]
- Xiong J, Zeng P, Cheng X, Miao S, Wu L, Zhou S, Wu P, Ye D. Lipoxin A4 blocks embryo implantation by controlling estrogen receptor alpha activity. Reproduction 2013;145:411–420. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhao F, Lin F, Chen J, Huang Y. Lipoxin A4 inhibits the development of endometriosis in mice: the role of anti-inflammation and anti-angiogenesis. Am J Reprod Immunol 2012;67:491–497. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhao J, Zhang H, Ke T, Xu P, Cai W, Katirai F, Ye D, Huang Y, Huang B. Spontaneous miscarriages are explained by the stress/glucocorticoid/lipoxin A4 axis. J Immunol 2013;190:6051–6058. [DOI] [PubMed] [Google Scholar]
- Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod 2002;66:445–449. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang X, Wu P, Li H, Jin S, Zhou X, Li Y, Ye D, Chen B, Wan J. BML-111, a lipoxin receptor agonist, modulates the immune response and reduces the severity of collagen-induced arthritis. Inflamm Res 2008;57:157–162. [DOI] [PubMed] [Google Scholar]
- Zheng S, Wang Q, He Q, Song X, Ye D, Gao F, Jin S, Lian Q. Novel biphasic role of LipoxinA(4) on expression of cyclooxygenase-2 in lipopolysaccharide-stimulated lung fibroblasts. Mediators Inflamm 2011;2011:745340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Chen B, Sun H, Deng Z, Andersson R, Zhang Q. The protective effects of Lipoxin A4 during the early phase of severe acute pancreatitis in rats. Scand J Gastroenterol 2011;46:211–219. [DOI] [PubMed] [Google Scholar]