Abstract
Purpose
Dual-specificity protein phosphatase 4 (DUSP4), also known as mitogen-activated protein kinase phosphatase (MKP) 2 is a member of the inducible nuclear MKP group. The role of DUSP4 in cancer development and progression appears to vary with the type of malignancy. The purpose of this study was to investigate DUSP4 expression in a case series of invasive ductal carcinoma of the breast.
Methods
We constructed tissue microarrays consisting of 16, 14, 47, and 266 cases of normal breast tissue, usual ductal hyperplasia, ductal carcinoma in situ, and invasive ductal carcinoma, respectively. DUSP4 expression was investigated by immunohistochemistry.
Results
Cytoplasmic DUSP4 expression was observed. DUSP4 was more frequently expressed in malignant than in benign cases (p=0.024). The mean DUSP4 expression score was significantly higher in malignant tumors than in benign lesions (p=0.019). DUSP4 expression was significantly correlated with a larger tumor size (>2 cm, p=0.015). There was no significant correlation between overall survival or disease-free survival and DUSP4 expression in all 266 patients. We evaluated the impact of DUSP4 expression on the survival of 120 patients with T1-stage tumors. Interestingly, Kaplan-Meier survival curves revealed that DUSP4 expression had a significant effect on both overall patient survival (p=0.034, log-rank test) and disease-free survival (p=0.045, log-rank test). In early T-stage breast cancer, DUSP4 expression was associated with a worse prognosis.
Conclusion
DUSP4 is frequently upregulated in breast malignancy, and may play an important role in cancer development and progression. In addition, it may be a marker of adverse prognosis, especially in patients with early T1-stage cancer.
Keywords: Breast neoplasms, Dual-specificity phosphatase 4, Prognosis
INTRODUCTION
Breast cancer is the most common malignant neoplasm among women in Western Europe and North America, and is a frequent cause of cancer death in recent years [1,2]. It is a genetically and clinically heterogeneous cancer, and the most common histologic subtype is invasive ductal carcinoma, which accounts for 70% to 80% of all breast cancer cases [3]. There have been advances in understanding carcinogenesis and breast cancer biology; however, treatment problems persist [4]. Using proven clinicopathological prognostic parameters, various proteins with essential cellular functions have been proposed as potential predictors of breast cancer [5].
Dual-specificity protein phosphatases (DUSPs), members of the type I cysteine-based protein-tyrosine phosphatase superfamily, are a heterogeneous group of protein phosphatases that can dephosphorylate both phosphotyrosine and phosphoserine/phosphothreonine residues within one substrate [6,7]. The DUSP family regulates members of the mitogen-activated protein (MAP)-kinase superfamily. A subgroup of DUSPs, mitogen-activated protein-kinase phosphatases (MKPs), plays an important role in regulating the tumor relevant MAP-kinase pathways. These pathways are associated with cellular proliferation, differentiation, apoptosis, and inflammation [8,9].
DUSP4, also known as MKP2, is a part of the inducible nuclear MKP group and specifically dephosphorylates the MAP kinases, ERK1/2, p38, and JNK [8]. It is expressed in many different tumor types including colorectal and pancreatic cancer, malignant melanoma, ovarian serous borderline tumors, lung cancer, glioblastomas, and breast cancer [9]. The role of DUSP4 in cancer development and progression appears to vary with the type of malignancy. It is thought that DUSP4 acts either as a tumor suppressor or as a cancer progression factor [8]. Its exact role is still controversial.
In the present study, we investigated DUSP4 expression by immunohistochemistry in a series of invasive ductal carcinoma cases and evaluated its association with clinicopathological variables. In addition, we assessed the impact of DUSP4 expression on the survival of patients with breast cancer.
METHODS
Patients and tumor samples
A consecutive series of 266 patients with invasive ductal carcinoma were enrolled in this study. All cases were diagnosed and underwent surgery at the Hanyang University Hospital (Seoul, Korea) between August 2000 and January 2009. This study was approved by the Institutional Review Board of the Hanyang University Hospital (HYU 2014-11-005-002). The mean age of the patients was 50 years and the mean follow-up period was 60 months. Of the 266 cases, 42, 152, and 72 were histological grades 1, 2, and 3, respectively. According to the seventh edition of the American Joint Committee on Cancer (AJCC) staging system, 194 cases were stage I or II and 72 were stage III or IV. In addition, 16, 12, and 47 samples of normal breast tissue, usual ductal hyperplasia, and ductal carcinoma in situ, respectively, were randomly selected to evaluate the role of DUSP4 expression in carcinogenesis and tumor progression. We reviewed all hematoxylin and eosin (H&E)-stained slides, pathology reports, and other medical records to confirm the diagnosis. The pathological parameters assessed included age, tumor size, tumor grade, perinodal tumor extension, lymph node metastasis, estrogen receptor (ER) and progesterone receptor (PR) status, c-erbB-2 expression, and patient survival.
Tissue microarray construction
We used a manual tissue microarrayer (Unitama, Seoul, Korea) for tissue microarray construction from archival formalin-fixed, paraffin-embedded tissue blocks. As previously described [10], we selected areas rich in tumor cells by light microscopy of H&E-stained sections. Tissue cylinders of 2 mm diameter were punched from a previously marked lesion on each donor block and transferred to the recipient block (Unitama). Each tissue microarray was comprised of 5×10 samples.
Immunohistochemical staining
Antibodies, polyclonal rabbit anti-DUSP4 (Abcam, Cambridge, UK), monoclonal mouse anti-ER (Novocastra Laboratories, Newcastle, UK), monoclonal mouse anti-PR (Novocastra Laboratories), and monoclonal mouse anti-c-erbB-2 (Novocastra Laboratories), were diluted 1:150, 1:50, 1:100, and 1:800 in goat serum, respectively. For immunohistochemical staining, 4-µm sections were cut from the tissue microarray block using a Leica microtome, transferred to adhesive-coated slides, and deparaffinized. The staining was performed using the Bond Max automated immunostainer (Vision Biosystems, San Francisco, USA). Before staining, heat-induced epitope retrieval was performed using the Bond epitope retrieval solution. We blocked endogenous peroxidase activity with 0.3% hydrogen peroxide. Slides were incubated in primary antibody for 30 minutes at room temperature and the slides were incubated with postprimary reagent for 15 minutes at room temperature. The reactions were developed using the Bond polymer refine detection kit and visualized with the chromogen, 3, 3'-diaminobenzidine tetrahydrochloride.
Interpretation of immunohistochemical staining
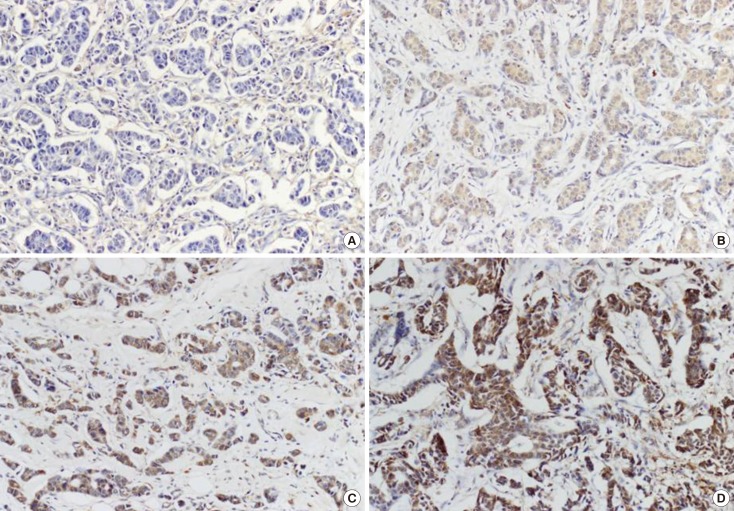
DUSP4 expression was evaluated semiquantitatively by two independent pathologists (H.K. and S.S.P.) who were blind to the patients' clinical outcome. We categorized cytoplasmic DUSP4 expression in terms of both staining intensity and extent, as described previously [11]. Staining intensity was graded as negative ( 0), weak ( 1), moderate ( 2), and strong ( 3), and staining extent was graded as 0% ( 0), 1%-25% ( 1), 26%-50% ( 2), 51%-75% ( 3), and 76%-100% ( 4). The product of intensity and extent grade was used as the final staining score. Thus, the maximum combined score was 12 and the minimum score was 0. Representative photomicrographs of DUSP4 immunostaining in invasive ductal carcinoma are shown in Figure 1. For the purpose of statistical analysis, a cutoff value of 4 was adopted according to the receiver operating characteristic curve. Therefore, the samples were finally classified as either negative (score 0-3) or positive (score 4-12) for DUSP4 expression. ER, PR, and c-erbB-2 expression status was interpreted according to the American Society of Clinical Oncology/College of American Pathologists guidelines for ER/PR/c-erbB-2 testing in breast cancer. When ≥1% of the tumor cell nuclei were stained, it was classified as ER or PR positive. Positive c-erbB-2 staining was determined by complete and intense membranous patterns in >10% of the tumor cells.
Figure 1.
Representative microphotographs of dual-specificity protein phosphatase 4 (DUSP4) immunostaining in invasive ductal carcinoma (×200). (A) Negative, (B) weak, (C) moderate, and (D) strong. The tumor cells showed cytoplasmic DUSP4 staining.
Statistical analysis
Statistical analysis was performed using the SPSS software version 19.0 (IBM Corp., Armonk, USA). The Mann-Whitney U test, chi-square test for linear trend, and chi-square test for independence were used to examine the association between DUSP4 expression and the clinicopathological parameters including age; histological grade; primary tumor (T) category; regional lymph nodes (N) category; AJCC stage; lymphatic invasion; perinodal tumor extension; expression of ER, PR, and c-erbB-2; and triple negativity. Spearman analysis was used to obtain the correlation coefficient. We used the Kaplan-Meier method with the log-rank test to perform analyses of overall and disease-free survival. To identify the independent prognostic factors, the Cox proportional hazards regression model was used in both univariable and multivariable analyses. A p-value <0.05 was considered significant.
RESULTS
Patterns of DUSP4 expression in breast tissue
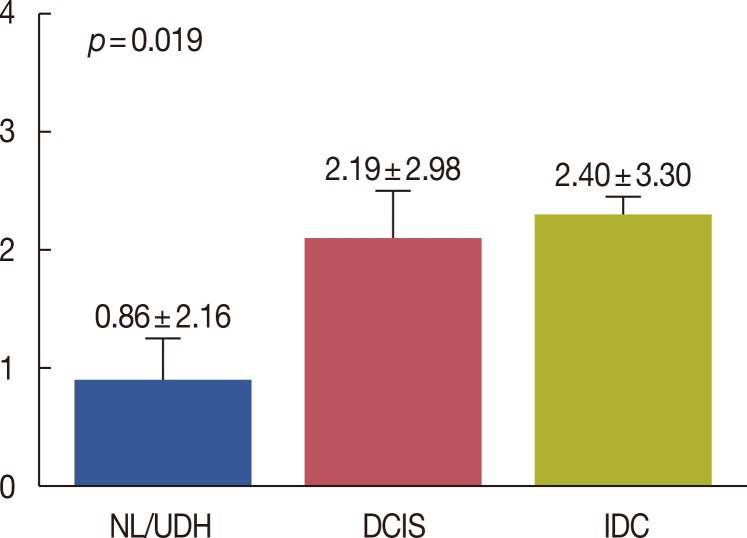
In this study, we evaluated DUSP4 expression in 16, 12, 47, and 266 cases of normal breast tissue, usual ductal hyperplasia, ductal carcinoma in situ, and invasive ductal carcinoma, respectively. Various grades of cytoplasmic DUSP4 expression were observed. DUSP4 expression was positive in 3 of 28 (10.7%) benign cases (normal breast tissue and usual ductal hyperplasia), 11 of 47 (23.4%) ductal carcinoma in situ cases, and 80 of 266 (30.1%) invasive ductal carcinoma cases (Table 1). DUSP4 was more frequently expressed in malignant than in benign cases (p=0.024). The mean DUSP4 expression score was 0.86±2.16 in normal breast tissue and usual ductal hyperplasia, 2.19±2.98 in ductal carcinoma in situ, and 2.40±3.30 in invasive ductal carcinoma (Figure 2). It was significantly higher in malignant tumors than in benign lesions (p=0.019, Kruskal-Wallis test).
Table 1.
Dual-specificity protein phosphatase 4 expression in normal breast tissue/usual ductal hyperplasia, ductal carcinoma in situ, and invasive ductal carcinoma (n=341)
| Tissue sample | DUSP4 expression | |||
|---|---|---|---|---|
| Negative (n = 247) No. (%) | Positive (n = 94) No. (%) | p-value* | rs | |
| NL/UDH | 25 (89.3) | 3 (10.7) | ||
| DCIS | 36 (76.6) | 11 (23.4) | 0.024 | 0.113 |
| IDC | 186 (69.9) | 80 (30.1) | ||
DUSP4=dual-specificity protein phosphatase 4; rs=Spearman rank correlation coefficient; NL=normal breast tissue; UDH=usual ductal hyperplasia; DCIS=ductal carcinoma in situ; IDC=invasive ductal carcinoma.
*Chi-square test for linear trend.
Figure 2.
Mean dual-specificity protein phosphatase 4 (DUSP4) expression score. Mean DUSP4 expression score was significantly higher in malignant tumors than in benign lesions (Kruskal-Wallis test). NL=normal breast tissue; UDH=usual ductal hyperplasia; DCIS=ductal carcinoma in situ; IDC=invasive ductal carcinoma.
Correlation between DUSP4 expression and clinicopathological parameters
We evaluated the correlation between DUSP4 expression and the clinicopathological parameters to assess the significance of its expression in invasive ductal carcinoma. DUSP4 expression was more frequent in the higher T-stage categories (T2 to T4) than in the lower one (T1) (Table 2), and significantly correlated with a larger tumor size (>2 cm, p=0.015). However, there was no correlation with histological grade, AJCC stage, lymphatic invasion, perinodal tumor extension, hormone receptor status, c-erbB-2 expression, or triple negativity.
Table 2.
Correlation between dual-specificity protein phosphatase 4 expression and clinicopathological factors in invasive ductal carcinoma
| Factor | DUSP4 expression | |||
|---|---|---|---|---|
| Negative (n = 186) No. (%) | Positive (n = 80) No. (%) | p-value | rs | |
| Age (yr)* | 50.75 ± 10.63 | 50.44 ± 10.56 | 0.700† | -0.024 |
| Histological grade | 0.410‡ | 0.052 | ||
| 1 | 30 (71.4) | 12 (28.6) | ||
| 2 | 109 (71.7) | 43 (28.3) | ||
| 3 | 47 (65.3) | 25 (34.7) | ||
| T category | 0.015 | 0.150 | ||
| T1 | 93 (77.5) | 27 (22.5) | ||
| T2, T3, T4 | 93 (63.7) | 53 (36.3) | ||
| N category | 0.913 | 0.007 | ||
| N0 | 99 (70.2) | 42 (29.8) | ||
| N1, N2, N3 | 87 (69.6) | 38 (30.4) | ||
| AJCC stage | 0.424 | -0.049 | ||
| I, II | 133 (68.6) | 61 (31.4) | ||
| III, IV | 53 (73.6) | 19 (26.4) | ||
| Lymphatic invasion | 0.557 | 0.036 | ||
| Absent | 91 (71.7) | 36 (28.3) | ||
| Present | 95 (68.3) | 44 (31.7) | ||
| Perinodal tumor extension | 0.406 | -0.086 | ||
| Absent | 54 (65.9) | 28 (34.1) | ||
| Present | 29 (74.4) | 10 (25.6) | ||
| ER expression | 0.205 | -0.078 | ||
| Negative | 78 (66.1) | 40 (33.9) | ||
| Positive | 107 (73.3) | 39 (26.7) | ||
| PR expression | 0.169 | -0.087 | ||
| Negative | 80 (66.1) | 41 (33.9) | ||
| Positive | 97 (74.0) | 34 (26.0) | ||
| c-erbB-2 expression | 0.069 | 0.114 | ||
| Negative | 140 (72.9) | 52 (27.1) | ||
| Positive | 37 (60.7) | 24 (39.3) | ||
| Triple negativity | 0.479 | 0.045 | ||
| Triple negative | 38 (74.5) | 13 (25.5) | ||
| Non-triple negative | 134 (69.4) | 59 (30.6) | ||
DUSP4=dual-specificity protein phosphatase 4; rs=Spearman rank correlation coefficient; AJCC=American Joint Committee on Cancer; ER=estrogen receptor; PR=progesterone receptor.
*Mean±SD; †Mann-Whitney U-test; ‡Chi-square test for linear trend.
Correlation between DUSP4 expression, and overall and disease-free survival
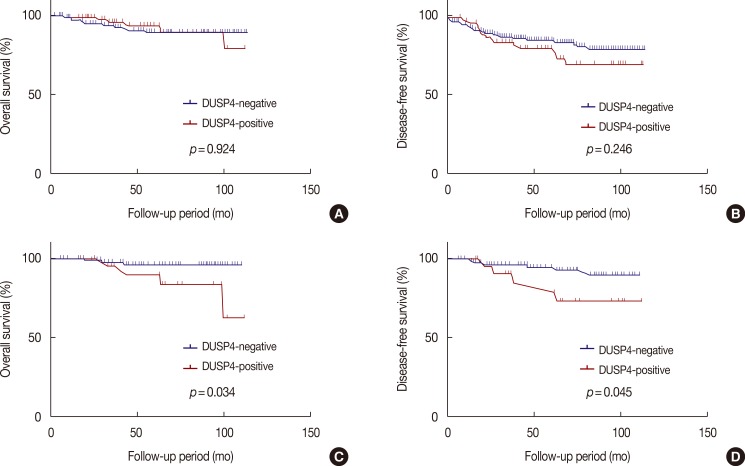
The impact of DUSP4 expression on survival in 266 patients with invasive ductal carcinoma was evaluated. We observed that AJCC stage, lymphatic invasion, perinodal tumor extension, ER/PR status, and triple negativity showed a significant effect on overall and disease-free survival in univariable and/or multivariable analyses (Table 3). However, there was no significant correlation between overall survival or disease-free survival and DUSP4 expression (p=0.924 and p=0.246, respectively; log-rank test) according to the Kaplan-Meier survival curves for all 266 patients with invasive ductal carcinoma (Figure 3A, B). We evaluated the impact of DUSP4 expression on survival in 120 patients with T1-stage tumors (Table 4). We found that DUSP4 expression had a significant effect on overall and disease-free survival in multivariable analysis. Kaplan-Meier survival curves revealed a significant effect of DUSP4 expression on overall survival (p=0.034, log-rank test) and disease-free survival (p=0.045, log-rank test) in early T-stage breast cancer (Figure 3C, D).
Table 3.
Variables associated with the risks of death and recurrence in invasive ductal carcinoma (n=266)
| Variable | Univariable analysis* | Multivariable analysis* | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Overall survival | ||||
| DUSP4 expression (negative vs. positive) | 0.955 (0.373-2.444) | 0.924 | 0.600 (0.200-1.803) | 0.363 |
| AJCC stage (I, II vs. III, IV) | 2.836 (1.230-6.543) | 0.015 | 1.448 (0.490-4.277) | 0.503 |
| Lymphatic invasion (absent vs. present) | 2.499 (0.978-6.386) | 0.056 | 1.604 (0.465-5.528) | 0.454 |
| Perinodal tumor extension (absent vs. present) | 2.957 (1.204-7.264) | 0.018 | 3.329 (1.105-10.030) | 0.033 |
| ER/PR status (all negative vs. one or both positive) | 0.266 (0.107-0.658) | 0.004 | 0.280 (0.080-0.981) | 0.047 |
| Triple negativity (triple negative vs. not) | 0.305 (0.126-0.735) | 0.008 | 0.624 (0.191-2.039) | 0.435 |
| Disease-free survival | ||||
| DUSP4 expression (negative vs. positive) | 1.434 (0.778-2.644) | 0.248 | 1.164 (0.587-2.311) | 0.663 |
| AJCC stage (I, II vs. III, IV) | 3.124 (1.741-5.606) | < 0.001 | 1.286 (0.612-2.702) | 0.508 |
| Lymphatic invasion (absent vs. present) | 3.531 (1.748-7.133) | < 0.001 | 2.551 (1.035-6.287) | 0.042 |
| Perinodal tumor extension (absent vs. present) | 3.813 (2.070-7.023) | < 0.001 | 3.030 (1.380-6.649) | 0.006 |
| ER/PR status (all negative vs. one or both positive) | 0.462 (0.252-0.846) | 0.012 | 0.552 (0.214-1.420) | 0.218 |
| Triple negativity (triple negative vs. not) | 0.448 (0.234-0.859) | 0.016 | 0.563 (0.215-1.469) | 0.240 |
HR=hazard ratio; CI=confidence interval; DUSP4=dual-specificity protein phosphatase 4; AJCC=American Joint Committee on Cancer; ER=estrogen receptor; PR=progesterone receptor.
*Cox proportional hazards model.
Figure 3.
Cumulative overall and disease-free survival curves according to dual-specificity protein phosphatase 4 (DUSP4) expression. There was no significant difference of overall and disease-free survival in all 266 patients with invasive ductal carcinoma (A, B). However, there was significant difference of overall and disease-free survival in 120 patients with the T1-stage tumor (C, D) (Kaplan-Meier method with log-rank test).
Table 4.
Variables associated with the risks of death and recurrence in T1-ranked invasive ductal carcinoma by the American Joint Committee on Cancer breast cancer staging system (n=120)
| Variable | Univariable analysis* | Multivariable analysis* | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Overall survival | ||||
| DUSP4 expression (negative vs. positive) | 4.450 (0.984-20.122) | 0.052 | 4.894 (1.035-23.142) | 0.045 |
| AJCC stage (I, II vs. III, IV) | 1.109 (0.133-9.248) | 0.924 | 0.953 (0.024-38.590) | 0.980 |
| Perinodal tumor extension (absent vs. present) | 1.441 (0.173-11.990) | 0.735 | 2.276 (0.056-92.063) | 0.663 |
| Disease-free survival | ||||
| DUSP4 expression (negative vs. positive) | 3.161 (0.964-10.367) | 0.058 | 4.503 (1.258-16.113) | 0.021 |
| AJCC stage (I, II vs. III, IV) | 2.567 (0.680-9.693) | 0.164 | 1.599 (0.176-14.501) | 0.676 |
| Perinodal tumor extension (absent vs. present) | 2.100 (1.024-4.307) | 0.043 | 3.924 (0.433-35.583) | 0.224 |
HR=hazard ratio; CI=confidence interval; DUSP4=dual-specificity phosphatase 4; AJCC=American Joint Committee on Cancer.
*Cox proportional hazards model.
DISCUSSION
In the present study, we investigated DUSP4 expression in 16, 12, 47, and 266 cases of normal breast tissue, usual ductal hyperplasia, ductal carcinoma in situ, and invasive ductal carcinoma, respectively. We evaluated the correlation between DUSP4 expression, and the clinicopathological parameters and survival of patients with invasive ductal carcinoma. DUSP4 was more frequently expressed in malignant (ductal carcinoma in situ and invasive ductal carcinoma) than in benign cases (normal breast tissue and usual ductal hyperplasia). The mean DUSP4 expression score was also significantly higher in malignant cases and DUSP4 expression was significantly correlated with a larger tumor size (>2 cm). There was a significant association between DUSP4 expression and overall and disease-free survival in patients with T1-stage tumors.
DUSPs are a heterogeneous group of protein phosphatases that can dephosphorylate both phosphotyrosine and phosphoserine/phosphothreonine residues [7]. They regulate members of the MAP-kinase superfamily. MKPs play an important role in regulating the tumor relevant MAP-kinase pathways. These pathways are associated with cellular proliferation, differentiation, apoptosis, and inflammation [9]. DUSP4 is a member of the inducible nuclear MKP group and specifically dephosphorylates the MAP kinases, ERK1/2, p38, and JNK [8]. DUSP4 is expressed in many different tumor types including colorectal and pancreatic cancer, malignant melanoma, ovarian serous borderline tumors, lung cancer, glioblastomas, and breast cancer [9]. The role of DUSP4 in cancer development and progression appears to vary with the type of malignancy.
Whether DUSP4 acts as a tumor promoter or tumor suppressor is still controversial and there is no consensus on the exact role of DUSP4 expression in human cancer. Recently, Saigusa et al. [8] suggested that DUSP4 might be involved in the suppression of tumor progression and metastasis in colorectal cancer. They showed that decreased DUSP4 expression was associated with advanced T-stage categories, lymphatic invasion, vascular invasion, advanced stage, and distant metastasis. Increased DUSP4 expression was associated with a better prognosis. Waha et al. [12] demonstrated that glioblastoma cell growth was inhibited by exogenous DUSP4 overexpression. Armes et al. [13] showed that DUSP4 was expressed in primary tumors, but could be lost in early-onset and highgrade breast cancers. Recently, Baglia et al. [14] described that low DUSP4 expression levels, particularly of variant 1, were associated with both increased recurrence/breast cancer mortality and increased overall mortality.
Some authors have reported that DUSP4 may play a role in promoting cancer progression. It has been proposed that DUSP4 may not act as a tumor suppressor factor, because its expression was found to be upregulated in some malignancies including breast and rectal cancer, and pancreatic and melanoma cell lines [15,16,17,18]. Liu et al. [9] demonstrated that overexpression of DUSP4 may play an important role in promoting the epithelial-mesenchymal transition in breast cancer, and suggested that it may be a marker of adverse prognosis. Gröschl et al. [6] found that DUSP4 was frequently overexpressed in colorectal cancer with high frequent microsatellite instability (MSI-H) compared to microsatellite-stable colorectal cancer. They posited that DUSP4 may act as an important regulator of cell growth within the MAPK pathway and may cause enhanced cell growth in MSI-H colorectal cancer. Wang et al. [15] reported that the expression of MKP1 and MKP2 displayed a significant increase in human breast cancer compared to normal breast tissue.
In our study, we found that DUSP4 was more frequently expressed in malignant (ductal carcinoma in situ and invasive ductal carcinoma) than in benign cases (normal breast tissue and usual ductal hyperplasia). The mean DUSP4 expression score was also significantly higher in malignant cases. These results suggest that DUSP4 may be involved in the carcinogenesis of breast cancer. DUSP4 expression was significantly correlated with a larger tumor size (>2 cm), indicating that it may be involved in breast cancer tumor progression. In survival analyses, the Kaplan-Meier survival curves revealed a significant effect of DUSP4 expression on both overall and diseasefree survival in T1-stage tumors. These results suggest that DUSP4 may be a marker of adverse prognosis, especially in patients with early-stage breast cancer. Our results imply that DUSP4 may play a role as a cancer promoter, not a tumor suppressor in invasive ductal carcinoma.
In conclusion, our findings show that DUSP4 is frequently upregulated in breast malignancy, and may play an important role in cancer development and progression. Furthermore, it may be a marker of adverse prognosis, especially in patients with early T1-stage cancer. The exact role of DUSP4 and its potential as a novel therapeutic target for breast cancer should be investigated in future studies.
Footnotes
This work was supported by the research fund of Hanyang University (HY-2013).
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt M, Voelker HU, Kapp M, Krockenberger M, Dietl J, Kammerer U. Glycolytic phenotype in breast cancer: activation of Akt, up-regulation of GLUT1, TKTL1 and down-regulation of M2PK. J Cancer Res Clin Oncol. 2010;136:219–225. doi: 10.1007/s00432-009-0652-y. [DOI] [PubMed] [Google Scholar]
- 3.Malhotra GK, Zhao X, Band H, Band V. Histological, molecular and functional subtypes of breast cancers. Cancer Biol Ther. 2010;10:955–960. doi: 10.4161/cbt.10.10.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang SM, Han H, Jang KS, Jun YJ, Jang SH, Min KW, et al. The glycolytic phenotype is correlated with aggressiveness and poor prognosis in invasive ductal carcinomas. J Breast Cancer. 2012;15:172–180. doi: 10.4048/jbc.2012.15.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolo C, Guerrero D, Vicente F, Cordoba A, Esteller M, Ropero S, et al. Differences and molecular immunohistochemical parameters in the subtypes of infiltrating ductal breast cancer. Am J Clin Pathol. 2008;130:414–424. doi: 10.1309/J3QV9763DYPV338D. [DOI] [PubMed] [Google Scholar]
- 6.Gröschl B, Bettstetter M, Giedl C, Woenckhaus M, Edmonston T, Hofstädter F, et al. Expression of the MAP kinase phosphatase DUSP4 is associated with microsatellite instability in colorectal cancer (CRC) and causes increased cell proliferation. Int J Cancer. 2013;132:1537–1546. doi: 10.1002/ijc.27834. [DOI] [PubMed] [Google Scholar]
- 7.De Vriendt V, De Roock W, Di Narzo AF, Tian S, Biesmans B, Jacobs B, et al. DUSP 4 expression identifies a subset of colorectal cancer tumors that differ in MAPK activation, regardless of the genotype. Biomarkers. 2013;18:516–524. doi: 10.3109/1354750X.2013.819038. [DOI] [PubMed] [Google Scholar]
- 8.Saigusa S, Inoue Y, Tanaka K, Toiyama Y, Okugawa Y, Shimura T, et al. Decreased expression of DUSP4 is associated with liver and lung metastases in colorectal cancer. Med Oncol. 2013;30:620. doi: 10.1007/s12032-013-0620-x. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Du F, Chen W, Yao M, Lv K, Fu P. Knockdown of dual specificity phosphatase 4 enhances the chemosensitivity of MCF-7 and MCF-7/ ADR breast cancer cells to doxorubicin. Exp Cell Res. 2013;319:3140–3149. doi: 10.1016/j.yexcr.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Jang SM, Sim J, Han H, Ahn HI, Kim H, Yi K, et al. Clinicopathological significance of CADM4 expression in invasive ductal carcinoma of the breast. J Clin Pathol. 2013;66:681–686. doi: 10.1136/jclinpath-2012-201405. [DOI] [PubMed] [Google Scholar]
- 11.Jang SM, Han H, Jun YJ, Jang SH, Min KW, Sim J, et al. Clinicopathological significance of CADM4 expression, and its correlation with expression of E-cadherin and Ki-67 in colorectal adenocarcinomas. J Clin Pathol. 2012;65:902–906. doi: 10.1136/jclinpath-2012-200730. [DOI] [PubMed] [Google Scholar]
- 12.Waha A, Felsberg J, Hartmann W, von dem, Mikeska T, Joos S, et al. Epigenetic downregulation of mitogen-activated protein kinase phosphatase MKP-2 relieves its growth suppressive activity in glioma cells. Cancer Res. 2010;70:1689–1699. doi: 10.1158/0008-5472.CAN-09-3218. [DOI] [PubMed] [Google Scholar]
- 13.Armes JE, Hammet F, de Silva M, Ciciulla J, Ramus SJ, Soo WK, et al. Candidate tumor-suppressor genes on chromosome arm 8p in earlyonset and high-grade breast cancers. Oncogene. 2004;23:5697–5702. doi: 10.1038/sj.onc.1207740. [DOI] [PubMed] [Google Scholar]
- 14.Baglia ML, Cai Q, Zheng Y, Wu J, Su Y, Ye F, et al. Dual specificity phosphatase 4 gene expression in association with triple-negative breast cancer outcome. Breast Cancer Res Treat. 2014;148:211–220. doi: 10.1007/s10549-014-3127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HY, Cheng Z, Malbon CC. Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett. 2003;191:229–237. doi: 10.1016/s0304-3835(02)00612-2. [DOI] [PubMed] [Google Scholar]
- 16.Gaedcke J, Grade M, Jung K, Camps J, Jo P, Emons G, et al. Mutated KRAS results in overexpression of DUSP4, a MAP-kinase phosphatase, and SMYD3, a histone methyltransferase, in rectal carcinomas. Genes Chromosomes Cancer. 2010;49:1024–1034. doi: 10.1002/gcc.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yip-Schneider MT, Lin A, Marshall MS. Pancreatic tumor cells with mutant K-ras suppress ERK activity by MEK-dependent induction of MAP kinase phosphatase-2. Biochem Biophys Res Commun. 2001;280:992–997. doi: 10.1006/bbrc.2001.4243. [DOI] [PubMed] [Google Scholar]
- 18.Teutschbein J, Haydn JM, Samans B, Krause M, Eilers M, Schartl M, et al. Gene expression analysis after receptor tyrosine kinase activation reveals new potential melanoma proteins. BMC Cancer. 2010;10:386. doi: 10.1186/1471-2407-10-386. [DOI] [PMC free article] [PubMed] [Google Scholar]