Abstract
Neurofibromatosis type 1 (NF1), which may occur as an autosom-al dominant disorder, is caused by the absence of neurofibromin protein due to somatic mutations in the NF1 gene, and it has been associated with an increased risk of breast cancer. Herein we describe a family with two women affected by both NF1 and early-onset breast cancer. We evaluated whether the concomitance of NF1 and early-onset breast cancer could be due to disease-causing mutations in both NF1 and BRCA1 gene in a Korean family with clinical features of both NF1 and hereditary breast cancer. Mutation analyses identified nonsense mutations in NF1 and BRCA1 genes. Our findings indicate that an awareness of the possible concomitance of NF1 and BRCA1 gene mutations is important for identifying the genetic origin of early-onset breast cancer in patients with NF1 to achieve early detection of cancers and decrease breast cancer-associated morbidity and mortality in these patients.
Keywords: BRCA1 genes, Breast neoplasms, Mutation, Neurofibromatosis 1
INTRODUCTION
Neurofibromatosis type 1 (NF1) is an autosomal dominant disorder with an approximate incidence ranging from 1/2,500 to 1/3,000 and a prevalence ranging from 1/2,000 to 1/5,000 in the United States and United Kingdom [1,2]. Patients with NF1 have various manifestations, such as café au lait spots, neurofibromas, intertriginous freckling, innocuous iris hamartomas termed Lisch nodules, and bony dysplasia of the long bones, which all result from the lack of neurofibromin protein. Importantly, NF1 gene is a tumor-suppressor gene, and the absence of neurofibromin resulting from somatic NF1 gene mutations has been associated with an increased risk of developing low- and high-grade gliomas and systemic malignancies, including gastrointestinal stromal tumors, somatostatinomas, pheochromocytomas, rhabdomyosarcomas, leukemias, and breast cancer [3,4,5].
Breast cancer has also been shown to be associated with NF1. Previous epidemiologic studies have demonstrated that women with NF1 have a three- to five-fold increased risk of breast cancer compared with the general population. In particular, the increased risk appears to be specific to women under 50 years of age [4,6,7,8]. Although women with NF1 have an increased risk of breast cancer, breast cancer in women with NF1 comprises only a small portion of all breast cancer cases. Furthermore, reports of hereditary breast cancer in women with NF1 in the literature are few. Among the few cases of an association between NF1 mutations and hereditary breast cancer identified through mutation analysis, none is from the Korean population [9,10,11].
We identified a Korean family with two women affected by both NF1 and hereditary breast cancer. Because both the NF1 and BRCA1 genes are located on the long arm of chromosome 17, we evaluated whether the concomitance of these two her-editary disorders could be due to deleterious mutations in both the NF1 and BRCA genes in a Korean family with clinical features of both NF1 and hereditary breast cancer.
CASE REPORT
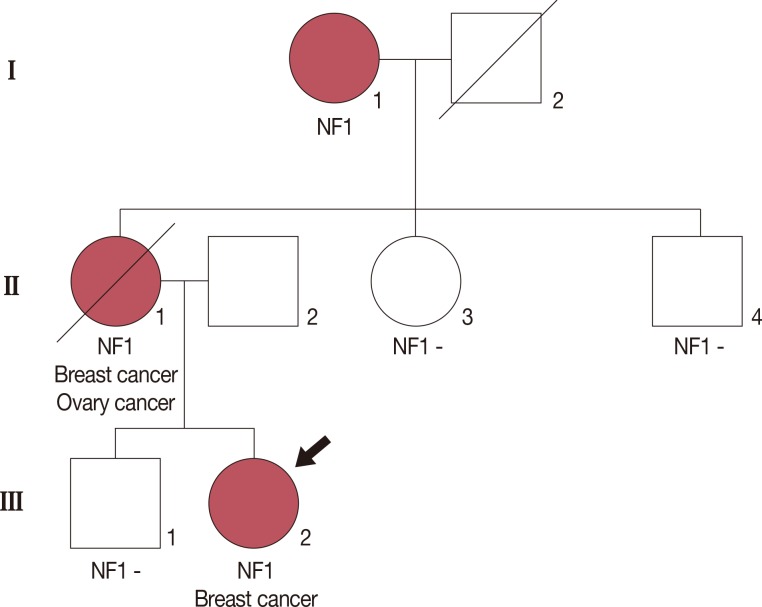
A pedigree revealing two women affected by both NF1 and early-onset breast cancer is presented in Figure 1. A 21-year-old woman (proband III:2) presenting with a palpable mass in her right breast was admitted to our institution. The proband was diagnosed with high-grade invasive lobular breast carcinoma (T2N1M0, estrogen receptor [ER] positive, progesterone receptor [PR] negative, human epidermal growth factor receptor 2 [HER2] negative, and Ki-67 25%) after breastconserving surgery. The proband was treated with adjuvant chemotherapy (six cycles of a regimen consisting of 5-fluorouracil, doxorubicin, and cyclophosphamide) and radiation therapy, and is currently receiving endocrine therapy (tamoxifen). NF1 was diagnosed at birth, as established by the presence of café au lait spots, intertriginous freckling, and cutaneous neurofibromas.
Figure 1. Pedigree of the family showing the index case (black arrow). The proband (III:2) was diagnosed with invasive breast carcinoma at 21 years of age and neurofibromatosis type 1 (NF1) at birth. Her mother (II:1) was affected with bilateral breast carcinoma, ovarian carcinoma and NF1. Although her grandmother (I:1) was affected by NF1, she did not have a history of carcinoma. Other familial members were not affected by cancer or NF1.
Her mother (II:1) was also diagnosed with NF1 that presented as café au lait spots and cutaneous neurofibromas at birth. She underwent a modified radical mastectomy for right invasive ductal breast carcinoma (T2N0M0, ER and PR positive, HER2 negative) at the age of 30 years. After surgery, she was treated with adjuvant chemotherapy (six cycles of a regimen consisting of 5-fluorouracil, methotrexate, and cyclophosphamide) and endocrine therapy. After 5 years, she underwent breast conserving surgery for left invasive ductal breast carcinoma (T2N2M0, ER and PR positive, HER2 negative). After the second surgery, she was treated with chemotherapy (six cycles of a regimen of taxotere, epirubicin, and cyclophosphamide) and radiation therapy. Two years after the second surgery, she underwent a radical hysterectomy and both oophorectomy due to ovarian cancer. Despite continuous chemotherapy, the ovarian carcinoma rapidly progressed and she died of metastatic ovarian carcinoma at 41 years of age.
No familial history of breast cancer or NF1 was noted in the proband's paternal pedigree. The mutational analysis results of her father were normal. In addition, her maternal aunt, uncle, and brother did not have a history of carcinoma or exhibit manifestations of NF1; these family members did not undergo mutation analyses because they did not provide informed consent. Although her grandmother (I:1) had NF1 and presented with café au lait spots and cutaneous neurofibromas, she had no history of carcinoma.
Given that two women in a single family were affected by both NF1 and hereditary breast/ovary cancer, we evaluated whether the concomitance of these two hereditary disorders was attributed to an NF1 gene mutation and its potentially associated increased risk of breast cancer or to disease-causing mutations in both NF1 and BRCA1/2 genes. We analyzed the DNA of the proband affected by NF1 and early-onset breast cancer. All DNA samples were extracted from peripheral blood according to standard procedures after informed consent was obtained. Specific NF1, BRCA1, and BRCA2 coding regions and intron-exon boundaries were amplified using polymerase chain reaction (PCR). Sequence alterations were confirmed at the genomic level with PCR amplification and direct full sequencing using sense and antisense primers. We used the sequence nomenclature of the Lab Genomics Mutation Database, which follows the recommendations of the Human Gene Mutation Database.
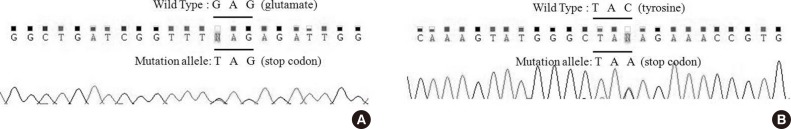
The NF1 gene sequence analysis identified a nonsense mutation in exon 27, c.3616G>T (p.Glu1206), in the index case (Figure 2A). This change (the 3616th nucleotide G is converted into T) results in the conversion of the 1,206th amino acid, a glutamate (GAG), into a premature stop codon (TAG). Our BRCA1 gene sequence analysis detected a nonsense mutation in exon 7, 509C>A (p.Tyr130X), in the same patient (Figure 2B). The 509th nucleotide C was converted into A, which causes the 130th amino acid, a tyrosine (TAC), to be converted into a premature stop codon (TAA). However, No BRCA2 gene mutations were identified in the same patient's peripheral blood DNA.
Figure 2. Mutation analysis in the proband. (A) The NF1 sequence analysis detected a nonsense mutation in exon27, c.3616G>T (p.Glu1206). (B) The BRCA1 sequence analysis detected a nonsense mutation in exon7, 509C>A (p.Tyr130X).
DISCUSSION
Three previous population-based studies have demonstrated that women <50 years of age with NF1 have an increased risk of breast cancer [4,6,7,8]. Walker et al. [4] found that women <50 years of age with NF1 had a standardized incidence ratio (SIR) of 4.0 (p=0.037; 95% confidence interval [CI], 1.1-10.3) for the diagnosis of breast cancer. Similarly, Sharif et al. [6] and Madanikia et al. [7] reported SIRs of 4.9 (p<0.05; 95% CI, 2.4-8.8) and 2.68 (p=0.076; 95% CI, 0.68-7.29), respectively, for breast cancer in women <50 years of age. Furthermore, Evans et al. [8] reported that there is not only an increased risk of breast cancer in women with NF1, but also a higher rate of death associated with the breast cancer diagnosis. The authors of these studies agree that women with NF1 are at a moderately increased risk of developing breast cancer and that early screening in these women is necessary.
Some authors have hypothesized that the concomitance of these two hereditary disorders could be attributed to disease-causing mutations in both the NF1 and BRCA1/2 genes. One study determined that two individuals, a mother and daughter affected by breast and peritoneal cancer, respectively, were carriers of a nonsense BRCA1 gene mutation [11]. However, this report did not include an analysis of the NF1 gene, and it had the limitation of a single-strand conformation polymorphism. Another study reported a mother and daughter affected by breast cancer at 23 and 58 years of age, respectively [12]. Moreover, the proband and her brother were diagnosed with NF1; however, no NF1, BRCA1, or BRCA2 gene mutations were observed in the proband's peripheral blood DNA. The authors reported that loss of heterozygosity (LOH) analyses of the breast tumor revealed LOH in the NF1 region. However, this report did not present the direct full sequencing analysis of the NF1 and BRCA genes, and the study neglected the fact that the breast tumor exhibited LOH at several loci [6].
Similar to our report, Campos et al. [13] presented a family with two women affected by both NF1 and early-onset breast cancer. Our study identified nonsense mutations in NF1 gene and BRCA1 gene, whereas Campos et al. identified a frame shift mutation in BRCA1 gene and a nonsense mutation in NF1 in the index case. Additionally, our study and that by Campos et al. did not identify mutations in NF1 and BRCA1 gene in the proband's mother, who was affected with NF1 and early-onset breast cancer, as she was deceased prior to the study. Although Campos et al. estimated that the proband's mother was probably a carrier of mutations in NF1 and BRCA1 gene based on her diagnoses of NF1 and early-onset breast cancer, early-onset breast cancer is not always associated with BRCA1 gene mutations. In the current study, we did not determine whether the BRCA1 gene mutation in the proband was vertically transmitted from her mother or was a new mutation in the proband.
To our knowledge, this study is the first report describing the concomitant presentation of NF1 and hereditary breast cancer in a Korean family. NF1 and BRCA gene analyses in the proband identified c.3646G>T (p.Glu1206) and 509C>A (p.Tyr130X) nonsense mutations, respectively. Although we could not analyze NF1 and BRCA genes in the proband's mother, we strongly suspect that her mother carried NF1 and BRCA1 gene mutations, given her development of bilateral breast/ovarian cancer syndrome at a young age. The proband and her mother were both affected by early-onset breast cancer, and the degree of the relationship via vertical transmission is suggestive of a genetic predisposition to hereditary breast cancer caused by a mutation in BRCA1 gene. Therefore, because the proband is likely to develop ovarian cancer, we will closely monitor this patient.
In conclusion, an awareness of the possibility of concomitant NF1 and BRCA1 gene mutations is important for the identification of the genetic origins of early-onset breast cancer in patients with NF1. Compared with the general population and women with BRCA1 gene mutations, breast cancer screening guidelines have not been established for patients with NF1 to decrease breast cancer-associated mortality through early diagnosis. Our findings suggest that early and annual clinical and radiographic screenings for breast cancer in women with NF1 may be important to achieve early detection of cancers and decrease breast cancer-associated morbidity and mortality.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol. 2000;151:33–40. doi: 10.1093/oxfordjournals.aje.a010118. [DOI] [PubMed] [Google Scholar]
- 2.Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A:327–332. doi: 10.1002/ajmg.a.33139. [DOI] [PubMed] [Google Scholar]
- 3.Korf BR. Malignancy in neurofibromatosis type 1. Oncologist. 2000;5:477–485. doi: 10.1634/theoncologist.5-6-477. [DOI] [PubMed] [Google Scholar]
- 4.Walker L, Thompson D, Easton D, Ponder B, Ponder M, Frayling I, et al. A prospective study of neurofibromatosis type 1 cancer incidence in the UK. Br J Cancer. 2006;95:233–238. doi: 10.1038/sj.bjc.6603227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brems H, Beert E, de Ravel T, Legius E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009;10:508–515. doi: 10.1016/S1470-2045(09)70033-6. [DOI] [PubMed] [Google Scholar]
- 6.Sharif S, Moran A, Huson SM, Iddenden R, Shenton A, Howard E, et al. Women with neurofibromatosis 1 are at a moderately increased risk of developing breast cancer and should be considered for early screening. J Med Genet. 2007;44:481–484. doi: 10.1136/jmg.2007.049346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madanikia SA, Bergner A, Ye X, Blakeley JO. Increased risk of breast cancer in women with NF1. Am J Med Genet A. 2012;158A:3056–3060. doi: 10.1002/ajmg.a.35550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans DG, O'Hara C, Wilding A, Ingham SL, Howard E, Dawson J, et al. Mortality in neurofibromatosis 1: in North West England: an assessment of actuarial survival in a region of the UK since 1989. Eur J Hum Genet. 2011;19:1187–1191. doi: 10.1038/ejhg.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura M, Tangoku A, Kusanagi H, Oka M, Suzuki T. Breast cancer associated with Recklinghausen's disease: report of a case. Nihon Geka Hokan. 1998;67:3–9. [PubMed] [Google Scholar]
- 10.el-Zawahry MD, Farid M, Abd el-Latif A, Horeia H, el-Gindy M, Twakal G. Breast lesions in generalized neurofibromatosis: breast cancer and cystosarcoma phylloides. Neurofibromatosis. 1989;2:121–124. [PubMed] [Google Scholar]
- 11.Ceccaroni M, Genuardi M, Legge F, Lucci-Cordisco E, Carrara S, D'Amico F, et al. BRCA1-related malignancies in a family presenting with von Recklinghausen's disease. Gynecol Oncol. 2002;86:375–378. doi: 10.1006/gyno.2002.6757. [DOI] [PubMed] [Google Scholar]
- 12.Güran S, Safali M. A case of neurofibromatosis and breast cancer: loss of heterozygosity of NF1 in breast cancer. Cancer Genet Cytogenet. 2005;156:86–88. doi: 10.1016/j.cancergencyto.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Campos B, Balmaña J, Gardenyes J, Valenzuela I, Abad O, Fàbregas P, et al. Germline mutations in NF1 and BRCA1 in a family with neurofibromatosis type 1 and early-onset breast cancer. Breast Cancer Res Treat. 2013;139:597–602. doi: 10.1007/s10549-013-2538-6. [DOI] [PubMed] [Google Scholar]