Highlights
-
•
Twenty-one species of Angiostrongylus are recognised from wildlife around the world.
-
•
Details of hosts, life cycles, pathogenesis, geographical range are known for nine.
-
•
Six species are spreading into new regions locally or globally.
-
•
Two species, A. cantonensis and A. costaricensis, are zoonotic.
-
•
A. mackerrasae, A. malaysiensis and A. siamensis are potentially zoonotic.
-
•
Debilitating disease occurs in avian and mammalian wildlife and humans in Australia.
Keywords: Angiostrongylus spp, Wildlife, Life cycles, Geographical distribution, Pathology, Zoonoses
Graphical Abstract
Abstract
Twenty-one species of Angiostrongylus plus Angiostrongylus sp. (Nematoda: Metastrongyloidea) are known currently in wildlife. These occur naturally in rodents, tupaiids, mephitids, mustelids, procyonids, felids, and canids, and aberrantly in a range of avian, marsupial and eutherian hosts including humans. Adults inhabit the pulmonary arteries and right atrium, ventricle and vena cava, bronchioles of the lung or arteries of the caecum and mesentery. All species pass first-stage larvae in the faeces of the host and all utilise slugs and/or aquatic or terrestrial snails as intermediate hosts. Gastropods are infected by ingestion or penetration of first-stage larvae; definitive hosts by ingestion of gastropods or gastropod slime. Transmission of at least one species may involve ingestion of paratenic hosts. Five developmental pathways are identified in these life cycles. Thirteen species, including Angiostrongylus sp., are known primarily from the original descriptions suggesting limited geographic distributions. The remaining species are widespread either globally or regionally, and are continuing to spread. Small experimental doses of infective larvae (ca. 20) given to normal or aberrant hosts are tolerated, although generally eliciting a granulomatous histopathological response; large doses (100–500 larvae) often result in clinical signs and/or death. Two species, A. cantonensis and A. costaricensis, are established zoonoses causing neurological and abdominal angiostrongliasis respectively. The zoonotic potential of A. mackerrasae, A. malaysiensis and A. siamensis particularly warrant investigation. Angiostrongylus cantonensis occurs in domestic animals, mammalian and avian wildlife and humans in the metropolitan areas of Brisbane and Sydney, Australia, where it has been suggested that tawny frogmouths and brushtail possums may serve as biosentinels. A major conservation issue is the devastating role A. cantonensis may play around zoos and fauna parks where captive rearing of endangered species programmes may exist and where Rattus spp. are invariably a problem.
1. Introduction
The parasitic nematode genus Angiostrongylus Kamensky, 1905 belongs to the superfamily Metastrongyloidea, the so-called “lungworms” of vertebrates. Species occur naturally in cricetid, heteromyid, soricid, glirid, sciurid and murid rodents, tupaiids, mephitids, mustelids, procyonids, felids, canids and aberrantly in a range of avian, marsupial and eutherian hosts including humans. Some species inhabit the pulmonary arteries and right ventricle, some develop in the brain and then migrate via the venous system to the heart and pulmonary arteries, some occur in mesenteric veins and others occur in the bronchioles of the lung. Regardless of site in the definitive host, all first-stage larvae pass through the gastrointestinal tract and exit with the faeces. All species in which the life cycle is known utilise gastropods (slugs and terrestrial and aquatic snails) as intermediate hosts and some species may also use paratenic or transport hosts.
2. Taxonomy
Readers are referred to Ubelaker (1986) and Costa et al. (2003) for a detailed taxonomic history of the genus Angiostrongylus and its treatment by numerous workers. Here, I offer only sufficient background to clarify the species I consider as occurring in wildlife.
The genus Angiocaulus was proposed by Schulz (1951) for Angiostrongylus gubernaculatus Dougherty, 1946 from mustelids; however, Chabaud (1965) placed Angiocaulus as a synonym of Angiostrongylus and this was followed by Dróźdź (1970) and Anderson (1978). Dróźdź (1970) recognised two subgenera, Angiostrongylus (Angiostrongylus) and Angiostrongylus (Parastrongylus). In the former, the externolateral (sometimes termed anterolateral) ray of the bursa of males is separate from the other two lateral rays, and in the latter the externolateral ray is joined to a common stalk with the other lateral rays. Chabaud (1972) recognised Angiostrongylus and Parastrongylus as separate genera based on the above features of the bursa, the former occurring in carnivores with A. vasorum, A. raillieti, A. chabaudi and A. gubernaculatus; the latter occurring in rodents with P. tateronae, P. cantonensis, P. mackerrasae, P. sandarsae, P.sciuri, P. dujardini and P. schmidti.
Anderson (1978) proposed the family Angiostrongylidae for the genus Angiostrongylus and 16 related genera. He followed Dróźdź (1970) in recognising two subgenera while appreciating that some authors (Chabaud, 1972) might prefer to regard these as separate genera. Ubelaker (1986) recognised separate genera, using morphological criteria of the male bursa which allowed separation along host groups as well. He relegated species often placed in the genus Angiostrongylus to six distinct genera all containing species located primarily in specific host groups. He recognised Angiostrongylus Kamensky, 1905 (syn. Haemostrongylus Railliet & Henry, 1907) parasitic in the arterial vasculature of the heart and lungs of carnivores and Parastrongylus Baylis, 1928 (syn. Pulmonema Chen, 1935, Rattostrongylus Schulz, 1951, Morerastronglyus Chabaud, 1972, Chabaudistrongylus Kontrimavichus and Delyamure, 1979) occurring in the arterial circulatory system of rodents (primarily Muridae). He placed the following species in each genus: Angiostrongylus chabaudi, A. raillieti, A. vasorum; Parastrongylus cantonensis, P. costaricensis, P. dujardini, P.mackerrasae, P. malaysiensis, P. petrowi, P.ryjikovi, P. sandarsae, P. schmidti, P. siamensis and P.tateronae. All the above species occur in the pulmonary arteries, right ventricle and lungs, with the exceptions of A. costaricensis and A. siamensis which occur in the mesenteric arteries of their hosts.
Ubelaker (1986) retained the genus Angiocaulus with type species A. gubernaculatus and placed Cardionema ten Yamaguti, 1941 from Martes malampus in Japan in Angiocaulus, while recognising its close relationship with Parastrongylus. I follow Chabaud (1965), Dróźdź (1970) and Anderson (1978) in recognising Angiocaulus as a synonym of Angiostrongylus and thus recognise Angiostrongylus gubernaculatus. Given the importance of the male bursa in defining genera in the Angiostrongylidae, I follow Yamaguti (1961) and recognise the species A. ten as incerta sedis, given it is based on female specimens only.
Yanchev and Genov (1988) described Angiostrongylus daskalovi from three mustelid species in Bulgaria incorrectly placing it in the Filaroididae rather than Angiostrongylidae and distinguishing it from A. chabaudi, A. gubernaculatus and A. vasorum. Subsequently, several new species of Angiostrongylus have been reported from members of the genus Akodon (Cricetidae) in South America; A. morerai from Ak. azarae in Argentina (Robles et al., 2008), A. lenzii from Ak. montensis in Brazil (Souza et al., 2009) and A. sp. from Ak. cursor and Ak. montensis in Brazil (Simões et al., 2011).
Angiocaulus raillieti was first described by Travassos (1927) as Haemostrongylus raillieti from the crab-eating fox, Cerdocyon thous azarae (as Canis azarae) in Rio de Janiero, Brazil. It was subsequently redescribed by Grisi (1971) from domestic dogs in Rio and he placed Angiostrongylus vasorum from dogs in Rio as a synonym of Angiocaulus raillieti. Dougherty (1946) reported Angiostrongylus raillieti in C. a. thous (as Dusicyonhous azarae), noting that the species was quite likely the same as A. vasorum from domestic dogs. Gonçalves (1961) identified Angiostrongylus vasorum in C. a. thous in Columbia and in domestic dogs in Brazil. Rosen et al. (1970) recognised Angiocaulus raillieti as a synonym of Angiostrongylus vasorum. Costa et al. (2003) redescribed A. vasorum from experimentally infected dogs in Brazil and concluded that Angiocaulus should be considered, if not a nomen nundum (sic), then at least a synonym of Angiostrongylus. Several other workers in South America have reviewed, in particular, morphological criteria of the male bursa of A. vasorum from dogs in Brazil (Robles et al., 2008) and foxes in Italy (Souza et al., 2009) demonstrating either that the lateral rays have a common trunk, although the externolateral ray is deeply cleft, a character shared by A. chabaudi and A. morerai (Robles et al., 2008), or that the right externolateral ray does not arise from a single trunk while the left externolateral ray does (Souza et al., 2009). Recently, Jefferies et al. (2009) looked at the molecular characterisation of isolates of A. vasorum from dogs in Europe and Brazil on the basis of the mitochondrial COI gene and the second ribosomal internal transcribed spacer. Sequence analyses revealed two distinct genotypes. Estimated rates of evolution based on the COI sequences for both nematodes and hosts were consistent with the hypothesis that the occurrence of A. vasorum in South America represents an ancient evolutionary event and may represent a cryptic species separate to that in Europe, implying that the synonymy of A. raillieti with A. vasorum may be premature.
Angiostrongylus felineus has recently been described from the puma, Puma (Herpailurus) yagouaroundi, in Brazil (Vieira et al., 2013).
As indicated above, features of the bursa and the morphology of its rays primarily define genera in the Angiostrongylidae including the genus Angiostrongylus. Despite the efforts to divide the genus Angiostrongylus into subgenera that conveniently separate species in carnivores from species in rodents, this separation now appears artificial rather than phylogenetic. With the exception of A. tens, incerta sedis, this review of Angiostrongylus in wildlife shall deal with each of the 21 above-mentioned species plus Angiostrongylus sp., ignoring the increasingly controversial issue of subgenera and recognising that members of the genus Angiostrongylus represent a group of species exhibiting great variation in the arrangement of the lateral rays of the male bursa.
3. Host and geographic distribution of species occurring in wildlife
Species of Angiostrongylus (n = 13) occurring in wildlife and known only from the original description and possibly an additional geographic record or brief discussion are listed in Table 1.
Table 1.
Species of Angiostrongylus (n = 13) from wildlife known only from the original description and possibly an additional geographic record or discussion.
| Parasite species | Host species | Site in host | Geographic locale | References |
|---|---|---|---|---|
| A.chabaudi | Felis silvestris | Lungs | Central Italy | Biocca, 1957 |
| A.daskalovi |
Meles meles, Martes martes, M. foina, |
Pulmonary arteries | Bulgaria, | Yanchev & Genov, 1988; Gerrikagoitia et al., 2010 |
| A.felineus | Puma (Herpailurus) yagouaroundi | Pulmonary arteries | Brazil, Iberian Peninsula, | Vieira et al., 2013 |
| A.gubernaculatus | Taxidea taxus, Mephitis mephitis, Urocyon littoralis | Heart (Right ventricle) | California, USA | Dougherty, 1946; Faulkner et al., 2001 |
| A.lenzii | Akodon montensis | Pulmonary arteries | Rio de Janiero, Brazil | Souza et al., 2009 |
| A.morerai | Akodon azarae | Pulmonary arteries | Buenos Aires, Argentina | Robles et al., 2008 |
| A.petrovi | Dryomys nitedula | Heart and bronchi | Azerbaidzhan, SSR | Tarzhimanova & Chertkova, 1969 |
| A.raillieti |
Cerdocyon thous azarae, Canis familiaris, Nasua nasua |
Pulmonary arteries (dubious, from mesenterium) |
Rio de Janiero, Brazil | Travassos, 1927; Grisi, 1971; Vieira et al., 2008; Jefferies et al., 2009; |
| A.ryjikovi | Clethrionomys rutilus | Northern Urals, USSR | Jushkov, 1971 | |
| A.sandarsae | Praomys (=Mastomys) natalensis, Gerbil tatera |
Pulmonary arteries | Mozambique, Kenya, Africa | Alicata, 1968; Kamiya & Fukumoto, 1988 |
| A.sciuri | Sciurus vulgaris | Pulmonary arteries | Turkey | Merdivenci, 1964 |
| Angiostrongylus sp. |
Cerdocyon thous, Eira barbara, Akodon cursor, A. montensis, |
Lungs | Brazil | Vieira et al., 2008; Simões et al., 2011 |
| A.tateronae | Apodemus mystacinus | Uncertain tissue site | Nigeria, Albania | Baylis, 1928; Erchardová, 1960 |
Angiostrongylus andersoni was described from large abscesses in the lungs of the African gerbillid rodents, Taterillus nigeriae and Tatera kempi from Upper Volta (Petter, 1972). First-stage larvae collected from the faeces of a host identified as being similar to (cf.) Tatera nigrita from Tchad were used to study the life cycle in molluscan intermediate hosts and in T. cf. nigrita and Taterillus cf. congicus from Tchad (Petter, 1974; Petter and Cassone, 1975).
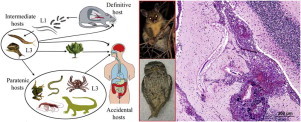
Angiostrongylus cantonensis is a parasite of the pulmonary arteries and right ventricle of numerous wild Rattus spp., Bandicota indica, Melomys burtoni, M. cervinipes and Suncus murinus in the Asian, Pacific and Australian regions (Mackerras and Sandars, 1955; Cross, 1979a; Smales et al., 2004). Discovered originally in the pulmonary arteries and right ventricle of Rattus rattus and R. norvegicus in Guangzhou, China (Chen, 1935), it spread subsequently to South Asia, the Pacific islands and Australia where it is endemic (Alicata, 1988). The first reports of A. cantonensis in rats and humans in the Western Hemisphere were from Cuba (Aguiar et al., 1981), then Puerto Rico (Andersen et al., 1986), followed by the southeastern United States (Campbell and Little, 1988; Kim et al., 2002) where it is now considered endemic in wildlife. It has subsequently been reported from Jamaica (Lindo et al., 2002), Haiti (Raccurt et al., 2003) and Brazil (Simões et al., 2011). Angiostrongylus cantonensis is the causative agent of eosinophilic meningoencephalitis, a zoonotic infection of humans (Fig. 1). Consequently, there is an extensive literature. Readers are referred to relevant literature reviews: historical events (Alicata, 1988), the complex life cycle of this nematode and its myriad of natural and experimental intermediate and wildlife definitive hosts (Anderson, 1968, 1992; Jindrak, 1968; Wallace and Rosen, 1969; Bhaibulaya, 1975), the situation in Southeast Asia and Australia (Alicata and Jindrak, 1970; Cross, 1979a) and documented worldwide cases in humans (Wang et al., 2008).
Fig. 1.
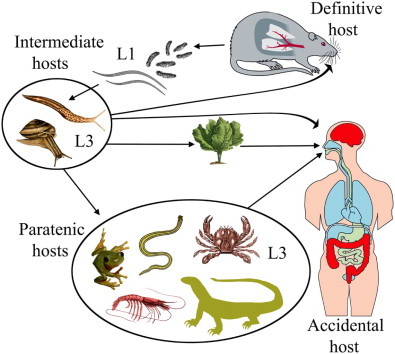
The life cycle of Angiostrongylus cantonensis. Rat definitive hosts acquire third-stage larvae by ingesting infected intermediate hosts, aquatic or terrestrial snails and slugs. Larvae penetrate the stomach, enter the hepatic portal and mesenteric lymphatic systems and are carried to the heart and lungs. They enter alveoli, invade the pulmonary veins, are returned to the left heart and distributed around the body by the arterial circulation. Larvae reach the CNS, predominantly the cerebrum and cerebellum, grow and moult twice in the parenchyma and young adults invade the subarachnoid space of the brain. After about two weeks they invade the cerebral vein and move to the heart and pulmonary arteries where they mature. Eggs are carried in the blood to the lungs where they embryonate. First-stage larvae escape up the bronchial escalator, are swallowed, pass out in the faeces, are ingested by intermediate hosts, snails and slugs, and develop to third-stage infective larvae. A broad spectrum of animals – planarians, prawns, crabs, frogs and lizards may serve as paratenic hosts in which infective larvae reside but undergo no further development. Humans are an accidental host and infection may occur through ingestion of intermediate or paratenic hosts, the latter often eaten raw or their juices used in preparation of local dishes. Infective larvae may also leave molluscs and contaminate vegetables such as lettuce (adapted from Wang et al., 2008, fig. 1).
Angiostrongylus costaricensis, like A. cantonensis, is a zoonotic infection and the causative agent of abdominal angiostrongyliasis in humans in Costa Rica, Honduras, Mexico, Nicaragua, Brazil, Guatemala, Columbia and islands of the Caribbean (Miller et al., 2006). It was first discovered in the small branches of the mesenteric artery of humans in Costa Rica (Morera and Céspedes, 1971). Granulomatous lesions with massive eosinophilic infiltrations occurred in the intestine and regional lymph nodes and contained encapsulated eggs and larvae, but these were never passed in faeces. Subsequently, A. costaricensis was found to occur commonly in arteries of the caecum and branches of the cranial mesenteric artery of rodent species in the families Cricetidae (Sigmodon hispidus, Oligoryzomys (=Oryzomys) fulvescens, Sooretamys angouya (syn. Oryzomys nigripes), Zygodontomys microtinus), Heteromyidae (Liomys adspersus) and Muridae (Rattus rattus, R. norvegicus ) in Costa Rica, Brazil, Ecuador, the Canal Zone and the Republic of Panama (Tesh et al., 1973; Monge et al., 1978; Morera, 1978, 1985; Morera et al., 1983; Graeff-Teixeira et al., 1990).
Angiostrongylus dujardini occurs in the heart and pulmonary arteries of Apodemus sylvaticus and Clethrionomys glareolus in southern France (Dróźdź and Doby, 1970a) and in A.sylvaticus dichrurus and Apodemus sp. in Portugal (Doby et al., 1971). Mészáros (1972) reported the species in these hosts plus A. flavicollis and Pitymys subterranus in Hungary. Tenora et al. (1983) recorded A. dujardini for the first time in Finland but only in C. glareolus; it was not found in C. rutilus, C. rufocanus, Microtus agrestis, M. oeconomus, Arvicola terrestris and Apodemus flavicollis. This species has also been reported in rodents in the Iberian Peninsula (Cordero del Campillo, 1994 cited in Gerrikagoitia et al., 2010).
Angiostrongylus mackerrasae is known from the right ventricle and pulmonary arteries of two indigenous rat species in Australia, the bush rat, Rattus fuscipes, in Queensland and New South Wales and the swamp rat, R. lutreolus, in Tasmania (Bhaibulaya, 1968; Prociv et al., 2000; Stokes et al., 2007). It also occurs in the introduced R. norvegicus and in mixed infections with A. cantonensis in this host in Queensland, but has not been reported in R. rattus (Bhaibulaya, 1968). The classic work by Mackerras and Sandars (1955) on the neurotropic behaviour of A. cantonensis in rats was, in fact, A. mackerrasae (see Bhaibulaya, 1975). Their status as distinct species was tested in a series of hybridization experiments (Bhaibulaya, 1974). Male F1 hybrids were sterile, but females were fertile. Although the author suggested that the species may cross-breed in nature, there has been no evidence forthcoming to date. Mackie et al. (2013) provided the first report of a patent infection of a species of Angiostrongylus in a non-murid host in Australia and the first report of A. mackerrasae in an accidental host, the black flying fox, Pteropus alecto. Taiwan monkeys (Macaca cyclopis) experimentally infected with A. mackerrasae showed no symptoms of infection and at necropsy no parasites or pathologic changes attributable to infection were observed (Cross, 1979b). Adult A. mackerrasae were recovered from the lungs of rat controls killed after several months. Despite its close similarity in morphology and life cycle to A. cantonensis, there is no evidence to date that this species is zoonotic.
Angiostrongylus malaysiensis was known originally as the Malaysian strain of A. cantonensis because of its difference from other strains of A. cantonensis when tested experimentally in Taiwan monkeys (Cross, 1979b). The Malaysian strain was capable of migrating to the central nervous system in monkeys in small numbers, causing some disease symptoms, but not death. In addition, the strain was able to immunise monkeys against the lethal effects of high numbers of larvae of geographic strains of A. cantonensis which were demonstrably pathogenic to monkeys (Cross, 1979b). Development of acquired immunity in rats to the Malaysian strain was minimal. It was subsequently described as a new species, A. malaysiensis, by Bhaibulaya and Cross (1971). Cross-breeding experiments by Cross and Bhaibulaya (1974) between A. cantonensis and A. malaysiensis, demonstrated that F1 hybrids developed to the adult stage but males were sterile, proving that A. malaysiensis was a valid species. It has been reported from the right ventricle and pulmonary arteries of Rattus tiomanicus (syn. jalorensis), R.rattus diardii, R. exulans, R. argentiventer, R. norvegicus, R. muelleri, R. bowersi, R. surifer, R. annandalei, R. cremoriventer, R. whiteheadi, R. sabanus, Suncus murinus and Tupaia glis in Malaysia (Lim and Ramachandran, 1979a), in R. tiomanicus, R. exulans and R. rattus diardii in Indonesia (Bhaibulaya and Cross, 1971; Carney and Stafford, 1979a; Lim and Ramachandran, 1979a) and in R. norvegicus, R. rattus and the snail, Achatina fulica, in Thailand (Bhaibulaya and Techasophonmani, 1972; Pipitgool et al., 1997). Mixed infections with A. cantonensis occurred in R. norvegicus and R. rattus in Bangkok (Bhaibulaya and Techasophonmani, 1972). Lim and Ramachandran (1979a) list a variety of terrestrial slugs and terrestrial and aquatic snails as natural intermediate hosts of A. malaysiensis in eight habitats in Malaysia. The high prevalence and widespread distribution of A. malaysiensis in rodents in Malaysia and development of a monoclonal antibody-ELISA system sensitive to detecting A. malaysiensis adult worm antigens have led to an assumption that this species is the causative agent of angiostrongyliasis in that country (Ambu et al., 1997). However, as with the previous species and despite the close similarity in morphology and life cycle of A. malaysiensis and A. cantonensis, unequivocal evidence that A. malaysiensis is zoonotic has not been forthcoming to date.
Angiostrongylus schmidti was described by Kinsella (1971) from the pulmonary artery and its branches of the rice rat, Oryzomys palustris, trapped in fresh water and saltwater marshes in Florida. He described the pathology associated with infection and subsequently described the complete life cycle and host specificity of A. schmidti (Kinsella, 1971, 1987).
Angiostrongylus siamensis was described from the mesenteric arteries of Rattus sabanus northeast of Bangkok, Thailand, and was the first mesenteric species of Angiostrongylus to be recorded from Eurasia (Ohbayashi et al., 1979). Subsequent investigations added R. berdmorei, R. rattus, R. surifer, Bandicota savilei and B. indica as natural definitive hosts (Kamiya et al., 1980; Ohbayashi et al., 1983). A suspected case was reported in a crab-eating monkey, Macaca fascicularis, imported to Japan from Malaysia (Oku et al., 1983).
Angiostrongylus vasorum was found in the right ventricle and pulmonary arteries of the dog in Toulouse, France by Serres (1854) and was subsequently described by Baillet (1866). It is now known from a wide spectrum of canid hosts in different parts of the world including red foxes (Vulpes vulpes) in Europe (Bolt et al., 1994), the Basque Country of the Iberian Peninsula (Gerrikagoitia et al., 2010), Great Britain (Morgan et al., 2008) and Newfoundland, Canada (Jeffery et al., 2004); pampas foxes (Pseudalopex gymnocerus) in Bolivia (Fiorello et al., 2006); hoary foxes (Pseudalopex vetulus) and crab-eating foxes (Cerdocyon thous) in Brazil (Lima et al., 1994); wolves (Canis lupus) in Spain (Segovia et al., 2001; Torres et al., 2001) and Italy (Eleni et al., 2014); coyotes (Canis latrans) in Newfoundland and Labrador (Bourque et al., 2005); and badgers (Meles meles ) in Spain (Miquel et al., 1993; Torres et al., 2001). Experimental infection of cats demonstrated that they are permissive rather than susceptible to infection and first-stage larvae were never passed in faeces of cats with adult female worms containing eggs (Guilhon and Cens, 1970; Dias et al., 2008).
4. Life cycles
There are two modalities of infection of the gastropod intermediate host, either first-stage larvae are ingested while the snails or slugs are feeding on the faeces from an infected definitive host, or the first-stage larvae penetrate the foot of the gastropod while it crawls across substrate .
Similarly, there are two forms of transmission to the definitive host, either infective third-stage larvae are ingested when the definitive host eats the gastropod intermediate host or third-stage larvae emerge spontaneously from the gastropod and are subsequently ingested. The latter has been proposed as one mechanism of transmission of A. cantonensis to humans on improperly washed vegetables such as lettuce (Heyneman and Lim, 1967). These authors demonstrated that infective larvae pass spontaneously from the slug, Microparmarion malayanus, the most common intermediate host of A. cantonensis (A. malaysiensis) in Malaya, while it is feeding. They remain viable for at least 72 hours embedded in the mucus trail of the slug. Barcante et al. (2003) experimentally infected Biomphalaria glabrata with first-stage larvae of A. vasorum and subjected them to four stimulus treatments to assess the emergence of third-stage larvae and then tested their infectivity in dogs. Most larvae emerged from snails in a water bath at 37 °C for 24 hours and from snails exposed to a 60W light bulb for 24 hours. Two dogs were infected demonstrating, rather artificially, that infection of dogs could occur without ingestion of infected gastropods. Brandao et al. (1998) experimentally infected veronicellid slugs of the genus Phylocaulis, described the kinetics of elimination of third-stage larvae in the mucus secretions of three species and confirmed the importance of P. variegatus as an intermediate host of A. costaricensis.
Transmission of at least one species of Angiostrongylus may involve paratenic hosts, hosts which ingest infected gastropods but in which no further larval development occurs. Land crabs, shrimp, frogs and monitor lizards may serve as paratenic hosts of A. cantonensis and are thought to be one of the major sources of human infection (Fig. 1). Ingestion of raw or undercooked meat of the invasive freshwater golden apple snail, Pomacea canaliculata, imported from South America as a food source, has become the major source of human infection in Taiwan, mainland China and possibly Japan, whereas ingestion of raw snails of the genus Pila is the major source of human infection in Thailand (Wang et al., 2008). In an experiment with surprising results, Bolt et al. (1993) exposed the common frog, Rana temporaria, to first-stage larvae; they developed to the infective stage in 30 days and were infective to a fox. Third-stage larvae of A. vasorum given to frogs remained viable for at least two weeks and these also were infective to a fox, demonstrating that R. temporaria could serve as both an intermediate and a paratenic host of this species.
The basic pattern of development in the definitive host is as follows. Hosts become infected by ingesting gastropods or a paratenic host and migrate, or are carried in the vascular system to a site where they undergo two moults from the third to the fourth and from the fourth to the fifth or sub-adult stage. Further movement may occur to a site where females lay eggs. The eggs become trapped in the arterioles of the lungs or the lower intestine and colon, hatch, and the first-stage larvae either emerge into the airways, move up the respiratory tree, are swallowed and pass out with faeces or escape into the intestinal lumen and pass out in faeces. Five types of development in definitive hosts are distinguished by the location of the third and fourth moults, where adult worms mature, and where first-stage larvae emerge from tissues.
4.1. Type I
Two moults occur in the lungs where adults deposit eggs; first-stage larvae emerge, move up the airways, are swallowed and pass in the faeces. This developmental pathway is exemplified by A. andersoni, A. dujardini and A. schmidti, parasites of rodents.
Larval development of A. andersoni occurs in experimentally infected aquatic snails, Limnea stagnalis and Planorbarius corneus (Petter, 1974; Petter and Cassone, 1975). Larvae developed equally well in these hosts and in the terrestrial snail Helix aspersa, but not in Physa acuta and Arion hortensis. Infection of aquatic snails was by ingestion. Larvae reached the third stage after 15 days, and occurred in nodules in the connective tissue and muscles throughout the body of the snails. Ingested third-stage larvae reached the liver of the definitive host in 14 hours, implying use of the hepatic portal system rather than direct migration through the peritoneal cavity. The majority of larvae were found in the lungs at 24 hours, again suggesting use of the circulatory system. Larvae moulted to fourth-stage between days 2 and 3 and to young adults between days 5 and 6 after infection. Eggs underwent development in the lung parenchyma and first-stage larvae were passed in the faeces of infected rodents 24 days after infection.
A variety of aquatic snails collected from the habitat of rodents infected with A. dujardini served as suitable experimental intermediate hosts as well as B. glabrata (Doby and Dróźdź, 1971). Terrestrial gastropods were either less suitable or unsuitable hosts. Natural infections with third-stage larvae were not found in gastropods collected from the habitat of infected hosts (Doby and Dróźdź, 1971; Dróźdź et al., 1971). Dróźdź and Doby (1970b) examined the development in five species of wild and three species of laboratory definitive hosts and showed that there was no obligatory period of development in the central nervous system. Rather, larvae of A. dujardini penetrated the intestine of A. sylvaticus and C. glareolus, reached the liver and were in the lungs as early as 28 hours post infection. Development in the lungs was rapid with the third moult at day 3 and young adults at day 7. Unsegmented eggs occurred in the lungs at 16 days and larvae in the faeces at 24–26 days post infection. Pitymys subterraneus, hamsters and white mice were receptive to experimental infection but surprisingly, laboratory rats were not, despite development occurring experimentally in three rodent families, Cricetidae, Microtidae and Muridae.
Kinsella (1987) used land snails, Polygyra septemvolva and the aquatic snail, B. glabrata, as experimental intermediate hosts of A. schmidti. Third-stage infective larvae were recovered 26–28 days later and used to infect marsh rice rats (Oryzomys palustris), cotton rats (Sigmodon hispidus), white-footed deer mice (Peromyscus leucopus), Mus musculus, Rattus norvegicus, gerbils (Meriones unguiculatus) and golden hamsters (Mesocricetus auratus). At 12 hours after infection, 50% of larvae were recovered from the lungs and 43% from the liver of O. palustris, implying migration via the hepatic portal system, although the small size of the hosts does not preclude an alternative route. At 24 hours, 80% of larvae were recovered from the lungs. Eggs were first seen in the lungs at day 26 and the first larvae were observed in the faeces at day 31. The number of larvae recovered from rice rats in comparison with the number dosed was low (9–30%), implying a degree of resistance and perhaps the reason for lack of mortality in this natural host. Peromyscus leucopus were refractory to infection; all white mice, and hamsters died from infection, although worms developed to adult in the lungs. Larvae appeared in the lungs of gerbils but the hosts died prior to larvae appearing in the faeces. Patent infections were established in rice rats, cotton rats and white rats, with the first larvae appearing in the faeces at days 30–31. However, none of the 86 wild cotton rats, many trapped in the same sites as infected wild rice rats, were infected (Kinsella, 1971, 1974).
4.2. Type II
Two moults occur in the visceral lymph nodes; sub-adults move via the venous system to the right ventricle and pulmonary arteries. Eggs hatch in the lungs and larvae emerge, pass up the airways, are swallowed and passed in the faeces. This developmental pathway is exemplified by Angiostrongylus vasorum, parasitic in canids.
Guilhon (1960, 1963) demonstrated that Arion ater and A. rufus were natural intermediate hosts of A. vasorum in France and infected dogs by forcing them to consume infected slugs. Rosen et al. (1970) conducted a number of experiments using aquatic and terrestrial gastropods. The slug, L. alte, contained large numbers of infective larvae, but there were relatively few in the other gastropods. Infective larvae were recovered from B. glabrata at 16 days. These migrated to the visceral lymph nodes of dogs where the third and fourth moults occurred at 4–5 days. Subadult worms then migrated via the hepatic portal vein, the liver and the caudal vena cava to reach the right ventricle and pulmonary arteries at days 9–10 after infection, where they matured. The prepatent period in experimentally infected dogs averaged 45 days. Guilhon and Cens (1973) confirmed the life cycle as reported by Rosen et al. (1970) and provided morphological descriptions of the various life stages. Of 17 terrestrial molluscs tested, 11 proved suitable intermediate hosts as well as the aquatic snails B. glabrata and Physa sp.
4.3. Type III
Two moults occur in the neural parenchyma of the central nervous system (primarily the brain); sub-adults relocate to the right heart and pulmonary arteries, eggs are filtered out in the lungs, hatch and larvae emerge, pass up the airways, are swallowed and passed in the faeces. This developmental pathway is exemplified by A. cantonensis, A. mackerrasae and A. malaysiensis, parasitic in rodents (Fig. 1).
The slug Deroceras laevae (=Agriolimax laevis) and an indigenous snail, Helicarion sp. have been used as experimental intermediate hosts for A. cantonensis and A. mackerrasae (Mackerras and Sandars, 1955; Bhaibulaya, 1974, 1975). The latter author reported the first moult of A. cantonensis and A. mackerrasae in snails at 7–10 days and the second at 12–16 days. At both moults, larvae retained the sheaths of the previous stage. Using a PCR-based detection assay, Teem et al. (2013) demonstrated that introduced apple snails, Pomacea maculata, were infected with A. cantonensis in Louisiana but not in Texas, Missisippi and Florida. However, the introduced giant African snail, Ac. fulica, was found infected in Florida, indicating that the parasite is now established in Florida as well as Louisiana. Angiostrongylus cantonensis developed to infective third-stage larvae in a broad range of aquatic and terrestrial gastropods in the southeastern USA (Richards and Merritt, 1967; Campbell and Little, 1988).
The aquatic snail, Lymnea rubiginosa, was used as an experimental intermediate host for A. malaysiensis and natural infections occurred primarily in the slugs Microparmarion malayanus, L. alte and Girasia peguensia; the terrestrial snails Macrochlamys resplendens and Ac. Fulica; and the aquatic snails of rice fields, Pila sututu, Bellamyia ingalisiana, Indoplanorbus exustus and L. rubiginosa (Lim and Ramachandran, 1979a). These authors recorded the first moult in experimentally infected snails at 5–7 days and the second at 9–12 days. Following ingestion by laboratory rats of infective third-stage larvae of A. cantonensis, A. mackerrasae and A. malaysiensis, most larvae penetrated the stomach and entered the hepatic portal and mesenteric lymphatic system carrying them to the heart and lungs (Bhaibulaya, 1975; Lim and Ramachandran, 1979a). They entered alveoli, invaded the pulmonary veins, were returned to the left heart and then distributed around the body in the arterial circulation. Larvae reached the central nervous system, primarily the cerebrum, 2–3 days post-infection where growth and both moults occurred in the neural parenchyma. Young adults invaded the subarachnoid space where they resided for 2 weeks before entering the cerebral vein and being carried to the right heart and pulmonary arteries where they matured. Oviposition occurred in the lungs and first-stage larvae occurred in the faeces. The following minor differences occurred in the respective life cycles: (i) the third moult in the definitive host occurred at the same time in A. cantonensis and A. malaysiensis at 4–6 days and a few days earlier than in A. mackerrasae at 6–10 days; (ii) the fourth moult occurred earlier in A. cantonensis at 7–9 days than in A. malaysiensis at 8–12 days and A. mackerrasae at 10–11 days; (iii) A. malaysiensis reached the pulmonary arteries at days 24–28, A. mackerrasae at days 25–26 and A. cantonensis at days 26–29; (iv) the prepatent period was 32 days in A. malaysiensis, 40–42 days in A. mackerrasae and 42–45 days in A. cantonensis (Bhaibulaya, 1979a). There were no differences in the migratory patterns of infective larvae in experimentally infected definitive hosts.
In addition to a period of larval development in terrestrial or aquatic gastropods, the life cycle of A. cantonensis may involve a range of paratenic hosts (freshwater prawns, land crabs, planarians, frogs, lizards) which feed on gastropods (Fig. 1). Human infection frequently involves these paratenic hosts which are eaten raw or the juices are used in the preparation of local dishes. Infective larvae may survive several days in fresh water and Heyneman and Lim (1967) have shown that larvae may leave molluscs and contaminate vegetables. The use of paratenic hosts has not been determined in A. mackerrasae and A. malaysiensis.
4.4. Type IV
Two moults occur in the mesenteric lymph nodes and vessels; sub-adults relocate to the arterioles of the colon and mesentery, adults occur in the mesenteric arteries of the lower small intestine and colon, eggs are lodged in the capillaries, first-stage larvae escape into the intestinal lumen and are passed in the faeces. This developmental pathway is exemplified by A. siamensis, parasitic in rodents.
Kamiya et al. (1980) examined 278 small mammals from six localities in Thailand for A. siamensis, noting infection in Rattus berdmorei, R. rattus, R.sabanus and R.surifer. Intestinal wall containing first-stage larvae was fed to B glabrata. Third-stage larvae were recovered 25 days later and fed to rats, cotton rats and Mongolian gerbils. First stage larvae were shed in the faeces of cotton rats and rats after 29 and 34 days, respectively. Two gerbils died at 21 and 25 days and contained adult worms, but larvae were not shed in faeces. Kudo et al. (1983) maintained A. siamensis in the laboratory using B. glabrata as the intermediate host and mice, laboratory rats, cotton rats and Mongolian gerbils as definitive hosts. They traced the development and migration route of the parasite in white mice. Third-stage larvae entered the wall of the colon and caecum, and migrated to the marginal and intermedial sinuses of the mesenteric lymph nodes and vessels where the third moult occurred between 30 and 72 hours and the fourth moult between 4 and 7 days post infection. Young adults moved from the lymphatic vessels to arterioles of the colon and mesentery, then reached the mesenteric arteries and branches supplying the lower small intestine and caecum. Oviposition commenced 22 days post infection. Eggs released into the blood lodged in the capillaries of the lower small intestine, caecum and colon. Larvae escaped into the lumen of the intestine and appeared in the faeces 31 days post infection.
4.5. Type V
Infective larvae undergo primarily a lymphatic/venous-arterial pathway and secondarily a venous portal pathway. In the former, one moult occurs in the abdominal lymphatic system and another in the arteries of the caecum and large intestine, subadults and adults occur in the arterial vessels of these organs, eggs are lodged in the arterioles, first-stage larvae escape into the intestinal lumen and are passed in the faeces. In the secondary pathway, two moults occur in venous intra-hepatic vessels; eggs laid here by females embolise, adults migrate to the mesenteric veins, first-stage larvae escape into the intestinal lumen and are passed in the faeces. This developmental pathway is exemplified by A. costaricensis, parasitic in rodents.
The first-stage larvae of A. costaricensis gain the molluscan intermediate host simultaneously by an oral and percutaneous route (Morera, 1973; Mendonça et al., 1999). Veronicellid slugs are the main intermediate hosts (Morera and Ash, 1970; Morera, 1973; Morera et al., 1988); however, the limacid slugs, Limax maximus and L. flavus, and Bradybaena similaris are also suitable intermediate hosts in nature (Graeff-Teixeira et al., 1993). I am not aware of a study of developmental stages and times in the slug intermediate host. Development in the definitive host was reported by Morera (1973) and Mota and Lenzi (1995, 2005). Following ingestion by mice or the natural definitive host, Sigmodon hispidus, infective larvae underwent two migratory routes, a lymphatic/venous-arterial pathway and a venous portal pathway, the former considered the principal one. In this pathway, larvae entered lymphatic vessels of the stomach, large intestine, mesentery and mesenteric lymph nodes where they were found from 3 hours to 11 days, preferentially in the vessels of the submucosa. The fourth moult occurred in the abdominal lymphatic system about day 5, and fourth-stage larvae were found in the arteries of the caecum and large intestine from day 6 onwards. Parasites moulted to subadult in the arterial vessels about day 7 and most were in the definitive site in arterial vessels of the caecum, large intestine and mesentery by day 11. Oviposition commenced 15 days post infection; first-stage larvae escaped into the lumen of the intestine and the prepatent period was 24 days. In the second pathway, development and maturation of A. costaricensis occurred in the liver together and independently of the parasites present in the lymphatic or arterial circulation. Third-stage larvae were found in venous intra-hepatic vessels 3 hours after infection; both moults occurred in this system and females laid eggs here, but these and first-stage larvae became embolised in intrahepatic venous vessels. Adults that developed in this pathway subsequently migrated to the mesenteric veins and first-stage larvae escaped into the lumen of the intestine.
5. Geographic distribution and epidemiology
The dearth of reports in the literature of many species of Angiostrongylus occurring in wildlife, A. andersoni, A. chabaudi, A.daskalovi, A. felineus, A. gubernaculatus, A. lenzii, A. morerai, A. petrovi, A. raillieti, A. ryjikovi, A. sandarsae, A. schmidti, A. sciuri, A. siamensis and A. tateronae, suggests that they may have a rather limited geographic distribution. Alternatively, it may reflect lack of opportunity or interest in examining non-urban and non-agricultural hosts. Others species, A. cantonensis, A. costaricensis, A. dujardini, A. mackerrasae, A. malaysiensis, and A. vasorum are widespread, either globally or regionally. These species appear to be species on the move, spreading into regions where previously they did not occur (Mészáros, 1972; Campbell and Little, 1988; Prociv and Carlisle, 2001; Kim et al., 2002; Morgan et al., 2005; Miller et al., 2006; Stokes et al., 2007; Jefferies et al., 2010; Simin et al., 2014 and references therein). This is epitomised by the global movement of A. cantonensis from China through Asia to the Pacific region and Australia, and thence to the western hemisphere and the Americas under the aegis either of transport of infected Rattus norvegicus on ships or of intermediate hosts. For example, the giant African snail, Ac. fulica, long known as a suitable intermediate host of A. cantonensis, was recently introduced to Brazil as an alternative for Helix aspersa, providing the escargot for traditional French cuisine (Teles et al., 1997). When some of these snail farms collapsed, Ac. fulica spread into the wild and is thought to be partially responsible for the spread of A. cantonensis into some parts of Brazil. Cowie (2013) presents a detailed picture of the recent geographic distribution of A. cantonensis.
6. Impacts on hosts – pathology and pathogenesis
Experimental infections of natural and aberrant definitive hosts with A. dujardini indicated that small doses of infective larvae (ca. 20) were tolerated while large doses (50–1500) were not and often resulted in clinical signs and/or death (Dróźdź and Doby, 1970b), a feature which may be general across species of Angiostrongylus. Nonetheless, studies of the routes taken by A. cantonensis and A. mackerrasae to the brain of rats (Bhaibulaya, 1975) and of A. costaricensis to the mesenteric arteries of cotton rats (Mota and Lenzi, 2005) have relied on large doses in the hope that some larvae would be found at examination of the various tissues post mortem. Mackerras and Sandars (1955) described swollen lobes with mottled appearance and a firm pleural covering in lungs of rats infected with A. mackerrasae (as A. cantonensis, see Bhaibulaya, 1975). The cut surface revealed normal alveoli between masses of developing larvae with no sign of reaction around them while the intervening tissue was emphysematous with an infiltration of leucocytes into the surrounding tissues. Arteries containing worms became hypertrophied to accommodate the increasing bulk. A similar macroscopic picture was described by Alicata (1968) for A. sandarsae. Microscopically, lung parenchyma in affected lobes was replaced by nodules containing eggs or hatched larvae and consisting of fibrous tissue arranged concentrically and containing multinuclear giant cells and histiocytes (Alicata, 1968). Some arteries were extremely dilated but the walls did not show hypertrophy, thrombosis or degeneration of the musculature. The adventitia of infected and uninfected arteries was infiltrated primarily by plasma cells. Okano et al. (2014) reported A. cantonensis in Ryukyu Islands tree rats, Diplothrix legata, noted that pathological observations were similar to those reported by Mackerras and Sandars (1955) for A. mackerrasae and suggested that they might be lethal in this rat species. Lung lobes of rice rats infected with A. schmidti were as described by Mackerras and Sandars (1955) for A. mackerrasae and by Alicata (1968) for A. sandarsae, i.e. swollen, mottled and firm to touch (Kinsella, 1971). The parenchyma of infected lobes was largely replaced by developing eggs and first-stage larvae. There was almost complete obliteration of the lumen of smaller arteries due to proliferation of the intima.
Natural infection with A. cantonensis in the USA, including deaths, has been reported in non-human primates (Gardiner et al., 1990), a miniature horse (Costa et al., 2000), a lemur (Varencia variegate rubra), wood rat (Neotoma floridanus) and opossums (Didelphis virginiana) (Kim et al., 2002). Angiostrongylus cantonensis has been reported from domestic animals, mammalian and avian wildlife and humans in eastern Australia (Table 2, Figs. 2 and 3). Some of these records and others from elsewhere in the world come from animals associated with zoos and nature parks where rat control, invariably, is a major issue.
Table 2.
Records of Angiostrongylus cantonensis in abnormal vertebrate hosts in Australia.
| Host: common name | Host: specific name | References |
|---|---|---|
| Eutheria | ||
| Human | Homo sapiens |
Gutteridge et al., 1972; Saltos, 1975; Cassar 1979; Heaton & Gutteridge 1980; Prociv & Tiernan, 1987; Prociv, 1999; Cooke-Yarborough et al., 1999a (N4422)b; Senanayake et al., 2003; Blair et al., 2013; Morton et al., 2013. |
| Dog | Canis lupus familiaris | Mason et al., 1976 (N250); Mason, 1987; Collins et al., 1992 (N3433); Lunn et al., 2012. |
| Horse | Equus caballus | Wright et al., 1991 (N3123) |
| Alpaca | Vicugna pacos | Hooper & Spratt, 1997 pers. obs. (N5231; P142) |
| Barbary sheep | Ammotragus lervia | Rose & Spratt, 2000 pers. obs. |
| Grey-headed flying fox | Pteropus poliocephalus | Reddacliff et al., 1999 (N4468); Barrett et al., 2002 |
| Black flying fox | Pteropus alecto | Barrett et al., 2002 |
| Tamarins |
Sanguinus spp Sanguinus oedipus |
Carlisle et al., 1998 Rose & Spratt, 2002 pers. obs. (N5248) |
| Squirrel monkey | Saimiri sciureus | Rose & Spratt, 2011 pers. obs. (N5236) |
| Marsupialia | ||
| Bilby | Macrotis lagotis | Rose & Spratt, 2005 pers. obs. |
| Rufous bettong | Aepyprymnus rufescens | Higgins et al., 1997 (N4347) |
| Purple-necked rock wallaby | Petrogale purpureicollis | Rose & Spratt, 2001 pers. obs. (N5233) |
| Parma wallaby |
Macropus parma/eugenii (hybrid) |
Rose & Spratt, 2001 pers. obs. (N5235) |
| Bennett's wallaby | Macropus rufogriseus | McKenzie et al., 1978 (N395) |
| Western grey kangaroo | Macropus fuliginosus | Rose & Spratt, 2003 pers. obs.(N5315) |
| Common ringtail possum | Pseudocheirus peregrinus | Prociv pers. obs. |
| Brushtail possum | Trichosurus vulpecula | Rose, 1999 pers. obs.; Spratt, 2001 pers. obs.; Ma et al., 2013. |
| Aves | ||
| Yellow-tailed black cockatoo |
Calyptorhynchus funereus | Monks et al., 2005 |
| Tawny frogmouth | Podargus strigoides | Rose & Spratt, 2000,2001,2002 pers. obs. (N5116, P139; N5210, P143 ); Monks et al., 2005; Gelis et al.; 2011; Ma et al., 2013. |
| Brolga | Grus rubicunda | Rose & Walker, 2001 pers. obs. |
| Channel-billed cuckoo | Scythrops novaehollandiae | Rose, 2006 pers. obs. |
First identification in humans based on nematodes recovered from brains and lungs.
Specimens held in Wildlife Parasite (N) and Wildlife Pathology (P) Collections, Australian National Wildlife Collection CSIRO Canberra.
Fig. 2.

Tawny frogmouth, Podargus strigoides, with severe posterior paresis and unable to right itself due to infection with Angiostrongylus cantonensis.
Fig. 3.
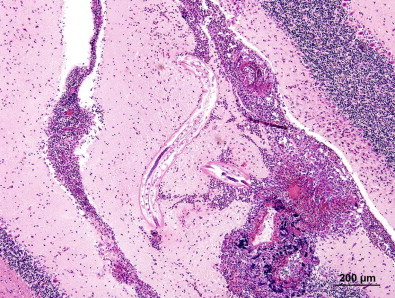
Immature Angiostrongylus cantonensis in the cerebellum of a brushtail possum, Trichosurus vulpecula, with extensive granulomatous and eosinophilic meningoencephalitis and malacia (adapted from Ma et al., 2013, fig. 7).
The clinical signs of neck stiffness, headache, vomiting, paresis, paralysis and sometimes death in domestic animals, wildlife and humans infected with A. cantonensis are induced as a consequence of its obligatory period of development, two moults and maturation in the parenchyma, and the meninges of the central nervous system. Young adults invade and reside in the subarachnoid space of the brain before moving to the right heart and pulmonary arteries via the cerebral vein. Most of the paresis and paralysis observed in aberrant wildlife hosts in Australia occurs when the nematodes are still in the central nervous system and are late third-, fourth- or young adult stage (Spratt, 2005a, 2005b) (Table 2) (Figs. 2 and 3).
Robles et al. (2012) described thrombosis of the pulmonary artery and complete obliteration of the lumen in Akodon dolores infected with A. morerai. Macroscopic lesions of verminous pneumonia in the lungs were similar to those described for A. mackerrasae by Mackerras and Sandars (1955) and A. sandarsae by Alicata (1968). Histopathological examination revealed nodules formed as a result of larvae being surrounded by granulocytes and mononuclear cells.
Tesh et al. (1973) reported little or no inflammatory reaction to adults and eggs of A. costaricensis in moderately parasitised S. hispidus, L. adspersus, O. fulvescens and R. rattus. They noted that a mild inflammatory reaction with plasma cells and pigment-laden macrophages may occur and occasionally acute or granulomatous inflammation occurs around degenerating eggs or larvae. In severely infected individuals, regional lymph nodes were replaced by eosinophilic fibrogranulomatous tissue. In contrast to other infected rodent species, only 3 of 104 Z. microtinus were infected with A. costaricensis, few eggs were present in the caecum, those present were undeveloped and tissues surrounding eggs contained numerous eosinophils and multinucleated giant cells; some eggs were undergoing mineralisation (Tesh et al., 1973). Additional aberrant hosts of the parasite were the carnivorous coatimundi, Nasua narica, and the marmoset, Saguinus mystax (Monge et al., 1978). Ubelaker and Hall, 1979 reported A. costaricensis in two cotton rats from Texas which stood as the only endemic record of the parasite in the United States until Miller et al., ( 2006) reported it in a siamang, Hylobates syndactylus, born at the Miami MetroZoo; two Ma's night monkeys, Aotus nancymaae, from the DuMond Conservancy at Monkey Jungle in Miami; four wild raccoons, Procyon lotor, trapped near the Metro Zoo; and an opossum, Didelphis virginiana, trapped at the Zoo. The primates were zoo-born, the raccoons and opossum native, indicating that the parasite is now endemic at two sites. Postulated sources of introduction of the parasite include (i) infected primates introduced to the zoo and subsequently infecting endemic molluscs and thus racoons and opossums; (ii) infected Rattus spp. introduced through the Miami seaport; (iii) third-stage larvae from molluscs contaminating imported food supplies fed to the primates.
Mota and Lenzi (2005), using doses of 100 to 500 larvae, reported inflammatory reactions in the abdominal lymphatic circulation of experimentally infected cotton rats to the presence of larval A. costaricensis. From day 9 onwards, larvae in the arterial system were accompanied by increasing eosinophil and macrophage infiltration and fibrinoid necrosis of the muscular layer with microhaemorrhages in the arterial wall. Immature and embryonated eggs carried by the blood lodged in the mucosal layer of the small and large intestine and caecum. By day 27, eggs and first-stage larvae were present in all layers of the intestinal and gastric wall surrounded by an inflammatory reaction. They also found eggs and first-stage larvae embolised in intra-hepatic venous vessels.
Ohbayashi et al. (1979) reported granulomatous thickening of the upper colon and granulomatous reactions against larval A. siamensis in infected R. sabanus.
7. Biodiversity and conservation issues
A major conservation issue with species of Angiostrongylus pertains primarily to the potentially devastating role A. cantonensis may play in and around zoos and fauna parks where control of Rattus spp. is invariably a problem (Table 2). It is potentially an important issue for wildlife rehabilitators and in the captive rearing and release of endangered species, programmes often associated with zoos and fauna parks. The reports of debilitating infection with A. cantonensis in tawny frogmouths, Podargus strigoides (Monks et al., 2005; Spratt, 2005b); yellow-tailed black cockatoos, Calyptorhynchus funereus (Monks et al., 2005); gang gang cockatoos, Callocephalon fimbriatum (Reece et al., 2013); and brushtail possums, Trichosurus vulpecula (Ma et al., 2013), in Brisbane and Sydney, Australia, serve as a further reminder that this nematode is well established in some parts of these capital cities and that neuro-angiostrongyliasis can be expected in aberrant species (Figs. 2 and 3). These include domestic, captive and free-living mammals and birds, and humans, especially children who deliberately or accidentally ingest snails or slugs containing infective larvae (Prociv and Tiernan, 1987; Prociv, 1999; Morton et al., 2013) or foolish young adults who do so for a bet (Senanayake et al., 2003; Blair et al., 2013). Tawny frogmouths, P. strigoides, and subadult brushtail possums, T. vulpecula, infected with A. cantonensis exhibit conspicuous neurological signs. They have been identified as potential biosentinels in the Sydney region (Spratt, 2005a, 2005b; Ma et al., 2013) (Figs. 2 and 3) and these hosts may be useful in monitoring the continuing southward spread of A. cantonensis in eastern Australia (Stokes et al., 2007).
8. Species in need of further investigation
The life cycles of A. cantonensis, A. mackerrasae and A. malaysiensis are extremely similar; all require a developmental phase, moults and growth in the central nervous system of the definitive host. Angiostrongylus mackerrasae occurs in two native rats in Australia, R. fuscipes and R. lutreolus, and in co-infections with A. cantonensis in R. norvegicus but not in R. rattus (Mackerras and Sandars, 1955; Bhaibulaya, 1968; Prociv et al., 2000). Its zoonotic potential is unknown. This is similar to A. malaysiensis occurring in many native rodent species in Malaysia, Indonesia and Central Thailand, as well as in R. norvegicus and R.rattus and co-occurring with A. cantonensis in some rodent species (Bhaibulaya and Cross, 1971; Bhaibulaya, 1979a; Carney and Stafford, 1979a; Lim and Ramachandran, 1979a). Angiostrongylus malaysiensis has not been incriminated definitively in human infection but causes neural disease in experimentally infected monkeys (Cross, 1979b). It occurs more commonly than A. cantonensis in rats in Malaysia where the incidence of human angiostrongyliasis is relatively high (Lim and Ramachandran, 1979a). Close attention must be paid to the zoonotic potential of A. mackerrasae and A. malaysiensis. Oku et al. (1981) suggested that A. siamensis and A. costaricensis were closely related because they occupy the same niche in the definitive host, have similar life cycles and because F1 hybrids could be produced experimentally from both of them. Here again, the zoonotic predilection of A. costaricensis demands focus on the potential of A. siamensis to occur as a zoonosis.
9. Conclusions
Twenty one species of Angiostrongylus and Angiostrongylus sp. are reported here from wildlife around the world, thirteen of them with no further details concerning intermediate and definitive host, geographic range, pathogenesis nor life cycle. Angiostrongulus cantonensis, A. costaricensis, A. dujardini, A. mackerrasae, A. malaysiensis and A. vasorum appear to be species on the move, spreading into regions where previously they did not occur. The life cycles, pathogenesis and geographic distributions of the two zoonotic species, A. cantonensis and A. costaricensis, have been studied in detail. The zoonotic potential of other species with complex migratory pathways in definitive hosts, like these three species, i.e. A. mackerrasae, A. malaysiensis and A. siamensis, are deserving of further study. It is surprising, given the increasing occurrence and debilitating effects of A. cantonensis in mammalian and avian wildlife in Australia, that the same is not happening in wildlife species elsewhere in the world, or is it a case of such occurrences simply going undetected?
Acknowledgements
The author expresses his thanks to Professor Ian Beveridge and an anonymous reviewer for critical evaluation of an earlier draft of the manuscript, to Dr. Crystal Kelehear for preparation of the Graphical abstract, to Drs. Richard Montali and Karrie Rose for provision of Fig. 2, and to Gemma Ma for provision of Fig. 3. Fig. 1 is adapted from The Lancet Infectious Diseases Vol. 8, Wang, Q-P., Lai, D-H, Zhu, X-Q, Chen, X-G, Lun, Z-R. Human angiostrongyliasis, pp. 621–630, October 2008, with permission from Elsevier, License number 3511700383518.
References
- Aguiar P.H., Morera P., Pascual J.E. First record of Angiostrongylus cantonensis in Cuba. Am. J. Trop. Med. Hyg. 1981;30:963–965. doi: 10.4269/ajtmh.1981.30.963. [DOI] [PubMed] [Google Scholar]
- Alicata J.E. Angiostrongylus sandarsae sp. n. (Nematoda: Metastrongyloidea), a lungworm of rodents in Mozambique, East Africa. J. Parasitol. 1968;54:896–899. [Google Scholar]
- Alicata J.E. Angiostrongylus cantonensis (eosinophilic meningitis): historical events in its recognition as a new parasitic disease of man. J. Wash. Acad. Sci. 1988;78:38–46. [Google Scholar]
- Alicata J.E., Jindrak K. Charles C. Thomas Publisher; New York: 1970. Angiostrongylosis in the Pacific and Southeast Asia. [Google Scholar]
- Ambu S., Noor Rain A., Mak J.W., Maslah D., Maidah S. Detection of Angiostrongylus malaysiensis circulating antigen using monoclonal antibody-based enzyme-linked immuosorbent assay (Mab-ELISA) Southeast Asian J. Trop. Med. Public Health. 1997;28(Suppl. 1):143–147. [PubMed] [Google Scholar]
- Andersen E., Gubler D.J., Sorenson K., Beddard J., Ash L.R. First report of Angiostrongylus cantonensis in Puerto Rico. Am. J. Trop. Med. Hyg. 1986;35:319–322. doi: 10.4269/ajtmh.1986.35.319. [DOI] [PubMed] [Google Scholar]
- Anderson R.C.A. The pathogenesis and transmission of neurotropic and accidental nematode parasites of the central nervous system of mammals and birds. Helm. Abs. 1968;37:191–210. [Google Scholar]
- Anderson R.C.A. Keys to genera of the superfamily Metastrongyloidea. In: Anderson R.C., Chabaud A.G., Willmott S., editors. vol. 5. Commonwealth Agricultural Bureaux; Farnham Royal, Bucks, England: 1978. (C.I.H. Keys to the Nematode Parasites of Vertebrates). [Google Scholar]
- Anderson R.C.A. CAB International; Wallingford U.K: 1992. Nematode Parasites of Vertebrates. Their development and Transmission. [Google Scholar]
- Baillet C.C. 1866. Strongyle des vaisseaux et du coeur du chien. Strongylus vasorum (Nobis) Nouveau Dictionnaire Practique de Médecine, de Chirurgie et d'Hygiène vétérinaire, v. 8, pp. 587–588. [Google Scholar]
- Barcante T.A., Barcante J.M., Dias S.R., Lima Wdos S. Angiostrongylus vasorum (Bailliet, 1866) Kamensky, 1905: emergence of third-stage larvae from infected Biomphalaria glabrata snails. Parasitol. Res. 2003;91:471–475. doi: 10.1007/s00436-003-1000-9. [DOI] [PubMed] [Google Scholar]
- Barrett J.L., Carlisle M.S., Prociv P. Neuro-angiostrongylosis in wild black and grey-headed flying foxes (Pteropus spp. Aust. Vet. J. 2002;80:554–558. doi: 10.1111/j.1751-0813.2002.tb11039.x. [DOI] [PubMed] [Google Scholar]
- Baylis H.A. On a collection of nematodes from Nigerian mammals, chiefly rodents. Parasitology. 1928;20:280–304. [Google Scholar]
- Bhaibulaya M. A new species of Angiostrongylus in an Australian rat, Rattus fuscipes. Parasitology. 1968;58:789–799. [Google Scholar]
- Bhaibulaya M. Experimental hybridization of Angiostrongylus mackerrasae Bhaibulaya, 1968 and Angiostrongylus cantonensis (Chen, 1935) Int. J. Parasitol. 1974;4:567–573. doi: 10.1016/0020-7519(74)90020-4. [DOI] [PubMed] [Google Scholar]
- Bhaibulaya M. Comparative studies of the life history of Angiostrongylus mackerrasae Bhaibulaya, 1968 and A. cantonensis (Chen, 1935) Int. J. Parasitol. 1975;5:7–20. doi: 10.1016/0020-7519(75)90091-0. [DOI] [PubMed] [Google Scholar]
- Bhaibulaya M. Morphology and taxonomy of major Angiostrongylus species of Eastern Asia and Australia. In: Cross J.H., editor. Studies on Angiostrongyliasis in Eastern Asia and Australia. 1979. pp. 4–13. Taipei, Taiwan; NAMRU-2 Special Publication No. 44. [Google Scholar]
- Bhaibulaya M. Geographical distribution of Angiostrongylus and angiostrongyliasis in Thailand, Indo-China and Australia. In: Cross J.H., editor. Studies on Angiostrongyliasis in Eastern Asia and Australia. 1979. pp. 49–52. Taipei, Taiwan; NAMRU-2 Special Publication No. 44. [Google Scholar]
- Bhaibulaya M., Cross J.H. Angiostrongylus malaysiensis (Nematoda: Metastrongylidae), a new species of rat lung-worm from Malaysia. Southeast Asian J. Trop. Med. Public Health. 1971;2:527–533. [PubMed] [Google Scholar]
- Bhaibulaya M., Techasophonmani V. Mixed infections of Angiostrongylus spp. in rats. Southeast Asian J. Trop. Med. Public Health. 1972;3:451. [PubMed] [Google Scholar]
- Biocca E. Angiostrongylus chabaudi n. sp. parassita del Cuore e dei vasi polmonari del gatto selvatico (Felis silvestris) Atti. Accad. Naz. Lincei Rc. 1957;22:526–532. [Google Scholar]
- Blair N.F., Orr C.F., Delaney A.P., Herkes G.K. Angiostrongylus meningoencephalitis: survival from minimally conscious state to rehabilitation. Med. J. Aust. 2013;198:440–442. doi: 10.5694/mja12.11085. [DOI] [PubMed] [Google Scholar]
- Bolt C., Monrad J., Frandsen F., Henriksen P., Dietz H.H. The common frog (Rana temporaria) as a potential paratenic and intermediate host for Angiostrongylus vasorum. Parasitol. Res. 1993;79:428–430. doi: 10.1007/BF00931834. [DOI] [PubMed] [Google Scholar]
- Bolt G., Monrad J., Koch J., Jensen A.L. Canine angiostrongylosis: a review. Vet. Rec. 1994;135:447–452. doi: 10.1136/vr.135.19.447. [DOI] [PubMed] [Google Scholar]
- Bourque A., Whitney H., Conboy G. Angiostrongylus vasorum infection in coyote (Canis latrans) from Newfoundland and Labrador. Can. J. Wildl. Dis. 2005;41:816–819. doi: 10.7589/0090-3558-41.4.816. [DOI] [PubMed] [Google Scholar]
- Brandao V.C., Bonetti D.O., Graeff-Teixeira C. Angiostrongylus costaricensis and the intermediate hosts: observations on elimination of L3 in the mucus and inoculation of L1 through the tegument of molluscs. Rev. Soc. Bras. Med. Trop. 1998;31:289–294. doi: 10.1590/s0037-86821998000300006. [DOI] [PubMed] [Google Scholar]
- Campbell B.G., Little M.D. The finding of Angiostrongylus cantonensis in rats in New Orleans. Am. J. Trop. Med. Hyg. 1988;38:568–573. doi: 10.4269/ajtmh.1988.38.568. [DOI] [PubMed] [Google Scholar]
- Carlisle M.S., Prociv P., Grennan J., Pass M.A., Campbell G.A., Mudie A. Cerebrospinal angiostrongyliasis in five captive tamarins (Sanguinus spp) Aust. Vet. J. 1998;76:167–170. doi: 10.1111/j.1751-0813.1998.tb10121.x. [DOI] [PubMed] [Google Scholar]
- Carney W.P., Stafford E.E. Angiostrongyliasis in Indonesia – a review. In: Cross J.H., editor. Studies on Angiostrongyliasis in Eastern Asia and Australia. 1979. pp. 14–25. Taipei, Taiwan; NAMRU-2 Special Publication No. 44. [Google Scholar]
- Cassar E.J. Eosinophilic meningitis. Aust. N. Z. J. Med. 1979;9:600–603. doi: 10.1111/j.1445-5994.1979.tb03402.x. [DOI] [PubMed] [Google Scholar]
- Chabaud A.G. Superfamille des Metastrongyloidea. In: Grassé P.P., editor. Traité de Zoologie. Masson et Cie; Paris: 1965. pp. 921–931. [Google Scholar]
- Chabaud A.G. Description de Stefanskostrongylus dubosti n. sp. parasite de Potomagale et essai de classification des Nématodes Angiostrongylinae. Ann. Parasitol. Hum. Comp. 1972;47:735–744. [PubMed] [Google Scholar]
- Chen H.T. A new pulmonary nematode of rats, Pulmonema cantonensis n g, n sp from Canton. Ann. Parasitol. Hum. Comp. 1935;13:312–317. [Google Scholar]
- Collins G.H., Rothwell T.L.W., Malik R., Church D.B., Dowden M.K. Angiostrongylosis in dogs in Sydney. Aust. Vet. J. 1992;69:170–171. doi: 10.1111/j.1751-0813.1992.tb07505.x. [DOI] [PubMed] [Google Scholar]
- Cooke-Yarborough C.M., Kornberg A.J., Hogg G.G., Spratt D.M., Forsyth J.R.L. A fatal case of angiostrongyliasis in an 11 month old infant. Med. J. Aust. 1999;170:541–543. doi: 10.5694/j.1326-5377.1999.tb127880.x. [DOI] [PubMed] [Google Scholar]
- Costa J.O., de Araujo Costa H.M., Guimarães M.P. Redescription of Angiostrongylus vasorum (Baillet, 1866) and systematic revision of species assigned to the genera Angiostrongylus Kamensky, 1905 and Angiocaulus Schulz, 1951. Revue Méd. Vét. 2003;154:9–16. [Google Scholar]
- Costa L.R.R., McClure J.J., Snider T.G., III, Stewart T.B. Verminous meningoencephalitis due to Angiostrongylus cantonensis in an American miniature horse. Eq. Vet. Edu. 2000;12:2–6. [Google Scholar]
- Cowie R.H. Biology, systematics, life cycle and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawaii J. Med. Public Health. 2013;72:6–9. [PMC free article] [PubMed] [Google Scholar]
- Cross J.H., editor. Studies on Angiostrongyliasis in Eastern Asia and Australia. US Naval Medical Research Unit; Taipei, Taiwan: 1979. NAMRU-2 Special Publication No. 44. [Google Scholar]
- Cross J.H. Experimental studies of Angiostrongylus species and strains in monkeys and laboratory animals. In: Cross J.H., editor. Studies on Angiostrongyliasis in Eastern Asia and Australia. US Naval Medical Research Unit; Taipei, Taiwan: 1979. pp. 118–137. NAMRU-2 Special Publication No. 44. [Google Scholar]
- Cross J.H., Bhaibulaya M. Validity of Angiostrongylus malaysiensis Bhaibulaya and Cross, 1971. Southeast Asian J. Trop. Med. Public Health. 1974;5:374–378. [PubMed] [Google Scholar]
- Dias S.R.C., Oliveira E.L., Viana M.H., Lima W.S. Permissivity of the domestic cat (Felis catus) to infection by Angiostrongylus vasorum (Nematoda: Protostrongylidae) Revue Méd. Vét. 2008;159:87–90. [Google Scholar]
- Doby J.M., Dróźdź J. Cycle évolutif de Angiostrongylus (Parastrongylus) dujardini Dróźdź et Doby, 1970 (Nematoda: Metastrongyloidea) C.R. Acad. Sci. Paris. 1971;272:604–607. [PubMed] [Google Scholar]
- Doby J.M., Piedade-Guerreiro J., Dróźdź J. Répartition géographique de Angiostrongylus (Parastrongylus) dujardini Dróźdź et Doby, 1970 nématode parasite des petits rongeurs sauvages. Présence au Portugal. An. Esc. Nac. Saude Publ. Med. Trop. (Lisboa) 1971;5:59–60. [PubMed] [Google Scholar]
- Dougherty E.C. The genus Aelurostrongylus Cameron, 1927 (Nematoda: Metastrongylidae), and its relatives; with descriptions of Parafilaroides gen. nov., and Angiostrongylus gubernaculatus sp. nov. Proc. Helm. Soc. Wash. 1946;13:16–26. [PubMed] [Google Scholar]
- Dróźdź J. Révision de la systématique du genre Angiostrongylus Kamensky, 1905 (Nematoda: Metastrongyloidea) Ann. Parasitol. Hum. Comp. 1970;45:597–603. doi: 10.1051/parasite/1970455597. [DOI] [PubMed] [Google Scholar]
- Dróźdź J., Doby J.M. Angiostrongylus dujardini sp. n. (Nematoda: Metastrongyloidea) parasite de Apodemus sylvaticus et Clethrionomys glareolus. Bull. Soc. Zool. Fr. 1970;95:659–668. [Google Scholar]
- Dróźdź J., Doby J.M. Evolution, morphologique, migrations et chronologie du cycle de Angiostrongylus (Parastrongylus) dujardini Dróźdź et Doby, 1970 (Nematoda: Metastrongyloidea) chez ses hôtes définitifs. Bull. Soc. Scient. Bretagne. 1970;45:229–239. [Google Scholar]
- Dróźdź J., Doby J.M., Mandahl-Barth G. Étude des morphologie et evolution larvaires de Angiostrongylus (Parastrongylus) dujardini Dróźdź et Doby, 1970 Nematoda: Metastrongyloidea. Infestation des mollusques hôtes intermédiares. Ann. Parasitol. Hum. Comp. 1971;46:265–276. [PubMed] [Google Scholar]
- Eleni C., De Liberato C., Azam D., Morgan E.R., Traversa D. Angiostrongylus vasorum in wolves in Italy. Int. J. Parasitol. Parasites Wildl. 2014;3:12–14. doi: 10.1016/j.ijppaw.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erchardová B. K voszporu gelmintofaunü müsevidnüh grüzunov v Albanii. Ceskoslov. Parasitol. 1960;7:91–96. [Google Scholar]
- Faulkner C.T., Patton S., Munson L., Johnson E.M., Coonan T.J. Angiocaulus gubernaculatus in the Island fox (Urocyon littoralis) from the California Channel Islands and comments on the diagnosis of Angiostrongylidae nematodes in canid and mustelid hosts. J. Parastiol. 2001;87:1174–1176. doi: 10.1645/0022-3395(2001)087[1174:AGITIF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Fiorello C.V., Robbins R.G., Maffei L., Wade S.E. Parasites of free-ranging small canids and felids in Bolivian Chaco. J. Zoo Wildl. Med. 2006;37:130–134. doi: 10.1638/05-075.1. [DOI] [PubMed] [Google Scholar]
- Gardiner C.H., Wells S., Gutter A.E., Fitzgerald L., Anderson D.C., Harris R.K. Eosinophilic meningoencephalitis due to Angiostrongylus cantonensis as the cause of death in captive non-human primates. Am. J. Trop. Med. Hyg. 1990;42:70–74. doi: 10.4269/ajtmh.1990.42.70. [DOI] [PubMed] [Google Scholar]
- Gelis S., Spratt D.M., Raidal S.R. Neuroangiostrongyliasis and other parasites in Tawny Frogmouths (Podargus strigoides) in south-eastern Queensland. Aust. Vet. J. 2011;89:47–50. doi: 10.1111/j.1751-0813.2010.00660.x. [DOI] [PubMed] [Google Scholar]
- Gerrikagoitia X., Barral M., Juste R.A. Angiostrongylus species in wild carnivores in the Iberian Peinsula. Vet. Parasitol. 2010;174:175–180. doi: 10.1016/j.vetpar.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Gonçalves P.C. Angiostrongylus vasorum (Baillet, 1866), novo parasite do cao no Rio Grande do Sul (Brazil). (Nematoda: Metastrongyloidea) Rev. Fac. Agron. Vet. Univ. Rio Grande do Sul, Porto Alegre. 1961;4:35–40. [Google Scholar]
- Graeff-Teixeira C., Thiengo S.C., Thomé J.W., Medeiros A.B., Camillo-Coura L., Agostini A.A. On the diversity of mollusc intermediate hosts of Angiostrongylus costaricensis Morera & Cespedes, 1971 in southern Brazil. Mem. Inst. Oswaldo Cruz. 1993;88:487–489. doi: 10.1590/s0074-02761993000300020. [DOI] [PubMed] [Google Scholar]
- Graeff-Teixeira C.L., De Avila-Pires F.D., Machado R.C.C., Camillo-Coura L., Lenzi L. Identificaçao de roedores silvestres como hospedeiros de Angiostrongylus costaricensis no sul do Brasil. Rev. Inst. Med. Trop. Sao Paulo. 1990;32:147–150. [PubMed] [Google Scholar]
- Grisi L. Ocorrência de Angiocaulus raillieti (Travassos, 1927) comb. n. em. Canis familiaris L. Nematoda, Protostrongylidae. Revta. Bras. Biol. 1971;31:27–32. [Google Scholar]
- Guilhon J. Rôle des limacides dans le cycle évolutif d'Angiostrongylus vasorum (Baillet), 1866) C.R. Acad. Sci. 1960;251:2252–2253. [PubMed] [Google Scholar]
- Guilhon J. Recherches sur le cycle évolutif du strongyle des vaisseaux du chien. Bull. Acad. Vét. Fr. 1963;36:431–442. [Google Scholar]
- Guilhon J., Cens B. Essais de transmission d'Angiostrongylus vasorum (Baillet, 1866) du chat. C.R. Acad. Sci. 1970;271:936–939. [PubMed] [Google Scholar]
- Guilhon J., Cens B. Angiostrongylus vasorum (Baillet, 1866): Étude biologique et morphologique. Ann. Parasitol. Hum. Comp. 1973;48:567–596. [PubMed] [Google Scholar]
- Gutteridge B.H., Bhaibulaya M., Findlater C. Human larval meningitis possibly following lettuce ingestion in Brisbane. Pathology. 1972;4:63–64. [Google Scholar]
- Heaton D.C., Gutteridge B.H. Angiostrongyliasis in Australia. Aust. N. Z. J. Med. 1980;10:255–256. doi: 10.1111/j.1445-5994.1980.tb03725.x. [DOI] [PubMed] [Google Scholar]
- Heyneman D., Lim B.L. Angiostrongylus cantonensis: proof of direct transmission with its epidemiological implications. Science. 1967;158:1057–1058. doi: 10.1126/science.158.3804.1057. [DOI] [PubMed] [Google Scholar]
- Higgins D.P., Carlisle-Nowak M.S., Mackie J. Neural angiostrongylosis in three captive rufous bettongs (Aepyprymnus rufescens) Aust. Vet. J. 1997;75:564–566. doi: 10.1111/j.1751-0813.1997.tb14194.x. [DOI] [PubMed] [Google Scholar]
- Jefferies R., Shaw S.E., Viney M.E., Morgan E.R. Angiostrongylus vasorum from South America and Europe represent distinct lineages. Parasitology. 2009;136:107–115. doi: 10.1017/S0031182008005258. [DOI] [PubMed] [Google Scholar]
- Jefferies R., Shaw S.E., Willesen J., Viney M.E., Morgan E.R. Elucidating the spread of the emerging canid nematode Angiostrongylus vasorum between Palaearctic and Nearctic ecozones. Infect. Genet. Evol. 2010;10:561–568. doi: 10.1016/j.meegid.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Jeffery R.A., Lankester M.W., McGrath M.J., Whitney H.G. Angiostrongylus vasorum and Crenosoma vulpis in red foxes (Vulpes vulpes) in Newfoundland, Canada. Can. J. Zool. 2004;82:66–74. [Google Scholar]
- Jindrak K. Early migration and pathogenicity of Angiostrongylus cantonensis in laboratory rats. Ann. Trop. Med. Parasitol. 1968;62:506–517. doi: 10.1080/00034983.1968.11686591. [DOI] [PubMed] [Google Scholar]
- Jushkov V.F. Angiocaulus ryjikovi sp. n. (Nematoda: Strongylata), a parasite of the northern redbacked vole (Clethrionomys rutilus Pallas) from northern Ural. Parazitologiva. 1971;4:344–346. [Google Scholar]
- Kamiya M., Fukumoto S. Angiostrongylus sandarsae Alicata, 1968 (Nematoda: Metastrongyloidea) from Praomys natalensis in Kenya. Jpn. J. Vet. Res. 1988;36:47–52. [PubMed] [Google Scholar]
- Kamiya M., Oku Y., Kamiya H., Ohbayashi M., Abe H., Suzuki H. Report on the prevalence and experimental infections of Angiostrongylus siamensis Ohbayashi, Kamiya et Bhaibulaya, 1979, parasitic in the mesenteric arteries of rodents in Thailand. Jpn. J. Vet. Res. 1980;28:114–121. [PubMed] [Google Scholar]
- Kim D.Y., Stewart T.B., Bauer R.W., Mitchell M. Parastrongylus (=Angiostrongylus) cantonensis now endemic in Louisiana wildlife. J. Parasitol. 2002;88:1024–1026. doi: 10.1645/0022-3395(2002)088[1024:PACNEI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kinsella J.M. Angiostrongylus schmidti sp. n. (Nematoda: Metastrongyloidea) from the rice rat, Oryzomys palustris, in Florida, with a key to the species of Angiostrongylus Kamensky, 1905. J. Parasitol. 1971;57:494–497. [PubMed] [Google Scholar]
- Kinsella J.M. Comparison of helminth parasites of the cotton rat, Sigmodon hispidus, from several habitats in Florida. Amer. Mus. Novitat. 1974;2540:1–12. [Google Scholar]
- Kinsella J.M. Studies on the life cycle and host specificity of Parastrongylus schmidti (Nematoda: Angiostrongylidae) Proc. Helm. Soc. Wash. 1987;54:245–248. [Google Scholar]
- Kontrimavichus V.L., Delyamure S.L. Nauka Publishers; Moscow: 1979. Osnovy Nematodologii, Tom. XXIX, Filyaroididy Domashnikh i Dikikh Zhivot nykh. [Filaroids of domestic and wild animals] Fundamentals of nematology volume 29. Published for the U.S. Department of Agriculture and the National Science Foundation, Washington, D.C., by Amerind Publishing Co. Pvt. Ltd., New Delhi, India, 1985, 183 p. [Google Scholar]
- Kudo N., Oku Y., Kamiya M., Ohbayashi M. Development and migration route of Angiostrongylus siamensis in mice. Jpn. J. Vet. Res. 1983;31:151–163. [PubMed] [Google Scholar]
- Lim B.L., Ramachandran C.P. Ecological studies on Angiostrongylus malaysiensis (Nematoda: Metastrongylidae) in Malaysia. In: Cross J.H., editor. Studies on Angiostrongyliasis in Eastern Asia and Australia. 1979. pp. 26–48. Taipei, Taiwan; NAMRU-2 Special Publication No. 44. [Google Scholar]
- Lima W.S., Guimãraes M.P., Lemos I.S. Occurrence of Angiostrongylus vasorum in the lungs of the Brazilian fox Dusicyon vetulus. J. Helminthol. 1994;68:87. doi: 10.1017/s0022149x00013547. [DOI] [PubMed] [Google Scholar]
- Lindo J., Waugh C., Hall J., Cunningham-Myrie C., Ashley D., Eberhard M. Enzootic Angiostrongylus cantonensis in rats and snails after outbreak of human eosinophilic meningitis in Jamaica. Emerg. Infect. Dis. 2002;8:324–326. doi: 10.3201/eid0803.010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn J.A., Lee R., Smaller J., MacKay B.M., King T., Hunt G.B. Twenty two cases of canine neural angiostronglyosis in eastern Australia (2002–2005) and a review of the literature. Parasit. Vectors. 2012;5:70. doi: 10.1186/1756-3305-5-70. Apr 5, 2012]. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G., Dennis M., Rose K., Spratt D., Spielman D. Tawny frogmouths and brushtail possums as sentinels for Angiostrongylus cantonensis, the rat lungworm. Vet. Parasitol. 2013;192:158–165. doi: 10.1016/j.vetpar.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Mackerras M.J., Sandars D.F. The life history of the rat lung-worm Angiostrongylus cantonensis (Chen) (Nematoda: Metastrongylidae) Aust. J. Zool. 1955;3:1–21. [Google Scholar]
- Mackie J.T., Lacasse C., Spratt D.M. Patent Angiostrongylus mackerrasae infection in a black flying fox (Pteropus alecto) Aust. Vet. J. 2013;91:366–367. doi: 10.1111/avj.12082. [DOI] [PubMed] [Google Scholar]
- Mason K.V. Canine neural angiostrongylosis: the clinical and therapeutic features of 55 natural cases. Aust. Vet. J. 1987;64:201–203. doi: 10.1111/j.1751-0813.1987.tb15181.x. [DOI] [PubMed] [Google Scholar]
- Mason K.V., Prescott C.W., Kelly W.R., Waddell A.H. Granulomatous encephalomyelitis of puppies due to Angiostrongylus cantonensis. Aust. Vet. J. 1976;52:295. doi: 10.1111/j.1751-0813.1976.tb00124.x. [DOI] [PubMed] [Google Scholar]
- McKenzie R.A., Green P.E., Wood A.D. Angiostrongylus cantonensis infection of the brain of a captive Bennett's wallaby (Macropus rufogriseus) Aust. Vet. J. 1978;54:86–88. doi: 10.1111/j.1751-0813.1978.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Mészáros F. The occurrence of Angiostrongylus (P.) dujardini Drozdz et Doby, 1970 (Nematoda) in rodents in Hungary. Parasit. Hung. 1972;5:163–176. [Google Scholar]
- Mendonça C.L.G.F., Carvalho O.S., Mota E.M., Pelajo-Machado M., Caputo L.F.G., Lenzi H.L. Penetration sites and migratory routes of Angiostrongylus costaricensis in the experimental intermediate host (Sarasinula marginata) Mem. Inst. Oswaldo Cruz. 1999;94:549–556. doi: 10.1590/s0074-02761999000400022. [DOI] [PubMed] [Google Scholar]
- Merdivenci A. A new lungworm, Angiostrongylus sciuri n. sp. parasitising in the venae pulmonales of the squirrel, Sciurus vulgaris. Istanb. Univ. Fen. Fak. Meim. 1964;29:155–158. [Google Scholar]
- Miller C.L., Kinsella J.M., Garner M.M., Evans S., Gullett P.A., Schmidt R.E. Endemic infections of Parastrongylus (=Angiostrongylus) costaricensis in two species of nonhuman primates, raccoons, and an opossum from Miami, Florida. J. Parasitol. 2006;92:406–408. doi: 10.1645/GE-653R.1. [DOI] [PubMed] [Google Scholar]
- Miquel J., Feliú C., Torres J., Casanova J.C. Nematode species parasitic in wild carnivores of Catalonia (NE Iberian Peninsula) Misc. Zool. 1993;17:49–57. [Google Scholar]
- Monge E., Arroyo R., Solano E. A new definitive natural host of Angiostrongylus costaricensis (Morera and Céspedes, 1971) J. Parasitol. 1978;64:34. [PubMed] [Google Scholar]
- Monks D.J., Carlisle M.S., Carrigan M., Rose K., Spratt D.M., Gallagher A. Angiostrongylus cantonensis as a cause of cerebrospinal disease in a yellow-tailed black cockatoo (Calyptorhynchus funereus) and two tawny frogmouths (Podargus strigoides) J. Avian Med. Surg. 2005;19:289–293. [Google Scholar]
- Morera P. Life history and redescription of Angiostrongylus costaricensis Morera and Céspedes, 1971. Am. J. Trop. Med. Hyg. 1973;22:613–621. doi: 10.4269/ajtmh.1973.22.613. [DOI] [PubMed] [Google Scholar]
- Morera P. 1978. Definitive and intermediate hosts of Angiostrongylus costaricensis. 4th International Congress of Parasitology 19–26 August 1978, Warsaw. [Google Scholar]
- Morera P. Abdominal angiostrongyliasis: a problem of public health. Parasitol. Today. 1985;1:173–175. doi: 10.1016/0169-4758(85)90177-2. [DOI] [PubMed] [Google Scholar]
- Morera P., Ash L.R. Investigación del huésped intermediario de Angiostrongylus costaricensis (Morera y Céspedes, 1971) Bol. Chil. Parasitol. 1970;25:135. [PubMed] [Google Scholar]
- Morera P., Céspedes R. Angiostrongylus costaricensis n. sp. (Nematoda: Metastrongyloidea), a new lungworm occurring in man in Costa Rica. Rev. Biol. Trop. 1971;18:173–185. [PubMed] [Google Scholar]
- Morera P., Lazo R., Urquizo J., Llaguno M. First record of Angiostrongylus costaricensis Morera and Céspedes, 1971 in Ecuador. Am. J. Trop. Med. Hyg. 1983;32:1460–1461. doi: 10.4269/ajtmh.1983.32.1460. [DOI] [PubMed] [Google Scholar]
- Morera P., Andrews K.L., Rueda A.D. The intermediate host of Angiostrongylus costaricensis in Honduras. Rev. Biol. Trop. 1988;36:575–576. [PubMed] [Google Scholar]
- Morgan E.R., Shaw S.E., Brennan S.F., De Waal T.D., Jones B.R., Mulcahy G. Angiostrongylus vasorum: a real heartbreaker. Trends Parasitol. 2005;21:49–51. doi: 10.1016/j.pt.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Morgan E.R., Tomlinson A., Hunter S., Nichols T., Roberts E., Fox M.T. Angiostrongylus vasorum and Eucoleus aerophilus in foxes (Vulpes vulpes) in Great Britain. Vet. Parasitol. 2008;154:48–57. doi: 10.1016/j.vetpar.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Morton N.J., Britton P., Palasanthiran P., Bye A., Sugo E., Keeson A. Severe haemorrhagic meningoencephalitis due to Angiostrongylus cantonensis among young children in Sydney, Australia. Clin. Infect. Dis. 2013;57:1158–1161. doi: 10.1093/cid/cit444. [DOI] [PubMed] [Google Scholar]
- Mota E.M., Lenzi H.L. Angiostrongylus costaricensis life cycle: a new proposal. Mem.Inst. Owaldo Cruz. 1995;90:707–709. doi: 10.1590/s0074-02761995000600010. [DOI] [PubMed] [Google Scholar]
- Mota E.M., Lenzi H.L. Angiostrongylus costaricensis: complete redescription of the migratory pathways based on experimental Sigmodon hispidus infection. Mem. Inst. Oswaldo Cruz. 2005;100:407–420. doi: 10.1590/s0074-02762005000400012. [DOI] [PubMed] [Google Scholar]
- Ohbayashi M., Kamiya M., Bhaibulaya M. Studies on the parasite fauna of Thailand I. Two new metatsrongylid nematodes, Angiostrongylus siamensis sp. n. and Thaistrongylus harinasutai gen. et sp n. (Metastrongyloidea; Angiostrongylidae) from wild rats. Jpn. J. Vet. Res. 1979;27:5–10. [PubMed] [Google Scholar]
- Ohbayashi M., Machida M., Kamiya H., Yuzaburo O., Abe H., Klongkamnuankarn K. Incidence of Angiostrongylus siamensis in small mammals at Nakorn Nayok, Thailand. Jpn. J. Vet. Res. 1983;31:49–50. [PubMed] [Google Scholar]
- Okano T., Haga A., Mizuno E., Onuma M., Nakaya Y., Nagamine T. Angiostrongylus cantonensis (Nematoda: Metastrongylidae) in the Ryukyu Islands tree rat (Diplothrix legata) J. Wildl. Dis. 2014;50:322–325. doi: 10.7589/2013-03-050. [DOI] [PubMed] [Google Scholar]
- Oku Y., Kudo N., Kamiya M. Experimental hybridization of Angiostrongylus costaricensis and Angiostrongylus siamensis. Jpn. J. Parasitol. 1981;30(Suppl.):35. in Japanese. [Google Scholar]
- Oku Y., Kudo N., Ohbayashi M., Narama I., Umemura T. A case of abdominal angiostrongyliasis in a monkey. Jpn. J. Vet. Res. 1983;31:71–75. [PubMed] [Google Scholar]
- Petter A.J. Description d'une nouvelle espèce d'Aelurostrongylus parasite de rongeur africain. Ann. Parasitol. Hum. Comp. 1972;47:131–137. [PubMed] [Google Scholar]
- Petter A.J. Le cycle évolutif de Morerastrongylus andersoni (Petter, 1972) Ann. Parasitol. Hum. Comp. 1974;49:69–82. [Google Scholar]
- Petter A.J., Cassone J. Mode de pénétration et localisation des larves de Morerastrongylus andersoni (Petter, 1972) (Metastrongyloidea, Nematoda) chez l'hôte intermédiare. Ann. Parasitol. Hum. Comp. 1975;50:469–475. doi: 10.1051/parasite/1975504469. [DOI] [PubMed] [Google Scholar]
- Pipitgool V., Sithithaworn P., Pongmuttasaya P., Hinz E. Angiostrongylus infections in rats and snails in northeast Thailand. Southeast Asian J. Trop. Med. Public Health. 1997;28(Suppl. 1):190–193. [PubMed] [Google Scholar]
- Prociv P. Parasitic meningitis. Crossing paths with the rat lungworm (Angiostrongylus cantonensis) Med. J. Aust. 1999;170:517–518. doi: 10.5694/j.1326-5377.1999.tb127872.x. [DOI] [PubMed] [Google Scholar]
- Prociv P., Carlisle M.S. The spread of Angiostrongylus cantonensis in Australia. Southeast Asian J. Trop. Med. Public Health. 2001;32(Suppl. 2):126–128. [PubMed] [Google Scholar]
- Prociv P., Tiernan J.R. Eosinophilic meningoencephalitis with permanent sequelae. Med. J. Aust. 1987;147:294–295. doi: 10.5694/j.1326-5377.1987.tb133461.x. [DOI] [PubMed] [Google Scholar]
- Prociv P., Spratt D.M., Carlisle M.S. Neuro-angiostrongyliasis: unresolved issues. Int. J. Parasitol. 2000;30:1295–1303. doi: 10.1016/s0020-7519(00)00133-8. [DOI] [PubMed] [Google Scholar]
- Raccurt C., Blaise J., Durette-Desset M. Présence d'Angiostrongylus cantonensis en Haiti. Trop. Med. Int. Health. 2003;8:423–426. doi: 10.1046/j.1365-3156.2003.01035.x. [DOI] [PubMed] [Google Scholar]
- Railliet A., Henry A.C.L. Sur les variations des strongyles de l'appareil respiratoire des mammiferes. C. R. Soc. Biol. 1907;63(38):751–753. [Google Scholar]
- Reddacliff L.A., Bellamy T.A., Hartley W.J. Angiostrongylus cantonensis infection in grey-headed fruit bats (Pteropus poliocephalus) Aust. Vet. J. 1999;77:466–468. doi: 10.1111/j.1751-0813.1999.tb12095.x. [DOI] [PubMed] [Google Scholar]
- Reece R.L., Perry R.A., Spratt D.M. Neuroangiostrongyliasis due to Angiostrongylus cantonensis in gang-gang cockatoos (Callocephalon fimbriatum) Aust. Vet. J. 2013;91:477–481. doi: 10.1111/avj.12116. [DOI] [PubMed] [Google Scholar]
- Richards C.S., Merritt J.W. Studies on Angiostrongylus cantonensis in molluscan intermediate hosts. J. Parasitol. 1967;53:382–388. [PubMed] [Google Scholar]
- Robles M.R., Navone G.T., Kinsella J.M. A new angiostrongylid (Nematoda) species from the pulmonary arteries of Akodon azarae (Rodentia: Cricetidae) in Argentina. J. Parasitol. 2008;94:515–519. doi: 10.1645/GE-1340.1. [DOI] [PubMed] [Google Scholar]
- Robles M.R., Perfumo C., Kinsella J.M., Navonne G.T. Histopathology associated with angiostrongylosis in Akodon species (Rodentia: Sigmodontinae) from Sierra de la Ventana, Buenos Aires, Argentina. J. Parasitol. 2012;98:1133–1138. doi: 10.1645/GE-3128.1. [DOI] [PubMed] [Google Scholar]
- Rosen L., Ash L.R., Wallace G.D. Life history of the canine lungworm Angiostrongylus vasorum (Bailliet) Am. J. Vet. Res. 1970;31:131–143. [PubMed] [Google Scholar]
- Saltos N. Eosinophilic meningitis (Angiostrongylosis): a probable case. Med. J. Aust. 1975;1:561–562. [PubMed] [Google Scholar]
- Schulz R.E. [Phylogenesis of strongylid nematodes and reconstruction of the Metastrongyloidea.] Dokl. Akad. Nauk SSSR. 1951;80:293–296. in Russian. [PubMed] [Google Scholar]
- Segovia J.M., Torres J., Miquel J., Llaneza L., Felie C. Helminths in the wolf, Canis lupus, from north-western Spain. J. Helminthol. 2001;75:183–192. [PubMed] [Google Scholar]
- Senanayake S.N., Pryor D.S., Walker J., Konecny P. First case of human angiostrongyliasis acquired in Sydney. Med. J. Aust. 2003;179:430–431. doi: 10.5694/j.1326-5377.2003.tb05623.x. [DOI] [PubMed] [Google Scholar]
- Serres E. Entozoaires trouvés dans l'oreille droite, le ventricule correspondant et l'artères pulmonaire d'un chien. J. Vét. du Midi. 1854;7:72–73. [Google Scholar]
- Simin S., Kosiƈ L.S., Kuruca L., Pavloviƈ I., Savoviƈ M., Laloševiƈ V. Moving the boundaries to the South-East: first record of autochthonous Angiostrongylus vasorum infection in a dog in Vojvodina province, northern Serbia. Parasit. Vectors. 2014;7:396–402. doi: 10.1186/1756-3305-7-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões R.O., Souza J.G.R., Malconado A., Jr., Luque J.L. Variation in the helminth community structure of three sympatric sigmodontine rodents from the coastal Atlantic forest of Rio de Janiero, Brazil. J. Helminthol. 2011;85:171–178. doi: 10.1017/S0022149X10000398. [DOI] [PubMed] [Google Scholar]
- Smales L.R., Heinrich B., McKillup S.C. The helminth parasites of Melomys cervinipes (Rodentia: Muridae: Hydromyinae) Aust. J. Zool. 2004;52:65–80. [Google Scholar]
- Souza J.G.R., Simoés R.O., Thiengo S.A.R.C., Lima W.S., Mota E.M., Rodrigues-Silva R. A new metastrongylid species (Nematoda: Metastrongylidae): a lungworm from Akodon montensis (Rodentia: Sigmodontinae) in Brazil. J. Parastiol. 2009;95:1507–1511. doi: 10.1645/GE-2013.1. [DOI] [PubMed] [Google Scholar]
- Spratt D.M. Australian ecosystems, capricious food chains and parasitic consequences for people. Int. J. Parasitol. 2005;35:717–724. doi: 10.1016/j.ijpara.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Spratt D.M. Neuroangiostrongyliasis: disease in wildlife and humans. Microbiol. Aust. 2005:64–65. [Google Scholar]
- Stokes V.L., Spratt D.M., Banks P.B., Pech R.P., Williams R. Occurrence of species of Angiostrongylus (Nematoda) in populations of Rattus rattus and Rattus fuscipes in coastal forests of south-eastern Australia. Aust. J. Zool. 2007;55:177–184. [Google Scholar]
- Tarzhimanova R.A., Chertkova A.N. Rattostrongylus petrovi (spelt petrowi in text) n. sp., a new nematode from Dryomys nitedula. Trudy Azerb. Nauch.-issled. Inst. Medit. Parazit. Trop. Medit. SM Kirova. 1969;7:307–310. [Google Scholar]
- Teem J.L., Qvarnstrom Y., Bishop H.S., Alexandre J., da Silva B.S., Carter J. The occurrence of the rat lungworm, Angiostrongylus cantonensis, in nonindigenous snails in the Gulf of Mexico Region of the United States. Hawaii J. Med. Public Health. 2013;72:11–14. [PMC free article] [PubMed] [Google Scholar]
- Teles H.M.S., Vaz J.F., Fontes L.R., Domingos M.F. Registro de Achatina fulica Bowdich, 1822 (Mollusca, Gastropoda) no Brasil: caramujo hospedeiro intermediário da angiostrongilíase. Rev. Saude Pública. 1997;31:310–312. [PubMed] [Google Scholar]
- Tenora F., Henttonen H., Haukisalmi V. On helminths of rodents in Finland. Ann. Zool. Fennici. 1983;20:37–45. [Google Scholar]
- Tesh R.B., Ackerman L.J., Dietz W.H., Williams J.A. Angiostrongylus costaricensis in Panama. Prevalence and pathologic findings in wild rodents infected with the parasite. Am. J. Trop. Med. Hyg. 1973;22:348–356. doi: 10.4269/ajtmh.1973.22.348. [DOI] [PubMed] [Google Scholar]
- Torres J., Miquel J., Motjé M. Helminth parasites of the Eurasian badger (Meles meles L.) in Spain: a biogeographic approach. Parasitol. Res. 2001;87:259–263. doi: 10.1007/s004360000316. [DOI] [PubMed] [Google Scholar]
- Travassos L. Nematódeos novos. Bol. Biolog. São Paulo. 1927;6:52–61. [Google Scholar]
- Ubelaker J.E. Systematics of species referred to the genus Angiostrongylus. J. Parasitol. 1986;72:237–244. [PubMed] [Google Scholar]
- Ubelaker J.E., Hall N.M. First report of Angiostrongylus costaricensis Morera and Céspedes, 1971 in the United States. J. Parasitol. 1979;65:307. [PubMed] [Google Scholar]
- Vieira F.M., Luque J.L., Muniz-Pereira L.C. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa. 2008;1721:1–23. [Google Scholar]
- Vieira F.M., Muniz-Pereira L.C., Lima S.S., Moraes Neto A.H.A., Guimãraes E.V., Luque J.L. A new metastrongyloidean species (Nematoda) parasitizing pulmonary arteries of Puma (Herpailurus) yagouaroundi (É. Geoffroy, 1803) (Carnivora: Felidae) from Brazil. J. Parasitol. 2013;99:327–331. doi: 10.1645/GE-3171.1. [DOI] [PubMed] [Google Scholar]
- Wallace G.D., Rosen L. Studies on eosinophilic meningitis. VI. Experimental infection of rats and other homiothermic vertebrates with Angiostrongylus cantonensis. Am. J. Epid. 1969;89:331–344. doi: 10.1093/oxfordjournals.aje.a120946. [DOI] [PubMed] [Google Scholar]
- Wang Q.-P., Lai D.-H., Zhu X.-Q., Chen X.-G., Lun Z.-R. Human angiostrongyliasis. Lancet Infect. Dis. 2008;8:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- Wright J.D., Kelly W.R., Wadell A.H., Hamilton J. Equine neural angiostrongylosis. Aust. Vet. J. 1991;68:58–60. doi: 10.1111/j.1751-0813.1991.tb03131.x. [DOI] [PubMed] [Google Scholar]
- Yamaguti S. Studies on the helminth fauna of Japan. Part 35. Mammalian nematodes II. Jap. J. Zool. 1941;9:400–439. [Google Scholar]
- Yamaguti S. Intersci. Publ. Inc; New York: 1961. Systema Helminthum Vol III. The Nematodes of Vertebrates. Pt. 1; p. 679. [Google Scholar]
- Yanchev J., Genov T. Angiostrongylus daskalovi (Nematoda: Filaroididae) from Mustelidae in Bulgaria. Helminthologia. 1988;25:81–88. [Google Scholar]


