Abstract
Noninvasive imaging has become the standard for hepatocellular carcinoma (HCC) diagnosis in cirrhotic livers. In this review paper, we go over the basics of MR imaging in cirrhotic livers and describe the imaging appearance of a spectrum of hepatic nodules marking the progression from regenerative nodules to low- and high-grade dysplastic nodules, and ultimately to HCCs. We detail and illustrate the typical imaging appearances of different types of HCC including focal, multi-focal, massive, diffuse/infiltrative, and intra-hepatic metastases; with emphasis on the diagnostic value of MR in imaging these lesions. We also shed some light on liver imaging reporting and data system, and the role of different magnetic resonance imaging (MRI) contrast agents and future MRI techniques including the use of advanced MR pulse sequences and utilization of hepatocyte-specific MRI contrast agents, and how they might contribute to improving the diagnostic performance of MRI in early stage HCC diagnosis.
Keywords: Magnetic resonance imaging, Hepatocellular carcinoma, Hepatic nodules, Liver imaging reporting and data system, Dysplastic nodules, Regenerative nodules
Core tip: Noninvasive imaging has become the standard for hepatocellular carcinoma (HCC) diagnosis in cirrhotic patients. Typical imaging features of HCC, including increased arterial enhancement and delayed washout, provide very high specificity and acceptable sensitivity in characterizing even very small nodules. Diagnostic limitations apply to detecting hypovascular HCCs and differentiating high-grade dysplastic nodules from early HCCs. New techniques such as diffusion-weighted images, T2*, and hepatocyte-specific magnetic resonance imaging contrast agents, are being currently evaluated, which might improve future detection and characterization of hepatic lesions when combined with the current standard imaging protocols with dynamic imaging.
INTRODUCTION
Every year, hepatocellular carcinoma (HCC) is diagnosed in more than 500000 people worldwide; with approximately 20000 new cases in the United States[1,2]. HCC is already the fifth most common neoplasm worldwide and is the third most common cause of cancer-related death, after lung and stomach cancers[1].
HCC rarely occurs before the age of 40 years, reaching a peak at approximately 70 years of age, and is two to four times more prevalent in men[3].
Most of the burden of disease (85%) occurs in developing countries, with the highest incidence rates reported in regions such as Southeast Asia and sub-Saharan Africa; where infection with hepatitis B virus (HBV) is endemic[1]. On the other hand, HCC related to hepatitis C virus (HCV) infection and secondary cirrhosis has become the fastest-rising cause of cancer-related death in the developed countries[2].
Patients diagnosed at an early stage are eligible for potentially curative therapies; including surgery (resection and liver transplantation) and locoregional ablative options (radiofrequency, microwave ablation, or ethanol injection). With this stage-driven strategies, 5-year survival rates range between 50%-70%[4]. However, very poor prognosis is observed with advanced HCC.
Therefore, an effort to diagnose HCC at early stages is being taken with the implementation of screening programs that may lead to earlier implementation of treatment.
Correlation with alpha-fetoprotein levels may sometimes be useful; however, not all tumors express alpha-fetoprotein. Additionally, mildly elevated alpha-fetoprotein levels may be seen in patients with chronic liver disease or in patients with cirrhosis but no HCC[5].
Ultrasound (US) is widely used, and represents the first imaging modality of screening by various international society consensuses; essentially because of the ease of access, lack of ionizing radiation, and lower cost compared with computed tomography (CT) and magnetic resonance imaging (MRI). The role of gray-scale US in cirrhotic patients in clinical practice is screening and surveillance, rather than accurate diagnosis of HCC (Figure 1). According to the updated American Association for the Study of Liver Diseases (AASLD) guidelines[6], the diagnostic algorithm of HCC starts from suspected nodules found on US surveillance. However, reported sensitivity and specificity is variable[7] and studies have shown a significant lower detection rate of HCC compared with CT and MRI[8]. Additionally, the technique is poor to detect small HCCs. At present, the real cost-effectiveness of US is not known, as it is common to find HCC in patients with prior negative US while receiving appropriate surveillance[9,10].
Figure 1.
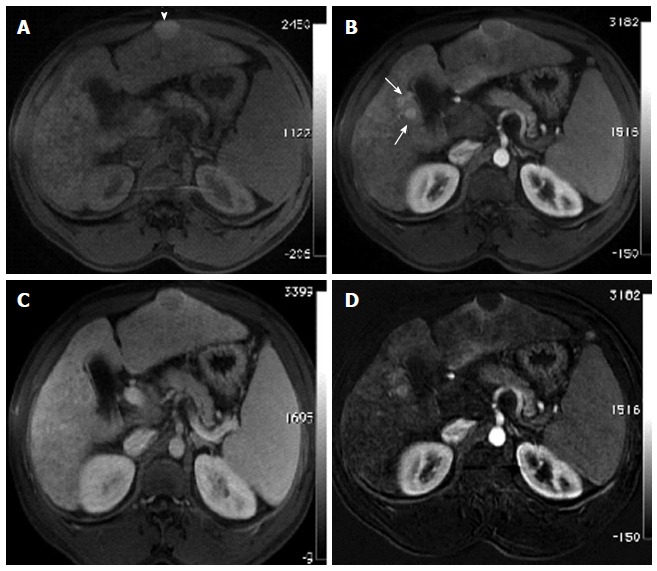
Incidentally discovered solitary left hepatic lobe nodule on screening ultrasound, referred as suspicious nodule for HCC, for MRI evaluation. A: Pre- and post-contrast fat-suppressed 3D-GRE T1-weighted images during the (B) late hepatic arterial and (C) delayed phases; D: Post-processed subtracted arterial image. The known left hepatic lobe nodule demonstrate increased intrinsic T1 signal (arrowhead, A), without appreciable increased arterial enhancement (B), confirmed on subtraction images (D), in keeping with a macro-regenerative nodule. However, the MRI reveals multiple small foci at hepatic segment #5 that show increased arterial enhancement (arrows, B) and delayed washout (D) in keeping with multiple small HCCs. HCC: Hepatocellular carcinoma; MRI: Magnetic resonance imaging; GRE: Gradient recalled echo.
The use of CT for the detection of HCC requires intravenous iodinated-contrast administration and a minimum of a triphasic technique to evaluate the characteristic findings of increased arterial enhancement and late washout of typical HCCs. Several studies have shown higher sensitivities of gadolinium-enhanced MR imaging compared to CT for the detection of HCC of all sizes[11], while other studies have suggested a lower sensitivity of CT for detecting dysplastic nodules, small HCCs, and diffuse HCC compared with MRI[7,10].
The relatively short interval follow-up that is advocated for this patient population raises concern regarding the cumulative radiation dose and increased risk of worsening renal function due to the necessary repeated administration of intravenous contrast material[12].
Recent technological development of MRI scanners allowed high-quality multiphasic dynamic imaging of the entire liver[13]. Additionally, the superb contrast resolution and development of liver specific contrast agents rendered MR an important imaging modality for assessing cirrhosis and its complications, especially HCC. However, despite being an optimal imaging technique for the comprehensive evaluation of the liver[14,15], MRI has been used mainly as a problem solving technique[14].
Several studies have demonstrated a trend to increased sensitivity and specificity of dynamic MRI over dynamic CT for the detection and characterization of HCC of all sizes with reported sensitivities of 76%, 61%, 90% and 77% for MRI vs 61%, 52%, 78% and 54% for CT, respectively[11,16-18]. An optimized, dynamic T1-weighted gradient recalled echo (GRE) with individually tailored arterial phase timing, has shown very high sensitivity and specificity (> 90%-95%)[19].
The MRI sensitivity vary with tumor size; however, it was estimated to be about 100% in HCCs larger than 2 cm[20]. The detection of small tumors remains challenging, and MRI also outperforms CT in this area, with reported sensitivities for the detection of HCCs measuring 1-2 cm of 84% and 47% for MRI vs 85% and 68% for CT, respectively[11,21].
To date, validated CT and MRI criteria for the diagnosis of HCC are based on the hemodynamic features of the nodules and include arterial hyper-enhancement and delayed washout[22]. The most recent recommendations by the AASLD state that a diagnosis of HCC can be made if a nodule larger than 1 cm shows typical hemodynamic features of HCC on either dynamic CT or MRI[6]. In our opinion, this reduces MRI and CT to a minimum common denominator; because despite the greater sensitivity of dynamic MRI for the detection of small HCCs; which might be explained by inherit superior contrast resolution of MRI and superior paramagnetic effect of intravenous gadolinium-based contrast agents, the diagnosis of HCC using only hemodynamic criteria is not without its limitation, as small HCCs frequently show atypical enhancement patterns[23]. One study showed that the majority of HCCs less than 2 cm showed arterial hypervascularity regardless of washout[24].
MRI provides multi-parametric data on anatomical abnormality with both T1- and T2-weighted sequences, and provides functional sequences such as diffusion-weighted images (DWI) and contrast uptake with the use of liver-specific hepatobiliary contrast agents, providing cellular information of the hepatocellular nodules that can improve lesion detection and characterization. Table 1 shows a summary of a wide-spectrum of lesions in cirrhotic liver and their imaging appearances on MRI.
Table 1.
Summary of a wide-spectrum of lesions in cirrhotic liver and their imaging appearances on magnetic resonance imaging
| Imaging sequences | RNs | Siderotic nodules (RNs or LGDNs) | LGDNs1 | AP Shunts2 | HGDNs | HCCs |
| T1-weighted images | Iso- or hyperintense3 | Hypointense4 | Iso- or hyperintense | Isointense | Iso- or slightly hyperintense | Ranging from hypo- to hyperintense |
| T2-weighted images | Iso- or hypointense | Hypointense | Iso- or hypointense | Isointense | Isointense | Iso- to mildly hyperintense |
| DWI | Isointense | Hypointense | Iso- or hypointense | Isointense | Isointense | Iso- to hyperintense5 |
| Post-Gadolinium Dynamic images (arterial and delayed images) | Iso-enhancement on the hepatic arterial phase, and no delayed washout | Iso-enhancement on the hepatic arterial phase, and no delayed washout | Iso-enhancement on the hepatic arterial phase, and no delayed washout | Hyper-enhancement on the arterial phase, and no show delayed washout6 | Usually hyper-enhancement on the arterial phase and can be mistaken for HCC. These nodules do not show delayed washout | Usually hyper-enhancement on the arterial phase and delayed washout (hypointense), with or without pseudocapsule enhancement7 |
| Hepatobiliary phase images | Iso- to slightly hyperintense | Hypointense | Iso- to slightly hyperintense | Isointense | Isointense | Hypointense8 |
RNs and LGDNs tend to be indistinguishable on MRI;
AP shunts might be indistinguishable from HGNDs and small, early HCCs. Relying on additional features and short-term follow-up can help in making this distinction;
The exact cause for this hyperintensity is believed to be due to the presence of binding proteins;
These nodules show lower signal intensity on longer TE T1-weighted GRE sequences, due to susceptibility artifact;
Usually hyperintense, especially if > 2 cm;
AP shuns are usually easy to differentiate from other hypervascular lesions when they show a triangular or linear configuration. When AP shunts show round configuration, they can be indistinguishable from HGDNs and HCCs;
HCCs ≤ 1.5 cm are frequently isointense on T1- and T2-weighted images and are detected only on the arterial phase. Early stage HCC, especially tumors ≤ 2 cm, may also appear isointense, or less likely, hypointense on the arterial phase;
Some HCCs may appear isointense or hyperintense on the hepatobiliary phase; especially well-differentiated and moderately-differentiated HCCs. LGDNs: Low-grade dysplastic nodules; AP: Arterio-portal; HGDNs: High-grade dysplastic nodules; HCCs: Hepatocellular carcinomas; RNs: Regenerative nodules; DWI: Diffusion weighted images.
In this article, we provide an overview of the basic MRI techniques used for assessment of cirrhotic nodules. We also shed some light on liver imaging reporting and data system (Li-RADS), and the role of different MRI contrast agents and future MR imaging techniques including the use of advanced MR pulse sequences and utilization of hepatocyte-specific MRI contrast agents, and how they might contribute to improving the diagnostic performance of MRI in early stage HCC diagnosis.
PROTOCOL
An adequate imaging protocol has to be standardized to allow repeatability and consistency. The standard imaging techniques are based on dynamic fat-suppressed post-contrast T1-weighted 3D GRE sequences, combined with fat-suppressed and non fat-suppressed T2-weighted sequences. T2-weighted images are usually acquired with single-shot fast spin-echo (SSFSE) technique due to its robustness to motion. Chemical shift imaging is acquired with breath-hold dual-echo spoiled GRE. Additional sequences may be added to the protocol (see below).
Since detection of HCC relies on dynamic fat-suppressed post-contrast T1-weighted 3D GRE sequences and proper timing of the arterial phase is critical for optimizing sensitivity for HCC detection (Figure 2). We routinely use real time bolus-triggering method in order to consistently achieve adequate arterial phase images. An optimal arterial phase is recognized when contrast is present in the portal veins and absent in the hepatic veins; referred to as late-hepatic arterial phase or hepatic-arterial dominant phase. Post-processed subtraction arterial imaging may be utilized and may carry an additional value for detecting subtle early enhancement; which can be observed in cases of nodules with increased intrinsic signal on T1-weighted images, nodules with microscopic fat (Figure 3), or small lesions in a background of heterogeneous background hepatic parenchymal enhancement.
Figure 2.
Value of proper timing for detecting hypervascular hepatic lesions. A-B: Post-contrast fat-suppressed 3D-GRE T1-weighted images acquired 4 mo apart. A: Initial scanning shows contrast in the portal vain branches (arrowhead, A), without opacification of the hepatic veins (arrow, A), suggesting late hepatic arterial phase timing; the optimal time for detecting hypervascular pathologies, with demonstration of multiple lesions; B: A subsequent scan acquired 4 mo later shows contrast in the hepatic artery without opacification of the portal vein branches (arrowhead, B), suggesting an early arterial timing, without evidence of hypervascular lesions. A subsequent scan was acquired (not shown); which confirmed the persistence of these hypervascular lesions. GRE: Gradient recalled echo.
Figure 3.
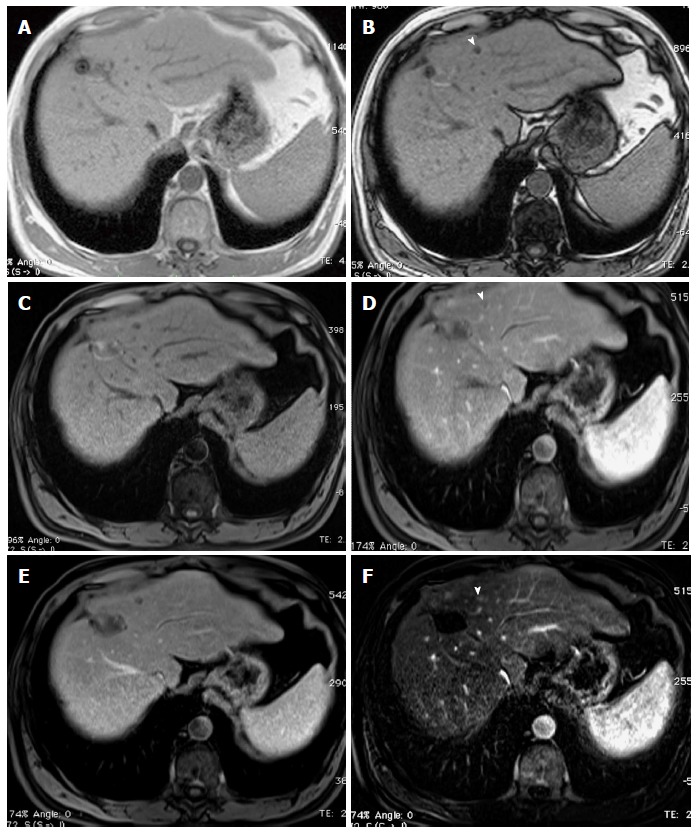
Small fat-containing hepatocellular carcinoma; the value of subtraction images. A: In-phase; B: Opposed-phase GRE T1 weighted images; C-E: Pre- and post-contrast fat-suppressed 3D-GRE T1-weighted images during the (D) late hepatic arterial and (E) delayed phases; F: Post-processed subtractions arterial phase image. There is a small left hepatic nodule, which demonstrates drop of signal intensity on opposed-phase (arrowhead, B) and pre-contrast images (C) compared to the in-phase images (A), suggesting the presence of fat, with possible minimal increased arterial enhancement (arrowhead, D), confirmed on subtraction images (arrowhead, F), and washout on delayed images (E) in keeping with a small fat-containing hepatocellular carcinoma. GRE: Gradient recalled echo.
HEPATOCARCINOGENESIS
In cirrhotic liver the stepwise development of cancer from areas of regeneration to overt development of HCC is called “multistep hepatocarcinogenesis” and is the widely accepted main mechanism of hepatocarcinogenesis. De novo hepatocarcinogenesis also is presumed to occur as an alternative pathway. Even in such cases, later progression to overt HCC takes place in a multistep fashion[25]. Accepted imaging diagnosis of HCC is primarily based on sequential changes in the intra-nodular blood supply during hepatocarcinogenesis; regenerative nodules (RN) show similar blood supply to normal liver, borderline lesions such as dysplastic nodules (DN) or early HCCs show wide variations of blood supply, and advanced HCCs are supplied by abnormal arteries alone[25,26].
High-grade DN is a lesion with strong malignant potential, being recognized as a precursor of HCC. DN and early HCCs are recognized as lesions in the “gray zone”[27] as although usually being hypervascular they tend to show no washout on late phases, hindering the diagnosis[24].
Another source of HCC misinterpretation is on iso-enhancement of HCC on arterial phase images due to the iso or hypovascularity of the lesion[28] (Figure 4). Additionally, misdiagnosed HCCs on MRI may be due to poor patient compliance, especially from the inability to suspend respiration, which is becoming less of a problem with the new advancement in developing faster and motion robust sequences.
Figure 4.
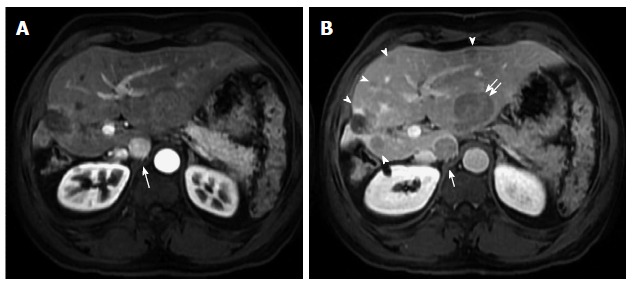
Hypervascular and non-hypervascular hepatocellular carcinomas. Post contrast fat-suppressed 3D-GRE T1-weighted images during the (A) late hepatic arterial and (B) delayed phases. There is a focal hepatic lesion medial to the inferior vena cava, which demonstrates intensely increased arterial enhancement (arrow, A) and washout on delayed images (arrow, B) in keeping with a hypervascular HCC. Additionally, there are multiple foci of delayed washout throughout the liver (arrowheads, B), the largest of which is seen at the left hepatic lobe (double-arrow, B), with variable degrees of arterial enhancement, in keeping with multiple hypo- and iso- vascular HCCs. Of note are the hypertrophic changes of the left hepatic lobe as well as atrophic and post-interventional changes of the right hepatic lobe. HCC: Hepatocellular carcinoma; GRE: Gradient recalled echo.
MRI FEATURES OF CIRRHOTIC NODULES
Dominant nodules are frequently identified during an imaging surveillance program in patients with liver cirrhosis. The change in vascularity observed in hepatic nodules during the multistep hepatocarcinogenesis correlates with the development of malignancy and determines their distinguishing imaging characteristics.
RN
RNs consist of proliferating normal liver cells surrounded by a fibrous stroma[29]. A RN is described as containing one or more portal tracts located in a liver that is abnormal whether because of cirrhosis or other disease[30]. The blood supply of a RN continues to be largely from the portal vein, with minimal contribution from the hepatic artery[31]. This explains why there is no hyper-enhancement on the hepatic arterial phase on MR images. Because of their histopathological nature, as described above, RNs are often indistinct on T1- and T2-weighted images. However, they can have higher T1 signal intensity compared to background liver tissue. The explanation for this increase in signal is not exactly known; it has been proposed to be due to the presence of metal-binding proteins, proteins per se, or lipid[32,33] (Figure 5). RNs may occasionally contain iron (siderotic nodules), which will show decreased T1- and T2 signal intensities due to susceptibility effects[34].
Figure 5.
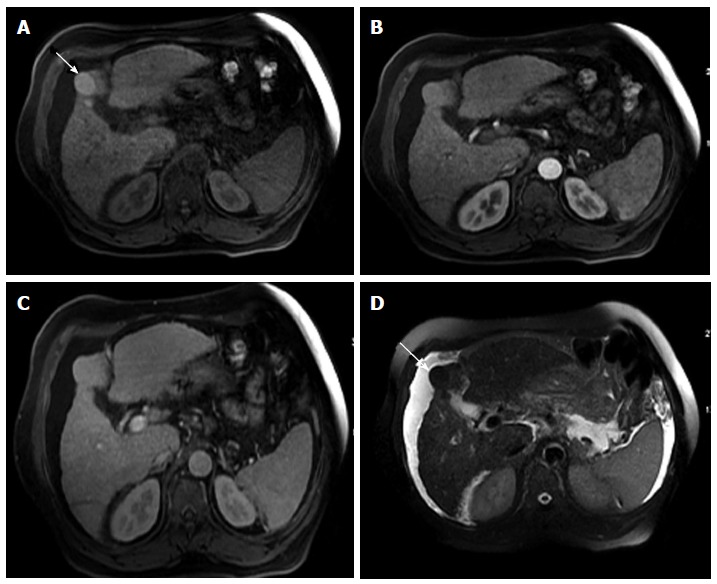
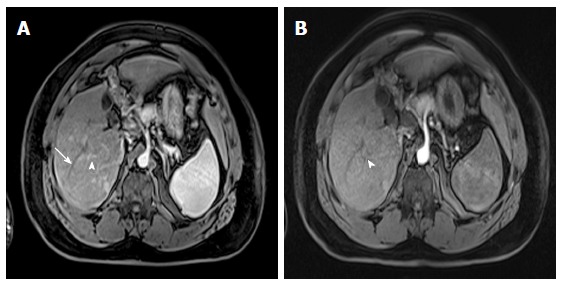
Dominant regenerative hepatic nodule. A-C: Pre- and post-contrast fat-suppressed 3D-GRE T1-weighted images during the (B) late hepatic arterial and (C) poral venous phases; D: Fat-suppressed SSFSE T2-weighted image. There is a subcapsular, partially exophytic nodule at hepatic segment #5, which demonstrates increased intrinsic T1 signal on pre-contrast images (arrow, A) and isosignal intensity to background liver parenchyma on post-contrast images (C), without appreciable increased arterial enhancement (B) or increased T2 signal intensity (arrow, D) in keeping with a dominant regenerative nodule. GRE: Gradient recalled echo; SSFSE: Single-shot fast spin-echo.
Chemical shift imaging aids in the characterization of hyperintense T1-weighted nodules. Fatty nodules show drop of signal intensity on the opposed-phase T1-weighted sequence, due to destruction of the magnitude vector within the same voxel, exerted by fat and water molecules having opposite directions and resulting in decreased signal intensity; indicative of intracellular (microscopic fat).
Chemical shift imaging aids also in the diagnosis of siderotic nodules, showing drop of signal on the sequence with the longer echo-time (TE), which could be during the in-phase or opposed phase, depending on the MR machine used for imaging and its field strength, due to susceptibility effects resulting from proton de-phasing exerted by the presence of iron (Figure 6).
Figure 6.
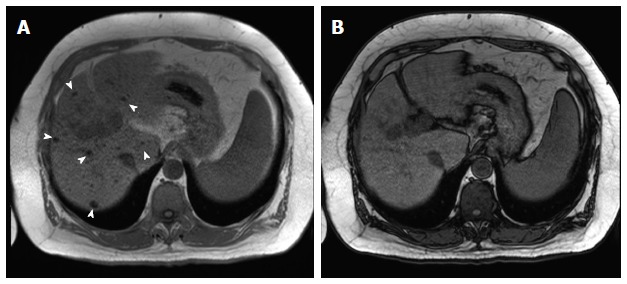
Multiple siderotic hepatic nodules. In-phase (TE = 4.9 ms) (A) and opposed-phase (TE = 2.4 ms) (B) GRE T1 weighted images. There are multiple small nodules seen through out the liver, which demonstrate isosignal intensity on the in-phase images (arrowheads, A), without corresponding abnormalities on the opposed-phase images in keeping with Multiple siderotic hepatic nodules. GRE: Gradient recalled echo.
Several studies have shown that nodules with high signal intensity on T1-weighted images are in most cases benign. In younger patients with numerous macro-nodules, almost all of these lesions follow a benign course[35]. In patients with cirrhosis, small hyperintense hepatic lesions on T1-weighted images without hyper-enhancement on the arterial-phase images usually show no interval growth or disappear during serial imaging[36]. Regardless of their intrinsic signal features, a reliable finding of RNs is the absence of enhancement on the arterial phase, compared with the background hepatic parenchyma.
A notable exception are fat-containing, large size (> 1.5 cm) nodules (hyperintense on T1-weighted in-phase images with drop of signal on the opposed-phase T1-weighted images), which strongly suggest malignancy (Figure 7). Otherwise, the presence of numerous nodules < 1 cm suggests benignity[37].
Figure 7.
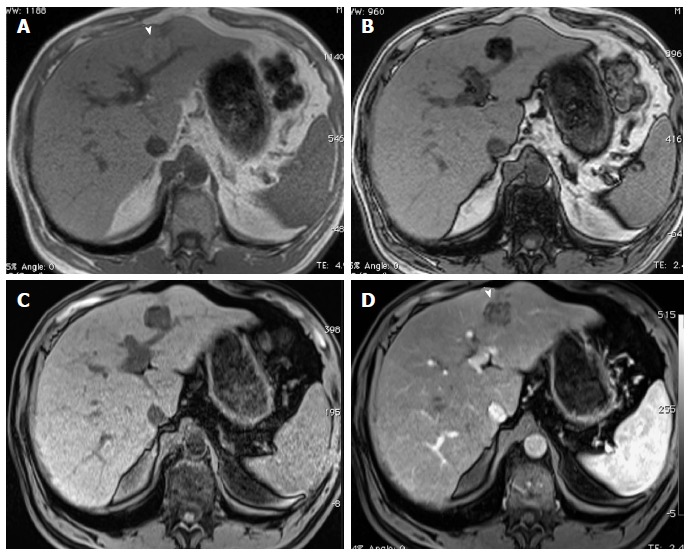
Large, fat-containing hepatocellular carcinoma. In-phase (A) and opposed-phase (B) GRE T1 weighted images. Pre- (C) and post-contrast fat-suppressed 3D-GRE T1-weighted images during late hepatic arterial phase (D). There is a prominent left hepatic nodule, which demonstrates minimally increased intrinsic T1 signal on the in-phase images (arrowhead, A) and low signal intensity on the opposed-phase (B) and pre-contrast images (C), indicating the presence of fat. The lesion demonstrates heterogeneous mildly increased arterial enhancement (arrowhead, D) in keeping with a fat-containing HCC. HCC: Hepatocellular carcinoma; GRE: Gradient recalled echo.
DNs
DNs are defined as regenerative nodules containing atypical cells with nuclear crowding and architectural derangement and a variable number of unpaired arterioles or capillaries without definite histologic signs of malignancy[38]. High-grade dysplastic nodules (HGDNs) display at least moderate atypia and occasional mitosis[30]. DNs primarily display T1 and T2 isointensity to the background liver parenchyma, but T1 hyperintensity is also possible as described above with RNs[39]. Low-grade dysplastic nodules (LGDNs) primarily display enhancement characteristics similar to that of the background liver parenchyma on all dynamic phases; because they remain mainly supplied by the portal circulation. LGDNs are not considered premalignant lesions. As lesions progress, their blood supply becomes more arterialized, giving the typical hypervascular features of HCC[40]. Unfortunately, the portal and arterial supply to LGDNs and HGDNs is variable and inconsistent[39]. They may even be associated with increased alpha-fetoprotein despite not being malignant[41].
HGDNs are considered premalignant lesions[30] and tend to show intense early enhancement after gadolinium injection and fade to isointensity[42], without washout (Figure 8), because supply from the portal venous system remains comparable with the background liver[43,44].
Figure 8.
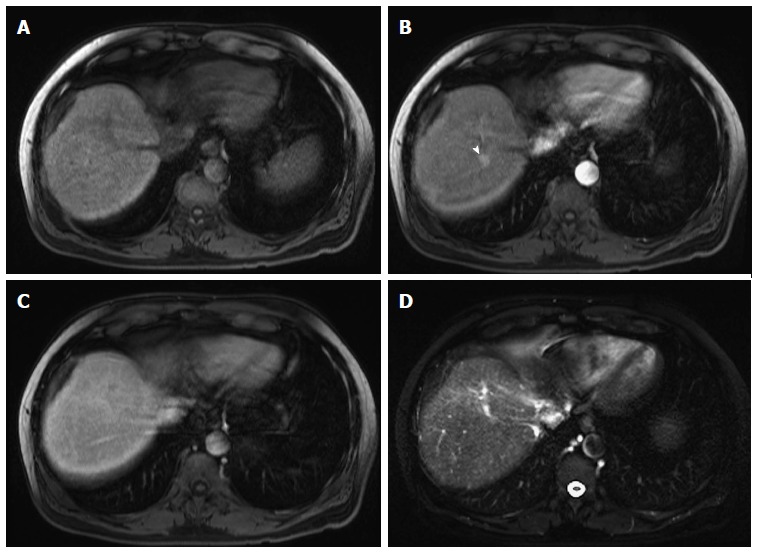
High-grade dysplastic nodules. Pre- (A) and post-contrast fat-suppressed 3D-GRE T1-weighted images during the (B) Late hepatic arterial; and (C) Delayed phases; (D) Fat-suppressed SSFSE T2-weighted image. There is a nodule at hepatic segment #7, which demonstrates iso T1 signal intensity (A), increased arterial enhancement (arrowhead, B), without definite washout or corresponding T2 signal abnormality in keeping with a HGDN. However, early HCC cannot be totally excluded and short-term follow-up should be recommended, especially in patients with hepatitis C infection. HGDN: High-grade dysplastic nodules; HCC: Hepatocellular carcinoma; GRE: Gradient recalled echo; SSFSE: Single-shot fast spin-echo.
The development of HCC within a DN has been reported within as short as 4 mo[45]. Usually it is seen as an increase in size and development of washout on delayed imaging, allowing definite diagnosis of HCC (Figure 9). Early studies have also reported DNs with “a nodule within a nodule” appearance. This classic MR description is a focus of increased T2 signal intensity within a T2 low-signal-intensity nodule, which may or may not demonstrate arterial hyper-enhancement on dynamic MR images[46].
Figure 9.
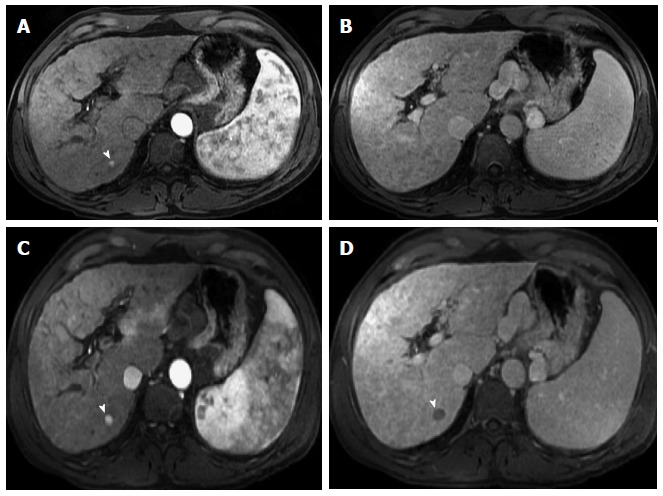
Dysplastic nodule progressing into an hepatocellular carcinoma. Post-contrast fat-suppressed 3D-GRE T1-weighted images during the late hepatic arterial (A and C) and delayed phases (B and D). There is a small right hepatic lobe nodule, which demonstrates increased arterial enhancement (arrowhead, A) and fades out on the delayed images (B) on the initial examination. On the 4-month follow-up study, there is evidence of interval growth (arrowhead, C, D) and development of clear delayed washout (arrowhead, D) both of which are signs of progression into HCC. HCC: Hepatocellular carcinoma; GRE: Gradient recalled echo.
According to the latest guidelines from the EASL and AASLD practice guidelines, DNs should not be treated or managed as cancers[47]. In our clinical practice, we advise more frequent surveillance imaging (usually 3 mo) as there is an increased risk of progression to HCC.
ARTERIOPORTAL SHUNTS
Arterioportal (AP) shunts usually demonstrate enhancement on the arterial phase and mostly fade back to isointensity on the portal venous or delayed images (Figure 10). They are sometimes easily distinguished from HGDNs/early HCCs by their subcapsular location and wedge- or comma shaped configuration. However, they may sometimes become main mimickers of HGDN/early HCCs; posing as a potential differential diagnosis when they are round or oval in configuration[48].
Figure 10.
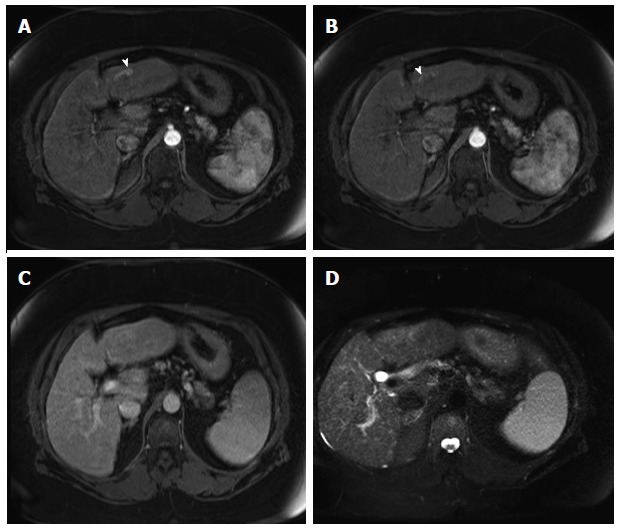
Arterioportal shunt. Post-contrast fat-suppressed 3D-GRE T1-weighted images during the late hepatic arterial (A and B) and delayed phases (C); D: Fat-suppressed SSFSE T2-weighted image. There is a convoluted linear area of increased arterial enhancement with a vessel leading to it (arrowhead, A, B), which does not demonstrate delayed washout (C), or corresponding T2 signal abnormality in keeping with an AP shunt. SSFSE: Single-shot fast spin-echo; GRE: Gradient recalled echo.
HCC
The EASL and AASLD have proposed and validated imaging criteria for the diagnosis of HCC in cirrhotic patients, which correspond to the typical HCC features including arterial hyper-enhancement and delayed washout[49] (Figure 11). HCCs may show a variety of MR imaging features; reflective of the variable characteristics of the tumor’s architecture, grading, stromal components, and intracellular content[22].
Figure 11.
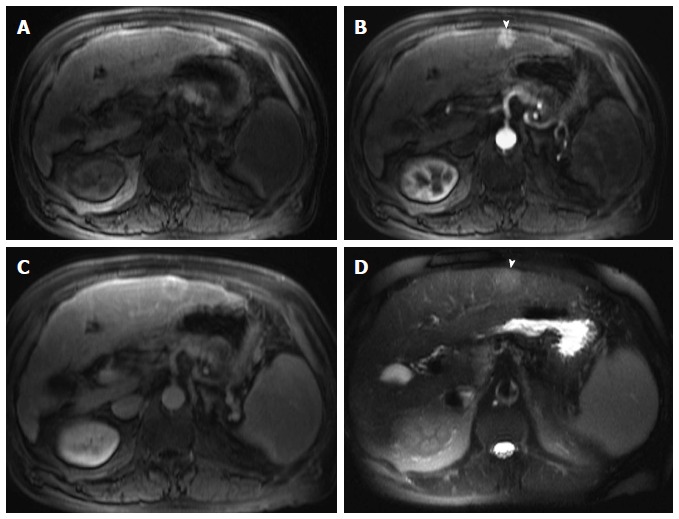
Classical hepatocellular carcinoma. A: Pre- and post-contrast fat-suppressed 3D-GRE T1-weighted images during the late hepatic arterial (B) and delayed phases (C); D: Fat-suppressed SSFSE T2-weighted image. There is a peripheral well defined left hepatic lobe nodule, which demonstrates iso T1 signal intensity (A), increased arterial enhancement (arrowhead, B), delayed washout and pseudocapsule enhancement (C), and mildly increased T2 signal (arrowhead, D) in keeping with classical features of HCC. HCC: Hepatocellular carcinoma; GRE: Gradient recalled echo; SSFSE: Single-shot fast spin-echo.
Arterial hyper-enhancement is the most common and important imaging finding in the diagnosis of HCC[50]. While considered a reliable feature, it can be seen in HGDNs and AP shunts. Arterial hyper-enhancement can also be seen in a variety of benign and malignant hepatic lesions, including hemangiomas and focal nodular hyperplasia and hypervascular metastases. However, these liver lesions are infrequent in the setting of hepatic cirrhosis[51].
Because arterial hyper-enhancement can be observed with other lesions and nodules, additional imaging criteria are needed to decrease the false-positive rate and increase sensitivity, while maintaining high specificity for the diagnosis of HCC[49]. Therefore, delayed washout, among other secondary features, is used for this purpose.
The key distinguishing feature of HCC is the development of delayed “washout”; defined as arterially enhancing nodules becoming hypointense compared to the background liver on the delayed phase imaging (not to be confused with “fade out”, which is defined as arterially enhancing nodules becoming isointense to background liver on delayed phase imaging).
HCCs greater than 2 cm in size tend to show washout[52,53], which explains the high diagnostic sensitivity for tumors this size. However, for HCCs smaller than 2 cm the sensitivity is lower. This is not due to hypovascular HCCs, which are uncommon, but rather to hypervascular HCCs that do not show washout on delayed images[24,49,54] (Figure 12). In one series of 60 HCCs, smaller than 2 cm, 85% of these lesions were hypervascular, and only 61.7% of which showed washout[24]. Similarly, in another series, 51 out of 131 HCCs showed arterial hyper-enhancement without clear wash-out on delayed images[54].
Figure 12.
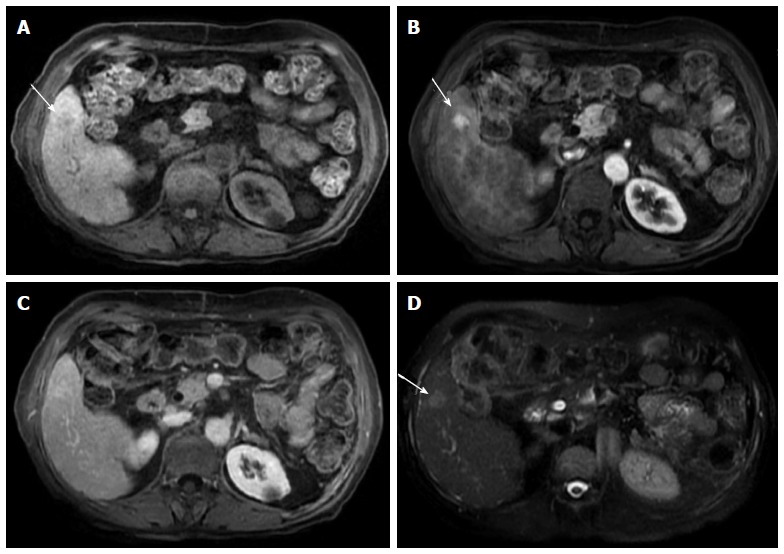
Small, non-washing-out hepatocellular carcinoma. A: Pre- and post-contrast fat-suppressed 3D-GRE T1-weighted images during the late hepatic arterial (B) and delayed phases (C). D: Fat-suppressed SSFSE T2-weighted image. There is a small nodule at hepatic segment #5, which demonstrates minimally decreased T1 signal on pre-contrast images (arrow, A) and increased arterial enhancement (arrow, B). The nodule demonstrates iso to slightly increased signal on the delayed phase images, without clear washout (C), but mildly increased T2 signal intensity (arrow, D) in keeping with an HCC. Note that T2 signal alteration increased the accuracy of diagnosing HCC in this patient, despite the lack of delayed washout (also see Figure 17). HCC: Hepatocellular carcinoma; GRE: Gradient recalled echo; SSFSE: Single-shot fast spin-echo.
Delayed pseudo-capsule enhancement of hepatic nodules aids in the diagnosis of HCC, and can be helpful in lesions that do not show classical features of HCC on dynamic imaging (Figure 13).
Figure 13.
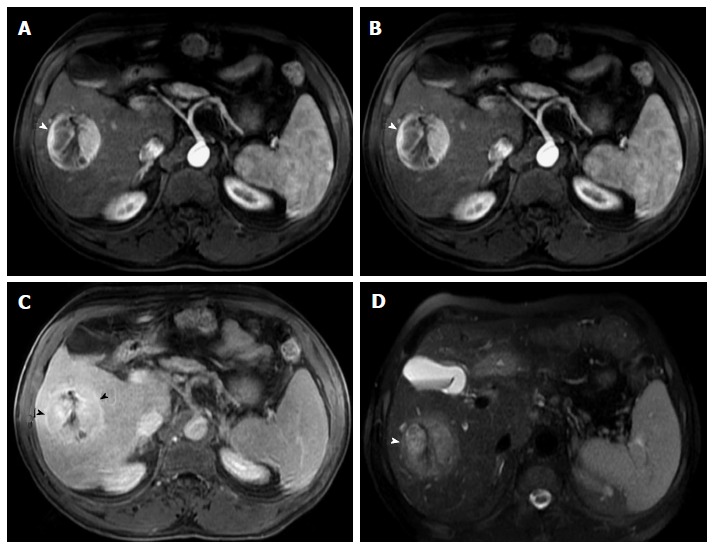
Large hepatocellular carcinoma with delayed pseudocapsule enhancement but no intralesional washout. Post-contrast fat-suppressed 3D-GRE T1-weighted images during the (A) late hepatic arterial, (B) poral venous, and (C) delayed phases; D: Fat-suppressed SSFSE T2-weighted image. There is a large right hepatic lobe mass, which demonstrates heterogeneous, intensely increased arterial enhancement (arrowhead, A) with progressive fading throughout the subsequent images (B), but no clear washout (C). The lesion demonstrates delayed pseudocapsular enhancement (black arrowheads, C) and mildly increased T2 signal intensity (arrowhead, D) in keeping with a large HCC. HCC: Hepatocellular carcinoma; GRE: Gradient recalled echo; SSFSE: Single-shot fast spin-echo.
Since it is extremely difficult to perform biopsy of small nodules that are only visible on arterial phase images, we usually prefer close follow-up. Generally we advocate that lesions measuring 1-2 cm are re-imaged at a 3-mo interval to assess for lesion interval growth or development of washout. The lack of interval growth on short-term follow-ups does not exclude the possibility of malignancy, as HCC may demonstrate slow growth. Therefore, only nodules that are stable for 2 years are considered benign[14]. However, it is worth emphasizing the value of direct comparison and lesion measurement between both the current and older prior examination to demonstrate undetected subtle changes in size on short-term followups; which is indicative of slow growth, a feature of early well-differentiated HCC (Figure 14).
Figure 14.
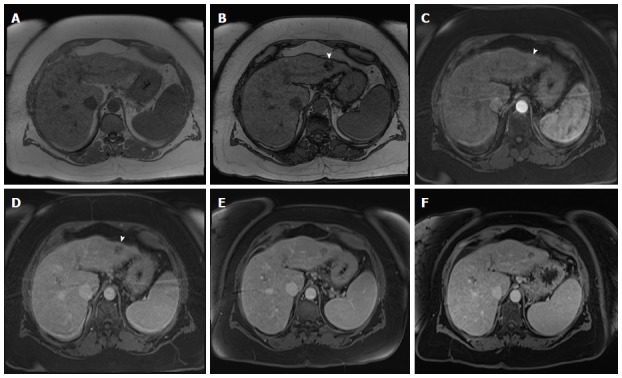
Slow growing, well-differentiated, fat-containing, small hepatocellular carcinoma. In-phase (A) and opposed-phase GRE T1 weighted images (B); Post-contrast fat-suppressed 3D-GRE T1-weighted images during the late hepatic (C) arterial and delayed phases (D). E, F: Post-contrast fat-suppressed 3D-GRE T1-weighted delayed images from prior examinations performed 1 and 2 years prior, respectively. The is a small left hepatic lobe nodule, which demonstrates drop of signal on the opposed-phase image (arrowhead, B) compared to the in-phase image (A), increased arterial enhancement (arrowhead, C), and delayed washout (arrowhead,D). The lesion does not demonstrate significant change in size from the immediate prior examination (E). However, when compared with a more remote examination (F), substantial interval growth can be appreciated consistent with a slow growing, well-differentiated, fat-containing, small HCC. HCC: Hepatocellular carcinoma; GRE: Gradient recalled echo.
MORPHOLOGIC HCC SUB-TYPES
HCCs can manifest as different morphologic types including focal (nodular), massive, and diffuse/infiltrative[55,56].
Nodular type is the most common encountered type and usually presents as encapsulated focal nodule with well-defined margins. Nodular type can be further classified as solitary or multi-focal.
Massive tumors are well-defined tumors large enough to often render these patients non-eligible for loco-regional ablative therapies or hepatic transplantation.
Multi-focal nodular subtype is an advanced type and shows similar features to solitary nodular subtype on conventional and dynamic MRI. Additional features that are not commonly seen with solitary focal lesions, but are noted with multi-focal HCC and other aggressive subtypes include portal venous thrombosis and in intrahepatic metastases[26].
Diffuse HCCs are usually large and have ill-defined boundaries without clear demarcation. They usually present with very high alpha-fetoprotein levels and are almost always associated with portal venous thrombus; which can be bland or most of the time tumoral in nature; based on the presence of neovascularity on the arterial imaging. Diffuse HCCs can be extremely subtle, and therefore difficult to demonstrate by imaging alone as they can blend with the background cirrhotic parenchyma; preventing early diagnosis and leading to advanced disease at presentation with often distant metastatic disease.
One study by Kneuertz et al[56] evaluated 147 patients with advanced HCCs (75 with infiltrative disease and 72 patients with multi-focal disease). In that study, failure to display a discrete mass was observed in 42.7% of patients, low signal on T1-weighted images was observed in 55.7%, high signal on T2- weighted images was observed in 80.3% of patients. They also demonstrated mild miliary pattern of enhancement on arterial phase imaging in 16.4% of patients, with delayed washout in 50.8%.
Diffuse HCCs can be difficult to differentiate from areas of confluent fibrosis on CT. However, the combined additive advantage of T2-weighted imaging, DWI, and delayed imaging can be used to enhance the diagnostic accuracy of diagnosis on MRI, which display more distinct lobulated margins, with poorly defined amorphous infiltration surrounding thrombosed portal veins, and clearly depict internal reticulation throughout the tumor[57,58].
Additionally, post-contrast delayed imaging demonstrates heterogeneous washout[59] , allowing differentiation between confluent fibrosis as this shows increase enhancement over time Another distinctive feature from confluent fibrosis is the presence of regional tumor thrombus that is almost invariably present in patients with diffuse HCC[57,60,61] (Figure 15).
Figure 15.
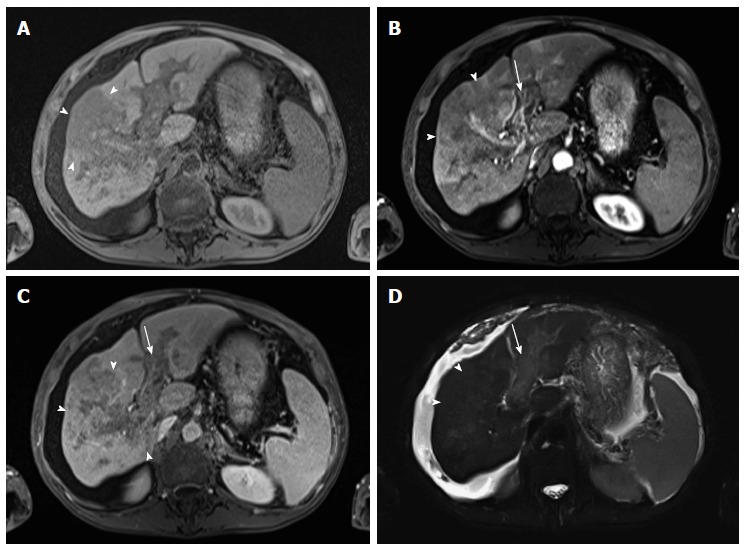
Diffuse hepatocellular carcinoma associated with tumor thrombus. (A) Pre- and post-contrast fat-suppressed 3D-GRE T1-weighted images during the (B) late hepatic arterial and (C) delayed phases; (D) Fat-suppressed SSFSE T2-weighted image. There is a large ill-defined area involving the right hepatic lobe, which shows decreased T1 signal in pre-contrast images (arrowheads, A), heterogeneous mildly increased arterial enhancement (arrowheads, B), and heterogeneous delayed washout with permeative appearance (arrowheads, C), and heterogeneous mildly increased T2 signal (arrowhead, D) in keeping with HCC. There is also evidence of expansion of the portal vein branches, abnormal increased arterial enhancement (arrow, B), lack of opacification and washout on the delayed images (arrow, C), and mildly increased T2 signal (arrow, D) simulating the behavior of the primary tumor in keeping with diffuse tumor thrombus, a finding almost invariably associated with diffuse HCC subtype. Also of note is the mild to moderate ascites and omental hypertrophy secondary to portal hypertension. HCC: Hepatocellular carcinoma; GRE: Gradient recalled echo.
Multiple small satellite nodules associated with the main tumor or multiple small recurrent tumors of moderate or poor differentiation are regarded as intrahepatic metastases[62]. The clinical significance about intrahepatic metastases is that they require immediate curative or palliative interventions even when smaller than 1 cm; as such lesions are likely to display aggressive behavior, unlike single or multicentric primary tumors of the same size[63].
A rare variant of nodular morphologic subtype is lesions with rim-enhancement on arterial imaging on initial MRI (Figure 16) has been described in the literature[64], suggesting a more progressive behavior with rapid interval growth and disease worsening; therefore, requiring prompt therapy and short-term follow-up.
Figure 16.
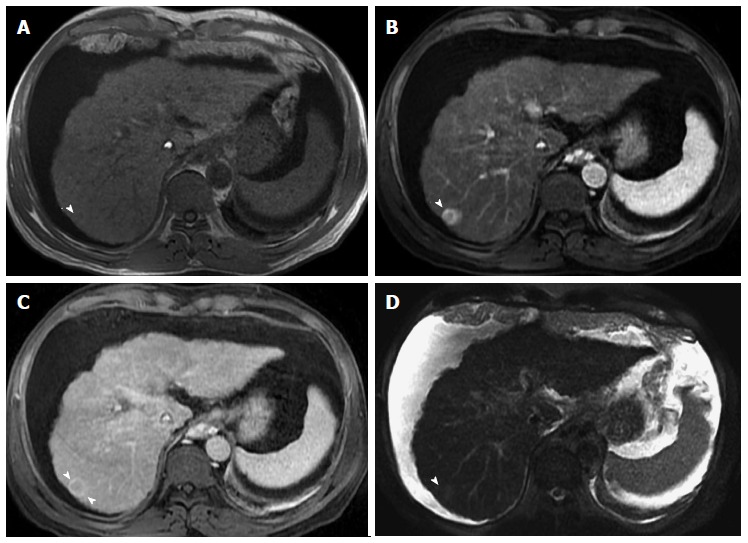
Ring-enhancing hepatocellular carcinoma, indicative of a more aggressive course. A: In-phase GRE T1 weighted image; B: Post-contrast fat-suppressed 3D-GRE T1-weighted images during the (B) late hepatic arterial and (C) delayed phases; (D) Fat-suppressed SSFSE T2-weighted image. There is a small nodule at hepatic segment #7, which demonstrates iso to slightly low T1 signal (arrowhead, A), heterogeneous increased arterial enhancement, predominantly peripheral (arrowhead, B), washout and pseudocapsule enhancement on delayed images (arrowhead, C), and mildly increased T2 signal intensity (arrowhead, D) in keeping with ring-enhancing HCC. HCC: Hepatocellular carcinoma; GRE: Gradient recalled echo; SSFSE: Single-shot fast spin-echo.
FUTURE DIRECTIONS
T2-weighted imaging
The appearance of HCC on T2-weighted images is variable. Early reports suggested that HCC displayed high or equivalent signal intensity compared to the liver parenchyma on T2-weighted images[65,66].
Other researchers reported that both non-enhanced T1- and T2-weighted sequences may contribute in the characterization of cirrhotic nodules; however, minimally increasing the detection rate[67].
More recent studies have shown that the addition of T2-weighted imaging to gadolinium-enhanced T1-weighted 3D-GRE dynamic imaging improves the diagnostic performance of MRI in the detection of HCC compared to dynamic MR imaging alone. This is especially true for lesions smaller than 1 or 2 cm (Figure 12), which may show hypervascularity but might not display any washout, distinguishing them from HGDNs[54,68-70] (Figure 17).
Figure 17.
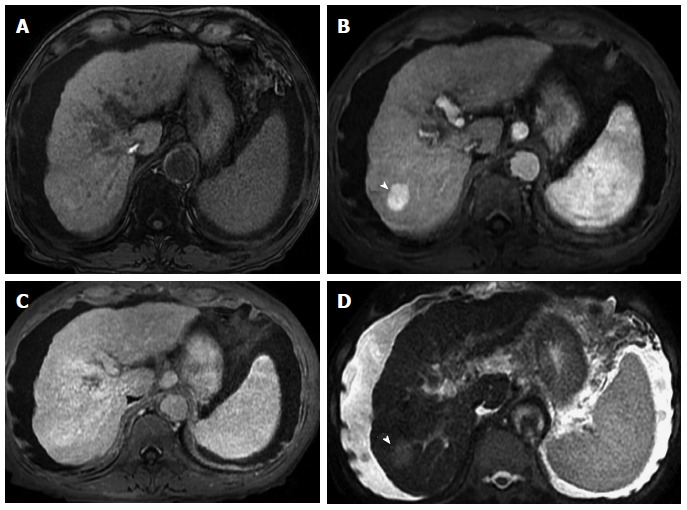
Large, non-washing-out hepatocellular carcinoma. A: Pre- and post-contrast fat-suppressed 3D-GRE T1-weighted images during the (B) late hepatic arterial and (C) delayed phases; D: Fat-suppressed SSFSE T2-weighted image. There is a sizable right hepatic lobe nodule, which demonstrates iso T1 signal intensity on pre-contrast images (A); increased arterial enhancement (arrowhead, B); becomes iso to slightly hyperintense compared to background liver, without definite washout, on the delayed images (C); and demonstrates mildly increased T2 signal intensity in keeping with HCC. Note that T2 sinal alteration increased the accuracy of diagnosing HCC in this patient, despite the lack of delayed washout (also see Figure 12). HCC: Hepatocellular carcinoma; GRE: Gradient recalled echo; SSFSE: Single-shot fast spin-echo.
HCCs tend to show minimal to mildly increased signal intensity on T2-weighted images, as opposed to intra-hepatic cholangiocarcinoma or mixed HCC-cholangiocarcinoma; both of which are increasingly being reported in patients with cirrhosis, and tend to show moderately increased signal intensity on T2-weighted images with evidence of increased vascularity on arterial phase imaging and progressive contrast enhancement throughout subsequence phases. Such distinction is clinically important as those lesions are associated with a poor prognosis and a high rate of tumor recurrence after liver transplantation, and have higher risk of nodal and distant metastatic disease[71].
T2-weighted imaging is also helpful in the detection of lymphadenopathy in patients with focal hepatic lesions[70].
Diffusion-weighted imaging
The possibility of performing functional imaging sequences is an additional advantage of MRI over CT[72]. With technological advances in hardware and software, DWI can be readily applied to liver imaging with improved image quality. DWI is an imaging technique based on differences in the Brownian motion (diffusibility) of water molecules within tissues. In highly cellular tissues such as tumors, the diffusion of water protons is restricted. Therefore, both qualitative and quantitative variables reflect tissue cellularity and cellular membrane integrity[49,73-75]. DWI is useful for detecting small focal liver lesions in general[49,73-75].
A limited number of small studies have shown encouraging results suggesting that DWI has a good diagnostic performance in the detection of HCC in patients with chronic liver disease and equivalent to conventional contrast-enhanced for lesions greater than 2 cm in size[49,76]. Currently, the limitation of DWI is primary lesion characterization rather than lesion detection[49,76].
The greatest benefit relies on the combined use of DWI with conventional dynamic MRI; providing higher sensitivities than dynamic MRI alone in the detection of small HCC lesions in patients with chronic liver disease[77,78] (Figure 18). Therefore, an additional acquisition of DWI is being implemented in abdominal protocols[77].
Figure 18.
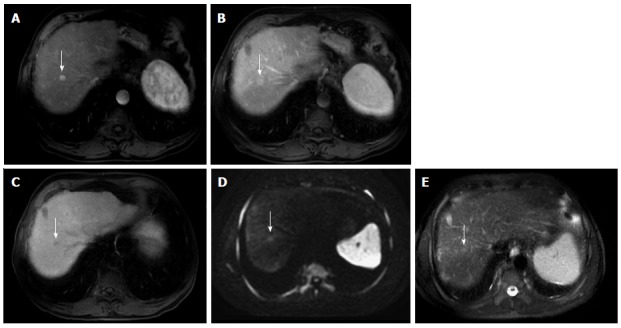
Small hepatocellular carcinoma showing the value of diffusion weighted image and hepatocyte-specific agents. A: Post-contrast fat-suppressed 3D-GRE T1-weighted images during the (A) late hepatic arterial, (B) delayed, and (C) hepatobiliary phases; D: Diffusion weighted image (DWI) (b = 50); E: Fat-suppressed SSFSE T2-weighted image. There is a small right hepatic lobe nodule, which demonstrates increased arterial enhancement (arrow, A), fading out on the delayed images (arrow, B), and demonstrates significantly decreased uptake on the hepatobiliary phase (arrow, C). The lesion also demonstrates clear increased signal on DWI (arrow, D) and subtle increased T2 signal (arrow, E). The constellation of findings is consistent with HCC. The decreased uptake on the hepatobiliary phase and increased signal on DWI increased the accuracy of HCC diagnosis in this patient with a small hypervascular nodule. HCC: Hepatocellular carcinoma; GRE: Gradient recalled echo; SSFSE: Single-shot fast spin-echo.
In a recent study a new MRI criteria was proposed, combining the features of lesions after gadolinium-based contrast agents administration and hyperintensity on DWI[49]. This significantly increased the sensitivity for the diagnosis of HCC compared to conventional hemodynamic criteria, irrespective of tumor size. However, further larger prospective studies are still needed to establish its definitive role for detecting HCC in patients with chronic liver diseases.
T2*-weighted imaging
The performance of liver MRI is highly dependent on gadolinium administration[79]. The revised recommendations refrain from the utilization of intravenous gadolinium-based contrast agents in patients with poor renal function[80]. One recent report has suggested that T2*-weighted MRI may offer the potential for diagnosing HCC in patients with liver cirrhosis[81].
The proposed mechanism for the visualization of HCC on the T2*-weighted sequence is attributed to the combination of the high sensitivity of this sequence to the presence of iron and iron differential deposition in the hepatic parenchyma. On T2*-weighted MRI, hepatic iron causes progressive signal loss with longer TEs, whereas HCCs demonstrate only slight signal loss[81].
One limitation of this sequence is the appearance of lesions after chemoembolization, which potentially reduces the diagnostic performance of the sequence[82].
The addition of a T2*-weighted sequence to a routine liver MRI protocol might lead to additional improved specificity[83], although future studies are likely indicated to determine the full diagnostic performance of T2*-weighted MRI in a larger patient population.
MRI CONTRAST AGENTS
Contrast agents used in cirrhosis-associated hepatic nodules MR evaluation are divided into three types: extracellular Gadolinium-based contrast agents (GBCAs), super-paramagnetic iron-oxide (SPIO) particles, and Gadolinium-based hepatobiliary contrast agents.
Extracellular GBCAs are paramagnetic contrast agents that generate T1-shortening and provide information about tissue vascularity[38]. SPIO particles and hepatobiliary agents are liver-specific contrast agents. SPIO particles are taken up by Kupffer cells within the reticuloendothelial system (RES), and the hepatobiliary agents are taken up by hepatocytes and are excreted via the bile ducts[84].
Despite early promising results of SPIO particles for diagnosing HCC, later evidence reveal that is less efficient than dynamic MRI using conventional extracellular GBCAs in the detection and characterization of HCC[85]. Additionally, there are currently no commercially available intravenous SPIO particles contrast agents in the market.
More recently, two hepatobiliary agents; gadobenate dimeglumine and gadoxetic acid were introduced to the market, combining extracellular properties with liver-specific properties, allowing both dynamic and hepatobiliary imaging. Gadoxetic acid is more highly liver-specific; approximately 50% of the injected dose is taken up by functioning hepatocytes and is excreted in bile, allowing delayed uptake imaging within 20 min from the time of injection, compared with an uptake of 3%-5% for gadobenate dimeglumine, which allows for delayed uptake imaging within two hours[13].
The hepatocyte uptake manifests as an increased signal in the hepatic parenchyma on T1-weighted images resulting in improved lesion-to-liver contrast as less well-differentiated HCCs contain hampered functioning hepatocytes. HCCs exhibit hypointensity on hepatobiliary phase images (Figure 18), except for some well-differentiated HCCs that may retain the contrast agent. Nevertheless, characterization of liver lesions depicted with hepatobiliary phase imaging must be performed in conjunction with routine dynamic sequences to improve accuracy[86].
Gadoxetic acid-enhanced MRI has several advantages in imaging the cirrhotic liver including: (1) higher sensitivity for the diagnosis of HCC, especially for lesions ≤ 2 cm[86-91]; (2) improved characterization of arterially enhancing lesions without definite washout on subsequent imaging[89,92]; (3) distinguishing arterially enhancing pseudo-lesions from HCC[92,93]; and (4) detection of lesions that are isointense to the background hepatic parenchyma on all sequences, apart from the hepatobiliary phase, that are at high risk of transforming to hypervascular HCC[94,95].
However, some limitations to the use of gadoxetic acid-enhanced MRI in the liver cirrhosis have been proposed, especially pertaining to the fact that some patients with cirrhosis can show less optimal lesion-to-liver contrast on early dynamic imaging and poor venous enhancement, which may hamper the diagnosis of HCC and assessment of the porto-spleno-mesenteric venous system patency[86].
Despite the recognized potential advantages of combined morphological and functional analysis of the liver, the inclusion of hepatobiliary contrast agents in international guidelines, besides the Japan Society of Hepatology, is still pending. Recently updated guidelines from the EASL[47] and the AASLD[6] make no contrast agent recommendations.
Overall, continued investigations with more direct comparative analysis between gadoxetic acid and other extracellular agents are warrant.
LI-RADS
LI-RADS was developed by the American College of Radiology[96]; with the aim of standardizing terminology and criteria for interpreting and reporting findings of CT and MRI examinations of the liver in patients with cirrhosis or increased risk of HCC; by using use a carefully chosen and agreed-on vocabulary, or lexicon, that differentiates hepatic histologic entities. It has been developed to provide a framework for assigning degrees of concern on imaging findings[97]. The LI-RADS classifies lesions to five categories ranging from definitely benign to definitely HCC. It uses arterial hyper-enhancement, washout, capsule, and interval growth as ancillary findings[96]. It currently, however, does not apply to hepatobiliary gadolinium-based agents[97].
CONCLUSION
Noninvasive imaging has become the standard for HCC diagnosis in cirrhotic patients. Typical imaging features of HCC such as increased arterial enhancement and delayed washout provide very high specificity and acceptable sensitivity even in nodules ranging from 1-2 cm in diameter. However, limitations apply specifically to hypovascular HCCs and in the differentiating HGDNs from early HCCs. In this review paper, we went over the basics of MR imaging of cirrhotic livers and described future directions, including the addition of new techniques such as DWI, T2*, and hepatocyte-specific MRI contrast agents, in order to improve HCC detection rate in conjunction with the reference standard of optimized dynamic GRE T1-weighted imaging, with individually tailored arterial phase timing.
Footnotes
Conflict-of-interest: The authors declare that they have no competing interests.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 30, 2014
First decision: September 30, 2014
Article in press: December 3, 2014
P- Reviewer: Kaya M, Lee YYV S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer;; 2013. Available from: http://globocan.iarc.fr, accessed on day/month/year. [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) Program. SEERStat database: incidence - SEER 9 Regs research data, Nov 2009 Sub (1973-2007) Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373:614–616. doi: 10.1016/S0140-6736(09)60381-0. [DOI] [PubMed] [Google Scholar]
- 5.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Digumarthy SR, Sahani DV, Saini S. MRI in detection of hepatocellular carcinoma (HCC) Cancer Imaging. 2005;5:20–24. doi: 10.1102/1470-7330.2005.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu NC, Chaudhari V, Raman SS, Lassman C, Tong MJ, Busuttil RW, Lu DS. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:161–167. doi: 10.1016/j.cgh.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Kim KA, Kim MJ, Choi JY, Chung YE. Development of hepatocellular carcinomas in patients with absence of tumors on a prior ultrasound examination. Eur J Radiol. 2012;81:1450–1454. doi: 10.1016/j.ejrad.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 10.Arif-Tiwari H, Kalb B, Chundru S, Sharma P, Costello J, Guessner RW, Martin DR. MRI of hepatocellular carcinoma: an update of current practices. Diagn Interv Radiol. 2014;20:209–221. doi: 10.5152/dir.2014.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, Caralt T, Ayuso JR, Solé M, Sanchez M, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034–1042. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 12.Solomon R. Contrast-induced acute kidney injury: is there a risk after intravenous contrast? Clin J Am Soc Nephrol. 2008;3:1242–1243. doi: 10.2215/CJN.03470708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JM, Choi BI. Hepatocellular nodules in liver cirrhosis: MR evaluation. Abdom Imaging. 2011;36:282–289. doi: 10.1007/s00261-011-9692-2. [DOI] [PubMed] [Google Scholar]
- 14.Willatt JM, Hussain HK, Adusumilli S, Marrero JA. MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008;247:311–330. doi: 10.1148/radiol.2472061331. [DOI] [PubMed] [Google Scholar]
- 15.Semelka RC, Martin DR, Balci NC. Magnetic resonance imaging of the liver: how I do it. J Gastroenterol Hepatol. 2006;21:632–637. doi: 10.1111/j.1440-1746.2006.04279.x. [DOI] [PubMed] [Google Scholar]
- 16.de Lédinghen V, Laharie D, Lecesne R, Le Bail B, Winnock M, Bernard PH, Saric J, Couzigou P, Balabaud C, Bioulac-Sage P, et al. Detection of nodules in liver cirrhosis: spiral computed tomography or magnetic resonance imaging? A prospective study of 88 nodules in 34 patients. Eur J Gastroenterol Hepatol. 2002;14:159–165. doi: 10.1097/00042737-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi Y, Murakami T, Kim T, Hori M, Osuga K, Kawata S, Okada A, Sugiura T, Tomoda K, Narumi Y, et al. Detection of hypervascular hepatocellular carcinoma by dynamic magnetic resonance imaging with double-echo chemical shift in-phase and opposed-phase gradient echo technique: comparison with dynamic helical computed tomography imaging with double arterial phase. J Comput Assist Tomogr. 2002;26:981–987. doi: 10.1097/00004728-200211000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Rode A, Bancel B, Douek P, Chevallier M, Vilgrain V, Picaud G, Henry L, Berger F, Bizollon T, Gaudin JL, et al. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327–336. doi: 10.1097/00004728-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Becker-Weidman DJ, Kalb B, Sharma P, Kitajima HD, Lurie CR, Chen Z, Spivey JR, Knechtle SJ, Hanish SI, Adsay NV, et al. Hepatocellular carcinoma lesion characterization: single-institution clinical performance review of multiphase gadolinium-enhanced MR imaging--comparison to prior same-center results after MR systems improvements. Radiology. 2011;261:824–833. doi: 10.1148/radiol.11110157. [DOI] [PubMed] [Google Scholar]
- 20.Krinsky GA, Lee VS, Theise ND, Weinreb JC, Morgan GR, Diflo T, John D, Teperman LW, Goldenberg AS. Transplantation for hepatocellular carcinoma and cirrhosis: sensitivity of magnetic resonance imaging. Liver Transpl. 2002;8:1156–1164. doi: 10.1053/jlts.2002.35670. [DOI] [PubMed] [Google Scholar]
- 21.Ayuso C, Rimola J, García-Criado A. Imaging of HCC. Abdom Imaging. 2012;37:215–230. doi: 10.1007/s00261-011-9794-x. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 23.Yoon SH, Lee JM, So YH, Hong SH, Kim SJ, Han JK, Choi BI. Multiphasic MDCT enhancement pattern of hepatocellular carcinoma smaller than 3 cm in diameter: tumor size and cellular differentiation. AJR Am J Roentgenol. 2009;193:W482–W489. doi: 10.2214/AJR.08.1818. [DOI] [PubMed] [Google Scholar]
- 24.Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 25.Kudo M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J Gastroenterol. 2009;44 Suppl 19:112–118. doi: 10.1007/s00535-008-2274-6. [DOI] [PubMed] [Google Scholar]
- 26.Matsui O. Detection and characterization of hepatocellular carcinoma by imaging. Clin Gastroenterol Hepatol. 2005;3:S136–S140. doi: 10.1016/s1542-3565(05)00707-x. [DOI] [PubMed] [Google Scholar]
- 27.International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 28.Karadeniz-Bilgili MY, Braga L, Birchard KR, Gerber D, Firat Z, Woosley JT, Shrestha R, Semelka RC. Hepatocellular carcinoma missed on gadolinium enhanced MR imaging, discovered in liver explants: retrospective evaluation. J Magn Reson Imaging. 2006;23:210–215. doi: 10.1002/jmri.20491. [DOI] [PubMed] [Google Scholar]
- 29.Quaia E, De Paoli L, Pizzolato R, Angileri R, Pantano E, Degrassi F, Ukmar M, Cova MA. Predictors of dysplastic nodule diagnosis in patients with liver cirrhosis on unenhanced and gadobenate dimeglumine-enhanced MRI with dynamic and hepatobiliary phase. AJR Am J Roentgenol. 2013;200:553–562. doi: 10.2214/AJR.12.8818. [DOI] [PubMed] [Google Scholar]
- 30.Lim JH, Kim EY, Lee WJ, Lim HK, Do YS, Choo IW, Park CK. Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983–993. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 31.Lim JH, Kim EY, Lee WJ, Lim HK, Do YS, Choo IW, Park CK. Regenerative nodules in liver cirrhosis: findings at CT during arterial portography and CT hepatic arteriography with histopathologic correlation. Radiology. 1999;210:451–458. doi: 10.1148/radiology.210.2.r99fe04451. [DOI] [PubMed] [Google Scholar]
- 32.Krinsky GA, Lee VS. MR imaging of cirrhotic nodules. Abdom Imaging. 2000;25:471–482. doi: 10.1007/s002610000015. [DOI] [PubMed] [Google Scholar]
- 33.Krinsky GA, Israel G. Nondysplastic nodules that are hyperintense on T1-weighted gradient-echo MR imaging: frequency in cirrhotic patients undergoing transplantation. AJR Am J Roentgenol. 2003;180:1023–1027. doi: 10.2214/ajr.180.4.1801023. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Krinsky GA. Iron-containing nodules of cirrhosis. NMR Biomed. 2004;17:459–464. doi: 10.1002/nbm.926. [DOI] [PubMed] [Google Scholar]
- 35.Yu JS, Lee JH, Park MS, Kim KW. Hyperintense nodules on non-enhanced T1-weighted gradient-echo magnetic resonance imaging of cirrhotic liver: fate and clinical implications. J Magn Reson Imaging. 2006;24:630–636. doi: 10.1002/jmri.20674. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu A, Ito K, Sasaki K, Hayashida M, Tanabe M, Shimizu K, Matsunaga N. Small hyperintense hepatic lesions on T1-weighted images in patients with cirrhosis: evaluation with serial MRI and imaging features for clinical benignity. Magn Reson Imaging. 2007;25:1430–1436. doi: 10.1016/j.mri.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 37.Yu JS, Chung JJ, Kim JH, Kim KW. Fat-containing nodules in the cirrhotic liver: chemical shift MRI features and clinical implications. AJR Am J Roentgenol. 2007;188:1009–1016. doi: 10.2214/AJR.06.0756. [DOI] [PubMed] [Google Scholar]
- 38.Hanna RF, Aguirre DA, Kased N, Emery SC, Peterson MR, Sirlin CB. Cirrhosis-associated hepatocellular nodules: correlation of histopathologic and MR imaging features. Radiographics. 2008;28:747–769. doi: 10.1148/rg.283055108. [DOI] [PubMed] [Google Scholar]
- 39.Krinsky GA, Lee VS, Nguyen MT, Rofsky NM, Theise ND, Morgan GR, Teperman LW, Weinreb JC. Siderotic nodules in the cirrhotic liver at MR imaging with explant correlation: no increased frequency of dysplastic nodules and hepatocellular carcinoma. Radiology. 2001;218:47–53. doi: 10.1148/radiology.218.1.r01ja4047. [DOI] [PubMed] [Google Scholar]
- 40.Lim JH, Choi D, Cho SK, Kim SH, Lee WJ, Lim HK, Park CK, Paik SW, Kim YI. Conspicuity of hepatocellular nodular lesions in cirrhotic livers at ferumoxides-enhanced MR imaging: importance of Kupffer cell number. Radiology. 2001;220:669–676. doi: 10.1148/radiol.2203001777. [DOI] [PubMed] [Google Scholar]
- 41.Ward J, Robinson PJ. How to detect hepatocellular carcinoma in cirrhosis. Eur Radiol. 2002;12:2258–2272. doi: 10.1007/s00330-002-1450-y. [DOI] [PubMed] [Google Scholar]
- 42.Hussain SM, Zondervan PE, IJzermans JN, Schalm SW, de Man RA, Krestin GP. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics. 2002;22:1023–1036; discussion 1037-1039. doi: 10.1148/radiographics.22.5.g02se061023. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita Y, Mitsuzaki K, Yi T, Ogata I, Nishiharu T, Urata J, Takahashi M. Small hepatocellular carcinoma in patients with chronic liver damage: prospective comparison of detection with dynamic MR imaging and helical CT of the whole liver. Radiology. 1996;200:79–84. doi: 10.1148/radiology.200.1.8657948. [DOI] [PubMed] [Google Scholar]
- 44.Oi H, Murakami T, Kim T, Matsushita M, Kishimoto H, Nakamura H. Dynamic MR imaging and early-phase helical CT for detecting small intrahepatic metastases of hepatocellular carcinoma. AJR Am J Roentgenol. 1996;166:369–374. doi: 10.2214/ajr.166.2.8553950. [DOI] [PubMed] [Google Scholar]
- 45.Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Okazaki N, Takayasu K, Kosuge T, Motoo Y, Yamazaki S, Hasegawa H. Malignant transformation of adenomatous hyperplasia to hepatocellular carcinoma. Lancet. 1990;336:1150–1153. doi: 10.1016/0140-6736(90)92768-d. [DOI] [PubMed] [Google Scholar]
- 46.Goshima S, Kanematsu M, Matsuo M, Kondo H, Kato H, Yokoyama R, Hoshi H, Moriyama N. Nodule-in-nodule appearance of hepatocellular carcinomas: comparison of gadolinium-enhanced and ferumoxides-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2004;20:250–255. doi: 10.1002/jmri.20100. [DOI] [PubMed] [Google Scholar]
- 47.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Hwang SH, Yu JS, Kim KW, Kim JH, Chung JJ. Small hypervascular enhancing lesions on arterial phase images of multiphase dynamic computed tomography in cirrhotic liver: fate and implications. J Comput Assist Tomogr. 2008;32:39–45. doi: 10.1097/RCT.0b013e318064c76b. [DOI] [PubMed] [Google Scholar]
- 49.Piana G, Trinquart L, Meskine N, Barrau V, Beers BV, Vilgrain V. New MR imaging criteria with a diffusion-weighted sequence for the diagnosis of hepatocellular carcinoma in chronic liver diseases. J Hepatol. 2011;55:126–132. doi: 10.1016/j.jhep.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 50.Kelekis NL, Semelka RC, Worawattanakul S, de Lange EE, Ascher SM, Ahn IO, Reinhold C, Remer EM, Brown JJ, Bis KG, et al. Hepatocellular carcinoma in North America: a multiinstitutional study of appearance on T1-weighted, T2-weighted, and serial gadolinium-enhanced gradient-echo images. AJR Am J Roentgenol. 1998;170:1005–1013. doi: 10.2214/ajr.170.4.9530051. [DOI] [PubMed] [Google Scholar]
- 51.Baron RL, Peterson MS. From the RSNA refresher courses: screening the cirrhotic liver for hepatocellular carcinoma with CT and MR imaging: opportunities and pitfalls. Radiographics. 2001;21 Spec No:S117–S132. doi: 10.1148/radiographics.21.suppl_1.g01oc14s117. [DOI] [PubMed] [Google Scholar]
- 52.Krinsky GA, Lee VS, Theise ND, Weinreb JC, Rofsky NM, Diflo T, Teperman LW. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445–454. doi: 10.1148/radiology.219.2.r01ma40445. [DOI] [PubMed] [Google Scholar]
- 53.Yamashita Y, Fan ZM, Yamamoto H, Matsukawa T, Yoshimatsu S, Miyazaki T, Sumi M, Harada M, Takahashi M. Spin-echo and dynamic gadolinium-enhanced FLASH MR imaging of hepatocellular carcinoma: correlation with histopathologic findings. J Magn Reson Imaging. 1994;4:83–90. doi: 10.1002/jmri.1880040117. [DOI] [PubMed] [Google Scholar]
- 54.Kim YK, Lee YH, Kim CS, Han YM. Added diagnostic value of T2-weighted MR imaging to gadolinium-enhanced three-dimensional dynamic MR imaging for the detection of small hepatocellular carcinomas. Eur J Radiol. 2008;67:304–310. doi: 10.1016/j.ejrad.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Trevisani F, Caraceni P, Bernardi M, D’Intino PE, Arienti V, Amorati P, Stefanini GF, Grazi G, Mazziotti A, Fornalè L. Gross pathologic types of hepatocellular carcinoma in Italian patients. Relationship with demographic, environmental, and clinical factors. Cancer. 1993;72:1557–1563. doi: 10.1002/1097-0142(19930901)72:5<1557::aid-cncr2820720512>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 56.Kneuertz PJ, Demirjian A, Firoozmand A, Corona-Villalobos C, Bhagat N, Herman J, Cameron A, Gurakar A, Cosgrove D, Choti MA, et al. Diffuse infiltrative hepatocellular carcinoma: assessment of presentation, treatment, and outcomes. Ann Surg Oncol. 2012;19:2897–2907. doi: 10.1245/s10434-012-2336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim S, Kim YK, Park HJ, Lee WJ, Choi D, Park MJ. Infiltrative hepatocellular carcinoma on gadoxetic acid-enhanced and diffusion-weighted MRI at 3.0T. J Magn Reson Imaging. 2014;39:1238–1245. doi: 10.1002/jmri.24265. [DOI] [PubMed] [Google Scholar]
- 58.Park YS, Lee CH, Kim BH, Lee J, Choi JW, Kim KA, Ahn JH, Park CM. Using Gd-EOB-DTPA-enhanced 3-T MRI for the differentiation of infiltrative hepatocellular carcinoma and focal confluent fibrosis in liver cirrhosis. Magn Reson Imaging. 2013;31:1137–1142. doi: 10.1016/j.mri.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Kanematsu M, Semelka RC, Leonardou P, Mastropasqua M, Lee JK. Hepatocellular carcinoma of diffuse type: MR imaging findings and clinical manifestations. J Magn Reson Imaging. 2003;18:189–195. doi: 10.1002/jmri.10336. [DOI] [PubMed] [Google Scholar]
- 60.Halıloğlu N, Özkavukcu E, Erden A, Erden İ. MR imaging in diffuse-type hepatocellular carcinoma with synchronous portal vein thrombi. Turk J Gastroenterol. 2011;22:158–164. doi: 10.4318/tjg.2011.0185. [DOI] [PubMed] [Google Scholar]
- 61.Rosenkrantz AB, Lee L, Matza BW, Kim S. Infiltrative hepatocellular carcinoma: comparison of MRI sequences for lesion conspicuity. Clin Radiol. 2012;67:e105–e111. doi: 10.1016/j.crad.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 62.Yu JS, Chung JJ, Kim JH, Cho ES, Kim DJ, Ahn JH, Kim KW. Detection of small intrahepatic metastases of hepatocellular carcinomas using diffusion-weighted imaging: comparison with conventional dynamic MRI. Magn Reson Imaging. 2011;29:985–992. doi: 10.1016/j.mri.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 63.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- 64.Kierans AS, Leonardou P, Hayashi P, Brubaker LM, Elazzazi M, Shaikh F, Semelka RC. MRI findings of rapidly progressive hepatocellular carcinoma. Magn Reson Imaging. 2010;28:790–796. doi: 10.1016/j.mri.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Ebara M, Ohto M, Watanabe Y, Kimura K, Saisho H, Tsuchiya Y, Okuda K, Arimizu N, Kondo F, Ikehira H. Diagnosis of small hepatocellular carcinoma: correlation of MR imaging and tumor histologic studies. Radiology. 1986;159:371–377. doi: 10.1148/radiology.159.2.3008213. [DOI] [PubMed] [Google Scholar]
- 66.Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Arai K, Gabata T, Takashima T, Nakanuma Y, Terada T, Ida M. Adenomatous hyperplastic nodules in the cirrhotic liver: differentiation from hepatocellular carcinoma with MR imaging. Radiology. 1989;173:123–126. doi: 10.1148/radiology.173.1.2550995. [DOI] [PubMed] [Google Scholar]
- 67.Coulam CH, Chan FP, Li KC. Can a multiphasic contrast-enhanced three-dimensional fast spoiled gradient-recalled echo sequence be sufficient for liver MR imaging? AJR Am J Roentgenol. 2002;178:335–341. doi: 10.2214/ajr.178.2.1780335. [DOI] [PubMed] [Google Scholar]
- 68.Kim JE, Kim SH, Lee SJ, Rhim H. Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196:W758–W765. doi: 10.2214/AJR.10.4394. [DOI] [PubMed] [Google Scholar]
- 69.Guo L, Liang C, Yu T, Wang G, Li N, Sun H, Gao F, Liu C. 3 T MRI of hepatocellular carcinomas in patients with cirrhosis: does T2-weighted imaging provide added value? Clin Radiol. 2012;67:319–328. doi: 10.1016/j.crad.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 70.Kondo H, Kanematsu M, Itoh K, Ito K, Maetani Y, Goshima S, Matsuo M, Matsunaga N, Konishi J, Hoshi H, et al. Does T2-weighted MR imaging improve preoperative detection of malignant hepatic tumors? Observer performance study in 49 surgically proven cases. Magn Reson Imaging. 2005;23:89–95. doi: 10.1016/j.mri.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Sapisochin G, Fidelman N, Roberts JP, Yao FY. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl. 2011;17:934–942. doi: 10.1002/lt.22307. [DOI] [PubMed] [Google Scholar]
- 72.Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47–66. doi: 10.1148/radiol.09090021. [DOI] [PubMed] [Google Scholar]
- 73.Coenegrachts K, Orlent H, ter Beek L, Haspeslagh M, Bipat S, Stoker J, Rigauts H. Improved focal liver lesion detection: comparison of single-shot spin-echo echo-planar and superparamagnetic iron oxide (SPIO)-enhanced MRI. J Magn Reson Imaging. 2008;27:117–124. doi: 10.1002/jmri.21247. [DOI] [PubMed] [Google Scholar]
- 74.Nasu K, Kuroki Y, Tsukamoto T, Nakajima H, Mori K, Minami M. Diffusion-weighted imaging of surgically resected hepatocellular carcinoma: imaging characteristics and relationship among signal intensity, apparent diffusion coefficient, and histopathologic grade. AJR Am J Roentgenol. 2009;193:438–444. doi: 10.2214/AJR.08.1424. [DOI] [PubMed] [Google Scholar]
- 75.Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, Taouli B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246:812–822. doi: 10.1148/radiol.2463070432. [DOI] [PubMed] [Google Scholar]
- 76.Wu LM, Xu JR, Lu Q, Hua J, Chen J, Hu J. A pooled analysis of diffusion-weighted imaging in the diagnosis of hepatocellular carcinoma in chronic liver diseases. J Gastroenterol Hepatol. 2013;28:227–234. doi: 10.1111/jgh.12054. [DOI] [PubMed] [Google Scholar]
- 77.Xu PJ, Yan FH, Wang JH, Shan Y, Ji Y, Chen CZ. Contribution of diffusion-weighted magnetic resonance imaging in the characterization of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver. J Comput Assist Tomogr. 2010;34:506–512. doi: 10.1097/RCT.0b013e3181da3671. [DOI] [PubMed] [Google Scholar]
- 78.Xu PJ, Yan FH, Wang JH, Lin J, Ji Y. Added value of breathhold diffusion-weighted MRI in detection of small hepatocellular carcinoma lesions compared with dynamic contrast-enhanced MRI alone using receiver operating characteristic curve analysis. J Magn Reson Imaging. 2009;29:341–349. doi: 10.1002/jmri.21650. [DOI] [PubMed] [Google Scholar]
- 79.Choi SH, Lee JM, Yu NC, Suh KS, Jang JJ, Kim SH, Choi BI. Hepatocellular carcinoma in liver transplantation candidates: detection with gadobenate dimeglumine-enhanced MRI. AJR Am J Roentgenol. 2008;191:529–536. doi: 10.2214/AJR.07.2565. [DOI] [PubMed] [Google Scholar]
- 80.Abu-Alfa A. The impact of NSF on the care of patients with kidney disease. J Am Coll Radiol. 2008;5:45–52. doi: 10.1016/j.jacr.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 81.Hardie AD, Romano PB. The use of T2*-weighted multi-echo GRE imaging as a novel method to diagnose hepatocellular carcinoma compared with gadolinium-enhanced MRI: a feasibility study. Magn Reson Imaging. 2010;28:281–285. doi: 10.1016/j.mri.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 82.Hardie AD, Nance JW, Boulter DJ, Kizziah MK. Assessment of the diagnostic accuracy of T2*-weighted MR imaging for identifying hepatocellular carcinoma with liver explant correlation. Eur J Radiol. 2011;80:e249–e252. doi: 10.1016/j.ejrad.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 83.Hardie AD, Kizziah MK, Rissing MS. Can the patient with cirrhosis be imaged for hepatocellular carcinoma without gadolinium?: Comparison of combined T2-weighted, T2*-weighted, and diffusion-weighted MRI with gadolinium-enhanced MRI using liver explantation standard. J Comput Assist Tomogr. 2011;35:711–715. doi: 10.1097/RCT.0b013e31823421ac. [DOI] [PubMed] [Google Scholar]
- 84.Zech CJ, Reiser MF, Herrmann KA. Imaging of hepatocellular carcinoma by computed tomography and magnetic resonance imaging: state of the art. Dig Dis. 2009;27:114–124. doi: 10.1159/000218343. [DOI] [PubMed] [Google Scholar]
- 85.Kim YK, Kim CS, Kwak HS, Lee JM. Three-dimensional dynamic liver MR imaging using sensitivity encoding for detection of hepatocellular carcinomas: comparison with superparamagnetic iron oxide-enhanced mr imaging. J Magn Reson Imaging. 2004;20:826–837. doi: 10.1002/jmri.20188. [DOI] [PubMed] [Google Scholar]
- 86.Sirlin CB, Hussain HK, Jonas E, Kanematsu M, Min Lee J, Merkle EM, Peck-Radosavljevic M, Reeder SB, Ricke J, Sakamoto M. Consensus report from the 6th International forum for liver MRI using gadoxetic acid. J Magn Reson Imaging. 2014;40:516–529. doi: 10.1002/jmri.24419. [DOI] [PubMed] [Google Scholar]
- 87.Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology. 2010;255:459–466. doi: 10.1148/radiol.10091388. [DOI] [PubMed] [Google Scholar]
- 88.Di Martino M, Marin D, Guerrisi A, Baski M, Galati F, Rossi M, Brozzetti S, Masciangelo R, Passariello R, Catalano C. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the Detection of hepatocellular carcinoma in patients with cirrhosis. Radiology. 2010;256:806–816. doi: 10.1148/radiol.10091334. [DOI] [PubMed] [Google Scholar]
- 89.Golfieri R, Renzulli M, Lucidi V, Corcioni B, Trevisani F, Bolondi L. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to Dynamic MRI in the detection of hypovascular small (≤ 2 cm) HCC in cirrhosis. Eur Radiol. 2011;21:1233–1242. doi: 10.1007/s00330-010-2030-1. [DOI] [PubMed] [Google Scholar]
- 90.Haradome H, Grazioli L, Tinti R, Morone M, Motosugi U, Sano K, Ichikawa T, Kwee TC, Colagrande S. Additional value of gadoxetic acid-DTPA-enhanced hepatobiliary phase MR imaging in the diagnosis of early-stage hepatocellular carcinoma: comparison with dynamic triple-phase multidetector CT imaging. J Magn Reson Imaging. 2011;34:69–78. doi: 10.1002/jmri.22588. [DOI] [PubMed] [Google Scholar]
- 91.Kim SH, Kim SH, Lee J, Kim MJ, Jeon YH, Park Y, Choi D, Lee WJ, Lim HK. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2009;192:1675–1681. doi: 10.2214/AJR.08.1262. [DOI] [PubMed] [Google Scholar]
- 92.Sun HY, Lee JM, Shin CI, Lee DH, Moon SK, Kim KW, Han JK, Choi BI. Gadoxetic acid-enhanced magnetic resonance imaging for differentiating small hepatocellular carcinomas (& lt; or =2 cm in diameter) from arterial enhancing pseudolesions: special emphasis on hepatobiliary phase imaging. Invest Radiol. 2010;45:96–103. doi: 10.1097/RLI.0b013e3181c5faf7. [DOI] [PubMed] [Google Scholar]
- 93.Motosugi U, Ichikawa T, Sou H, Sano K, Tominaga L, Muhi A, Araki T. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology. 2010;256:151–158. doi: 10.1148/radiol.10091885. [DOI] [PubMed] [Google Scholar]
- 94.Kobayashi S, Matsui O, Gabata T, Koda W, Minami T, Ryu Y, Kawai K, Kozaka K. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic Acid-enhanced magnetic resonance imaging findings of borderline lesions at high risk for progression to hypervascular classic hepatocellular carcinoma. J Comput Assist Tomogr. 2011;35:181–186. doi: 10.1097/RCT.0b013e3182026f3b. [DOI] [PubMed] [Google Scholar]
- 95.Kumada T, Toyoda H, Tada T, Sone Y, Fujimori M, Ogawa S, Ishikawa T. Evolution of hypointense hepatocellular nodules observed only in the hepatobiliary phase of gadoxetate disodium-enhanced MRI. AJR Am J Roentgenol. 2011;197:58–63. doi: 10.2214/AJR.10.5390. [DOI] [PubMed] [Google Scholar]
- 96.American College of Radiology (ACR) Liver Imaging Reporting and Data System (LI-RADS) [accessed 2014 August 24] Bethesda, MD: National Cancer Institute; 2013. Available from: http://www.acr.org/Quality-Safety/Resources/LIRADS. [Google Scholar]
- 97.Jha RC, Mitchell DG, Weinreb JC, Santillan CS, Yeh BM, Francois R, Sirlin CB. LI-RADS categorization of benign and likely benign findings in patients at risk of hepatocellular carcinoma: a pictorial atlas. AJR Am J Roentgenol. 2014;203:W48–W69. doi: 10.2214/AJR.13.12169. [DOI] [PubMed] [Google Scholar]


