Abstract
Cirrhotic cardiomyopathy is a disease that has only recently been recognised as a definitive clinical entity. In the setting of liver cirrhosis, it is characterized by a blunted inotropic and chronotropic response to stress, impaired diastolic relaxation of the myocardium and prolongation of the QT interval in the absence of other known cardiac disease. A key pathological feature is the persistent over-activation of the sympathetic nervous system in cirrhosis, which leads to down-regulation and dysfunction of the β-adrenergic receptor. Diagnosis can be made using a combination of echocardiography (resting and stress), tissue Doppler imaging, cardiac magnetic resonance imaging, 12-lead electrocardiogram and measurement of biomarkers. There are significant implications of cirrhotic cardiomyopathy in a number of clinical situations in which there is an increased physiological demand, which can lead to acute cardiac decompensation and heart failure. Prior to transplantation there is an increased risk of hepatorenal syndrome, cardiac failure following transjugular intrahepatic portosystemic shunt insertion and increased risk of arrhythmias during acute gastrointestinal bleeding. Liver transplantation presents the greatest physiological challenge with a further risk of acute cardiac decompensation. Peri-operative management should involve appropriate choice of graft and minimization of large fluctuations in preload and afterload. The avoidance of cardiac failure during this period has important prognostic implications, as there is evidence to suggest a long-term resolution of the abnormalities in cirrhotic cardiomyopathy.
Keywords: Cirrhotic cardiomyopathy, Liver transplantation, Diastolic dysfunction, Electrophysiological abnormalities, Perioperative care
Core tip: Cirrhotic cardiomyopathy is characterised by a blunted inotropic and chronotropic response to stress, impaired diastolic relaxation and prolongation of the QT interval. It is only recently that it has been recognised as a definitive clinical entity, and yet it has significant implications in a number of clinical situations in which there is increased physiological demand, which can lead to acute cardiac decompensation and heart failure. Liver transplantation is one such situation, and in this review we discuss criteria for diagnosis, possible methods to limit further deterioration and the perioperative management of these patients.
INTRODUCTION
Cirrhotic cardiomyopathy (CCM) in a patient with chronic liver disease is a clinical entity characterised by a blunted inotropic and chronotropic response to stress, impaired diastolic relaxation and prolongation of the QT interval. These abnormalities occur in the absence of other known cardiac disease, are independent of liver aetiology, and occur to a variable degree in up to 40%-50% of patients with cirrhosis.
Currently, CCM is often undetected, as it is not widely recognised and is largely asymptomatic at rest, with overt heart failure being rare in cirrhosis. This latent cardiomyopathy classically only manifests itself during periods of physiological or pharmacological stress, but it assumes clinical importance in the setting of events that challenge the cardiovascular system. It is associated with an increased risk of hepatorenal syndrome, particularly following sepsis, and insertion of transjugular intrahepatic portosystemic shunts (TIPS) can precipitate acute cardiac failure following the sudden delivery of an increased volume load to the heart. Liver transplantation presents the greatest physiological challenge, with the significant fluctuations in preload and afterload during the peroperative period. Cardiac decompensation at this stage can lead to graft failure, multi-organ failure and death.
Long term however, there is a reversal of the abnormalities evident in cirrhotic cardiomyopathy, with both structural and functional improvements seen by six to twelve months post-transplantation. It is important therefore to recognise and diagnose this condition of impaired cardiac reserve function to minimise the incidence of decompensation during periods of increased demand. Careful patient selection and additional monitoring for invasive procedures pre-transplantation, followed by appropriate graft allocation and tailoring of both anaesthetic and surgical techniques at the time of transplant may improve postoperative outcomes with improved longer-term survival. The key points are summarised in Table 1.
Table 1.
Cirrhotic cardiomyopathy and liver transplantation
| Key points |
| CCM is a latent cardiac dysfunction that is independent of aetiology and may be unmasked during periods of increased cardiovascular demand |
| It is characterised by systolic incompetence to stress, diastolic dysfunction and electrophysiological abnormalities |
| The persistent over-activation of the SNS in cirrhosis leads to down-regulation and dysfunction of the β-adrenergic receptor, a key pathological feature in CCM |
| Clinical implications include an increased risk of hepatorenal syndrome, cardiac failure following TIPS insertion and increased risk of arrhythmias during acute gastrointestinal bleeding |
| Diagnosis can be made using a combination of echocardiography (resting and stress), tissue Doppler imaging, cardiac MRI, 12 lead ECG and measurement of biomarkers |
| Cardiac status should be re-evaluated regularly until liver transplant |
| Peri-operative management at transplantation should involve careful choice of graft and minimisation of large fluctuations in preload and afterload |
| Long term there is a resolution of the abnormalities in CCM |
CCM: Cirrhotic cardiomyopathy; SNS: Sympathetic nervous system; TIPS: Transjugular intrahepatic portosystemic shunts; ECG: Electrocardiogram.
CLINICAL FEATURES
Cirrhotic cardiomyopathy is characterised by an impaired contractile response to stress, diastolic dysfunction and electrophysiological abnormalities. It is a spectrum of cardiological impairment that has its origin in the haemodynamic changes that accompany end stage liver disease (ESLD). The definition and diagnostic criteria were defined by an international expert consensus committee at the World Congress of Gastroenterology in 2005[1] (Table 2).
Table 2.
2005 World Congress of Gastroenterology diagnostic and supportive criteria for cirrhotic cardiomyopathy[1]
| A working definition of cirrhotic cardiomyopathy |
| A cardiac dysfunction in patients with cirrhosis characterised by impaired contractile responsiveness to stress and/or altered diastolic relaxation with electrophysiological abnormalities in the absence of other known cardiac disease |
| Diagnostic criteria |
| Systolic dysfunction |
| Blunted increase in cardiac output on exercise, volume challenge or pharmacological stimuli |
| Resting LVEF < 55% |
| Diastolic dysfunction |
| E/A ratio < 1 (age-corrected) |
| Prolonged deceleration time (> 200 ms) |
| Prolonged isovolumetric relaxation time (> 80 ms) |
| Supportive criteria |
| Electrophysiological abnormalities |
| Abnormal chronotropic response |
| Electromechanical uncoupling |
| Prolonged QTc interval |
| Enlarged left atrium |
| Increased myocardial mass |
| Increased BNP (brain natriuretic peptide) and pro-BNP |
| Increased troponinI |
BNP: B-type natriuretic peptide; LVEF: Left ventricular ejection fraction.
Hyperdynamic circulation of cirrhosis
Kowalski et al[2] were the first to report the existence of an altered circulation in 1953, describing an increased cardiac output at rest, with an inverse relationship to systemic vascular resistance. In addition, they noted that the increase in cardiac output was not accompanied by a parallel increase in oxygen consumption and that in one third of their study population, the QT interval was prolonged. Their findings were consistently reproduced in subsequent studies[3-7] and the concept of the “hyperdynamic circulation of cirrhosis” was established.
An altered vascular resistance and redistribution of plasma volume has been implicated as the cause of this hyperdynamic circulation[8]. The increase in intrahepatic resistance due to fibrosis causes hypertension in the portal circulation, which stimulates the release of circulating and endothelial vasodilators (both due to a compensatory release and an impaired hepatic degradation). The resulting peripheral arterial vasodilatation and subsequent volume expansion leads to an initially appropriate response of hyperkinesis in the circulatory system.
Over time, with worsening hepatic dysfunction, the increased volume is redistributed to the splanchnic bed. There is a resultant relative reduction in central blood volume (despite an increase in absolute volume), triggering an activation of the SNS and the renin-angiotensin-aldosterone system in an effort to counteract the low arterial blood pressure and volume reduction. Thus patients with cirrhosis exhibit enhanced activity of the SNS with increased circulating catecholamines in direct relation to the severity of the disease[9,10].
These findings of an increased cardiac output at rest and reduced systemic vascular resistance are not present in all patients with ESLD, with the degree of hyperkinesis correlating with worsening hepatocellular insufficiency and portal hypertension[11].
Systolic dysfunction under stress
Left ventricular ejection fraction (LVEF) at rest in the context of chronic liver disease has been reported to be normal[12,13] and often higher than controls[14]. In patients without ascites, the increased pre-load can compensate for cardiac dysfunction, whereas in patients with ascites, the reduced afterload secondary to the systemic arterial vasodilatation compensates for both a decreased preload and contractile dysfunction[15]. Thus, the majority of patients are asymptomatic for heart failure at rest.
When subjected to physiological[5,6,12-14,16] or pharmacological[17,18] stress however, the increase in contractility and cardiac output is significantly blunted in comparison to matched controls. There is an abnormal ventricular response to an increased ventricular filling pressure, which correlates with the severity of liver disease. During certain treatment interventions therefore, such as liver transplantation and TIPS, the volume and pressure load stresses may be significant enough to overcome the “auto-protection” provided by the low systemic vascular resistance and acutely unveil previously asymptomatic latent cardiac disease.
Diastolic dysfunction
Left ventricular diastolic dysfunction (LVDD) is the most prominent characteristic of cirrhotic cardiomyopathy and is thought to precede systolic dysfunction. Its prevalence has been found to be over 50% in cirrhotic patients[19-21] and can be detected at rest using trans-thoracic echocardiography (TTE).
In the early stages of cirrhosis, the expanded blood volume and subsequent increased cardiac preload causes overloading of the left ventricle (LV) and can lead to impaired contractility. There is a resultant increase in LV mass[21] with decreased compliance and relaxation, resulting in abnormal filling of the ventricle. The presence of LVDD has not been found to correlate with the aetiology of liver disease[22], however, the severity of LVDD correlates with worsening liver disease[22,23]. Its prevalence is higher in patients with ascites[19,21,23] and there is evidence to suggest that paracentesis induces an improvement (Figure 1)[24].
Figure 1.
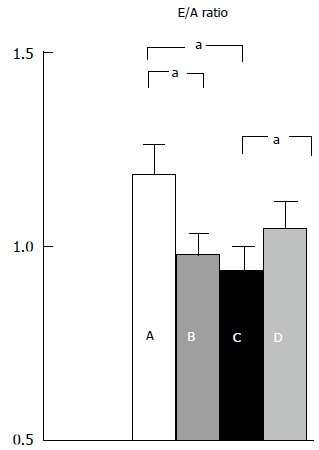
Left ventricular diastolic function in normal subjects (A); cirrhosis (B); cirrhosis with tense ascites (C); cirrhosis with ascites after paracentesis (D). aP < 0.05, E/A ratio: (early peak: late peak filling velocities). Ratio declines with worsening LVDD. Pozzi et al[24].
The presence and severity of LVDD prior to transplantation has been found to be associated with an increased mortality[22,23,25]. Karagiannakis et al[22] evaluated the 2-year probability of patients’ survival classified by the presence of diastolic dysfunction and reported survival rates of patients with and without LVDD to be 88.2% vs 96.4% at 6 mo; 70.6% vs 89.3% at 12 mo; 53% vs 85.7% at 18 mo and 47% vs 82.1% at 24 mo (Figure 2).
Figure 2.
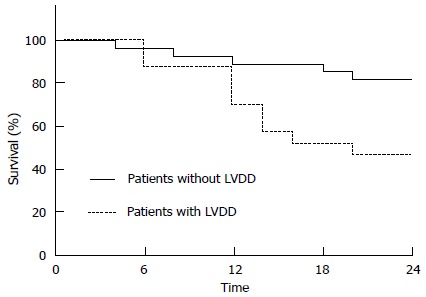
Differences in survival of patients according to the presence of left ventricular diastolic dysfunction. LVDD: Left ventricular diastolic dysfunction. Karagiannakis et al[22].
The difference in survival becomes more significant after the first year of follow-up, which is presumably why some shorter studies cannot elucidate a trend. In addition, survival is also related to the severity of LVDD. Ruíz-del-Árbol et al[23] reported that after a twelve month follow up period from baseline investigations, survival was 95% in those without LVDD, 79% in those with grade 1 LVDD and 39% in those with grade 2 LVDD.
Electrophysiological abnormalities
Three main electrophysiological abnormalities exist in cirrhotic cardiomyopathy: (1) Prolongation of the QT interval; (2) electromechanical dyssynchrony; and (3) chronotropic incompetence.
A prolonged QTc interval (> 440 ms) is reported to be present in up to 50% of patients with cirrhosis. It is not related to aetiology of liver disease, worsens in parallel with the severity of disease (Figure 3) and may be associated with a reduced survival[26,27]. This abnormality is also frequently prolonged in non-cirrhotic patients with portal hypertension[28].
Figure 3.
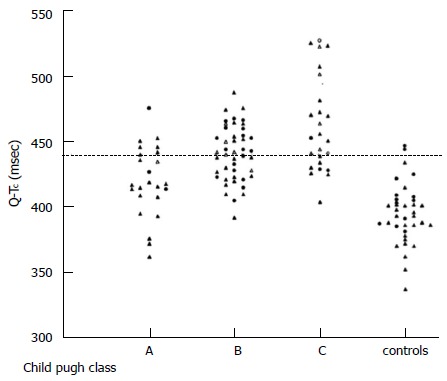
Individual values of QTc interval in patients with cirrhosis (divided according to Child-Pugh classes) and controls. Bernardi et al[26].
The QT interval represents length of ventricular electric systole and a prolongation can lead to severe ventricular arrhythmias, syncope and sudden death.
The coupling of electrical depolarisation to ventricular contraction is also abnormal in cirrhosis. Henriksen and colleagues[27] found this electromechanical dyssynchrony to be greater in patients with a prolonged QTc interval, which in turn was related to systemic circulatory dysfunction. The resultant effect of this abnormality is a further impairment of cardiac contractility.
Finally, in CCM there is an impaired ability to increase heart rate in response to activation of the sympathetic nervous system and an increased demand in cardiac output[13,16,29,30]. The decrease in heart rate variability has been found to be associated with increasing severity of cirrhosis and additionally to poor prognosis and increased mortality[31]. It represents an impaired ability to maintain cardiac output at a level that matches cellular demands.
Cardiac autonomic dysfunction
Autonomic dysfunction is common in cirrhosis and is characterised by an increase in baseline sympathetic nervous system (SNS) activity[10] and decreased baroreflex sensitivity[32]. The baroreceptor and volume receptor stimulation caused by the low arterial blood pressure and blood volume drives this over-activity, causing an increased cellular exposure to noradrenaline and an eventual impairment in β-adrenergic function[9]. These changes play an important role in the development of the hyperdynamic circulation and its adverse effects.
PATHOPHYSIOLOGY
Multiple pathogenic mechanisms have been implicated in the development of cirrhotic cardiomyopathy[33,34]. The diminished inotropic and chronotropic responses to sympathetic stimulation are mainly secondary to a significant down-regulation (reduced density and function) of the β-adrenergic receptors[35] following long-term exposure to persistently high levels of catecholamines[36].
In addition, there is a decrease in the fluidity of the cardiomyocyte plasma membrane caused by an accumulation of cholesterol and bile acid. This adversely affects the receptor-agonist interaction of not only the β-adrenergic receptors[37], but also the other protein receptors embedded in the plasma membrane, ultimately having the effect of compromising cardiomyocyte activation.
There is also increased activity of the cardiac inhibitory systems in cirrhosis, involving substances such as nitric oxide (NO)[38], carbon monoxide (CO)[39] and endogenous cannabinoids (endocannabinoids)[40,41]. The resultant effect is negative inotropy, which further exacerbates the cardiac dysfunction in cirrhotic cardiomyopathy.
DIAGNOSIS
There is no single diagnostic test that can identify patients with cirrhotic cardiomyopathy and predict who will develop postoperative complications[42,43]. Furthermore, there is no comprehensive data that exists to guide the preoperative cardiac assessment of patients undergoing consideration for liver transplantation.
Diastolic dysfunction
Two-dimensional transthoracic echocardiography: A number of studies to date have used the E/A ratio to determine the presence of diastolic dysfunction, with a ratio < 1 being considered as a positive finding[15,24,44,45]. It represents the pattern of blood flow through the mitral valve. Under normal conditions, early rapid passive filling causes a peak in the transmitral flow profile, known as the E wave, and late rapid filling due to atrial contraction results in a second smaller peak known as the A wave. In the presence of diastolic dysfunction, early passive filling (E wave) is reduced due to an increasingly non-compliant left ventricle, with a greater contribution to filling from atrial contraction during the late phase of diastole (represented by an abnormally large A wave). The overall effect is a reduction in the E wave to A wave ratio.
The E/A ratio may be insufficient as a single parameter to characterise LVDD as it can normalise despite increasing severity of dysfunction. As LV stiffness increases and impairs passive filling, left atrial pressure rises (along with increasing LA dilatation) and eventually drives the filling of the ventricle in early diastole, thereby increasing the E wave. This initially restores the E/A ratio to the normal range, with eventual elevation to supra-normal values. Other features of the TTE are subsequently used to supplement the evaluation of LV relaxation, such as the deceleration time (DT) and isovolumetric relaxation time (IVRT), both of which are initially prolonged, then shortened with increasing severity of dysfunction. Interventricular septal and posterior wall thickness have also been found to be increased in LVDD[14,15,24,46].
In the general population left ventricular stiffness and hence diastolic dysfunction increase with age, so interpretation of TTE findings should be made in context of this variable[47].
Tissue Doppler imaging: The parameters that are measured on conventional two-dimensional (2D) ECHO are dependent on cardiac loading conditions and describe fluid movements rather than tissue dynamics. Tissue Doppler echocardiography has now been suggested as a more accurate modality to assess for diastolic dysfunction[8,47].
Cardiac MRI: Cardiovascular magnetic resonance (CMR) has become the gold standard method for assessing function and morphology in diseases of the myocardium[48], and is of particular use in obese patients where TTE may be suboptimal. It allows repeated evaluation of disease course and detection of subclinical changes prior to dysfunction. The addition of gadolinium contrast (late gadolinium enhancement) has allowed analysis of the intercellular matrix in post-infarct scars, non-ischaemic cardiomyopathies and amyloidosis[48,49]. This ability to analyse myocardial infiltration and fibrosis has diagnostic potential in the early recognition of cirrhotic cardiomyopathy.
Systolic incompetence
Dobutamine and exercise stress echocardiography: In addition to assessing for inducible ischaemia secondary to coronary artery disease, stress echocardiography can be used to detect underlying chronotropic and inotropic incompetence under conditions of increased cardiovascular demand. They are useful screening tools prior to transplantation, but the criteria for undertaking these tests remains varied between institutions.
In a review of 157 pre-transplant dobutamine stress echocardiographys (DSEs), Umphrey et al[50] found 37% to be inconclusive due to failure to reach 85% of their maximum predicted heart rate.
Furthermore, the inability to achieve > 82% of this target correlated with an increased risk of having an adverse cardiovascular event up to 4 mo post-OLT. Another group found the incidence of chronotropic incompetence on DSE in cirrhosis to be over 85%[51], when defined by a heart rate reserve of less than 80%. No patient had taken medication that had an effect on haemodynamics until the study was completed. In contrast to this, out of the 58 patients with an inconclusive test in the previous study, 50% had taken a beta-blocker within 12 h of the investigation.
Since beta blockade is responsible for moderation of the heart rate, it raises the question as to whether or not patients undergoing DSE, in part to assess for chronotropic incompetence, should have this drug withheld for 24 h prior to this investigation to enable a more accurate and comparable evaluation of their underlying cardiac reserve function.
STRAIN AND STRAIN RATE IMAGING BY ECHOCARDIOGRAPHY
Strain imaging using either tissue doppler or myocardial speckle tracking and strain rate imaging are becoming increasingly recognised as valuable non-invasive tools for a more comprehensive and reliable assessment of myocardial function[52]. These modalities can detect early changes in systolic function at rest. The long axis sub-endocardial fibres are thought to be more susceptible to damage than the radial-orientated ones in the midwall[20] so may convey the first manifestation of cardiac impairment. In addition, peak systolic strain rate has been found to be more closely related to contractility than ejection fraction determined by TTE[52].
Electrophysiological abnormalities
Twelve lead Electrocardiogram: Prolongation of the QT interval, when corrected for heart rate (QTc) is commonly quoted to be > 440 ms. In normal subjects, QT interval is also affected by age and gender, with the length being longer in older subjects and females. However, in cirrhosis this gender difference is abolished[53].
Biomarkers
Atrial natriuretic peptide (ANP) is secreted in response to atrial stretch, with the resultant effect of lowering blood pressure and cardiac preload. It is stored as pro-ANP, which is cleaved to ANP and NT-proANP, the latter of which is thought to be a better marker of LV dysfunction[54,55]. ANP levels reflect volume overload and are increased in patients with ascites[46].
B-type natriuretic peptide (BNP) is similarly cleaved from its pro-peptide and released from ventricular myocytes in response to ventricular wall stretch or myocardial ischaemia[56], with the primary purpose of reducing cardiac hypertrophy and fibrosis[57]. Increased concentrations have been detected in cirrhosis, with levels correlating with the severity of liver disease, presence of diastolic dysfunction, myocardial hypertrophy and survival[46,49,58,59]. Preoperative levels of BNPs (BNP and NT-proBNP) are powerful independent predictors of cardiovascular events and mortality in patients undergoing non-cardiac surgery[60,61], with further enhancement of this risk stratification seen with the additional measurement of postoperative levels[62,63].
High sensitivity Troponin T (hs-TnT) is also increased in decompensated cirrhosis with evidence emerging for it to be a strong independent prognostic marker for survival. Wiese et al[64] found that the risk of dying within 1 year predicted by the MELD score is increased by a factor of 1.6 if the hsTnT is 4-8 ng/L and by a factor of 2.7 if hsTnT is more than 8 ng/L (Figure 4).
Figure 4.
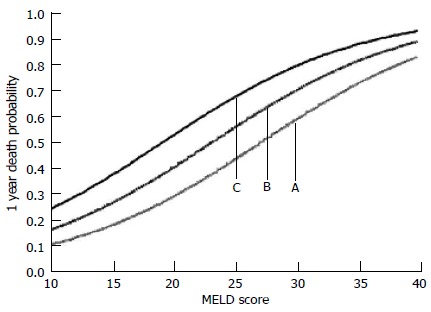
Estimated 1 year probability of dying as a function of: (A) MELD score alone; (B) MELD score and hs-TnT of 4-8 ng/L; and (C) MELD score and hs-TnT of > 8 ng/L. Taken from Wiese et al[64].
Proinflammatory markers: Inflammatory activation has been shown to aggravate the circulatory failure and worsen the prognosis in cirrhosis. There is evidence to show that levels of soluble urokinase-type plasminogen activator receptor (suPAR) and high-sensitive C-reactive protein (hsCRP) are potential markers of this interaction. Levels have been found to correlate significantly with Child class and haemodynamic derangement[64].
Currently, the commonly used scoring systems for assessment of severity of liver disease such as MELD and Child Pugh do not incorporate the circulatory or inflammatory state. Therefore, these markers may in the future enable a more comprehensive assessment of the liver transplant candidate.
CLINICAL IMPLICATIONS
Pre-transplant
TIPS: Prior to liver transplantation, additional events that take place in the clinical course of a patient with end stage liver disease may be impacted on by the presence of cirrhotic cardiomyopathy. In particular, diastolic dysfunction may become clinically problematic following procedures that involve a sudden volume load to a stiff, non-compliant ventricle.
One such procedure is the insertion of a TIPS, in which a high splanchnic blood flow is rapidly delivered to the systemic circulation, resulting in an almost two fold increase in central pressures and pulmonary capillary wedge pressure[65,66]. This not only aggravates the hyperdynamic circulation, with a sustained rise in cardiac output for up to 1 year post procedure[49], but also several cases of heart failure following TIPS have been reported[67-69]. In addition, there is evidence to suggest that those patients with diastolic dysfunction defined by an E/A ratio ≤ 1 have a lower post-TIPS ascites clearance and probability of survival at one year than those without[44,45] (Figures 5 and 6).
Figure 5.
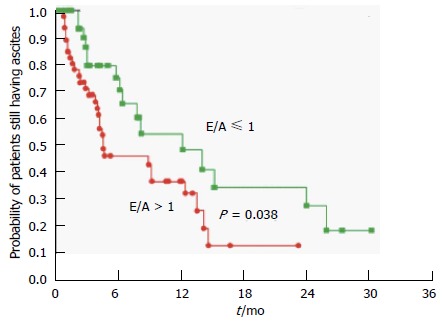
Probability of patients still having ascites after transjugular intrahepatic portosystemic shunts insertion in the group with diastolic dysfunction (E/A ≤ 1) and the group without (E/A > 1). Rabie et al[45].
Figure 6.
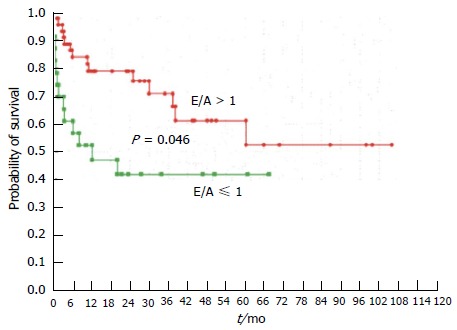
Probability of survival in patients with (E/A ≤ 1) or without (E/A > 1) diastolic dysfunction. Rabie et al[45].
The non-compliant left ventricle renders the patient less able to adequately increase their cardiac output in response to the sudden volume load which, in addition to the worsening of the vasodilation following the procedure, results in a relative under-filling of the central blood volume and poor clearance of the ascites. The dumping of cardioactive substances from the splanchnic to systemic circulation post-TIPS also causes a further prolongation of the QT interval, which worsens post procedure at 1-3 mo and stays elevated above the baseline value[28].
The above findings suggest that careful patient selection for TIPS is needed and a consideration of referral directly to transplantation may be warranted in those with diastolic dysfunction. If TIPS is undertaken, increased cardiac monitoring post-procedure may be beneficial in those patients with suspected cirrhotic cardiomyopathy, for early recognition and treatment of potential cardiovascular complications.
Hepatorenal syndrome
This is a functional renal failure that occurs in the presence of liver dysfunction, splanchnic vasodilatation and intense activation of the endogenous vasoconstrictor systems, with important prognostic implications in a patient with decompensated cirrhosis. There is increasing evidence to suggest that inotropic and chronotropic insufficiency in CCM may have a role in the progression to hepatorenal syndrome (HRS). Krag et al[70] demonstrated that the number of cirrhotic patients with ascites who developed HRS type 1 within three months of initial assessment was higher in the group with a low cardiac index (< 1.5 L/min per square meter) than in the high cardiac index group (43% vs 5% respectively). Furthermore, patients with the lowest cardiac index at baseline had significantly poorer survival at 3, 9 and 12 mo.
Likewise a cardiac output < 6.0 L/min was found to be an independent predictor of HRS in a group of patients with cirrhosis and tense ascites who, at baseline had normal creatinine levels (Figure 7)[71]. The implication is that the patients who are unable to maintain cardiac output in the presence of systemic arterial vasodilatation at a level that maintains a sufficient renal perfusion pressure are predisposed to the development of renal dysfunction and HRS.
Figure 7.
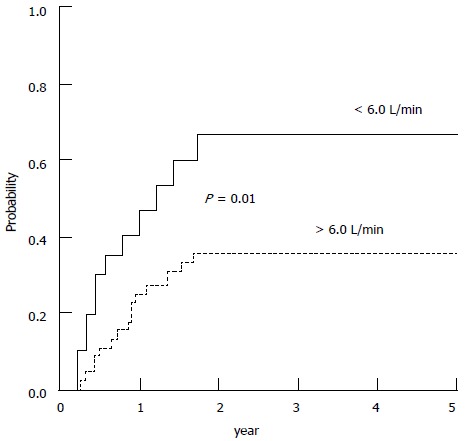
Probability of developing hepatorenal syndrome during follow-up in patients with baseline cardiac output higher and lower than 6 L/min. Ruiz-del-Arbol et al[71].
Spontaneous bacterial peritonitis
The susceptibility of a patient with a decreased cardiac reserve to further organ impairment becomes more pronounced in the face of systemic infection. In a study of patients with a diagnosis of spontaneous bacterial peritonitis (SBP), those that subsequently developed renal failure had a reduced cardiac output both at the time of infection diagnosis and at infection resolution[72]. If the additional physiological demands caused by the inflammatory mediated vasodilatation and negative inotropy could not be met, a rapidly progressive impairment of systemic haemodynamics followed, with severe renal and hepatic failure, aggravation of portal hypertension, encephalopathy and death.
Gastrointestinal bleeding
QT prolongation in cirrhosis is common and one of the postulated causes is the baseline sympathetic nervous system hyperactivity. Therefore, a sudden increase in this activity caused by acute gastrointestinal bleeding further lengthens this time interval with potential adverse outcomes. Trevisani et al[73] showed that in a group of cirrhotic patients with this complication, the QT interval lengthened at the time of bleeding and all those that died had a longer QTc than survivors. In addition, QTc was able to independently predict survival, with a best cut-off value of ≥ 460 ms.
IMPLICATIONS OF DIAGNOSIS AND MANAGEMENT
When a patient displays features of CCM, this is an indication to perform more frequent cardiovascular reassessment, with regular TTE studies perhaps every three months prior to transplantation. Deterioration can then be detected earlier, enabling prompt management of symptomatic heart failure, appropriate cardiovascular monitoring at the time of TIPs or acute gastrointestinal bleeding and appropriate fluid therapy and circulatory support on diagnosis of SBP.
Positive diagnosis may also affect the decision to proceed with TIPS, as underlying CCM has been associated with not only suboptimal ascites clearance and LV dysfunction, but also increased mortality. In this situation the decision may be to refer straight to transplantation.
At the time of transplantation, the presence of an underlying latent cardiac failure may also affect the choice of graft. Mittal et al[74] followed up 970 liver transplant recipients over an average of 5.3 years, and found that those with pre-transplant diastolic dysfunction were significantly more likely than those without to develop acute cellular rejection (58.6% vs 31% respectively), graft failure and all-cause mortality, making the initial quality of graft ever more important (Figures 8 and 9).
Figure 8.
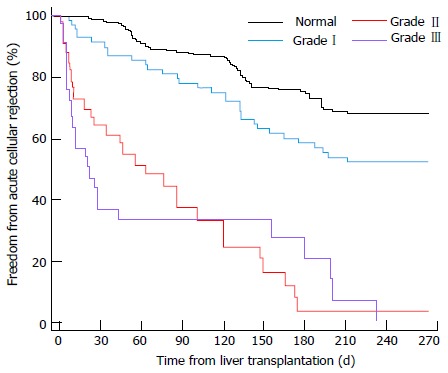
Time to acute rejection for various grades of left ventricular diastolic dysfunction. Mittal et al[74].
Figure 9.
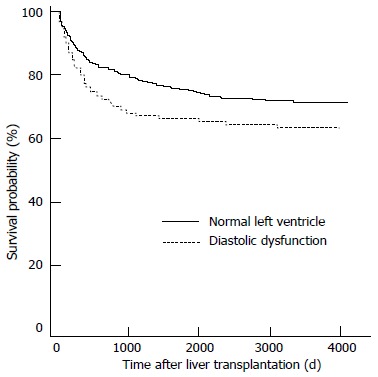
Survival analysis for patients with left ventricular diastolic dysfunction vs patients without. Mittal et al[74].
Management
There is currently no specific treatment for cirrhotic cardiomyopathy. Patients are managed as for heart failure due to other aetiologies, with sodium restriction, diuretics and careful management of their preload and afterload. Vasodilators such as ACE inhibitors are avoided as they exacerbate the reduced systemic vascular resistance.
Many patients with cirrhosis are beta-blocked for the management of portal hypertension. It is still unclear what impact this has on CCM, but the additional negative inotropic and chronotropic effects may have an important role in the development of and outcome from adverse cardiac sequelae during periods of increased physiological demand. On the other hand, both the acute and chronic administration of beta-blockers have been shown to shorten the QT interval in cirrhotic patients who have elevated baseline values[75,76]. Although the improved QT interval potentially has preventative benefits in the context of life-threatening arrhythmias, the deleterious effect of beta blockade on cardiac output and hence clinical outcome remains to be studied.
Long-term aldosterone blockade may also have potential therapeutic benefits. In preliminary studies it has been shown to reduce left ventricular wall thickness and may potentially improve diastolic dysfunction[77].
INTRAOPERATIVE
Maintaining preload and fluid management
During liver transplantation there are significant haemodynamic fluctuations and immense physiological demands on the recipient. The third space losses over the long procedure can be copious with the added effect of substantial blood loss and on-going ascites production[43]. If a patient has cirrhotic cardiomyopathy, an important intraoperative aim should be to minimise excessive fluctuations in preload and afterload, to avoid acute cardiac decompensation.
Surgical technique can greatly influence physiological status, with cross clamping of the inferior vena cava causing the greatest cardiovascular instability. In a patient with cirrhotic cardiomyopathy the use of caval preservation techniques (piggyback), or the addition of veno-venous bypass if caval cross clamp is employed, will minimise the large variations in preload with the maintenance of caval flow during explantation[78,79]. This would decrease the reliance on positive chronotropy to maintain cardiac output in the face of reduced venous return, a compensation mechanism that may be impaired in CCM. It could also attenuate the sudden change in preload on reperfusion of the new liver, which can precipitate acute left ventricular failure and pulmonary oedema if diastolic dysfunction is present.
Reperfusion also involves the release of cold, ischaemic metabolites and vasoactive mediators from the graft that can further exacerbate systemic vasodilatation and cause myocardial depression. An impaired contractile response to stress may render the patient unable to overcome these effects and result in an inadequate cardiac output for tissue perfusion, including the newly implanted liver. Techniques to minimise reperfusion “hit” are especially important in the context of CCM, including normalising acid base and electrolyte status prior to reperfusion, and the use of as good quality grafts as possible.
Haemodynamic monitoring
In light of the special considerations in CCM during OLT, additional haemodynamic monitoring may be warranted for the early detection of cardiac decompensation and to guide therapy. Both aggressive fluid administration and excessive vasoconstriction can precipitate acute cardiac failure in this group, so careful titration of inotropic support and the avoidance of excess intravenous fluids are necessary.
The use of pulmonary artery flotation catheters (PAFC) enables measurement of continuous cardiac output and the derivation of systemic vascular resistance and hence afterload. In addition many PAFCs allow measurement of right ventricular end diastolic pressures and volume. Other methods of continuous non-invasive cardiac output (CO) monitoring are less accurate, unless recalibrated at times when there are significant changes in CO, i.e., after caval cross clamping and following reperfusion.
Transesophageal echocardiography (TOE) is an imaging modality that is becoming increasingly popular in evaluating intraoperative haemodynamic status during liver transplantation[80]. It provides the added benefit of real time assessment of the response to fluid, direct visualisation of global and regional myocardial performance and detection of air and thromboembolism[81]. In addition, it can allow an earlier visual detection of right ventricular failure, as the substantial compliance of the right ventricle allows significant dilation before any pressure changes that would be detected with a PAFC[82]. It also permits re-evaluation of the recipient’s cardiac function at the start of surgery, which would highlight deterioration since previous assessment. A limiting factor over its use however, is the training required for competent scanning and interpretation of images[83]. There is also the small risk of bleeding from oesophageal varices.
Both PAFC and TOE have important advantages in the guidance of therapy for the CCM patient. The choice between the two remains operator dependent and reliant on skill base and experience.
Electrolytes/arrhythmias
During liver transplantation, there can be further prolongation of the QT interval with the associated increased risk of ventricular arrhythmias. One study reported that 54% of recipients showed a marked prolongation of QTc (≥ 500 msec) during the anhepatic phase, with values remaining prolonged at each stage compared to baseline[84]. 77% of the patients with a pre-operative increased QTc and 36% of the group with a normal starting value showed this abnormality.
Electrolyte imbalance is common during this procedure and can further precipitate cardiac instability. Ionised hypocalcaemia can occur as a result of citrate toxicity following the transfusion of large amounts of citrated blood products causing further depression of myocardial contractility. Reperfusion of the donor liver can acutely raise serum potassium to levels that can potentially result in fatal arrhythmias. Additionally, hypomagnesaemia has been shown to precipitate cardiac arrhythmias that are refractory to conventional treatment and can also lead to hypokalaemia and hypocalcaemia[85]. Careful monitoring of these electrolytes and judicious replacement should be employed to minimise further cardiac instability in a condition with pre-existing limited cardiac reserve.
POST OPERATIVE COURSE
Immediately after liver transplantation there is a significant increase in blood pressure and peripheral vascular resistance with restoration of normal liver function and portal pressure. This increase in preload and afterload can be another stage at which cardiac de-compensation in CCM occurs, typically in the first week after OLT. Various authors have reported cases of pulmonary oedema following this procedure, a complication that can impair gas exchange and stress critical oxygen delivery to the new organ[86,87], with incidences being reported at between 30% and 50%[88-90].
Peri-transplant heart failure and myocardial depression have been found to correlate with both a preoperative impaired cardiac reserve function and increased morbidity and mortality post-operatively[25,91]. Josefsson et al[25] found it to be associated with a longer ITU stay (14.5 d vs 4 d), a longer inpatient stay (33 d vs 21 d), a higher peri-transplant infection rate (42% vs 19.9%) and higher long term patient and graft mortalities (Figure 10).
Figure 10.
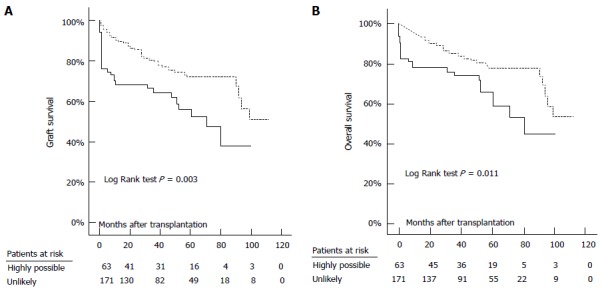
Graft (A) and overall (B) survival in patients with highly possible (continuous line) and those with unlikely (dashed line) peri-transplant heart failure. Josefsson et al[25].
In a search to identify predictors of heart failure after liver transplantation, Dowsley et al[92] found that markers of diastolic dysfunction on pre-operative TTE (E/e’ > 10 and LA volume index ≥ 40 mL/m2) were associated with a 3.4 fold and 2.9 fold increase in risk respectively. Furthermore, Kaplan-Meier analysis of LA volume index below and above 40 mL/m2 revealed survival differences of 82% vs 54% at 1 year and 71% vs 50% at 5 years respectively. Therapondos et al[93] found elevated baseline BNP levels in those who went on to develop pulmonary oedema in the first 3 mo after transplant. These pre-transplant markers that are associated with an increased risk of heart failure may aid the prediction of post liver transplant outcomes.
LONG TERM POST OLT
After transplantation, there is a triphasic course of myocardial recovery in CCM. The first phase is the aforementioned perioperative cardiac failure. Over the next few weeks, the restoration of the portal circulation and the hypertensive side effects of the calcineurin inhibitors administered for immunosuppression, result in an additional increase in afterload, potentially leading to further decompensation. LV diastolic function (as measured by E/A and IVRT) has been shown to deteriorate for up to 3 mo post transplant[93].
In the final phase, there appears to be a recovery of functional, structural and electrophysiological abnormalities. Whereas the hyperdynamic syndrome normalizes in some patients over time[94], it persists in others[95]. There is a significant improvement in myocardial performance, with normalization of the systolic response to stress, improvement of diastolic dysfunction and regression of ventricular wall thickness between 6-12 mo after transplantation[30,49]. In addition, there is an improvement of QT prolongation in approximately 50% of patients from as early as three months[96-98].
CONCLUSION
Cirrhotic cardiomyopathy describes a condition of impaired cardiac reserve function. It is characterised by a systolic incompetence to stress, diastolic dysfunction and electrophysiological abnormalities. Although mostly asymptomatic at rest, it can be unveiled during periods of physiological or pharmacological stress and predisposes the patient to complications such as hepatorenal syndrome, acute cardiac failure and life-threatening arrhythmias. Regular re-evaluation of cardiac status should be undertaken to minimise the risk of decompensation. Management at the time of liver transplantation should involve careful selection of donor grafts and tailoring of the anaesthetic and surgical techniques to avoid large fluctuations in preload and afterload. If cardiac failure in the peri-operative period can be avoided, there is potentially a good outcome with long term reversal of the cardiovascular abnormalities.
Footnotes
Conflict-of-interest: The authors of this manuscript have no conflicts of interest to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 11, 2014
First decision: November 3, 2014
Article in press: December 16, 2014
P- Reviewer: Gara N, Petrikovics I, Ramsay M S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;57:268–278. doi: 10.1136/gut.2006.112177. [DOI] [PubMed] [Google Scholar]
- 2.Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec’s cirrhosis. J Clin Invest. 1953;32:1025–1033. doi: 10.1172/JCI102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kontos HA, Shapiro W, Mauck HP, Patterson JL. General and regional circulatory alterations in cirrhosis of the liver. Am J Med. 1964;37:526–535. doi: 10.1016/0002-9343(64)90066-x. [DOI] [PubMed] [Google Scholar]
- 4.Murray JF, Dawson AM, Sherlock S. Circulatory changes in chronic liver disease. Am J Med. 1958;24:358–367. doi: 10.1016/0002-9343(58)90322-x. [DOI] [PubMed] [Google Scholar]
- 5.Caramelo C, Fernandez-Muñoz D, Santos JC, Blanchart A, Rodriguez-Puyol D, López-Novoa JM, Hernando L. Effect of volume expansion on hemodynamics, capillary permeability and renal function in conscious, cirrhotic rats. Hepatology. 1986;6:129–134. doi: 10.1002/hep.1840060125. [DOI] [PubMed] [Google Scholar]
- 6.Gould L, Shariff M, Zahir M, Di Lieto M. Cardiac hemodynamics in alcoholic patients with chronic liver disease and a presystolic gallop. J Clin Invest. 1969;48:860–868. doi: 10.1172/JCI106044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maroto A, Ginès P, Arroyo V, Ginès A, Saló J, Clària J, Jiménez W, Bru C, Rivera F, Rodés J. Brachial and femoral artery blood flow in cirrhosis: relationship to kidney dysfunction. Hepatology. 1993;17:788–793. [PubMed] [Google Scholar]
- 8.Wiese S, Hove JD, Bendtsen F, Møller S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol. 2014;11:177–186. doi: 10.1038/nrgastro.2013.210. [DOI] [PubMed] [Google Scholar]
- 9.Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9–15. doi: 10.1136/heart.87.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henriksen JH, Møller S, Ring-Larsen H, Christensen NJ. The sympathetic nervous system in liver disease. J Hepatol. 1998;29:328–341. doi: 10.1016/s0168-8278(98)80022-6. [DOI] [PubMed] [Google Scholar]
- 11.Braillon A, Cales P, Valla D, Gaudy D, Geoffroy P, Lebrec D. Influence of the degree of liver failure on systemic and splanchnic haemodynamics and on response to propranolol in patients with cirrhosis. Gut. 1986;27:1204–1209. doi: 10.1136/gut.27.10.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelbaek H, Eriksen J, Brynjolf I, Raboel A, Lund JO, Munck O, Bonnevie O, Godtfredsen J. Cardiac performance in patients with asymptomatic alcoholic cirrhosis of the liver. Am J Cardiol. 1984;54:852–855. doi: 10.1016/s0002-9149(84)80220-9. [DOI] [PubMed] [Google Scholar]
- 13.Kelbaek H, Rabøl A, Brynjolf I, Eriksen J, Bonnevie O, Godtfredsen J, Munck O, Lund JO. Haemodynamic response to exercise in patients with alcoholic liver cirrhosis. Clin Physiol. 1987;7:35–41. doi: 10.1111/j.1475-097x.1987.tb00631.x. [DOI] [PubMed] [Google Scholar]
- 14.Wong F, Girgrah N, Graba J, Allidina Y, Liu P, Blendis L. The cardiac response to exercise in cirrhosis. Gut. 2001;49:268–275. doi: 10.1136/gut.49.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong F, Liu P, Lilly L, Bomzon A, Blendis L. Role of cardiac structural and functional abnormalities in the pathogenesis of hyperdynamic circulation and renal sodium retention in cirrhosis. Clin Sci (Lond) 1999;97:259–267. [PubMed] [Google Scholar]
- 16.Grose RD, Nolan J, Dillon JF, Errington M, Hannan WJ, Bouchier IA, Hayes PC. Exercise-induced left ventricular dysfunction in alcoholic and non-alcoholic cirrhosis. J Hepatol. 1995;22:326–332. doi: 10.1016/0168-8278(95)80286-x. [DOI] [PubMed] [Google Scholar]
- 17.Krag A, Bendtsen F, Mortensen C, Henriksen JH, Møller S. Effects of a single terlipressin administration on cardiac function and perfusion in cirrhosis. Eur J Gastroenterol Hepatol. 2010;22:1085–1092. doi: 10.1097/MEG.0b013e32833a4822. [DOI] [PubMed] [Google Scholar]
- 18.Møller S, Hansen EF, Becker U, Brinch K, Henriksen JH, Bendtsen F. Central and systemic haemodynamic effects of terlipressin in portal hypertensive patients. Liver. 2000;20:51–59. doi: 10.1034/j.1600-0676.2000.020001051.x. [DOI] [PubMed] [Google Scholar]
- 19.Wong F, Villamil A, Merli M, Romero G, Angeli P, Caraceni P, Steib CJ, Baik SK, Spinzi G, Colombato LA, et al. Prevalence of diastolic dysfunction in cirrhosis and its clinical significance. Hepatology. 2011;54 Suppl 1:A475–A476. [Google Scholar]
- 20.Kazankov K, Holland-Fischer P, Andersen NH, Torp P, Sloth E, Aagaard NK, Vilstrup H. Resting myocardial dysfunction in cirrhosis quantified by tissue Doppler imaging. Liver Int. 2011;31:534–540. doi: 10.1111/j.1478-3231.2011.02468.x. [DOI] [PubMed] [Google Scholar]
- 21.Merli M, Calicchia A, Ruffa A, Pellicori P, Riggio O, Giusto M, Gaudio C, Torromeo C. Cardiac dysfunction in cirrhosis is not associated with the severity of liver disease. Eur J Intern Med. 2013;24:172–176. doi: 10.1016/j.ejim.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Karagiannakis D, Vlachogiannakos J, Anastasiadis G, Vafiadis-Zouboulis I, Ladas S. Diastolic cardiac dysfunction is a predictor of dismal prognosis in patients with liver cirrhosis. Hepatology International. 2014;8:588–594. doi: 10.1007/s12072-014-9544-6. [DOI] [PubMed] [Google Scholar]
- 23.Ruíz-del-Árbol L, Achécar L, Serradilla R, Rodríguez-Gandía MÁ, Rivero M, Garrido E, Natcher JJ. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatology. 2013;58:1732–1741. doi: 10.1002/hep.26509. [DOI] [PubMed] [Google Scholar]
- 24.Pozzi M, Carugo S, Boari G, Pecci V, de Ceglia S, Maggiolini S, Bolla GB, Roffi L, Failla M, Grassi G, et al. Evidence of functional and structural cardiac abnormalities in cirrhotic patients with and without ascites. Hepatology. 1997;26:1131–1137. doi: 10.1002/hep.510260507. [DOI] [PubMed] [Google Scholar]
- 25.Josefsson A, Fu M, Allayhari P, Björnsson E, Castedal M, Olausson M, Kalaitzakis E. Impact of peri-transplant heart failure & amp; left-ventricular diastolic dysfunction on outcomes following liver transplantation. Liver Int. 2012;32:1262–1269. doi: 10.1111/j.1478-3231.2012.02818.x. [DOI] [PubMed] [Google Scholar]
- 26.Bernardi M, Calandra S, Colantoni A, Trevisani F, Raimondo ML, Sica G, Schepis F, Mandini M, Simoni P, Contin M, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27:28–34. doi: 10.1002/hep.510270106. [DOI] [PubMed] [Google Scholar]
- 27.Henriksen JH, Fuglsang S, Bendtsen F, Christensen E, Møller S. Dyssynchronous electrical and mechanical systole in patients with cirrhosis. J Hepatol. 2002;36:513–520. doi: 10.1016/s0168-8278(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 28.Trevisani F, Merli M, Savelli F, Valeriano V, Zambruni A, Riggio O, Caraceni P, Domenicali M, Bernardi M. QT interval in patients with non-cirrhotic portal hypertension and in cirrhotic patients treated with transjugular intrahepatic porto-systemic shunt. J Hepatol. 2003;38:461–467. doi: 10.1016/s0168-8278(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 29.Nazar A, Guevara M, Sitges M, Terra C, Solà E, Guigou C, Arroyo V, Ginès P. LEFT ventricular function assessed by echocardiography in cirrhosis: relationship to systemic hemodynamics and renal dysfunction. J Hepatol. 2013;58:51–57. doi: 10.1016/j.jhep.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 30.Torregrosa M, Aguadé S, Dos L, Segura R, Gónzalez A, Evangelista A, Castell J, Margarit C, Esteban R, Guardia J, et al. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol. 2005;42:68–74. doi: 10.1016/j.jhep.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Ates F, Topal E, Kosar F, Karincaoglu M, Yildirim B, Aksoy Y, Aladag M, Harputluoglu MM, Demirel U, Alan H, et al. The relationship of heart rate variability with severity and prognosis of cirrhosis. Dig Dis Sci. 2006;51:1614–1618. doi: 10.1007/s10620-006-9073-9. [DOI] [PubMed] [Google Scholar]
- 32.Møller S, Iversen JS, Henriksen JH, Bendtsen F. Reduced baroreflex sensitivity in alcoholic cirrhosis: relations to hemodynamics and humoral systems. Am J Physiol Heart Circ Physiol. 2007;292:H2966–H2972. doi: 10.1152/ajpheart.01227.2006. [DOI] [PubMed] [Google Scholar]
- 33.Milani A, Zaccaria R, Bombardieri G, Gasbarrini A, Pola P. Cirrhotic cardiomyopathy. Dig Liver Dis. 2007;39:507–515. doi: 10.1016/j.dld.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Alqahtani SA, Fouad TR, Lee SS. Cirrhotic cardiomyopathy. Semin Liver Dis. 2008;28:59–69. doi: 10.1055/s-2008-1040321. [DOI] [PubMed] [Google Scholar]
- 35.Lee SS, Marty J, Mantz J, Samain E, Braillon A, Lebrec D. Desensitization of myocardial beta-adrenergic receptors in cirrhotic rats. Hepatology. 1990;12:481–485. doi: 10.1002/hep.1840120306. [DOI] [PubMed] [Google Scholar]
- 36.Hausdorff WP, Caron MG, Lefkowitz RJ. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 37.Ma Z, Lee SS, Meddings JB. Effects of altered cardiac membrane fluidity on beta-adrenergic receptor signalling in rats with cirrhotic cardiomyopathy. J Hepatol. 1997;26:904–912. doi: 10.1016/s0168-8278(97)80259-0. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Ma Z, Lee SS. Contribution of nitric oxide to the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Gastroenterology. 2000;118:937–944. doi: 10.1016/s0016-5085(00)70180-6. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Song D, Lee SS. Role of heme oxygenase-carbon monoxide pathway in pathogenesis of cirrhotic cardiomyopathy in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G68–G74. doi: 10.1152/ajpgi.2001.280.1.G68. [DOI] [PubMed] [Google Scholar]
- 40.Fernández-Rodriguez CM, Romero J, Petros TJ, Bradshaw H, Gasalla JM, Gutiérrez ML, Lledó JL, Santander C, Fernández TP, Tomás E, et al. Circulating endogenous cannabinoid anandamide and portal, systemic and renal hemodynamics in cirrhosis. Liver Int. 2004;24:477–483. doi: 10.1111/j.1478-3231.2004.0945.x. [DOI] [PubMed] [Google Scholar]
- 41.Bonz A, Laser M, Küllmer S, Kniesch S, Babin-Ebell J, Popp V, Ertl G, Wagner JA. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J Cardiovasc Pharmacol. 2003;41:657–664. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Therapondos G, Flapan AD, Plevris JN, Hayes PC. Cardiac morbidity and mortality related to orthotopic liver transplantation. Liver Transpl. 2004;10:1441–1453. doi: 10.1002/lt.20298. [DOI] [PubMed] [Google Scholar]
- 43.Myers RP, Lee SS. Cirrhotic cardiomyopathy and liver transplantation. Liver Transpl. 2000;6:S44–S52. doi: 10.1002/lt.500060510. [DOI] [PubMed] [Google Scholar]
- 44.Cazzaniga M, Salerno F, Pagnozzi G, Dionigi E, Visentin S, Cirello I, Meregaglia D, Nicolini A. Diastolic dysfunction is associated with poor survival in patients with cirrhosis with transjugular intrahepatic portosystemic shunt. Gut. 2007;56:869–875. doi: 10.1136/gut.2006.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rabie RN, Cazzaniga M, Salerno F, Wong F. The use of E/A ratio as a predictor of outcome in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2009;104:2458–2466. doi: 10.1038/ajg.2009.321. [DOI] [PubMed] [Google Scholar]
- 46.Wong F, Siu S, Liu P, Blendis LM. Brain natriuretic peptide: is it a predictor of cardiomyopathy in cirrhosis? Clin Sci (Lond) 2001;101:621–628. [PubMed] [Google Scholar]
- 47.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Lossnitzer D, Steen H, Zahn A, Lehrke S, Weiss C, Weiss KH, Giannitsis E, Stremmel W, Sauer P, Katus HA, et al. Myocardial late gadolinium enhancement cardiovascular magnetic resonance in patients with cirrhosis. J Cardiovasc Magn Reson. 2010;12:47. doi: 10.1186/1532-429X-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Møller S, Hove JD, Dixen U, Bendtsen F. New insights into cirrhotic cardiomyopathy. Int J Cardiol. 2013;167:1101–1108. doi: 10.1016/j.ijcard.2012.09.089. [DOI] [PubMed] [Google Scholar]
- 50.Umphrey LG, Hurst RT, Eleid MF, Lee KS, Reuss CS, Hentz JG, Vargas HE, Appleton CP. Preoperative dobutamine stress echocardiographic findings and subsequent short-term adverse cardiac events after orthotopic liver transplantation. Liver Transpl. 2008;14:886–892. doi: 10.1002/lt.21495. [DOI] [PubMed] [Google Scholar]
- 51.Kim MY, Baik SK, Won CS, Park HJ, Jeon HK, Hong HI, Kim JW, Kim HS, Kwon SO, Kim JY, et al. Dobutamine stress echocardiography for evaluating cirrhotic cardiomyopathy in liver cirrhosis. Korean J Hepatol. 2010;16:376–382. doi: 10.3350/kjhep.2010.16.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N, Hetzer R. Strain and strain rate imaging by echocardiography - basic concepts and clinical applicability. Curr Cardiol Rev. 2009;5:133–148. doi: 10.2174/157340309788166642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adigun AQ, Pinto AG, Flockhart DA, Gorski JC, Li L, Hall SD, Chalasani N. Effect of cirrhosis and liver transplantation on the gender difference in QT interval. Am J Cardiol. 2005;95:691–694. doi: 10.1016/j.amjcard.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 54.Lerman A, Gibbons RJ, Rodeheffer RJ, Bailey KR, McKinley LJ, Heublein DM, Burnett JC. Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction. Lancet. 1993;341:1105–1109. doi: 10.1016/0140-6736(93)93125-k. [DOI] [PubMed] [Google Scholar]
- 55.Davidson NC, Naas AA, Hanson JK, Kennedy NS, Coutie WJ, Struthers AD. Comparison of atrial natriuretic peptide B-type natriuretic peptide, and N-terminal proatrial natriuretic peptide as indicators of left ventricular systolic dysfunction. Am J Cardiol. 1996;77:828–831. doi: 10.1016/S0002-9149(97)89176-X. [DOI] [PubMed] [Google Scholar]
- 56.Rodseth RN. B type natriuretic peptide--a diagnostic breakthrough in peri-operative cardiac risk assessment? Anaesthesia. 2009;64:165–178. doi: 10.1111/j.1365-2044.2008.05689.x. [DOI] [PubMed] [Google Scholar]
- 57.Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011;278:1808–1817. doi: 10.1111/j.1742-4658.2011.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henriksen JH, Gøtze JP, Fuglsang S, Christensen E, Bendtsen F, Møller S. Increased circulating pro-brain natriuretic peptide (proBNP) and brain natriuretic peptide (BNP) in patients with cirrhosis: relation to cardiovascular dysfunction and severity of disease. Gut. 2003;52:1511–1517. doi: 10.1136/gut.52.10.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saner FH, Neumann T, Canbay A, Treckmann JW, Hartmann M, Goerlinger K, Bertram S, Beckebaum S, Cicinnati V, Paul A. High brain-natriuretic peptide level predicts cirrhotic cardiomyopathy in liver transplant patients. Transpl Int. 2011;24:425–432. doi: 10.1111/j.1432-2277.2011.01219.x. [DOI] [PubMed] [Google Scholar]
- 60.Karthikeyan G, Moncur RA, Levine O, Heels-Ansdell D, Chan MT, Alonso-Coello P, Yusuf S, Sessler D, Villar JC, Berwanger O, et al. Is a pre-operative brain natriuretic peptide or N-terminal pro-B-type natriuretic peptide measurement an independent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery? A systematic review and meta-analysis of observational studies. J Am Coll Cardiol. 2009;54:1599–1606. doi: 10.1016/j.jacc.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 61.Young YR, Sheu BF, Li WC, Hsieh TM, Hung CW, Chang SS, Lee CC. Predictive value of plasma brain natriuretic peptide for postoperative cardiac complications--a systemic review and meta-analysis. J Crit Care. 2014;29:696.e1–696.10. doi: 10.1016/j.jcrc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 62.Rodseth RN, Biccard BM, Le Manach Y, Sessler DI, Lurati Buse GA, Thabane L, Schutt RC, Bolliger D, Cagini L, Cardinale D, et al. The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide: a systematic review and individual patient data meta-analysis. J Am Coll Cardiol. 2014;63:170–180. doi: 10.1016/j.jacc.2013.08.1630. [DOI] [PubMed] [Google Scholar]
- 63.Rodseth RN, Biccard BM, Chu R, Lurati Buse GA, Thabane L, Bakhai A, Bolliger D, Cagini L, Cahill TJ, Cardinale D, et al. Postoperative B-type natriuretic peptide for prediction of major cardiac events in patients undergoing noncardiac surgery: systematic review and individual patient meta-analysis. Anesthesiology. 2013;119:270–283. doi: 10.1097/ALN.0b013e31829083f1. [DOI] [PubMed] [Google Scholar]
- 64.Wiese S, Mortensen C, Gøtze JP, Christensen E, Andersen O, Bendtsen F, Møller S. Cardiac and proinflammatory markers predict prognosis in cirrhosis. Liver International. 2014;34:e19–e30. doi: 10.1111/liv.12428. [DOI] [PubMed] [Google Scholar]
- 65.Kovács A, Schepke M, Heller J, Schild HH, Flacke S. Short-term effects of transjugular intrahepatic shunt on cardiac function assessed by cardiac MRI: preliminary results. Cardiovasc Intervent Radiol. 2010;33:290–296. doi: 10.1007/s00270-009-9696-2. [DOI] [PubMed] [Google Scholar]
- 66.Huonker M, Schumacher YO, Ochs A, Sorichter S, Keul J, Rössle M. Cardiac function and haemodynamics in alcoholic cirrhosis and effects of the transjugular intrahepatic portosystemic stent shunt. Gut. 1999;44:743–748. doi: 10.1136/gut.44.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma Z, Lee SS. Cirrhotic cardiomyopathy: getting to the heart of the matter. Hepatology. 1996;24:451–459. doi: 10.1002/hep.510240226. [DOI] [PubMed] [Google Scholar]
- 68.Franco D, Vons C, Traynor O, de Smadja C. Should portosystemic shunt be reconsidered in the treatment of intractable ascites in cirrhosis? Arch Surg. 1988;123:987–991. doi: 10.1001/archsurg.1988.01400320073015. [DOI] [PubMed] [Google Scholar]
- 69.Braverman AC, Steiner MA, Picus D, White H. High-output congestive heart failure following transjugular intrahepatic portal-systemic shunting. Chest. 1995;107:1467–1469. doi: 10.1378/chest.107.5.1467. [DOI] [PubMed] [Google Scholar]
- 70.Krag A, Bendtsen F, Henriksen JH, Møller S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59:105–110. doi: 10.1136/gut.2009.180570. [DOI] [PubMed] [Google Scholar]
- 71.Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439–447. doi: 10.1002/hep.20766. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, Albillos A, Jiménez W, Arroyo V. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210–1218. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- 73.Trevisani F, Di Micoli A, Zambruni A, Biselli M, Santi V, Erroi V, Lenzi B, Caraceni P, Domenicali M, Cavazza M, et al. QT interval prolongation by acute gastrointestinal bleeding in patients with cirrhosis. Liver Int. 2012;32:1510–1515. doi: 10.1111/j.1478-3231.2012.02847.x. [DOI] [PubMed] [Google Scholar]
- 74.Mittal C, Qureshi W, Singla S, Ahmad U, Huang MA. Pre-transplant left ventricular diastolic dysfunction is associated with post transplant acute graft rejection and graft failure. Dig Dis Sci. 2014;59:674–680. doi: 10.1007/s10620-013-2955-8. [DOI] [PubMed] [Google Scholar]
- 75.Henriksen JH, Bendtsen F, Hansen EF, Møller S. Acute non-selective beta-adrenergic blockade reduces prolonged frequency-adjusted Q-T interval (QTc) in patients with cirrhosis. J Hepatol. 2004;40:239–246. doi: 10.1016/j.jhep.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 76.Zambruni A, Trevisani F, Di Micoli A, Savelli F, Berzigotti A, Bracci E, Caraceni P, Domenicali M, Felline P, Zoli M, et al. Effect of chronic beta-blockade on QT interval in patients with liver cirrhosis. J Hepatol. 2008;48:415–421. doi: 10.1016/j.jhep.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 77.Pozzi M, Grassi G, Ratti L, Favini G, Dell’Oro R, Redaelli E, Calchera I, Boari G, Mancia G. Cardiac, neuroadrenergic, and portal hemodynamic effects of prolonged aldosterone blockade in postviral child A cirrhosis. Am J Gastroenterol. 2005;100:1110–1116. doi: 10.1111/j.1572-0241.2005.41060.x. [DOI] [PubMed] [Google Scholar]
- 78.Jovine E, Mazziotti A, Grazi GL, Ercolani G, Masetti M, Morganti M, Pierangeli F, Begliomini B, Mazzetti PG, Rossi R, et al. Piggy-back versus conventional technique in liver transplantation: report of a randomized trial. Transpl Int. 1997;10:109–112. doi: 10.1007/pl00003824. [DOI] [PubMed] [Google Scholar]
- 79.Hosein Shokouh-Amiri M, Osama Gaber A, Bagous WA, Grewal HP, Hathaway DK, Vera SR, Stratta RJ, Bagous TN, Kizilisik T. Choice of surgical technique influences perioperative outcomes in liver transplantation. Ann Surg. 2000;231:814–823. doi: 10.1097/00000658-200006000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soong W, Sherwani SS, Ault ML, Baudo AM, Herborn JC, De Wolf AM. United States practice patterns in the use of transesophageal echocardiography during adult liver transplantation. J Cardiothorac Vasc Anesth. 2014;28:635–639. doi: 10.1053/j.jvca.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 81.Biancofiore G, Mandell MS, Rocca GD. Perioperative considerations in patients with cirrhotic cardiomyopathy. Curr Opin Anaesthesiol. 2010;23:128–132. doi: 10.1097/ACO.0b013e328337260a. [DOI] [PubMed] [Google Scholar]
- 82.Burtenshaw AJ, Isaac JL. The role of trans-oesophageal echocardiography for perioperative cardiovascular monitoring during orthotopic liver transplantation. Liver Transpl. 2006;12:1577–1583. doi: 10.1002/lt.20929. [DOI] [PubMed] [Google Scholar]
- 83.Wax DB, Torres A, Scher C, Leibowitz AB. Transesophageal echocardiography utilization in high-volume liver transplantation centers in the United States. J Cardiothorac Vasc Anesth. 2008;22:811–813. doi: 10.1053/j.jvca.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Shin WJ, Kim YK, Song JG, Kim SH, Choi SS, Song JH, Hwang GS. Alterations in QT interval in patients undergoing living donor liver transplantation. Transplant Proc. 2011;43:170–173. doi: 10.1016/j.transproceed.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Ranasinghe DN, Mallett SV. Hypomagnesaemia, cardiac arrhythmias and orthotopic liver transplantation. Anaesthesia. 1994;49:403–405. doi: 10.1111/j.1365-2044.1994.tb03472.x. [DOI] [PubMed] [Google Scholar]
- 86.Sampathkumar P, Lerman A, Kim BY, Narr BJ, Poterucha JJ, Torsher LC, Plevak DJ. Post-liver transplantation myocardial dysfunction. Liver Transpl Surg. 1998;4:399–403. doi: 10.1002/lt.500040513. [DOI] [PubMed] [Google Scholar]
- 87.Stewart KS, Rhim CH, Bahrain ML, Ashkezari ZD, Ozdemirli M, Fishbein TM, Johnson LB, Lu AD, Plotkin JS. Nonischemic cardiomyopathy after orthotopic liver transplantation: a report of three cases and a review of the literature. Liver Transpl. 2005;11:573–578. doi: 10.1002/lt.20410. [DOI] [PubMed] [Google Scholar]
- 88.Donovan CL, Marcovitz PA, Punch JD, Bach DS, Brown KA, Lucey MR, Armstrong WF. Two-dimensional and dobutamine stress echocardiography in the preoperative assessment of patients with end-stage liver disease prior to orthotopic liver transplantation. Transplantation. 1996;61:1180–1188. doi: 10.1097/00007890-199604270-00011. [DOI] [PubMed] [Google Scholar]
- 89.Snowden CP, Hughes T, Rose J, Roberts DR. Pulmonary edema in patients after liver transplantation. Liver Transpl. 2000;6:466–470. doi: 10.1053/jlts.2000.7580. [DOI] [PubMed] [Google Scholar]
- 90.Fouad TR, Abdel-Razek WM, Burak KW, Bain VG, Lee SS. Prediction of cardiac complications after liver transplantation. Transplantation. 2009;87:763–770. doi: 10.1097/TP.0b013e318198d734. [DOI] [PubMed] [Google Scholar]
- 91.Nasraway SA, Klein RD, Spanier TB, Rohrer RJ, Freeman RB, Rand WM, Benotti PN. Hemodynamic correlates of outcome in patients undergoing orthotopic liver transplantation. Evidence for early postoperative myocardial depression. Chest. 1995;107:218–224. doi: 10.1378/chest.107.1.218. [DOI] [PubMed] [Google Scholar]
- 92.Dowsley TF, Bayne DB, Langnas AN, Dumitru I, Windle JR, Porter TR, Raichlin E. Diastolic dysfunction in patients with end-stage liver disease is associated with development of heart failure early after liver transplantation. Transplantation. 2012;94:646–651. doi: 10.1097/TP.0b013e31825f0f97. [DOI] [PubMed] [Google Scholar]
- 93.Therapondos G, Flapan AD, Dollinger MM, Garden OJ, Plevris JN, Hayes PC. Cardiac function after orthotopic liver transplantation and the effects of immunosuppression: a prospective randomized trial comparing cyclosporin (Neoral) and tacrolimus. Liver Transpl. 2002;8:690–700. doi: 10.1053/jlts.2002.34381. [DOI] [PubMed] [Google Scholar]
- 94.Gadano A, Hadengue A, Widmann JJ, Vachiery F, Moreau R, Yang S, Soupison T, Sogni P, Degott C, Durand F. Hemodynamics after orthotopic liver transplantation: study of associated factors and long-term effects. Hepatology. 1995;22:458–465. [PubMed] [Google Scholar]
- 95.Henderson JM, Mackay GJ, Hooks M, Chezmar JL, Galloway JR, Dodson TF, Kutner MH. High cardiac output of advanced liver disease persists after orthotopic liver transplantation. Hepatology. 1992;15:258–262. doi: 10.1002/hep.1840150214. [DOI] [PubMed] [Google Scholar]
- 96.García González M, Hernandez-Madrid A, Lopez-Sanromán A, Candela A, Nuño J, Barcena R. Reversal of QT interval electrocardiographic alterations in cirrhotic patients undergoing liver transplantation. Transplant Proc. 1999;31:2366–2367. doi: 10.1016/s0041-1345(99)00381-4. [DOI] [PubMed] [Google Scholar]
- 97.Mohamed R, Forsey PR, Davies MK, Neuberger JM. Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end-stage liver disease. Hepatology. 1996;23:1128–1134. doi: 10.1002/hep.510230529. [DOI] [PubMed] [Google Scholar]
- 98.Bal JS, Thuluvath PJ. Prolongation of QTc interval: relationship with etiology and severity of liver disease, mortality and liver transplantation. Liver Int. 2003;23:243–248. doi: 10.1034/j.1600-0676.2003.00833.x. [DOI] [PubMed] [Google Scholar]


