Abstract
Oxidative stress has been investigated in the context of alcoholic liver injury for many years and shown to be a causal factor of chronic hepatitis C (CHC), nonalcoholic steatohepatitis (NASH), drug-induced liver injury, Wilson’s disease, and hemochromatosis. In CHC, it has been demonstrated that oxidative stress plays an important role in hepatocarcinogenesis. In cases with persistent hepatitis due to failure of hepatitis C virus eradication, or chronic liver disease, such as NASH, the treatment of which remains unestablished, it is important to reduce serum alanine aminotransferase levels and prevent liver fibrosis and development of hepatocellular carcinoma. This also suggests the importance of antioxidant therapy. Among treatment options where it would be expected that anti-inflammatory activity plays a role in their confirmed efficacy for chronic hepatitis, iron depletion therapy, glycyrrhizin, ursodeoxycholic acid, Sho-Saiko-To, and vitamin E can all be considered antioxidant therapies. To date, however, the ability of these treatments to prevent cancer has been confirmed only in CHC. Nevertheless, anti-inflammatory and anti-fibrotic effects have been demonstrated in other liver diseases and these therapies may potentially be effective for cancer prevention.
Keywords: Chronic hepatitis, Antioxidant therapy, Hepatocellular carcinoma, Prevention, Iron depletion therapy
Core tip: Among treatment options where it would be expected that anti-inflammatory activity plays a role in their confirmed efficacy for chronic hepatitis, iron depletion therapy, glycyrrhizin, ursodeoxycholic acid, Sho-Saiko-To, and vitamin E can all be considered antioxidant therapies. In chronic liver diseases, it has been demonstrated that antioxidant therapy may potentially be effective for suppressing inflammation and liver fibrosis and expected to prevent carcinogenesis.
INTRODUCTION
Oxidative stress has been investigated for many years as a possible cause of alcoholic liver injury. Recently, it has attracted attention as one of the causal factors for a variety of liver diseases, such as chronic hepatitis C (CHC), nonalcoholic steatohepatitis (NASH), drug-induced liver injury, Wilson’s disease, and hemochromatosis. Furthermore, it has been demonstrated that oxidative stress plays an important role in hepatocarcinogenesis in CHC.
Recent studies have shown that excess hepatic iron accumulation in CHC patients contributes to liver injury[1-3]. It is believed that free iron in the liver facilitates the formation of reactive oxygen species (ROS), including hydroxyl radicals (•OH), which cause oxidative damage to numerous cellular components, including lipids, proteins and nucleic acids, and also cause an up-regulation of collagen synthesis[4]. Further, •OH is known to generate promutagenic bases such as 8-hydroxy-2’-deoxyguanosine (8-OHdG), which has been implicated in spontaneous DNA mutagenesis and carcinogenesis[5,6]. Although the mechanism of hepatocarcinogenesis due to hepatitis C virus (HCV) infection remains unclear, long-term follow-up studies indicate that most patients with progressive liver disease who develop cirrhosis and/or hepatocellular carcinoma (HCC) have persistently elevated or fluctuating serum alanine aminotransferase (ALT) levels, suggesting that they have a background of chronic active inflammation and regeneration of the liver[7]. Further, we have demonstrated in a 6-year follow-up study of CHC patients that iron depletion therapy, consisting of intermittent phlebotomies and a low-iron diet, significantly reduced serum ALT levels, the histological hepatic fibrosis grade, and hepatic 8-OHdG levels[3].
In cases with persistent hepatitis due to failure of HCV eradication and chronic liver disease, such as NASH, the treatment of which remains unestablished, it is important to reduce serum ALT levels and prevent liver fibrosis and development of HCC. This also suggests the importance of antioxidant therapy. To date, reported effective treatment options expected to exert anti-inflammatory activity for chronic hepatitis include iron depletion therapy, glycyrrhizin, ursodeoxycholic acid, and Sho-Saiko-To, which can be considered as antioxidant therapies. Cancer prevention by antioxidants such as vitamin E has also been investigated (Table 1). Here, we review iron depletion as an antioxidant therapy for the treatment of the inflammatory effects of chronic hepatitis to reduce fibrosis and prevent cancer, as illustrated in this paper by an analysis of own cases.
Table 1.
Clinical trials of chemoprevention effects in hepatocarcinogenesis
| Therapy | Ref. | Year | Study design | Treated patients/control | Disease | Combined medication | Hepatocarcinogenesis rate |
| Phlebotomy | Kato et al[16] | 2007 | Open labeled | 35/40 | Chronic hepatitis C | None | Hepatocarcinogenesis rates in iron depletion and control were 5.7% and 17.5% at the end of the fifth year, and 8.6% and 39% in the tenth year, respectively (P = 0.018) |
| Glycyrrhizin | Ikeda[29] | 2007 | Case-control | 244/102 | Chronic hepatitis C | None | Crude carcinogenesis rates in the treated and untreated group were 13.3%, 26.0% at the fifth year, and 21.5% and 35.5% at the 10th year, respectively (P = 0.021) |
| Glycyrrhizin | Arase et al[32] | 1997 | Case-control | 84/109 | Chronic hepatitis C | None | The 10th-year rates of cumulative HCC incidence for the treated and untreated group were 7% and 12%, and the 15th-yr rates were 12% and 25%, respectively (P = 0.032) |
| Ursodeoxycholic Acid | Tarao et al[44] | 2005 | Case-control | 56/46 | Hepatitis C virus -associated liver cirrhosis | Sho-saiko-to, Ursodeoxycholic acid | The cumulative 5-yr incidence of HCC in the patients treated with UDCA was 17.9% and was significantly lower than that in patients not treated with UDCA (39.1%; P = 0.025) |
| Vitamin E | Kakizaki et al[48] | 2001 | Randomized controlled | 44/39 | Chronic hepatitis C | None | Cumulative tumor-free survival tended to be higher in the Vit E group than in controls, albeit statistically insignificant |
| Sho-saiko-to | Oka et al[64] | 1995 | Randomized open controlled | 130/130 | Cirrhosis from chronic liver disease | None | The cumulative incidence curve for 5 yr of the trial group was lower than that of the control group (P = 0.071), albeit statistically insignificant |
HCC: Hepatocellular carcinoma; UDCA: Ursodeoxycholic acid.
IRON DEPLETION THERAPY
The liver is the major iron storage organ in the body; thus, it is not surprising that disorders of iron metabolism are involved in chronic liver diseases. We have shown previously that in Long-Evans cinnamon rats, an abrupt accumulation of iron in the liver causes spontaneous hepatitis and subsequent development of HCC[8]. Free iron in the liver is believed to catalyze the formation of ROS[4]. In particular, the Fenton reaction, in which Fe3+, •OH and OH- are produced in the presence of Fe2+ and H2O2, generates large amounts of highly toxic promutagenic ROS hydroxyl radicals (•OH)[9,10].
Iron overload in the setting of hereditary hemochromatosis has long been known to be associated with an increased risk for HCC[11]. Standard of care is phlebotomy to reduce total body iron levels and achieve normal ferritin levels. Although for ethical reasons the beneficial effect of phlebotomy has never been formally demonstrated in controlled trials, Bomford et al[12] reported that the percentage survival 5 years after diagnosis was 66% in 85 patients treated by phlebotomy, and 18% in 26 untreated patients who died before phlebotomy had become widely accepted. Hepatic iron accumulation has also been reported in patients with CHC[13] but the mechanisms responsible for this have not been fully elucidated. Possibly, inflammatory cytokines stimulate iron uptake via up-regulation of transferrin receptor expression in hepatocytes, as described previously[14]. Nishina et al[15] demonstrated in mice that HCV-induced reactive oxygen species may down-regulate hepcidin transcription which leads to increased duodenal iron transport and macrophage iron release, causing hepatic iron accumulation. We have reported previously that iron depletion improves serum ALT levels as well as hepatic oxidative DNA damage in patients with CHC, and that long-term phlebotomy together with a low-iron diet lowers the risk of developing HCC[3,16]. In this cohort study, we undertook weekly phlebotomy (200 g) until the patients achieved a state of mild iron deficiency, and we followed this by monthly maintenance phlebotomy for 107 mo (median). Patients were advised to consume a low-iron diet (5-7 mg iron/d). We have continuously followed these patients, with the result shown in Figure 1. If dietary iron intake is not restricted, phlebotomy may lead to enhanced iron absorption; therefore, a low-iron diet is essential for a successful outcome of this treatment.
Figure 1.
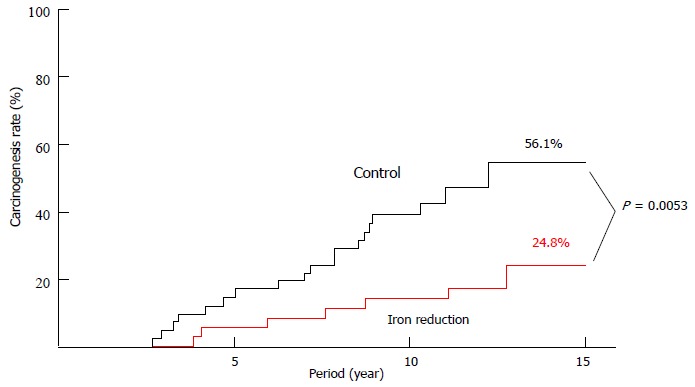
Crude hepatocarcinogenesis rate in iron reduction and control groups.
It was recently reported that a high frequency of patients with NASH develop HCC. NASH is a severe form of nonalcoholic fatty liver disease (NAFLD)[17] suggested by Day et al[18] to require two hits for its development, (1) excess accumulation of triglyceride in the hepatocyte; and (2) factors such as free radicals capable of inducing oxidative stress. Slight increases of hepatic iron concentration have been reported in NAFLD/NASH patients[19]. Although the exact mechanisms involved in iron overload remain to be clarified, it can be hypothesized that insulin plays a role by stimulating cellular iron uptake through increased transferrin receptor expression[20]. Facchini et al[21] reported an improvement in ALT levels and plasma insulin concentrations following phlebotomy in 17 NAFLD patients with impaired glucose tolerance. Riquelme et al[22] reported histological resolution of NASH after iron depletion therapy in a case report. According to Fargion et al[23], HOMA-IR and ALT were significantly reduced after phlebotomy in 42 patients with NAFLD. Sumida et al[24] also reported that aspartate aminotransferase (AST) and ALT were reduced by phlebotomy in 9 Japanese patients with NASH. Valenti et al[25] reported that 64 NAFLD patients treated by phlebotomy achieved significant reduction in insulin resistance compared with 64 NAFLD patients who underwent lifestyle modifications only. Fujita et al[26] showed that iron reduction resulting from a-combination of phlebotomy and a low iron diet resulted not only in improvement of ALT levels but also normalization of hepatic levels of 8-OHdG in 11 NASH patients. In a phase II trial on 31 patients with NAFLD, phlebotomy resulted in a significant improvement in the NAFLD activity score (NAS), AST and ALT[27]. In a phase III trial, Valenti et al[28] studied 38 NAFLD patients randomized to phlebotomy (n = 21) or lifestyle changes alone (n = 17). It was concluded that phlebotomy was associated with improvement in NAS, AST, ALT and γGT without adverse events.
Because it has been reported that iron depletion therapy has anti-inflammatory effects in NASH, it may also contribute to the prevention of hepatocarcinogenesis in these patients. However, it has been reported that it is not effective in all cases[25]. It would therefore be valuable to establish a method for selecting those NASH patients most likely to benefit clinically from iron depletion therapy.
GLYCYRRHIZIN (GLYCYRRHIZIC ACID), STRONGER NEO-MINOPHAGEN C
Glycyrrhizin is a triterpene glycoside from licorice root (Glycyrrhiza glabra) and consists of one molecule of glycyrrhetinic acid (GA) and two molecules of glucuronic acid. Glycyrrhizin is widely used in patients with chronic viral hepatitis because of its anti-inflammatory action and beneficial effects on ALT levels and histology[29]. The anti-inflammatory action of glycyrrhizin is believed to be due to its protective effect on the hepatic cellular membrane, which may explain its ability to lower the serum transaminase level in patients with chronic hepatitis. Kiso et al[30] demonstrated that GA inhibited free radical generation and lipid peroxidation in vitro. Stronger neo-minophagen C (SNMC, Minophagen Pharmaceutical, Tokyo, Japan), was first reported by Yamamoto et al in 1958, and has now been used in the treatment of chronic liver disease for more than 50 years. SNMC is a compound GA tablet that includes GA (2 mg) together with glycine acid (20 mg) and L-cysteine hydrochloride (1 mg)[31]. In 1977, Arase et al[32] confirmed its ability to reduced aminotransferase levels in patients with histologically-documented chronic hepatitis in a double-blind randomized controlled trial using a dose of 40 mL daily for a month. According to a retrospective study of 84 patients (Group A) who had been treated with SNMC at a dose of 100 mL daily and 109 patients (Group B) who could not be treated with SNMC or interferon, 36% of Group A achieved ALT normalization. The 10-year HCC rates in Groups A and B were 7% and 12%, respectively, and the 15-year rates 12% and 25%[32]. van Rossum et al[33] performed a double-blind randomized placebo-controlled trial in which glycyrrhizin was administered three times per week for 4 wk, but reported that only 10% of the European patients so treated normalized their ALT levels. These investigators also performed an open study in which SNMC was administered six times per week at a dose of 100 mg for 4 wk. At the end of treatment, 20% (3 of 15) of the patients achieved normal ALT levels[34]. Shiota et al[35] demonstrated in mice treated with diethylnitrosamine as a model of hepatocarcinogenesis due to viral hepatitis that AST and albumin values were significantly improved and the occurrence of HCC decreased in the glycyrrhizin group. A long-term prospective randomized trial in humans is actually difficult from both ethical and medical viewpoints. Therefore, Ikeda[29] retrospectively analyzed 346 patients with chronic hepatitis with high alanine transaminase, 244 of whom had received glycyrrhizin injections. Carcinogenesis rates in the treated and untreated groups were 13.3% and 26.0% at the fifth year, and 21.5% and 35.5% at the 10th year, respectively.
URSODEOXYCHOLIC ACID
Ursodeoxycholic acid (UDCA) is a hydrophilic bile acid which has cytoprotective effects not only in chronic cholestatic liver disease, but also in various other liver diseases. The therapeutic properties of UDCA include hypercholeresis, protection of cell membranes by replacing hydrophobic bile acids, and immunomodulation[36-38]. Ljubuncic et al[39] reported that UDCA can act as an antioxidant blocking hydrophobic bile acids which otherwise oxidatively activate Kupffer cells to generate reactive oxygen species in vitro. Mitsuyoshi et al[40] also proposed the antioxidant effect of UDCA in cultured rat hepatocytes. They demonstrated that UDCA increased thiol-containing proteins such as metallothionein, and activated γ-glutamylcysteine synthetase, which regulates glutathione.
A decrease of serum transaminase levels in patients with chronic hepatitis after UDCA administration was first reported from a pilot study by Leuschner et al[41]. The effect was confirmed in a double-blind study by Crosignani et al[42] and Bellentani et al[43], who also established efficacy in long-term treatment. A retrospective study by Tarao et al[44] implied an association of UDCA use with lower incidence of hepatocellular carcinoma in hepatitis C virus-associated liver cirrhosis.
Crosignani et al[42] reported that 250 mg/d of UDCA was effective in improving biochemical markers of liver function, but no further improvement could be attained with higher doses in patients with chronic hepatitis. By contrast, according to a large multicenter randomized controlled dose study of UDCA for chronic hepatitis C, Takano et al[45] suggested that UDCA at a dose of 600 mg/d was optimal.
VITAMIN E (α-TOCOPHEROL)
Vitamin E, an essential lipid-soluble nutrient, is a potent peroxyl radical scavenger that prevents the propagation of free radicals in membranes and in plasma lipoproteins[46]. Vitamin E has been shown to protect against liver damage induced by oxidative stress in animal experiments[47,48]. In 1997, von Herbay et al[49] treated 23 chronic hepatitis C patients refractory to interferon therapy with high doses of vitamin E (2 x 400 IU α-tocopherol/d) for 12 wk. In 11 of these patients, ALT and AST levels improved during treatment. Mahmood et al[50] also suggested that Vitamin E can act as a supportive therapy to protect the liver from damage caused by oxidative stress. In their study, 17 CHC patients, receiving anti-inflammatory drug therapy at least 6 mo prior to Vitamin E administration, were given α-tocopherol 500 mg/d, orally, for a period of 3 mo. The ALT level was lowered in those patients initially with high levels (ALT > 70 IU/L). The thioredoxin (TRX) level was reduced in all patients. Houglum et al[51] showed that treatment of 6 interferon-refractory patients with α-tocopherol (1200 IU/d for 8 wk) decreased the level of carbonyl modification of plasma proteins, a sensitive index of oxidative stress, but it did not significantly affect serum ALT levels, hepatitis C virus titers, or the histologically-determined degree of hepatocellular inflammation or fibrosis. These results suggest that the treatment may need to be prolonged. In 2000, the effect of vitamin E against NASH/NAFLD was first reported in a cohort[52]. Eleven children < 16 years old with NAFLD were prescribed oral vitamin E (400-1200 IU/d for 4-10 mo) with the result that serum ALT, AST and alkaline phosphatase decreased significantly during treatment. However, liver histology was not assessed. In a small, uncontrolled pilot trial, Hasegawa et al[53] demonstrated improvement in fibrosis in 66% of NASH patients who took vitamin E in doses of 300 mg/d for 1 year. In 2003, according to a prospective, double-blind, randomized, placebo-controlled trial with 45 NASH patients, combination vitamin E and C (1000 IU and 1000 mg per day, respectively) was well tolerated and effective in improving fibrosis scores[54]. Nonetheless, no improvement in inflammatory activity or ALT was seen with this combination. Two subsequent studies by Kugelmas et al[55] in 16 NASH patients and Vajro et al[56] in 28 NAFLD children also showed no improvement in ALT levels[55,56]. Dufour et al[57] showed that two years of treatment with UDCA (12-15 mg/kg per day) and vitamin E (400 IU twice a day) improved laboratory values (AST, ALT) and hepatic steatosis of NASH patients compared with UDCA alone or placebo. Sanyal et al[58] concluded that vitamin E therapy was associated with a significantly higher rate of improvement in hepatic steatosis, lobular inflammation, ALT and AST.
SHO-SAIKO-TO (TJ-9, XIAO-CHAI-HU-TANG)
Sho-saiko-to is a traditional Chinese herbal medicine derived from seven species of medicinal plants[59]. Although the mechanism by which Sho-saiko-to protects hepatocytes against liver disease is not fully elucidated, clinical trials have shown its efficacy in patients with chronic hepatitis and liver cirrhosis in Japan, Korea and China. Some plausible actions as an antioxidant have been reported, for example, scavenging free radicals[60,61] and reducing the hepatic level of malondialdehyde, a product of lipid peroxidation[62]. Shiota et al[61] demonstrated that TJ-9 lowered diethylnitrosamine-induced ROS, resulting in reduction of 8-OHdG formation and hepatocarcinogenesis in rats. A double-blind multicenter trial reported an improvement in AST and ALT values in 116 chronic hepatitis patients treated with TJ-9 for 12 wk[63]. Oka et al[64] showed a weak not statistically significant benefit of TJ-9 treatment at a daily dose of 7.5 g and decreased hepatic carcinogenesis rate in a randomized study of patients with cirrhotic chronic liver disease.
CONCLUSION
In CHC cases with persistent inflammation where HCV eradication is difficult, it has been reported that a combination of antioxidant therapies is an effective method to prevent the onset of liver cirrhosis and HCC. In other chronic liver diseases, in particular NASH, it has been demonstrated that antioxidant therapy may potentially be effective for suppressing inflammation and liver fibrosis and expected to prevent carcinogenesis.
Footnotes
Conflict-of-interest: The authors have no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 23, 2014
First decision: September 28, 2014
Article in press: December 29, 2014
P- Reviewer: Hu HP S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
References
- 1.Hayashi H, Takikawa T, Nishimura N, Yano M, Isomura T, Sakamoto N. Improvement of serum aminotransferase levels after phlebotomy in patients with chronic active hepatitis C and excess hepatic iron. Am J Gastroenterol. 1994;89:986–988. [PubMed] [Google Scholar]
- 2.Bassett SE, Di Bisceglie AM, Bacon BR, Sharp RM, Govindarajan S, Hubbard GB, Brasky KM, Lanford RE. Effects of iron loading on pathogenicity in hepatitis C virus-infected chimpanzees. Hepatology. 1999;29:1884–1892. doi: 10.1002/hep.510290623. [DOI] [PubMed] [Google Scholar]
- 3.Kato J, Kobune M, Nakamura T, Kuroiwa G, Takada K, Takimoto R, Sato Y, Fujikawa K, Takahashi M, Takayama T, et al. Normalization of elevated hepatic 8-hydroxy-2’-deoxyguanosine levels in chronic hepatitis C patients by phlebotomy and low iron diet. Cancer Res. 2001;61:8697–8702. [PubMed] [Google Scholar]
- 4.Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287–305. doi: 10.1016/s0891-5849(96)00327-9. [DOI] [PubMed] [Google Scholar]
- 5.Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992;267:166–172. [PubMed] [Google Scholar]
- 6.Floyd RA. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990;11:1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- 7.Benvegnù L, Alberti A. Risk factors and prevention of hepatocellular carcinoma in HCV infection. Dig Dis Sci. 1996;41:49S–55S. doi: 10.1007/BF02087876. [DOI] [PubMed] [Google Scholar]
- 8.Kato J, Kobune M, Kohgo Y, Sugawara N, Hisai H, Nakamura T, Sakamaki S, Sawada N, Niitsu Y. Hepatic iron deprivation prevents spontaneous development of fulminant hepatitis and liver cancer in Long-Evans Cinnamon rats. J Clin Invest. 1996;98:923–929. doi: 10.1172/JCI118875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Liu J, Wen J. Elevation of hydrogen peroxide after spinal cord injury detected by using the Fenton reaction. Free Radic Biol Med. 1999;27:478–482. doi: 10.1016/s0891-5849(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 10.Fenton HJH. Oxidation of tartaric acid in presence of iron. J Chem Soc. 1894;65:899–910. [Google Scholar]
- 11.Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology. 2004;127:S79–S86. doi: 10.1016/j.gastro.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Bomford A, Williams R. Long term results of venesection therapy in idiopathic haemochromatosis. Q J Med. 1976;45:611–623. [PubMed] [Google Scholar]
- 13.Farinati F, Cardin R, De Maria N, Della Libera G, Marafin C, Lecis E, Burra P, Floreani A, Cecchetto A, Naccarato R. Iron storage, lipid peroxidation and glutathione turnover in chronic anti-HCV positive hepatitis. J Hepatol. 1995;22:449–456. doi: 10.1016/0168-8278(95)80108-1. [DOI] [PubMed] [Google Scholar]
- 14.Hirayama M, Kohgo Y, Kondo H, Shintani N, Fujikawa K, Sasaki K, Kato J, Niitsu Y. Regulation of iron metabolism in HepG2 cells: a possible role for cytokines in the hepatic deposition of iron. Hepatology. 1993;18:874–880. doi: 10.1002/hep.1840180420. [DOI] [PubMed] [Google Scholar]
- 15.Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, Furutani T, Sakai A, Okuda M, Hidaka I, et al. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226–238. doi: 10.1053/j.gastro.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Kato J, Miyanishi K, Kobune M, Nakamura T, Takada K, Takimoto R, Kawano Y, Takahashi S, Takahashi M, Sato Y, et al. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42:830–836. doi: 10.1007/s00535-007-2095-z. [DOI] [PubMed] [Google Scholar]
- 17.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 18.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 19.George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311–318. doi: 10.1016/s0016-5085(98)70482-2. [DOI] [PubMed] [Google Scholar]
- 20.Davis RJ, Corvera S, Czech MP. Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. J Biol Chem. 1986;261:8708–8711. [PubMed] [Google Scholar]
- 21.Facchini FS, Hua NW, Stoohs RA. Effect of iron depletion in carbohydrate-intolerant patients with clinical evidence of nonalcoholic fatty liver disease. Gastroenterology. 2002;122:931–939. doi: 10.1053/gast.2002.32403. [DOI] [PubMed] [Google Scholar]
- 22.Riquelme A, Soza A, Nazal L, Martínez G, Kolbach M, Patillo A, Arellano JM, Duarte I, Martínez J, Molgó M, et al. Histological resolution of steatohepatitis after iron depletion. Dig Dis Sci. 2004;49:1012–1015. doi: 10.1023/b:ddas.0000034564.68307.39. [DOI] [PubMed] [Google Scholar]
- 23.Fargion S, Dongiovanni P, Guzzo A, Colombo S, Valenti L, Fracanzani AL. Iron and insulin resistance. Aliment Pharmacol Ther. 2005;22 Suppl 2:61–63. doi: 10.1111/j.1365-2036.2005.02599.x. [DOI] [PubMed] [Google Scholar]
- 24.Sumida Y, Kanemasa K, Fukumoto K, Yoshida N, Sakai K, Nakashima T, Okanoue T. Effect of iron reduction by phlebotomy in Japanese patients with nonalcoholic steatohepatitis: A pilot study. Hepatol Res. 2006;36:315–321. doi: 10.1016/j.hepres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Valenti L, Fracanzani AL, Dongiovanni P, Bugianesi E, Marchesini G, Manzini P, Vanni E, Fargion S. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case-control study. Am J Gastroenterol. 2007;102:1251–1258. doi: 10.1111/j.1572-0241.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 26.Fujita N, Miyachi H, Tanaka H, Takeo M, Nakagawa N, Kobayashi Y, Iwasa M, Watanabe S, Takei Y. Iron overload is associated with hepatic oxidative damage to DNA in nonalcoholic steatohepatitis. Cancer Epidemiol Biomarkers Prev. 2009;18:424–432. doi: 10.1158/1055-9965.EPI-08-0725. [DOI] [PubMed] [Google Scholar]
- 27.Beaton MD, Chakrabarti S, Levstik M, Speechley M, Marotta P, Adams P. Phase II clinical trial of phlebotomy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37:720–729. doi: 10.1111/apt.12255. [DOI] [PubMed] [Google Scholar]
- 28.Valenti L, Fracanzani AL, Dongiovanni P, Rovida S, Rametta R, Fatta E, Pulixi EA, Maggioni M, Fargion S. A randomized trial of iron depletion in patients with nonalcoholic fatty liver disease and hyperferritinemia. World J Gastroenterol. 2014;20:3002–3010. doi: 10.3748/wjg.v20.i11.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda K. Glycyrrhizin injection therapy prevents hepatocellular carcinogenesis in patients with interferon-resistant active chronic hepatitis C. Hepatol Res. 2007;37 Suppl 2:S287–S293. doi: 10.1111/j.1872-034X.2007.00199.x. [DOI] [PubMed] [Google Scholar]
- 30.Kiso Y, Tohkin M, Hikino H, Hattori M, Sakamoto T, Namba T. Mechanism of antihepatotoxic activity of glycyrrhizin. I: Effect on free radical generation and lipid peroxidation. Planta Med. 1984;50:298–302. doi: 10.1055/s-2007-969714. [DOI] [PubMed] [Google Scholar]
- 31.Li JY, Cao HY, Liu P, Cheng GH, Sun MY. Glycyrrhizic acid in the treatment of liver diseases: literature review. Biomed Res Int. 2014;2014:872139. doi: 10.1155/2014/872139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494–1500. doi: 10.1002/(sici)1097-0142(19970415)79:8<1494::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 33.van Rossum TG, Vulto AG, Hop WC, Brouwer JT, Niesters HG, Schalm SW. Intravenous glycyrrhizin for the treatment of chronic hepatitis C: a double-blind, randomized, placebo-controlled phase I/II trial. J Gastroenterol Hepatol. 1999;14:1093–1099. doi: 10.1046/j.1440-1746.1999.02008.x. [DOI] [PubMed] [Google Scholar]
- 34.van Rossum TG, Vulto AG, Hop WC, Schalm SW. Glycyrrhizin-induced reduction of ALT in European patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:2432–2437. doi: 10.1111/j.1572-0241.2001.04049.x. [DOI] [PubMed] [Google Scholar]
- 35.Shiota G, Harada K, Ishida M, Tomie Y, Okubo M, Katayama S, Ito H, Kawasaki H. Inhibition of hepatocellular carcinoma by glycyrrhizin in diethylnitrosamine-treated mice. Carcinogenesis. 1999;20:59–63. doi: 10.1093/carcin/20.1.59. [DOI] [PubMed] [Google Scholar]
- 36.Renner EL, Lake JR, Cragoe EJ, Van Dyke RW, Scharschmidt BF. Ursodeoxycholic acid choleresis: relationship to biliary HCO-3 and effects of Na+-H+ exchange inhibitors. Am J Physiol. 1988;254:G232–G241. doi: 10.1152/ajpgi.1988.254.2.G232. [DOI] [PubMed] [Google Scholar]
- 37.Güldütuna S, Zimmer G, Imhof M, Bhatti S, You T, Leuschner U. Molecular aspects of membrane stabilization by ursodeoxycholate [see comment] Gastroenterology. 1993;104:1736–1744. doi: 10.1016/0016-5085(93)90653-t. [DOI] [PubMed] [Google Scholar]
- 38.Yoshikawa M, Tsujii T, Matsumura K, Yamao J, Matsumura Y, Kubo R, Fukui H, Ishizaka S. Immunomodulatory effects of ursodeoxycholic acid on immune responses. Hepatology. 1992;16:358–364. doi: 10.1002/hep.1840160213. [DOI] [PubMed] [Google Scholar]
- 39.Ljubuncic P, Fuhrman B, Oiknine J, Aviram M, Bomzon A. Effect of deoxycholic acid and ursodeoxycholic acid on lipid peroxidation in cultured macrophages. Gut. 1996;39:475–478. doi: 10.1136/gut.39.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitsuyoshi H, Nakashima T, Sumida Y, Yoh T, Nakajima Y, Ishikawa H, Inaba K, Sakamoto Y, Okanoue T, Kashima K. Ursodeoxycholic acid protects hepatocytes against oxidative injury via induction of antioxidants. Biochem Biophys Res Commun. 1999;263:537–542. doi: 10.1006/bbrc.1999.1403. [DOI] [PubMed] [Google Scholar]
- 41.Leuschner U, Leuschner M, Sieratzki J, Kurtz W, Hübner K. Gallstone dissolution with ursodeoxycholic acid in patients with chronic active hepatitis and two years follow-up. A pilot study. Dig Dis Sci. 1985;30:642–649. doi: 10.1007/BF01308413. [DOI] [PubMed] [Google Scholar]
- 42.Crosignani A, Battezzati PM, Setchell KD, Camisasca M, Bertolini E, Roda A, Zuin M, Podda M. Effects of ursodeoxycholic acid on serum liver enzymes and bile acid metabolism in chronic active hepatitis: a dose-response study. Hepatology. 1991;13:339–344. [PubMed] [Google Scholar]
- 43.Bellentani S, Podda M, Tiribelli C, Callea F, Marazzi M, Sodde M, Merlini R, Batezzati PM, Crosignani A, Zuin M. Ursodiol in the long-term treatment of chronic hepatitis: a double-blind multicenter clinical trial. J Hepatol. 1993;19:459–464. doi: 10.1016/s0168-8278(05)80558-6. [DOI] [PubMed] [Google Scholar]
- 44.Tarao K, Fujiyama S, Ohkawa S, Miyakawa K, Tamai S, Hirokawa S, Masaki T, Tanaka K. Ursodiol use is possibly associated with lower incidence of hepatocellular carcinoma in hepatitis C virus-associated liver cirrhosis. Cancer Epidemiol Biomarkers Prev. 2005;14:164–169. [PubMed] [Google Scholar]
- 45.Takano S, Ito Y, Yokosuka O, Ohto M, Uchiumi K, Hirota K, Omata M. A multicenter randomized controlled dose study of ursodeoxycholic acid for chronic hepatitis C. Hepatology. 1994;20:558–564. [PubMed] [Google Scholar]
- 46.Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000–1013. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Factor VM, Laskowska D, Jensen MR, Woitach JT, Popescu NC, Thorgeirsson SS. Vitamin E reduces chromosomal damage and inhibits hepatic tumor formation in a transgenic mouse model. Proc Natl Acad Sci USA. 2000;97:2196–2201. doi: 10.1073/pnas.040428797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kakizaki S, Takagi H, Fukusato T, Toyoda M, Horiguchi N, Sato K, Takayama H, Nagamine T, Mori M. Effect of alpha-tocopherol on hepatocarcinogenesis in transforming growth factor-alpha (TGF-alpha) transgenic mice treated with diethylnitrosamine. Int J Vitam Nutr Res. 2001;71:261–267. doi: 10.1024/0300-9831.71.5.261. [DOI] [PubMed] [Google Scholar]
- 49.von Herbay A, Stahl W, Niederau C, Sies H. Vitamin E improves the aminotransferase status of patients suffering from viral hepatitis C: a randomized, double-blind, placebo-controlled study. Free Radic Res. 1997;27:599–605. doi: 10.3109/10715769709097863. [DOI] [PubMed] [Google Scholar]
- 50.Mahmood S, Yamada G, Niiyama G, Kawanaka M, Togawa K, Sho M, Ito T, Sasagawa T, Okita M, Nakamura H, et al. Effect of vitamin E on serum aminotransferase and thioredoxin levels in patients with viral hepatitis C. Free Radic Res. 2003;37:781–785. doi: 10.1080/1071576031000102141. [DOI] [PubMed] [Google Scholar]
- 51.Houglum K, Venkataramani A, Lyche K, Chojkier M. A pilot study of the effects of d-alpha-tocopherol on hepatic stellate cell activation in chronic hepatitis C. Gastroenterology. 1997;113:1069–1073. doi: 10.1053/gast.1997.v113.pm9322499. [DOI] [PubMed] [Google Scholar]
- 52.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–738. [PubMed] [Google Scholar]
- 53.Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667–1672. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 54.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 55.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 56.Vajro P, Mandato C, Franzese A, Ciccimarra E, Lucariello S, Savoia M, Capuano G, Migliaro F. Vitamin E treatment in pediatric obesity-related liver disease: a randomized study. J Pediatr Gastroenterol Nutr. 2004;38:48–55. doi: 10.1097/00005176-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, Zala JF, Helbling B, Steuerwald M, Zimmermann A. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–1543. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 58.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morgan TR. Chemoprevention of hepatocellular carcinoma in chronic hepatitis C. Recent Results Cancer Res. 2011;188:85–99. doi: 10.1007/978-3-642-10858-7_7. [DOI] [PubMed] [Google Scholar]
- 60.Sakaguchi S, Tsutsumi E, Yokota K. Preventive effects of a traditional Chinese medicine (sho-saiko-to) against oxygen toxicity and membrane damage during endotoxemia. Biol Pharm Bull. 1993;16:782–786. doi: 10.1248/bpb.16.782. [DOI] [PubMed] [Google Scholar]
- 61.Shiota G, Maeta Y, Mukoyama T, Yanagidani A, Udagawa A, Oyama K, Yashima K, Kishimoto Y, Nakai Y, Miura T, et al. Effects of Sho-Saiko-to on hepatocarcinogenesis and 8-hydroxy-2’-deoxyguanosine formation. Hepatology. 2002;35:1125–1133. doi: 10.1053/jhep.2002.33066. [DOI] [PubMed] [Google Scholar]
- 62.Shimizu I, Ma YR, Mizobuchi Y, Liu F, Miura T, Nakai Y, Yasuda M, Shiba M, Horie T, Amagaya S, et al. Effects of Sho-saiko-to, a Japanese herbal medicine, on hepatic fibrosis in rats. Hepatology. 1999;29:149–160. doi: 10.1002/hep.510290108. [DOI] [PubMed] [Google Scholar]
- 63.Hirayama C, Okumura M, Tanikawa K, Yano M, Mizuta M, Ogawa N. A multicenter randomized controlled clinical trial of Shosaiko-to in chronic active hepatitis. Gastroenterol Jpn. 1989;24:715–719. doi: 10.1007/BF02774173. [DOI] [PubMed] [Google Scholar]
- 64.Oka H, Yamamoto S, Kuroki T, Harihara S, Marumo T, Kim SR, Monna T, Kobayashi K, Tango T. Prospective study of chemoprevention of hepatocellular carcinoma with Sho-saiko-to (TJ-9) Cancer. 1995;76:743–749. doi: 10.1002/1097-0142(19950901)76:5<743::aid-cncr2820760506>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]


